Abstract
Background
Neuroblastoma is a paediatric cancer which originates from precursor cells of the sympathetic nervous system. Previous studies have shown that miR-184 expression has anti-proliferative effects in neuroblastoma cells grown in culture. Therefore, it was of interest to evaluate this effect in vivo.
Materials and Methods
Neuroblastoma cells overexpressing miR-184 were injected retroperitoneally into CB17-SCID mice and tumour burden was assessed by measuring bioluminescence. Overall survival was also evaluated.
Results
Ectopic overexpression of miR-184 in neuroblastoma cell lines is anti-proliferative. In addition, overexpression of miR-184 led to a significant reduction in tumour growth relative to negative control-treated cohorts in a xenograft model of neuroblastoma.
Conclusion
This study demonstrated for the first time that miR-184 significantly reduces tumour growth and increases overall survival in an orthotopic murine model of neuroblastoma through assessment of tumour growth and moribundity relative to control miRNA-treated cohorts.
Keywords: Mouse models, targeted therapeutics, miR-184 transfection, neuroblastoma
Mature microRNAs (miRNAs) are noncoding RNA molecules which act as post-transcriptional regulators of specific mRNA transcripts, leading to the targeted degradation and suppression of gene expression. MiRNAs are known to play major roles in normal developmental processes (1, 2), and their dysregulation has been found to significantly contribute to various aspects of carcinogenesis in nearly all forms of cancer (3), including neuroblastoma (4-7). Neuroblastoma is a paediatric cancer derived from the precursor cells of the sympathetic nervous system which displays extreme heterogeneity in clinical behaviour, ranging from spontaneous regression without therapy to rapid progression and death due to disease despite intensive therapy (8).
MiRNA expression profiling studies have demonstrated that expression of many miRNAs is dysregulated in neuroblastoma and that the expression levels of specific miRNAs can be significantly associated with clinical outcome (5, 6, 9). Previous in vivo and in vitro functional studies have identified a number of miRNAs as having either oncogenic or tumour suppressor effects in neuroblastoma (9-14). Recently, it has been shown that miR-184 expression levels are inversely correlated with MYCN amplification and that low expression of this miRNA is highly associated with poor clinical outcome in neuroblastoma (13). It was also determined that ectopic overexpression of miR-184 in cultured neuroblastoma cell lines activated a caspase dependent apoptotic pathway specifically through direct targeting of the serine-threonine kinase AKT2 3’ UTR. This study provides the first in vivo functional data demonstrating the biological effects of this miRNA in neuroblastoma using a mouse orthotopic xenograft model of the disease.
Materials and Methods
Cell lines
NB1691luc and SK-N-ASluc cell lines were maintained in RPMI-1640 supplemented with heat-inactivated foetal bovine serum (10%), L-glutamine (1%) and 100 μg/ml Zeocin (InVivoGen, San Diego, CA, USA). The Pre-miR™ to miR-184 (30 μM) and the premiR-negative control miRNA (negative control 1; Applied Biosystems/Ambion, Austin, TX, USA) were reverse transfected into NB1691luc and SK-N-ASluc cell lines using the transfection agent siPORT™ NeoFX™ (Applied Biosystems/Ambion). Cell culture-transfection media were changed after 24 h and replaced with pre-warmed standard cell culture media. In experiments where quantitative PCR (qPCR) was intended for analysis, total RNA/miRNA was extracted 48 h post-transfection using RNeasy Kit/miRNeasy© kit (Qiagen Inc, Valencia, CA, USA).
Reverse transcription and real-time qPCR
Reverse transcription was carried out using 50 ng of total RNA with a primer specific for miR-184 and the TaqMan microRNA reverse transcription kit (Applied Biosystems/Ambion). qPCR was carried out on a 7900 HT Fast Real-time System (Applied Biosystems/Ambion). RNU66, a small RNA encoded in the intron of RPL5 (chr1:93,018,360-93,018,429; 1p22.1), was used for normalisation in miRNA studies. A relative fold-change in expression of the target miRNA transcript was determined using the comparative cycle threshold method (2–ΔΔCT).
