Cha et al. show that Plasmodium GAPDH on the sporozoite surface acts as a ligand for binding Kupffer cell CD68, an interaction that is critical for parasite liver invasion. Thus, Plasmodium GAPDH is a candidate antigen for a prehepatic malaria vaccine.
Abstract
Malaria transmission begins when an infected mosquito delivers Plasmodium sporozoites into the skin. The sporozoite subsequently enters the circulation and infects the liver by preferentially traversing Kupffer cells, a macrophage-like component of the liver sinusoidal lining. By screening a phage display library, we previously identified a peptide designated P39 that binds to CD68 on the surface of Kupffer cells and blocks sporozoite traversal. In this study, we show that the P39 peptide is a structural mimic of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) on the sporozoite surface and that GAPDH directly interacts with CD68 on the Kupffer cell surface. Importantly, an anti-P39 antibody significantly inhibits sporozoite liver invasion without cross-reacting with mammalian GAPDH. Therefore, Plasmodium-specific GAPDH epitopes may provide novel antigens for the development of a prehepatic vaccine.
INTRODUCTION
Malaria, caused by parasites of the genus Plasmodium, is among the most devastating parasitic diseases worldwide. Efforts to control malaria have been hampered by the lack of an effective vaccine, by the constant resurgence of drug-resistant parasites, and by the development of insecticide resistance by mosquito vectors (WHO, 2016). The bite of an infected Anopheles spp. mosquito releases <100 sporozoites in the skin (Medica and Sinnis, 2005; Kebaier et al., 2009), but after liver infection, up to 10,000 merozoites per sporozoite are released into the circulation (Sturm et al., 2006). Therefore, the prehepatic stages represent a severe bottleneck in parasite numbers and constitute a prime target for induction of sterile immunity. Sporozoites released by a mosquito migrate in the skin to find a blood vessel and enter the circulation. To continue its cycle, the sporozoite must egress in the liver. What are the cues used by the sporozoite to leave the liver? Circulating sporozoites bind to the sinusoidal wall via interaction between the parasite surface circumsporozoite protein (CSP) and heparan sulfate proteoglycans produced by liver stellate cells (Frevert et al., 1993; Cerami et al., 1994; Coppi et al., 2007). To infect hepatocytes, sporozoites must exit the sinusoids by traversing Kupffer cells surrounded by a parasitophorous vacuole (Danforth et al., 1980; Meis et al., 1983; Vreden, 1994; Pradel and Frevert, 2001) or via endothelial cells by breaching their cell membrane (Tavares et al., 2013). Each infected hepatocyte generates thousands of merozoites that are released into the circulation and initiate the blood cycle that is responsible for disease symptoms (Sturm et al., 2006).
Research to elucidate how Plasmodium sporozoites navigate from the mosquito to their final destination in the liver has identified several antigens that are potential candidates for a prehepatic vaccine (Draper et al., 2015). In addition, attenuated live sporozoites have also been tested as vaccine candidates (Richie et al., 2015). Although most candidates were shown to be ineffective in phase II a/b efficacy trials, additional candidate parasite proteins are under active development or in clinical trials (Draper et al., 2015). RTS,S/AS01, a vaccine using Plasmodium falciparum CSP antigens, is the most advanced preerythrocytic vaccine candidate (Tran et al., 2015; WHO, 2016). It affords up to 50% protection for clinical malaria in older children but only ∼30% in infants and no significant protection from severe malaria at 18 mo after vaccination (Tran et al., 2015). Whole-sporozoite immunization approaches such as radiation-attenuated sporozoites have shown promising results (Seder et al., 2013; Richie et al., 2015), but many hurdles remain to be resolved (Richie et al., 2015; Tran et al., 2015). Understanding the mechanisms of parasite liver invasion may provide crucial insights for the development of additional prehepatic vaccine antigens. Recently, Yilmaz et al. (2014) showed that an antibody against the α-Gal glycan on sporozoite surface proteins inhibits the parasite life cycle in its mammalian host. However, the anti–α-Gal antibody was effective only for the skin stages, and once sporozoites entered the blood circulation, no protection was observed (Yilmaz et al., 2014). In addition, Kaushansky et al. (2015) identified EphA2 as a hepatocyte receptor for sporozoite invasion. A novel vaccine candidate targeting the parasite ligand for EphA2 recognition is eagerly awaited.
Our study focuses on mechanisms of sporozoite exit from the liver circulation via traversal of Kupffer cells (Danforth et al., 1980; Meis et al., 1983; Vreden, 1994; Frevert et al., 2005), a macrophage-type cell that lines the liver blood vessels (sinusoids). Using a phage display peptide library, we identified three peptides that bind to Kupffer cells and strongly inhibit sporozoite–Kupffer cell interactions (Cha et al., 2015). The peptides bind to CD68 on the Kupffer cell surface and, by doing so, inhibit sporozoite traversal. CD68 is a surface marker specific for phagocytic macrophages (Kinoshita et al., 2010). Knockout of the mouse CD68 gene reduced sporozoite liver invasion by >70%. Here, we show that antibodies against these Kupffer cell–binding peptides recognize GAPDH on the sporozoite surface. Furthermore, we show that Plasmodium sporozoite GAPDH is a ligand of CD68 and that this interaction is critical for Kupffer cell traversal and liver infection. GAPDH from other pathogens has been shown to serve as a ligand for host cell recognition and therefore has been assessed as a vaccine candidate (Perez-Casal and Potter, 2016). Our experiments suggest that Plasmodium GAPDH may also be developed into a prehepatic malaria vaccine antigen.
RESULTS
Antibodies against mimotope peptides recognize a putative sporozoite ligand protein and significantly inhibit sporozoite Kupffer cell entry
Previously, we reported that a phage display library screen led to the identification of three peptides, P39, P61, and P52, that not only bind to Kupffer cells but also, importantly, inhibit sporozoite entry. Given that P39 binds to the Kupffer cell surface protein CD68 (Cha et al., 2015), we hypothesized that P39 is a structural mimic (mimotope) of a sporozoite ligand to the CD68 receptor and that inhibition of sporozoite Kupffer cell entry occurs by competition between P39 and a sporozoite CD68 ligand. To test this hypothesis, we generated antibodies against each of the three phages displaying the peptides, as well as against the P39 peptide itself. Western blot assays revealed that all candidate antibodies recognize an ∼40 kD Plasmodium berghei sporozoite protein band (Fig. 1 A, left). Moreover, immunofluorescence assays indicated that every candidate antibody recognizes a sporozoite surface protein (Fig. 1 A, right). The P39 antibody appears to recognize a conserved protein, as it recognizes an ∼40 kD surface protein from both the human P. falciparum and rodent P. berghei parasites (Fig. 1 B). All immunofluorescence assays were done with freshly isolated sporozoites without permeabilization or freeze/thawing. The antibodies stained sporozoites with the same punctuated pattern. Importantly, binding of the antimimotope antibodies to the sporozoite surface significantly inhibited sporozoite entry into CD68+/+ macrophages, whereas no inhibition of sporozoite entry into CD68−/− macrophages was observed (Fig. 2). These results are consistent with the hypothesis that the antibodies bind to the ligand that the parasite uses for CD68 recognition. Moreover, more sporozoites preincubated with antimimotope antibodies remained attached to the outside of the cell (Fig. 2 B, right), possibly a consequence of the inability of sporozoites to enter the cell. This result also suggests that the antimimotope antibodies do not interfere with sporozoite attachment to their host cells. Additional experiments confirmed that the anti-P39 antibody strongly inhibits P. berghei and P. falciparum sporozoite traversal of rodent and human macrophages (Fig. 3, A and B). In separate experiments, we verified that inhibition of sporozoite macrophage entry by the anti-P39 antibody is not caused by inhibition of sporozoite movement. Only anti-CSP antibody significantly inhibited sporozoite movement (Fig. 3, C and D), which explains complete inhibition of P. falciparum sporozoite macrophage traversal by anti-CSP antibody (Fig. 3 B) and inhibition of empty Transwell traversal (Fig. 3 D).
