Abstract
Importance
Long-acting opioids increase the risk of unintentional overdose deaths, but also may increase mortality from cardiorespiratory and other causes.
Objective
Compare all-cause mortality for chronic noncancer pain patients prescribed either long-acting opioids or alternative medications for moderate to severe chronic pain.
Design, Setting, and Participants
Retrospective cohort study between 1999 and 2012 of Tennessee Medicaid patients with chronic noncancer pain and no evidence of palliative or end-of-life care.
Exposures
Propensity-score-matched new episodes of prescribed therapy for long-acting opioids or either analgesic anticonvulsants or low dose cyclic antidepressants (control medications).
Main Outcomes and Measures
Total and cause-specific mortality as determined from death certificates. Adjusted hazard ratios (HR) and risk differences (RD, difference in incidence of death between patients with long-acting opioid and control-drug therapy) were calculated for long-acting-opioid versus control-medication patients.
Results
There were 22,912 new episodes of prescribed therapy for both long-acting opioids and control medications (mean age 48, 60% women) with respective mean follow-up of 176 and 128 days and 185 and 87 deaths, respectively. The HR for total mortality was 1.64 (95% CI, 1.26–2.12) with an RD of 69 excess deaths (28–121) per 10,000 person-years. Increased risk was due to out-of-hospital deaths (154 long-acting opioid, 60 control deaths; HR = 1.90 [1.40–2.58], RD = 67 [30–117] excess deaths per 10,000 person-years). For out-of-hospital deaths other than unintentional overdose (120 long-acting opioid, 53 control deaths) the HR was 1.72 (1.24–2.39) with an RD of 47 excess deaths (16–91) per 10,000 person years. The HR for cardiovascular deaths (79 long-acting opioid, 36 control deaths) was 1.65 (1.10–2.46) with an RD of 29 excess deaths (5–65) per 10,000 person-years. The HR during the first 30 days of therapy (53 long-acting opioid, 13 control deaths) was 4.16 (2.27–7.63) with an RD of 200 excess deaths (80–420) per 10,000 person-years.
Conclusions and Relevance
Prescription of long-acting opioids for chronic noncancer pain, compared with anticonvulsants or cyclic antidepressants, was associated with a significantly increased risk for all-cause mortality, including deaths from causes other than overdose, with a modest absolute risk difference. These findings should be considered when evaluating harms and benefits of treatment.
The pronounced increase in the prescribing of opioid analgesics for chronic noncancer pain has led to escalating concern regarding their potential harms.1 The increase in opioid prescribing is paralleled by an increase in overdose deaths1–3 and there is a dose-related elevation in the risk of overdose hospitalization or death.4;5 However, the focus on drug overdose may underestimate the harms of opioid analgesics. Opioids can cause or exacerbate sleep-disordered breathing,6 potentially increasing the risk for adverse cardiovascular events.7 Opioids also have adverse psychomotor,8 endocrine,8 gastrointestinal9 and immunologic10 effects. Long-acting opioids, included in chronic pain guidelines and recommended for patients with frequent or constant pain,11 are of particular concern, given prolonged drug levels and the link between the increase in their use and that for opioid overdose deaths.1;3
Thus, comparative studies of the safety of long-acting opioids relative to other therapy for chronic noncancer pain are needed. Common alternative medications for moderate to severe chronic pain include analgesic anticonvulsants12;13 and low-dose cyclic antidepressants.14 Although these drugs are thought to be relatively safe, they do have potentially serious adverse effects.12;15 However, there are limited data from population-based studies regarding the comparative safety of long-acting opioids.
This study compared the risk of death among patients initiating long-acting opioid therapy for chronic noncancer pain with that for matched patients initiating therapy with either an analgesic anticonvulsant or a low-dose cyclic antidepressant. There were three questions. First, did total mortality differ between the two groups? Second, what was the relative risk for deaths outside of the hospital, which are less likely to be due to existing conditions and most plausibly related to opioid adverse effects?16 Third, were there differences in the risk of deaths other than those from unintentional medication overdose?
Methods
Cohort
This retrospective cohort study included Tennessee Medicaid enrollees initiating therapy with a study drug from 1999 through 2012. The Medicaid files provided an efficient source of data for identifying the cohort, determining periods of probable exposure to medications, and ascertaining deaths.17;18 The Medicaid data included enrollment, pharmacy, hospital, outpatient, and nursing home files, augmented with linkage to death certificates17;19 and a statewide hospital discharge database. The study was reviewed and approved by the IRBs of Vanderbilt University and the State of Tennessee Health Department, which waived informed consent.
To improve study capacity to identify medication-related deaths, thus reducing the potential for confounding, the cohort was limited to patients without evidence of cancer, palliative, or end-of-life care (Appendix Tables 1–2).20 Thus, the cohort excluded persons 75 years of age or older, patients with cancer, other life-threatening diseases or evidence of hospice or other terminal care, and nursing home residents. Hospitalized patients could not enter the cohort until 30 days after discharge, because deaths during this period may have been related to the reasons for the hospitalization. Persons with recorded evidence of drug abuse were excluded, given the increased risk for abuse-related medication overdose.
The cohort consisted of qualifying patients initiating therapy21 with the study drugs who had a diagnosis of chronic pain (back, other musculoskeletal, abdominal, headache, other neurologic) in the past 90 days. The study drugs were the long-acting opioids (morphine SR, oxycodone CR, transdermal fentanyl, methadone) and the control drugs: anticonvulsants indicated for chronic pain (gabapentin, pregabalin, carbamazepine), or low-dose cyclic antidepressants (Appendix Table 5). Patients had a new episode of therapy when they filled a study drug prescription with no prior fill for a drug in that class for the previous year (except for the past 30 days, permitting inclusion of persons starting drug after hospitalization). They could not have had a prescription filled in the prior year for any of the other study drugs. Patients were excluded if the starting daily dose (Appendix Tables 3–5) was not recommended for chronic pain (cyclic antidepressants > 150 mg amitriptyline equivalents) or was unusually high (long-acting opioids > 180 mg morphine-equivalents22 or anticonvulsants > 1800 mg gabapentin equivalents).