Growth curve
In vitro experiments were carried out in triplicate in 6-well plates. Neuroblastoma cells (3×105) were reverse transfected with premiR-184 or premiR-negative control and an additional set of wells remained untreated (non-transfected). At the appropriate time point, 24, 48 and 72 h post-transfection, cells were trypsinised and re-suspended in 1 ml of medium and nuclei were counted in triplicate for each sample using a Beckman Coulter Cell counter (Beckman Coulter Inc, Brea, CA, USA).
In vivo tumour establishment and imaging
All animal experiments were carried out in six-week-old CB-17/SCID mice (Charles’ River Laboratories, Wilmington, MA, USA) and were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of St Jude Children's Research Hospital, Memphis, TN, USA. Retroperitoneal tumours were established by injection of 4.4×105 NB1691luc or SK-N-ASluc cells behind the left adrenal gland via a left subcostal incision during administration of isoflurane (2%). For tumour imaging, mice received an intraperitoneal injection of D-Luciferin (150 mg/kg, Caliper Life Sciences, Hopkinton, MA, USA) and, five minutes after substrate injection, in vivo bioluminescence images were obtained using an IVIS Imaging System 100 Series (Xenogen Corporation, Alameda, CA, USA). All mice were imaged at a range of 25 cm and acquired images were analysed using Living Image Software version 2.5 (Xenogen). In vivo bioluminescence measurements were recorded as photons/s and the automatic range of interest function of the Living Image Software was used to analyse tumour bioluminescence in the retroperitoneal tumours resulting in a value of photons per second per centimetre squared (photons/s/cm2). Mice were initially imaged for 1 min and if an image were saturated, the image time was reduced by 10 s intervals until saturation was eliminated.
Statistical analysis
Bioluminescence intensities are reported as the mean photons/s/cm2±SEM. The GraphPad Prism program (Prism 5, GraphPad Software Inc., La Jolla, CA, USA) was used to analyse and graphically present all in vitro and in vivo data. Student's t-test analysis of cell growth 48 and 72 h post-transfection was carried out for each cell line relative to the premiR-negative control miRNA-treated samples. Two-way ANOVA analysis was used to analyse significance of tumour bioluminescence over time and Mantle-Cox analysis was used to compare overall survival in xenograft cohorts.
Results
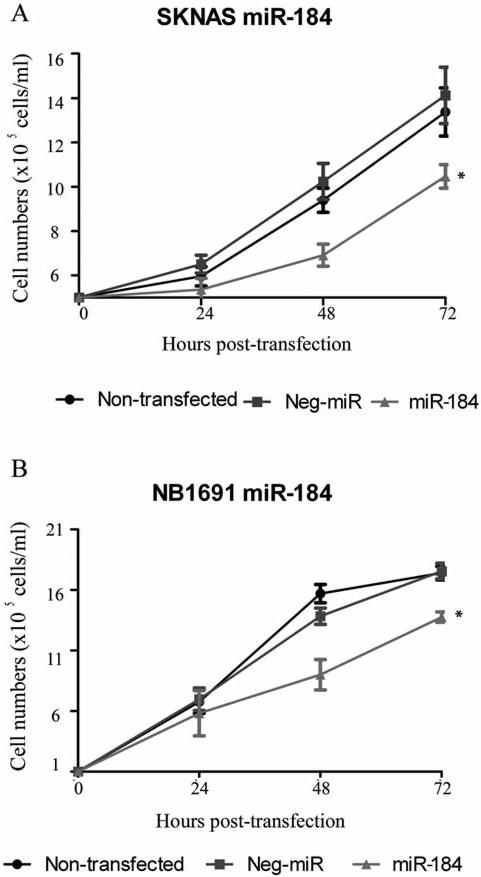
The in vitro effects of miR-184 ectopic overexpression were initially analysed on each of the cell lines. Mature miRNA-184 mimics (premiR-184) or a negative control oligonucleotide (premiR-negative control) were transiently transfected into SKN-ASluc or NB1691luc cells resulting in significantly enhanced expression of miR-184. These initial experiments confirm that miR-184 overexpression has a pronounced anti-proliferative effect on NB1691luc and SK-N-ASluc cell lines cultured in vitro (Figure 1A and B), consistent with previous publications (13).
