Abstract
Background: The incidence of carpal tunnel syndrome (CTS) is 48 million patients in the United States. The purpose of this longitudinal study was to determine whether Dynasplint stretching (immediately after diagnosis) had an effect on a patient’s decision to seek surgical treatment for CTS. Methods: Fifty patients (10 men, 40 women, mean age 51.2 ± 12 years) were recruited for this randomized, controlled, longitudinal trial. Patients were diagnosed with CTS by physical examination and nerve conduction studies. The intervention used was Dynasplint stretching that delivered a prolonged duration of low load stretching. Patients who were randomly chosen for the Experimental category wore the device for two 30-minute sessions per day with regular increases in splint tension for 60 days. Control patients received nonsteroidal anti-inflammatory medication plus instructions on daily home stretching. Results: The final, longitudinal outcome showed a 72% reduction in surgery chosen by the experimental group (n = 25), compared with 38% reduction for control patients (n = 25). Conclusions: Immediate treatment with Dynasplint stretching showed a 2 to 1 reduction in surgery, with abundant financial savings.
Keywords: contracture reduction, Dynasplint, home therapy, pain management
Introduction
This is an investigation of a nonsurgical treatment for carpal tunnel syndrome (CTS) that has a suggested lifetime occurrence of 48 million patients in the United States.1,3-9,13,16-24,26-29 CTS is defined as compression and/or entrapment of the median nerve as it traverses through the carpal tunnel that causes impairment of motor and/or sensory nerve conduction. The resulting symptoms include numbness, paresthesia, and pain in the median nerve distribution, occurring as a result of compression of the median nerve at the wrist, frequently due to hypertrophy or edema of the flexor synovium. The differential diagnosis assessment includes patient history, physical examination, and electrodiagnostic testing (motor and sensory nerve conduction).2,6,9-19,25-29
Current treatments for CTS still often require postoperative sick leave that exceeds 2 weeks,10,13,21,24,26 and Dynasplint splinting uses a prolonged duration of passive, joint-specific, end-range (of motion) stretching with low loads of force. Dynamic splinting has been shown to reduce sick-leave time.3,4,8 The Dynasplint modality for CTS works by stretching the transverse carpal ligament with a protocol of prolonged, low-intensity stretching (see Figure 1.) Berner et al conducted the initial randomized, controlled trial to examine this modality and therapeutic protocol,4 and this current study was the prolonged follow-up.
Figure 1.

Carpal tunnel Dynasplint.
The results from that randomized, controlled trial showed a significant reduction in Levine-Katz symptom scores (40% reduction, P < .001) in only 60 days for experimental patients, but control participants displayed a mean 1-point increase in Levine-Katz symptom scores. The purpose of this longitudinal outcome study was to determine whether Dynasplint stretching (immediately after diagnosis) had effects on the choices for surgical treatment of CTS.
Methods
This longitudinal examination of patients’ choices for surgery was completed by tracking medical records following completion of the initial randomized controlled trial. The trial measured change in symptoms with Levine-Katz symptom survey and changes in nerve conduction (see Table 1). This survey is a 100-point questionnaire divided into 2 sections. The first section addresses frequency, intensity, and duration of pain, whereas the second section examines functional abilities hindered by pain. This study was approved by the LifeBridge Hospital’s Institutional Review Board (including medical record examinations), and informed consent was obtained from all individual participants included in the study.
Table 1.
Demographics.
| N = 50 | 10 men | 40 women |
| Experimental | 7 men | 18 women |
| Control | 3 men | 22 women |
| Mean age 51.2 ± 12, - 48 Caucasian, 1 Asian, 1 Indian | ||
CTS diagnosis was confirmed with physical examination and a nerve conduction test of the median nerve (following electrodiagnostic standards established by the American Association for Hand Surgery). Patients then were qualified for participation based on the inclusion/exclusion requirements given in Table 2. (Ten patients were prohibited from participation because they met one of the exclusion criteria.) Subject categories were calculated randomly by the SPSS program (Chicago, Illinois; see Figure 2).
Table 2.
Inclusion/Exclusion Criteria.
|
Note. CTS, carpal tunnel syndrome.
Figure 2.
