Abstract
Ingestion of innocuous antigens, including food proteins, normally results in local and systemic immune nonresponsiveness in a process termed oral tolerance. Oral tolerance to food proteins is likely to be intimately linked to mechanisms that are responsible for gastrointestinal tolerance to large numbers of commensal microbes. Here, we review our current understanding of the immune mechanisms responsible for oral tolerance and how perturbations in these mechanisms may promote the loss of oral tolerance and development of food allergies. Roles for the commensal microbiome in promoting oral tolerance, and the association of intestinal dysbiosis with food allergy, are discussed. Growing evidence supports cutaneous sensitization to food antigens as one possible mechanism leading to the failure to develop or loss of oral tolerance. A goal of immunotherapy for food allergies is to induce sustained desensitization, or even true long-term oral tolerance, to food allergens through mechanisms that may in part overlap with those associated with the development of natural oral tolerance.
Keywords: Food allergy, microbiome, sensitization, desensitization, immunotherapy, tolerance, Tregs, basophils, mast cells, dendritic cells
To maintain immune tolerance, the immune system must not only be able to distinguish self from non-self, but also to discriminate between innocuous non-self and threatening non-self. The gastrointestinal (GI) tract represents a unique challenge to the immune system in making these distinctions and in maintaining tolerance, for several reasons. It is the largest interface between the body and the external environment, with the intestinal mucosa having a surface area of over 300 m2.1 As such, it encounters a huge quantity and diversity of foreign antigens (Ags) representing non-self, over 30 kg of food proteins each year,2 as well as the products of trillions of resident bacteria representing more than 1000 species.3 Maintaining tolerance requires complex interactions between non-immune cells, and cells making up the gut-associated lymphoid tissue (GALT), which contains 1012 lymphoid cells per meter of gut and more immunoglobulin (Ig) producing cells than the rest of the body.4,5 These cells must act in concert to limit inflammatory responses to resident bacteria and food proteins that could lead to tissue injury, keep microbes confined to the gut, and recognize and respond to pathogens that can cause tissue injury or disease. Failure to achieve an appropriate balance in these roles can lead to a loss of tolerance, resulting in inflammatory diseases such as inflammatory bowel disease or responses to innocuous food Ags such as those occurring in celiac disease and IgE-mediated food allergies. This review will focus on the complex mechanisms underlying the development of natural tolerance to food Ags and how these may break down in individuals who develop IgE-mediated food allergies and anaphylaxis. We also will discuss how experimental immunotherapeutic approaches, some of them currently in clinical trials, have the potential to restore food tolerance.
ANTIGEN UPTAKE, DISSEMINATION AND PRESENTATION
Potentially immunogenic proteins are first subject to denaturation and degradation by digestion in the gut. The fact that these processes may play a role in preventing sensitization to food Ags has been shown in several models. Co-administration of antacids with fish proteins to mice resulted in increased levels of IgE reactive with fish proteins and increased T cell reactivity compared to mice administered fish proteins alone,6 implying that acid either directly decreases protein antigenicity by denaturing the protein or indirectly influences antigenicity by affecting protein proteolysis or uptake.7 Similar observations were made in a mouse model for hazelnut allergy, and there is a positive correlation in humans with antacid use and sensitization to food allergens.8 Ag placed in acrylic microspheres, and thereby protected from both acid denaturation and enzymatic proteolysis, can induce allergy in animals previously tolerant to ovalbumin (OVA).9
Proteins and peptides that survive denaturation and digestion in the gut can pass through the epithelial barrier via several potential mechanisms including paracellular diffusion, transcytosis through intestinal epithelial cells, endocytosis by microfold cells (M cells) and sampling by luminal processes of CX3CR1+ cells (Figure 1A).10-14 Intestinal epithelial cells may also directly present Ag to T cells in the gut as they can express MHC II on basolateral surfaces under some conditions.15-17 It is unclear at this point which mechanisms are the most important in promoting oral tolerance and food sensitization.
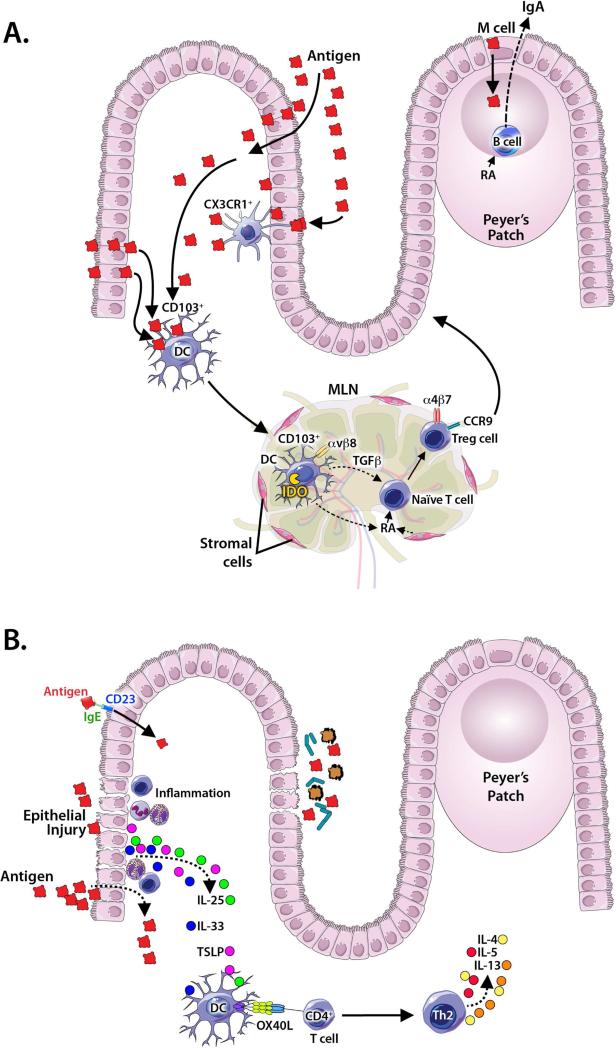
Figure 1. A model of how the gut promotes tolerance or sensitization.
Protein antigens (Ags) pass through the epithelial barrier via multiple mechanisms including capture by transluminal processes of CX3CR1+ cells. CD103+ dendritic cells (DCs) then capture Ag, migrate to the mesenteric lymph nodes (MLNs), and present Ag to naïve T cells. In tolerance (A), this interaction promotes the generation of Tregs via 1) production of retinoic acid (RA) by MLN, 2) DC expression of IDO, 3) DC secretion of TGFβ, and 4) DC upregulation of αvβ8 to activate latent TGFβ. Gut homing receptors, CCR9 and α4β7, are upregulated on newly formed Tregs. RA and DC interactions also stimulate differentiation of IgA producing B cells. In sensitization (B), epithelial disruption allows increased antigen penetration, and promotes production/release of epithelial cytokines (IL-33, TSLP and IL-25) that upregulate OX40 ligand (OX40L) on DCs. DCs then promote differentiation of naïve T cells to Th2 cells producing cytokines that recruit eosinophils (IL-5) and promote IgE class switching in B cells (IL-4 and IL-13). IgE may facilitate Ag uptake through CD23.
Certain specialized cells are implicated in Ag sampling from the gut via distinct mechanisms. M cells are a type of epithelial cell overlying the GALT (including Peyer's patches) that have a reduced glycocalyx, irregular brush border, and reduced microvilli. M cells actively engage in phagocytosis and transcytosis of particulate Ags (including microbes) and, less efficiently, soluble macromolecules from the gut lumen.15,18,19 Although one study demonstrated that targeting of soluble protein Ags to M cells facilitated tolerance induction to OVA,20 other studies have demonstrated that tolerance to soluble Ags could be induced even in the absence of Peyer's patches found beneath M cells, implying that M cell-facilitated transport to Peyer's patches does not play an essential role in oral tolerance.21-23 A population of CD11c+ myeloid cells in the lamina propria that express CX3CR1 can extend cellular processes into the intestinal lumen and sample Ags without compromising tight junctions or epithelial integrity.10,12 These cells do not migrate to mesenteric lymph nodes (MLNs) and cannot activate naïve T cells, but may pass Ag to neighboring migratory dendritic cells (DCs).13,24-26
It has been estimated that 2% of gut luminal proteins may pass through the epithelial barrier intact27 and then be disseminated locally or systemically through blood or lymph. Food proteins can be detected in the blood of mice and humans shortly after eating.28,29 Food Ags may then be presented by conventional antigen-presenting cells (APCs; eg, DCs) or unconventional APCs (eg, liver-sinusoidal endothelial cells, Kupffer cells, or plasmacytoid DCs) where, in the absence of costimulatory signals, Ag is likely to induce tolerance.13,30,31 A potential role for systemic dissemination of Ag in tolerance is supported by studies demonstrating that transfer of serum from fed mice can induce tolerance32 and that shunting of the portal blood flow can inhibit development of oral tolerance.33,34 Locally, in the gut, CD11c+ CD103+ DCs migrate from the lamina propria to the MLNs in a CCR7-dependent manner, carrying Ag that appears critical for the development of oral tolerance.35-37 Inhibition of normal lymph node drainage and lymphocyte trafficking through mesenteric lymphadenectomy, small bowel transplantation (without ligation of lymphatic vessels), or CCR7 deficiency all prevented induction of oral tolerance in mice.37
How Ag uptake, dissemination and/or presentation may differ in those who develop food allergy rather than food tolerance, and the importance of such differences in the development of the disorder, is not well understood. Patients with food allergy are known to have increased gut permeability at baseline,38 that may be further exacerbated by cytokines and chemokines produced during an allergic reaction (eg, TNF-α) which can act to reduce tight junction integrity or otherwise increase gut permeability.39-41 In rodent models of food allergy, increased specific Ag uptake may occur through IgE binding to Ag in the lumen, followed by transcytosis through intestinal epithelia via the low affinity IgE receptor, CD23.42,43 Notably, while CD103+ DC migration is necessary for induction of oral tolerance, adoptive transfer of CD11c+B220− splenic and Peyer's patch cells (including DC populations) from mice with cow's milk allergy to naïve recipient mice was sufficient to induce milk-specific IgE production.44 These observations indicate that DCs are likely to play critical roles in induction of both oral tolerance and allergic sensitization.