Matching
To reduce potential confounding, new episodes of therapy for long-acting opioids were matched to new episodes of therapy for the control drugs according to propensity score, the probability of long-acting opioid use, given the study covariates on cohort entry. These were factors with a plausible direct or indirect relation to both study drug use and mortality (Appendix Table 6). The 122 covariates included demographic characteristics, diagnoses related to chronic pain, use of short-acting opioids and other medications for pain, benzodiazepines and other psychotropic medications linked with risk of overdose death,23 psychiatric diagnoses, cardiovascular conditions, respiratory diseases, other illnesses, and medical care utilization.
The frequency matching was performed by dividing the cohort into centiles (1%) according to the long-acting opioid propensity score distribution. Within each centile, one control drug patient was randomly selected for every opioid patient, randomly discarding opioid patients if there were too few control drug patients. The random frequency matching increased the likelihood that all matches were of equal quality and permitted an unmatched analysis. The final cohort consisted of 1-1 frequency-matched new episodes of therapy with the long-acting opioids and the control drugs (Appendix Table 6).
Follow-up
Patients entered the cohort on the date of the filling of the first study drug prescription. They left the cohort on the earliest of: one year with no filled prescription, filling of a prescription for a drug in a different class (e.g., a long-acting opioid patient or an anticonvulsant patient starts a cyclic antidepressant, regardless of dose), death, failure to meet inclusion-exclusion criteria, or end of the study. Patients who left the cohort could reenter if they subsequently became eligible. Since the episodes were non-overlapping and the end point occurred only once, statistical independence assumptions were satisfied.24
Follow-up was further restricted to the dispensed days of medication therapy included in each study prescription, the time during which patients were most likely to be taking the drug. This period was defined as the interval between the filling of the prescription and the earliest of the end of the days of supply, filling of a subsequent prescription for a drug in the same class, or end of study follow-up. Person-time from the day after hospital admission through the 30 days following discharge was not considered active medication therapy because in-hospital medication data were unavailable and post-discharge medication changes could take up to one refill interval to become known.
End points
The study end point was all deaths during study follow-up. Hospital deaths were those for patients hospitalized on a day of current study drug use who died within 30 days of admission. All other deaths were considered out-of-hospital deaths (including patients who died in the emergency department) and were further classified as unintentional medication overdose or other deaths. The latter included cardiovascular, respiratory, other injury, or other deaths (Appendix Table 7). In one analysis, we examined cardiovascular mortality for the subgroups defined by specific cardiovascular diagnoses (Appendix Table 8).
Statistical Analysis
The statistical analysis compared the adjusted risk of death during follow-up for long-acting opioid patients to that for control medication patients. Relative risk was estimated with the hazard ratio (HR), calculated from Cox regression models (Appendix §6). To adjust for residual confounding, regression models were stratified according to deciles of the baseline propensity score.25 The primary models included age, calendar year, and study medication as time-dependent covariates, estimated via a counting process formulation that accommodates non-proportional hazards (see Allison,26 p.172). Other time-dependent covariates were not included in the primary analysis because these might be on the causal pathway for mortality (e.g., non-fatal injury). A sensitivity analysis included a time-dependent propensity score27 that accounted for changes in study covariates during follow-up (Appendix §6).
The adjusted risk difference (RD), or difference in the incidence of death between patients with long-acting opioid and control-drug therapy, was estimated. The RD was calculated as I0*(HR-1), where HR is the adjusted hazard ratio and I0 the unadjusted incidence for control medication patients. 95% confidence intervals were calculated analogously.
The analysis included a time-dependent analysis of the relation of duration of study drug therapy and dose during follow-up to total study mortality. Duration was defined as cumulative days of prior use on the day a study drug prescription was filled. Cutpoints for low (≤ cutpoint) versus high dose (> cutpoint) were the approximate median time-dependent doses: 60 mg/day morphine equivalents, 600 mg/day gabapentin equivalents and 40 mg/day amitriptyline equivalents. The regression analysis was stratified by 20 quantiles of a time-dependent disease risk score (Appendix §6).28–30 The disease risk score, the risk of death as a function of the study covariates given the reference exposure category, facilitates analyses for multiple exposure categories, given that propensity scores are less suited to non-binary comparisons.28–30
Sensitivity analyses that assessed populations of particular interest or tested study assumptions were performed. These included exclusion of patients prescribed methadone, restriction of the cohort to patients with a diagnosis of neurologic pain, use of control groups consisting exclusively of propensity-score matched anticonvulsant or cyclic antidepressant patients, exclusion of patients entering the cohort before 2003, restriction of analysis to the first 180 days of therapy, exclusion of deaths with unknown cause from the cardiovascular death category, and expansion of the non-overdose and cardiovascular death categories to include hospital deaths.
The effect of cardiovascular death misclassification was assessed (Appendix §8) by adjudication of a convenience sample of 50 deaths from a previous study of long-acting opioids for which medical records had been reviewed.16 The analysis made the conservative assumption that control medication deaths were not misclassified.
All analyses were done with SAS version 9.4. All p-values are two-sided, with a p-value less than .05 indicating statistical significance.
