Abstract
The impact of JAK1/2 inhibitor therapy prior to allogeneic hematopoietic cell transplantation (HCT) has not been studied in a large cohort in myelofibrosis (MF). In this retrospective multicenter study, we analyzed outcomes of patients who underwent HCT for MF with prior exposure to JAK1/2 inhibitors.
One hundred consecutive patients from participating centers were analyzed, and based on clinical status and response to JAK1/2 inhibitors at the time of HCT, patients were stratified into five groups: (a) clinical improvement (n=23), (b) stable disease (n=31), (c) new cytopenia/increasing blasts/intolerance (n=15), (d) progressive disease: splenomegaly (n=18), and (e) progressive disease: leukemic transformation (LT) (n=13). Overall survival (OS) at two years was 61% (95%CI, 49–71). This was 91% (95% CI, 69–98) for those who experienced clinical improvement, and 32% (95% CI, 8–59) for those who developed LT on JAK1/2 inhibitors. In multivariable analysis, response to JAK1/2 inhibitors (p=0.03), DIPSS score (p=0.003), and donor type (p=0.006) were independent predictors of survival. Among the 66 patients who remained on JAK1/2 inhibitors until stopped for HCT, two patients developed serious adverse events necessitating delaying of HCT, and another 8 patients had symptoms with lesser severity. Adverse events were more common in patients who started tapering or abruptly stopped their regular dose ≥6 days prior to conditioning therapy.
We conclude that prior exposure to JAK1/2 inhibitors did not adversely affect post-transplant outcomes. Our data suggest that JAK1/2 inhibitors should be continued near to the start of conditioning therapy. The favorable outcomes of patients who experienced clinical improvement with JAK1/2 inhibitor therapy prior to HCT were particularly encouraging, and need further prospective validation.
Keywords: JAK1/2 inhibitors, ruxolitinib, myelofibrosis, allogeneic transplantation, survival
Introduction
Myelofibrosis (MF) is a group of neoplasms characterized by aberrant hematopoiesis, splenomegaly, inflammation-related symptoms, and an increased risk of leukemic transformation (LT).1–3 Dysregulation of JAK-STAT pathway is the hallmark of MF, and JAK1/2 inhibitors have shown clinical benefit with reduction of splenomegaly and MF-related symptoms irrespective of JAK2 V617F mutation status.4,5 However, JAK1/2 inhibitors have limited activity on the neoplastic clones, and do not reduce the risk of LT. At present, allogeneic hematopoietic cell transplantation (HCT) remains the only potentially curative therapy for MF.6–8
A high incidence of non-relapse mortality (NRM) arising from graft failure (GF), regimen-related toxicities (RRT) and graft versus host disease (GVHD) remain the major barriers to the success of HCT in MF.9–13 Theoretically, JAK1/2 inhibitor therapy may help in overcoming some of these barriers.7,14,15 Its potential benefits in this setting include:(a) reduction in splenomegaly, which may facilitate engraftment, (b) decreasing symptoms due to pro-inflammatory cytokines, (c) improvement in performance status prior to HCT, (d) and a possible beneficial effect on GVHD.16 However, conflicting data have emerged in the last two years on the safety of JAK1/2 inhibitors prior to HCT. Preliminary results of a prospective multicenter JAK-Allo study from French researchers reported several serious adverse events such as tumor lysis syndrome, cardiogenic shock and sepsis, resulting in temporary hold on recruitment.17 On the contrary, small retrospective studies did not observe such events. 18–22 Additionally, there is a concern about potential risk of opportunistic infections due to the immunomodulatory effects of JAK inhibitors.23,24
Another clinical dilemma faced by patients and treating physicians is the appropriate timing of HCT in a patient responding well to JAK1/2 inhibitor therapy: should one proceed with HCT while the patient is responding to JAK1/2 inhibitor therapy, or reserve HCT at the time of loss of response or intolerance to JAK1/2 inhibitors? At present, there is an equipoise in this area, no data to guide these decisions, and practice patterns vary. To understand some of the issues involved with the use of JAK1/2 inhibitors in the HCT setting, we conducted a retrospective multicenter study of MF patients who underwent HCT with a prior exposure to JAK1/2 inhibitors.
Patients and methods
Patients
This study was coordinated by the Myeloproliferative Neoplasm (MPN) program of the Princess Margaret Cancer Centre, Toronto. We contacted 20 centers with a major interest in MPN, and, among these, 16 centers from Canada, United States, and United Kingdom participated in this study. Institutional Research and Ethics Boards of respective centers approved this study. All centers reported data on consecutive patients who met eligibility criteria as below.
Inclusion criteria were: (a) Adult patients who received first HCT for primary MF (PMF) or MF secondary to polycythemia vera (PPV-MF), or essential thrombocythemia (PET-MF); and (b) had received treatment with either experimental or commercially available JAK1/2 inhibitors prior to HCT. Patients who had developed LT prior to starting JAK1/2 inhibitors were excluded. The primary endpoint was overall survival (OS). Secondary endpoints included the difference in OS between the groups based on response to JAK1/2 inhibitors, and other transplant outcomes.
Definitions
Dynamic International Prognostic Scoring System (DIPSS) prior to starting JAK1/2 inhibitor was used for disease specific risk stratification.25 Comorbidities were scored using Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI).26 Cytogenetics were delineated as normal, abnormal standard risk, and abnormal high-risk, using an adaptation of the classification published by Caramazza et al.27 Conditioning intensity was classified as full-intensity conditioning (FIC) or reduced-intensity conditioning (RIC) according to the CIBMTR classification.28
Patients who survived more than 14 days after HCT were evaluable for assessment of hematologic recovery. Dates of platelet and neutrophil recovery were defined as the first of three consecutive days of unsupported platelet count ≥20 x109/L and absolute neutrophil count (ANC) ≥0.5 x109/L respectively. Primary graft failure was defined as failure to recover ANC by day +35 as reported previously.6 Regimen-related toxicity (RRT) was defined as per Bearman’s criteria.29
Classification of responses to JAK1/2 inhibitors prior to HCT
A working definition of response to JAK1/2 inhibitors was established inspired by revised response criteria by International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT)30 as follows:
Group-A: Clinical improvement; defined as ≥50% improvement in palpable spleen length for spleen palpable by ≥10 cm, or complete resolution of splenomegaly for palpable spleen <10 cm.