Environmental Exposure to Food Allergens through the Skin
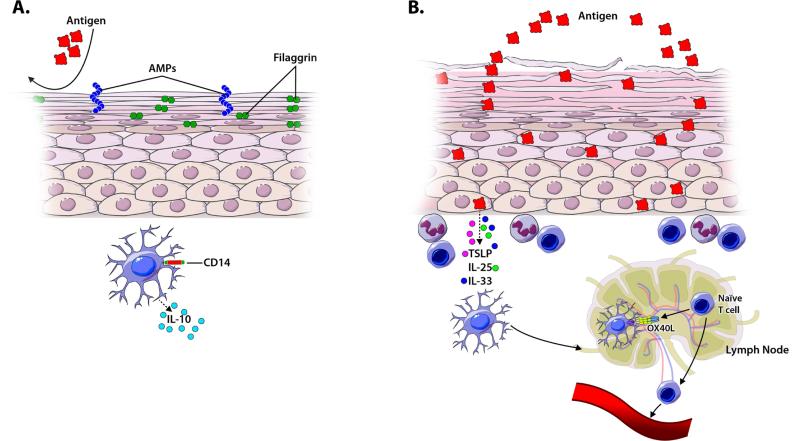
Even before introduction to complementary or solid foods, infants may be exposed to food proteins (eg, in household dust) through cutaneous contact.45,46 Like the gut, the skin is one of the largest immune organs and provides not only a physical and chemical barrier, but also serves as a protective immunological barrier in maintaining immune homeostasis between the environment and the host's deeper tissues. The ability of the skin to constitute a protective barrier to environmental insults and Ag exposure is the result of a complex constellation of its properties, including proper epidermal cell differentiation, a hydrolipidic milieu due to lipids, sebum, sweat, and antimicrobial peptides, and features of the normal dermis (Figure 2A).47 Healthy skin normally represents a non-inflammatory environment with numerous resident APCs. One such APC, the CD14+ DC, shares features of both CX3CR1+ and CD103+ cells in the lamina propria. It phagocytoses large quantities of Ag (similar to CX3CR1+ cells), but produces large amounts of IL-10 and effectively induces T regulatory cell (Treg) differentiation.48 Loss of epithelial integrity and skin inflammation can predispose individuals to allergic sensitization (Figure 2B), as discussed below.
Figure 2. A model of how the skin promotes tolerance or sensitization.
Keratinocyte differentiation, an intact uppermost stratum corneum layer with epidermal proteins that maintain barrier function (eg. filaggrin), antimicrobial peptides (AMPs), and tolerogenic APCs, such as CD14+ DCs producing IL-10, are important for promoting tolerance in the skin barrier (A). Loss of barrier function in the stratum corneum, allowing increased Ag penetration, can occur as a result of genetically-determined defects in factors necessary for keratinocyte differentiation (eg. mutations in filaggrin) or as a result of inflammatory skin diseases (eg. atopic dermatitis). In response to injury, activation by microbial or food antigens, or inflammatory signals TSLP, IL-33 and/or IL-25, produced by keratinocytes, can upregulate OX40L on APCs to promote Th2 differentiation (B).
ORAL TOLERANCE
Dendritic Cells in Oral Tolerance
It still is unclear if there is a critical time period for encountering Ag, and an ideal type of Ag exposure, for the development of tolerance. Many believe that first encountering Ag at the GI mucosa or GALT can promote tolerogenic responses to food proteins.13 Indeed, a recent clinical trial has indicated that earlier exposure in infancy to one potentially allergenic food, ie, peanut, can decrease rates of clinical food allergy.49 Tolerance may largely be driven by APCs within the lamina propria that sample Ags in the lumen and promote T cell differentiation. Intriguingly, diet may influence the development of APCs in lamina propria, as mice fed an elemental diet exhibited differences in lamina propria DC subsets.50 As mentioned above, mouse studies have revealed that at least two distinct CD11c+ APC populations exist: CX3CR1+CD103− and CX3CR1−CD103+ phagocytes. CX3CR1+CD103− phagocytes are derived from monocytes and extend processes through the epithelium to sample Ags. They do not migrate or activate naïve T cells, but they influence early immunological responses to Ag and are involved in the restimulation of T cells.51 In contrast, CX3CR1−CD103+ DCs capture Ag in the lamina propria and migrate to draining MLNs to present Ag to T cells.
In the MLNs, migratory CD103+ DCs from the lamina propria can promote the development of gut-homing Tregs through multiple mechanisms. CD103+ DCs produce TGF-β and retinoic acid (derived from vitamin A), driving Treg differentiation.52 Moreover, upregulation of αvβ8 on CD103+ DCs is important for activating latent TGF-β and generating Tregs during the induction of tolerance to intestinal Ags in mice.53,54 Additionally, CD103+ DCs can express indoleamine 2,3-dioxygenase (IDO), an enzyme involved in tryptophan catabolism. Inhibition of IDO diminishes Treg conversion and favors Th1 and Th17 induction.55
The cooperation of CD103+ DCs and MLN stromal cells is important for inducing the expression of gut homing receptors on activated T cells.56 CD103+ DCs induce expression of gut homing receptors CCR9 and α4β7 on T cells primed in the MLNs to facilitate migration of T cells to the small intestine.57 However, other mechanisms may also be capable of inducing gut tropism. In vitro activation of T cells by intestinal DCs, retinoic acid alone, and stromal cells isolated from MLNs were sufficient for induction of gut tropism. High levels of retinoic acid producing enzymes are unique to MLNs (as compared to peripheral lymph nodes) and support induction of the chemokine receptor CCR9 on activated T cells; CCR9 expression is further enhanced by bone marrow-derived DCs in vitro.58
In addition to driving gut tropism of T cells, retinoic acid derived from GALT-associated DCs has also been shown to imprint gut tropism on B cells and to act synergistically with DC-derived IL-6 or IL-5 to induce IgA secretion; mice deprived of vitamin A, and thus retinoic acid, lacked IgA secreting cells in the small intestine.59 Clearly, such studies indicate that retinoic acid, derived from DCs and MLN stromal cells, is important for inducing tolerogenic responses in B cells and T cells, and for directing these cells to the small intestine in mice.
Tregs in Oral Tolerance
Tregs play a central role in oral tolerance. In patients with immunodysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX syndrome), a rare disease linked to the dysfunction of the transcription factor Foxp3, which is essential for Treg development, there is an increased incidence of food allergies.60 Several mouse models support a role for Foxp3+ T cells in oral tolerance. In a model using the hapten 2,4-dinitrofluorobenzene (DNFB), antibody depletion of CD25+ cells (a marker for Tregs) impaired the oral tolerance normally induced by feeding DNFB.61 Transfer of CD4+CD25+ cells to CD4+ T cell-deficient mice, which do not normally develop oral tolerance after feeding DNFB, is sufficient to restore oral tolerance induced by feeding.61 Similarly in a model of OVA-induced allergic diarrhea, Foxp3+ Ag-specific cells proliferated in the lamina propria during oral tolerance induction and depletion of Foxp3+ cells abrogated oral tolerance.62 Dietary antigens may promote differentiation of Tregs and devlopment of oral tolerance. Mice fed an elemental diet had reduced numbers of lamina propria Tregs, increased proliferation of Ag-specific T cells upon Ag feeding, and increased susceptibility to a model of allergic diarrhea when compared to control mice fed normal chow.50
Although it is now appreciated that there are multiple subtypes of Tregs,63,64 and that these populations can exhibit phenotypic plasticity, the roles of individual Treg subtypes in oral tolerance is less well defined. Both Foxp3+ and Foxp3− Tregs producing IL-10 can be found in the gut and many of the Foxp3− Tregs are likely peripherally-induced Tr1 cells that can produce large amounts of IL-10 and TGF-β.34,65,66 In two models of oral tolerance to OVA, peripheral conversion of naïve T cells to Foxp3+ inducible Tregs was necessary for tolerance induction.62, 67 Deficiency of CCR9 or α4β7 integrin on T cells, or a deficiency of the α4β7 ligand MAdCAM-1 on gut endothelial cells, inhibited Treg homing to the gut, which is essential for induction of oral tolerance.62,68 These observations indicate that Tregs can act locally in the gut and GALT, rather than (or in addition to) acting at peripheral sites. As a prominent source of TGF-β, Tregs that have homed to the GALT may also promote B cell production of non-inflammatory IgA.69
Allergic Sensitization—A Breakdown in Oral Tolerance
The inciting events leading to the breakdown of oral tolerance, allergic sensitization, and the development of food allergies in a subset of such sensitized individuals, are poorly understood. It is likely that multiple pathways could ultimately lead to a failure to develop or loss of oral tolerance (Figure 1B). Epithelial cells produce thymic stromal lymphopoietin, IL-25, and/or IL-33 in response to injury, inflammation, and innate immune activation, and these cytokines can drive Th2 inflammation.70 Such epithelial cytokines promote Th2 inflammation through activating innate lymphoid cells (ILCs), mast cells, basophils, and DCs to produce cytokines that drive Th2 immunity.70 IL-33 was shown to be critical in the development of allergy in a cholera toxin-induced mouse model of peanut allergy.71 Intriguingly, both cholera toxin and IL-33 upregulate OX40L on DCs, driving differentiation of naïve CD4+ T cells to Th2 cells.71,72 We are only beginning to understand the full spectrum of factors that interact to regulate epithelial cell production of cytokines in food allergy.
