Abstract
Background
Central vision loss caused by age‐related macular degeneration (AMD) is the leading cause of blindness among the elderly in developed countries. Neovascular AMD is characterized by choroidal neovascularization (CNV). Growth of new blood vessels in patients with neovascular AMD is driven by a complex process that involves a signal protein called vascular endothelial growth factor A (VEGF‐A). Anti‐VEGF drugs that block this protein include ranibizumab, bevacizumab, and aflibercept.
Objectives
To assess and compare the effectiveness and safety of intravitreal injections of aflibercept versus ranibizumab, bevacizumab, or sham for treatment of patients with neovascular AMD.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (Issue 11, 2015), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to November 2015), EMBASE (January 1980 to November 2015), PubMed (1948 to November 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to November 2015), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com) (last searched December 4, 2014), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic search for trials. We last searched the electronic databases on November 30, 2015.
Selection criteria
We included randomized controlled trials (RCTs) in which aflibercept monotherapy was compared with ranibizumab, bevacizumab, or sham for participants with neovascular AMD who were treatment‐naive.
Data collection and analysis
We used standard methodological procedures of The Cochrane Collaboration for screening, data abstraction, and study assessment. Two review authors independently screened records, abstracted data, and assessed risk of bias of included studies; we resolved discrepancies by discussion or with the help of a third review author when needed.
Main results
We included two RCTs (total of 2457 participants, 2457 eyes). Trial participants had neovascular AMD with active subfoveal choroidal neovascular lesions. Both trials followed the same protocol and compared aflibercept at various doses versus ranibizumab, but they were carried out in different countries. One trial enrolled participants from the United States and Canada, and the second trial was conducted at 172 sites in Europe, Asia Pacific, Latin America, and the Middle East. The overall quality of the evidence was high, and included trials were at low risk for most bias domains assessed; however, both trials were funded by the manufacturers of aflibercept. For the purposes of analysis, we combined aflibercept groups regardless of dosing and analyzed them as a single group.
Visual acuity outcomes were similar between aflibercept and ranibizumab groups; at one year, participants in the aflibercept groups showed mean change in best‐corrected visual acuity (BCVA) from baseline similar to that of participants in the ranibizumab groups (mean difference (MD) ‐0.15 Early Treatment Diabetic Retinopathy Study (ETDRS) letters, 95% confidence interval (95% CI) ‐1.47 to 1.17; high‐quality evidence). At two years, the mean change in BCVA from baseline was 7.2 ETDRS letters for aflibercept groups versus 7.9 for ranibizumab groups. Sufficient data were not available for calculation of confidence intervals.
The proportion of participants who gained 15 or more letters of BCVA by one year of follow‐up was approximately 32% for both aflibercept and ranibizumab (RR 0.97, 95% CI 0.85 to 1.11; high‐quality evidence), and by two years of follow‐up was approximately 31% (RR 0.98, 95% CI 0.85 to 1.12; high‐quality evidence). Similar small proportions of participants in the aflibercept and ranibizumab groups lost 15 or more letters of BCVA at one year (RR 0.89, 95% CI 0.61 to 1.30; high‐quality evidence); this outcome was not reported for two‐year follow‐up. Data were not reported on the proportion of participants with BCVA worse than 20/200 at one‐ or two‐year follow‐up.
Participants treated with aflibercept or ranibizumab showed similar improvement in morphological outcomes, as assessed from images (central retinal thickness and CNV size). At one year, the proportion of eyes that achieved dry retina was similar between aflibercept and ranibizumab groups (absence of cystic intraretinal fluid and subretinal fluid on optical coherence tomography (OCT); RR 1.06, 95% CI 0.98 to 1.14; high‐quality evidence). In addition, investigators reported no difference in reduction of CNV area between aflibercept‐ and ranibizumab‐treated eyes at one year (MD ‐0.24 mm2, 95% CI ‐0.78 to 0.29; high‐quality evidence). Data were not reported for the proportion of eyes with absence of leakage on fluorescein angiography at one‐ or two‐year follow‐up.
Overall, occurrence of serious systemic adverse events was similar and comparable in aflibercept‐ and ranibizumab‐treated groups at one year (RR 0.99, 95% CI 0.79 to 1.25). Risk of any serious ocular adverse event was lower in the aflibercept group than in the ranibizumab group, but the risk estimate is imprecise (RR 0.62, 95% CI 0.36 to 1.07). As the result of imprecision, we graded the quality of evidence for all adverse events as moderate.
Authors' conclusions
Results of this review document the comparative effectiveness of aflibercept versus ranibizumab for visual acuity and morphological outcomes in eyes with neovascular AMD. Current available information on adverse effects of each medication suggests that the safety profile of aflibercept is comparable with that of ranibizumab; however, the number of participants who experienced adverse events was small, leading to imprecise estimates of absolute and relative effect sizes. The eight‐week dosing regimen of aflibercept represents reduced treatment requirements in comparison with monthly dosing regimens and thus has the potential to reduce treatment burden and risks associated with frequent injections.
Keywords: Humans; Angiogenesis Inhibitors; Angiogenesis Inhibitors/therapeutic use; Choroidal Neovascularization; Choroidal Neovascularization/complications; Choroidal Neovascularization/drug therapy; Macular Degeneration; Macular Degeneration/drug therapy; Macular Degeneration/etiology; Ranibizumab; Ranibizumab/therapeutic use; Receptors, Vascular Endothelial Growth Factor; Receptors, Vascular Endothelial Growth Factor/therapeutic use; Recombinant Fusion Proteins; Recombinant Fusion Proteins/therapeutic use; Visual Acuity
Plain language summary
Aflibercept for neovascular age‐related macular degeneration
Review question How well does aflibercept work, and how safe is it for the treatment of individuals with neovascular age‐related macular degeneration compared with other currently used treatments (ranibizumab and bevacizumab) or no treatment?
Background One cause of loss of sight in the center of the eye is a disease called age‐related macular degeneration (AMD). AMD causes blindness in many older people in developed countries. Around the world, nearly 9% (one in 11) of people 45 to 85 years of age are estimated to have AMD. About 10% of people with AMD develop a type of AMD called neovascular (wet) AMD, which results from new blood vessels that develop in an inner layer of the eye called the choroid. If a patient with this type of AMD is not treated, the affected eye may lose sight and may develop other problems related to blindness.
New blood vessels grow when they are signaled by a protein called vascular endothelial growth factor A (VEGF‐A). Medicines that block this protein, called anti‐VEGF drugs, are injected into the eye and have been shown to reduce fluid in the back of the eye while causing new blood vessels to slow down growth or to shrink. Ranibizumab and bevacizumab were the most commonly used anti‐VEGF drugs from 2006 to 2011, when aflibercept became available. Aflibercept was created to bond more strongly with VEGF; therefore, aflibercept acts longer after an injection, so patients should need fewer injections of aflibercept than of ranibizumab or bevacizumab.
Study details We found two trials that enrolled a total of 2457 participants with neovascular AMD. These trials were similar in most respects, but they were conducted at different locations. One was completed only in North America, and the other took place in many countries in many parts of the world. Both trials compared injections of aflibercept into the eye versus ranibizumab injections and were funded by the company that makes aflibercept. No trial was found that compared aflibercept versus bevacizumab injections into the eye. We last searched for trials on November 30, 2015.
Key results Participants treated with aflibercept or ranibizumab experienced similar improvements in visual acuity one year after the start of treatment. Serious vision loss did not occur in many eyes given either treatment and did not occur more often with either aflibercept or ranibizumab. Serious adverse effects in the eye were reported rarely in both aflibercept and ranibizumab groups. The incidence of serious adverse health effects, such as internal bleeding, stroke, and high blood pressure, was comparable between aflibercept and ranibizumab groups; however, such reported events were few. Thus, we are uncertain about possible differences in adverse effects reported among individuals given aflibercept or ranibizumab.
Quality of the evidence The two identified trials were well designed, and we judged the quality of evidence as high for vision‐related outcomes. A judgement of high quality means that we believe future research is very unlikely to change our conclusions. Because of uncertainty regarding adverse effects due to the small number of occurrences, we judged the quality of evidence for adverse effects as moderate. A judgement of moderate quality means that we believe future research may have an important impact on our conclusions.
Summary of findings
for the main comparison.
| Aflibercept vs ranibizumab for neovascular age‐related macular degeneration | ||||||
|
Patient or population: people with age‐related macular degeneration Settings: clinical centers Intervention: intravitreal injections of aflibercept Comparison: intravitreal injections of ranibizumab | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Ranibizumab | Aflibercept | |||||
|
Mean change in BCVA in ETDRS letters at 1 year (number of letters) |
Mean change in visual acuity across ranibizumab groups ranged from gains of 8.57 letters to 8.71 letters | Mean change in visual acuity in aflibercept groups was on average 0.15 fewer letters gained (95% CI 1.47 fewer letters to 1.17 more letters) | MD ‐0.15 (‐1.47 to 1.17) | 2412 (2) | ⊕⊕⊕⊕ High | |
| Gain of ≥ 15 letters of BVCA at 1 year | 324 per 1000 | 314 per 1000 (275 to 360) | RR 0.97 (0.85 to 1.11) | 2412 (2) |
⊕⊕⊕⊕ High | |
| Absence of fluid on optical coherence tomography (OCT) at 1 year | 595 per 1000 | 630 per 1000 (583 to 678) | RR 1.06 (0.98 to 1.14) | 2291 (2) |
⊕⊕⊕⊕ High | |
|
Quality‐of‐life measures at 1 year (National Eye Institute‐Visual Function Questionnaire [NEI‐VFQ]) |
Mean improvement in composite NEI‐VQF score ranged across control groups from 4.9 to 6.3 points | Mean improvement in composite NEI‐VQF score in intervention groups was on average 0.39 points lower (95% CI 1.71 points lower to 0.93 points higher) | MD ‐0.39 (‐1.71 to 0.93) | 2412 (2) |
⊕⊕⊕⊕ High | |
| Adverse events ‐ serious systemic events at 1 year | 139 per 1000 | 138 per 1000 (110 to 174) | RR 0.99 (0.79 to 1.25) | 2419 (2) |
⊕⊕⊕⊝ Moderatea | |
| Adverse events ‐ serious ocular events at 1 year | 32 per 1000 | 20 per 1000 (12 to 34) | RR 0.62 (0.36 to 1.07) | 2419 (2) |
⊕⊕⊕⊝ Moderatea | |
| *The basis for the assumed risk (eg, median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The unit of analysis is the individual (one study eye per person). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
Very low quality: We are very uncertain about the estimate aAdverse events downgraded to moderate quality as the number of events is small (wide confidence intervals) | ||||||
Background
Description of the condition
Age‐related macular degeneration (AMD) is a progressive degenerative disease of the central part of the retina (the macula) that causes central vision loss. It is most common among people 55 years of age and older and is the leading cause of blindness among the elderly in developed countries (Friedman 2004). Global prevalence of any type or stage of AMD is estimated at 8.69% (95% confidence interval (CI) 4.26% to 17.40%) among people 45 to 85 years of age (Wong 2014). As the world population ages, projections suggest that 196 million people will have AMD by the year 2020 (Wong 2014). The estimated global prevalence of neovascular AMD is 0.46% (95% CI 0.18% to 1.08%) (Wong 2014). It is estimated that 1.75 million people in the United States (US) are affected by geographic atrophy or neovascular (wet) AMD (Friedman 2004). Risk factors implicated for AMD include older age, smoking, genetic predisposition and family history, and cardiovascular disease (Chakravarthy 2010; Hyman 2002; Schultz 2003; Thornton 2005).
The two main types of AMD are dry AMD and neovascular (wet) AMD (Bird 1995). Dry AMD is characterized by drusen (yellow deposits under the retina), changes in the retinal pigment epithelium (RPE), or both; it causes gradual vision loss until the fovea is affected by atrophy of the RPE (Bird 1995). Geographic atrophy is a form of dry AMD. Neovascular AMD, a form of wet AMD, is characterized by choroidal neovascularization (CNV). Associated clinical manifestations of neovascular AMD include subretinal fluid; intraretinal fluid; and retinal, subretinal, or sub‐RPE hemorrhage (Bird 1995). Approximately 10% of eyes with AMD manifest the neovascular type of AMD. If left untreated, neovascular AMD can cause severe vision loss (20/200 or worse) in most eyes (Wong 2008). This disease affects quality of life with consequent loss of independence among elderly people (AREDS 2005; Childs 2004; Clemons 2003; Dong 2004; Mangione 1999; Miskala 2004). Blurred vision, distortion of an image (metamorphopsia), and scotoma (a visual field defect or 'blind spot') are the most commonly reported symptoms among people with neovascular AMD (Fine 1986). Loss of visual acuity may not be noticed by the patient until both eyes are affected, or until or unless neovascularization is subfoveal.
Diagnostic evaluation of a patient with symptoms suggestive of neovascular AMD includes stereoscopic biomicroscopic examination of the macula, fluorescein angiography, and optical coherence tomography (OCT). Fluorescein angiography has been the gold standard for detecting and confirming the presence of choroidal neovascularization. Fluorescein angiography is an invasive imaging test in which serial retinal photographs are taken after fluorescein dye is injected into a vein. OCT is a noninvasive method of imaging posterior structures of the eye. This imaging technique is frequently used to detect and monitor morphological changes associated with choroidal neovascularization. However, fluorescein angiography is the best method for detecting new‐onset CNV (Do 2012).
