Abstract
Background
Neuroendocrine markers, which could indicate for aggressive variants of prostate cancer and Ki67 (a well-known marker in oncology for defining tumor proliferation), have already been associated with clinical outcome in prostate cancer. The aim of this study was to investigate the prognostic value of those markers in primary prostate cancer patients.
Patients and methods
NSE (neuron specific enolase), ChrA (chromogranin A), Syp (Synaptophysin) and Ki67 staining were performed by immunohistochemistry. Then, the prognostic impact of their expression on overall survival was investigated in 166 primary prostate cancer patients by univariate and multivariate analyses.
Results
NSE, ChrA, Syp and Ki67 were positive in 50, 45, 54 and 146 out of 166 patients, respectively. In Kaplan-Meier analysis only diffuse NSE staining (negative vs diffuse, p = 0.004) and Ki67 (≤ 10% vs > 10%, p < 0.0001) were significantly associated with overall survival. Ki67 expression, but not NSE, resulted as an independent prognostic factor for overall survival in multivariate analysis.
Conclusions
A prognostic model incorporating Ki67 expression with clinical-pathological covariates could provide additional prognostic information. Ki67 may thus improve prediction of prostate cancer outcome based on standard clinical-pathological parameters improving prognosis and management of prostate cancer patients.
Key words: primary prostate cancer, prognosis, Ki67, NSE
Introduction
Conventional clinical parameters alone are inadequate for differentiating indolent and aggressive prostate cancer. Therefore, molecular biomarkers are needed to better define prognosis of prostate cancer patients.
Neuroendocrine markers could be used to detect particularly aggressive variants of prostate cancer. Typical markers used to identify neuroendocrine differentiation (NED) in tumor tissue are neuron specific enolase (NSE), chromogranin A (CgA) and synaptophysin (Syp).1-3 Neuroendocrine differentiation, measured by one or more of those markers, has been associated with disease progression4 or poor survival in prostate cancer5, but up to now its prognostic value has not been clarified because of controversial results6;7,8. However, Epstein et al.9 have recently suggested using neuroendocrine markers to better characterize and classify NED in prostate cancer. They also outlined Ki67 ranges in those tumors, which usually have a high proliferative index.4,9-13 Ki67 is a well-known marker in oncology for defining tumor proliferation. Expression of Ki67 detection by immunohistochemistry14 is used as a prognostic marker for cell proliferation in many tumors, especially in breast carcinoma15 and cervical cancer.16 In prostate cancer it has also been associated with clinical outcome, irrespective of treatment.4,17-27
The aims of this study were to 1) investigate the immunohistochemical expression of neuroendocrine and Ki67 markers in primary prostate cancer patients in order to identify tumors characterized by biological aggressiveness and poor prognosis, 2) evaluate neuroendocrine expression with respect to Ki67 staining.
Patients and methods
Patients
Detailed histopathological and clinical data were retrospectively collected for 166 patients, who were diagnosed with primary prostate cancer in a single institution of the North-eastern Italy from January 1992 to December 1994, therefore associated to a long follow-up period. Inclusion criteria for this study were: a) diagnosis of prostate cancer and b) availability of formalin-fixed and paraffin-embedded tissues for immunohistochemical staining and molecular analyses. Only TURP (N = 122, 73.9%) and prostatectomy (N = 43, 26.1%) specimens were used (missing information for one patient). Fine needle biopsies were excluded because of the low amount of tissue. Patients did not receive any treatment before diagnosis. The use of formalin-fixed and paraffin-embedded prostate cancer tissues and their related clinical information were approved by the Ethical Committee of the University of Trieste (Report 23; 5.10.2009) before the beginning of the study.
Tissue microarray and immunohistochemical staining
Representative multiple areas of the primary tumours were selected by two pathologists (G.S. and R.B.) for TMA construction. Tissue cores were chosen at the border of the primary tumour. Tissue cylinders of 1.0 mm in diameter were taken from the selected regions of the donor’s paraffin block and were placed into a recipient paraffin block using a tissue-arrayer (Galileo TMA CK3500; Integrated Systems Engineering, Milano, Italy), as previously described.28 Multiple cores were taken for cases as representative of heterogeneous histological areas. Neuroendocrine differentiation (NED) was evaluated using NSE, ChrA, Syp as neuroendocrine markers.
Immunostainings for Ki67 (clone MIB-1; DakoDenmark A/S, Glostrup, Denmark), 1:200 dilution; NSE (clone E27; NeoMarker; ThermoScientific, Waltham, MA, USA) 1:2500; ChrA (clone LK2H10; NeoMarker; ThermoScientific, Waltham, MA, USA), 1:500; Syp (clone SY 38; Thermo-Fisher; ThermoScientific, Waltham, MA, USA) 1:75 were performed in a Lab Vision Autostainer 480S (Thermo Scientific, Waltham, MA, USA) with the UltraVision LP Large Volume Detection System HRP Polymer (Lab Vision Corporation, Thermo Scientific) according to manufacturer’s recommended protocol. For evaluation of the immunostaining, positively stained cells were counted across 3 high-power fields. Staining intensity was not taken into consideration. Due to technical issues related to the detachment of tissue cores it was not possible to analyze the four biomarkers in all samples (Figure 1). The percentage of the positively stained cells was reported for each specimen. Ki67 expression was dichotomized for assessing its prognostic value using a cut-off of 10%.7,29
Figure 1.