Figure 1.
Antibodies against Kupffer cell–binding peptides recognize a conserved protein on the sporozoite surface. (A, left) Western blotting assays show that antibodies against the three recombinant phages recognize an ∼40-kD P. berghei sporozoite protein band (arrowheads). Antibodies against recombinant phages (Ø) displaying the P39, P61, and P52 peptides were tested with blots of enriched salivary gland P. berghei (Pb) sporozoite lysates. Mock lysates of salivary gland preparations from uninfected mosquitoes served as controls (m). An anti-CSP antibody served as a positive control. Preimmune serum (PIS) and antibodies against wild-type (Wt) phages served as negative controls. The left two lanes show a Coomassie blue–stained gel of the same preparations that were run in the remaining lanes. (Right) Immunofluorescence assays show that the antibodies against recombinant phages bind to the surface of P. berghei sporozoites. Nonpermeabilized P. berghei sporozoites were incubated with antisera raised against selected phages (Ø) or with an anti-CSP antibody as a positive control. No antibody (Ab) or anti–wild-type phage antibody (α-ØWt) served as negative controls. Differential interference contrast (DIC) images superimposed with DAPI nuclear staining (blue) are also shown. (B, left) Western blotting assays show that an anti-P39 peptide antibody recognizes an ∼40-kD protein band (arrowhead) from P. berghei as well as P. falciparum (Pf) sporozoite lysates. An anti-P39 antibody was tested with blots of enriched salivary gland P. berghei or P. falciparum sporozoite lysates or lysates of mock salivary gland preparations (m). An anti-CSP antibody and an anti-KLH antibody served as positive and negative controls, respectively. Incubation with an antiactin antibody served as a loading control. (Right) Immunofluorescence assays show that the anti-P39 antibody binds to the surface of nonpermeabilized P. berghei and P. falciparum sporozoites. An anti-CSP antibody and an anti-KLH antibody served as positive and negative controls, respectively. (A and B) Western blotting and immunofluorescence assays represent two or more independent experiments. Bars, 10 µm.
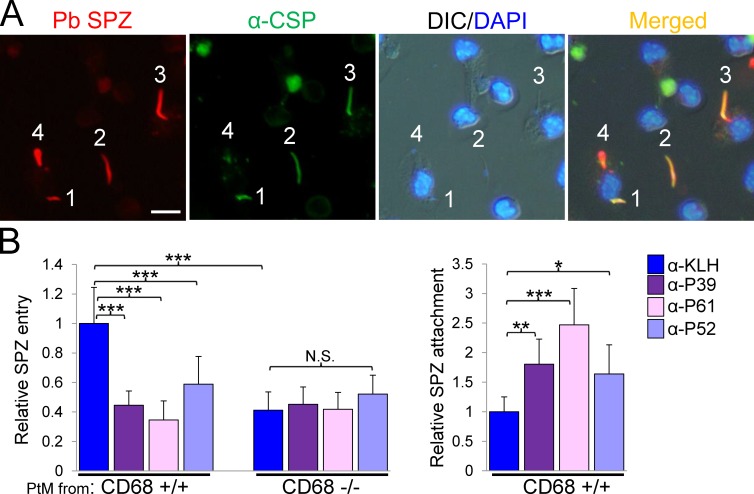
Figure 2.
The antimimotope antibodies inhibit sporozoite entry but not attachment of CD68+/+ Kupffer cells. (A) Immunofluorescence assay using anti-CSP antibody differentially labels extra versus intracellular sporozoites (SPZ). P. berghei (Pb) sporozoites expressing tdTomato (red; Graewe et al., 2009) were incubated with a primary mouse peritoneal macrophage culture and then fixed with paraformaldehyde. Anti-CSP antibody labeled only extracellular sporozoites with Alexa Fluor 488–conjugated secondary antibody (green): extracellular sporozoites 1 and 2 appear orange when merged. Sporozoite 3 is entering the cell and appears partially orange (top) and partially red (bottom tip). Sporozoite 4 is inside the host cell and appears completely red. The immunofluorescence assay represents two or more independent experiments. Bar, 10 µm. DIC, differential interference contrast. (B, left) Antimimotope antibodies (indicated to the right) inhibit sporozoite entry into CD68+/+ macrophages but not into CD68−/− cells. Relative sporozoite entry into primary peritoneal macrophages (PtM) isolated from either CD68+/+ or CD68−/− mice is shown. (Right) Inhibition of sporozoite entry by antimimotope antibodies resulted in increased numbers of sporozoites attached to wild-type mouse peritoneal macrophages. The data from two independent experiments were combined. P-values were calculated using Mann-Whitney U tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Error bars indicate standard deviation.
Figure 3.
The anti-P39 antibody inhibits sporozoite Kupffer cell traversal and does not affect sporozoite movement. (A) Schematic diagram of the sporozoite neutralization assay using Transwells (Corning) with 5-µm pore supports (estimated sporozoite diameter: 1 µm). (B) Quantification of sporozoite (spz) traversal using the assay illustrated in (A). A total of 4 × 105 sporozoites per well were preincubated with the indicated antibody and transferred to Transwell supports. After incubation for 4 h at 37°C, the number of P. berghei (left) and P. falciparum (right) sporozoites that traversed Transwell supports covered with Kupffer cells (KC; left) or differentiated (PMA treated) THP1 human macrophages (right) was determined. Data from two independent experiments were pooled, and each group represents counts of three wells. The percent inhibition relative to the anti-KLH antibody (Ab)–treated group is indicated at the bottom of each panel. (C) P. berghei sporozoites were incubated for 30 min at 4°C in buffer or 0.2 mg/ml anti-KLH, anti-P39, or anti-CSP antibody and then transferred into 8-well chamber slides. After 30-min incubation at 37°C in RPMI medium with 10% FBS, sporozoites were fixed, and their movement was detected by labeling the CSP trails with an anti-CSP antibody (top). The immunofluorescence assay represents two independent experiments. Alternatively, sporozoite moving distances were measured at 2-min intervals (bottom). Sporozoite positions at 0 min are shown in white, at 2 min in yellow, and at 4 min in green. Resting sporozoites appear orange after merge. White lines connect the positions of each sporozoite at the different time points. The calculated sporozoite movement for each experimental group is summarized on the right. n = moving sporozoite count/total sporozoite count. Data from two independent experiments were combined. No, no antibody added. (D) Antibody-treated P. berghei sporozoites as described in C transferred to Transwell supports with 3-µm pores (arrow) and 4 × 104 sporozoites per well. The Transwell supports were not covered by cells. After incubation for 2 h at 37°C, the number of P. berghei sporozoites that traversed the Transwell supports was determined. Data from two independent experiments were combined, and each group represents counts of six wells. The percent inhibition relative to the buffer-treated group is indicated at the bottom. (Right) CSP trails on the bottom of the Transwell support by sporozoites preincubated with anti-P39 antibody. The immunofluorescence assay represents two independent experiments. (B–D) P-values were calculated using Mann-Whitney U tests. *, P < 0.05; **, P < 0.01. Bars, 10 µm.