Figure 1.
MiR-184 reduces neuroblastoma cell proliferation in vitro. SK-N-ASluc and NB1691luc (1×106) cells transfected with synthetic miR-184 showed a marked reduction in cell growth relative to premiR-negative control-treated groups in both SK-N-ASluc (A, p=0.005 48 h post-transfection and p=0.023 72 h post-transfection) and NB1691luc cells (B, p=0.027 48 h post-transfection and p=0.007 72 h post-transfection).
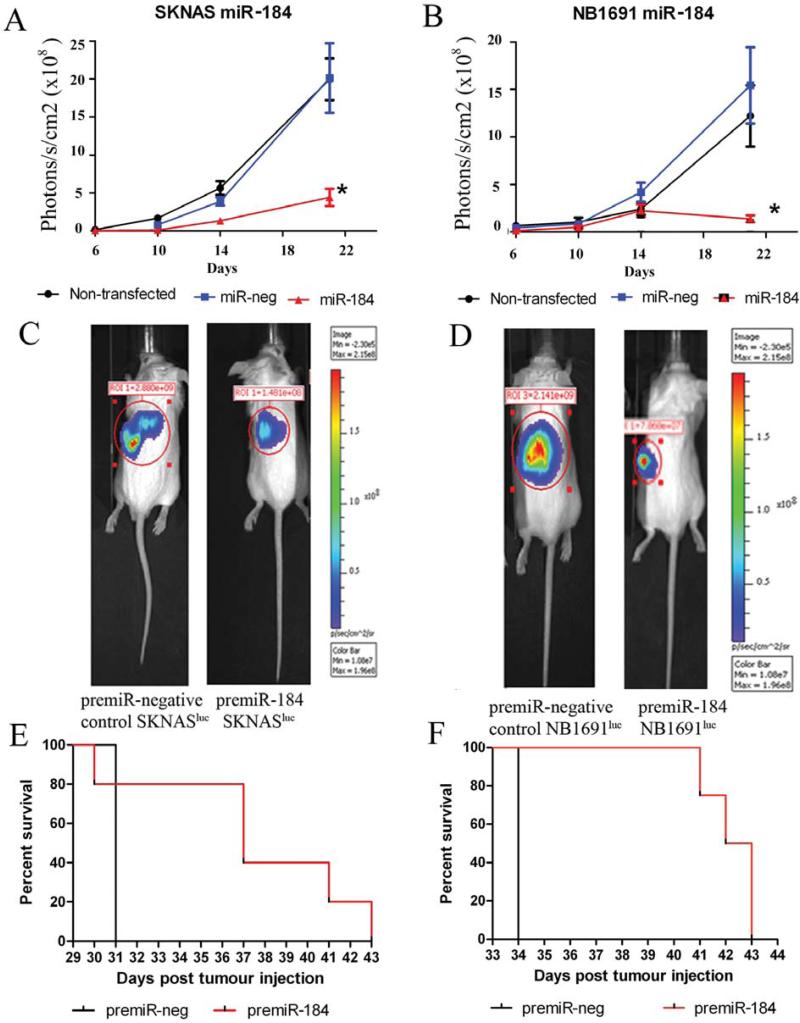
Several factors affecting tumour growth cannot be investigated through cell culture alone; therefore the effects of miR-184 overexpression in a fully established xenograft model of neuroblastoma were investigated (16). Cells pre-transfected with miR-184 or the premiR-negative control were injected retroperitoneally into CB17-SCID mice (n=4-6 per group) and tumour growth was detected by bioluminescence imaging facilitated by stable expression of the firefly luciferase gene in SK-N-ASluc and NB1691luc cells. Bioluminescent data indicates that expression of miR-184 in these murine models resulted in significant reduction in tumour volume up to 21 days post-injection relative to premiR-negative control-treated groups (*p=0.01, Figure 2A and B, respectively). Control-treated cells (premiR-negative control transfections) did not yield any significant variations in tumour volume relative to non-transfected cell induced tumours. Bioluminescence data from tumour growth was collected up to 21 days post-tumour induction in each animal cohort. Images obtained at day 21 from mice injected with miR-184-transfected cells demonstrated a reduction in bioluminescence in both SK-N-ASluc and NB1691luc tumours relative to premiR-negative control cohorts (Figure 2C and 2D are representative bioluminescence scans showing SK-N-ASluc and NB1691luc tumours, respectively). Subsequent to this time point, animals were sacrificed at moribundity. Notably, animals with miR-184-treated tumours survived longer than cohorts with premiR-negative control-treated tumours (Figure 2E, p=0.064 for SK-N-ASluc and Figure 2F p=0.0047 for NB1691luc tumour models).