Description of the intervention
Vascular endothelial growth factor‐A (VEGF‐A), a signal protein produced by cells in the back of the eye, acts as the driving force behind the development of new vessels in neovascular AMD. Drugs that can block this protein successfully have been shown to be effective not only by decreasing accumulated fluid in the back of the eye, but by causing regression of the new fragile vessels (Ferrara 2009). Available medications in this class include ranibizumab (Lucentis, Genentech Inc.), bevacizumab (Avastin, Genentech Inc.), and aflibercept (Eylea, Regeneron Pharmaceuticals, Inc.). These medications are given through intravitreal injection into the eye. Ranibuzimab is a recombinant, humanized, monoclonal, VEGF‐specific antibody fragment that inhibits all VEGF‐A isoforms. Monthly injections of ranibizumab have been widely used for treatment of patients with neovascular AMD since several clinical trials showed substantial visual gain with its use (Brown 2006;Brown 2011; Rosenfeld 2006). Bevacizumab is a monoclonal, humanized, VEGF‐specific full‐length antibody that was developed for use in various cancers and has been used off‐label for treatment of patients with neovascular AMD (CATT Research Group 2011;IVAN Trial 2013; Jansen 2013). Treatment with ranibizumab or bevacizumab usually requires monthly injections into the eye or injections given as needed on the basis of monthly assessments to monitor disease activity (Brown 2011; CATT Research Group 2011; IVAN Trial 2013; Rosenfeld 2006). Frequent injections and assessments place a significant burden on patients and caregivers and carry the risk of rare but serious adverse events associated with an injection into the eye (Haller 2013). The effectiveness (Solomon 2014) and safety (Moja 2014) of ranibizumab and bevacizumab for the treatment of patients with neovascular AMD have been analyzed in published Cochrane reviews and documented to be similar; thus, this review focuses on a comparison of outcomes of aflibercept treatment versus outcomes with other agents or no anti‐VEGF agent.
Aflibercept is a relatively new medication for neovascular AMD that was approved by the US Food and Drug Administration in November 2011 (FDA 2011). It had been known in the scientific literature as VEGF‐Trap (Stewart 2008). Aflibercept is a fusion protein made of key domains of VEGF receptors 1 (VEGFR1) and 2 (VEGFR2) fused with a portion of human antibody. Unlike ranibizumab and bevacizumab, aflibercept binds VEGF‐A, VEGF‐B, and another protein ‐ placental growth factor (PIGF) ‐ that is believed to play a role in progression of neovascular AMD. The binding affinity of aflibercept for VEGF (Kd = 0.5 pM) is substantially stronger than that of bevacizumab (Kd = 58 pM) or ranibizumab (Kd = 46 pM) (Holash 2002), leading to potentially longer duration of action in the eye that allows longer intervals between treatments. Less frequent dosing should lead to reduction in the risk of harm associated with intraocular injections (Brown 2011; Heier 2011; Heier 2012).
How the intervention might work
Aflibercept acts as a decoy receptor for VEGF. It binds both ends of activated dimerized VEGF very tightly between its arms and prevents it from interacting and activating native VEGFR1 and VEGFR2 receptors and cross linking. This binding results in blockage of the biological activity of VEGF and inhibits abnormal growth of blood vessels. It binds to PIGF in a similar fashion and prevents it from activating VEGFR1 receptors (Rudge 2007).
Why it is important to do this review
Several systematic reviews have assessed and compared the advantages and disadvantages of different dosing regimens of ranibizumab and bevacizumab tested in different clinical trials for the treatment of individuals with neovascular AMD (Mitchell 2011; Moja 2014; Schmucker 2010; Schmucker 2012; Solomon 2014). Data regarding the benefits and harms of aflibercept for treatment of neovascular AMD and comparison of its effects versus those of ranibizumab or bevacizumab are sparse. Synthesis and analysis of available data will help clinicians, patients, and caregivers when they choose among treatment options.
Objectives
To assess and compare the effectiveness and safety of intravitreal injections of aflibercept versus ranibizumab, bevacizumab, or sham for treatment of patients with neovascular AMD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) only.
Types of participants
We included trials of participants with diagnosed subfoveal neovascular AMD, confirmed by fluorescein angiography, who received no previous treatment for AMD in the study eye.
Types of interventions
We included trials in which aflibercept monotherapy was compared with ranibizumab, bevacizumab, or sham. We excluded studies in which aflibercept was evaluated as part of combination therapy versus other active treatments, such as laser photocoagulation.
Types of outcome measures
Primary outcomes
The primary outcome for comparison of interventions was the mean change from baseline in number of letters of best‐corrected visual acuity (BCVA) at one year, as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) chart or equivalent.
Secondary outcomes
We included the following secondary outcomes for comparison of interventions.
Mean change in number of letters of BCVA at two years.
Proportion of participants who gained 15 or more letters of BCVA at one year and at two years.
Proportion of participants who lost 15 or more letters of BCVA at one year and at two years.
Proportion of participants with BCVA worse than 20/200 at one year and at two years.
Proportion of eyes with absence of fluid on optical coherence tomography (OCT) at one year and at two years.
Proportion of eyes with absence of leakage on fluorescein angiography at one year and at two years.
Mean number of injections received by one year and by two years.
Mean change in central retinal thickness from baseline to one year and to two years.
Mean change in extent of choroidal neovascularization (CNV) from baseline at one year and at two years.
Quality‐of‐life outcomes
We included quality‐of‐life outcomes as measured by a validated scale, such as the National Eye Institute Visual Function Questionnaire (NEI‐VFQ), at one year and at two years.
Adverse events
We included the following adverse events for comparison of interventions.
Proportion of participants with arterial thrombotic events at one year and at two years.
Proportion of participants with serious systemic adverse events at one year and at two years.
Proportion of eyes with serious ocular adverse events at one year and at two years.
Follow‐up
In our protocol (Sarwar 2014), we specified that we would not consider outcomes at less than one year of follow‐up and planned to use outcome data collected between nine and 18 months to estimate one‐year outcomes when 12‐month data were not reported from an individual study. It was not necessary to use this backup strategy, as investigators for each of the included studies reported data at one year of follow‐up. Similarly, we planned to use outcome data collected between 18 and 30 months to estimate two‐year outcomes. Therefore, we used 96‐week data published for the two included studies to estimate two‐year outcomes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (Issue 11, 2015), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to November 2015), EMBASE (January 1980 to November 2015), PubMed (1948 to November 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to November 2015), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com) (last searched December 4, 2014), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic search for trials. We last searched the electronic databases on November 30, 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), PubMed (Appendix 4), LILACS (Appendix 5), mRCT (Appendix 6), ClinicalTrials.gov (Appendix 7), and the ICTRP (Appendix 8).
Searching other resources
We searched the reference lists of included trials and contacted investigators to request additional published and unpublished studies. We did not handsearch conference proceedings for the specific purposes of this review, as CEV personnel annually search major ophthalmology conference abstracts and add pertinent ones to the CENTRAL database.
Data collection and analysis
Selection of studies
We employed a two‐stage selection process. At the first stage, two review authors independently reviewed the titles and abstracts of all records identified by the searches. We classified each record as "definitely relevant," "possibly relevant," or "definitely not relevant." At the second stage, we obtained the full‐text reports of all records classified as "definitely relevant" or "possibly relevant" by both review authors following adjudication by discussion at the first stage. We grouped study reports by trial, and two review authors independently assessed each trial for relevance to the review by classifying it as "include," "exclude," or "unclear." We documented in the Characteristics of excluded studies table our assessments of studies excluded after review of full‐text reports. We assessed all studies as "include" or "exclude" after review of full‐text reports. Thus we did not have to contact study authors to ask for information as we had anticipated when we prepared our protocol. The two review authors resolved differences in study selection through discussion.
Data extraction and management
Two review authors independently extracted participant and intervention characteristics, details of study design and methods, and primary and secondary outcome data by using forms adapted from others developed by Cochrane Eyes and Vision. One review author entered data into RevMan (RevMan 2014), and a second review author verified the data entered. We resolved differences in data extraction by discussion. When desired data had not been reported or were represented only in graphic form, we had planned to contact trial report authors to request relevant data, or to use graph digitization software to estimate the numbers; however, it was not necessary to implement these strategies.
Assessment of risk of bias in included studies
Two review authors assessed each trial for potential bias according to the methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered the following "Risk of bias" domains.
Selection bias – random sequence generation and allocation concealment before randomization.
Performance bias – masking of participants and study personnel to the allocated intervention.
Detection bias – masking of outcome assessors during follow‐up examination for data related to primary and secondary outcomes.
Attrition bias – quantity of and reasons for incomplete or missing outcome data for each treatment group.
Reporting bias – evidence of selective outcome reporting.
Other sources of bias – support or funding for the study and potential conflicts of interests that could bias study results; other design flaws.
We classified each trial with respect to each "Risk of bias" domain as having "unclear risk," "low risk," or "high risk" of bias and provided documentation from trial reports to support our judgments. The two review authors resolved discrepancies in judgment through discussion and by asking a third review author to make the final judgment when they could not reach consensus.
Measures of treatment effect
We analyzed the primary and secondary outcome data analysis according to the methods described in our protocol (Sarwar 2014), which are in accordance with the guidance set out in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). For the primary outcome (mean change in number of letters of BCVA at one year), we used the mean difference (MD) and corresponding 95% confidence interval (CI) to compare the mean change from baseline versus specific follow‐up time points between intervention groups. We calculated MDs and corresponding 95% CIs for other continuous outcomes, including mean change in number of letters of BCVA at two years; mean number of injections received during first year of the study; mean change in central retinal thickness at one year and at two years; and mean change in extent of CNV from baseline at one year and at two years.
We calculated risk ratios (RRs) and corresponding 95% CIs for dichotomous outcomes, including the proportion of participants who gained 15 or more letters of BCVA from baseline BCVA at one year and at two years; proportion of participants who lost 15 or more letters of BCVA from baseline BCVA at one year and at two years; proportion of participants with BCVA worse than 20/200 at one year and at two years; proportion of eyes with absence of fluid on OCT at one year and at two years; proportion of eyes with absence of leakage on fluorescein angiography at one year and at two years; proportion of participants with arterial thrombotic events at one year and at two years; proportion of participants with serious systemic adverse events at one year and at two years; and proportion of eyes with serious ocular adverse events at one year and at two years.
Unit of analysis issues
The unit of analysis was the individual participant (one study eye per participant).
Dealing with missing data
We planned to contact trial authors to ask for unreported or unclearly reported outcome data, such as data reported only in graph format, but it was not necessary to contact investigators of included trials. We did not impute data for the purposes of this review. Instead we documented outcomes with missing data and potential implications when we could not assume that data were missing at random.
Assessment of heterogeneity
We assessed clinical and methodological diversity among studies by reviewing participant characteristics and trial methods. We checked for statistical heterogeneity by using the I² statistic, which provides the percentage of variability in effect estimates that is due to heterogeneity rather than to sampling error (chance). We considered a value greater than 50% to indicate substantial statistical heterogeneity.
Assessment of reporting biases
We assessed risk of bias due to selective outcome reporting by comparing outcomes that the trial investigators intended to measure versus outcomes reported by trial authors. When available, we reviewed protocols, reports on trial design and methods, and clinical trial registers to determine intended outcomes. Otherwise, we compared outcomes measured as described in the Methods section versus outcomes reported in the Results section of published reports.
We planned to use funnel plots to assess for potential publication bias when 10 or more trials were included in a meta‐analysis; as this review included only two studies, it was not possible to implement this method.
Data synthesis
We used a fixed‐effect model, as only two trials were included in the meta‐analysis. If three or more trials had been included in a meta‐analysis, we planned to use a random‐effects model. We performed all statistical analyses using RevMan 5.3 (RevMan 2014). If we had detected considerable clinical, methodological, or statistical heterogeneity (I² > 50%), we planned not to perform a meta‐analysis but rather provide a narrative summary of study results. However, because two trials using identical study protocols were included, we conducted meta‐analysis even when statistical heterogeneity (I² > 50%) was indicated.
Subgroup analysis and investigation of heterogeneity
We planned to analyze different dose regimens of aflibercept versus the comparison intervention; however, we did not perform these subgroup analyses because different dose regimens were not evaluated by independent trials. We planned to perform subgroup analysis according to the comparison intervention reported in the included trials (eg, aflibercept vs placebo or sham injections, aflibercept vs ranibizumab); however, we did not perform these subgroup analyses, as only one comparison intervention (ranibizumab) was used in trials included in this review.
Sensitivity analysis
As included trials were too few, we did not conduct planned sensitivity analyses to assess the effects of excluding (1) trials judged to have high risk of bias as the result of incomplete outcome data or selective reporting, (2) unpublished trials, or (3) industry‐funded trials.
Summary of findings
We produced a "Summary of findings" table for outcomes at one year of follow‐up (Table 1). Two review authors graded independently the overall certainty of evidence for each outcome using the GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) classification (www.gradeworkinggroup.org/). The tables that we prepared include results for the following seven outcomes.
Mean change in number of letters from baseline BCVA.
Proportion of participants who gained 15 or more letters of BCVA from baseline.
Proportion of eyes with absence of fluid on OCT.
Proportion of eyes with absence of dye leakage on fluorescein angiography.
Quality of life.
Proportion of participants with serious systemic adverse events.
Proportion of eyes with serious ocular adverse events.