More than Your Average Food Protein: A Role for Allergens in Sensitization
Although ample evidence supports a role for aeroallergens in promoting allergic sensitization and Type 2 immunity, similar evidence for food allergens is more limited. Aeroallergens have been shown to foster allergic sensitization and production of epithelial cell cytokines through multiple mechanisms, including activation of innate pattern recognition receptors, activation of protease-activated receptors, and through direct injury to epithelia.73 For example, the proteolytic activity of the dust mite protein Der p 1 has been extensively studied and shown to disrupt bronchial epithelial integrity and enhance allergen uptake from the lumen,74 cleave receptors from the surface of immune cells (CD25 and CD23), and enhance IgE production.75 Among food Ags, Ara h1 binds to CD209 on DCs and milk sphingomyelin activates invariant NKT cells, effectively acting as adjuvants that enhance type 2 cytokine production.76,77
Many have tried to identify other characteristics of proteins that promote loss of tolerance and/or allergic sensitization. Features of proteins such as disulfide bonds, resistance to enzymatic proteolysis or thermal degradation, biological functional activity, and glycosylation of proteins may contribute to allergenicity and elicit more avid IgE binding.78 Disulfide bonds preserve protein structure and stability. For example, amongst aeroallergens, disrupting the disulfide bonds in dust mite proteins, Der p 1 and Lep d 2, reduced binding of IgE derived from allergic patients.79,80 Similarly, common food allergens resisted proteolysis in gastric fluid,81 though there are many proteins resistant to digestion that are not ordinarily allergenic. Sensitization to food proteins that are resistant to thermal degradation during cooking is associated with more severe and persistent milk and egg allergies,82-85 whereas sensitization to heat labile PR-10 proteins in oral allergy syndrome is associated with symptoms that are generally more mild and rarely systemic.86 Finally, glycosylation of Ags may prevent proteolysis and form neoantigens, potentially affecting food protein allergenicity. The Maillard reaction is a non-enzymatic chemical reaction between amino acids and reducing sugars occurring at high temperatures, which leads to the “browning” of food. Studies of peanut allergenicity have shown that dry roasting can increase sensitization to peanut in mouse models.87 Indeed, when the major peanut epitopes Ara h 1 and 2 were subjected to Maillard reactions, they bound higher levels of IgE from peanut allergic patients and were resistant to digestion.88 It may be that the allergenicity of various proteins from the environment and in food operate in concert to induce allergenic responses.
Strange Encounters: Food Allergen Sensitization through Skin
Atopic dermatitis is a chronic inflammatory disorder of the skin in which defects in the epidermal epithelium can lead to systemic allergen sensitization often preceding other atopic diseases such as food allergy, asthma, and allergic rhinitis, a phenomenon known as the atopic march. Recent studies have highlighted the impact of both defective skin epithelium and the presence of food Ag in household dust in leading to sensitization to food allergens. Filaggrin is an epidermal protein involved in maintenance of skin barrier function, and patients with loss of function mutations of filaggrin are at higher risk of developing eczema and atopy.89,90 Patients with loss of function filaggrin mutations had higher prevalence of food sensitization and development of food allergy by the age of 10.91 Given this observation, Brough et al. investigated exposure of peanut protein in the dust of households consuming peanut and the development of peanut allergy in patients with skin barrier defects. In children with filaggrin loss of function mutations, peanut allergy was 3-fold higher in 8-11 year olds compared to those without filaggrin mutations.45 As with filaggrin, polymorphisms of SPINK5, a protease regulating keratinocyte differentiation and affecting epithelial integrity, are associated with severe atopic dermatitis and increased incidence of food allergy.92 Further supporting a role for cutaneous sensitization in food allergies, increased exposure to peanut protein in dust increased the risk of peanut sensitization in children, especially those with atopic dermatitis.93
Since atopic dermatitis has been identified as an instigating event predisposing to further sensitization, many have tried to find therapies to prevent eczema and potentially food sensitization. Simply applying moisturizers or emollients from birth has been shown to reduce the development of atopic dermatitis.94,95 One of these two studies also examined whether emollient use would influence allergic sensitization to egg, but observed no differences between those receiving emollient and control groups.95 Larger long-term studies are needed to see if this approach can prevent the further development of additional atopic diseases in patients with atopic dermatitis.
Just as some allergens can have an adjuvant effect in the respiratory and GI tracts, some allergens may have adjuvant effects in the skin. Peanut extract, and specifically Ara h2, were shown to have adjuvant activity in the mouse skin.96 In vitro peanut extract induced IL-33 and IL-6 expression in keratinocytes and upregulated OX40L expression on bone marrow-derived DCs, while in vivo peanut extract enhanced cutaneous responses to a bystander Ag, OVA, and promoted Th2 T cell development.96 These observations suggest that future studies examining food allergenicity and the propensity to develop clinical food allergies may need to examine both the skin and the gut.
MICROBIOME IN TOLERANCE AND ALLERGY
The communities of bacteria comprising the gut microbiome are complex and dynamic. They are influenced by the environment in which individuals live, and they evolve as people age from infancy to adulthood.97-99 Living in a rural vs. an urban environment likely influences the composition of an individual's microbiome,97,100 but the underlying causes of these differences are not fully understood. An increased diversity of bacteria in household dust in farm homes inversely correlated with risk of asthma and atopy,101 but it is unclear if this was related to any differences in individuals’ microbiomes. It is possible that additional factors can contribute to variations in microbial communities between urban and rural dwellers, such as diet.102,103 For instance, plant-based diets promote growth of phyla capable of fermenting plant polysaccharides.102 As factors affecting the diversity and development of the gut microbiome are being elucidated, it is also becoming clear that the microbiome can dramatically influence the development of immune responses in the gut, including those to food Ags (Figure 3).
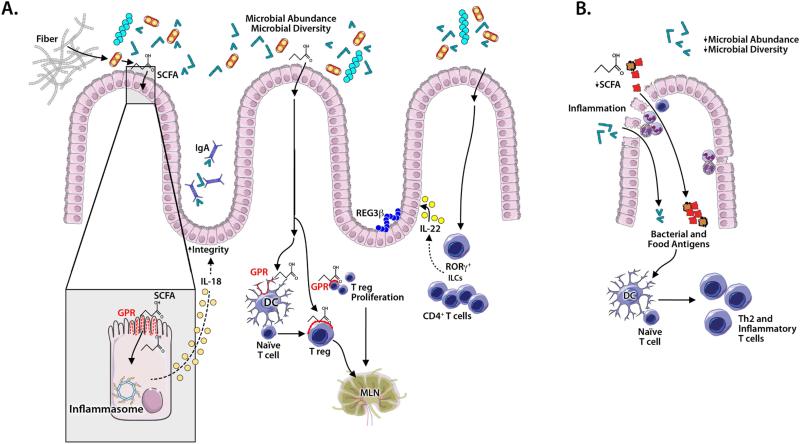
Figure 3. Microbial mechanisms contributing to oral tolerance and allergic sensitization in the colon.
Microbial diversity and abundance promote tolerance (A). Microbes ferment fiber to produce short chain fatty acids (SCFAs) that bind G-protein coupled receptors (GPRs) on: 1) intestinal epithelial cells (IECs) to activate inflammasome production of IL-18 that promotes epithelial barrier integrity; 2) dendritic cells (DCs) to drive naïve T cells to become Tregs; 3) Tregs to induce proliferation. Additionally, SCFAs promote acetylation of histone H3 to preserve or induce FoxP3+ Tregs. Microbe induced IL-22 production by RORγt+ innate lymphocytes and CD4+ T cells promotes barrier integrity and IEC synthesis of antimicrobial peptides and mucus. “Tolerogenic” colonic DCs and lymphocytes likely migrate to mesenteric lymph nodes. In allergic sensitization (B), changes in microbial abundance and diversity (eg, after antibiotic exposure) decrease SCFA, IL-18 and IL-22 levels, compromising epithelial integrity, thereby facilitating epithelial passage of microbial and food antigens. DC activation promotes inflammation, the development of Th2 cell-associated immune responses (including production of allergen-specific IgE antibodies), and allergic sensitization.