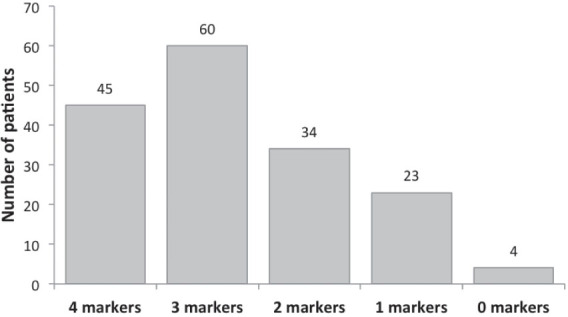
Determination of Ki67, NSE, CgA, SYP in primary prostate cancer patients. Number of concurrent biomarkers evaluated. The number of patients is reported above each column.
Statistical analysis
The Kaplan-Meier method was used to generate overall survival (OS) curves, which was defined as the time between the date of diagnosis and the date of death or the last follow-up (FU) observation. Patients were censored if they were still alive or they were lost to FU. The log-rank test was used to evaluate differences between groups. Association of OS with each prognostic factor was evaluate in univariate and multivariate analyses by using the Cox proportional hazards model. All variables associated with univariate value of p ≤ 0.05 were included in the multivariate model using a stepwise method. The proportional hazards assumptions were checked before applying the Cox regression model. The goodness of fit was assessed using a likelihood-ratio test. The discrimination ability was quantified by calculating the concordance index that ranges from 0.5 (no discrimination) to 1 (perfect discrimination). Possible correlations of the expression of Ki67 and neuroendocrine markers with the other prognostic variables were assessed by χ2 test or Wilcoxon rank sum. All statistical tests were performed using STATA software (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP) and p values ≤; 0.05 were considered as statistically significant.
Results
Neuroendocrine marker staining
NSE expression was assessable in 89 prostate cancer cases. Of those, 28 cases (31.5%) were completely negative, whereas 61 (68.5%) revealed a cytoplasmic positivity, referring to diffuse, focal and spotty staining (Table 1). Detachment of tissue cores did not allow analyzing NSE in some cases. ChrA was negative in 49 out of 121 cores (40.5%) and it was positive on the cytoplasmic level in 72 cores (59.5%). Positive cases had weakly to highly diffused, or focal or spotty reactivity (Table 1). Syp was analyzed in 127 cases of which 44 (34.6%) were negative and 83 (65.4%) showed a cytoplasmic positivity, from spotty to focal to diffused (Table 1). Only diffused expression was considered as positive for each marker. Focal or spotty stainings were evaluated as negative.
Table 1.
Neuroendocrine marker staining in our cohort of primary prostate cancer patients
| Staining | Neuroendocrine markers | ||
|---|---|---|---|
| NSE | CgA | Syp | |
| Negative | 28 (31.5) | 49 (40.5) | 44 (34.6) |
| Diffuse | 50 (56.1) | 45 (37.2) | 54 (42.5) |
| Focal | 3 (3.4) | 19 (15.7) | 17 (13.4) |
| Spotty | 8 (9) | 8 (6.6) | 12 (9.5) |
| Total | 89 | 121 | 127 |
| missing | 77 | 45 | 39 |
Data reported as N (%)
No significant associations were found between neuroendocrine markers and clinicopathological variables. A slight association was revealed between NSE expression and Gleason score (p = 0.04). Moreover, a positive relationship was found between CgA and SYP expression (p = 0.01). The type of intervention was significantly associated with CgA (p = 0.009) and NSE (p = 0.001) expression.
Ki67 staining
Ki67 staining analysis was measured in 146 out of 166 prostatic cases. The median Ki67 staining score was 5% with an IQR of 9 whereas the mean value was 10.3% with a standard deviation of 14.2% (range of 0 – 90%). The percentage of the positively stained cells was recorded for each sample (Table 2, Figures 2,3).
Table 2.
Distribution of Ki67 positively stained cells in our cohort of primary prostate cancer patients
| % Ki67-positive cells | Number of samples | |
|---|---|---|
| N | % | |
| 0 | 14 | 9.6 |
| ≤ 5 | 70 | 47.9 |
| > 5 and ≤ 10 | 24 | 16.5 |
| > 10 and ≤ 20 | 18 | 12.3 |
| > 20 and ≤ 30 | 13 | 8.9 |
| > 30 | 7 | 4.8 |
| Total | 146 | 100% |
Figure 2.
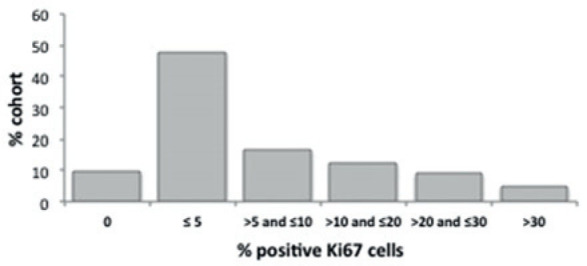
Distribution of Ki67 staining score. Positive Ki67 cells in TURP and prostatectomy specimens from a cohort of 166 primary prostate cancer patients.