Group-B: Stable disease.
Group-C: Increase in blasts to 10–19%, intolerance to treatment due to hematologic / non-hematologic side effects, or new onset transfusion-requiring anemia.
Group-D: Disease progression manifesting as; appearance of new splenomegaly palpable ≥5 cm below costal margin (BCM), or ≥100% increase in palpable distance BCM for baseline splenomegaly of 5–10 cm BCM, ≥50% increase in palpable distance BCM for baseline splenomegaly of ≥10 cm BCM, loss of spleen response, or symptomatic splenomegaly requiring splenectomy.
Group-E: Disease progression manifesting as leukemic transformation defined as a peripheral blood or bone marrow blast count of ≥20%.
Responses were assessed centrally based on the hematologic parameters and spleen sizes provided by the individual centers, and patients were stratified as above with the additional input from study investigators.
Definitions and classification of symptoms during pre-transplant JAK1/2 inhibitor discontinuation
As the definition of “rebound symptoms”, “withdrawal symptom” or return of MF-related symptoms remain highly observer dependent,17,31–33 we reported all the potential new symptoms that occurred during pre-transplant discontinuation of JAK1/2 inhibitors, and their relationship with timing of drug discontinuation was explored. Symptoms were graded as following using clinical judgment:
Mild: symptoms not requiring any medical interventions.
Moderate: symptoms requiring medical interventions including: restarting of JAK1/2 inhibitors, unplanned use of steroids, oral analgesics for spleen pain; however, did not require hospitalization or intravenous medications.
Severe: symptoms requiring intravenous medications, hospital admissions, splenectomy, or delaying of HCT
Fatal: Death attributable to withdrawal symptoms.
Statistical methods
Differences in continuous variables were tested using the Wilcoxon rank sum test, and that of categorical variables with Chi-Square or Fishers exact test as appropriate. Patients who received a subsequent HCT were censored on the date of second HCT. The probabilities of overall survival were calculated by Kaplan–Meier method, and differences between groups were estimated by log rank test. Incidences of acute and chronic GVHD, neutrophil recovery, platelet recovery, NRM, and relapse/ progression were generated using cumulative incidence method with competing risks. Hazard ratio of pre-transplant variables on survival was calculated using Cox regression analysis. Considering small number of events, a limited multivariable analysis was performed for OS using variables, which were found to be significant (p<0.05) at univariable level. There were two exceptions to this: (1) Age was not included in the multivariable model, as it was already represented by DIPSS score. (2) Performance status (ECOG) was not included in multivariable model, as there was a significant co-linearity between it and response to JAK1/2 inhibitors.
All P-values were two-sided and for the statistical analyses, P< 0.05 was considered to indicate a statistically significant result. Statistical analyses were performed using version 9.4 of the SAS system for Windows, Copyright © 2002–2012 SAS Institute, Inc., Cary, NC, and the open source statistical software R version-3.0 (The R Foundation for Statistical Computing, Vienna, Austria, 2013).
Results
Between 2009–2014, 100 patients with prior exposure to JAK1/2 inhibitors underwent HCT at the 16 participating centers in this study. Among these, 66 patients continued JAK1/2 inhibitors until HCT, whereas drug was discontinued in other patients at least four weeks prior to HCT due to intolerance or disease progression. Baseline characteristics at HCT are shown in Table 1. The median follow up from the date of HCT was 17 months (range 3–53 months).
Table 1.
Patient, disease and transplant characteristics (n=100)
| Variables | Frequency |
|---|---|
| Age at HCT, y, median (range) | 59 (32–72) |
| Gender, Male: Female | 59:41 |
| Diagnosis, n | |
| Primary MF | 57 |
| PET-MF | 21 |
| PPV- MF | 22 |
| Cytogenetics, n | |
| Normal karyotype | 49 |
| Abnormal, Standard Risk* | 26 |
| Abnormal, High Risk* | 18 |
| Not available | 7 |
| JAK2 V617 F status | |
| Mutated | 62 |
| Wild type | 37 |
| Not available | 1 |
| JAK# used prior to HCT** | |
| Ruxolitinib | 90 |
| CYT 387 (momelitinib) | 6 |
| Others | 4 |
| Duration of JAK#, months, median (range) ** | 5 (1–56) |
| Timing of JAK# in relation to HCT** | |
| Continued until stopped for HCT | 66 |
| Discontinued at least 1 month before HCT | 30 |
| Not available | 4 |
| DIPSS Risk groups at initiation of JAK#, n | |
| Intermediate 1 | 40 |
| Intermediate 2 | 48 |
| High | 6 |
| Not available | 6 |
| DIPSS plus Risk groups at initiation of JAK#, n | |
| Intermediate 1 | 27 |
| Intermediate 2 | 45 |
| High | 22 |
| Not available | 6 |
| Splenomegaly at HCT, n | |
| Not palpable | 14 |
| ≤10 cm below costal margin | 39 |
| >10 cm below costal margin | 33 |
| Splenectomized | 13 |
| Not available | 1 |
| Constitutional Symptoms at initiation of JAK#, n | |
| Present | 35 |
| Absent | 65 |
| Response to JAK#, n | |
| Group A: Clinical Improvement | 23 |
| Group B: Stable disease | 31 |
| Group C: New onset cytopenia or increasing blasts | 15 |
| Group D: Progressive disease: Splenomegaly | 18 |
| Group E: Progressive disease: Leukemic Transformation | 13 |
| ECOG at HCT, n | |
| 0 | 20 |
| 1 | 70 |
| ≥2 | 9 |
| Not available | 1 |
| HCT-CI, n | |
| 0 | 30 |
| 1–2 | 28 |
| ≥3 | 42 |
| Type of Donor, n | |
| Matched sibling donor | 36 |
| Well matched unrelated donor | 50 |
| 1 antigen or allele mismatched unrelated /Haploidentical donor | 14 |
| Graft Source, n | |
| Peripheral blood | 93 |
| Bone marrow | 6 |
| Cord | 1 |
| Preparatory Regimen, n | |
| Full intensity Conditioning | 44 |
| Flu Bu +/− TBI | 36 |
| Flu Bu Mel | 5 |