The anti-P39 antibody recognizes Plasmodium GAPDH, a protein that interacts with CD68
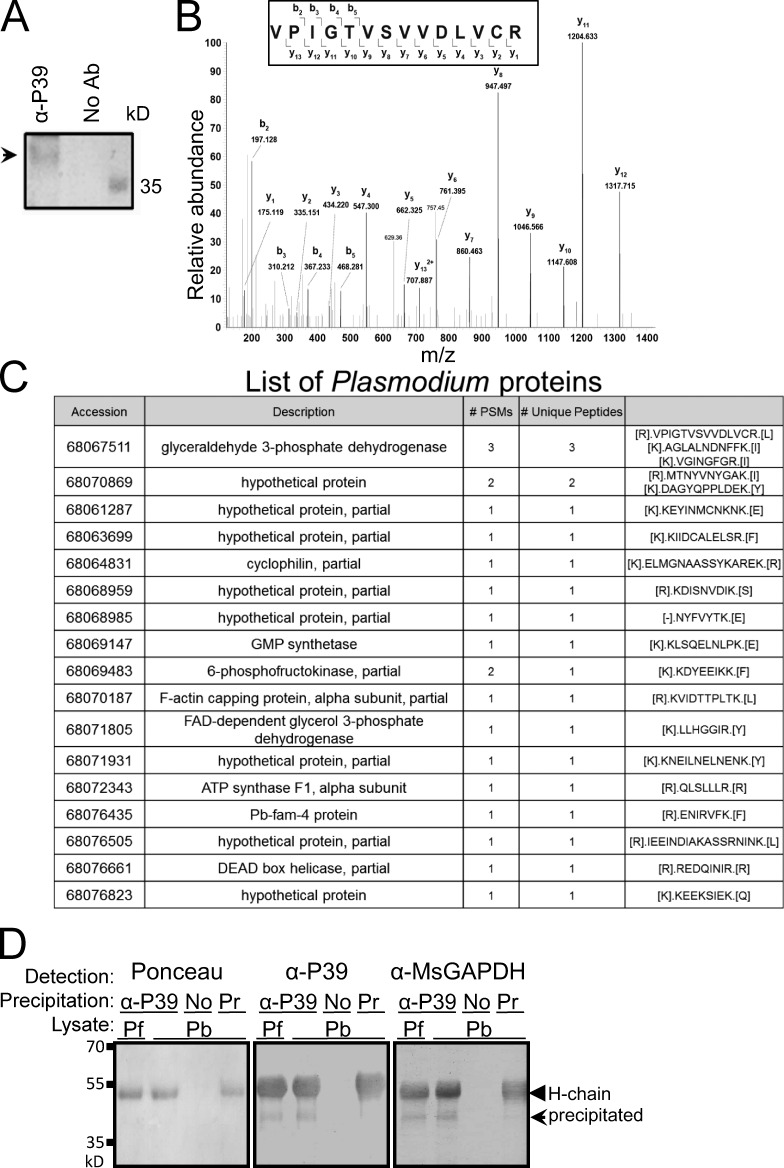
We used the anti-P39 antibody to precipitate an ∼40 kD putative sporozoite ligand protein (Fig. 4 A), and mass spectrometry (MS) determined that P. berghei GAPDH (PbGAPDH) is the likely protein recognized by the antibody (Fig. 4, B and C), with three unique peptide hits including VPIGTVSVVDLVCR. The three peptides were 100% identical to PbGAPDH amino acids 5–14, 237–252, and 301–313 but only partially identical to Anopheles gambiae GAPDH (90%, 68.8%, and 53%, respectively). Western blotting assays confirmed that the P. falciparum and P. berghei sporozoite proteins immunoprecipitated by the anti-P39 antibody were recognized by anti-GAPDH antibody (Fig. 4 D). To characterize PbGAPDH as a putative sporozoite ligand, we produced recombinant PbGAPDH protein using the pET32b expression system. The empty tag protein of the pET32b expression system was used as a negative control. The recombinant PbGAPDH was recognized by an anti–mouse GAPDH antibody and by the three antimimotope peptide antibodies (Fig. 5 A). Control anti-KLH and anti–wild type phage antibodies did not bind. Far-Western blotting assays using recombinant CD68 showed that PbGAPDH and CD68 directly interact (Fig. 5 A, right). Direct molecular interaction between PbGAPDH and CD68 was further confirmed by ELISA and pull-down assays. Membrane proteins from Cos7 cells engineered to express or not express CD68 on their surface were incubated with recombinant PbGAPDH or control tag protein that had been attached to wells of an ELISA plate. Only proteins from CD68-expreessing cells bound to PbGAPDH (Fig. 5 B). In addition, recombinant CD68 bound to protein A beads via an anti-CD68 antibody was incubated with either PbGAPDH or control tag protein. PbGAPDH and not control tag protein bound to the CD68–protein A beads (Fig. 5 C). In additional tests, PbGAPDH bound to CD68+/+ macrophages but not to CD68−/− macrophages as measured by both immunofluorescence and flow cytometry assays (Fig. 5 D). Moreover, recombinant CD68 bound to the surface of sporozoites (Cha et al., 2015), and this binding was competitively inhibited by recombinant PbGAPDH but not by control tag protein (Fig. 5 E).
Figure 4.
The P39 peptide is a mimotope of the Plasmodium GAPDH protein. (A) A P. berghei sporozoite lysate was incubated with either anti-P39 antibody (Ab) or, as a control, no antibody (protein A–agarose beads only). Sporozoite proteins precipitated with the anti-P39 antibody were fractionated by SDS-PAGE and stained with Coomassie blue. The ∼40-kD sporozoite protein band (same size as in Fig. 1) was excised for MS. Arrowhead, precipitated sporozoite protein. (B) MS/MS spectrum of the VPIGTVSVVDLVCR peptide that was assigned to GAPDH. The cysteine in the peptide sequence was alkylated. m/z, mass-to-charge ratio: in MS, the ratio of an ion's mass (m) in atomic mass units to its formal charge (z). (C) A full list of proteins identified from P. berghei along with the number of peptide-spectrum matches (PSMs) and unique peptides. GAPDH was identified with three unique peptides. FAD, flavin adenine dinucleotide; GMP, granulocyte-macrophage progenitor. (D) Western blot assays of the P. falciparum (Pf) or P. berghei (Pb) sporozoite proteins immunoprecipitated by the anti-P39 antibody (arrow) were tested for anti–mouse GAPDH (MsGAPDH) antibody recognition. No antibody (protein A agarose beads only) or IgG purified from preimmune sera (Pr) were used as negative immunoprecipitation controls. H-chain, IgG heavy chain (arrowhead). (A and D) Western blotting assays represent two independent experiments.
Figure 5.
Plasmodium GAPDH interacts with CD68. (A) Recombinant PbGAPDH was produced using the pET32b (pET) expression system. Purified recombinant PbGAPDH and empty pET32b tag protein (negative control) were fractionated by gel electrophoresis and transferred to membranes. The stained gel shows the 56-kD recombinant PbGAPDH (fused to the pET32b tag) and the 19-kD pET32b empty tag protein. Antibodies against mouse GAPDH (Ms GAPDH), against the P39 peptide, and against recombinant phages 61 and 52 (compare with Fig. 1) all recognized the recombinant PbGAPDH but not the control tag protein. Recombinant CD68 protein from transfected 293T cells (Cha et al., 2015) also bound to PbGAPDH and not to the control tag protein, suggesting direct interaction between the two proteins. Binding of CD68 to the PbGAPDH protein was visualized with an anti-CD68 antibody. Control anti-KLH antibody did not bind. The Western blotting assay represents two independent experiments. Arrows: (top) PbGAPDH; (bottom) pET. (B) Recombinant PbGAPDH or pET32b tag protein bound to an ELISA plate were incubated with membrane proteins from Cos7 cells engineered to express rat CD68 on their surface (CD68-Cos7) or from nonengineered cells (negative control [Cont]). Binding of CD68 to the proteins attached to the wells was detected with an anti–rat CD68 antibody. Error bars indicate standard deviation. Data from two independent experiments were pooled. The p-value was calculated using a Mann-Whitney U test. *, P < 0.05. (C) Recombinant rat CD68 was bound to protein A beads using an anti–rat CD68 antibody. These beads were incubated with either PbGAPDH or pET32b tag protein and washed, and the bound protein was eluted by boiling in Laemmli buffer. After gel electrophoresis, proteins were detected by Western blotting using an anti-thioredoxin (Trx) tag antibody. (Left) Input proteins (either recombinant PbGAPDH or pET32b tag protein). (Right) Bound proteins. The box at the bottom shows CD68 protein detected with an anti-CD68 antibody. The Western blotting assay represents two independent experiments. (D) Primary cultures of peritoneal macrophages (PtM) from wild-type mice (+/+) or from CD68 KO mice (−/−) were incubated with recombinant PbGAPDH. Binding of PbGAPDH was visualized with an anti–His tag antibody in immunofluorescence (middle) and flow cytometry (bottom) assays. Differential interference contrast (DIC) images superimposed with DAPI nuclear staining (blue) are also shown (top). Flow cytometry data illustrate an experiment that yielded similar results to a repeat experiment. GAP, PbGAPDH. Bar, 20 µm. (E) P. berghei sporozoites were incubated with the supernatant of cultured 293T cells engineered to secrete the CD68 protein. Binding of CD68 to sporozoites was competed either with pET32 tag protein (negative control) or with recombinant PbGAPDH. Nonengineered 293T cell culture supernatant served as a negative control. CD68 binding to the P. berghei sporozoite surface was visualized with an anti-CD68 antibody in immunofluorescence assays (middle) and flow cytometry assays (bottom). Flow cytometry data represent two independent experiments with similar results. Differential interference contrast images superimposed with DAPI nuclear staining (blue) are also shown (top). CD68 binding to the sporozoite surface was competitively inhibited by PbGAPDH but not by the pET32b tag protein. Cont Sup, supernatant from untransfected 293T cell culture. (D and E) Immunofluorescence and flow cytometry assays represent two independent experiments. Bars, 10 µm.