Data suggest that particular bacteria, most notably from the Clostridia class, may promote the development of tolerance in the gut. Colonization of antibiotic-treated mice with Clostridia-enriched microbiota prevented allergen absorption and allergic sensitization, restoring oral tolerance.104 Clostridia may promote tolerance in the gut through several mechanisms. Colonization of germ-free mice with Clostridia-enriched microbiota promoted IgA production and Foxp3+ cell numbers in the colon.104 IgA in the intestinal lumen (Figure 3A) may regulate the composition of the microbiome, and inhibit inflammation induced by the bacterial species and Ags to which it binds.105,106 Although the mechanisms underlying these changes are unclear, Clostridium clusters IV, XIVa and XVIII are known to promote a TGF-β and IL-10 rich environment in the mouse colon.107,108 In addition, Clostridia promote IL-22 production by RAR-related orphan receptor gamma-positive ILCs and T cells in the intestinal lamina propria, promoting epithelial integrity, and upregulating expression of antimicrobial peptides, including REG3β, and mucus (Figure 3A).104 Injection of anti-IL-22 in mice enhanced absorption of peanut Ag, but did not lead to significantly increased levels of peanut-specific IgE or IgG.104
Exactly how Clostridia may promote these effects is unknown, but evidence now supports a role for bacterial metabolites in regulating epithelial integrity and immune responses in the gut. Clostridial families Lachnospiraceae and Ruminococaceae are among prominent bacterial groups in the proximal colon that ferment dietary fiber to produce short chain fatty acids (SCFAs), including acetate, propionic acid, and most notably butyric acid, which can have multiple effects on the immune response (Figure 3A).109-112 SCFAs bind to G protein coupled receptors (GPRs) GPR43 and GPR109A on mouse enterocytes, activating the inflammasome and promoting production of IL-18; IL-18 fosters epithelial integrity, repair and homeostasis.113,114 SCFAs, particularly butyric acid, can increase numbers of colonic Foxp3+ Tregs when administered to mice in drinking water, via enema, or as dietary precursors.115-117 Inhibition of histone deacetylase activity by SCFAs may promote acetylation of histone H3 at the Foxp3 promoter, thereby promoting Treg differentiation.115,116 Signaling through GPR43 by propionate may promote expansion of Tregs,117 whereas there is conflicting evidence for whether GPR109a expression on APCs has a role in promoting Treg differentiation.114,115 How the gut microbiome, largely resident in the colon, affects immune responses in the small intestine is unclear. Possible mechanisms include the migration of colonic immune cells (APCs, T cells and B cells) to the MLNs where they interact with cells from the small intestine, or transport of bacterial metabolites or cytokines to distant sites via blood or lymph where they can exert their effects; there also may be direct effects of the less abundant microbiota in the small intestine.110
Consistent with observations that the gut microbiome may normally promote oral tolerance, growing evidence suggests that perturbations of the microbiome may correlate with, or even predispose to, food allergy (Figure 3B). Most notably in humans, early antibiotic use has been linked to alterations in microbiome composition and the development of food allergies. Intrapartum antibiotics were associated with changes in infant microbiome composition at 3 and 12 months.118 Similarly, other studies have shown that effects of antibiotics on human microbiome composition can be persistent, even in older individuals.119,120 Dysbiosis has also been observed in neonatal mice treated with antibiotics, and these perturbations also may persist.104,121 Maternal use of antibiotics during human pregnancy or infant antibiotic use in the first month of life is associated with increased risk of cow's milk allergy,122 and higher urinary levels of triclosan are found in children that are sensitized to food and aeroallergens.123 In mice, it has been similarly observed that neonatal antibiotic administration promotes allergic sensitization to peanut.104 In one study, disturbances in the microbiome occurred at doses of antibiotics of only 1/50-1/100th of treatment doses,121 raising the possibility that even exposure to low doses of antibiotics may affect human microbiome composition.
Given the large number of other factors that can affect microbial composition, as discussed above, identification of consistent patterns of microbiome features in individuals with food allergy has been difficult. In a prospective study, an increased Enterobacteriaceae to Bacteroidaceae ratio and low Ruminococcaceae abundance in the context of low microbiota richness at 3 months was associated with sensitization to 1 or more foods at 12 months of age.118 In contrast, 4-month-old infants with milk allergy identified by double blind oral food challenge were found to have increased microbial diversity with an increased abundance of Ruminococcaceae and Lachnospiraceae compared to healthy age-matched controls.124 In that study, the microbiota of healthy infant controls had lower diversity, dominated by Bifidobacteriaceae, Enterobacteriaceae and Enterococaceae.124 Chinese infants with both IgE- and non-IgE-mediated food allergy had similar overall microbial diversity when compared to control infants, but showed alterations in particular phylotypes.125 Notably, infants with IgE-mediated allergy had decreased levels of Bacteroides and Clostridium XVIII.125 Given the many factors that can influence microbiome composition in the gut, many more large longitudinal studies will be needed to identify if reproducible patterns are associated with Type I food allergy.
Skin Microbiota in Food Allergy
With growing evidence that food sensitization can occur through the skin, as discussed above, it is possible that dysbiosis of skin microbiomes also may contribute to food sensitization. Alterations of the skin microbiome have been observed in patients with atopic dermatitis and can drive atopic dermatitis in a mouse model,126,127 and therefore it is reasonable to hypothesize that such changes may promote food sensitization through the skin. Staphylococcal enterotoxin B acts as adjuvant in the skin to drive Th2 responses and follicular helper T cell (Tfh) development.96 Indeed, Staphylococcal enterotoxin B is used in animal models of food allergy as an oral adjuvant to promote allergic sensitization in the gut, and can enhance Th2 responses to peanut in mouse skin.128,129 Clearly, the relationship between dysbiosis of the skin microbiota and food allergy needs to be more closely examined.
Probiotic Therapy
Probiotic administration for the prevention or treatment of allergic disease has yielded conflicting results to date. A meta-analysis of trials of prenatal and neonatal probiotic treatment found reduced total IgE levels and atopic sensitization, but no reductions in asthma or wheezing.130 Few trials of probiotics in the prevention or treatment of food-challenge-verified food allergies have been published. In food challenge-proven cow's milk allergy, treatment with Lactobacillus casei and Bifidobacterium lactis for 12 months did not affect rates of milk allergy resolution, however treatment with Lactobacillus rhamnosus in combination with extensively hydrolyzed casein formula increased rates of milk allergy resolution compared to a control group receiving hydrolyzed formula alone.131-133 Notably, treatment with Lactobacillus rhamnosus correlated with increased levels of fecal butyrate.124 Similarly, co-administration of peanut oral immunotherapy (OIT) with Lactobacillus rhamnosus for 18 months resulted in nonresponsiveness in 82% of treated individuals at 2-5 weeks after cessation of OIT, vs. 3% of those receiving placebo.134 However, because no OIT alone or probiotic alone control groups were included, it is unclear what the benefit of probiotic + OIT would be over OIT alone or probiotic alone. It is likely that the benefits of probiotic supplementation are phyla specific, but insufficient data are currently available to support the use of probiotics containing particular phyla at this time. Additional data on microbiota dysbiosis in food allergy will allow the design of appropriate randomized control trials of probiotics and prebiotics (dietary substances promoting the growth of beneficial microorganisms) in food allergy. Given current data discussed above, it will be important to evaluate critically whether probiotic supplementation with specific Clostridia can have benefits for treating or preventing food allergy.
IMMUNOTHERAPY: MECHANISMS OF DESENSITIZATION AND LONG-TERM TOLERANCE
Multiple approaches have been attempted to regain or induce tolerance to foods. OIT was first reported by Schofield in 1908 with successful desensitization by incorporation of egg into a child's diet.135 Note that we are using the term “desensitized” herein to refer to the ability of a subject to ingest the offending food without clinical reactivity to it, but requiring continued consumption of that food to maintain this state of non-reactivity. Nelson and colleagues attempted subcutaneous desensitization to peanut in 1997 in a small cohort and noted significantly increased systemic reactions requiring epinephrine during build up and maintenance periods, impelling others to find alternative routes and safer approaches.136 Further attempts at desensitization have involved trials of OIT, sub-lingual immunotherapy (SLIT), or epicutaneous immunotherapy (EPIT). Although SLIT and EPIT are regarded as safer approaches compared with OIT as subjects typically only experience mild, local oral and cutaneous reactions respectively,137-140 one subject undergoing SLIT for peanut allergy required epinephrine for urticaria and coughing.141 OIT also can be done safely, but subjects experience increased reactions (the majority are mild GI complaints) with daily dosing during build up and maintenance periods.142 However, these approaches differ significantly in the amount of food to which the subject is effectively desensitized, with OIT achieving desensitization to serving sizes and SLIT and EPIT substantially less.138,140,142-144 Recently, omalizumab, a monoclonal antibody against IgE, has been explored as an adjunctive therapy with OIT with multiple studies showing safe and faster desensitization rates to milk, peanut, or multiple foods simultaneously.145-148 While many questions remain in optimizing OIT regarding the optimal dose of the offending food allergen to be used for maintenance, the maintenance time period, and the sustainability of the desensitization process, OIT can achieve desensitization rates on average of 80-85%.149 Whether these therapies produce long lasting non-reactivity to the offending allergen (either with or without the continued intentional ingestion of those allergens) has only been analyzed in a few studies. Re-challenge after varying periods of avoidance has shown rates of sustained unresponsiveness (defined here as non-reactivity to a food challenge after avoidance of the offending allergen for periods of 1 week to 6 months) ranging from 13%-36%.149 While these rates seem sub-optimal, most subjects still maintained a state of desensitization to a threshold level higher than their screening challenge. Whether any of these patients will maintain “long-term tolerance” to that allergen, which we propose to define as experiencing years of unresponsiveness to the food in the absence of intentional ingestion of the offending allergens, remains to be seen. It also will be important to determine the extent to which the mechanisms underlying “desensitization” vs. “long-term tolerance” are similar or different, and to develop tests that can reliably determine the immune status of food allergic subjects.