Results
There were 23,308 new episodes of prescriptions for long-acting opioids and 131,883 new episodes of prescriptions for control medications (Table 1). These groups differed with regard to baseline characteristics, with standardized differences exceeding 10% for most study covariates (Table 1). After matching, the cohort included 22,912 long-acting opioid episodes and an equal number of control medication episodes. The matched long-acting opioid and control medication groups were more comparable, with no standardized difference exceeding 3% and the majority less than 1%. The mean age of the matched patients was 48 years and 60% were female. The most common chronic pain diagnoses were back pain (75%), other musculoskeletal pain (63%), and abdominal pain (18%). More than 96% of study patients had filled a prescription for a short-acting opioid in the prior year and 68% had a current prescription for these drugs at the beginning of follow-up. Patients frequently filled prescriptions for other pain medications and psychotropic drugs, including skeletal muscle relaxants (63%), non-steroidal antiinflammatory drugs (70%), benzodiazepines (52%), and selective serotonin or serotonin and norepinephrine reuptake inhibitor antidepressants (45%).
Table 1.
Selected baseline characteristicsa for new episodes of study drug therapy. Abbreviations: SD, standard deviation; SSRI, selective serotonin uptake inhibitor; SNRI, selective norepinephrine uptake inhibitor; COPD, chronic obstructive pulmonary disease; ED, emergency department.
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Anticonvulsant or Cyclic Antidepressant | Long-Acting Opioid | Standardized difference, % | Anticonvulsant or Cyclic Antidepressant | Long-Acting Opioid | Standardized difference, % | |
| N in cohort | 131,883 | 23,308 | 22,912 | 22,912 | ||
| Demographics | ||||||
| Age, mean (SD), y | 46.7 (11.0) | 47.9 (10.5) | 11.2% | 47.9 (10.7) | 47.9 (10.5) | 0.0% |
| Female sex | 96,163 (73%) | 13,878 (60%) | 28.6% | 13,696 (60%) | 13,738 (60%) | 0.4% |
| Medicaid disability enrollment | 61,948 (47%) | 13,701 (59%) | 23.8% | 13,421 (59%) | 13,385 (58%) | 0.3% |
| Chronic pain past 90 days | ||||||
| Back pain | 65,880 (50%) | 17,462 (75%) | 53.4% | 17,333 (76%) | 17,071 (75%) | 2.6% |
| Other musculoskeletal pain | 75,103 (57%) | 14,796 (63%) | 13.4% | 14,601 (64%) | 14,512 (63%) | 0.8% |
| Abdominal pain | 26,577 (20%) | 4,167 (18%) | 5.8% | 4,093 (18%) | 4,108 (18%) | 0.2% |
| Headache | 29,782 (23%) | 2,783 (12%) | 28.4% | 2,663 (12%) | 2,773 (12%) | 1.5% |
| Other neurologic pain | 25,343 (19%) | 3,909 (17%) | 6.4% | 3,736 (16%) | 3,855 (17%) | 1.4% |
| Short-acting opioid use | ||||||
| Any use past year | 110,487 (84%) | 22,468 (96%) | 43.2% | 22,203 (97%) | 22,072 (96%) | 3.2% |
| >270 days use past year | 13,937 (11%) | 6,430 (28%) | 44.4% | 6,025 (26%) | 6,192 (27%) | 1.6% |
| Current use | 55,652 (42%) | 15,733 (68%) | 36.6% | 15,629 (68%) | 15,361 (67%) | 2.5% |
| Current use >60 mg morphine equivalents | 6,315 (5%) | 3,440 (15%) | 34.1% | 3,012 (13%) | 3,239 (14%) | 2.9% |
| Other analgesic or psychotropic drug past year | ||||||
| Skeletal muscle relaxant | 67,544 (51%) | 14,659 (63%) | 23.8% | 14,378 (63%) | 14,361 (63%) | 0.2% |
| Non-steroidal antiinflammatory drug | 95,366 (72%) | 16,099 (69%) | 7.1% | 16,008 (70%) | 15,886 (69%) | 1.2% |
| Benzodiazepine | 46,613 (35%) | 12,338 (53%) | 36.0% | 11,774 (51%) | 11,986 (52%) | 1.9% |
| SSRI or SNRI | 52,751 (40%) | 10,641 (46%) | 11.4% | 10,360 (45%) | 10,436 (46%) | 0.7% |
| Trazodone | 15,529 (12%) | 3,046 (13%) | 3.9% | 2,987 (13%) | 2,997 (13%) | 0.1% |
| Other comorbidity past year | ||||||
| AMI, revascularization, or angina | 7,002 (5%) | 1,426 (6%) | 3.5% | 1,398 (6%) | 1,402 (6%) | 0.1% |
| Congestive heart failure | 5,219 (4%) | 1,269 (5%) | 7.0% | 1,239 (5%) | 1,237 (5%) | 0.0% |
| Cerebrovascular disease | 6,979 (5%) | 1,236 (5%) | 0.0% | 1,239 (5%) | 1,213 (5%) | 0.5% |
| COPD | 18,696 (14%) | 4,751 (20%) | 16.5% | 4,593 (20%) | 4,611 (20%) | 0.2% |
| Asthma | 14,501 (11%) | 2,620 (11%) | 0.8% | 2,488 (11%) | 2,578 (11%) | 1.3% |
| Home oxygen | 5,305 (4%) | 1,416 (6%) | 9.4% | 1,389 (6%) | 1,372 (6%) | 0.3% |
| Hospital stay | 18,740 (14%) | 4,108 (18%) | 9.3% | 3,986 (17%) | 4,025 (18%) | 0.4% |
| Injury ED visit | 37,430 (28%) | 7,676 (33%) | 9.9% | 7,635 (33%) | 7,531 (33%) | 1.0% |
The characteristics were selected as those the authors considered to most likely to be associated with greater risk of mortality and with the decision to prescribe a long-acting opioid. Appendix Table 6 lists the distribution of all study covariates.
The most commonly prescribed medications in the cohort were morphine SR, gabapentin, and amitriptyline (Table 2). The median doses at the time of cohort entry were 50 mg morphine-equivalents for the long-acting opioids, 600 mg gabapentin-equivalents for the analgesic anticonvulsants, and 25 mg amitriptyline-equivalents for the cyclic antidepressants.