Results
Description of studies
Results of the search
Electronic searches yielded 9961 total records as of November 30, 2015. After removal of duplicates, we reviewed 7633 unique records. We assessed 13 records as "definitely relevant" or "possibly relevant" after screening titles and abstracts. Eight of these records were reports from two trials (VIEW 1; VIEW 2), both of which we included in the review (Figure 1). We excluded five records and provided reasons for exclusion in the Characteristics of excluded studies table.
1.
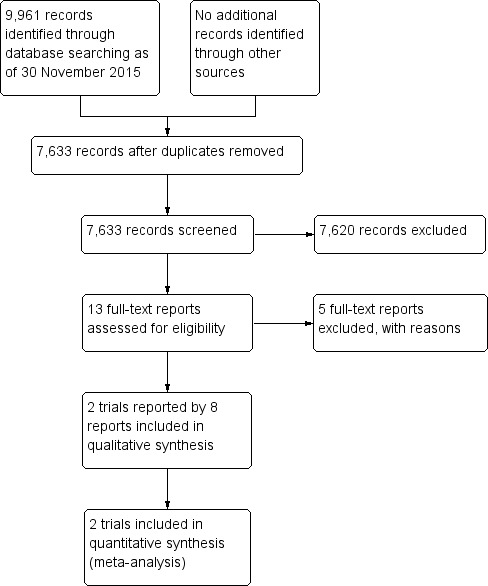
Study flow diagram.
The VIEW 1 and VIEW 2 trials were two randomized, double‐masked, active‐controlled, phase 3 trials designed to assess the safety and efficacy of intravitreal aflibercept when used to treat patients with neovascular AMD. These two trials followed the same protocol; VIEW 1 included participants from 154 sites in Canada and the United States, and VIEW 2 included participants from 172 sites located elsewhere. Although data from both trials were reported in a single paper, these data were used to calculate separate estimates of one‐year outcomes. Two‐year outcomes were combined when reported.
We found no trials that compared aflibercept versus bevacizumab or sham intravitreal injections and met the inclusion criteria for this review.
Included studies
Types of participants
This review includes 2458 participants from two RCTs (VIEW 1, registered at clinicaltrials.gov NCT00509795; VIEW 2, registered at clinicaltrials.gov NCT00637377). VIEW 1 enrolled 1217 participants, and VIEW 2 enrolled 1240 participants. Criteria for participant selection common to the two RCTs included age 50 years or older, CNV lesions confirmed by fluorescein angiography, and BCVA score equivalent to 20/40 or worse. Both trials included one study eye per participant. Specific inclusion and exclusion criteria are described in greater detail in the Characteristics of included studies tables.
Types of interventions
Participants in VIEW 1 and VIEW 2 were randomly assigned to four cohorts: 0.5 mg aflibercept every four weeks (0.5q4), 2 mg aflibercept every four weeks (2q4), 2 mg aflibercept every eight weeks (2q8) following three monthly injections, and 0.5 mg ranibizumab every four weeks (Rq4). Both aflibercept and ranibizumab were administered by intravitreal injections, and the primary treatment period lasted one year (52 weeks). During the follow‐up phase ‐ from 52 weeks to 96 weeks ‐ all regimens were switched from a fixed monthly or bimonthly regimen to an as‐needed regimen with a minimum quarterly dosing ("capped PRN").
Types of outcome measures
Visual acuity outcomes
Both trials reported outcomes for best‐corrected visual acuity (BCVA), measured by using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart, at one year and at two years. Investigators assessed BCVA outcomes by using multiple methods of aggregation, including mean change in number of letters, proportion of participants who gained 15 or more letters, and proportion of participants who maintained BCVA, defined by both trials as losing fewer than 15 letters. Neither trial reported the proportion of participants with BCVA worse than 20/200 at any time point.
Anatomic outcomes
Both trials used optical coherence tomography (OCT) to assess the proportion of eyes with absence of fluid at one‐year and two‐year follow‐up and mean change in central retinal thickness from baseline to one year and to two years. The two trials used fluorescein angiography to assess changes in CNV area from baseline to one year and two years. Neither trial reported the proportion of eyes with absence of leakage at any time point.
Mean number of injections
Both trials reported the mean number of injections received by each treatment group by one‐year and two‐year follow‐up. Treatment schedules were fixed for the first year in both trials (every four or eight weeks); therefore, this outcome will be biased on the basis of treatment group. For the second year of the trials, in which treatment schedules were switched to as‐needed dosing, this outcome was reported as the mean number of injections received between one‐year and two‐year follow‐up.
Quality‐of‐life outcomes
Both trials measured vision‐related quality‐of‐life outcomes by using the National Eye Institute Visual Function Questionnaire (NEI‐VFQ), and investigators measured outcomes as mean change from baseline in composite scores at one‐year follow‐up. They reported no data for two‐year follow‐up.
Adverse events
Both VIEW 1 and VIEW 2 reported information related to adverse events, including serious systemic adverse events (eg, death, arteriothrombotic events) and serious ocular adverse events (eg, severe visual acuity loss, retinal hemorrhage). We noted no variation between trials in the types of adverse events reported because the trials were designed similarly and used the same protocol.
Excluded studies
We excluded five studies after full‐text assessment. CLEAR‐AMD 1 examined various doses of aflibercept versus placebo following intravenous administration. This route of treatment is not used in practice, and although this study did compare aflibercept versus placebo ‐ a comparison we sought to review when drafting our protocol (Sarwar 2014) ‐ this comparison was not relevant to this review. We excluded one study because participants were not treatment‐naive (Zehetner 2015) and three studies that were not RCTs (Elshout 2014; Yoshida 2014; Zinkernagel 2015).
Risk of bias in included studies
Because the two included RCTs used the same protocol and methods in different populations, "Risk of bias" assessments are the same for both trials (Figure 2). We assessed studies at low risk of bias for most domains. However, both trials were sponsored by the manufacturer of aflibercept; therefore, we assessed these trials at high risk of bias because of the funding source.
2.
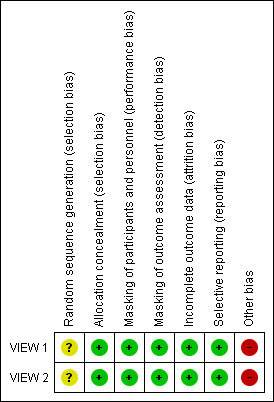
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed these trials at low risk of selection bias. Participants were assigned to treatment groups according to a predetermined centralized randomization scheme with balanced allocation and managed by an interactive voice response system, minimizing the possibility of selection bias.
Masking (performance bias and detection bias)
We assessed these trials at low risk of performance and detection bias. Participants were masked to their particular treatment group. The only unmasked investigator was responsible for receipt, tracking, preparation, destruction, and administration of study drugs, as well as for safety assessments. All other study site personnel were masked to treatment assignment by separating of study records and masking of drug packaging.
Incomplete outcome data
The percentage of participants in each treatment group completing 52 weeks of follow‐up ranged from 91.1% to 96.4% in VIEW 1, and from 88.1 to 96.4% in VIEW 2. Trial investigators reported outcomes based on a full analysis set and a per protocol set by using the last observation carried forward (LOCF) approach to impute missing values. On the basis of these considerations, we assessed these trials at low risk of attrition bias.
Selective reporting
Both trials were registered on clinicaltrials.gov, where primary and secondary outcome measures were prespecified. Final reports of the two trials included all outcomes listed in the clinical trial register. Therefore, we assessed these trials at low risk of reporting bias.
Other potential sources of bias
Many of the corresponding trial authors were employees of or consultants to, or had received research funding from, the manufacturer of aflibercept, Regeneron Pharmaceuticals. Also, the manufacturer of aflibercept participated in trial design, collected and analyzed data, and prepared study reports. We judged this level of involvement to confer high risk of bias because the company may have been motivated to find aflibercept as an equal or superior treatment over ranibizumab, which is manufactured by a different company.
Effects of interventions
See: Table 1
Aflibercept vs no treatment, placebo, or sham
We found no trials in which aflibercept had been compared with no treatment or with placebo or sham intravitreal injections.
Aflibercept vs ranibizumab
To compare aflibercept versus ranibizumab for the treatment of patients with neovascular AMD, we performed meta‐analyses of one‐year results from VIEW 1 and VIEW 2. For these meta‐analyses, we combined data from all aflibercept treatment groups and compared them with data from ranibizumab groups. We reported together and summarized below two‐year results for VIEW 1 and VIEW 2.
Visual acuity outcomes
Mean change in BCVA
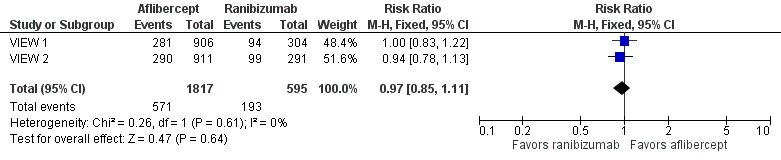
Both trials measured visual acuity using the ETDRS protocol. The mean difference (MD) in mean change in number of letters of BCVA from baseline to one year was less than one letter when aflibercept was compared with ranibizumab (MD ‐0.15, 95% CI ‐1.47 to 1.17; Analysis 1.1;Figure 3). Thus, eyes treated with aflibercept and ranibizumab showed similar gains in visual acuity at one year. We graded the quality of evidence for this outcome as high.
1.1. Analysis.
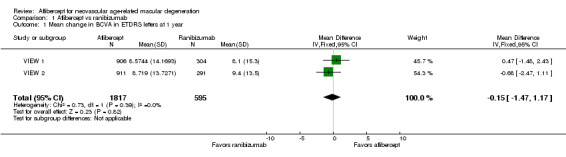
Comparison 1 Aflibercept vs ranibizumab, Outcome 1 Mean change in BCVA in ETDRS letters at 1 year.
3.
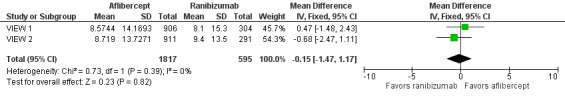
Forest plot of comparison: 1 Aflibercept vs ranibizumab, outcome: 1.1 Mean change in BCVA in ETDRS letters at 1 year.
At two years, the mean change in BCVA from baseline was 7.2 letters for the aflibercept groups versus 7.9 letters for the ranibizumab groups. Additional data regarding two‐year outcomes, such as standard deviation for the mean BCVA change, were not available for further analysis of this outcome.
Gain of 15 or more letters of BCVA
At one‐year follow‐up, the proportion of participants who gained 15 or more letters of BCVA was 31.4% in the aflibercept groups and 32.4% in the ranibizumab groups. For this outcome, a risk ratio (RR) greater than 1 favors treatment with aflibercept. The RR for the combined aflibercept groups versus the ranibizumab groups was 0.97 (95% CI 0.85 to 1.11), which indicates that similar proportions of participants in the aflibercept and ranibizumab groups showed large visual acuity gains (Analysis 1.2;Figure 4). We graded the quality of evidence for this outcome as high.
1.2. Analysis.
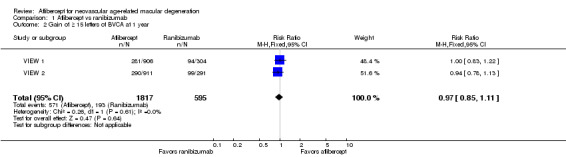
Comparison 1 Aflibercept vs ranibizumab, Outcome 2 Gain of ≥ 15 letters of BVCA at 1 year.
4.
Forest plot of comparison: 1 Aflibercept vs ranibizumab, outcome: 1.2 Gain of ≥ 15 letters of BVCA at 1 year.
At two‐year follow‐up, 562 (30.9%) of 1817 participants in the aflibercept groups and 188 (31.6%) of 595 participants in the ranibizumab groups gained 15 or more letters from baseline. This outcome was comparable between the two groups (RR 0.98, 95% CI 0.85 to 1.12). We graded the quality of evidence for this outcome as high.
Loss of 15 or more letters of BCVA
At one‐year follow‐up, the proportion of participants who lost 15 or more letters of BCVA was 5.1% in the aflibercept groups and 5.7% in the ranibizumab groups. For this outcome, an RR less than 1 favors treatment with aflibercept, as it indicates that a higher proportion of participants lost letters of visual acuity ‐ a negative outcome ‐ in the ranibizumab groups (RR 0.89, 95% CI 0.61 to 1.30; Analysis 1.3). We graded the quality of evidence for this outcome as moderate due to imprecision.
1.3. Analysis.
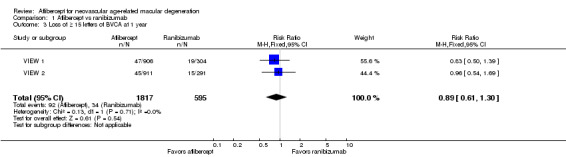
Comparison 1 Aflibercept vs ranibizumab, Outcome 3 Loss of ≥ 15 letters of BVCA at 1 year.
Two‐year follow‐up reports for the combined VIEW 1 and VIEW 2 trials did not report on the proportion of participants who lost 15 or more letters of BCVA.
BCVA worse than 20/200
This outcome was not reported in the included studies at one year or at two years.
Anatomic outcomes
Absence of fluid on optical coherence tomography (OCT)
At one year, researchers found no significant difference between aflibercept and ranibizumab in the proportion of eyes who achieved dry retinas (absence of cystic intraretinal fluid and subretinal fluid on OCT). In VIEW 1, 61.0% of participants in the aflibercept groups and 63.6% of those in the ranibizumab group had no fluid evident on OCT images at one year; in VIEW 2, 64.4% in aflibercept groups and 55.7% in ranibizumab groups had no fluid. The RR for comparison of aflibercept and ranibizumab groups was 1.06 (95% CI 0.98 to 1.14; Analysis 1.4;Figure 5). We graded the quality of evidence for this outcome as high.