Figure 3.
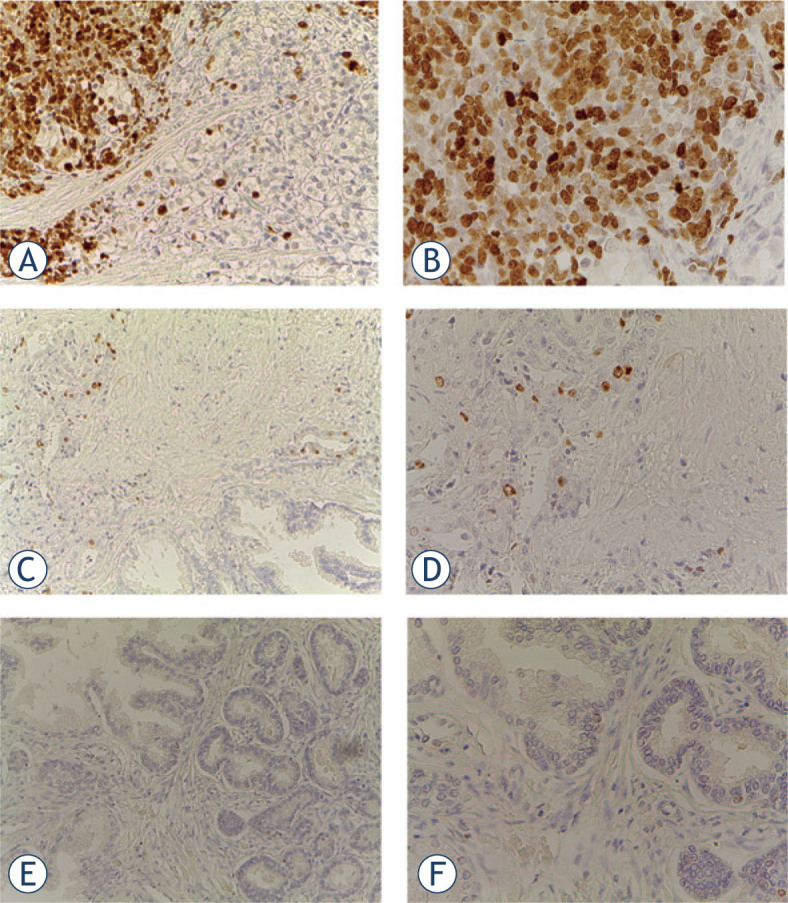
Representative immunohistochemical staining for Ki67. (A) prostate adenocarcinoma with Ki67 > 10% 20 x and 40 x (B) magnification; (C) prostate adenocarcinoma with Ki67 ≤ 10% 20 x and 40 x (D) magnification; (E) prostate adenocarcinoma negative for Ki67 20 x and 40 x (F) magnification.
Ki67 was scored and stratified into two groups (low ≤ 10%; high > 10%) as already reported.23,24 No statistically significant difference was observed for Ki67 staining between TURP and prostatectomy specimens (p = 0.08). Ki67 expression was significantly associated with Gleason score and nuclear grading, but not with age at diagnosis or with neuroendocrine markers (Table 3).
Table 3.
Association between Ki67 expression and clinicopathological variables (N = 146)
| Variable | Ki67 expression | |||
|---|---|---|---|---|
| ≤ 10% | > 10% | Tot | P-value | |
| Nuclear grading | ||||
| 1 | 22 | 1 | 23 | |
| 2 | 72 | 23 | 95 | 0.001* |
| 3 | 14 | 14 | 28 | |
| Gleason Score | ||||
| < 7 | 56 | 8 | 64 | 0.001* |
| ≥ 7 | 51 | 30 | 81 | |
| missing 1 | ||||
| Age at diagnosis (median, years) | ||||
| ≤ 71 | 53 | 19 | 72 | 0.922 |
| > 71 | 55 | 19 | 74 | |
| Age at diagnosis (continuous) | 108 | 38 | 146 | 0.973 |
| NSE expression | ||||
| Negative | 21 | 4 | 25 | 0.073 |
| Positivea | 28 | 16 | 44 | |
| missing 77 | ||||
| CgA expression | ||||
| Negative | 37 | 10 | 47 | 0.217 |
| Positivea | 24 | 12 | 36 | |
| missing 40 | ||||
| Syp expression | ||||
| Negative | 31 | 8 | 39 | 0.326 |
| Positivea | 33 | 14 | 47 | |
| missing 60 | ||||
| Type of intervention | ||||
| TURP | 78 | 33 | 111 | 0.081 |
| Prostatectomy | 29 | 5 | 34 | |
| missing 1 | ||||
| Total | 108 | 38 | 146 | |
Significant value
Only diffuse expression was considered as positive staining;
Univariate analysis
In univariate analysis, diffuse expression of NSE, Ki67 expression > 10%, Gleason score ≥ 7 and nuclear grading ≥ 2 predicted for shorter OS (Table 4).
Table 4.