| Bu CY | 3 |
| Reduced intensity Conditioning | 56 |
| Flu Bu +/− TBI | 29 |
| Flu Mel +/− TBI | 16 |
| Flu Cy TBI | 4 |
| Flu BCNU Mel | 5 |
| Flu Bu Mel | 1 |
| Flu TBI | 1 |
| T cell depletion | |
| In vivo ATG / alemtuzumab | 46 |
| In vitro TCD | 5 |
| No TCD | 49 |
HCT denotes, allogeneic hematopoietic cell transplantation; MF, myelofibrosis; PET-MF, post essential thrombocythemia myelofibrosis; PPV-MF, post polycythemia myelofibrosis; JAK#, JAK1/2 inhibitors; DIPSS, Dynamic International Prognostic Scoring System; ECOG, Eastern Cooperative Oncology Group Scoring; HCT-CI, hematopoietic cell transplantation comorbidity index; Flu, Fludarabine; Bu, busulfan; TBI, total body irradiation; Mel, melphalan; Cy, cyclophosphamide; BCNU, carmustine; ATG, antithymocyte globulin; and TCD, T cell depletion
High risk and standard risk classification adopted from criteria proposed by Caramazza et al. 27
In 8 patients who had more than one JAK#, the one which was used closest to HCT was reported
Disease response and adverse effects during JAK1/2 inhibitor therapy
At the time of HCT, 23 patients met criteria for inclusion in group-A, 31 in group-B, 15 in group-C, 18 in group-D, and 13 in group-E. After starting JAK1/2 inhibitors, 10 patients underwent splenectomy (n=8) or splenic radiation (n=2); due to progression (n=6), or lack of response to medical therapy (n=4). Additionally, one patient underwent splenectomy due to rebound splenomegaly during tapering of JAK1/2 inhibitors. Fifteen patients who progressed while on JAK1/2 inhibitors (13 patients with LT, two patients with blast count between 10–19%) received intensive chemotherapy or hypomethylating agents prior to HCT.
The major adverse effects reported during JAK1/2 inhibitor therapy were cytopenias and atypical infections. Grade ≥3 anemia, thrombocytopenia, and neutropenia were reported in 29, 17, and 3 patients respectively. Five cases of opportunistic infections were reported: Two patients contracted atypical mycobacterial infection; and invasive fungal infection, listeriosis, and varicella zoster reactivation were each diagnosed in a single patient. In addition to these, one patient developed an unexplained skin rash and leg pain, and another patient developed significant diarrhea and vomiting resulting in weight loss.
Pre-transplant discontinuation of JAK1/2 inhibitors and reported symptoms
Sixty-six patients who remained on JAK1/2 inhibitors underwent scheduled discontinuation prior to HCT. Strategies of tapering, and intervals between stopping JAK1/2 inhibitor therapy and the start of conditioning therapy were variable according to center practices. Among these patients, 10 (15%) reported new symptoms attributable to drug discontinuation. The symptoms were severe in 2 and mild to moderate in 8 patients.
It is noteworthy that HCT was delayed in two patients due to significant clinical events occurring during drug discontinuation (Table 2). One of these patients developed pulmonary infiltrates and rebound splenomegaly necessitating restarting of JAK1/2 inhibitors and postponement of HCT. This patient underwent an elective splenectomy prior to re-scheduled HCT and tolerated discontinuation on that occasion without significant symptoms. A second patient experienced fever and hypoxic respiratory failure during planned discontinuation of JAK1/2 inhibitor resulting in re-scheduling of HCT. In this patient similar symptoms, albeit of milder severity, recurred during second time also. A notable features in both these patients was abrupt discontinuation of JAK1/2 inhibitors ≥6 days prior to start of conditioning therapy.
Table 2.
“Withdrawal symptoms” as reported by investigators*
| No | Age, Gender Disease | JAK# used, duration of therapy, and response | Tapering strategy (days) | Interval between last dose of JAK# & start of conditioning therapy | New symptoms during discontinuation | Severity*** | HCT delayed? | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Female, 68 JAK-2neg PMF | Ruxolitinib 6 months SD | 14 | 1 | Recurrence of CS | Mild | No | Alive at 11 months post HCT |
| 2 | Male, 55 JAK-2neg PMF | Ruxolitinib 4 months SD | 14 | 3 | Hemolytic anemia | Moderate | No | Alive at 15 months post HCT |
| 3 | Male, 61 JAK-2neg PMF | Ruxolitinib 9 months SD | 14 | 1 | Bone pain and splenic pain | Moderate | No | Alive at 16 months post HCT |
| 4 | Female, 64 JAK-2pos PPV-MF | Ruxolitinib 9 months CI | Abrupt discontinuation | 10 | Rebound splenomegaly, Pulmonary infiltrate and respiratory failure | Severe | Yes | Underwent splenectomy and delayed HCT. Alive at 16 months post HCT |
| 5 | Male 63 JAK-2pos PPV-MF | Ruxolitinib 2 months SD | 4 | 7 | Tumor lysis | Mild | No | Relapsed and died at 7 months post HCT |
| 6 | Female 52 JAK-2pos PPV-MF | Ruxolitinib 16 months SD | Abrupt discontinuation | ≥ 6days | Fever, hypoxic respiratory failure | Severe | Yes | Delayed HCT by 4 months. Symptoms recurred during second time. Died on day+37 due to respiratory arrest |
| 7 | Male, 61 JAK-2neg PMF | Ruxolitinib 5 months CI | 7 | 9 | Recurrence of CS | Mild | No | Alive at 24 months post HCT |
| 8 | Male, 51 JAK-2neg PMF | Ruxolitinib 6 months SD | Unknown | 14 | Fatigue and discomfort in spleen area | Moderate | No | Alive at 22 months post HCT |
| 9 | Female, 56 JAK-2pos PMF | Ruxolitinib 2 months SD | Abrupt discontinuation | 10 | Fatigue and early satiety | Mild | No | Alive at 17 months post HCT |
| 10 | Male, 64 JAK-2pos PET-MF | Ruxolitinib 1 months Blast >10% | 6 | 3 | Recurrence of CS and muscle pain | Moderate | No | Alive at 10 months post HCT |
JAK# denotes, JAK1/2 inhibitors; HCT, hematopoietic cell transplantation; PMF, primary myelofibrosis; PPV-MF, post polycythemia myelofibrosis; PET-MF, post essential thrombocythemia myelofibrosis; SD, stable disease; CI, clinical improvement; and CS, constitutional symptoms.