Early responses to antigen specific immunotherapy
Mechanisms of action in allergen specific immunotherapy have been explored for allergic rhinitis and stinging insect hypersensitivity150 and are likely to be similar in food allergy immunotherapy (IT). Protection from reactions in the early stages of IT are due to decreased activation of mast cells and basophils, which has been seen as early as in the first 3-4 months of OIT.143,151-153 This may in part be due to reduced levels of Ag-specific IgE on the surfaces of these effector cells.154,155 OIT in a mouse model of egg allergy did not result in desensitization of blood basophils or peritoneal mast cells or protection from challenge by intraperitoneal injection despite protection from oral challenge, implying that desensitization may occur locally in the GI tract.156 However, other mechanisms also may be involved. It has long been speculated that desensitization may deplete certain mediators of effector cells (eg, by inducing the release of histamine from granules), and stimulate the release of leukotrienes, but in amounts that are small and below the threshold for causing anaphylaxis. In patients undergoing rush desensitization for venom allergy, decreased levels of histamine were observed in whole blood, suggesting degranulation of basophils or decreased basophil numbers.157 In contrast, patients undergoing standard (not rush) IT for venom allergy had normal histamine content in blood leukocytes.158 Notably, mouse studies of oral desensitization for penicillin demonstrated Ag-specific desensitization with no evidence for mediator depletion.156,159
In addition to basophils and mast cells, many other cell types may contribute to early IT responses. During venom desensitization, monocytes increased expression of immunoglobulin-like transcript 3 (ILT3) and ILT4 receptors, which are crucial to the tolerogenic function of monocytes.160 Tolerogenic APCs, such as CD103+ DCs as described above, can aid in the differentiation of T cells into Tregs. Oral mucosal Langerhans cells have been shown to bind grass pollen in an ex vivo model, which enhanced their migratory capacity and promoted the secretion of the tolerogenic cytokines, TFG-β1 and IL-10.161 Grass pollen SCIT has been shown to mitigate seasonal increases in peripheral ILC2s,162 potent potential producers of the type 2 cytokines, IL-4, IL-13, and IL-5, that can enhance inflammation in asthma, allergic rhinitis and atopic dermatitis, through activation of mast cells, basophils and eosinophils and promotion of B cell class switching to produce IgE antibodies.163-166
Skin-derived APCs are important in directing the initial immune response. When OVA is applied to the intact skin of mice, it is taken up in the superficial layers of the stratum corneum and transported to draining lymph nodes. After repeated epicutaneous delivery of OVA, local and systemic Th2 responses are down regulated with associated upregulation of T regs.167 Consistent with this observation in mice, EPIT requires an intact stratum corneum layer.168
T cell responses to antigen specific immunotherapy
The induction of peripheral T cell tolerance is a crucial step induced by IT, and in different models various changes in Ag specific T cell populations correlated with tolerance, including increased Tregs,169 decreased Th2 cells,170 and increased anergic T cells.171 The proportion of allergen-specific T cell subsets and the change in the dominant subset may skew towards allergy vs. tolerance.169 In a cholera toxin induced mouse model of milk allergy, treatment with milk OIT increased levels of IL-10 and TGF-β in the jejunum, likely produced by Tregs within the gut.170 Using allergen-MHC tetramers to track allergen-specific T cells during the course of wasp-venom immunotherapy, clinical tolerance was associated with a loss of IL-4-producing T cells and increased IL-10-producing Foxp3+ Ag-specific T cells that might share a common precursor with IL-4-producing T cells specific for the same epitope.171 Recently, we used peanut-MHC dextramers to sort peanut-specific T cells from subjects with peanut allergy and analyzed changes in gene expression of individual CD4+ T cells during the course of OIT. Our evidence indicated that increased length of treatment with OIT induced peanut-specific T cells to shift towards an anergic, memory T cell phenotype (CD28loKi67lo). Moreover, sustained nonresponsiveness to peanut, even after a 3-month period of withdrawal from peanut, was associated with induction and maintenance of naïve and memory peanut-specific T cells that were detectable even 3 months after therapy.172 The failure to maintain tolerance to the offending allergen may be due to the induction of Tregs that are short lived or epigenetically modified. In our cohort of subjects who completed 24 months of peanut OIT (20/23 subjects), we have shown that 7/20 subjects were still “immune tolerant” after a 3 month period of withdrawal, and 3/7 remained “immune tolerant” after an additional 3 month withdrawal period (totaling 6 months of withdrawal from therapy).151 All subjects were found to have an increase in peanut specific Tregs after 12 months of OIT; in those that were immune tolerant, there was significant hypomethylation of CpG sites in peanut specific Tregs at 24 months and 27 months.151 In the 4 subjects who “lost” their tolerant status after 6 months of peanut withdrawal, there was increased methylation of their peanut specific Tregs.151 These findings suggest that epigenetic changes of Ag-specific immune cells may, at least in part, explain desensitization and tolerance. However, achieving sustained responses to therapy may depend on whether such epigenetic changes can be maintained.
B cell responses to antigen-specific immunotherapy
OIT can induce changes in immunoglobulin subsets in subjects with peanut allergy. Patients undergoing peanut OIT for a median of 41 months exhibited increased levels of peanut-specific IgG4 with de novo specificities associated with reduced serum levels of peanut IgE.173 In our own cohort of patients, peanut OIT was associated with increases in the frequency of peanut-specific B cells in the blood.174 The allergen-specific B cells were mainly of the memory phenotype, with lower numbers of plasmablasts, and predominantly expressed somatically mutated class-switched antibodies of IgG and IgA subtypes, with lower numbers of IgM-expressing cells also noted.174 Antibodies from these cells recognized both conformational and linear epitopes.174 Notably, during the course of OIT, more highly-mutated IgG4–expressing members of a peanut specific clone were observed; in contrast, the somatic mutation levels in IgE members of the clone did not increase, suggesting that ongoing somatic mutation of IgG4–expressing B cells may contribute to the increased effectiveness of peanut OIT over time, perhaps by increasing IgG4 affinity for allergen.174
Although increasing evidence supports a role for Ag-specific IgG4 in directly promoting tolerance, IgG4 levels may also correlate with other mechanisms responsible for inducing tolerance. Ratios of peanut-specific IgG4 to peanut-specific IgE were higher in sensitized (ie, skin test or specific IgE positive) but clinically tolerant patients than in patients with clinical peanut allergy.175 Sera from sensitized but clinically tolerant patients, or patients undergoing peanut OIT, inhibited activation of peanut sensitized mast cells or basophils by peanut extract, and the inhibitory activity of sera was decreased if IgG4 was depleted.175,176 The mechanisms by which IgG4 inhibited mast cell or basophil activation may include IgG4 activity as a “blocking antibody” binding allergen before it encounters IgE bound to the surface of basophils or mast cells, or alternatively IgG4-dependent activation of inhibitory Fcγ receptors.150,177 Studies using blocking antibodies for CD32 with human basophils, or in a mouse model for peanut OIT, demonstrated that IgG binding to inhibitory Fcγ receptors is at least partially responsible for the observed inhibitory effects.176 In a study of egg OIT, children with egg allergy had lower egg white specific IgA compared to healthy controls; in most who became tolerant to egg there was a significant increase of over 28% of egg white specific IgA over time, suggesting a role of allergen specific IgA in food tolerance.178 Analysis of tolerant beekeepers and patients following venom IT demonstrated that venom specific IgG4 production was predominately by IL-10-secreting B regulatory 1 cells, a population that was observed to increase following IT, and to express high levels of IL-10 on a per-cell basis.179 Large amounts of IL-10 produced by B cells could suppress effector T cell function or promote differentiation of Tregs. Overall, global epitope-specific shifts from IgE to IgG4 binding occur over the course of immunotherapy, and may often be attributed to B cells of a regulatory type. Such changes may act in concert with regulatory T cell alterations and tolerogenic APC functions to promote long-term tolerance.
SUMMARY
Oral tolerance to food is a result of a complicated interaction between the Ags in the food we consume, the microbiome inhabiting our guts, non-immune cells in the gut, and specialized APCs and lymphocytes found in the gut and associated lymphatic tissues. A failure to develop or breakdown in tolerance leading to food allergy could occur at multiple points, and possibly in multiple tissues including the gut or the skin. Future progress in our understanding of oral tolerance in humans will be greatly advanced by new techniques allowing detailed analysis of innate and Ag-specific responses in the blood and in small samples of affected tissues, complemented by using genetic models in mice to analyze in mechanistic detail immune responses in the gut which contribute to sensitization, desensitization, and tolerance. Such studies will offer crucial insights into factors determining development of natural or therapy induced “long-lasting tolerance”.