Univariate analysis of prognostic factors in patients with primary prostate cancer based on Cox proportional hazards regression model
| Variable | UNIVARIATE | |
|---|---|---|
| HR (95% CI) | P-value | |
| Nuclear grading | ||
| 2 vs 1 | 1.45 (0.90-2.34) | 0.125 |
| 3 vs 1 | 3.49 (1.94-6.27) | < 0.001* |
| Gleason score | ||
| ≥ 7 vs < 7 | 2.97 (2.07-4.26) | < 0.001* |
| Ki67 expression | ||
| > 10% vs ≤ 10% | 2.75 (1.85-4.09) | < 0.001* |
| Age at diagnosis (continuous) | 1.04 (1.02-1.07) | < 0.001* |
| NSE expression | ||
| Positiveavs negative | 1.99 (1.19-3.34) | 0.009* |
| CgA expression | ||
| Positivea vs negative | 0.96 (0.62-1.47) | 0.840 |
| Syp expression | ||
| Positivea vs negative | 1.18 (0.77-1.81) | 0.447 |
| Type of intervention | ||
| Prostatectomy vs TURP | 0.45 (0.30-0.67) | < 0.001* |
Significant value
Only diffuse expression was considered as positive staining;
CI = confidence interval; HR = hazard ratio
Considering neuroendocrine markers, only NSE staining was significantly associated with OS. Patients with diffuse NSE expression had a reduced survival time (median OS 2 years; 95% CI, 2–4) compared to patients with negative expression (median OS 7; 95% CI, 5–10; p = 0.004), showing nearly double-fold increased risk of death (Table 4, Figure 4). Additionally, NED measured as positive at least at one of the three markers did not result significant.
Figure 4.
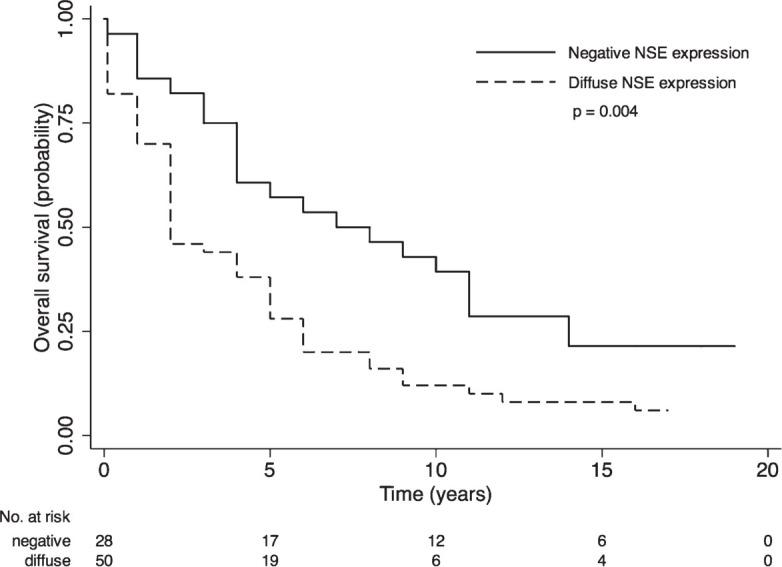
Survival curves by NSE staining in patients with primary prostate cancer. p-value from log-rank test is reported. Numbers of at risk (still alive) patients are indicated below the x-axis.
Kaplan-Meier survival curves showed a significant difference (p < 0.001) between low and high levels of Ki67 staining with median survival time of 6 years (95% CI, 5–9) and 2 years (95% CI, 1–2) for patients with ≤; 10% and > 10% of positive cells, respectively (Figure 5A). Moreover, splitting group four categories according to Ki67 staining were obtained: negative, 1–10%, 11–20%, > 20%. Negative patients showed a median overall survival (10 years, 95% CI: 4–9) which was four years longer than in patients with Ki67 staining of 1–10% (6 years, 95% CI: 5–8; p < 0.001) (Figure 5B). An improved discrimination was also reached in the category > 10%. Patients with Ki67 staining > 20% were associated with a 2.5-fold increased risk of death, compared with patients showing Ki67 expression of 10–20% (the overall 2-year survival rate of 50%–95% CI: 25–70 vs 20%, 95% CI: 6–39, p < 0.001). These data further support the potential role of Ki67 immunostaining in selecting patients according to proliferation rate, and thus to tumor aggressiveness. However, we decided to assess the prognostic value of Ki67 for overall survival of prostate cancer patients by using the previous binary variable because of harmonization with already published studies.7,29
Figure 5.