Table 2.
Study drugs before and after matching. Abbreviations: IQR, interquartile range, SR, sustained release, CR, controlled release.
| Before Matching | After Matching | |
|---|---|---|
| N (%) | N (%) | |
| Patients | ||
| Long-acting opioids | ||
| All | 23,308 (100%) | 22,912 (100%) |
| Morphine SR | 12,891 (55%) | 12,667 (55%) |
| Oxycodone CR | 5,539 (24%) | 5,446 (24%) |
| Fentanyl transdermal | 3,377 (14%) | 3,323 (14%) |
| Methadone | 1,501 (6%) | 1,476 (6%) |
| Anticonvulsant or cyclic antidepressant | ||
| All | 131,883 (100%) | 22,912 (100%) |
| Gabapentin | 53,078 (40%) | 10,879 (47%) |
| Pregabalin | 7,272 (6%) | 1,403 (6%) |
| Carbamazepine | 3,884 (3%) | 579 (3%) |
| Amitriptyline | 48,072 (36%) | 6,959 (30%) |
| Doxepin | 7,382 (6%) | 1,266 (6%) |
| Nortriptyline | 7,075 (5%) | 1,071 (5%) |
| Other cyclic antidepressant | 5,120 (4%) | 755 (3%) |
| Dose, mg, median (IQR) | Median (IQR) | Median (IQR) |
| Long-acting opioids, morphine equivalents | 50 (30–60) | 50 (30–60) |
| Analgesic anticonvulsants, gabapentin equivalents | 600 (300–900) | 600 (300–900) |
| Cyclic antidepressants, amitriptyline equivalents | 25 (25–50) | 25 (25–50) |
Long-acting opioid patients had 185 deaths during 11,070 person-years of follow-up (167 per 10,000 person-years), whereas there were 87 deaths during 8,066 person-years of follow-up for control medication patients (108 per 10,000). The adjusted HR for death from any cause during follow-up was 1.64 (1.26–2.12), and the RD was 69 (28–121) excess deaths per 10,000 person-years (Table 3). The elevated risk of death for long-acting opioids was confined to the out-of-hospital deaths (HR = 1.90 [1.40–2.58], RD = 67 [30–117] per 10,000 person-years), which constituted 79% of study deaths. There was no increased risk for in-hospital deaths (HR = 1.00 [0.59–1.69], RD = 0 [−14–23] per 10,000 person-years). The HR for out-of-hospital deaths with a cause of death other than unintentional overdose was 1.72 (1.24–2.39) with an RD of 47 (16–91) per 10,000 person-years. The most frequent category of non-overdose deaths was cardiovascular deaths, with an HR of 1.65 (1.10–2.46) and an RD of 29 (5–65) per 10,000 person-years. Long-acting opioid patients had elevated cardiovascular mortality for all of the subgroups defined by specific cardiovascular diagnoses, with the exception of diabetes (Appendix Table 8).
Table 3.
Mortality according to underlying cause of death.
| Anticonvulsant or Cyclic Antidepressant (8,066 person-years follow-up) | Long-acting Opioid (11,070 person-years follow-up) | Adjusted Hazard Ratioa (95% CI) | Adjusted Risk Differencea,b (95% CI) | p | |||
|---|---|---|---|---|---|---|---|
| Deaths | Incidence/10,000 Person-Years | Deaths | Incidence/10,000 Person-Years | ||||
| All deaths | 87 | 107.9 | 185 | 167.1 | 1.64 (1.26–2.12) | 68.5 (28.2–120.7) | <.001 |
| Out-of-hospital deaths | 60 | 74.4 | 154 | 139.1 | 1.90 (1.40–2.58) | 67.1 (30.1–117.3) | <.001 |
| Unintentional overdose deathsc | 7 | 8.7 | 34 | 30.7 | 3.37 (1.47–7.70) | 20.6 (4.1–58.1) | 0.004 |
| Other causes of death | 53 | 65.7 | 120 | 108.4 | 1.72 (1.24–2.39) | 47.4 (15.7–91.4) | 0.001 |
| Cardiovascular | 36 | 44.6 | 79 | 71.4 | 1.65 (1.10–2.46) | 28.9 (4.6–65.3) | 0.015 |
| Respiratory | 3 | 3.7 | 10 | 9.0 | 3.00 (0.81–11.09) | 7.4 (−0.7–37.5) | 0.100 |
| Other Injury | 11 | 13.6 | 19 | 17.2 | 1.15 (0.54–2.47) | 2.1 (−6.3–20.0) | 0.716 |
| Other | 3 | 3.7 | 12 | 10.8 | 3.72 (1.04–13.30) | 10.1 (0.2–45.7) | 0.043 |
| Hospital Deaths | 27 | 33.5 | 31 | 28.0 | 1.00 (0.59–1.69) | 0.0 (−13.6–23.1) | 0.996 |
Adjusted for baseline propensity score decile and age and calendar year during followup.
Risk differences for the specific causes of death do not sum because the regression model parameters are estimated separately for each cause.
The cohort excluded patients with a diagnosis of or procedure for treatment of substance abuse other than nicotine or alcohol as well as those prescribed buprenorphine. Because such patients would plausibly have increased risk for overdose, overdose mortality in the study cohort is likely to be lower than that in a more general patient population.