1.4. Analysis.
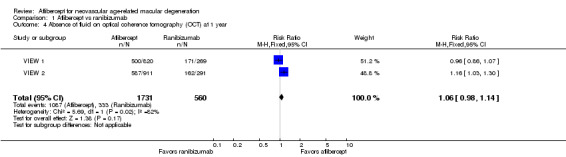
Comparison 1 Aflibercept vs ranibizumab, Outcome 4 Absence of fluid on optical coherence tomography (OCT) at 1 year.
5.
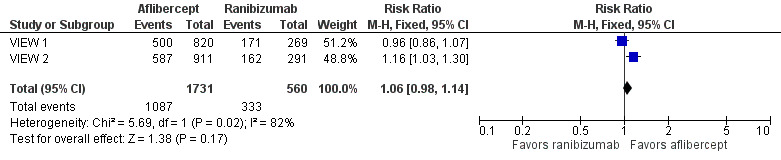
Forest plot of comparison: 1 Aflibercept vs ranibizumab, outcome: 1.4 Absence of fluid on optical coherence tomography (OCT) at 1 year.
At two years, only combined data from VIEW 1 and VIEW 2 were available. The proportion of participants with no retinal fluid evident on OCT images decreased in all treatment groups from one year to two years. A higher proportion of participants in the aflibercept groups (757/1520, 49.8%) showed absence of fluid on OCT compared with participants in the ranibizumab groups (231/508, 45.5%) (RR 1.10, 95% CI 0.98 1.22). We graded the quality of evidence for this outcome as high.
Absence of leakage on fluorescein angiography
The included studies reported no data for this outcome at one year or at two years.
Mean change in extent of choroidal neovascularization (CNV)
In these two trials, change in CNV area from baseline was measured in mm2. At one year, the MD in change in CNV area between aflibercept and ranibizumab groups was ‐0.24 mm2 (95% CI ‐0.78 to 0.29; Analysis 1.5). We graded the quality of evidence for this outcome as high.
1.5. Analysis.
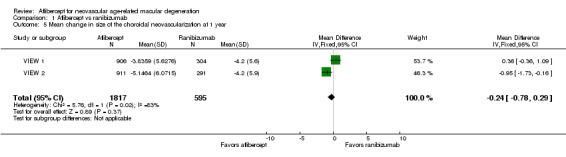
Comparison 1 Aflibercept vs ranibizumab, Outcome 5 Mean change in size of the choroidal neovascularization at 1 year.
Published analyses of combined data from VIEW 1 and VIEW 2 showed mean decreases in CNV area that were maintained from one‐year to two‐year follow‐up in all treatment groups; however, no data were provided for calculating the mean difference in changes in CNV area between groups at two years.
Mean change in central retinal thickness (CRT)
At one‐year follow‐up, the MD between aflibercept and ranibizumab was ‐4.94 µm (95% CI ‐15.48 to 5.61), which is neither a clinically nor statistically important difference (Analysis 1.6). We graded the quality of evidence for this outcome as high.
1.6. Analysis.
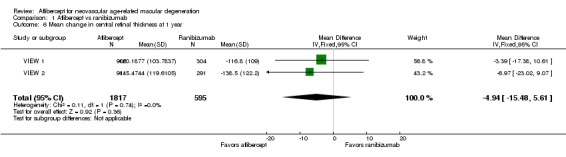
Comparison 1 Aflibercept vs ranibizumab, Outcome 6 Mean change in central retinal thickness at 1 year.
Investigators provided no data for calculating the mean difference in change in CRT between groups at two years.
Mean number of injections
The dosing schedule for all treatment groups was fixed for the first year of the trial (administered every four weeks or every eight weeks); therefore, we did not analyze data on the mean number of injections for one‐year follow‐up.
The mean number of injections during the "capped PRN" period of these trials (from the end of year one to the end of year two) was within one injection when aflibercept was compared with ranibizumab (MD ‐0.40, 95% CI ‐0.62 to ‐0.19). We graded the quality of evidence for this outcome as high.
Vision‐related quality‐of‐life (VRQoL)
In both VIEW 1 and VIEW 2, researchers assessed VRQoL by using the National Eye Institute Visual Function Questionnaire‐25 (NEI‐VFQ‐25). Composite scores range from 0 to 100, and higher scores represent higher visual functioning. Similar changes in NEI‐VFQ‐25 composite scores from baseline to one year were reported for both aflibercept and ranibizumab (MD ‐0.39, 95% CI ‐1.71 to 0.93; Analysis 1.7;Figure 6). We graded the quality of evidence for this outcome as high.
1.7. Analysis.
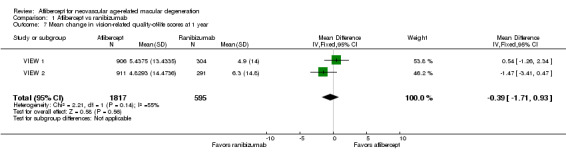
Comparison 1 Aflibercept vs ranibizumab, Outcome 7 Mean change in vision‐related quality‐of‐life scores at 1 year.
6.
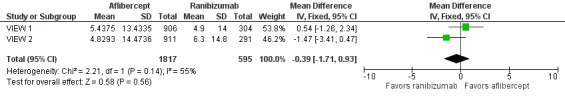
Forest plot of comparison: 1 Aflibercept vs ranibizumab, outcome: 1.7 Mean change in vision‐related quality‐of‐life scores at 1 year.
VRQoL data for two‐year follow‐up were not available.
Adverse events
For the VIEW studies (combined data from VIEW 1 and VIEW 2), 1.75% of participants in the aflibercept groups and 1.68% of those in the ranibizumab groups experienced any arterial thrombotic event, as defined by the Antiplatelet Trialists’ Collaboration (APTC), during the first year of follow‐up (RR 1.04, 95% CI 0.52 to 2.11; Analysis 1.8). At one year, the risk for vascular death was small (< 1%), with uncertainty regarding the effect between aflibercept groups and ranibizumab groups (RR 1.47, 95% CI 0.32 to 6.78; Analysis 1.8). Risks of non‐fatal myocardial infarction (RR 0.81, 95% CI 0.32 to 2.09) and non‐fatal stroke (RR 1.11, 95% CI 0.27 to 4.50) were also uncertain when aflibercept groups were compared with ranibizumab groups (Analysis 1.8).
1.8. Analysis.
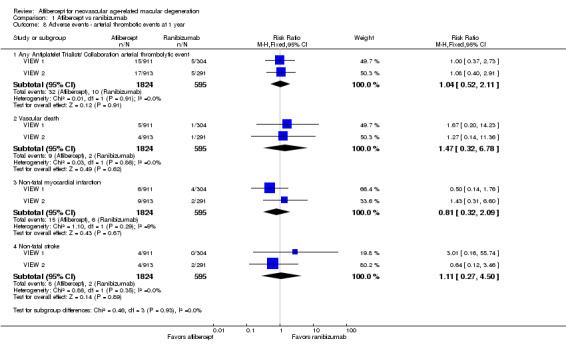
Comparison 1 Aflibercept vs ranibizumab, Outcome 8 Adverse events ‐ arterial thrombotic events at 1 year.
At one‐year follow‐up, the risk of any serious systemic adverse event was similar between aflibercept and ranibizumab groups (RR 0.99, 95% CI 0.79 to 1.25; Analysis 1.9). Congestive heart failure events were more frequent in the aflibercept group than in the ranibizumab group (RR 0.77, 95% CI 0.20 to 2.97; Analysis 1.9). Risk of a non‐ocular hemorrhagic event was lower in the aflibercept group than in the ranibizumab group (RR 2.30, 95% CI 0.42 to 12.70; Analysis 1.9).
1.9. Analysis.
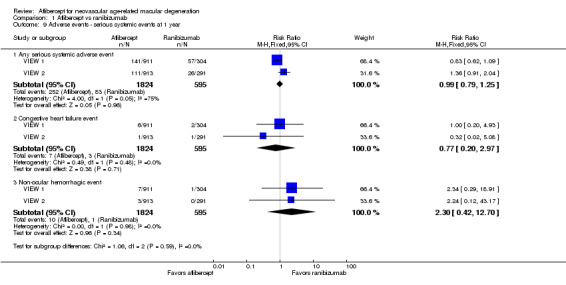
Comparison 1 Aflibercept vs ranibizumab, Outcome 9 Adverse events ‐ serious systemic events at 1 year.
At one‐year follow‐up, risk of any serious ocular adverse event was lower in the aflibercept group than in the ranibizumab group (RR 0.62, 95% CI 0.36 to 1.07; Analysis 1.10). Risk of visual acuity loss was similar between aflibercept and ranibizumab groups (RR 1.08, 95% CI 0.30 to 3.93), and risk of retinal hemorrhage was greater in the aflibercept group than in the ranibizumab group (RR 0.65, 95 % CI 0.16 to 2.60; Analysis 1.10).
1.10. Analysis.
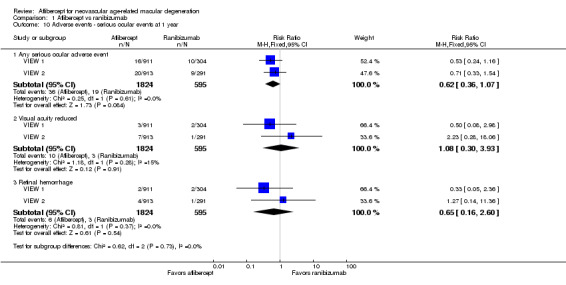
Comparison 1 Aflibercept vs ranibizumab, Outcome 10 Adverse events ‐ serious ocular events at 1 year.
Adverse event data from individual VIEW 1 and VIEW 2 trials were not available for analysis for two‐year outcomes. By two years, 3.3% (60/1824) of participants in the aflibercept group and 3.2% (19/595) of those in the ranibizumab group had experienced any arterial thrombotic event (RR 1.03, 95% CI 0.62 to 1.71). Risk for vascular death was 1.3% (24/1824) in the aflibercept group versus 0.5% (3/595) in the ranibizumab group (RR 2.61, 95% CI 0.79 to 8.64). Comparative risks of non‐fatal myocardial infarction (RR 0.68, 95% CI 0.34 to 1.34) and non‐fatal stroke (RR 0.85, 95% CI 0.30 to 2.37) were uncertain when aflibercept groups were compared with ranibizumab groups.
At two‐year follow‐up, the risk of any serious systemic adverse event was similar between aflibercept and ranibizumab groups (RR 0.98, 95% CI 0.83 to 1.15). Congestive heart failure events occurred in similar proportions in the aflibercept group and the ranibizumab group (RR 1.04, 95% CI 0.38 to 2.84). Non‐ocular hemorrhagic events occurred in less than one per cent of participants; these data were not reported for two‐year outcomes.
At two‐year follow‐up, the risk of any serious ocular adverse event was lower in the aflibercept group than in the ranibizumab group (RR 0.82, 95% CI 0.52 to 1.27). Risks of visual acuity loss (RR 0.91, 95% CI 0.33 to 2.53) and retinal hemorrhage (RR 1.06, 95 % CI 1.06 0.35 to 3.24) were similar between aflibercept and ranibizumab groups.
The small numbers of events led to risk estimates for these adverse events that are imprecise. As the result of imprecision, we graded the quality of evidence for all adverse events as moderate.
Aflibercept vs bevacizumab
We found no trials in which aflibercept had been compared with bevacizumab.
Discussion
Summary of main results
The two trials included in this systematic review were at low risk for most bias domains and demonstrated that benefits and harms of therapy with intravitreal aflibercept were similar to those with ranibizumab among participants with neovascular age‐related macular degeneration (AMD). Changes in visual acuity ‐ both gains and losses ‐ were similar among aflibercept‐ and ranibizumab‐treated eyes. Overall improvement in visual acuity correlated with anatomic improvements (eg, retinal thickening, choroidal neovascularization [CNV] size) for both agents. Although safety profiles (ocular and systemic) for aflibercept and ranibizumab were similar, and no safety signals were identified, neither VIEW 1 nor VIEW 2, alone or together, provided adequate power for precise evaluation of safety outcomes. Vision‐related quality‐of‐life outcomes correlated with clinical and anatomic outcomes. Scores and one‐year changes in scores were similar among aflibercept‐ and ranibizumab‐treated participants.
Aflibercept treatment regimens currently in use typically allow for intravitreal injections at eight‐week intervals with monthly monitoring. The decreased frequency of injections in comparison with ranibizumab and bevacizumab thus decreases the treatment burden and the potential for injection‐related complications. Ranibizumab and bevacizumab were the most commonly used treatments for neovascular AMD at the time the VIEW 1 and VIEW 2 studies were conducted. Analyses of available data indicate that aflibercept is equally effective and safe and may be considered as a first‐line treatment for patients with neovascular AMD because of the potential for fewer injections needed to achieve similar results.
Overall completeness and applicability of evidence
The goal of this review was to compare the effectiveness and safety of aflibercept for the treatment of patients with neovascular AMD versus ranibizumab, bevacizumab, or sham. Only data from randomized controlled trials (RCTs) with a minimum follow‐up of one year were selected for inclusion in this review. We found no trial in which aflibercept had been compared with bevacizumab or sham that met the eligibility criteria for this review. Thus, our analysis was limited to comparison of aflibercept and ranibizumab.