GAPDH on the surface of Plasmodium sporozoites plays important roles in liver infection
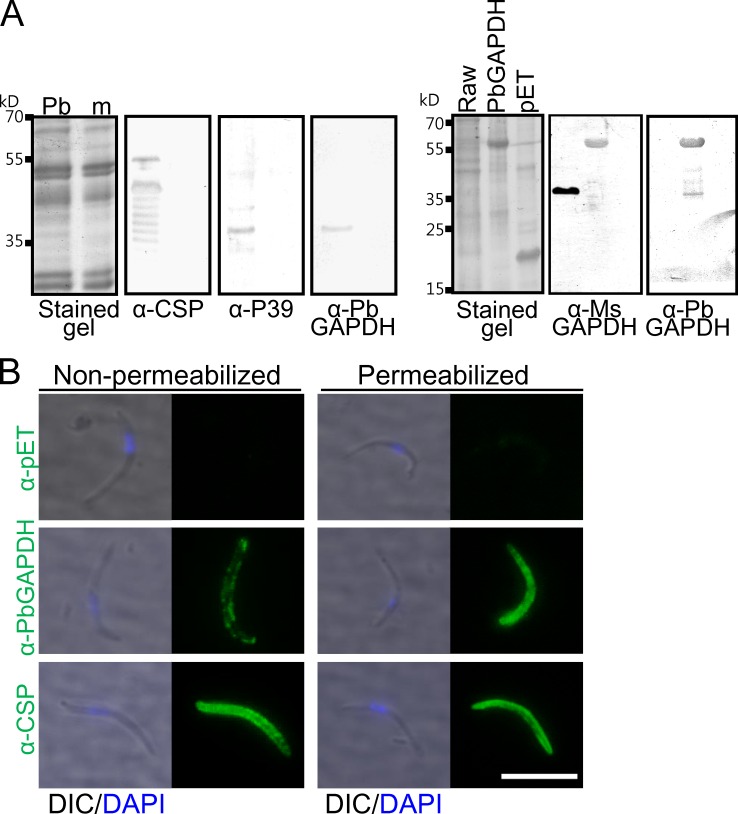
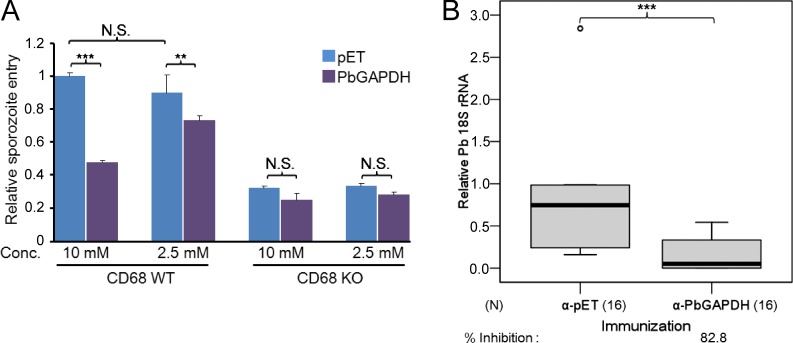
To test the role played by PbGAPDH on the sporozoite surface, we generated an anti-PbGAPDH antibody by immunizing mice with recombinant PbGAPDH. Western blotting assays showed that the anti-PbGAPDH antibody specifically recognizes PbGAPDH but not mouse GAPDH (Fig. 6 A). Additional assays supported the notion that PbGAPDH is a sporozoite ligand for liver infection. First, immunofluorescence assays provided evidence that GAPDH is on the sporozoite surface. Anti-PbGAPDH antibody detected the protein on nonpermeabilized sporozoites, and as expected, the intensity of the signal increased greatly after permeabilization. In contrast, the intensity of the anti-CSP signal was similar before and after sporozoite permeabilization (Fig. 6 B). Second, in an in vitro assay, recombinant PbGAPDH competitively inhibited sporozoite entry into CD68+/+ but not CD68−/− macrophages in a dose-dependent manner, presumably by interfering with PbGAPDH–CD68 interactions (Fig. 7 A). Third, liver infection of mice passively immunized with an anti-PbGAPDH antibody was reduced by ∼83% relative to the group treated with an unrelated antitag protein antibody control (Fig. 7 B). Because the anti-PbGAPDH antibody does not cross-react with mouse GAPDH, the 83% reduction of parasite liver invasion can be attributed to the neutralization of sporozoite surface GAPDH by the anti-PbGAPDH antibodies. In these experiments, parasite liver burden in 3 out of 16 mice passively immunized with anti-PbGAPDH antibody was not detectable with a quantitative RT-PCR assay, whereas all mice immunized with the control antibody were positive. Protection was not absolute, as mice passively immunized with the anti-PbGAPDH antibody developed blood-stage parasite infection (unpublished data).
Figure 6.
Anti-PbGAPDH antibody detects GAPDH on the sporozoite surface. (A, left) Anti-PbGAPDH antibody specifically detects Plasmodium GAPDH and does not cross react with mouse (m) GAPDH. Western blot assays with sporozoite lysate show that the anti-PbGAPDH antibody recognizes the same molecular weight band as does the anti-P39 antibody. (Right) The anti-PbGAPDH antibody does not cross-react with mouse GAPDH when tested with a Raw cell (a mouse macrophage cell line) lysate. The Western blotting assay represents two independent experiments. α-Ms GAPDH, anti-mouse GAPDH antibody. pET, pET32b. (B) Nonpermeabilized or permeabilized P. berghei sporozoites, as indicated, were incubated with an anti-PbGAPDH or an anti-CSP antibody. An anti-pET32b tag antibody served as a negative control. Whereas both antibodies bound to the sporozoite surface, permeabilization significantly increased the intensity of the anti-PbGAPDH but not of the anti-CSP signal. Differential interference contrast (DIC) images superimposed with DAPI nuclear staining (blue) are also shown. The immunofluorescence assay represents two independent experiments. Bar, 10 µm.
Figure 7.
PbGAPDH plays a critical role in P. berghei sporozoite entry into macrophages in vitro and in mouse liver invasion in vivo. (A) Primary cultures of peritoneal macrophages from wild-type mice or from CD68 KO mice were incubated in 8-well chamber slides with 2 × 104 P. berghei sporozoites per well for 1 h in the presence of 10 mM or 2.5 mM recombinant PbGAPDH or pET32 (pET) tag protein. Data are pooled from two independent experiments. Sporozoite entry of KO macrophages (no surface CD68) was less efficient than of WT macrophages. Error bars indicate standard deviation. Conc., concentration. (B) Mice were passively immunized by injection of 0.2 mg anti-pET tag or anti-PbGAPDH antibodies. After 1 d, each mouse was challenged by intravenous injection of 2 × 103 P. berghei sporozoites. Relative parasite liver burden was determined as previously described (Cha et al., 2015). Data are pooled from three independent experiments. N, number of mice assayed. Percent inhibition relative to the anti-pET32b tag antibody–treated group is provided at the bottom. (A and B) P-values were calculated using Mann-Whitney U tests. **, P < 0.01; ***, P < 0.001.