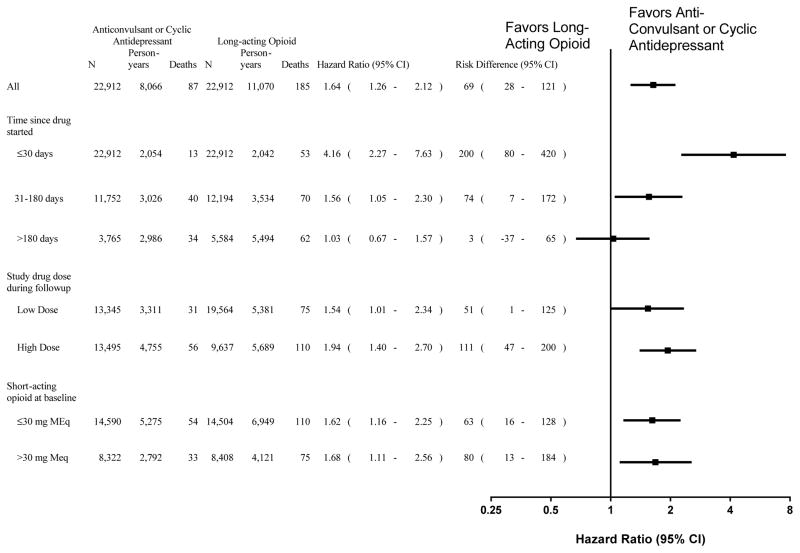
The increased mortality for long-acting opioid patients was limited to the first 180 days of prescribed therapy (Figure). During the first 30 days of therapy, the HR was 4.16 (2.27–7.63) and the RD was 200 (80–420) per 10,000 person-years; for the remainder of the first 180 days the HR was 1.56 (1.05–2.30) and the RD was 74 (7–172) per 10,000 person-years. By contrast, once long-acting opioid patients had more than 180 days of therapy, their risk of death did not differ significantly from that of comparable control drug patients (HR = 1.03 [0.67–1.57], RD = 3 [−37–65] per 10,000 person-years).
Figure. Mortality according to study drug duration, dose, and baseline use short-acting opioids.
N indicates number of patients. An individual patient can be in multiple duration and dose categories during followup; thus, the numbers do not sum to the total cohort size. Adjusted hazard ratios and risk differences are shown are shown (95% confidence interval in parentheses) for current use of long-acting opioids versus current use of analgesic anticonvulsants or cyclic antidepressants. The estimates according to duration of use and study drug dose during follow-up are adjusted for a time-dependent disease risk score; those for baseline use of short-acting opioids are adjusted for baseline propensity score and age and calendar year during followup. Cutpoints for low (≤ cutpoint) versus high (> cutpoint) study drug dose were: 60 mg/day morphine equivalents, 600 mg/day gabapentin equivalents and 40 mg/day amitriptyline equivalents. For short-acting opioids, doses are in morphine equivalents.
For both low and high doses of study drugs, total mortality for long-acting opioid patients was greater than that for comparable control drug patients (Figure). For low doses (≤60 mg of morphine or its equivalent) the HR was 1.54 (1.01–2.34) and the RD was 51 (1–126) per 10,000 person-years; for high doses (>60 mg morphine or its equivalent) the HR was 1.94 (1.40–2.70) and the RD was 111 (47–200) per 10,000 person-years. Similarly, long-acting opioid patients had greater mortality within groups defined by baseline short-acting opioid doses of ≤30 mg or >30 mg morphine-equivalents.
Sensitivity analyses that assessed populations of particular interest or tested study assumptions were performed (Table 4). In each case, findings were similar to those from the primary analysis.
Table 4.
Sensitivity analyses. CI = confidence interval.
| Anticonvulsant (AC) or Cyclic Antidepressant (TCA) | Long-acting Opioids (LAO) | Adjusted Hazard Ratioa (95% CI) | Adjusted Risk Differencea,b (95% CI) | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths | Incidence/10,000 Person-Years | Deaths | Incidence/10,000 Person-Years | ||||||
| Methadone excluded (AC or TCA: 22,912 patients, 8,066 person-years; LAO: 21,436 patients, 10,255 person-years) | |||||||||
| All deaths | 87 | 107.9 | 166 | 161.9 | 1.56 | (1.20–2.04) | 60.9 | (21.7–111.9) | 0.001 |
| Out of hospital deaths | 60 | 74.4 | 135 | 131.6 | 1.77 | (1.30–2.41) | 57.3 | (22.2–105.1) | <.001 |
| Not overdose death | 53 | 65.7 | 106 | 103.4 | 1.62 | (1.16–2.26) | 40.6 | (10.3–83.1) | 0.005 |
| Cardiovascular deaths | 36 | 44.6 | 70 | 68.3 | 1.56 | (1.04–2.35) | 25.1 | (1.7–60.4) | 0.033 |
| Neurologic pain diagnosis (AC or TCA: 14,316 patients, 4,923 person-years; LAO: 14,021 patients, 7,013 person-years) | |||||||||
| All deaths | 50 | 101.6 | 101 | 144.0 | 1.54 | (1.09–2.18) | 54.9 | (9.1–119.6) | 0.015 |
| Out of hospital deaths | 35 | 71.1 | 90 | 128.3 | 1.91 | (1.28–2.85) | 64.8 | (20.2–131.2) | 0.001 |
| Not overdose death | 32 | 65.0 | 64 | 91.3 | 1.52 | (0.98–2.34) | 33.6 | (−1.1–87.2) | 0.059 |
| Cardiovascular deaths | 19 | 38.6 | 40 | 57.0 | 1.60 | (0.92–2.79) | 23.2 | (−3.2–69.2) | 0.098 |
| Anticonvulsant-only control group (AC:20,296 patients, 7,991 person-years; LAO: 20,296 patients, 9,441 person-years) | |||||||||
| All deaths | 84 | 105.1 | 148 | 156.8 | 1.56 | (1.19–2.05) | 58.8 | (19.8–110.0) | 0.001 |
| Out of hospital deaths | 61 | 76.3 | 126 | 133.5 | 1.79 | (1.31–2.43) | 60.0 | (23.7–109.5) | <.001 |
| Not overdose death | 48 | 60.1 | 98 | 103.8 | 1.80 | (1.27–2.55) | 48.0 | (16.1–93.3) | 0.001 |
| Cardiovascular deaths | 29 | 36.3 | 66 | 69.9 | 1.97 | (1.27–3.07) | 35.3 | (9.6–75.2) | 0.003 |
| Cyclic antidepressant-only control group (TCA:18,106 patients, 5,650 person-years; LAO: 18,106 patients, 8,626 person-years) | |||||||||
| All deaths | 52 | 92.0 | 123 | 142.6 | 1.84 | (1.32–2.56) | 77.3 | (29.7–143.7) | <.001 |