The primary outcome of this review was mean change from baseline in best‐corrected visual acuity (BCVA) (Early Treatment Diabetic Retinopathy Study [ETDRS] letters) to one‐year of follow‐up. Secondary outcomes included other visual acuity outcomes, morphologic characteristics assessed by optical coherence tomography (OCT) and fluorescein angiography, ocular and systemic adverse events, and vision‐related quality of life. We searched journal publications, conference abstracts, US Food and Drug Administration (FDA) documents, and clinical trial registers to identify relevant data.
For this review, we analyzed outcome data on 2457 participants from two large trials. VIEW 1 and VIEW 2 enrolled both men and women 50 years of age or older who had subfoveal neovascular AMD in the study eye, and were conducted in 174 countries on all continents except Africa and Antarctica.
Quality of the evidence
These studies were well designed; they evaluated both clinically relevant outcomes and patient‐important outcomes. We assessed the quality of evidence as high for most outcomes. Our analyses of outcomes consistently found no meaningful differences between aflibercept and ranibizumab. However, the numbers of participants included in the analyses of some infrequent outcomes, such as adverse events, were insufficient to rule out possible differences between treatments.
Potential biases in the review process
Two authors for this review were also investigators for the VIEW 1 and VIEW 2 trials and were authors of the reports from those studies. Other authors of this review were not affiliated with the included studies but are members of the department chaired by one of the VIEW 1 and VIEW 2 investigators/authors. Thus, this review has substantial potential for bias despite our efforts to provide an objective assessment and interpretation of available data.
It is important to note that both trials included in this review were sponsored by Regeneron Pharmaceuticals, the manufacturer of aflibercept. The company participated in trial design, collected and analyzed the data, and prepared the study reports for both trials.
Agreements and disagreements with other studies or reviews
Our clinical experience with aflibercept in the treatment of patients with neovascular AMD is consistent with the conclusions of this review ‐ that aflibercept provides similar visual acuity and morphologic outcomes with no excess of adverse events in comparison with ranibizumab. A review of the literature that summarized VIEW 1 and VIEW 2 findings concluded that "compared to current treatments, aflibercept has shown equal efficacy and safety" to ranibizumab (Thomas 2013). As of 2015, the American Academy of Ophthalmology has recommended anti‐vascular endothelial growth factor (VEGF) agents, including ranibizumab, bevacizumab, and aflibercept, as first‐line treatment for individuals with neovascular AMD (AAO 2015). A network meta‐analysis by Schmid et al comparing various anti‐VEGF agents and dosages estimated that standard doses of aflibercept (2 mg) and ranibizumab (0.5 mg) may perform slightly better than other doses of these agents (0.5 mg and 0.3 mg, respectively), bevacizumab (1.25 mg), and placebo with respect to visual acuity outcomes and serious adverse events at one‐year follow‐up (Schmid 2015).
Authors' conclusions
Implications for practice.
Results of this review indicate that effects of intravitreal aflibercept in terms of improvement and stability in visual acuity after one and two years of treatment are similar to those observed when ranibizumab is used in a substantial number of treated eyes. The beneficial effects of both aflibercept and ranibizumab with respect to visual acuity are consistent with changes in retinal thickness and CNV lesion size observed on OCT imaging. Available information on adverse effects associated with the use of aflibercept or ranibizumab does not suggest that either drug produces a greater incidence of systemic or vision‐threatening complications. Advantages of aflibercept include the possibility of longer intervals between injections, which may result in decreased treatment burden.
Implications for research.
We found no clinical trial that compared aflibercept versus bevacizumab for the treatment of individuals with neovascular AMD. Several studies have compared ranibizumab versus bevacizumab for outcomes of neovascular AMD (Solomon 2014). A comparative study of aflibercept, ranibizumab, and bevacizumab for vision outcomes of neovascular AMD may be unnecessary, given the findings of this review and others. However, a comparison of the three drugs as currently used in clinical practice would be useful to show whether the frequency of injection and the long‐term costs associated with use of each drug are similar or different. A very large trial, including tens of thousands of participants, would be needed to provide acceptably precise estimates of undesirable outcomes and adverse events based on estimates of the incidence of such outcomes already available.
Research is under way to develop anti‐VEGF agents with longer duration of action and to identify new methods of delivery with long‐term release of anti‐VEGF medications, such as intraocular implants. Future studies will be needed to evaluate new treatments and methods.
Acknowledgements
We acknowledge Lori Rosman, CEV Trials Search Co‐ordinator, for developing the search strategy for this review and executing it. We thank Sueko Ng for assistance provided with screening and data abstraction.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Macular Degeneration] explode all trees #2 MeSH descriptor: [Retinal Degeneration] explode all trees #3 MeSH descriptor: [Retinal Neovascularization] explode all trees #4 MeSH descriptor: [Choroidal Neovascularization] explode all trees #5 MeSH descriptor: [Macula Lutea] explode all trees #6 ((macul* or retina* or choroid*) near/4 degener*) #7 ((macul* or retina* or choroid*) near/4 neovasc*) #8 maculopath* #9 (macul* near/2 lutea*) #10 (macul* near/3 dystroph*) #11 (macul* near/2 syndrome) #12 ((macul* or geographic) near/2 atroph*) #13 ((macul* or retina*) near/2 edema*) #14 (AMD or ARMD or CNV) #15 {or #2‐#14} #16 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees #17 MeSH descriptor: [Angiogenesis Inducing Agents] explode all trees #18 MeSH descriptor: [Endothelial Growth Factors] explode all trees #19 MeSH descriptor: [Vascular Endothelial Growth Factors] explode all trees #20 (anti* near/2 VEGF*) #21 (endothelial near/2 growth near/2 factor*) #22 (aflibercept or VEGF Trap* or Trap Eye or "AVE 005" or AVE005 or Zaltrap or ZIV‐aflibercept or "AVE 0005" or AVE0005 or eylea or vasculotropin trap) #23 {or #16‐#22} #24 #15 and #23
Appendix 2. MEDLINE (OvidSP) search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomized).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Macular Degeneration/ 13. exp Retinal Degeneration/ 14. exp Retinal Neovascularization/ 15. exp Choroidal Neovascularization/ 16. exp Macula Lutea/ 17. ((macul* or retina* or choroid*) adj4 degener*).tw. 18. ((macul* or retina* or choroid*) adj4 neovasc*).tw. 19. Maculopath*.tw. 20. (macul* adj2 lutea*).tw. 21. (macul* adj3 dystroph*).tw. 22. (macul* adj2 syndrome).tw. 23. ((macul* or geographic) adj2 atroph*).tw. 24. ((macul* or retina*) adj2 edema*).tw. 25. (AMD or ARMD or CNV).tw. 26. or/13‐24 27. exp angiogenesis inhibitors/ 28. exp angiogenesis inducing agents/ 29. exp endothelial growth factors/ 30. exp vascular endothelial growth factors/ 31. (anti* adj2 VEGF*).tw. 32. (endothelial adj2 growth adj2 factor*).tw. 33. (aflibercept or VEGF Trap* or Trap Eye or "AVE 005" or AVE005 or Zaltrap or ZIV‐aflibercept or "AVE 0005" or AVE0005 or eylea or vasculotropin trap).tw. 34. (15C2VL427D or 845771‐78‐0 or 862111‐32‐8).rn. 35. or/27‐34 36. 11 and 26 and 35
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'retina maculopathy'/exp #34 'retina degeneration'/exp #35 'retina macula degeneration'/exp #36 'retina neovascularization'/exp #37 'subretinal neovascularization'/exp #38 'retina macula lutea'/exp #39 ((macul* OR retina* OR choroid*) NEAR/4 degener*):ab,ti #40 ((macul* OR retina* OR choroid*) NEAR/4 neovasc*):ab,ti #41 maculopath*:ab,ti #42 (macul* NEAR/2 lutea*):ab,ti #43 (macul* NEAR/3 dystroph*):ab,ti #44 (macul* NEAR/2 syndrome):ab,ti #45 ((macul* OR geographic) NEAR/2 atroph*):ab,ti #46 ((macul* OR retina*) NEAR/2 edema*):ab,ti #47 amd:ab,ti OR armd:ab,ti OR cnv:ab,ti #48 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 #49 'angiogenesis'/exp #50 'angiogenesis inhibitor'/exp #51 'angiogenic factor'/exp #52 'endothelial cell growth factor'/exp #53 'vasculotropin'/exp #54 (anti* NEAR/2 vegf*):ab,ti #55 endothelial:ab,ti AND growth:ab,ti AND factor*:ab,ti #56 'aflibercept'/exp #57 aflibercept:ab,ti OR (vegf NEXT/1 trap*):ab,ti OR 'trap eye':ab,ti OR 'ave 005':ab,ti OR ave005:ab,ti OR zaltrap:ab,ti OR 'ziv aflibercept':ab,ti OR 'ave 0005':ab,ti OR ave0005:ab,ti OR eylea:ab,ti OR 'vasculotropin trap':ab,ti #58 15c2vl427d:rn OR '845771 78 0':rn OR '862111 32 8':rn #59 '301253 48 5':rn #60 #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 #61 #32 AND #48 AND #60
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 ((macul*[tw] OR retina*[tw] OR choroid*[tw]) AND degener*[tw]) NOT Medline[sb] #3 ((macul*[tw] OR retina*[tw] OR choroid*[tw]) AND neovasc*[tw]) NOT Medline[sb] #4 Maculopath*[tw] NOT Medline[sb] #5 (macul*[tw] AND lutea*[tw]) NOT Medline[sb] #6 (macul*[tw] AND dystroph*[tw]) NOT Medline[sb] #7 (macul*[tw] AND syndrome[tw]) NOT Medline[sb] #8 ((macul*[tw] OR geographic[tw]) AND atroph*[tw]) NOT Medline[sb] #9 ((macul*[tw] OR retina*[tw]) AND edema*[tw]) NOT Medline[sb] #10 (AMD[tw] OR ARMD[tw] OR CNV[tw]) NOT Medline[sb] #11 #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 #12 (anti VEGF*[tw] OR antiVEGF*[tw]) NOT Medline[sb] #13 (endothelial[tw] AND growth[tw] AND factor*[tw]) NOT Medline[sb] #14 (aflibercept[tw] OR VEGF Trap*[tw] OR Trap Eye[tw] OR "AVE 005"[tw] OR AVE005[tw] OR Zaltrap[tw] OR ZIV‐aflibercept[tw] OR "AVE 0005"[tw] OR AVE0005[tw] OR eylea[tw] OR vasculotropin trap[tw]) NOT Medline[sb] #15 #12 OR #13 OR #14 #16 #1 AND #11 AND #15
Appendix 5. LILACS search strategy
((Macul$ OR Mácul$ OR Retina$ OR Retiniana OR Choroid$ OR Coroide) AND (Degenera$ OR Neovasculariza$) OR MH:C11.768.585$ OR MH:C11.768.585.439$ OR MH: C11.768.725$ OR MH:C23.550.589.500.725$ OR MH:C11.941.160.244$ OR MH:C23.550.589.500.145$ OR MH:A09.371.729.522$ OR maculopath$ OR AMD OR ARMD OR CNV) AND ("Recombinant Fusion Proteins" OR "Proteínas Recombinantes de Fusión" OR "Proteínas Recombinantes de Fusão" OR MH:D12.776.828.300$ OR "Angiogenesis Inhibitors" OR "Inhibidores de la Angiogénesis" OR "Inibidores da Angiogênese" OR "Angiogenic Antagonists" OR MH:D27.505.696.377.077.099$ OR MH:D27.505.696.377.450.100$ OR MH:D27.505.954.248.025$ OR "Angiogenesis Inducing Agents" OR "Inductores de la Angiogénesis" OR "Indutores da Angiogênese" OR MH:D27.505.696.377.077.077$ OR "Factores de Crecimiento Endotelial" OR "Fatores de Crescimento Endotelial" OR MH:D12.644.276.390$ OR MH:D12.776.467.390$ OR MH:D23.529.390$ OR MH:D12.644.276.100.800$ OR MH:D12.776.467.100.800$ OR MH:D23.529.100.800$ OR antiVEGF$ OR anti‐VEGF$ OR (endothelial AND growth AND factor$))
Appendix 6. metaRegister of Controlled Trials search strategy
aflibercept or VEGF Trap* or Trap Eye OR AVE 005 or AVE005 or Zaltrap or ZIV‐aflibercept or AVE 0005 or AVE0005 OR eylea or vascular endothelial growth factor trap or vasculotropin trap or anti‐VEGF
Appendix 7. ClinicalTrials.gov search strategy
(anti‐VEGF AND ("Macular Degeneration" OR AMD OR ARMD OR "Retinal Degeneration" OR "Retinal Neovascularization" OR "Choroidal Neovascularization" OR CNV)) OR (aflibercept OR "VEGF Trap" OR "Trap Eye" OR "AVE 005" OR AVE005 OR Zaltrap OR ZIV‐aflibercept OR "AVE 0005" OR AVE0005 OR eylea OR "vascular endothelial growth factor trap" OR "vasculotropin trap")
Appendix 8. ICTRP search strategy
aflibercept OR VEGF Trap* OR "Trap Eye" OR "AVE 005" OR AVE005 OR Zaltrap OR ZIV‐aflibercept OR "AVE 0005" OR AVE0005 OR eylea OR "vascular endothelial growth factor trap" OR "vasculotropin trap" OR Macular degeneration AND anti‐VEGF OR AMD AND anti‐VEGF OR ARMD AND anti‐VEGF OR "Retinal Degeneration" AND anti‐VEGF OR "Retinal Neovascularization" AND anti‐VEGF OR "Choroidal Neovascularization" AND anti‐VEGF OR CNV AND anti‐VEGF
Data and analyses
Comparison 1. Aflibercept vs ranibizumab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in BCVA in ETDRS letters at 1 year | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐1.47, 1.17] |
| 2 Gain of ≥ 15 letters of BVCA at 1 year | 2 | 2412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.11] |
| 3 Loss of ≥ 15 letters of BVCA at 1 year | 2 | 2412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.30] |
| 4 Absence of fluid on optical coherence tomography (OCT) at 1 year | 2 | 2291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.14] |
| 5 Mean change in size of the choroidal neovascularization at 1 year | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.78, 0.29] |
| 6 Mean change in central retinal thickness at 1 year | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐4.94 [‐15.48, 5.61] |
| 7 Mean change in vision‐related quality‐of‐life scores at 1 year | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.71, 0.93] |
| 8 Adverse events ‐ arterial thrombotic events at 1 year | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Any Antiplatelet Trialists' Collaboration arterial thrombolytic event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.52, 2.11] |
| 8.2 Vascular death | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.32, 6.78] |
| 8.3 Non‐fatal myocardial infarction | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.32, 2.09] |
| 8.4 Non‐fatal stroke | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.27, 4.50] |
| 9 Adverse events ‐ serious systemic events at 1 year | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Any serious systemic adverse event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.79, 1.25] |
| 9.2 Congestive heart failure event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.20, 2.97] |
| 9.3 Non‐ocular hemorrhagic event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.42, 12.70] |
| 10 Adverse events ‐ serious ocular events at 1 year | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Any serious ocular adverse event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.07] |
| 10.2 Visual acuity reduced | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.30, 3.93] |
| 10.3 Retinal hemorrhage | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.16, 2.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
VIEW 1.