A Plasmodium GAPDH mimotope for CD68 binding is a prehepatic malaria vaccine candidate
Next, we tested the hypothesis that P39 mimics a sporozoite PbGAPDH epitope involved in CD68 recognition. For this test, we immunized mice with the P39 peptide conjugated to the KLH carrier protein. Fig. 8 A shows that the anti-P39 antibody recognizes PbGAPDH and does not cross-react with mouse or human GAPDH, suggesting that the PbGAPDH epitope involved in CD68 recognition is parasite specific. Next, we challenged P39-immunized mice by intravenous sporozoite injection or by the bite of infected mosquitoes (Fig. 8 B). A total of 87.5% (seven mice out of eight challenged) of P39-immunized mice were protected when challenged with intravenous injection of 100 P. berghei sporozoites (similar to the number of sporozoites deposited in the skin by an infected mosquito; Medica and Sinnis, 2005; Kebaier et al., 2009). Moreover, 80% (four mice out of five challenged) were protected when challenged by mosquito bites. When challenged with 1,000 sporozoites, protection was 22.2%. Our finding strongly implies that the Plasmodium GAPDH epitope that P39 mimics may serve as a vaccine candidate for protective immunization against malaria liver infection. The GAPDH sequences are well conserved among Plasmodium species when compared with mammalian GAPDH (Fig. 9). Multiple sequence alignment shows candidate parasite-specific peptides (Fig. 9, red boxes) that were highly conserved (identity >87%) among Plasmodium species and divergent (identity <63%) from mammalian GAPDH (Fig. 9 A). GAPDH is an essential housekeeping gene and therefore is not expected to have strong antigenic variation. 204 P. falciparum field isolates show only seven nonsynonymous single nucleotide polymorphisms (SNPs; Fig. 9 A). The Q140K allele has a 43.1% frequency. However, the other six SNPs occurred with a frequency of <1% among the 204 field isolates. The phylogenetic tree suggests that Plasmodium GAPDH may serve as a suitable vaccine candidate for prevention of malaria liver infection (Fig. 9 B).
Figure 8.
An anti-P39 antibody binds to a Plasmodium-specific GAPDH epitopes and protects mice from sporozoite liver invasion. (A) Raw cell (mouse macrophage cell line) lysate, HepG2 cell (a human hepatoma cell line) lysate, and recombinant PbGAPDH protein were fractionated by gel electrophoresis (left) and transferred to blots. An anti–mouse GAPDH antibody (α-Ms GAPDH; middle) recognized mouse and human GAPDH (arrowhead) as well as PbGAPDH (arrow). However, an anti-P39 antibody (right) recognized only PbGAPDH but not mouse or human GAPDH. The Western blotting assay represents two independent experiments. (B) Mice were challenged 7 d after the third immunization boost (KLH or KLH-P39). Challenge was either by intravenous injection of the indicated number of sporozoites (spz) or by the bite of a single-infected mosquito. The numbers in each cell indicate the number of infected mice/the number challenged. Protected mice were those that had no detectable parasites by day 28 after challenge. The two experiments were performed at different times with different sporozoite preparations and cannot be directly compared. Percent protection is denoted at the bottom.
Figure 9.
Polymorphism of the GAPDH gene. (A) Multiple sequence alignment of GAPDH among Plasmodium species (P) and pairwise alignment with mammalian (human) GAPDH (M) were compared. Red boxes indicate peptides that are well conserved among Plasmodium species (identity >87%) and divergent from mammalian GAPDH (identity <63%). Nonsynonymous SNPs detected in 204 field isolates of P. falciparum (from PlasmoDB) are shown in red above the alignment. (B) Rooted phylogenetic tree of GAPDH sequence among species. Branch length shows relative evolutionary distance of GAPDH among species. The percent identity with P. falciparum GAPDH sequence is shown in parenthesis.
DISCUSSION
Whereas GAPDH plays a key role in glycolysis, evidence suggests that it also can serve multiple other functions (Pancholi and Fischetti, 1992, 1997; Alvarez-Dominguez et al., 1997; Daubenberger et al., 2003; Bergmann et al., 2004; Boël et al., 2005; Terao et al., 2006; Jin et al., 2011). In particular, GAPDH has been implicated as a ligand for cell invasion present on the surface of several pathogenic microbes and has been tested as a vaccine candidate (Gil et al., 1999; Argiro et al., 2000; Rosinha et al., 2002; Lama et al., 2009; Perez-Casal and Potter, 2016). Recent studies have identified surface expression of GAPDH on the parasite in multiple stages of the Plasmodium life cycle (Lindner et al., 2013; Sangolgi et al., 2016). We confirmed expression of Plasmodium GAPDH on the sporozoite surface and show that interfering with its interaction with the Kupffer cell CD68 receptor confers protection from malaria sporozoite challenge. Thus, interference of parasite–Kupffer cell recognition may be used as an alternate strategy for the development of a preerythrocytic vaccine. This approach could be used to complement and improve the efficacy of the existing (RTS,S) vaccine candidate presently in stage III trials (Tran et al., 2015).
Our results have shown that PbGAPDH plays vital roles in sporozoite macrophage entry both in vitro, with dose-dependent competition assay (Fig. 7 A), and in vivo, with passive immunization assays using anti-PbGAPDH antibody (Fig. 7 B). These findings strengthen our previous results suggesting that CD68-dependent sporozoite liver invasion is a major pathway of malaria infection. However, the anti-PbGAPDH antibody could not confer sterile protection. Only 3 mice out of 16 challenged showed undetectable parasite liver burden (Fig. 7 B), and no protection was found using the criterion of blood-stage parasite development. It is possible that PbGAPDH has poor immunogenicity because of a highly conserved amino acid sequence (64% identity with the mouse protein). Another possibility is that the PbGAPDH epitope for CD68 recognition is not an immune-dominant antigen. In addition, the sporozoite may have alternate routes for exit from the sinusoid such as traversal of endothelial cells (Tavares et al., 2013) or via as yet unidentified ligand–receptor combinations other than GAPDH–CD68. Nonetheless, immunization with P39 peptide elicited ≥80% sterile protection when challenged with low parasite numbers, similar to natural numbers delivered by a mosquito bite (Medica and Sinnis, 2005; Kebaier et al., 2009).
As proposed by Perez-Casal and Potter (2016), protective GAPDH epitopes unique to the pathogen protein must be identified. The initial phage library screen identified three 12–amino acid peptides that competitively inhibit Plasmodium sporozoite Kupffer cell entry (Cha et al., 2015). These peptides may mimic small moieties of the Plasmodium GAPDH protein important for CD68 recognition. As shown in Fig. 2 B, antimimotope antibodies only inhibited sporozoite entry into the CD68+/+ macrophages and had no effect on sporozoite entry of macrophages lacking CD68. The Transwell assays using an anti-P39 antibody (Fig. 3) and the mouse immunization experiment (Fig. 8) further support this proposition. The finding that the anti-P39 antibody does not cross-react with mammalian GAPDHs (Figs. 6 A and 8 A) suggests that parasite-specific GAPDH epitopes for CD68 interaction may be developed for generating protective immunization. Whereas each of the three peptides has a unique amino acid sequence, they all appear to mimic GAPDH (Figs. 1 and 5), suggesting either that the conformation of the three peptides mimics a common sporozoite GAPDH epitope or, alternatively, that they mimic different epitopes of the GAPDH protein. We have shown that P39 can provide protective immunity against Plasmodium liver invasion (Fig. 8 B). If P61 and/or P52 mimic GAPDH epitopes different from P39, they would constitute additional epitopes candidates that may act additively or synergistically in enhancing protective efficacy.