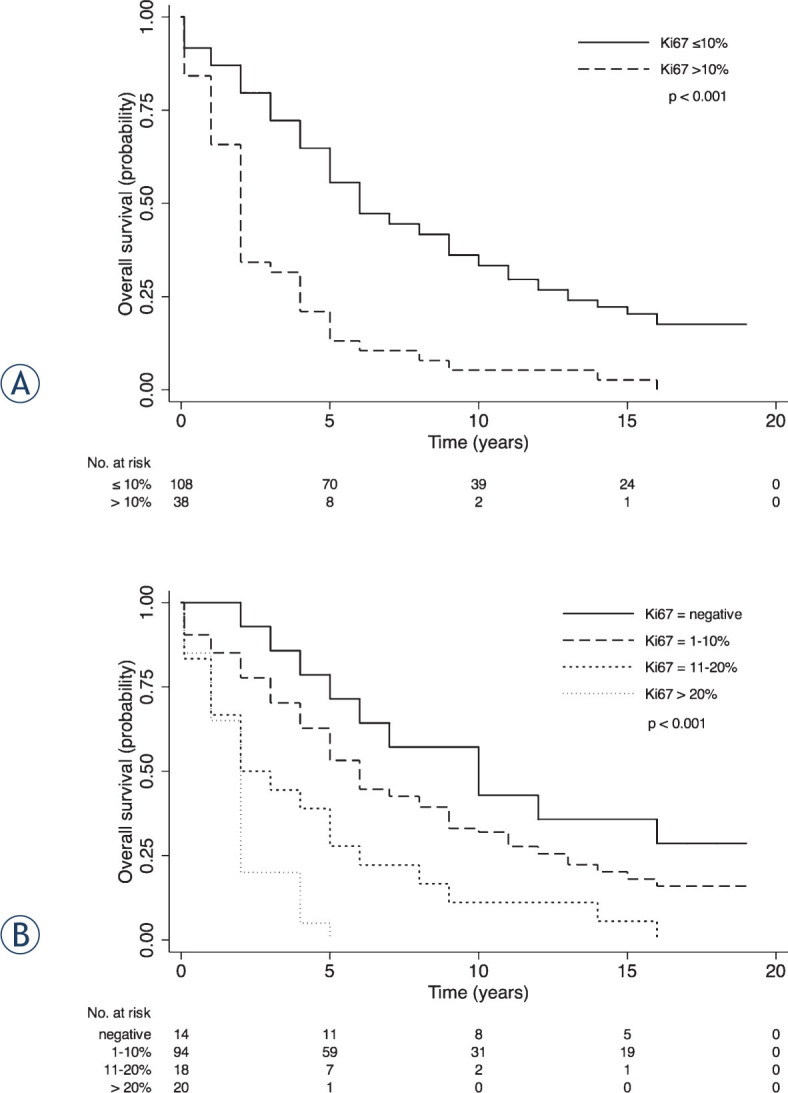
Survival curves by Ki67 expression in patients with primary prostate cancer. (A) Ki67 staining dichotomized in ≤; 10% and > 10%; (B) Ki67 staining divided into negative, 1–10%, 11%–20%, > 20%. p-value from log-rank test is reported. Numbers of at risk (still alive) patients are indicated below the x-axis.
Age at diagnosis and type of intervention significantly impacted on OS (p < 0.001). Thus, considering the relationship existing with other variables, they were included in multivariate analysis to take into account their possible confounding effect.
Multivariate analysis
Multivariate analysis was done by stratifying according to type of intervention, because of no proportional risks between TURP and prostatectomy groups (Test of proportional-hazards assumption, χ2 = 8.86, df = 1, p = 0.003). Ki67 expression, but not NSE, resulted as an independent prognostic factor for OS. In the final multivariable model the risk of death was higher for older patients (p = 0.001) with a nuclear grading of 3 (p = 0.04), a Gleason score ≥ 7 (p < 0.001) and Ki67 expression > 10% (p < 0.001) (Table 5). Unfortunately, our dataset did not include information on PSA before surgical intervention for all patients, because they were diagnosed many years ago before the routinely application of PSA screening.
Table 5.
Multivariate analysis of prognostic factors in patients with primary prostate cancer based on Cox proportional hazards regression model stratified by type of intervention (N = 144)
| Variable | MULTIVARIATE | |
|---|---|---|
| Adjusted HR (95%CI) | Adjusted P-value | |
| Nuclear grading | ||
| 2 vs 1 | 1.27 (0.75-2.13) | 0.373 |
| 3 vs 1 | 1.93 (1.01-3.68) | 0.045* |
| Gleason score | ||
| ≥ 7 vs < 7 | 2.41 (1.56-3.74) | < 0.001* |
| Ki67 expression | ||
| > 10% vs ≤ 10% | 2.14 (1.41-3.25) | < 0.001* |
| Age at diagnosis (continuous) | 1.04 (1.02-1.07) | 0.001* |
Significant value
CI = confidence interval; HR = hazard ratio;
Comparison of the multivariate model incorporating Ki67 expression with a base model including conventional variables only (nuclear grading, Gleason score, age at diagnosis stratified by type of intervention) showed an improved fit which suggested an enhanced prognostic ability over the models containing clinicopathological variables only (χ2 = 11.33, df = 1, p = 0.0006). These data indicated that in a multivariate analysis Ki67 is a relevant and independent prognostic factor for OS of primary prostate cancer patients undergoing TURP or radical prostatectomy. The concordance index (0.72) revealed a good accuracy of the model in predicting OS.
Discussion
This study shows that Ki67 expression, but not NED, is an independent prognostic factor for OS in primary prostate cancer patients who underwent TURP or prostatectomy.
Our data demonstrate that NED, as measured by NSE, CgA and SYP immunohistochemistry, is present at the time of diagnosis in a large proportion of our cohort (over 50%), but without influence on OS. NSE staining seems to influence OS, but it was not confirmed as an independent prognostic factor in the multivariate analysis. These results are consistent with current published literature where strong evidence of NSE as potential prognostic factor is lacking (reviewed in 6,8), especially at early stages.30 NED in PCa increases with higher histological grades31 and disease progression, especially in response to androgen deprivation therapy.32-34 It seems that androgen deprivation therapy may promote the transformation of prostate adenocarcinoma into a neuroendocrine cancer, defined as t-NEPC (transformed neuroendocrine prostate cancer), as a mechanism of resistance.35 Ki67 expression has already been proposed as a candidate marker for gastroenteropancreatic36 and lung neuroendocrine cancers.37 An increased proliferation using Ki67 expression has been shown in prostate tumors with high NED compared to tumors with low or without NED.4,10,11 In this study we did not find any association between NED and Ki67 expression, although a trend for a positive relationship between NSE staining and Ki67 expression (p = 0.08) has been observed.