| Methods |
Study design: parallel‐group randomized controlled trial Number randomly assigned: 1217 total participants (1217 eyes) · 304 in the aflibercept 0.5 mg every 4 weeks group · 304 in the aflibercept 2.0 mg every 4 weeks group · 303 in the aflibercept 2.0 mg every 8 weeks group · 306 in the ranibizumab group Exclusions after randomization: Full analysis: 7 total participants · 3 in the aflibercept 0.5 mg every 4 weeks group, 0 in the aflibercept 2.0 mg every 4 weeks group, 2 in the aflibercept 2.0 mg every 8 weeks group, and 2 in the ranibizumab group Safety analysis: 2 total participants (both in the ranibizumab group) Losses to follow‐up: 103 participants discontinued treatment at 1‐year follow‐up · 30 in the aflibercept 0.5 mg every 4 weeks group · 16 in the aflibercept 2.0 mg every 4 weeks group · 30 in the aflibercept 2.0 mg every 8 weeks group · 27 in the ranibizumab group Number analyzed: Full analysis ‐ 1210 total participants at 1‐year follow‐up · 301 in the aflibercept 0.5 mg every 4 weeks group · 304 in the aflibercept 2.0 mg every 4 weeks group, · 301 in the aflibercept 2.0 mg every 8 weeks group · 304 in the ranibizumab group Safety analysis ‐ 1215 total participants at 1‐year follow‐up · 304 in the aflibercept 0.5 mg every 4 weeks group · 304 in the aflibercept 2.0 mg every 4 weeks group · 303 in the aflibercept 2.0 mg every 8 weeks group · 304 in the ranibizumab group Unit of analysis: individual (1 study eye per participant) How were missing data handled? missing values imputed using last observation carried forward approach Power calculation: none reported |
|
| Participants |
Country: United States and Canada (154 study sites) Mean age (range not reported): 78 years in the aflibercept 0.5 mg every 4 weeks group, 78 years in the aflibercept 2.0 mg every 4 weeks group, 78 years in the aflibercept 2.0 mg every 8 weeks group, and 78 years in the ranibizumab group Gender: 134 men (44.5%) and 167 women (55.5%) in the aflibercept 0.5 mg every 4 weeks group, 110 men (36.2%) and 194 women (63.8%) in the aflibercept 2.0 mg every 4 weeks group, 123 men (40.9%) and 178 women (59.1%) in the aflibercept 2.0 mg every 8 weeks group, and 132 men (43.4%) and 172 women (56.6%) in the ranibizumab group Inclusion criteria: 50 years of age or older; diagnosed with neovascular AMD in the study eye; active subfoveal CNV lesions of any subtype (12 optic disc areas or smaller) constituting ≥ 50% of total lesion size; BCVA between 73 and 25 Early Treatment Diabetic Retinopathy Study (ETDRS) chart letters (20/40 to 20/320 Snellen equivalent); willingness and ability to return for clinic visits and complete study‐related procedures; ability to provide informed consent Exclusion criteria: prior or concomitant treatment for AMD in the study eye; prior treatment with anti‐VEGF therapy; subretinal hemorrhage or scar or fibrosis constituting > 50% of total lesion size or involving the center of the fovea in the study eye; retinal pigment epithelial tears or rips involving the macula in the study eye; history of other ocular conditions such as vitreous hemorrhage, retinal detachment, macular hole, corneal transplant, corneal dystrophy, diabetic retinopathy, diabetic macular edema, uveitis, scleromalacia; presence of other ocular conditions such as uncontrolled glaucoma, significant media opacities, phakia or pseudophakia with absence of posterior capsule, intraocular inflammation or infection; prior vitrectomy, trabeculectomy, or other filtration surgery or therapy in the study eye Equivalence of baseline characteristics: yes; "Baseline demographics and disease characteristics were evenly balanced among all treatment groups" |
|
| Interventions |
Intervention 1: intravitreal aflibercept 0.5 mg every 4 weeks Intervention 2: intravitreal aflibercept 2.0 mg every 4 weeks Intervention 3: intravitreal aflibercept 2.0 mg every 8 weeks after 3 initial doses at weeks 0, 4, and 8 (to maintain masking, sham injections were given at the interim 4‐week visits after week 8) Intervention 4: intravitreal ranibizumab 0.5 mg every 4 weeks Length of follow‐up: 1 year for primary end point; dosing for all groups changed to as needed (PRN) after 1 year and follow‐up at 2 years from baseline |
|
| Outcomes |
Primary outcome, as defined in study reports: "proportion of patients maintaining vision at week 52 (losing < 15 letters on Early Treatment Diabetic Retinopathy Study [ETDRS] chart)" Secondary outcomes, as defined in study reports: change in BCVA, proportion gaining ≥ 15 letters, change in total National Eye Institute 25‐Item Visual Function Questionnaire (NEI‐VFQ‐25) score, change in CNV area on fluorescein angiography, retinal thickness and persistent fluid as assessed by OCT, mean number of intravitreal injections, adverse events Intervals at which outcomes assessed: every 4 weeks through 96 weeks; week 1 after first treatment for safety assessment; weeks 12, 24, 36, and 52 for the NEI‐VFQ‐25 assessment |
|
| Notes |
Type of study reports: published journal articles; clinical trial registration Funding sources: "Sponsored by Regeneron Pharmaceuticals, Inc, Tarrytown, New York, and Bayer HealthCare, Berlin Germany. The sponsors participated in the design and conduct of the study, analysis of the data, and preparation of the manuscript" Disclosures of interest: "J.S.H. is a consultant to and has received research funding from Alimera, Allergan, Fovea, Genentech, Genzyme, GlaxoSmithKline, Neovista, and Regeneron Pharmaceuticals. He has also received travel support from Regeneron Pharmaceuticals. D.M.B. is a consultant to Alimera, Allergan, Bayer, Genentech/Roche, Novartis, Regeneron Pharmaceuticals, and Thrombogenics and has received research funding from Alcon, Alimera, Allergan, Eli Lilly, Genentech, GlaxoSmithKline, Novartis, Regeneron Pharmaceuticals, and Thrombogenics. He has also received travel support from Regeneron Pharmaceuticals and lecture fees from Genentech. V.C. is a consultant to Alimera and Bayer and has received research funding from Alcon, Allergan, Bayer, Novartis, and Pfizer. He is an advisory board member for Allergan and Novartis and has also received travel support from Bayer. J.‐F.K. is a consultant to Alcon, Bayer, and Thea and an advisory board member for Allergan, Bayer, and Novartis. He has received travel support from Regeneron Pharmaceuticals. P.K.K. is a consultant to Bayer, Genentech, Novartis, and Regeneron Pharmaceuticals. He has received research funding from Regeneron Pharmaceuticals. Q.D.N. is a consultant to Bausch & Lomb and Santen and has received research funding from Genentech, Novartis, and Pfizer. B.K. has received travel support from Bayer. A.H. is a consultant to Alcon, Allergan, Centocor, Johnson & Johnson, Neovista, Merck, Ophthotech, Oraya, Paloma, P.R.N., Q.L.T., Regeneron Pharmaceuticals, and Thrombogenics. He has received research funding and lecture fees from Alcon, Allergan, Genentech, Neovista, Ophthotech, Oraya, P.R.N., Q.L.T., Regeneron Pharmaceuticals, and Second Sight. Y.O. is a consultant to Alcon and Bayer and has received travel support from Bayer. G.D.Y., N.S., R.V., A.J.B., and Y.S. are employees of Regeneron Pharmaceuticals. M.A., G.G., B.S., and R.S. are employees of Bayer HealthCare. C.S.’s institution has received payments from the Medical University of Vienna for data monitoring/reviewing and statistical analysis. U.S.‐E. is a consultant to Alcon, Allergan, Bayer HealthCare, and Novartis, and an advisory board member for Alcon and Novartis. She has received travel support from Bayer HealthCare and lecture fees from Bayer HealthCare and Novartis" Study period: July 2007 to September 2010 Subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation was unclear. “Consecutively enrolled patients were assigned to treatment groups on the basis of a predetermined central randomization scheme with balanced allocation, managed by an interactive voice response system” |
| Allocation concealment (selection bias) | Low risk | Central randomization: “Consecutively enrolled patients were assigned to treatment groups on the basis of a predetermined central randomization scheme with balanced allocation, managed by an interactive voice response system” |
| Masking of participants and personnel (performance bias) | Low risk | “Patients were masked as to treatments. An unmasked investigator also was responsible for the receipt, tracking, preparation, destruction, and administration of study drug, as well as safety assessments both pre‐ and post‐dose...All other study site personnel were masked to treatment assignment by separating study records or masked packaging” |
| Masking of outcome assessment (detection bias) | Low risk | “A separate masked physician assessed adverse events and supervised the masked assessment of efficacy. All other study site personnel were masked to treatment assignment by separating study records or masked packaging. Optical coherence tomography technicians and visual acuity examiners remained masked relative to treatment assignment” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A full analysis set and a per protocol set were reported. Last observation carried forward (LOCF) approach was used to impute missing values; 91.1% to 96.4% of participants per treatment group completed 52 weeks of follow‐up |
| Selective reporting (reporting bias) | Low risk | The study was registered at clinicaltrials.gov; intended outcomes were reported |
| Other bias | High risk | Many authors are employees of, consultants to, or have received research funding from Regeneron Pharmaceuticals, which manufactures aflibercept and participated in the design of the trial, collected and analyzed data, and prepared the study reports |
VIEW 2.