A previous study suggested a Plasmodium sporozoite is taken up in a normal phagocytic way by the Kupffer cells based on the ultrastructure of a phagocytic vacuole containing a sporozoite and the surrounding microfilaments (Meis et al., 1985). A further study has shown that Plasmodium sporozoites traverse Kupffer cells within a vacuole without fusing with lysosomes and exit the macrophages unharmed (Pradel and Frevert, 2001). We previously suggested that sporozoite liver invasion is mainly mediated by the active CD68 transport pathway in phagocytic Kupffer cells (Cha et al., 2015). Here, we identified that Plasmodium surface GAPDH plays an important role for interaction with the Kupffer cell sporozoite receptor CD68. Interestingly, Plasmodium GAPDH has been suggested to play an important role for membrane fusion and trafficking (Daubenberger et al., 2003). Interaction between Plasmodium GAPDH and Kupffer cell CD68 may facilitate safe Kupffer cell traversal of the Plasmodium sporozoite within a vacuole. Furthermore, surface GAPDH localization in Plasmodium blood-stage forms was reported by Daubenberger et al. (2003), of which immunofluorescence assays showed concentrated GAPDH localization at the apical end of merozoites. If Plasmodium surface GAPDH plays important roles for host cell invasion, understanding the function of surface GAPDH and mechanisms of its surface localization may lead to new multistage strategies to contain the malaria parasite.
MATERIALS AND METHODS
Cell culture and sporozoite isolation
Isolation and primary culture procedures of rat Kupffer cells were as described previously (Cha et al., 2015). Primary mouse peritoneal macrophages were isolated from CD68 wild-type (+/+) or KO (−/−) mice (Song et al., 2011) and incubated in RPMI medium supplemented with 10% FBS. All procedures were executed in accordance with a protocol approved by the Johns Hopkins University Animal Care and Use Committee. THP1 human monocyte cells were activated to develop into macrophage-like cells by treatment with PMA (1,000-fold dilution of a 0.1 mM stock in DMSO; Sigma-Aldrich) for 4 d. Control groups were treated with the same concentration of DMSO (Traore et al., 2005). Sporozoites were isolated from infected Anopheles stephensi mosquitoes using salivary gland dissection or density gradient centrifugation as described previously (Cha et al., 2015).
Recombinant protein
Recombinant rat CD68 was generated as a membrane-bound protein (whole protein) in Cos7 cells or as a secreted protein (missing the C′-transmembrane domain) in 293T cells (Cha et al., 2015). The GFP expression cassette of the pMaxGFP (Amaxa) plasmid was replaced with the rat CD68 coding sequence (RefSeq accession no. NM_001031638.1) and transfected into target cells using Xfect transfection reagent (Takara Bio Inc.). For preparation of membrane proteins, ∼1–2 × 107 Cos7 cells were harvested with buffer 1 (0.01% digitonin, 10 mM Pipes, pH 6.8, 300 mM sucrose, 100 mM NaCl, 3 mM MgCl2, and 5 mM EDTA) and rotated for 10 min at 4°C. After 1-min centrifugation (16,900 g), the supernatant (cytosolic fraction) was discarded, and the cell pellet was resuspended with buffer 2 (0.5% Triton X-100, 10 mM Pipes, pH 7.4, 300 mM sucrose, 100 mM NaCl, 3 mM MgCl2, and 3 mM EDTA) and rotated for 20 min at 4°C. After 1-min centrifugation (16,900 g), the supernatant (membrane fraction) was collected. For collecting secreted CD68, transfected 293T cells were incubated in RPMI medium supplemented with 10% FBS for 1 d, and then culture medium was exchanged with fresh RPMI medium without FBS. The culture supernatant was harvested on day 3 after transfection and further concentrated using a 100-kD centrifugal filter (Amicon-Ultra; EMD Millipore; Fig. 6; Cha et al., 2015). Recombinant PbGAPDH was generated using the pET32b expression system (EMD Millipore). PbGAPDH (fused to pET32b tag protein) or empty tag protein (thioredoxin-tag, His-tag, and S-tag) was purified with NiNTA agarose (QIAGEN).
MS analysis
Protein lysate was immunoprecipitated by the antibody against P39, and the immunoprecipitate was resolved by SDS-PAGE. One gel band that was visualized by colloidal Coomassie staining was excised and processed for in-gel tryptic digestion as described previously (Dinglasan et al., 2007; Ghosh et al., 2009; Chaerkady et al., 2011). The digested peptides were analyzed on a high-resolution mass spectrometer (LTQ-Orbitrap Elite; Thermo Fisher Scientific) by liquid chromatography tandem MS (MS/MS), as described previously (Kim et al., 2014). The MS/MS data were searched using Proteome Discoverer (version 2.1.0.60) using the SequestHT database search algorithm against a combined protein database of P. berghei (RefSeq71; NCBI) and A. stephensi (AsteS1.2; VectorBase) along with common contaminants. Using the Percolator algorithm (Käll et al., 2007), proteins were considered identified if they contained two or more unique peptides at ≤1% false discovery rate at the peptide and protein levels.
Transwell experiments
Experiments for the sporozoite neutralization assay used 105 primary rat Kupffer cells or PMA-treated THP1 cells cultured in a 6.5-mm Transwell support (Corning) with 5-µm pores. A total of 4 × 105 P. berghei or P. falciparum sporozoites were preincubated in 20 µl of antibody (0.5 mg/ml) at 4°C for 1 h. Sporozoites were transferred to a Transwell with 100 µl of medium. After 4-h incubation at 37°C, Transwell supports were removed, and sporozoites that had traversed to the bottom chamber (Fig. 3 A) were counted with the aid of a hemocytometer (model 63512-10; Electron Microscopy Sciences). For sporozoite movement inhibition assay, a total of 4 × 104 P. berghei sporozoites were preincubated with each antibody (0.2 mg/ml) at 4°C for 30 min. Sporozoites were transferred to a Transwell with 3-µm pores in 100 µl of medium. After 2-h incubation at 37°C, Transwell supports were removed, and sporozoites that had traversed to the bottom chamber (Fig. 3 A) were counted.
PbGAPDH–CD68 interaction measurements with ELISA and pull-down assays
PbGAPDH or pET32b tag protein was attached to a Ni-coated ELISA plate (Thermo Fisher Scientific). After washing, the coated wells were further incubated with the membrane protein fraction of Cos7 cells (a monkey kidney cell line), which were either transfected to express rat CD68 or not transfected as a negative control. An anti–thioredoxin tag antibody determined the amount of PbGAPDH or pET32b tag protein attached to the wells. The amount of CD68 binding to the attached protein was determined with an anti–rat CD68 antibody (AbD Serotec) and normalized to the amount of attached protein.
Recombinant rat CD68 was bound to protein A beads (Invitrogen) using anti–rat CD68 antibody (AbD Serotec). After incubation with either PbGAPDH or pET32b tag protein, the CD68–protein A beads were boiled in Laemmli buffer to elute bound proteins. Western blotting assays with an anti–thioredoxin tag antibody visualized the recombinant PbGAPDH and pET32b tag proteins.
Immunofluorescence and flow cytometry assays
For binding assays of PbGAPDH or CD68 to macrophages or sporozoites, primary mouse peritoneal macrophages and Plasmodium sporozoites were harvested and fixed in 4% paraformaldehyde for 30 min. Mouse macrophages were initially treated with an Fc receptor–blocking antibody (eBioscience) for 10 min. For the PbGAPDH binding assay, mouse peritoneal macrophages were incubated in 5 mM PbGAPDH or pET32b tag protein (Fig. 5 D). For CD68 binding assay, P. berghei sporozoites were incubated in the culture supernatant of CD68-secreting 293T cells in presence of 18 mM PbGAPDH or pET32b tag protein (Fig. 5 E). Binding of PbGAPDH or CD68 was visualized with anti–His tag antibody (GenScript) and anti-CD68 antibody (AbD Serotec), respectively. Flow cytometry data were acquired with a FACSCalibur cell analyzer (BD) and analyzed using the FlowJo software (v8.7; Tree Star).