| Out of hospital deaths | 29 | 51.3 | 100 | 115.9 | 2.52 | (1.65–3.83) | 77.8 | (33.5–145.4) | <.001 |
| Not overdose death | 26 | 46.0 | 80 | 92.7 | 2.31 | (1.47–3.63) | 60.4 | (21.8–121.0) | <.001 |
| Cardiovascular deaths | 17 | 30.1 | 50 | 58.0 | 2.25 | (1.28–3.94) | 37.6 | (8.5–88.5) | 0.005 |
| Cohort entry 2003 or later (AC or TCA: 15,209 patients, 5,080 person-years; LAO: 15,030 patients, 6,150 person-years) | |||||||||
| All deaths | 49 | 96.5 | 103 | 167.5 | 1.82 | (1.29–2.56) | 78.8 | (27.8–150.7) | <.001 |
| Out of hospital deaths | 37 | 72.8 | 88 | 143.1 | 1.98 | (1.34–2.92) | 71.5 | (25.1–140.0) | <.001 |
| Not overdose death | 31 | 61.0 | 66 | 107.3 | 1.80 | (1.17–2.77) | 48.9 | (10.4–108.3) | <.001 |
| Cardiovascular deaths | 19 | 37.4 | 43 | 69.9 | 1.84 | (1.06–3.18) | 31.3 | (2.4–81.4) | 0.029 |
| Duration therapy <180 days (AC or TCA: 22,912 patients, 5,081 person-years; LAO: 22,912 patients, 5,576 person-years) | |||||||||
| All deaths | 53 | 104.3 | 123 | 220.6 | 2.16 | (1.56–2.98) | 121.0 | (58.8–206.8) | <.001 |
| Out of hospital deaths | 39 | 76.8 | 101 | 181.1 | 2.39 | (1.65–3.46) | 106.5 | (49.8–188.6) | <.001 |
| Not overdose death | 36 | 70.9 | 77 | 138.1 | 1.98 | (1.33–2.94) | 69.4 | (23.4–137.6) | <.001 |
| Cardiovascular deaths | 22 | 43.3 | 50 | 89.7 | 2.12 | (1.28–3.50) | 48.4 | (12.1–108.2) | 0.004 |
| Deaths with unknown cause not considered cardiovascular deaths (AC or TCA: 22,912 patients, 8,066 person-years; LAO: 22,912 patients, 11,070 person-years) | |||||||||
| Not overdose death | 53 | 65.7 | 120 | 108.4 | 1.72 | (1.24–2.39) | 47.4 | (15.7–91.4) | 0.001 |
| Cardiovascular deaths | 35 | 43.4 | 72 | 65.0 | 1.55 | (1.03–2.34) | 23.8 | (1.2–58.0) | 0.037 |
| Specific death categories include hospital deaths (AC or TCA: 22,912 patients, 8,066 person-years; LAO: 22,912 patients, 11,070 person-years) | |||||||||
| Not overdose death | 77 | 95.5 | 151 | 136.4 | 1.55 | (1.17–2.05) | 52.4 | (16.4–100.1) | 0.002 |
| Cardiovascular deaths | 51 | 63.2 | 96 | 86.7 | 1.46 | (1.03–2.07) | 29.2 | (2.2–67.5) | 0.031 |
Adjusted for baseline propensity score decile and age and calendar year during followup.
Risk differences for the specific causes of death do not sum because the regression model parameters are estimated separately for each cause
The medical-records based cardiovascular death misclassification analysis (Appendix §8) found that 44% of total out-of-hospital deaths in the convenience sample met the criteria for cardiovascular death, slightly lower than the 46% based on the death certificate underlying cause of death. This degree of misclassification would decrease the observed HR for cardiovascular deaths from 1.65 (1.10–2.46) to 1.58 (1.05–2.36), although, depending on the specific adjudication criteria, the HR could have been as small as 1.36 (0.90–2.06, no longer statistically significant) or as large as 1.79 (1.21–2.66) (Appendix §8).
Discussion
Although long-acting opioids increase the risk of unintentional overdose,1–3;5 their overall safety relative to other medications commonly prescribed to treat noncancer pain has not been previously well quantified. This study found that patients prescribed therapy for a long-acting opioid had a risk of all-cause mortality 1.64 times greater than that for matched patients starting an analgesic anticonvulsant or a low-dose cyclic antidepressant, corresponding to 69 excess deaths per 10,000 person-years of therapy. This difference was explained by a 1.90 times greater risk of out-of-hospital deaths. More than two-thirds of the excess deaths were due to causes other than unintentional overdose; of these, more than one-half were cardiovascular deaths. The increased risk was confined to the first 180 days of prescribed therapy, but was present for long-acting opioid doses of ≤60 mg morphine-equivalents.
The study was designed to reduce confounding by factors associated with starting a long-acting opioid. The cohort excluded patients with evidence of palliative or end-of-life care. It was restricted to those initiating therapy with study medications, managing the bias inherent in study of those who survive a high-risk early exposure period.21 Patients in the two study groups were tightly matched according to potential confounders, including chronic pain diagnoses, patterns of prior use of short-acting opioids and other analgesics, use of benzodiazepines and other psychotropic drugs associated with increased risk of overdose deaths,23 and cardiovascular, respiratory and other somatic comorbidity.