Figure 2.
miR-184 expression reduces tumour burden and increases overall survival in a xenograft model of neuroblastoma. MiR-184 or premiR-negative control-treated SK-N-ASluc and NB1691luc cells (4.4×105), which were stably transfected with luciferase, were introduced into the retroperitoneal space of CB17-SCID mice (n=4-6). Pre-treatment of both SK-N-ASluc cells (A and C) and NB1691luc cells (B and D) with synthetic miR-184 led to significant reduction in tumour volume relative to premiR-negative control-treated cell tumours (*p<0.02). Animals were sacrificed at moribundity and Mantle-Cox analysis was used to compare overall survival in xenograft cohorts (E represents SK-N-ASluc and F represents the data obtained for NB1691luc animals).
Discussion
Targeted therapeutics utilising the efficacy of miR-184 is a novel area of research in terms of the treatment of both neuroblastoma and alternative forms of cancer. MiR-184 has been shown to directly bind to the 3’UTR of AKT2, a downstream protein in the PI3K pathway (13). Activation of the PI3K pathway is considered a highly potent pro-survival mechanism through which cancer progresses (15). Specific degradation of a key element of this cascade by miR-184 supports the use of this miRNA as a potential cancer therapeutic. This study provides the first evidence of in vivo functionality of miR-184 in neuroblastoma or any form of cancer. These findings provide a rationale for further analysis of miR-184-mediated treatment of pre-established neuroblastoma tumours; a line of research currently being undertaken by our research group.
Conclusion
Transient transfection of both MYCN-amplified (NB1691luc) and non-MYCN amplified (SK-N-ASluc) neuroblastoma cells with miR-184 led to a significant reduction in neuroblastoma tumour growth in vivo. This finding indicates that miR-184 replacement therapy could be of benefit in the treatment of neuroblastoma.
Acknowledgements
This work was supported in part by a Science Foundation Ireland Short Term Travel Fellowship (AT), a Science Foundation Ireland Principal Investigator Award (07/IN.1/B1776)(RLS), the Children's Medical and Research Foundation (RLS), the NIH (5R01CA127496) (RLS), the Assisi Foundation of Memphis (AMD), the US Public Health Service Childhood Solid Tumour Program Project Grant No. CA23099 (AMD), the Cancer Centre Support Grant No. 21766 from the National Cancer Institute (AMD), and by the American Lebanese Syrian Associated Charities (ALSAC) (AMD). We thank Dr. Christopher Calabrese and the staff of the Small Animal Imaging facility, and all technicians and staff associated with the Department of Surgery, St. Jude Children's Research Hospital, Memphis, TN 38105, USA.