For immunofluorescence assays with antimimotope and control antibodies, sporozoites were fixed in 4% paraformaldehyde. For immunofluorescence assays with anti-PbGAPDH and control antibodies, sporozoites were incubated in Cytofix/Cytoperm solution (BD) per the manufacturer’s directions or in PBS as a control (Fig. 6 B). Alexa Fluor 488 (green)– or rhodamine-conjugated (red) secondary antibodies (Invitrogen) visualized sporozoites or peritoneal macrophages.
Sporozoite entry inhibition assay and determination of the parasite liver burden
For in vitro sporozoite entry inhibition assays, mouse peritoneal macrophages were isolated from CD68 wild-type (+/+) or KO (−/−) mice and incubated in 8-well chamber slides (Lab-Tek II). A total of 2 × 104 P. berghei sporozoites were added in presence of PbGAPDH or pET32b tag protein at 10 mM or 2.5 mM concentration. After 2-h incubation, relative sporozoite entry was determined as described previously (Cha et al., 2015). Relative sporozoite entry into macrophages and percent inhibition were calculated from the mean of three wells.
For in vivo sporozoite neutralization assay (Fig. 7 B), 0.2 mg of purified anti-PbGAPDH or anti-pET32b tag protein antibody was injected intravenously into a mouse. 1 d after antibody transfer, mice were challenged with 2 × 103 P. berghei sporozoites/mouse by intravenous injection. Before purification using protein A beads (Invitrogen), anti-PbGAPDH antisera were preincubated with pET32b tag protein bound to NiNTA beads (QIAGEN) to remove anti-pET32b tag protein antibodies.
Immunization and challenge
For generating antibodies, ∼21–25-g Swiss Webster mice were immunized by injection of 1011 of each selected phage, 50 µg of KLH-conjugated P39 peptide (Bio-Synthesis, Inc.), or 50 µg of recombinant PbGAPDH. Immunization with 50 µg of nonconjugated KLH (Sigma-Aldrich) or 50 µg pET32b tag protein was used for controls. Prime injection in Freund’s complete adjuvant was followed by three boosts at 14-d intervals with the same amount of antigen in incomplete Freund’s adjuvant. 7 d after the last boost, mice were bled to collect antisera or challenged with P. berghei sporozoites. For mouse challenges, either 100 or 1,000 P. berghei salivary gland sporozoites were injected through the tail vein. Alternatively, mice were challenged with the bite of a single infected A. stephensi mosquito. The salivary gland of this mosquito was dissected after the bite to ascertain that sporozoites were present.
Statistics for data analysis
Nonparametric comparisons between groups were performed using the Mann-Whitney U test.
ACKNOWLEDGMENTS
We thank the Johns Hopkins Malaria Research Institute mosquito and parasite core facilities for help with mosquito rearing and with P. falciparum gametocyte culture.
This work was supported by the National Institute of Allergy and Infectious Diseases (grant AI080668). Support from the Johns Hopkins Malaria Research Institute and the Bloomberg Family Foundation is gratefully acknowledged. Supply of human blood was supported by the National Institutes of Health (grant RR00052).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- CSP
- circumsporozoite protein
- MS
- mass spectrometry
- PbGAPDH
- P. berghei GAPDH
- SNP
- single nucleotide polymorphism
References
- Alvarez-Dominguez C., Roberts R., and Stahl P.D.. 1997. Internalized Listeria monocytogenes modulates intracellular trafficking and delays maturation of the phagosome. J. Cell Sci. 110:731–743. [DOI] [PubMed] [Google Scholar]
- Argiro L.L., Kohlstädt S.S., Henri S.S., Dessein H.H., Matabiau V.V., Paris P.P., Bourgois A.A., and Dessein A.J.. 2000. Identification of a candidate vaccine peptide on the 37 kDa Schistosoma mansoni GAPDH. Vaccine. 18:2039–2048. 10.1016/S0264-410X(99)00521-6 [DOI] [PubMed] [Google Scholar]
- Bergmann S., Rohde M., and Hammerschmidt S.. 2004. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pneumoniae is a surface-displayed plasminogen-binding protein. Infect. Immun. 72:2416–2419. 10.1128/IAI.72.4.2416-2419.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boël G., Jin H., and Pancholi V.. 2005. Inhibition of cell surface export of group A streptococcal anchorless surface dehydrogenase affects bacterial adherence and antiphagocytic properties. Infect. Immun. 73:6237–6248. 10.1128/IAI.73.10.6237-6248.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami C., Frevert U., Sinnis P., Takacs B., and Nussenzweig V.. 1994. Rapid clearance of malaria circumsporozoite protein (CS) by hepatocytes. J. Exp. Med. 179:695–701. 10.1084/jem.179.2.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S.J., Park K., Srinivasan P., Schindler C.W., van Rooijen N., Stins M., and Jacobs-Lorena M.. 2015. CD68 acts as a major gateway for malaria sporozoite liver infection. J. Exp. Med. 212:1391–1403. 10.1084/jem.20110575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaerkady R., Kelkar D.S., Muthusamy B., Kandasamy K., Dwivedi S.B., Sahasrabuddhe N.A., Kim M.S., Renuse S., Pinto S.M., Sharma R., et al. 2011. A proteogenomic analysis of Anopheles gambiae using high-resolution Fourier transform mass spectrometry. Genome Res. 21:1872–1881. 10.1101/gr.127951.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppi A., Tewari R., Bishop J.R., Bennett B.L., Lawrence R., Esko J.D., Billker O., and Sinnis P.. 2007. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe. 2:316–327. 10.1016/j.chom.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth H.D., Aikawa M., Cochrane A.H., and Nussenzweig R.S.. 1980. Sporozoites of mammalian malaria: attachment to, interiorization and fate within macrophages. J. Protozool. 27:193–202. 10.1111/j.1550-7408.1980.tb04680.x [DOI] [PubMed] [Google Scholar]
- Daubenberger C.A., Tisdale E.J., Curcic M., Diaz D., Silvie O., Mazier D., Eling W., Bohrmann B., Matile H., and Pluschke G.. 2003. The N′-terminal domain of glyceraldehyde-3-phosphate dehydrogenase of the apicomplexan Plasmodium falciparum mediates GTPase Rab2-dependent recruitment to membranes. Biol. Chem. 384:1227–1237. 10.1515/BC.2003.135 [DOI] [PubMed] [Google Scholar]
- Dinglasan R.R., Kalume D.E., Kanzok S.M., Ghosh A.K., Muratova O., Pandey A., and Jacobs-Lorena M.. 2007. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc. Natl. Acad. Sci. USA. 104:13461–13466. 10.1073/pnas.0702239104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper S.J., Angov E., Horii T., Miller L.H., Srinivasan P., Theisen M., and Biswas S.. 2015. Recent advances in recombinant protein-based malaria vaccines. Vaccine. 33:7433–7443. 10.1016/j.vaccine.2015.09.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert U., Sinnis P., Cerami C., Shreffler W., Takacs B., and Nussenzweig V.. 1993. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J. Exp. Med. 177:1287–1298. 10.1084/jem.177.5.1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert U., Engelmann S., Zougbédé S., Stange J., Ng B., Matuschewski K., Liebes L., and Yee H.. 2005. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 3:e192 10.1371/journal.pbio.0030192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A.K., Devenport M., Jethwaney D., Kalume D.E., Pandey A., Anderson V.E., Sultan A.A., Kumar N., and Jacobs-Lorena M.. 2009. Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the Anopheles saglin proteins. PLoS Pathog. 5:e1000265 10.1371/journal.ppat.1000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M.L., Villamón E., Monteagudo C., Gozalbo D., and Martínez J.P.. 1999. Clinical strains of Candida albicans express the surface antigen glyceraldehyde 3-phosphate dehydrogenase in vitro and in infected tissues. FEMS Immunol. Med. Microbiol. 23:229–234. 10.1111/j.1574-695X.1999.tb01243.x [DOI] [PubMed] [Google Scholar]