Acknowledgments
Supported by The Sean N. Parker Center for Allergy and Asthma Research; NIH grants R01 AR067145 (to SJG) and U19AI10420901 (to SJG, KCN, SDB and RSC); AAAAI Mylan Anaphylaxis Award, Child Health Research Institute/Lucile Packard Foundation for Children's Health awards (to JDH); Stanford CTSA (UL1 TR001085), and the Department of Pathology, Stanford University.
Abbreviations
- Ag
Antigen
- APC
Antigen-presenting cell
- DC
Dendritic cell
- DNFB
2,4-dinitrofluorobenzene
- EPIT
Epicuteanous immunotherapy
- GALT
Gut-associated lymphoid tissue
- GI
Gastrointestinal
- GPR
G protein coupled receptors
- IDO
Indoleamine 2,3-dioxygenase
- Ig
Immunoglobulin
- ILC
Innate lymphoid cell
- IT
Immunotherapy
- M Cell
Microfold cell
- MLN
Mesenteric lymph node
- OIT
Oral immunotherapy
- OVA
Ovalbumin
- SCFA
Short-chain fatty acid
- SLIT
Sub-lingual immunotherapy
- Tfh
Follicular helper T cell
- Tregs
T regulatory cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Moog F. The lining of the small intestine. Sci Am. 1981;245:154–8. 60, 62. doi: 10.1038/scientificamerican1181-154. et passiom. [DOI] [PubMed] [Google Scholar]
- 2.Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr Rev. 1998;56:S5–18. doi: 10.1111/j.1753-4887.1998.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 3.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 5.van der Heijden PJ, Stok W, Bianchi AT. Contribution of immunoglobulin-secreting cells in the murine small intestine to the total 'background' immunoglobulin production. Immunology. 1987;62:551–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Untersmayr E, Scholl I, Swoboda I, Beil WJ, Forster-Waldl E, Walter F, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol. 2003;112:616–23. doi: 10.1016/s0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- 7.Untersmayr E, Jensen-Jarolim E. The role of protein digestibility and antacids on food allergy outcomes. J Allergy Clin Immunol. 2008;121:1301–8. doi: 10.1016/j.jaci.2008.04.025. quiz 9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Untersmayr E, Bakos N, Scholl I, Kundi M, Roth-Walter F, Szalai K, et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J. 2005;19:656–8. doi: 10.1096/fj.04-3170fje. [DOI] [PubMed] [Google Scholar]
- 9.Barone KS, Reilly MR, Flanagan MP, Michael JG. Abrogation of oral tolerance by feeding encapsulated antigen. Cell Immunol. 2000;199:65–72. doi: 10.1006/cimm.1999.1603. [DOI] [PubMed] [Google Scholar]
- 10.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 11.Menard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 2010;3:247–59. doi: 10.1038/mi.2010.5. [DOI] [PubMed] [Google Scholar]
- 12.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 13.Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol. 2012;5:232–9. doi: 10.1038/mi.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6:666–77. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115:3–12. doi: 10.1016/j.jaci.2004.11.008. quiz 3. [DOI] [PubMed] [Google Scholar]
- 16.Hershberg RM, Cho DH, Youakim A, Bradley MB, Lee JS, Framson PE, et al. Highly polarized HLA class II antigen processing and presentation by human intestinal epithelial cells. J Clin Invest. 1998;102:792–803. doi: 10.1172/JCI3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 2011;32:412–9. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–5. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 19.Sicinski P, Rowinski J, Warchol JB, Jarzabek Z, Gut W, Szczygiel B, et al. Poliovirus type 1 enters the human host through intestinal M cells. Gastroenterology. 1990;98:56–8. doi: 10.1016/0016-5085(90)91290-m. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, Sekine S, Kataoka K, Pascual DW, Maddaloni M, Kobayashi R, et al. Ovalbumin-protein sigma 1 M-cell targeting facilitates oral tolerance with reduction of antigen-specific CD4+ T cells. Gastroenterology. 2008;135:917–25. doi: 10.1053/j.gastro.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraus TA, Brimnes J, Muong C, Liu JH, Moran TM, Tappenden KA, et al. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J Clin Invest. 2005;115:2234–43. doi: 10.1172/JCI19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spahn TW, Fontana A, Faria AM, Slavin AJ, Eugster HP, Zhang X, et al. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur J Immunol. 2001;31:1278–87. doi: 10.1002/1521-4141(200104)31:4<1278::aid-immu1278>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 23.Spahn TW, Weiner HL, Rennert PD, Lugering N, Fontana A, Domschke W, et al. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur J Immunol. 2002;32:1109–13. doi: 10.1002/1521-4141(200204)32:4<1109::AID-IMMU1109>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 24.Bain CC, Mowat AM. Intestinal macrophages - specialised adaptation to a unique environment. Eur J Immunol. 2011;41:2494–8. doi: 10.1002/eji.201141714. [DOI] [PubMed] [Google Scholar]
- 25.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–25. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–14. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warshaw AL, Walker WA, Isselbacher KJ. Protein uptake by the intestine: evidence for absorption of intact macromolecules. Gastroenterology. 1974;66:987–92. [PubMed] [Google Scholar]
- 28.Husby S, Jensenius JC, Svehag SE. Passage of undegraded dietary antigen into the blood of healthy adults. Quantification, estimation of size distribution, and relation of uptake to levels of specific antibodies. Scand J Immunol. 1985;22:83–92. doi: 10.1111/j.1365-3083.1985.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 29.Walker WA, Isselbacher KJ. Uptake and transport of macromolecules by the intestine. Possible role in clinical disorders. Gastroenterology. 1974;67:531–50. [PubMed] [Google Scholar]
- 30.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–75. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–66. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 32.Peng HJ, Turner MW, Strobel S. The generation of a ‘tolerogen’ after the ingestion of ovalbumin is time-dependent and unrelated to serum levels of immunoreactive antigen. Clin Exp Immunol. 1990;81:510–5. doi: 10.1111/j.1365-2249.1990.tb05365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callery MP, Kamei T, Flye MW. The effect of portacaval shunt on delayed-hypersensitivity responses following antigen feeding. J Surg Res. 1989;46:391–4. doi: 10.1016/0022-4804(89)90208-4. [DOI] [PubMed] [Google Scholar]
- 34.Yang R, Liu Q, Grosfeld JL, Pescovitz MD. Intestinal venous drainage through the liver is a prerequisite for oral tolerance induction. J Pediatr Surg. 1994;29:1145–8. doi: 10.1016/0022-3468(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 35.Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity. 2014;40:248–61. doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Milling S, Yrlid U, Cerovic V, MacPherson G. Subsets of migrating intestinal dendritic cells. Immunol Rev. 2010;234:259–67. doi: 10.1111/j.0105-2896.2009.00866.x. [DOI] [PubMed] [Google Scholar]
- 37.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ventura MT, Polimeno L, Amoruso AC, Gatti F, Annoscia E, Marinaro M, et al. Intestinal permeability in patients with adverse reactions to food. Dig Liver Dis. 2006;38:732–6. doi: 10.1016/j.dld.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–94. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perrier C, Corthesy B. Gut permeability and food allergies. Clin Exp Allergy. 2011;41:20–8. doi: 10.1111/j.1365-2222.2010.03639.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–19. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang PC, Berin MC, Yu LC, Conrad DH, Perdue MH. Enhanced intestinal transepithelial antigen transport in allergic rats is mediated by IgE and CD23 (FcepsilonRII). J Clin Invest. 2000;106:879–86. doi: 10.1172/JCI9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu LC, Yang PC, Berin MC, Di Leo V, Conrad DH, McKay DM, et al. Enhanced transepithelial antigen transport in intestine of allergic mice is mediated by IgE/CD23 and regulated by interleukin-4. Gastroenterology. 2001;121:370–81. doi: 10.1053/gast.2001.26470. [DOI] [PubMed] [Google Scholar]
- 44.Chambers SJ, Bertelli E, Winterbone MS, Regoli M, Man AL, Nicoletti C. Adoptive transfer of dendritic cells from allergic mice induces specific immunoglobulin E antibody in naive recipients in absence of antigen challenge without altering the T helper 1/T helper 2 balance. Immunology. 2004;112:72–9. doi: 10.1111/j.1365-2567.2004.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brough HA, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: Effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol. 134:867–75.e1. doi: 10.1016/j.jaci.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makinen-Kiljunen S, Mussalo-Rauhamaa H. Casein, an important house dust allergen. Allergy. 2002;57:1084–5. doi: 10.1034/j.1398-9995.2002.23836_6.x. [DOI] [PubMed] [Google Scholar]
- 47.Di Meglio P, Perera Gayathri K, Nestle Frank O. The Multitasking Organ: Recent Insights into Skin Immune Function. Immunity. 2011;35:857–69. doi: 10.1016/j.immuni.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du Toit G, Roberts G, Sayre PH, Plaut M, Bahnson HT, Mitchell H, et al. Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol. 2013;131:135–43. e12. doi: 10.1016/j.jaci.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016 doi: 10.1126/science.aac5560. [DOI] [PubMed] [Google Scholar]
- 51.Swiatczak B, Rescigno M. Semin Immunol. Elsevier; 2012. How the interplay between antigen presenting cells and microbiota tunes host immune responses in the gut. pp. 43–9. [DOI] [PubMed] [Google Scholar]
- 52.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paidassi H, Acharya M, Zhang A, Mukhopadhyay S, Kwon M, Chow C, et al. Preferential expression of integrin alphavbeta8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology. 2011;141:1813–20. doi: 10.1053/j.gastro.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Worthington JJ, Czajkowska BI, Melton AC, Travis MA. Intestinal dendritic cells specialize to activate transforming growth factor-beta and induce Foxp3+ regulatory T cells via integrin alphavbeta8. Gastroenterology. 2011;141:1802–12. doi: 10.1053/j.gastro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 56.Molenaar R, Greuter M, van der Marel AP, Roozendaal R, Martin SF, Edele F, et al. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J Immunol. 2009;183:6395–402. doi: 10.4049/jimmunol.0900311. [DOI] [PubMed] [Google Scholar]