Limitations of this study are: small sample size, missing data for neuroendocrine IHC, statistically significant results obtained by using the less specific marker for NED.9 Another limitation is the analysis based on mixed sample population of prostatectomy and TURP which was partially solved using a stratified Cox model to investigate the risk difference in two groups. Of relevance, our cohort represents a long-term time series which allows investigating OS in cancers with a long life expectancy.
The prognostic significance of concurrent presence of NED features in prostate adenocarcinoma is currently very controversial.6 Although NED may have an adverse effect on prognosis of newly diagnosed prostate cancer, other mechanisms probably influence the prognosis by favoring the selection of the neuroendocrine pattern transformation under a specific stimulus, such as the pressure by androgen deprivation therapy.38 The mechanisms that are currently involved are not known.
Interestingly, we found that cell proliferation measured by Ki67 staining scored as a dichotomous variable with a 10% cut-off is an independent prognostic factor for OS in our cohort. Comparing prognostic models with and without Ki67 demonstrated that Ki67 expression could yield additional prognostic information to that provided by conventional clinicopathological parameters improving prognosis of prostate cancer patients. Furthermore, our data show that Ki67 expression correlates with Gleason score and nuclear grading, highlighting its association with prostate cancer aggressiveness, in agreement with others.4,23,25,39,40 Several studies have shown that Ki67 is useful to predict prostate cancer prognosis either on TURP19,23 or needle biopsy21,23,25,40 specimens. Furthermore, it has been proposed as a candidate prognostic marker both for overall and specific survival endpoints17-22 as well as disease progression.4,17,18,20,22-27 Its prognostic relevance does not seem to be influenced by therapy, as it has been reported to predict prognosis in patients treated by prostatectomy, alone 4,24,25,27 or with adjuvant therapy26, or radiotherapy, alone17,22,23 or with androgen deprivation therapy17,18,20,22, or conservatively managed.19,21
Consistent with our results, Ki67 emerges as a powerful marker of biological aggressiveness that could provide supplemental prognostic information, concerning the cellular proliferation rate, in addition to that provided by currently used markers, which are related to tumor pattern and extension only. Therefore, Ki67 may improve prediction of prostate cancer outcome based on standard clinical parameters, and may help stratify and select patients for more aggressive treatments. The utility of Ki67 was also demonstrated in selecting candidates with clinically insignificant cancer suitable for active surveillance among patients with PSA < 4 ng/ml at diagnosis.40 The validity of Ki67 expression as an indicator of prognosis for prostate cancer patients in different treatment cohorts, including both radical4,17,18,20,22-27 and conservative therapies19,21, and in many investigative materials, such as biopsy21,23,25,40, TURP19,23 or prostatectomy4,24-27 specimens, further supports its implementation in clinical practice, as recently sustained also in a meta-analysis. 41 However, larger prospective studies are needed to validate its use in routine pathology.
Despite unresolved issues on cut-offs, we suggest the analysis of Ki67 in routine diagnostic practice as an additional factor for improving prognosis and management of prostate cancer patients.
Acknowledgments
This work was partially supported by grants from ABREOC 2011.
Disclosure: No potential conflicts of interest were disclosed.