It is important to consider whether the elevated risk for long-acting opioids is due to confounding by indication. Long-acting opioids have been widely recommended for chronic noncancer pain.11;22 Gabapentin and pregabalin are indicated for neuropathic pain and fibromyalgia,12;31;32 and low-dose cyclic antidepressants for chronic back pain, neuropathic pain, and fibromyalgia.14;32;33 In clinical practice all are widely prescribed for chronic back and other musculoskeletal pain,13;14;32;34 by far the most common recorded diagnosis in the study cohort. Thus, material confounding by indication seems unlikely, a conclusion supported by the essentially unchanged findings for control groups restricted to patients with a diagnosis of neurologic pain or consisting exclusively of patients prescribed either anticonvulsants or cyclic antidepressants alone. Patients starting a long-acting opioid may have had other unmeasured factors that increased risk of death; however, the absence of increased risk for the hospital deaths and the marked elevation in risk early in therapy argue against such confounding.
The increased risk for long-acting opioids was not confined to deaths identified as due to unintentional overdose. Thus, of the estimated 69 excess deaths per 10,000 person years of followup among long-acting opioid patients, 47 had an underlying cause of death other than unintentional overdose and 29 had a cardiovascular cause of death. The increased risk for cardiovascular death persisted when patients prescribed methadone, a known pro-arrhythmic drug,16 were excluded from the cohort.
The increased risk of cardiovascular death could be related to adverse respiratory effects of long-acting opioids. Opioids can cause or exacerbate sleep-disordered breathing, including both obstructive and central sleep apnea;35–37 and patients with sleep-disordered breathing have increased incidence of nocturnal arrhythmias, myocardial ischemia or infarction, and sudden death.7 Study findings are consistent with those of Solomon and colleagues, who found that older adults with arthritis prescribed opioids (predominantly short-acting) had nearly twice the risk of out-of-hospital cardiac death as did comparable patients prescribed non-selective NSAIDs.38
A study limitation was reliance on the death certificate to classify the cause of death, thus raising the possibility that the cardiovascular death finding was due to misclassification. A sensitivity analysis based upon a convenience sample of deaths for which medical records were reviewed provided evidence that misclassification was unlikely to explain the elevated cardiovascular death risk, although under a worst-case scenario the cardiovascular death HR was no longer statistically significant and the convenience sample analysis had several other limitations (Appendix §8). Furthermore, more than two-thirds of the excess deaths for long-acting opioid patients were not coded as due to unintentional overdose. If there is this degree of misclassification, then previous research on opioid mortality, most of which has focused on overdose deaths identified from death certificates,2;3;5 has substantially underestimated the true risks of opioids.
The study cohort differed from other populations of patients taking long-acting opioids. To improve study capacity to detect adverse effects of long-acting opioids, the cohort consisted of patients for whom illness-related deaths should be relatively infrequent. Thus, it excluded persons 75 years of age or older, patients with cancer, other life-threatening diseases, or evidence of palliative or end-of-life care, and nursing home residents. These restrictions were likely to reduce study cohort mortality, as the excluded patients would have higher baseline risk and could be more susceptible to adverse medication effects. The cohort also excluded patients with any recorded evidence of drug abuse, thus underestimating the potential for overdose. Conversely, the cohort consisted of Medicaid enrollees, who are likely to have had greater mortality than the population at large.39
The study findings reinforce the conclusion of the recent CDC guideline for prescribing opioids for chronic noncancer pain that “of primary importance, nonopoid therapy is preferred for treatment of chronic pain”.20 Although this study did not consider medication efficacy, the CDC’s synthesis of the available evidence suggests the efficacy of nonopoid pharmacotherapy for many chronic conditions is at least equal to that of opioids. The study finding that prescription of long-acting opioids was associated with increased cardiovascular and other non-overdose mortality adds to the already considerable known harms of the opioids and thus should be considered when assessing the benefits and harms of medications for chronic pain. Nevertheless, for some individual patients, the therapeutic benefits from long-acting opioid therapy may outweigh the modest increase in mortality risk. As the CDC guideline indicates, all prescribing decisions must be based on an evaluation of the source and severity of the patient’s pain and a discussion of the “known risks and realistic benefits of opioid therapy”.20
Conclusion
Prescription of long-acting opioids for chronic noncancer pain, compared with anticonvulsants or cyclic antidepressants, was associated with a significantly increased risk for all-cause mortality, including deaths from causes other than overdose, with a modest absolute risk difference. These findings should be considered when evaluating harms and benefits of treatment.
Supplementary Material
Key Points.
Question
What is the relative risk of death from any cause for patients with chronic noncancer pain and no evidence of end-of-life care who begin therapy with either long-acting opioids or analgesic anticonvulsants or cyclic antidepressants, alternative medications for moderate to severe chronic pain?
Findings
In this retrospective cohort study that included 22,912 new episodes of prescribed therapy for both long-acting opioids and alternative medications, the long-acting opioid patients had a 64% increased risk of all-cause mortality, including a 65% increased risk for cardiovascular death.
Meaning
These findings support recommendations from recent guidelines to avoid opioid therapy for chronic noncancer pain when possible.
Acknowledgments
Funding/Support: This work was supported by National Heart Lung, and Blood Institute grant 5R01HL081707 (to Dr. Ray). Dr. Chung was additionally supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases grant K23AR064768 as well as by a grant from the Rheumatology Research Foundation. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr. Ray had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Role of the Funder/Sponsor: The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Additional Contributions: We gratefully acknowledge the Tennessee Bureau of TennCare and the Tennessee Department of Health, which provided study data.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health, the Department of Health and Human Services, the Tennessee Bureau of TennCare, or the Tennessee Department of Health.