We are reporting all clinical findings, which could be attributable to stopping ofJAK1/2 inhibitors. These include, unexpected clinical features like tumor lysis syndrome, shock and cardio pulmonary dysfunctions, as well as reappearance of MF-related symptoms.
Interval between last day of regular dose or tapered dose of JAK1/2 inhibitor and start of transplant conditioning.
Withdrawal symptoms were arbitrarily graded as mild, if there were no medical interventions required; moderate, if medical interventions including: restartingJAK1/2 inhibitor, unplanned steroids; oral analgesia were required for symptom control; severe, if IV analgesia and or hospitalization was required for symptom control; or symptoms resulted in delaying of HCT; and fatal if patient died within 2 weeks of HCT in the presence of above defined clinical features.
Drug discontinuation symptoms were more common in patients who had a longer interval between last dose of JAK1/2 inhibitor and beginning of conditioning regimen. Among 21 patients with ≥6 days interval, 6 (29%) patients developed symptoms; on the other hand, among 45 patients with <6 days interval, only 3 (7%) patients developed any symptoms.
Early transplant outcomes: RRT, hematopoietic recovery and graft-failure
Grade ≥2 RRT were reported as below: stomatitis (40%), hepatic toxicity including veno-occlusive disease (31%), GI (19%), renal (13%), pulmonary (13%), cardio-vascular (7%), CNS (3%), and bladder (2%) toxicities. Two patients died of veno-occlusive disease, on day +13 and +52.
Four patients experienced primary graft-failure and died due to hemorrhage (n=2) or sepsis (n=2). The remaining 96 patients achieved neutrophil engraftment by day 35. Cumulative incidence of platelet count recovery by day 100 was 74% (95% CI, 66–82). (Figure 1)
Fig 1. Allogeneic hematopoietic cell transplantation (HCT) outcomes in myelofibrosis (MF) patients with prior exposure to JAK1/2 inhibitors.
(A) Probability of overall survival (OS), cumulative incidence of non-relapse mortality (NRM) and cumulative incidence of relapse / progression (CIR). (B) Cumulative incidence of neutrophil count ≥ 0.5x 109 / L and platelet count ≥20 x 109 / L. (C) Cumulative incidence of acute graft versus host disease (GVHD). (D) Cumulative incidence of chronic GVHD
Four patients developed secondary graft-failure, and three of these were successfully salvaged by: donor lymphocyte infusion (n=1), unmodified stem cell boost (n=1), and a second HCT (n=1). The fourth patient recovered normal blood counts and regained donor chimerism after withdrawal of immunosuppression.
GVHD
Cumulative incidences of grade II-IV, and III-IV acute GVHD by 100 days were 37% (95%CI, 27–47), and 16% (95%CI, 8–24) respectively. Acute GVHD was fatal in three patients. There was no difference in incidence of grade II-IV acute GVHD between various disease response groups (p=0.3) Cumulative incidence of chronic GVHD by two years was 48% (95% CI, 35–62) and that of extensive chronic GVHD was 23% (95% CI, 10–36).
Opportunistic infections and viral reactivations after HCT
Among 60 CMV seropositive recipients, 26 (43%) developed CMV reactivations, but no CMV disease was reported. EBV reactivation was reported in six patients, and EBV-positive post-transplant lymphoproliferative disorder was diagnosed in one patient. Other viral infections reported were: BKV hemorrhagic cystitis (n=6), mucocutaneous HSV (n=1), and rhinovirus pneumonitis (n=2).
Invasive fungal infections were reported in seven patients, and these were: mucormycosis (n=2), invasive aspergillosis (n=1), pulmonary candidiasis (n=1), central nervous system candidiasis (n=1), and unspecified (n=2). One patient was diagnosed with central nervous system toxoplasmosis. Culture positive bacterial infections were reported in 29 patients. Mycobacterial infections were not reported.
Relapse/progression and NRM after HCT
Relapse or progression was observed in 16 patients, and resulted in death in 12 patients. Cumulative incidence of relapse at two years was 17% (95%CI, 6–27). (Fig 1) Twenty-five patients died without relapse or progression, and the causes were: GVHD (n=6), sepsis (n=11), organ failure (n=5), intracranial bleed (n=2), and secondary myelodysplastic syndrome (n=1). The cumulative incidence of NRM at two years was 28% (95% CI, 17–39)
Survival after HCT
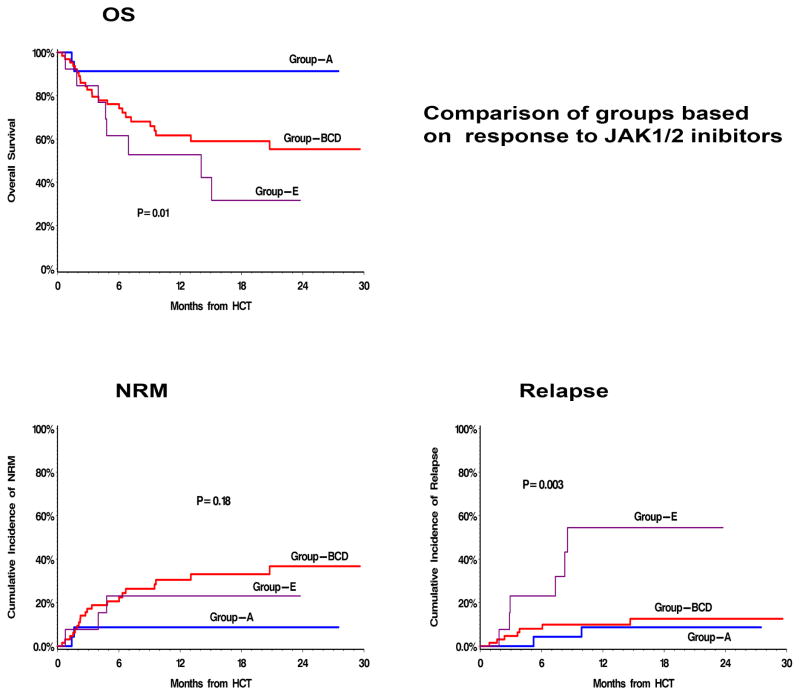
The probability of OS at two years for the whole cohort was 61% (95% CI, 49–71). When analyzed separately for groups based on pre-transplant response to JAK1/2 inhibitors, this was 91% (95% CI, 69–98) for patients with clinical improvement (group-A), 54% (95% CI, 32–72) for patients with stable disease (group-B), 54% (95% CI, 24–76) in patients with blast in the range of 10–19% or new onset transfusion requiring anemia or intolerance (group-C), 60% (95% CI, 30–80) for patients with progressive disease: splenomegaly (group-D), and 32% (95% CI, 8–59) in patients with history of leukemic transformation while on JAK1/2 inhibitors (group-E) (Supplemental figure 1). As the survival probabilities were comparable in group-B, group-C, and group-D; we combined these groups for further analysis as group-BCD, and the 2-yr OS of combined group was 55% (95%CI, 39–68). The difference between OS of group-A, group-BCD, and groupE were statistically significant (log rank p=0.01) (Fig 2A).