References
- 1.Kim VN. Small RNAs: classification, biogenesis, and function. Mol Cells. 2005;19(1):1–15. [PubMed] [Google Scholar]
- 2.Breving K, Esquela-Kerscher A. The complexities of microRNA regulation: mirandering around the rules. Int J Biochem Cell Biol. 2009;42(8):1316–1329. doi: 10.1016/j.biocel.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 4.Buckley PG, Alcock L, Bryan K, Bray I, Schulte JH, Schramm A, Eggert A, Mestdagh P, De Preter K, Vandesompele J, Speleman F, Stallings RL. Chromosomal and microRNA expression patterns reveal biologically distinct subgroups of 11qneuroblastoma. Clin Cancer Res. 2010;16(11):2971–2978. doi: 10.1158/1078-0432.CCR-09-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mestdagh P, Fredlund E, Pattyn F, Schulte JH, Muth D, Vermeulen J, Kumps C, Schlierf S, De Preter K, Van Roy N, Noguera R, Laureys G, Schramm A, Eggert A, Westermann F, Speleman F, Vandesompele J. MYCN/c-MYC-induced microRNAs repress coding gene networks associated with poor outcome in MYCN/c-MYC-activated tumours. Oncogene. 2010;29(9):1394–1404. doi: 10.1038/onc.2009.429. [DOI] [PubMed] [Google Scholar]
- 6.Schulte JH, Horn S, Otto T, Samans B, Heukamp LC, Eilers UC, Krause M, Astrahantseff K, Klein-Hitpass L, Buettner R, Schramm A, Christiansen H, Eilers M, Eggert A, Berwanger B. MYCN regulates oncogenic MicroRNAs in neuroblastoma. Int J Cancer. 2008;122(3):699–704. doi: 10.1002/ijc.23153. [DOI] [PubMed] [Google Scholar]
- 7.Stallings RL, Foley NH, Bryan K, Buckley PG, Bray I. Therapeutic targeting of miRNAs in neuroblastoma. Expert Opin Ther Targets. 2010;14(9):951–962. doi: 10.1517/14728222.2010.510136. [DOI] [PubMed] [Google Scholar]
- 8.Hoehner JC, Gestblom C, Hedborg F, Sandstedt B, Olsen L, Pahlman S. A developmental model of neuroblastoma: differentiating stroma-poor tumors’ progress along an extra-adrenal chromaffin lineage. Lab Invest. 1996;75(5):659–675. [PubMed] [Google Scholar]
- 9.Bray I, Bryan K, Prenter S, Buckley PG, Foley NH, Murphy DM, Alcock L, Mestdagh P, Vandesompele J, Speleman F, London WB, McGrady PW, Higgins DG, O'Meara A, O'Sullivan M, Stallings RL. Widespread dysregulation of MiRNAs by MYCN amplification and chromosomal imbalances in neuroblastoma: association of miRNA expression with survival. PLoS One. 2009;4(11):e7850. doi: 10.1371/journal.pone.0007850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stallings RL, Howard J, Dunlop A, Mullarkey M, McDermott M, Breatnach F, O'Meara A. Are gains of chromosomal regions 7q and 11p important abnormalities in neuroblastoma? Cancer Genet Cytogenet. 2003;140(2):133–137. doi: 10.1016/s0165-4608(02)00681-7. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Stallings RL. Differential patterns of microRNA expression in neuroblastoma are correlated with prognosis, differentiation, and apoptosis. Cancer Res. 2007;67(3):976–83. doi: 10.1158/0008-5472.CAN-06-3667. [DOI] [PubMed] [Google Scholar]
- 12.Cole KA, Attiyeh EF, Mosse YP, Laquaglia MJ, Diskin SJ, Brodeur GM, Maris JM. A functional screen identifies miR-34a as a candidate neuroblastoma tumour suppressor gene. Mol Cancer Res. 2008;6(5):735–742. doi: 10.1158/1541-7786.MCR-07-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley NH, Bray IM, Tivnan A, Bryan K, Murphy DM, Buckley PG, Ryan J, O'Meara A, O'Sullivan M, Stallings RL. MicroRNA-184 inhibits neuroblastoma cell survival through targeting the serine/threonine kinase AKT2. Mol Cancer. 2010;9(1):83. doi: 10.1186/1476-4598-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontana L, Fiori ME, Albini S, Cifaldi L, Giovinazzi S, Forloni M, Boldrini R, Donfrancesco A, Federici V, Giacomini P, Peschle C, Fruci D. Antagomir-17-5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS One. 2008;3(5):e2236. doi: 10.1371/journal.pone.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 16.Dickson PV, Hamner B, Ng CY, Hall MM, Zhou J, Hargrove PW, McCarville MB, Davidoff AM. In vivo bioluminescence imaging for early detection and monitoring of disease progression in a murine model of neuroblastoma. J Pediatr Surg. 2007;42(7):1172–1179. doi: 10.1016/j.jpedsurg.2007.02.027. [DOI] [PubMed] [Google Scholar]