References
- 1.Okie S. A flood of opioids, a rising ride of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 2.Vital signs: overdoses of prescription opioid pain relievers---United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–1492. [PubMed] [Google Scholar]
- 3.Dhalla IA, Mamdani MM, Sivilotti ML, Kopp A, Qureshi O, Juurlink DN. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ. 2009;181:891–896. doi: 10.1503/cmaj.090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 6.Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120:1273–1285. doi: 10.1213/ANE.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 7.Mansukhani MP, Wang S, Somers VK. Sleep, death, and the heart. Am J Physiol Heart Circ Physiol. 2015;309:H739–H749. doi: 10.1152/ajpheart.00285.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 9.Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473:2402–2412. doi: 10.1007/s11999-015-4173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brack A, Rittner HL, Stein C. Immunosuppressive effects of opioids--clinical relevance. J Neuroimmune Pharmacol. 2011;6:490–502. doi: 10.1007/s11481-011-9290-7. [DOI] [PubMed] [Google Scholar]
- 11.McCarberg BH, Barkin RL. Long-acting opioids for chronic pain: pharmacotherapeutic opportunities to enhance compliance, quality of life, and analgesia. Am J Ther. 2001;8:181–186. doi: 10.1097/00045391-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011:CD007938. doi: 10.1002/14651858.CD007938.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen L, Hansen AB, Svendsen K, Skurtveit S, Borchgrevink PC, Fredheim OM. Reimbursement of analgesics for chronic pain. Tidsskr Nor Laegeforen. 2012;132:2489–2493. doi: 10.4045/tidsskr.11.1214. [DOI] [PubMed] [Google Scholar]
- 14.Carragee EJ. Clinical practice. Persistent low back pain. N Engl J Med. 2005;352:1891–1898. doi: 10.1056/NEJMcp042054. [DOI] [PubMed] [Google Scholar]
- 15.Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther. 2003;75:234–241. doi: 10.1016/j.clpt.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Ray WA, Chung CP, Murray KT, Cooper WO, Hall K, Stein CM. Out-of-hospital mortality among patients receiving methadone for noncancer pain. JAMA Intern Med. 2015;175:420–427. doi: 10.1001/jamainternmed.2014.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. Am J Epidemiol. 1989;129:837–849. doi: 10.1093/oxfordjournals.aje.a115198. [DOI] [PubMed] [Google Scholar]
- 18.Ray WA. Population-based studies of adverse drug effects. N Engl J Med. 2003;349:1592–1594. doi: 10.1056/NEJMp038145. [DOI] [PubMed] [Google Scholar]
- 19.Piper JM, Ray WA, Griffin MR, Fought R, Daugherty JR, Mitchel E., Jr Methodological issues in evaluating expanded Medicaid coverage for pregnant women. Am J Epidemiol. 1990;132:561–571. doi: 10.1093/oxfordjournals.aje.a115692. [DOI] [PubMed] [Google Scholar]
- 20.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315:1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray WA. Evaluating medication effects outside of clinical trials: New-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 22.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 23.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westreich D, Cole SR, Tien PC, Chmiel JS, Kingsley L, Funk MJ, Anastos K, Jacobson LP. Time scale and adjusted survival curves for marginal structural cox models. Am J Epidemiol. 2010 Mar 15;171(6):691–700. doi: 10.1093/aje/kwp418. Epub 2010 Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shadish WR, Steiner PM. A primer on propensity score analysis. Newborn & Infant Nursing Reviews. 2010;10:19–26. [Google Scholar]
- 26.Allison PD. Survival Analysis Using SAS. A Practical Guide. 2. Cary, NC: SAS Institute; 2010. [Google Scholar]
- 27.Ray WA, Liu Q, Shepherd BE. Performance of time-dependent propensity scores: a pharmacoepidemiology case study. Pharmacoepidemiol Drug Saf. 2015;24:98–106. doi: 10.1002/pds.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arbogast PG, Kaltenbach L, Ding H, Ray WA. Adjustment of multiple cardiovascular risk factors with a summary risk score. Epidemiol. 2008;19:30–37. doi: 10.1097/EDE.0b013e31815be000. [DOI] [PubMed] [Google Scholar]
- 29.Arbogast PG, Ray WA. Use of disease risk scores in pharmacoepidemiologic studies. Statistical Meth Med Res. 2009;18:67–80. doi: 10.1177/0962280208092347. [DOI] [PubMed] [Google Scholar]
- 30.Arbogast PG, Ray WA. Performance of disease risk scores, propensity scores, and traditional multivariable outcome regression in the presence of multiple confounders. Am J Epi. 2011;174:613–620. doi: 10.1093/aje/kwr143. [DOI] [PubMed] [Google Scholar]
- 31.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505–514. doi: 10.7326/0003-4819-147-7-200710020-00008. [DOI] [PubMed] [Google Scholar]
- 33.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;12:CD008242. doi: 10.1002/14651858.CD008242.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Ghinea N, Lipworth W, Kerridge I. Evidence, regulation and ‘rational’ prescribing: the case of gabapentin for neuropathic pain. J Eval Clin Pract. 2015;21:28–33. doi: 10.1111/jep.12223. [DOI] [PubMed] [Google Scholar]
- 35.Yue HJ, Guilleminault C. Opioid medication and sleep-disordered breathing. Med Clin North Am. 2010;94:435–446. doi: 10.1016/j.mcna.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Zutler M, Holty JE. Opioids, sleep, and sleep-disordered breathing. Curr Pharm Des. 2011;17:1443–1449. doi: 10.2174/138161211796197070. [DOI] [PubMed] [Google Scholar]
- 37.Lee-Iannotti J, Parish JM. The epidemic of opioid use: implications for the sleep physician. J Clin Sleep Med. 2014;10:645–646. doi: 10.5664/jcsm.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170:1968–1976. doi: 10.1001/archinternmed.2010.391. [DOI] [PubMed] [Google Scholar]
- 39.Center for Disease Control. Overdose deaths involving prescription opioids among Medicaid enrollees - Washington, 2004–2007. MMWR. 2009;58:1171–1175. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.