Fig 2. Allogeneic hematopoietic cell transplantation (HCT) outcomes by response to prior JAK1/2 inhibitor therapy.
Group-A: patients who experienced clinical improvement with JAK1/2 inhibitors, Group -E: patients who developed leukemic transformation while on JAK1/2 inhibitor therapy, and Group -BCD: combined group of others. (A) Probability of overall survival (OS), (B) cumulative incidence of non relapse mortality (NRM) and, (C) cumulative incidence of relapse / progression
Univariable analysis of factors predictive of OS, relapse, and NRM are presented in Table 3, and that of a multivariable analysis of OS in Table 4. Pre-transplant response toJAK1/2 inhibitors (p=0.03), DIPSS score (p=0.003), and donor type (p=0.006) were the independent predictors of survival in multivariable analysis.
Table 3.
Univariate analysis of OS, NRM, Relapse
| Death | NRM | Relapse | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p |
|
| ||||||
| Age at HCT continuous | 1.06 (1.01–1.1) | 0.02 | 1.04 (0.98– 1.1) | 0.2 | 1.06 (0.99 – 1.1) | 0.1 |
|
| ||||||
| Age at HCT | 0.07 | 0.5 | 0.08 | |||
| Age<60 (n=54) | 1 | 1 | 1 | |||
| Age≥60 (n=46) | 1.8 (0.9–3.6) | 1.3 (0.6 – 2.9) | 2.5 (0.9 – 7.3) | |||
|
| ||||||
| Diagnosis | 0.2 | 0.3 | 0.7 | |||
| Primary MF (n=57) | 1 | 1 | 1 | |||
| Post ET MF (n=21) | 0.4 (0.2–1.2) | 0.4 (0.1– 1.4) | 0.6 (0.1 – 2.6) | |||
| Post PV MF (n=22) | 1.3 (0.6–2.7) | 0.9 (0.3 – 2.4) | 1.2 (0.4 – 4.0) | |||
|
| ||||||
| Cytogenetics | 0.6 | 0.6 | 0.06 | |||
| Normal (n=49) | 1 | 1 | 1 | |||
| Abnormal Std Risk (n=26) | 0.9 (0.4–2.0) | 0.6 (0.2 – 1.5) | 1.9 (0.5 – 6.8) | |||
| Abnormal High Risk (n=18) | 1.4 (0.6–3.2) | 0.7 (0.3 – 2.1) | 4.5 (1.4 – 14.4) | |||
|
| ||||||
| JAK2 mutation status | 0.2 | 0.5 | 0.6 | |||
| Mutated (n=62) | 1 | 1 | 1 | |||
| Wild type (n=37) | 0.6 (0.3–1.3) | 0.6 (0.3– 1.5) | 0.6 (0.2 – 1.8) | |||
|
| ||||||
| Response groups | 0.1 | 0.5 | 0.01 | |||
| Group-A (n=23) | 1 | 1 | 1 | |||
| Group-B (n=31) | 3.9 (1.1–13.7) | 2.8 (0.8 –10.4) | 1.3 (0.2 2– 7.2) | |||
| Group-C (n=15) | 4.4 (1.1–17.7) | 3.2 (0.8–12.7) | 0.9 (0.1– 10.2) | |||
| Group-D (n=18) | 4.3 (1.1–16.4) | 2.7 (0.7– 11.2) | 2.4 (0.4 – 13.4) | |||
| Group-E (n=13) | 6.4 (1.7–24.2) | 2.1 (0.4–10.1) | 7.5 (1.6 – 34.3) | |||
|
| ||||||
| Response: combined groups | 0.02 | 0.2 | 0.003 | |||
| Group-A (n=23) | 1 | 1 | 1 | |||
| Group-BCD (n=64) | 4.1 (1.2–13.5) | 2.9 (0.9–9.7) | 1.5 (0.3 – 6.8) | |||
| Group-E (n=13) | 6.4 (1.7–24.2) | 2.1 (0.4–10.1) | 7.4 (1.6 – 34.2) | |||
|
| ||||||
| DIPSS score prior to JAK# | 0.03 | 0.1 | 0.96 | |||
| Intermediate-1 (n=40) | 1 | 1 | 1 | |||
| Intermediate-2 (n=48) | 1.5 (0.7–3.3) | 1.2 (0.6 – 2.8) | 1.2 (0.4 – 3.4) | |||
| High-risk (n=6) | 5.0 (1.5–16.4) | 3.4 (0.7 –16.0) | 1.4 (0.2 – 12.6) | |||
|
| ||||||
| Palpable splenomegaly at HCT | 0.5 | 0.6 | 0.9 | |||
| Not palpable (n=14) | 1 | 1 | 1 | |||
| ≤10 cm BCM (n=39) | 0.8 (0.3–2.1) | 0.9 (0.3 – 2.9) | 0.7 (0.2 – 2.7) | |||
| > 10 cm BCM (n=33) | 1.01 (0.4–2.6) | 1.2 (0.4 – 3.9) | 0.6 (0. – 2.6) | |||
| Splenectomy (n=13) | 0.4 (0.1–1.5) | 0.2 (0.03 – 2.2) | 1.03 (0.2 – 4.7) | |||
|
| ||||||
| Circulating blast before JAK# | 0.2 | 0.5 | 0.0006 | |||
| <1% (n=32) | 1 | 1 | 1 | |||
| 1–9% (n=55) | 0.9 (0.4–2.0) | 1.2 (0.5 – 2.5) | 0.6 (0.2 – 1.8) | |||
| 10–19% (n=5) | 2.7 (0.8–8.6) | 0.0 (0.0 – 0.0) | 7.3 (2.2–24.0) | |||
|
| ||||||
| ECOG at HCT | 0.01 | 0.3 | 0.5 | |||
| 0 (n=20) | 1 | 1 | 1 | |||
| 1 (n=70) | 1.8 (0.6–5.1) | 2.0 (0.6 – 6.4) | 1.4 (0.3 – 6.8) | |||
| ≥2 (n=9) | 5.3 (1.6–18.2) | 3.8 (0.9 – 17.1) | 3.4 (0.6 – 20.6) | |||
|
| ||||||
| HCT-CI at HCT | 0.1 | 0.5 | 0.1 | |||
| 0 (n=30) | 1 | 1 | 1 | |||
| 1 or 2 (n=28) | 1.5 (0.5–4.3) | 1.7 (0.6 – 5.0) | 0.2 (0.02 – 1.6) | |||
| ≥3 (n=32) | 2.5 (1.0–6.2) | 1.9 (0.7 – 5.2) | 1.2 (0.4 – 3.5) | |||
|
| ||||||
| Donor | 0.004 | 0.005 | 0.01 | |||
| Matched Sibling (n=36) | 1 | 1 | 1 | |||
| Matched unrelated (n=50) | 1.9 (0.8–4.3) | 0.97 (0.4 – 2.4) | 10.4 (1.3 – 82.8) | |||
| Other (n=14) | 5.1 (1.9–13.4) | 4.0 (1.5–10.4) | 2.7 (0.2 – 45.4) | |||
|
| ||||||
| TCD | 0.8 | 0.4 | 0.09 | |||
| No (n=49) | 1 | 1 | 1 | |||
| Yes (n=51) | 0.9 (0.5–1.7) | 0.7 (0.3 – 1.5) | 2.6 (0.8 – 8.3) | |||
|
| ||||||
| Intensity of conditioning | 0.007 | 0.02 | 0.4 | |||
| Full intensity (n=44) | 1 | 1 | 1 | |||