| Methods |
Study design: parallel‐group randomized controlled trial Number randomly assigned: 1240 total participants (1240 eyes) · 311 in the aflibercept 0.5 mg every 4 weeks group · 313 in the aflibercept 2.0 mg every 4 weeks group · 313 in the aflibercept 2.0 mg every 8 weeks group · 303 in the ranibizumab group Exclusions after randomization: Full analysis ‐ 38 total participants: · 15 in the aflibercept 0.5 mg every 4 weeks group · 4 in the aflibercept 2.0 mg every 4 weeks group · 7 in the aflibercept 2.0 mg every 8 weeks group · 12 in the ranibizumab group Safety analysis ‐ 36 total participants: · 14 in the aflibercept 0.5 mg every 4 weeks group · 4 in the aflibercept 2.0 mg every 4 weeks group · 6 in the aflibercept 2.0 mg every 8 weeks group · 12 in the ranibizumab group Losses to follow‐up: 148 participants discontinued treatment at 1‐year follow‐up · 45 in the aflibercept 0.5 mg every 4 weeks group · 37 in the aflibercept 2.0 mg every 4 weeks group · 33 in the aflibercept 2.0 mg every 8 weeks group · 33 in the ranibizumab group Number analyzed: Full analysis ‐ 1202 total participants at 1‐year follow‐up · 296 in the aflibercept 0.5 mg every 4 weeks group · 309 in the aflibercept 2.0 mg every 4 weeks group · 306 in the aflibercept 2.0 mg every 8 weeks group · 291 in the ranibizumab group Safety analysis ‐ 1204 total participants at 1‐year follow‐up · 297 in the aflibercept 0.5 mg every 4 weeks group · 309 in the aflibercept 2.0 mg every 4 weeks group · 307 in the aflibercept 2.0 mg every 8 weeks group · 291 in the ranibizumab group Unit of analysis: individual (1 study eye per participant) How were missing data handled? missing values imputed using last observation carried forward approach Power calculation: none reported |
|
| Participants |
Country: Argentina; Australia; Austria; Brazil; Belgium; Colombia; Czech Republic; France; Germany; Hungary; India; Israel; Italy; Japan; Latvia; Mexico; Netherlands; Poland; Portugal; South Korea; Singapore; Slovakia; Spain; Sweden; Switzerland; United Kingdom (172 study sites) Mean age (range not reported): 75 years in the aflibercept 0.5 mg every 4 weeks group, 74 years in the aflibercept 2.0 mg every 4 weeks group, 74 years in the aflibercept 2.0 mg every 8 weeks group, and 73 years in the ranibizumab group Gender: 149 men (50.3%) and 147 women (49.7%) in the aflibercept 0.5 mg every 4 weeks group, 133 men (43.0%) and 176 women (57.0%) in the aflibercept 2.0 mg every 4 weeks group, 131 men (42.8%) and 175 women (57.2%) in the aflibercept 2.0 mg every 8 weeks group, and 122 men (41.9%) and 169 women (58.1%) in the ranibizumab group Inclusion criteria: 50 years or older; diagnosed with neovascular AMD in the study eye; active subfoveal CNV lesions of any subtype (12 optic disc areas or fewer) constituting ≥ 50% of total lesion size; BCVA between 73 and 25 Early Treatment Diabetic Retinopathy Study (ETDRS) chart letters (20/40 to 20/320 Snellen equivalent); willingness and ability to return for clinic visits and complete study‐related procedures; ability to provide informed consent Exclusion criteria: prior or concomitant treatment for AMD in the study eye; prior treatment with anti‐VEGF therapy; subretinal hemorrhage or scar or fibrosis constituting > 50% of total lesion size or involving the center of the fovea in the study eye; retinal pigment epithelial tears or rips involving the macula in the study eye; history of other ocular conditions such as vitreous hemorrhage, retinal detachment, macular hole, corneal transplant, corneal dystrophy, diabetic retinopathy, diabetic macular edema, uveitis, scleromalacia; presence of other ocular conditions such as uncontrolled glaucoma, significant media opacities, phakia or pseudophakia with absence of posterior capsule, intraocular inflammation or infection; prior vitrectomy, trabeculectomy, or other filtration surgery or therapy in the study eye Equivalence of baseline characteristics: yes; "Baseline demographics and disease characteristics were evenly balanced among all treatment groups" |
|
| Interventions |
Intervention 1: intravitreal aflibercept 0.5 mg every 4 weeks Intervention 2: intravitreal aflibercept 2.0 mg every 4 weeks Intervention 3: intravitreal aflibercept 2.0 mg every 8 weeks after 3 initial doses at weeks 0, 4, and 8 (to maintain masking, sham injections were given at the interim 4‐week visits after week 8) Intervention 4: intravitreal ranibizumab 0.5 mg every 4 weeks Length of follow‐up: 1 year for primary end point; dosing for all groups changed to as needed (PRN) after 1 year and follow‐up at 2 years from baseline |
|
| Outcomes |
Primary outcome, as defined in study reports: "proportion of patients maintaining vision at week 52 (losing < 15 letters on Early Treatment Diabetic Retinopathy Study [ETDRS] chart)" Secondary outcomes, as defined in study reports: change in BCVA and anatomic measures, proportion gaining ≥ 15 letters, change in total National Eye Institute 25‐Item Visual Function Questionnaire (NEI‐VFQ‐25) score, change in CNV area on fluorescein angiography, retinal thickness and persistent fluid as assessed by OCT, mean number of intravitreal injections, adverse events Intervals at which outcomes assessed: every 4 weeks through 96 weeks; week 1 after first treatment for safety assessment; weeks 12, 24, 36, and 52 for the NEI‐VFQ‐25 assessment |
|
| Notes |
Type of study reports: published journal articles; clinical trial registration Funding sources: "Sponsored by Regeneron Pharmaceuticals, Inc, Tarrytown, New York, and Bayer HealthCare, Berlin Germany. The sponsors participated in the design and conduct of the study, analysis of the data, and preparation of the manuscript" Disclosures of interest: "J.S.H. is a consultant to and has received research funding from Alimera, Allergan, Fovea, Genentech, Genzyme, GlaxoSmithKline, Neovista, and Regeneron Pharmaceuticals. He has also received travel support from Regeneron Pharmaceuticals. D.M.B. is a consultant to Alimera, Allergan, Bayer, Genentech/Roche, Novartis, Regeneron Pharmaceuticals, and Thrombogenics and has received research funding from Alcon, Alimera, Allergan, Eli Lilly, Genentech, GlaxoSmithKline, Novartis, Regeneron Pharmaceuticals, and Thrombogenics. He has also received travel support from Regeneron Pharmaceuticals and lecture fees from Genentech. V.C. is a consultant to Alimera and Bayer and has received research funding from Alcon, Allergan, Bayer, Novartis, and Pfizer. He is an advisory board member for Allergan and Novartis and has also received travel support from Bayer. J.‐F.K. is a consultant to Alcon, Bayer, and Thea and an advisory board member for Allergan, Bayer, and Novartis. He has received travel support from Regeneron Pharmaceuticals. P.K.K. is a consultant to Bayer, Genentech, Novartis, and Regeneron Pharmaceuticals. He has received research funding from Regeneron Pharmaceuticals. Q.D.N. is a consultant to Bausch & Lomb and Santen and has received research funding from Genentech, Novartis, and Pfizer. B.K. has received travel support from Bayer. A.H. is a consultant to Alcon, Allergan, Centocor, Johnson & Johnson, Neovista, Merck, Ophthotech, Oraya, Paloma, P.R.N., Q.L.T., Regeneron Pharmaceuticals, and Thrombogenics. He has received research funding and lecture fees from Alcon, Allergan, Genentech, Neovista, Ophthotech, Oraya, P.R.N., Q.L.T., Regeneron Pharmaceuticals, and Second Sight. Y.O. is a consultant to Alcon and Bayer and has received travel support from Bayer. G.D.Y., N.S., R.V., A.J.B., and Y.S. are employees of Regeneron Pharmaceuticals. M.A., G.G., B.S., and R.S. are employees of Bayer HealthCare. C.S.’s institution has received payments from the Medical University of Vienna for data monitoring/reviewing and statistical analysis. U.S.‐E. is a consultant to Alcon, Allergan, Bayer HealthCare, and Novartis, and an advisory board member for Alcon and Novartis. She has received travel support from Bayer HealthCare and lecture fees from Bayer HealthCare and Novartis" Study period: March 2008 to September 2010 Subgroup analyses: yes; Japanese subgroup |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation was unclear. “Consecutively enrolled patients were assigned to treatment groups on the basis of a predetermined central randomization scheme with balanced allocation, managed by an interactive voice response system” |
| Allocation concealment (selection bias) | Low risk | Central randomization: “Consecutively enrolled patients were assigned to treatment groups on the basis of a predetermined central randomization scheme with balanced allocation, managed by an interactive voice response system” |
| Masking of participants and personnel (performance bias) | Low risk | “Patients were masked as to treatments. An unmasked investigator also was responsible for the receipt, tracking, preparation, destruction, and administration of study drug, as well as safety assessments both pre‐ and post‐dose...All other study site personnel were masked to treatment assignment by separating study records or masked packaging” |
| Masking of outcome assessment (detection bias) | Low risk | “A separate masked physician assessed adverse events and supervised the masked assessment of efficacy. All other study site personnel were masked to treatment assignment by separating study records or masked packaging. Optical coherence tomography technicians and visual acuity examiners remained masked relative to treatment assignment” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A full analysis set and a per protocol set were reported. Last observation carried forward (LOCF) approach was used to impute missing values; 88.1% to 91.1% of participants per treatment group completed 52 weeks of follow‐up |
| Selective reporting (reporting bias) | Low risk | Study was registered at clinicaltrials.gov; intended outcomes were reported |
| Other bias | High risk | Many authors are employees of, consultants to, or have received research funding from Regeneron Pharmaceuticals, which manufactures aflibercept and participated in the design of the trial, collected and analyzed data, and prepared the study reports |
AMD: age‐related macular degeneration. BCVA: best‐corrected visual acuity. CNV: choroidal neovascularization. ETDRS: Early Treatment Diabetic Retinopathy Study. NEI‐VFQ‐25: National Eye Institute 25‐Item Visual Function Questionnaire. OCT: optical coherence tomography. VEGF: vascular endothelial growth factor.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| CLEAR‐AMD 1 | Study administered aflibercept and placebo via an intravenous injection. This method of administration is not used in clinical practice |
| Elshout 2014 | Not a randomized controlled trial; uses data from other trials to create a cost‐utility model comparing aflibercept vs other AMD drugs |
| Yoshida 2014 | Not a randomized controlled trial |
| Zehetner 2015 | Included participants who had been previously treated with other anti‐VEGF medications (not treatment‐naive); reported only outcomes for 4 weeks |
| Zinkernagel 2015 | Not a randomized controlled trial |
Differences between protocol and review
In our original protocol (Sarwar 2014), we did not specify methods for assessing the quality of the evidence using the GRADE approach. We added this assessment to our methods to comply with standard Cochrane methodological expectations. In our protocol, we planned to not perform a meta‐analysis when we had detected considerable clinical, methodological, or statistical heterogeneity (I² > 50%). However, because the two included trials were similar clinically and methodologically, we performed meta‐analysis even when statistical heterogeneity (I² > 50%) was indicated.
Contributions of authors
Conceived of the review: SS, YJS, DVD, QDN. Designed the review: SS, MH, YJS, DVD, QD. Co‐ordinated the review: SS, EC. Collected data for the review: Designed electronic search strategies: Lori Rosman (CEV Trials Search Co‐ordinator). Undertook electronic searches: Lori Rosman (CEV Trials Search Co‐ordinator). Screened search results: SS, MKS, MAS, MH, AA, EC. Organized retrieval of papers: EC. Screened retrieved papers against inclusion criteria: EC, SS, Sueko Ng (SN). Appraised quality of papers: EC, SS, SN. Extracted data from papers: EC, SS, SN. Managed data for the review: Entered data into RevMan 2014: EC. Verified data entered into RevMan 2014: SS. Analyzed data: SS, EC, MKS, AA, MH, YJS, DVD. Interpreted data: Provided a methodological perspective: SS, EC, AA, MAS, MKS, DVD, QDN. Provided a clinical perspective: SS, MKS, AB, AA, MKS, MH, DVD, QDN. Provided a policy perspective: EC, DVD, QDN. Assisted in writing the review: SS, EC, AB, MKS, MAS, AA, MH, DVD, QDN. Performed previous work that was the foundation of the current study: DVD, QDN.
Sources of support
Internal sources
No sources of support supplied
External sources
Elizabeth Clearfield works for the Cochrane Eyes and Vision US Project, supported by cooperative agreement 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
Dr. Salman Sarwar: none known. Ms. Elizabeth Clearfield: none known. Dr. Mohamed K Soliman: none known. Dr. Mohammad A Sadiq: none known. Dr. Andrew J Baldwin: none known. Dr. Mostafa Hanout: none known. Dr. Aniruddha Agarwal: none known. Dr. Yasir J Sepah: none known. Dr. Diana V. Do's institution has received research funding from Genentech and Regeneron. Dr. Do has received consulting fees or honoraria from Allergan, Bayer, Genentech, Oligasis, and Regeneron, and has served as a consultant to Genentech and Regeneron within the past three years. Dr. Do chairs the Steering Committee for the VISTA/VIVID Study. Dr. Quan Dong Nguyen's institution has received research funding from Genentech, Regeneron, AbbVie, Psivida, and XOMA. Dr. Nguyen has received consulting fees or honoraria from Allergan, Bausch and Lomb, Bayer, Genentech, Oligasis, Regeneron, and Santen. He has served on the Scientific Advisory Board for AbbVie, Genentech, Regeneron, Santen, and XOMA within the past three years. Dr. Nguyen chairs the Steering Committee for the RISE/RIDE Study, as well as the EYEGUARD, SAKURA, and VISUAL Studies.