References
- [1].Schmechel D, Marangos PJ, Brightman M. Neurone-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature. 1978;276:834–6. doi: 10.1038/276834a0. [DOI] [PubMed] [Google Scholar]
- [2].Kimura N, Funakoshi A, Aunis D, Tateishi K, Miura W, Nagura H. Immunohistochemical localization of chromostatin and pancreastatin, chromogranin A-derived bioactive peptides, in Normal and Neoplastic Neuroendocrine Tissues. Endocr Pathol. 1995;6:35–43. doi: 10.1007/BF02914987. [DOI] [PubMed] [Google Scholar]
- [3].Wiedenmann B, Franke WW, Kuhn C, Moll R, Gould VE. Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci USA. 1986;83:3500–4. doi: 10.1073/pnas.83.10.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].May M, Siegsmund M, Hammermann F, Loy V, Gunia S. Prognostic significance of proliferation activity and neuroendocrine differentiation to predict treatment failure after radical prostatectomy. Scand J Urol Nephrol. 2007;41:375–81. doi: 10.1080/00365590701224445. [DOI] [PubMed] [Google Scholar]
- [5].Krauss DJ, Amin M, Stone B, Ye H, Hayek S, Cotant M. et al. Chromogranin A staining as a prognostic variable in newly diagnosed Gleason score. Prostate. 2014;74:520–7. doi: 10.1002/pros.22771. [DOI] [PubMed] [Google Scholar]
- [6].Berruti A, Vignani F, Russo L, Bertaglia V, Tullio M, Tucci M. et al. Prognostic role of neuroendocrine differentiation in prostate cancer, putting together the pieces of the puzzle. Open Access J Urol. 2010;2:109–24. doi: 10.2147/rru.s6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cindolo L, Cantile M, Franco R, Chiodini P, Schiavo G, Forte I. et al. Parallel determination of NeuroD1, chromogranin-A, KI67 and androgen receptor expression in surgically treated prostate cancers. Int Braz J Urol. 2011;37:57–66. doi: 10.1590/s1677-55382011000100008. [DOI] [PubMed] [Google Scholar]
- [8].Jeetle SS, Fisher G, Yang ZH, Stankiewicz E, Moller H, Cooper CS. et al. Neuroendocrine differentiation does not have independent prognostic value in conservatively treated prostate cancer. Virchows Arch. 2012;461:103–7. doi: 10.1007/s00428-012-1259-2. [DOI] [PubMed] [Google Scholar]
- [9].Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera J-M, Reuter VE. et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38:756–67. doi: 10.1097/PAS.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Helpap B, Kollermann J. Undifferentiated carcinoma of the prostate with small cell features: immunohistochemical subtyping and reflections on histogenesis. Virchows Arch. 1999;434:385–91. doi: 10.1007/s004280050357. [DOI] [PubMed] [Google Scholar]
- [11].Grobholz R, Griebe M, Sauer CG, Michel MS, Trojan L, Bleyl U. Influence of neuroendocrine tumor cells on proliferation in prostatic carcinoma. Hum Pathol. 2005;36:562–70. doi: 10.1016/j.humpath.2005.02.019. [DOI] [PubMed] [Google Scholar]
- [12].Gunia S, Albrecht K, Koch S, Herrmann T, Ecke T, Loy V. et al. Ki67 staining index and neuroendocrine differentiation aggravate adverse prognostic parameters in prostate cancer and are characterized by negligible interobserver variability. World J Urol. 2008;26:243–50. doi: 10.1007/s00345-008-0257-0. [DOI] [PubMed] [Google Scholar]
- [13].Beltran H, Tomlins S, Aparicio A, Arora V, Rickman D, Ayala G. et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res. 2014;20:2846–50. doi: 10.1158/1078-0432.CCR-13-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- [15].Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J. et al. Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2011;103:1656–64. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ikenberg H, Bergeron C, Schmidt D, Griesser H, Alameda F, Angeloni C. et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550–7. doi: 10.1093/jnci/djt235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li R, Heydon K, Hammond ME, Grignon DJ, Roach M 3rd, Wolkov HB. et al. Ki-67 staining index predicts distant metastasis and survival in locally advanced prostate cancer treated with radiotherapy: an analysis of patients in radiation therapy oncology group protocol 86-10. Clin Cancer Res. 2004;10:4118–24. doi: 10.1158/1078-0432.CCR-1052-03. [DOI] [PubMed] [Google Scholar]
- [18].Pollack A, DeSilvio M, Khor LY, Li R, Al-Saleem TI, Hammond ME. et al. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92-02. J Clin Oncol. 2004;22:2133–40. doi: 10.1200/JCO.2004.09.150. [DOI] [PubMed] [Google Scholar]
- [19].Berney DM, Gopalan A, Kudahetti S, Fisher G, Ambroisine L, Foster CS. et al. Ki-67 and outcome in clinically localised prostate cancer: analysis of conservatively treated prostate cancer patients from the Trans-Atlantic Prostate Group study. Br J Cancer. 2009;100:888–93. doi: 10.1038/sj.bjc.6604951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Khor LY, Bae K, Paulus R, Al-Saleem T, Hammond ME, Grignon DJ. et al. MDM2 and Ki-67 predict for distant metastasis and mortality in men treated with radiotherapy and androgen deprivation for prostate cancer: RTOG 92-02. J Clin Oncol. 2009;27:3177–84. doi: 10.1200/JCO.2008.19.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fisher G, Yang ZH, Kudahetti S, Møller H, Scardino P, Cuzick J. et al. Prognostic value of Ki-67 for prostate cancer death in a conservatively managed cohort. Br J Cancer. 2013;108:271–7. doi: 10.1038/bjc.2012.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Verhoven B, Yan Y, Ritter M, Khor LY, Hammond E, Jones C. et al. Ki-67 is an independent predictor of metastasis and cause-specific mortality for prostate cancer patients treated on Radiation Therapy Oncology Group (RTOG) 94-08. Int J Radiat Oncol Biol Phys. 2013;86:317–23. doi: 10.1016/j.ijrobp.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cowen D, Troncoso P, Khoo VS, Zagars GK, von Eschenbach AC, Meistrich ML. et al. Ki-67 staining is an independent correlate of biochemical failure in prostate cancer treated with radiotherapy. Clin Cancer Res. 2002;8:1148–54. [PubMed] [Google Scholar]