| Reduced intensity (n=56) | 2.9 (1.3–6.1) | 2.8 (1.1 – 6.9) | 1.5 (0.6 – 4.6) | |||
HCT denotes, allogeneic hematopoietic cell transplantation; MF, myelofibrosis; ET, essential thrombocythemia; PV, polycythemia; DIPSS, Dynamic International Prognostic Scoring System; BCM, below left costal margin; JAK#, JAK1/2 inhibitor; ECOG, Eastern Cooperative Oncology Group Scoring; HCT-CI, hematopoietic cell transplantation comorbidity index; and TCD, T cell depletion.
Table 4.
Multivariable analysis of OS
| Variable | Death
|
|
|---|---|---|
| HR (95%CI) | p | |
|
| ||
| Response: 3 groups | 0.03 | |
| Group-A (n=23) | 1 | |
| Group-B+C+D (n=64) | 5.4 (1.5–20.0) | |
| Group-E (n=13) | 8.0 (1.6–39.6) | |
|
| ||
| DIPSS score prior to JAK# | 0.003 | |
| Intermediate-1 (n=40) | 1 | |
| Intermediate-2 (n=48) | 1.1 (0.5–2.6) | |
| High-risk (n=6) | 8.7 (2.4–31.8) | |
|
| ||
| Donor | 0.006 | |
| Matched Sibling (n=36) | 1 | |
| Matched unrelated (n=50) | 1.03 (0.4–2.6) | |
| Other (n=14) | 4.3 (1.5–12.4) | |
|
| ||
| Intensity of conditioning | 0.1 | |
| Full intensity (n=44) | 1 | |
| Reduced intensity (n=56) | 2.0 (0.9–4.4) | |
Exploratory analysis
As expected, the inferior survival of patients with history of leukemic transformation (gp-E) was due to higher relapse in this group (Fig 2C). The difference in survivals of gp-A and gp-BCD was due to difference in NRM (Fig 2B and supplementary table 1); 2-yr cumulative incidence; 9% (95%CI, 0–21) vs. 37% (95%CI, 22–52) (p=0.07). Further analysis of causes of NRM (GVHD / infection /others) was not performed due to low number of events.
Given these findings, we performed an exploratory analysis comparing baseline characteristics of gp-A with gp-BCD. The patient and disease characteristics prior to starting JAK1/2 inhibitors were similar for these groups (Supplemental table 2). As expected, gp-A had smaller spleen size and better performance status compared to gp-BCD at the time of HCT, but other characteristics were similar.
Discussion
We report the largest multicenter experience to date on the use of JAK1/2 inhibitor therapy prior to HCT in MF. Use of JAK1/2 inhibitors was variable at the participating centers: they were used either as a bridge to transplant or as a strategy to delay HCT, reflecting prevailing variations in clinical practice. Although retrospective in nature, several important observations can be made from this study, which can guide current clinical practices.
An important learning point is the timing of discontinuation of JAK1/2 inhibitors prior to conditioning therapy. Reports of serious adverse events during scheduled discontinuation of ruxolitinib in prospective JAK-Allo study have raised serious concerns about safety of JAK1/2 inhibitors prior to HCT.17 The events observed in that study included acute circulatory compromise, respiratory failure, and severe tumor lysis syndrome; and were similar to isolated events reported in non-HCT setting.31,33–35 It is postulated that these symptoms are probably due to deranged cytokine milieu secondary to withdrawal of JAK-STAT inhibition.31 Some investigators have referred these symptoms as return of MF-related symptoms in their description.32,36,37
Given the lack of a standard definition of “rebound symptoms” or “withdrawal symptoms” or “return of MF-related symptoms”, we adapted a conservative approach towards collecting data on new symptoms observed during the discontinuation of JAK1/2 inhibitor therapy, and as a result we may have overestimated these symptoms. A majority of these symptoms were consistent with MF-related symptoms, and were mild-to-moderate in severity. However, two patients experienced severe adverse events necessitating rescheduling of HCT. In both of these patients, JAK1/2 inhibitor therapy was discontinued ≥6 days prior to planned start of transplant conditioning. It is noteworthy that two small retrospective studies, who used a strategy of discontinuing ruxolitinib close to HCT also did not observe any untoward symptoms during discontinuation.19,20 Taken together, these data suggest that JAK1/2 inhibitors should be continued close to the start of conditioning therapy. This approach is being investigated in an ongoing prospective trial by Myeloproliferative Disorders-Research Consortium (NCT01790295).