New
References
References to studies included in this review
VIEW 1 {published data only}
- Freund KB, Hoang QV, Saroj N, Thompson D. Intraocular pressure in patients with neovascular age‐related macular degeneration receiving intravitreal aflibercept or ranibizumab. Ophthalmology 2015;122(9):1802‐10. [DOI] [PubMed] [Google Scholar]
- Heier JS, Brown DM, Chong V, Korobelnik J‐F, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF Trap‐Eye) in wet age‐related macular degeneration. Ophthalmology 2012;119(12):2537‐48. [DOI] [PubMed] [Google Scholar]
- Mitchell P, VIEW Study Group. Intravitreal aflibercept versus ranibizumab for neovascular age‐related macular degeneration: 52‐week subgroup analyses from the combined VIEW studies. Clinical and Experimental Ophthalmology 2012;40(Suppl 1):41‐56. [Google Scholar]
- Richard G, Monés J, Wolf S, Korobelnik JF, Guymer R, Goldstein M, et al. Scheduled versus pro re nata dosing in the VIEW trials. Ophthalmology 2015;122(12):2497‐503. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Erfurth U, Kaiser PK, Korobelnik J‐F, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age‐related macular degeneration. Ninety‐six week results of the VIEW studies. Ophthalmology 2014;121(1):193‐201. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Erfurth U, Waldstein SM, Deak GG, Kundi M, Simader C. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age‐related macular degeneration. Ophthalmology 2015;122(4):822‐32. [DOI] [PubMed] [Google Scholar]
- Yuzawa M, Fujita K, Wittrup‐Jensen KU, Norenberg C, Zeitz O, Adachi K, et al. Improvement in vision‐related function with intravitreal aflibercept. Data from phase 3 studies in wet age‐related macular degeneration. Ophthalmology 2015;122(3):571‐8. [DOI] [PubMed] [Google Scholar]
VIEW 2 {published data only}
- Freund KB, Hoang QV, Saroj N, Thompson D. Intraocular pressure in patients with neovascular age‐related macular degeneration receiving intravitreal aflibercept or ranibizumab. Ophthalmology 2015;122(9):1802‐10. [DOI] [PubMed] [Google Scholar]
- Heier JS, Brown DM, Chong V, Korobelnik J‐F, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF Trap‐Eye) in wet age‐related macular degeneration. Ophthalmology 2012;119(12):2537‐48. [DOI] [PubMed] [Google Scholar]
- Mitchell P, VIEW Study Group. Intravitreal aflibercept versus ranibizumab for neovascular age‐related macular degeneration: 52‐week subgroup analyses from the combined VIEW studies. Clinical and Experimental Ophthalmology 2012;40(Suppl 1):41‐56. [Google Scholar]
- Ogura Y, Terasaki H, Gomi F, Yuzawa M, Iida T, Honda M, et al. Efficacy and safety of intravitreal aflibercept injection in wet‐related macular degeneration: outcomes in the Japanese subgroup of the VIEW 2 study. British Journal of Ophthalmology 2015;99(1):92‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard G, Monés J, Wolf S, Korobelnik JF, Guymer R, Goldstein M, et al. Scheduled versus pro re nata dosing in the VIEW trials. Ophthalmology 2015;122(12):2497‐503. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Erfurth U, Kaiser PK, Korobelnik J‐F, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age‐related macular degeneration. Ninety‐six week results of the VIEW studies. Ophthalmology 2014;121(1):193‐201. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Erfurth U, Waldstein SM, Deak GG, Kundi M, Simader C. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age‐related macular degeneration. Ophthalmology 2015;122(4):822‐32. [DOI] [PubMed] [Google Scholar]
- Yuzawa M, Fujita K, Wittrup‐Jensen KU, Norenberg C, Zeitz O, Adachi K, et al. Improvement in vision‐related function with intravitreal aflibercept. Data from phase 3 studies in wet age‐related macular degeneration. Ophthalmology 2015;122(3):571‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
CLEAR‐AMD 1 {published data only}
- Nguyen QD, Shah SM, Hafiz G, Quinlan E, Sung J, Chu K, et al. A phase I trial of an IV‐administered vascular endothelial growth factor trap for treatment in patients with choroidal neovascularization due to age‐related macular degeneration. Ophthalmology 2006;113(9):1522.e1‐14. [DOI] [PubMed] [Google Scholar]
Elshout 2014 {published data only}
- Elshout M, Reis MI, Webers CA, Schouten JS. The cost‐utility of aflibercept for the treatment of age‐related macular degeneration compared to bevacizumab and ranibizumab and the influence of model parameters. Graefe's Archive for Clinical and Experimental Ophthalmology 2014;252(12):1911‐20. [DOI] [PubMed] [Google Scholar]
Yoshida 2014 {published data only}
- Yoshida I, Shiba T, Taniquchi H, Takahashi M, Murano T, Hiruta N, et al. Evaluation of plasma vascular endothelial growth factor levels after intravitreal injection of ranibizumab and aflibercept for exudative age‐related macular degeneration. Graefe's Archive for Clinical and Experimental Ophthalmology 2014;252(9):1483‐9. [DOI] [PubMed] [Google Scholar]
Zehetner 2015 {published data only}
- Zehetner C, Kralinger MT, Modi YS, Waltl I, Ulmer H, Kirchmair R, et al. Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age‐related macular degeneration: a randomised, prospective trial. Acta Ophthalmologica 2015;93(2):e154‐9. [DOI] [PubMed] [Google Scholar]
Zinkernagel 2015 {published data only}
- Zinkernagel MS, Schorno P, Ebneter A, Wolf S. Scleral thinning after repeated intravitreal injections of antivascular endothelial growth factor agents in the same quadrant. Investigative Ophthalmology & Visual Science 2015;56(3):1894‐900. [DOI] [PubMed] [Google Scholar]
Additional references
AAO 2015
- American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern® Guidelines. Age‐Related Macular Degeneration. San Francisco, CA: American Academy of Ophthalmology; 2015. www.aao.org/ppp.
AREDS 2005
- Age‐Related Eye Disease Study Research Group. Responsiveness of the National Eye Institute Visual Function Questionnaire to progression to advanced age‐related macular degeneration, vision loss, and lens opacity: AREDS report no. 14. Archives of Ophthalmology 2005;123(9):1207‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bird 1995
- Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, et al. An international classification and grading system for age‐related maculopathy and age‐related macular degeneration. The International ARM Epidemiological Study Group. Survey of Ophthalmology 1995;39(5):367‐74. [DOI] [PubMed] [Google Scholar]
Brown 2006
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age‐related macular degeneration. New England Journal of Medicine 2006;355(14):1432‐44. [DOI] [PubMed] [Google Scholar]
Brown 2011
- Brown DM, Heier JS, Ciulla T, Benz M, Abraham P, Yancopoulos G, et al. Primary endpoint results of a phase II study of vascular endothelial growth factor trap‐eye in wet age‐related macular degeneration. Ophthalmology 2011;118(6):1089‐97. [DOI] [PubMed] [Google Scholar]
CATT Research Group 2011
- CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, et al. Ranibizumab and bevacizumab for neovascular age‐related macular degeneration. New England Journal of Medicine 2011;364(20):1897‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chakravarthy 2010
- Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, et al. Clinical risk factors for age‐related macular degeneration: a systematic review and meta‐analysis. BMC Ophthalmology 2010;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Childs 2004
- Childs AL, Bressler NM, Bass EB, Hawkins BS, Mangione CM, Marsh MJ, et al. Surgery for hemorrhagic choroidal neovascular lesions of age‐related macular degeneration: quality‐of‐life findings: SST Report No. 14. Ophthalmology 2004;111(11):2007‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clemons 2003
- Clemons TE, Chew EY, Bressler SB, McBee W. National Eye Institute Visual Function Questionnaire in the Age‐Related Eye Disease Study (AREDS): AREDS Report No. 10. Archives of Ophthalmology 2003;121(2):211‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Do 2012
- Do DV, Gower EW, Cassard SD, Boyer D, Bressler NM, Bressler SB, et al. Detection of new‐onset choroidal neovascularization using optical coherence tomography: the AMD DOC Study. Ophthalmology 2012;119(4):771‐8. [DOI] [PubMed] [Google Scholar]
Dong 2004
- Dong LM, Childs AL, Mangione CM, Bass EB, Bressler NM, Hawkins BS, et al. Health‐ and vision‐related quality of life among patients with choroidal neovascularization secondary to age‐related macular degeneration at enrollment in randomized trials of submacular surgery: SST Report No. 4. American Journal of Ophthalmology 2004;138(1):91‐108. [DOI] [PubMed] [Google Scholar]
FDA 2011
- US Food, Drug Administration. FDA news release (2011): FDA approves Eylea for eye disorder in older people. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm280601.htm (accessed 16 December 2013).
Ferrara 2009
- Ferrara N. VEGF‐A: a critical regulator of blood vessel growth. European Cytokine Network 2009;20(4):158‐63. [DOI] [PubMed] [Google Scholar]
Fine 1986
- Fine AM, Elman MJ, Ebert JE, Prestia PA, Starr JS, Fine SL. Earliest symptoms caused by neovascular membranes in the macula. Archives of Ophthalmology 1986;104(4):513‐4. [DOI] [PubMed] [Google Scholar]
Friedman 2004
- Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, Jong PT, et al. Prevalence of age‐related macular degeneration in the United States. Archives of Ophthalmology 2004;122(4):564‐72. [DOI] [PubMed] [Google Scholar]
Haller 2013
- Haller JA. Current anti‐vascular endothelial growth factor dosing regimens: benefits and burden. Ophthalmology 2013;120(5 Suppl):S3‐7. [DOI] [PubMed] [Google Scholar]
Heier 2011
- Heier JS, Boyer D, Nguyen QD, Marcus D, Roth DB, Yancopoulos G, et al. The 1‐year results of CLEAR‐IT 2, a phase 2 study of vascular endothelial growth factor trap‐eye dosed as‐needed after 12‐week fixed dosing. Ophthalmology 2011;118(6):1098‐106. [DOI] [PubMed] [Google Scholar]
Heier 2012
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap‐eye) in wet age‐related macular degeneration. Ophthalmology 2012;119(12):2537‐48. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Holash 2002
- Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF‐Trap: a VEGF blocker with potent antitumor effects. Proceedings of the National Academy of Sciences of the United States of America 2002;99(17):11393‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyman 2002
- Hyman L, Neborsky R. Risk factors for age‐related macular degeneration: an update. Current Opinion in Ophthalmology 2002;13(3):171‐5. [DOI] [PubMed] [Google Scholar]
IVAN Trial 2013
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. Alternative treatments to inhibit VEGF in age‐related choroidal neovascularisation: 2‐year findings of the IVAN randomised controlled trial. Lancet 2013;382(9900):1258‐67. [DOI] [PubMed] [Google Scholar]
Jansen 2013
- Jansen RM. The off‐label use of medication: the latest on the Avastin ‐ Lucentis debacle. Medicine and Law 2013;32(1):65‐77. [PubMed] [Google Scholar]
Mangione 1999
- Mangione CM, Gutierrez PR, Lowe G, Orav EJ, Seddon JM. Influence of age‐related maculopathy on visual functioning and health‐related quality of life. American Journal of Ophthalmology 1999;128(1):45‐53. [DOI] [PubMed] [Google Scholar]
Miskala 2004
- Miskala PH, Bass EB, Bressler NM, Childs AL, Hawkins BS, Mangione CM, et al. Surgery for subfoveal choroidal neovascularization in age‐related macular degeneration: quality‐of‐life findings: SST Report No. 12. Ophthalmology 2004;111(11):1981‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2011
- Mitchell P. A systematic review of the efficacy and safety outcomes of anti‐VEGF agents used for treating neovascular age‐related macular degeneration: comparison of ranibizumab and bevacizumab. Current Medical Research and Opinion 2011;27(7):1465‐75. [DOI] [PubMed] [Google Scholar]
Moja 2014
- Moja L, Lucenteforte E, Kwag KH, Bertele V, Campomori A, Chakravarthy U, et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011230.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenfeld 2006
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age‐related macular degeneration. New England Journal of Medicine 2006;355(14):1419‐31. [DOI] [PubMed] [Google Scholar]
Rudge 2007
- Rudge JS, Holash J, Hylton D, Russell M, Jiang S, Leidich R, et al. VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proceedings of the National Academy of Sciences of the United States of America 2007;104(47):18363‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmid 2015
- Schmid MK, Bachmann LM, Fäs L, Kessels AG, Job OM, Thiel MA. Efficacy and adverse events of aflibercept, ranibizumab and bevacizumab in age‐related macular degeneration: a trade‐off analysis. British Journal of Ophthalmology 2015;99(2):141‐6. [DOI] [PubMed] [Google Scholar]
Schmucker 2010
- Schmucker C, Ehlken C, Hansen LL, Antes G, Agostini HT, Lelgemann M. Intravitreal bevacizumab (Avastin) vs. ranibizumab (Lucentis) for the treatment of age‐related macular degeneration: a systematic review. Current Opinion in Ophthalmology 2010;21(3):218‐26. [DOI] [PubMed] [Google Scholar]
Schmucker 2012
- Schmucker C, Ehlken C, Agostini HT, Antes G, Ruecker G, Lelgemann M, et al. A safety review and meta‐analyses of bevacizumab and ranibizumab: off‐label versus gold standard. PloS One 2012;7(8):e42701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schultz 2003
- Schultz DW, Klein ML, Humpert AJ, Luzier CW, Persun V, Schain M, et al. Analysis of the ARMD1 locus: evidence that a mutation in HEMICENTIN‐1 is associated with age‐related macular degeneration in a large family. Human Molecular Genetics 2003;12(24):3315‐23. [DOI] [PubMed] [Google Scholar]
Solomon 2014
- Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti‐vascular endothelial growth factor for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD005139.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2008
- Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. British Journal of Ophthalmology 2008;92(5):667‐8. [DOI] [PubMed] [Google Scholar]
Thomas 2013
- Thomas M, Mousa SS, Mousa SA. Comparrative effectiveness of aflibercept for the treatment of patients with neovascular age‐related macular degeneration. Clinical Opthahalmology 2013;7:495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thornton 2005
- Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age‐related macular degeneration: a review of association. Eye 2005;19(9):935‐44. [DOI] [PubMed] [Google Scholar]
Wong 2008
- Wong TY, Chakravarthy U, Kelin R, Mitchell P, Zlateva G, Buggage R, et al. The natural history and prognosis of neovascular age‐related macular degeneration: a systematic review of the literature and meta‐analysis. Ophthalmology 2008;115(1):116‐26. [DOI] [PubMed] [Google Scholar]
Wong 2014
- Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng CY, et al. Global prevalence of age‐related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta‐analysis. The Lancet Global Health 2014;2(2):e106‐16. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sarwar 2014
- Sarwar S, Maya JR, Hanout M, Sepah YJ, Do DV, Nguyen QD. Aflibercept for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD011346] [DOI] [PMC free article] [PubMed] [Google Scholar]