- 57.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–49. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–90. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 60.Torgerson TR, Linane A, Moes N, Anover S, Mateo V, Rieux-Laucat F, et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology. 2007;132:1705–17. doi: 10.1053/j.gastro.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 61.Dubois B, Chapat L, Goubier A, Papiernik M, Nicolas JF, Kaiserlian D. Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood. 2003;102:3295–301. doi: 10.1182/blood-2003-03-0727. [DOI] [PubMed] [Google Scholar]
- 62.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–46. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 63.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–7. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 65.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–41. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 66.Battaglia M, Gianfrani C, Gregori S, Roncarolo MG. IL-10-producing T regulatory type 1 cells and oral tolerance. Ann N Y Acad Sci. 2004;1029:142–53. doi: 10.1196/annals.1309.031. [DOI] [PubMed] [Google Scholar]
- 67.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–26. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Cassani B, Villablanca EJ, Quintana FJ, Love PE, Lacy-Hulbert A, Blaner WS, et al. Gut-tropic T cells that express integrin alpha4beta7 and CCR9 are required for induction of oral immune tolerance in mice. Gastroenterology. 2011;141:2109–18. doi: 10.1053/j.gastro.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–92. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 70.Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 71.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013;131:187–200. e1–8. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Blazquez AB, Berin MC. Gastrointestinal dendritic cells promote Th2 skewing via OX40L. J Immunol. 2008;180:4441–50. doi: 10.4049/jimmunol.180.7.4441. [DOI] [PubMed] [Google Scholar]
- 73.Lambrecht BN, Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol. 2014;134:499–507. doi: 10.1016/j.jaci.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 74.Herbert CA, King CM, Ring PC, Holgate ST, Stewart GA, Thompson PJ, et al. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am J Respir Cell Mol Biol. 1995;12:369–78. doi: 10.1165/ajrcmb.12.4.7695916. [DOI] [PubMed] [Google Scholar]
- 75.Shakib F, Schulz O, Sewell H. A mite subversive: cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol Today. 1998;19:313–6. doi: 10.1016/s0167-5699(98)01284-5. [DOI] [PubMed] [Google Scholar]
- 76.Jyonouchi S, Abraham V, Orange JS, Spergel JM, Gober L, Dudek E, et al. Invariant natural killer T cells from children with versus without food allergy exhibit differential responsiveness to milk-derived sphingomyelin. J Allergy Clin Immunol. 2011;128:102–9. e13. doi: 10.1016/j.jaci.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–85. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 78.Huby RD, Dearman RJ, Kimber I. Why are some proteins allergens? Toxicol Sci. 2000;55:235–46. doi: 10.1093/toxsci/55.2.235. [DOI] [PubMed] [Google Scholar]
- 79.Smith AM, Chapman MD. Reduction in IgE binding to allergen variants generated by site-directed mutagenesis: contribution of disulfide bonds to the antigenic structure of the major house dust mite allergen Der p 2. Mol Immunol. 1996;33:399–405. doi: 10.1016/0161-5890(95)00150-6. [DOI] [PubMed] [Google Scholar]
- 80.Olsson S, van Hage-Hamsten M, Whitley P. Contribution of disulphide bonds to antigenicity of Lep d 2, the major allergen of the dust mite Lepidoglyphus destructor. Mol Immunol. 1998;35:1017–23. doi: 10.1016/s0161-5890(98)00101-1. [DOI] [PubMed] [Google Scholar]
- 81.Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. 1996;14:1269–73. doi: 10.1038/nbt1096-1269. [DOI] [PubMed] [Google Scholar]
- 82.Nowak-Wegrzyn A, Fiocchi A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr Opin Allergy Clin Immunol. 2009;9:234–7. doi: 10.1097/ACI.0b013e32832b88e7. [DOI] [PubMed] [Google Scholar]
- 83.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, et al. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol. 2008;122:342–7. e2. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 84.Kim JS, Nowak-Węgrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow's milk allergy in children. J Allergy Clin Immunol. 2011;128:125–31. e2. doi: 10.1016/j.jaci.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leonard SA, Sampson HA, Sicherer SH, Noone S, Moshier EL, Godbold J, et al. Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol. 2012;130:473–80. e1. doi: 10.1016/j.jaci.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010;104:101–8. doi: 10.1016/j.anai.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 87.Moghaddam AE, Hillson WR, Noti M, Gartlan KH, Johnson S, Thomas B, et al. Dry roasting enhances peanut-induced allergic sensitization across mucosal and cutaneous routes in mice. J Allergy Clin Immunol. 2014;134:1453–6. doi: 10.1016/j.jaci.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maleki SJ, Chung S-Y, Champagne ET, Raufman J-P. The effects of roasting on the allergenic properties of peanut proteins. J Allergy Clin Immunol. 2000;106:763–8. doi: 10.1067/mai.2000.109620. [DOI] [PubMed] [Google Scholar]
- 89.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 90.Marenholz I, Nickel R, Rüschendorf F, Schulz F, Esparza-Gordillo J, Kerscher T, et al. Filaggrin loss-of-function mutations predispose to phenotypes involved in the atopic march. J Allergy Clin Immunol. 2006;118:866–71. doi: 10.1016/j.jaci.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 91.Venkataraman D, Soto-Ramírez N, Kurukulaaratchy RJ, Holloway JW, Karmaus W, Ewart SL, et al. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. J Allergy Clin Immunol. 134:876–82.e4. doi: 10.1016/j.jaci.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kusunoki T, Okafuji I, Yoshioka T, Saito M, Nishikomori R, Heike T, et al. SPINK5 polymorphism is associated with disease severity and food allergy in children with atopic dermatitis. J Allergy Clin Immunol. 2005;115:636–8. doi: 10.1016/j.jaci.2004.12.1114. [DOI] [PubMed] [Google Scholar]
- 93.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 135:164–70.e4. doi: 10.1016/j.jaci.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WHI, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 134:818–23. doi: 10.1016/j.jaci.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Horimukai K, Morita K, Narita M, Kondo M, Kitazawa H, Nozaki M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol. 134:824–30.e6. doi: 10.1016/j.jaci.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 96.Tordesillas L, Goswami R, Benede S, Grishina G, Dunkin D, Jarvinen KM, et al. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest. 2014;124:4965–75. doi: 10.1172/JCI75660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–45S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 99.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun. 2014;5:3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 102.David LA, Weil A, Ryan ET, Calderwood SB, Harris JB, Chowdhury F, et al. Gut microbial succession follows acute secretory diarrhea in humans. MBio. 2015;6:e00381–15. doi: 10.1128/mBio.00381-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A. 2014;111:13145–50. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Macpherson AJ, Koller Y, McCoy KD. The bilateral responsiveness between intestinal microbes and IgA. Trends Immunol. 2015;36:460–70. doi: 10.1016/j.it.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 106.Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, et al. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci U S A. 2004;101:1981–6. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 108.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berni Canani R, Gilbert JA, Nagler CR. The role of the commensal microbiota in the regulation of tolerance to dietary allergens. Curr Opin Allergy Clin Immunol. 2015;15:243–9. doi: 10.1097/ACI.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao S, Feehley TJ, Nagler CR. The role of commensal bacteria in the regulation of sensitization to food allergens. FEBS Lett. 2014;588:4258–66. doi: 10.1016/j.febslet.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014;40:833–42. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 112.Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio. 2014;5:e00889. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 114.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–39. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 117.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015;45:632–43. doi: 10.1111/cea.12487. [DOI] [PubMed] [Google Scholar]
- 119.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5:e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–21. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Metsala J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Mother's and offspring's use of antibiotics and infant allergy to cow's milk. Epidemiology. 2013;24:303–9. doi: 10.1097/EDE.0b013e31827f520f. [DOI] [PubMed] [Google Scholar]
- 123.Savage JH, Matsui EC, Wood RA, Keet CA. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J Allergy Clin Immunol. 2012;130:453–60. e7. doi: 10.1016/j.jaci.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2015 doi: 10.1038/ismej.2015.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ling Z, Li Z, Liu X, Cheng Y, Luo Y, Tong X, et al. Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol. 2014;80:2546–54. doi: 10.1128/AEM.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity. 2015;42:756–66. doi: 10.1016/j.immuni.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Williams MR, Gallo RL. The Role of the Skin Microbiome in Atopic Dermatitis. Curr Allergy Asthma Rep. 2015;15:65. doi: 10.1007/s11882-015-0567-4. [DOI] [PubMed] [Google Scholar]