- [24].Laitinen S, Martikainen PM, Tolonen T, Isola J, Tammela TL, Visakorpi T. EZH2, Ki-67 and MCM7 are prognostic markers in prostatectomy treated patients. Int J Cancer. 2008;122:595–602. doi: 10.1002/ijc.23145. [DOI] [PubMed] [Google Scholar]
- [25].Zellweger T, Günther S, Zlobec I, Savic S, Sauter G, Moch H. et al. Tumour growth fraction measured by immunohistochemical staining of Ki67 is an independent prognostic factor in preoperative prostate biopsies with small-volume or low-grade prostate cancer. Int J Cancer. 2009;124:2116–23. doi: 10.1002/ijc.24174. [DOI] [PubMed] [Google Scholar]
- [26].Antonarakis ES, Keizman D, Zhang Z, Gurel B, Lotan TL, Hicks JL. et al. An immunohistochemical signature comprising PTEN, MYC, and Ki67 predicts progression in prostate cancer patients receiving adjuvant docetaxel after prostatectomy. Cancer. 2012;118:6063–71. doi: 10.1002/cncr.27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rubio J, Ramos D, López-Guerrero JA, Iborra I, Collado A, Solsona E. et al. Immunohistochemical expression of Ki-67 antigen, cox-2 and Bax/Bcl-2 in prostate cancer; prognostic value in biopsies and radical prostatectomy specimens. Eur Urol. 2005;48:745–51. doi: 10.1016/j.eururo.2005.06.014. [DOI] [PubMed] [Google Scholar]
- [28].Pascale M, Pracella D, Barbazza R, Marongiu B, Roggero E, Bonin S. et al. Is human papillomavirus associated with prostate cancer survival? Dis Markers. 2013;35:607–13. doi: 10.1155/2013/735843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zellweger T, Ninck C, Mirlacher M, Annefeld M, Glass AG, Gasser TC. et al. Tissue microarray analysis reveals prognostic significance of syndecan-1 expression in prostate cancer. Prostate. 2003;55:20–9. doi: 10.1002/pros.10209. [DOI] [PubMed] [Google Scholar]
- [30].Surcel CI, van Oort IM, Sooriakumaran P, Briganti A, De Visschere PJL, Futterer JJ. et al. Prognostic effect of neuroendocrine differentiation in prostate cancer: A critical review. Urol Oncol. 20133:265.e1–7. doi: 10.1016/j.urolonc.2014.08.007. [DOI] [PubMed] [Google Scholar]
- [31].Hirano D, Jike T, Okada Y, Minei S, Sugimoto S, Yamaguchi K. et al. Immunohistochemical and ultrastructural features of neuroendocrine differentiated carcinomas of the prostate: an immunoelectron microscopic study. Ultrastruct Pathol. 2005;29:367–75. doi: 10.1080/019131290945718. [DOI] [PubMed] [Google Scholar]
- [32].Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004;45:586–92. doi: 10.1016/j.eururo.2003.11.032. discussion 592. [DOI] [PubMed] [Google Scholar]
- [33].Beltran H, Tagawa ST, Park K, MacDonald T, Milowsky MI, Mosquera JM. et al. Challenges in recognizing treatment-related neuroendocrine prostate cancer. J Clin Oncol. 2012;30:e386–9. doi: 10.1200/JCO.2011.41.5166. [DOI] [PubMed] [Google Scholar]
- [34].Komiya A, Yasuda K, Watanabe A, Fujiuchi Y, Tsuzuki T, Fuse H. The prognostic significance of loss of the androgen receptor and neuroendocrine differentiation in prostate biopsy specimens among castration-resistant prostate cancer patients. Mol Clin Oncol. 2013;1:257–62. doi: 10.3892/mco.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Aggarwal R, Zhang T, Small EJ, Armstrong AJ. Neuroendocrine prostate cancer: subtypes, biology, and clinical outcomes. J Natl Compr Canc Netw. 2014;12:719–26. doi: 10.6004/jnccn.2014.0073. [DOI] [PubMed] [Google Scholar]
- [36].Ezziddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, Grünwald F. et al. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-Octreotate. J Nucl Med. 2014;55:183–90. doi: 10.2967/jnumed.113.125336. [DOI] [PubMed] [Google Scholar]
- [37].Pelosi G, Rindi G, Travis WD, Papotti M. Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J Thorac Oncol. 2014;9:273–84. doi: 10.1097/JTO.0000000000000092. [DOI] [PubMed] [Google Scholar]
- [38].Mosquera JM, Beltran H, Park K, MacDonald TY, Robinson BD, Tagawa ST. et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia. 2013;15:1–10. doi: 10.1593/neo.121550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Khoo VS, Pollack A, Cowen D, Joon DL, Patel N, Terry NH. et al. Relationship of Ki-67 labeling index to DNA-ploidy, S-phase fraction, and outcome in prostate cancer treated with radiotherapy. Prostate. 1999;41:166–72. doi: 10.1002/(sici)1097-0045(19991101)41:3<166::aid-pros3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- [40].Nagao K, Yamamoto Y, Hara T, Komatsu H, Inoue R, Matsuda K. et al. Ki67 and BUBR1 may discriminate clinically insignificant prostate cancer in the PSA range < 4 ng/ml. Jpn J Clin Oncol. 2011;41:555–64. doi: 10.1093/jjco/hyq233. [DOI] [PubMed] [Google Scholar]
- [41].Zhao L, Yu N, Guo T, Hou Y, Zeng Z, Yang X. et al. Tissue biomarkers for prognosis of prostate cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1047–54. doi: 10.1158/1055-9965.EPI-13-0696. [DOI] [PubMed] [Google Scholar]