Another observation of this study is superior survival and lower NRM in patients who had clinical improvement with JAK1/2 inhibitors prior to HCT. This finding was also observed in a previous study on a small number of patients.20 Although one should be cautious in drawing strong conclusions due to retrospective nature of this study and the risk of selection bias, these data raise an important question: are the better outcomes in patients who had clinical improvement with JAK1/2 inhibitors a result of direct effect of JAK1/2 inhibitors, or does response to JAK1/2 inhibitors predict a favorable disease biology. In our study, the baseline characteristics (prior to JAK1/2 inhibitor therapy) of patients who responded to JAK1/2 inhibitor therapy were similar to those who did not; hence, do not explain the difference in post-transplant outcome. On the other hand, response to JAK1/2 inhibitor therapy resulted in better performance status at the time of HCT. A better performance status may have contributed towards improved survival, and may partially explain these results. It is noteworthy however that, though spleen size at HCT was smaller in responders, it was not associated with better survival. Patients with disease progression other than LT appear to have intermediate outcomes similar to those with stable disease.
In addition to response to JAK1/2 inhibitors and DIPSS score, the donor type influenced survival. Mismatched unrelated donors and haploidentical donors were associated with inferior outcomes, but we did not observe any significant difference between well-matched unrelated donors and matched sibling donors. This is in contrast to recent studies, which reported inferior outcomes with matched unrelated donors,10,12 and possibly could be due to continuously improving supportive therapies and wider use of high resolution HLA matching.
Other significant observations in this study include lower rate of graft failure. We have not observed any pattern or difference in graft failure based on response to JAK1/2 inhibitors, though the number of events was too small to draw any valid conclusions. Although difficult to compare between various studies, we have not observed any striking reduction in the incidence or severity of acute GVHD in this cohort. Similarly prior exposure to JAK1/2 inhibitors does not appear to increase the risk of opportunistic infections compared to other reported studies.
We conclude that prior treatment with JAK1/2 inhibitors did not adversely impact early post-transplant outcomes in MF. We recommend that JAK1/2 inhibitor therapy should be continued close to the start of transplant conditioning to minimize the risk of “rebound or withdrawal symptoms”. The favorable transplant outcomes in patients who had clinical improvement with JAK1/2 inhibitor therapy are particularly encouraging. These findings need further validation in well- designed prospective trials comparing the strategies of early versus delayed transplants in patients responding well to JAK1/2 inhibitor therapy.
Supplementary Material
Use of JAK1/2 inhibitors prior to HCT had no adverse impact on early outcomes.
Continuation of JAK1/2 inhibitors closer to start of conditioning is recommended.
Patients undergoing HCT when responding to JAK inhibitors had encouraging outcomes.
Footnotes
Previous presentation:
This study was presented in part as an oral abstract on June 13, 2015 at EHA in Vienna, Austria
Financial Disclosure Statement:
M.S has received travel grant from Novartis; U.P has received research funding from Otsuka and CTI for his institution; L.C.M has received honoraria for consulting / advisory role from Incyte, D.M has received research funding for his institution, is a member of speakers bureau, and has received honoraria for consulting / advisory role from Novartis; RK has received honoraria for consulting / advisory role from Gilead sciences, Teva, MorphoSys, and Karyopharm Therapeutics; M.O.A received research funding from Incyte, Gilead sciences, and Janssen oncology for his institution; U.G has stock or other ownership in Incyte, Agios, Pfizer, Exact sciences, Chimerix, Ariad, Juno therapeutics, Novavax, Alexion pharmaceuticals, and honoraria from Alexion pharmaceuticals, Merck, Bristol-Myers Squibb, and travel and accommodation expenses from Alexion pharmaceuticals; JMS has received honoraria from Celgene, Novartis, Bristol-Myers Squibb, Lundbeck, Sandoz, Seattle Genetics, and received research funding for his institution from Celgene, Seattle Genetics, Janssen oncology, and Boehringer-Ingelheim, and received travel and accommodation expenses from Celgene and Novartis; S.V received research funding from Incyte, AstraZeneca, Lilly, Roche, Geron, NS pharma, Bristol-Myers Squibb, Celgene, Gilead Sciences, Seattle Genetics, Promedior, CTI Biopharma, Galena Biopharma, and Pfizer; R.E.C received research funding from Otsuka, Celgene, and Sanofi; P.H received honoraria from Celgene, Millennium, Sanofi, Amgen, Spectrum Pharmaceuticals, Onyx, research funding for his institution from Millennium, Celgene, Onyx, Spectrum Pharmaceuticals, and has stock or other ownership in Pharmacyclic; V.G has received honoraria from Novartis and Incyte, and has received research funding through his institution from Novartis, Incyte, and Gilead sciences. V.F, J.M, R.T, J.D, O.C.U, R.T.K, N.S.M, R.R, A.P, S.V, B.E, E.G.A, and AH declares no confict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lundberg P, Karow A, Nienhold R, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123(14):2220–2228. doi: 10.1182/blood-2013-11-537167. [DOI] [PubMed] [Google Scholar]
- 2.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 3.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison C, Kiladjian J-J, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 6.Ballen KK, Shrestha S, Sobocinski KA, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2010;16(3):358–367. doi: 10.1016/j.bbmt.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta V, Hari P, Hoffman R. Allogeneic hematopoietic cell transplantation for myelofibrosis in the era of JAK inhibitors. Blood. 2012;120(7):1367–1379. doi: 10.1182/blood-2012-05-399048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLornan DP, Mead AJ, Jackson G, Harrison CN. Allogeneic stem cell transplantation for myelofibrosis in 2012. Br J Haematol. 2012;157(4):413–425. doi: 10.1111/j.1365-2141.2012.09107.x. [DOI] [PubMed] [Google Scholar]