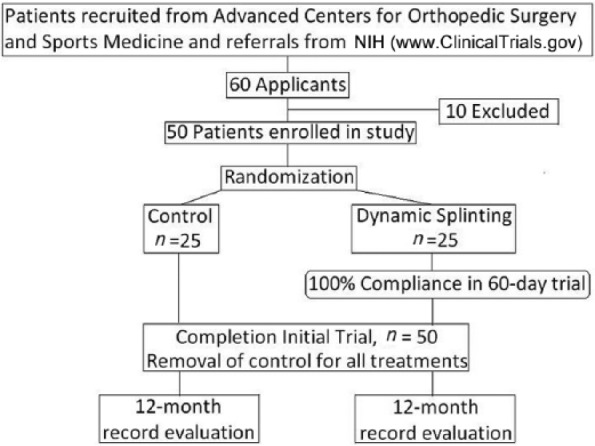
Experimental flowchart.
Note. NIH, National Institutes of Health.
The control patients were only treated with nonsteroidal anti-inflammatory medications, stretching exercises, and instructions for reducing the potential movement causing the pain. Steroid injections were not given during this period so that results would be uniform. The initial Levine-Katz symptom survey scores were 45.5 for experimental patients and 44.3 for control patients.
Intervention
The experimental patients were treated with Dynasplint stretching modality (Figure 1; Dynasplint Systems, Inc., Severna Park, Maryland). Each Dynasplint (DS) was customized by one technician to fit each patient’s hand length, width, and girth. The first week of DS use was an accommodation period for the patient, and patients were encouraged to wear the unit twice daily for 15 minutes each session. Time was then increased by 2 to 4 minutes each following session. After the patient comfortably wore the device for two 30-minute sessions each day for 1 week, instructions were given to increase the tension of the DS device once every 2 weeks, based on comfort and tolerance. If the new tension setting caused excess joint fatigue or “soreness,” the patient was instructed to reduce the time to 15 minutes, twice daily, and work the time back up to 30 minutes, twice daily.
The goal was to wear the modality for 30 minutes, twice a day and increase the tension twice a month, based on comfort and tolerance. All patients were instructed to communicate on compliance through weekly transfer of wearing diary records to the prescribing physician. (All patient records were held in confidence under the Helsinki Declaration by the World Medical Association.)
Duration
The controlled trial lasted 60 days to determine whether there were significant, immediate changes in pain, function, and nerve conduction. It was brief to ensure 100% compliance and completion. The dependent variables in that trial were change in the Levine-Katz symptom survey and change in nerve conduction (sensory and motor latency). These tests were conducted following enrollment and after completion of the 60-day phase. Tracking was measured by receipt of patients’ wear dairies that were e-mailed or faxed to the prescribing physician’s office weekly. The inclusion/exclusion criteria can be examined in Table 2 and the flowchart for that trial can be seen in Figure 2.
The longitudinal follow-up and tracking (12 months following the controlled trial) was chosen to measure permanence of changes but patients could choose surgery any time during that duration. After completion of the initial randomized trial, all control over treatments was removed. All patients were free to pursue any treatment methods they wished, including surgery, manual hand therapy, massage therapy, steroid injections, continued dynamic splinting, and so forth. Tracking of these treatment options were completed, and a Fisher’s analysis was completed on the follow-up, tracking data.
Results
The longitudinal outcome showed a statistically significant difference in choice of surgical outcome for experimental versus control patients (P = .425). In 12 months following completion of the controlled trial, 18 of 25 experimental patients (fit immediately with DS) avoided surgery, which was a 72% reduction. Only 9 of 25 control patients avoided surgery over 12 months following the initial trial, which was a 38% reduction. Following the 60-day trial, none of the secondary, unregulated treatments had a statistically significant effect on the outcomes of choice for surgery (P > .05) and this was calculated with repeated-measures analysis of variance (ANOVA).
In the previous controlled trial, the Levine-Katz symptom survey scores showed a significant reduction for experimental participants from 45.5 to 32.4 after 60 days (P < .001). Control group participants displayed increased symptom scores of 44.3 to 46.0 after 60 days.