- 128.Forbes-Blom E, Camberis M, Prout M, Tang SC, Le Gros G. Staphylococcal-derived superantigen enhances peanut induced Th2 responses in the skin. Clin Exp Allergy. 2012;42:305–14. doi: 10.1111/j.1365-2222.2011.03861.x. [DOI] [PubMed] [Google Scholar]
- 129.Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009;123:231–8. e4. doi: 10.1016/j.jaci.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Elazab N, Mendy A, Gasana J, Vieira ER, Quizon A, Forno E. Probiotic administration in early life, atopy, and asthma: a meta-analysis of clinical trials. Pediatrics. 2013;132:e666–76. doi: 10.1542/peds.2013-0246. [DOI] [PubMed] [Google Scholar]
- 131.Berni Canani R, Nocerino R, Terrin G, Coruzzo A, Cosenza L, Leone L, et al. Effect of Lactobacillus GG on tolerance acquisition in infants with cow's milk allergy: a randomized trial. J Allergy Clin Immunol. 2012;129:580–2. 2, e1–5. doi: 10.1016/j.jaci.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 132.Berni Canani R, Nocerino R, Terrin G, Frediani T, Lucarelli S, Cosenza L, et al. Formula selection for management of children with cow's milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. J Pediatr. 2013;163:771–7. e1. doi: 10.1016/j.jpeds.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 133.Hol J, van Leer EH, Elink Schuurman BE, de Ruiter LF, Samsom JN, Hop W, et al. The acquisition of tolerance toward cow's milk through probiotic supplementation: a randomized, controlled trial. J Allergy Clin Immunol. 2008;121:1448–54. doi: 10.1016/j.jaci.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 134.Tang ML, Ponsonby AL, Orsini F, Tey D, Robinson M, Su EL, et al. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. J Allergy Clin Immunol. 2015;135:737–44. e8. doi: 10.1016/j.jaci.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 135.Schofield A. A case of egg poisoning. The Lancet. 1908;171:716. [Google Scholar]
- 136.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99:744–51. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 137.Dupont C, Kalach N, Soulaines P, Legoué-Morillon S, Piloquet H, Benhamou P-H. Cow's milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol. 2010;125:1165–7. doi: 10.1016/j.jaci.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 138.Dupont C. Peanut epicutaneous immunotherapy (EPIT) in peanut-allergic children: 18 months treatment in the ARACHILD Study. 2014 AAAAI Annual Meeting:Aaaai. 2014 [Google Scholar]
- 139.De Boissieu D, Dupont C. Sublingual immunotherapy for cow's milk protein allergy: a preliminary report. Allergy. 2006;61:1238–9. doi: 10.1111/j.1398-9995.2006.01196.x. [DOI] [PubMed] [Google Scholar]
- 140.Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127:640–6. e1. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol. 2013;131:119–27. e1–7. doi: 10.1016/j.jaci.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bégin P, Winterroth LC, Dominguez T, Wilson SP, Bacal L, Mehrotra A, et al. Safety and feasibility of oral immunotherapy to multiple allergens for food allergy. Allergy Asthma Clin Immunol. 2014;10:1. doi: 10.1186/1710-1492-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–55. 55, e1–5. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Enrique E, Pineda F, Malek T, Bartra J, Basagaña M, Tella R, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005;116:1073–9. doi: 10.1016/j.jaci.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 145.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow's milk allergy. J Allergy Clin Immunol. 2011;127:1622–4. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol. 2013;132:1368–74. doi: 10.1016/j.jaci.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Begin P, Dominguez T, Wilson SP, Bacal L, Mehrotra A, Kausch B, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using Omalizumab. Allergy Asthma Clin Immunol. 2014;10:7. doi: 10.1186/1710-1492-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow's milk allergy. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Begin P, Chinthrajah RS, Nadeau KC. Oral immunotherapy for the treatment of food allergy. Hum Vaccin Immunother. 2014;10:2295–302. doi: 10.4161/hv.29233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133:621–31. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 151.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol. 2014;133:500–10. e11. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–43. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300, e1-97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Khodoun MV, Kucuk ZY, Strait RT, Krishnamurthy D, Janek K, Clay CD, et al. Rapid desensitization of mice with anti-FcgammaRIIb/FcgammaRIII mAb safely prevents IgG-mediated anaphylaxis. J Allergy Clin Immunol. 2013;132:1375–87. doi: 10.1016/j.jaci.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oka T, Rios EJ, Tsai M, Kalesnikoff J, Galli SJ. Rapid desensitization induces internalization of antigen-specific IgE on mouse mast cells. J Allergy Clin Immunol. 2013;132:922–32. e1–16. doi: 10.1016/j.jaci.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Leonard SA, Martos G, Wang W, Nowak-Wegrzyn A, Berin MC. Oral immunotherapy induces local protective mechanisms in the gastrointestinal mucosa. J Allergy Clin Immunol. 2012;129:1579–87. e1. doi: 10.1016/j.jaci.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jutel M, Muller U, Ericker M, Rihs S, Pichler W, Dahinden C. Influence of bee venom immunotherapy on degranulation and leukotriene generation in human blood basophils. Clin Exp Allergy. 1996;26:1112–8. [PubMed] [Google Scholar]
- 158.Eberlein - Konig B, Ullmann S, Thomas P, Przybilla B. Tryptase and histamine release due to a sting challenge in bee venom allergic patients treated successfully or unsuccessfully with hyposensitization*. Clin Exp Allergy. 1995;25:704–12. doi: 10.1111/j.1365-2222.1995.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 159.Woo HY, Kim YS, Kang NI, Chung WC, Song CH, Choi IW, et al. Mechanism for acute oral desensitization to antibiotics. Allergy. 2006;61:954–8. doi: 10.1111/j.1398-9995.2006.01147.x. [DOI] [PubMed] [Google Scholar]
- 160.Bussmann C, Xia J, Allam JP, Maintz L, Bieber T, Novak N. Early markers for protective mechanisms during rush venom immunotherapy. Allergy. 2010;65:1558–65. doi: 10.1111/j.1398-9995.2010.02430.x. [DOI] [PubMed] [Google Scholar]
- 161.Allam J-P, Würtzen PA, Reinartz M, Winter J, Vrtala S, Chen K-W, et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-β1 and IL-10–producing properties. J Allergy Clin Immunol. 2010;126:638–45. e1. doi: 10.1016/j.jaci.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 162.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134:1193. doi: 10.1016/j.jaci.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 163.Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–42. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 164.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33–driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–50. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Doherty TA, Scott D, Walford HH, Khorram N, Lund S, Baum R, et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol. 2014;133:1203–5. e7. doi: 10.1016/j.jaci.2013.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yu S, Kim HY, Chang Y-J, DeKruyff RH, Umetsu DT. Innate lymphoid cells and asthma. J Allergy Clin Immunol. 2014;133:943–50. doi: 10.1016/j.jaci.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 167.Dioszeghy V, Mondoulet L, Dhelft V, Ligouis M, Puteaux E, Benhamou P-H, et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol. 2011;186:5629–37. doi: 10.4049/jimmunol.1003134. [DOI] [PubMed] [Google Scholar]
- 168.Mondoulet L, Dioszeghy V, Puteaux E, Ligouis M, Dhelft V, Letourneur F, et al. Intact skin and not stripped skin is crucial for the safety and efficacy of peanut epicutaneous immunotherapy (EPIT) in mice. Clin Transl Allergy. 2012;2:22. doi: 10.1186/2045-7022-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Smaldini PL, Delgado MLO, Fossati CA, Docena GH. Orally-Induced Intestinal CD4+ CD25+ FoxP3+ Treg Controlled Undesired Responses towards Oral Antigens and Effectively Dampened Food Allergic Reactions. PLoS One. 2015;10:e0141116. doi: 10.1371/journal.pone.0141116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Aslam A, Chan H, Warrell DA, Misbah S, Ogg GS. Tracking antigen-specific T-cells during clinical tolerance induction in humans. PLoS One. 2010;5:e11028. doi: 10.1371/journal.pone.0011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ryan JF, Hovde R, Glanville J, Lyu SC, Ji X, Gupta S, et al. Successful immunotherapy induces previously unidentified allergen-specific CD4+ T-cell subsets. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1520180113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vickery BP, Lin J, Kulis M, Fu Z, Steele PH, Jones SM, et al. Peanut oral immunotherapy modifies IgE and IgG 4 responses to major peanut allergens. J Allergy Clin Immunol. 2013;131:128–34. e3. doi: 10.1016/j.jaci.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hoh RA, Joshi SA, Liu Y, Wang C, Roskin KM, Lee J-Y, et al. Single B-cell deconvolution of peanut-specific antibody responses in allergic patients. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Santos AF, James LK, Bahnson HT, Shamji MH, Couto-Francisco NC, Islam S, et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135:1249–56. doi: 10.1016/j.jaci.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Noval Rivas M, et al. Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. 2014;134:1310–7. e6. doi: 10.1016/j.jaci.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006;116:833–41. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Konstantinou GN, Nowak - Węgrzyn A, Bencharitiwong R, Bardina L, Sicherer SH, Sampson HA. Egg - white - specific IgA and IgA2 antibodies in egg - allergic children: Is there a role in tolerance induction? Pediatr Allergy Immunol. 2014;25:64–70. doi: 10.1111/pai.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG, et al. IgG 4 production is confined to human IL-10–producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131:1204–12. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]