- 9.Kröger N, Holler E, Kobbe G, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009;114(26):5264–5270. doi: 10.1182/blood-2009-07-234880. [DOI] [PubMed] [Google Scholar]
- 10.Gupta V, Malone AK, Hari PN, et al. Reduced-intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2014;20(1):89–97. doi: 10.1016/j.bbmt.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abelsson J, Merup M, Birgegård G, et al. The outcome of allo-HSCT for 92 patients with myelofibrosis in the Nordic countries. Bone Marrow Transplant. 2012;47(3):380–386. doi: 10.1038/bmt.2011.91. [DOI] [PubMed] [Google Scholar]
- 12.Rondelli D, Goldberg JD, Isola L, et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood. 2014;124(7):1183–1191. doi: 10.1182/blood-2014-04-572545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong KM, Atenafu EG, Kim D, et al. Incidence and risk factors for early hepatotoxicity and its impact on survival in patients with myelofibrosis undergoing allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(10):1589–1599. doi: 10.1016/j.bbmt.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Tamari R, Mughal TI, Rondelli D, et al. Allo-SCT for myelofibrosis: reversing the chronic phase in the JAK inhibitor era? Bone Marrow Transplant. 2015;50(5):628–636. doi: 10.1038/bmt.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta V, Gotlib J, Radich JP, et al. Janus kinase inhibitors and allogeneic stem cell transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2014;20(9):1274–1281. doi: 10.1016/j.bbmt.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballinger TJ, Savani BN, Gupta V, Kroger N, Mohty M. How we manage JAK inhibition in allogeneic transplantation for myelofibrosis. Eur J Haematol. 2015;94(2):115–119. doi: 10.1111/ejh.12455. [DOI] [PubMed] [Google Scholar]
- 17.Robin M, Francois S, Huynh A, et al. Ruxolitinib before allogeneic hematopoietic stem cell transplantation (HSCT) in patients with myelofibrosis: a preliminary descriptive report of the JAK ALLO study, a phase II trial sponsored By Goelams-FIM in collaboration With The Sfgmtc. Blood. 2013;122:21–306. ABSTRACT. [Google Scholar]
- 18.Shanavas M, Messner HA, Atenafu EG, et al. Allogeneic hematopoietic cell transplantation for myelofibrosis using fludarabine-, intravenous busulfan- and low-dose TBI-based conditioning. Bone Marrow Transplant. 2014;49(9):1162–1169. doi: 10.1038/bmt.2014.131. [DOI] [PubMed] [Google Scholar]
- 19.Jaekel N, Behre G, Behning A, et al. Allogeneic hematopoietic cell transplantation for myelofibrosis in patients pretreated with the JAK1 and JAK2 inhibitor ruxolitinib. Bone Marrow Transplant. 2014;49(2):179–184. doi: 10.1038/bmt.2013.173. [DOI] [PubMed] [Google Scholar]
- 20.Stübig T, Alchalby H, Ditschkowski M, et al. JAK inhibition with ruxolitinib as pretreatment for allogeneic stem cell transplantation in primary or post-ET/PV myelofibrosis. Leukemia. 2014;28(8):1736–1738. doi: 10.1038/leu.2014.86. [DOI] [PubMed] [Google Scholar]
- 21.Lebon D, Rubio MT, Legrand F, et al. Ruxolitinib for patients with primary or secondary myelofibrosis before allogeneic hematopoietic stem cell transplantation (allo-HSCT): a retrospective study of the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) Blood. 2013;122(21):2111. ABSTRACT. [Google Scholar]
- 22.Hanif A, Hari P, Atallah E, Carlson K-SB, Pasquini M, MIchaellis L. Ruxolitinib Prior to Allogeneic Stem Cell Transplantation Does Not Adversely Affect Post-Transplant Outcomes. Blood. 2014;124(21):1851. ABSTRACT. [Google Scholar]
- 23.Heine A, Held SAE, Daecke SN, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122(7):1192–1202. doi: 10.1182/blood-2013-03-484642. [DOI] [PubMed] [Google Scholar]
- 24.Heine A, Brossart P, Wolf D. Ruxolitinib is a potent immunosuppressive compound: is it time for anti-infective prophylaxis? Blood. 2013;122(23):3843–3844. doi: 10.1182/blood-2013-10-531103. [DOI] [PubMed] [Google Scholar]
- 25.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010;115(9):1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- 26.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caramazza D, Begna KH, Gangat N, et al. Refined cytogenetic-risk categorization for overall and leukemia-free survival in primary myelofibrosis: a single center study of 433 patients. Leukemia. 2011;25(1):82–88. doi: 10.1038/leu.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bearman SI, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6(10):1562–1568. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 30.Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122(8):1395–1398. doi: 10.1182/blood-2013-03-488098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tefferi A, Pardanani A. Serious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosis. Mayo Clin Proc. 2011;86(12):1188–1191. doi: 10.4065/mcp.2011.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verstovsek S, Mesa RA, Gotlib JR, et al. J Clin Oncol. 2012;(30) Abstract 6624. [Google Scholar]
- 33.Dai T, Friedman EW, Barta SK. Ruxolitinib withdrawal syndrome leading to tumor lysis. J Clin Oncol. 2013;31(29):e430–e432. doi: 10.1200/JCO.2012.47.6473. [DOI] [PubMed] [Google Scholar]
- 34.Tefferi A, Litzow MR, Pardanani A. Long-term outcome of treatment with ruxolitinib in myelofibrosis. N Engl J Med. 2011;365(15):1455–1457. doi: 10.1056/NEJMc1109555. [DOI] [PubMed] [Google Scholar]
- 35.Beauverd Y, Samii K. Acute respiratory distress syndrome in a patient with primary myelofibrosis after ruxolitinib treatment discontinuation. Int J Hematol. 2014;100(5):498–501. doi: 10.1007/s12185-014-1628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cervantes F, Vannucchi AM, Kiladjian J-J, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122(25):4047–4053. doi: 10.1182/blood-2013-02-485888. [DOI] [PubMed] [Google Scholar]
- 37.Verstovsek S, Kantarjian HM, Estrov Z, et al. Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controls. Blood. 2012;120(6):1202–1209. doi: 10.1182/blood-2012-02-414631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.