- Graewe S., Retzlaff S., Struck N., Janse C.J., and Heussler V.T.. 2009. Going live: a comparative analysis of the suitability of the RFP derivatives RedStar, mCherry and tdTomato for intravital and in vitro live imaging of Plasmodium parasites. Biotechnol. J. 4:895–902. 10.1002/biot.200900035 [DOI] [PubMed] [Google Scholar]
- Jin H., Agarwal S., Agarwal S., and Pancholi V.. 2011. Surface export of GAPDH/SDH, a glycolytic enzyme, is essential for Streptococcus pyogenes virulence. MBio. 2:e00068-11 10.1128/mBio.00068-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käll L., Canterbury J.D., Weston J., Noble W.S., and MacCoss M.J.. 2007. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods. 4:923–925. 10.1038/nmeth1113 [DOI] [PubMed] [Google Scholar]
- Kaushansky A., Douglass A.N., Arang N., Vigdorovich V., Dambrauskas N., Kain H.S., Austin L.S., Sather D.N., and Kappe S.H.. 2015. Malaria parasites target the hepatocyte receptor EphA2 for successful host infection. Science. 350:1089–1092. 10.1126/science.aad3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebaier C., Voza T., and Vanderberg J.. 2009. Kinetics of mosquito-injected Plasmodium sporozoites in mice: fewer sporozoites are injected into sporozoite-immunized mice. PLoS Pathog. 5:e1000399 10.1371/journal.ppat.1000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.S., Pinto S.M., Getnet D., Nirujogi R.S., Manda S.S., Chaerkady R., Madugundu A.K., Kelkar D.S., Isserlin R., Jain S., et al. 2014. A draft map of the human proteome. Nature. 509:575–581. 10.1038/nature13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M., Uchida T., Sato A., Nakashima M., Nakashima H., Shono S., Habu Y., Miyazaki H., Hiroi S., and Seki S.. 2010. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J. Hepatol. 53:903–910. 10.1016/j.jhep.2010.04.037 [DOI] [PubMed] [Google Scholar]
- Lama A., Kucknoor A., Mundodi V., and Alderete J.F.. 2009. Glyceraldehyde-3-phosphate dehydrogenase is a surface-associated, fibronectin-binding protein of Trichomonas vaginalis. Infect. Immun. 77:2703–2711. 10.1128/IAI.00157-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner S.E., Swearingen K.E., Harupa A., Vaughan A.M., Sinnis P., Moritz R.L., and Kappe S.H.. 2013. Total and putative surface proteomics of malaria parasite salivary gland sporozoites. Mol. Cell. Proteomics. 12:1127–1143. 10.1074/mcp.M112.024505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medica D.L., and Sinnis P.. 2005. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect. Immun. 73:4363–4369. 10.1128/IAI.73.7.4363-4369.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis J.F., Verhave J.P., Jap P.H., and Meuwissen J.H.. 1983. An ultrastructural study on the role of Kupffer cells in the process of infection by Plasmodium berghei sporozoites in rats. Parasitology. 86:231–242. 10.1017/S003118200005040X [DOI] [PubMed] [Google Scholar]
- Meis J.F., Verhave J.P., Brouwer A., and Meuwissen J.H.. 1985. Electron microscopic studies on the interaction of rat Kupffer cells and Plasmodium berghei sporozoites. Z. Parasitenkd. 71:473–483. 10.1007/BF00928350 [DOI] [PubMed] [Google Scholar]
- Pancholi V., and Fischetti V.A.. 1992. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J. Exp. Med. 176:415–426. 10.1084/jem.176.2.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V., and Fischetti V.A.. 1997. Regulation of the phosphorylation of human pharyngeal cell proteins by group A streptococcal surface dehydrogenase: signal transduction between streptococci and pharyngeal cells. J. Exp. Med. 186:1633–1643. 10.1084/jem.186.10.1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Casal J., and Potter A.A.. 2016. Glyceradehyde-3-phosphate dehydrogenase as a suitable vaccine candidate for protection against bacterial and parasitic diseases. Vaccine. 34:1012–1017. 10.1016/j.vaccine.2015.11.072 [DOI] [PubMed] [Google Scholar]
- Pradel G., and Frevert U.. 2001. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology. 33:1154–1165. 10.1053/jhep.2001.24237 [DOI] [PubMed] [Google Scholar]
- Richie T.L., Billingsley P.F., Sim B.K., James E.R., Chakravarty S., Epstein J.E., Lyke K.E., Mordmüller B., Alonso P., Duffy P.E., et al. 2015. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine. 33:7452–7461. 10.1016/j.vaccine.2015.09.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinha G.M., Myioshi A., Azevedo V., Splitter G.A., and Oliveira S.C.. 2002. Molecular and immunological characterisation of recombinant Brucella abortus glyceraldehyde-3-phosphate-dehydrogenase, a T- and B-cell reactive protein that induces partial protection when co-administered with an interleukin-12-expressing plasmid in a DNA vaccine formulation. J. Med. Microbiol. 51:661–671. 10.1099/0022-1317-51-8-661 [DOI] [PubMed] [Google Scholar]
- Sangolgi P.B., Balaji C., Dutta S., Jindal N., and Jarori G.K.. 2016. Cloning, expression, purification and characterization of Plasmodium spp. glyceraldehyde-3-phosphate dehydrogenase. Protein Expr. Purif. 117:17–25. 10.1016/j.pep.2015.08.028 [DOI] [PubMed] [Google Scholar]
- Seder R.A., Chang L.J., Enama M.E., Zephir K.L., Sarwar U.N., Gordon I.J., Holman L.A., James E.R., Billingsley P.F., Gunasekera A., et al. VRC 312 Study Team . 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 341:1359–1365. 10.1126/science.1241800 [DOI] [PubMed] [Google Scholar]
- Song L., Lee C., and Schindler C.. 2011. Deletion of the murine scavenger receptor CD68. J. Lipid Res. 52:1542–1550. 10.1194/jlr.M015412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A., Amino R., van de Sand C., Regen T., Retzlaff S., Rennenberg A., Krueger A., Pollok J.M., Menard R., and Heussler V.T.. 2006. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science. 313:1287–1290. 10.1126/science.1129720 [DOI] [PubMed] [Google Scholar]
- Tavares J., Formaglio P., Thiberge S., Mordelet E., Van Rooijen N., Medvinsky A., Ménard R., and Amino R.. 2013. Role of host cell traversal by the malaria sporozoite during liver infection. J. Exp. Med. 210:905–915. 10.1084/jem.20121130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao Y., Yamaguchi M., Hamada S., and Kawabata S.. 2006. Multifunctional glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pyogenes is essential for evasion from neutrophils. J. Biol. Chem. 281:14215–14223. 10.1074/jbc.M513408200 [DOI] [PubMed] [Google Scholar]
- Tran T.M., Portugal S., Draper S.J., and Crompton P.D.. 2015. Malaria vaccines: moving forward after encouraging first steps. Curr. Trop. Med. Rep. 2:1–3. 10.1007/s40475-015-0041-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore K., Trush M.A., George M. Jr., Spannhake E.W., Anderson W., and Asseffa A.. 2005. Signal transduction of phorbol 12-myristate 13-acetate (PMA)-induced growth inhibition of human monocytic leukemia THP-1 cells is reactive oxygen dependent. Leuk. Res. 29:863–879. 10.1016/j.leukres.2004.12.011 [DOI] [PubMed] [Google Scholar]
- Vreden S.G. 1994. The role of Kupffer cells in the clearance of malaria sporozoites from the circulation. Parasitol. Today (Regul. Ed.). 10:304–308. 10.1016/0169-4758(94)90084-1 [DOI] [PubMed] [Google Scholar]
- WHO 2016. Malaria. Available at: http://www.who.int/topics/malaria/en/ (accessed January 11, 2016).
- Yilmaz B., Portugal S., Tran T.M., Gozzelino R., Ramos S., Gomes J., Regalado A., Cowan P.J., d’Apice A.J., Chong A.S., et al. 2014. Gut microbiota elicits a protective immune response against malaria transmission. Cell. 159:1277–1289. 10.1016/j.cell.2014.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]