Discussion
This modality showed a 2 to 1 reduction in the patients’ choice of surgery over 12 months following the controlled trial. This longitudinal outcome reflects findings from other studies.2,15 Baker et al stated in their conclusion said that “Results do suggest that a combined splinting/stretching treatment may be effective in reducing the incidence of CTS surgery.” Their study showed that following 24 weeks of treatment, 74.5% of those participants avoided choosing surgery.2
Efficacy of nonsurgical interventions was also studied by Mishra et al who examined static splinting compared with oral steroids.18 Their study of 40 participants used a static, neutral position splint for 4 weeks, and their study showed benefits in reduced symptoms and improved nerve conduction for both groups in a 3-month outcome. Nobuta examined static splinting on 171 hands with parabolic outcomes following a 7-month study.19 Their calculations showed that 41 hands reported excellent outcomes, 110 hands were good, 45 hands fair, and 18 hands were graded as poor. They concluded that static splinting was the most effective in low to moderate levels of severity, but that surgical release was still needed for severe CTS. Static splinting was beneficial but it does nothing to elongate the connective tissue. Dynasplint uses sequential tension changes to achieve lengthening of connective tissues.4
A systematic review by Huisstede et al found that none of the Standard of Care (SOC) treatments examined (this included oral steroids, steroid injections, ultrasound, electromagnetic field therapy, and static splinting) displayed long-term effects in treating CTS.12 If surgery is selected the duration of postoperative sick leave is important to consider. Hansen et al completed a prospective surgical outcome study examining the difference in “sick days” following endoscopic carpal tunnel release surgery.10 In this study, 75 employed, Danish patients averaged 20 days of sick leave and reported symptoms causing reducing work productivity for a mean of 10 months. A similar study by Katz et al showed that a significant percentage of patients were absent for 21 days following endoscopic carpal tunnel release.14
The controlled trial was effective in reducing symptoms and achieved 100% compliance over 60 days. Patients reported feeling the benefits of stretching that may have encouraged their compliance. After completion of the controlled trial, treatments over the second phase (12 months) were intentionally unregulated but were tracked.
Kier and Rempel discussed the anatomical relationships in the carpal tunnel, and their description of how hydrostatic pressure can be measurably changed by either increasing or decreasing the volume of the contents explains why prolonged, passive stretching of the transverse ligament reduces the symptoms of CTS.15 They elaborated that the structure of the carpal tunnel is easily compressed because it has an hourglass-shaped structure. This further explains the need for prolonged stretching with sequential changes in tension on the transverse ligament to reduce symptoms of CTS.
The cost savings with dynamic splinting versus surgery were abundant. In the location of this study, one endoscopic surgical procedure for treating CTS was reported to have a total cost of $6000 compared with this unit’s 2-month rental cost of $900. (The normal prescription for full reduction of symptoms is 4 months.) The cost of Dynasplint versus surgery in this study (excluding treatments after 60 days) yielded total costs of $64 500 for all experimental subjects versus $96 000 for all control subjects (see Table 3). One can also infer the greater savings in pain and workplace absence.10,13
Table 3.
Cost Analysis.
| CONTROL GROUP (N=25) |
| Cost of Surgery, 16 × $6,000 = $96,000 |
| Total Cost----------------------------------------------- $96,000 |
| EXPERIMENTAL GROUP (N=25) |
| Cost of Dynasplint (2 mo.) 25 × $900 = $22,500 |
| Cost of Surgery, 7 × $6,000 = $42,000 |
| Total Cost----------------------------------------------- $64,500 |
| Savings------------------------------------------------ $31,500 |
The limitations of this study include that only a small population was tested and that this trial was conducted at only one site. Another limitation is that the experimental treatment duration was limited to 60 days. A recent article has reported an even higher incidence of CTS as 3.51 per 100 person-years,11 and this further mandates the need for research in prevention and nonsurgical treatments. Future research should be conducted in a multicenter trial to measure effects with longer, 10-year durations of Dynasplint stretching treatment regimes.
This modality showed reduction of symptoms and a 2 to 1 benefit ratio in reduction of surgery over 12 months following the controlled trial, and this therapeutic modality should now be considered in the treatment plan for CTS.
Acknowledgments
The authors would like to thank Dr. Stacey Berner for his insights and mentorship before retirement, and Abimael Ramirez (student at McMurry University) for his help and support in this research study.
Footnotes
Ethical Approval: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with animal subjects.
Statement of Informed Consent: Informed consent was obtained from all individual participants included in the study.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Neither author has conflict of interest at this time. Neither author has received any earnings or compensation for this publication; nor will any earnings be awarded in the future. Dr. F.B.W. was employed by Galveston Clinical Research Foundation at the time of this study. He had a previous affiliation with the parent company of Dynasplint Systems, Inc. but the affiliation including all compensation or earnings were completed in 2013. B.F. was previously employed by Dynasplint Systems but her employment was also completed in 2013.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding to operate Galveston Clinical Research is obtained through book revenues, allowing this to be completely independent research.
References
- 1. Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282(2):153-158. [DOI] [PubMed] [Google Scholar]
- 2. Baker NA, Moehling KK, Rubinstein EN, Wollstein R, Gustafson NP, Baratz M. The comparative effectiveness of combined lumbrical muscle splints and stretches on symptoms and function in carpal tunnel syndrome. Arch Phys Med Rehabil. 2012;93(1):1-10. [DOI] [PubMed] [Google Scholar]
- 3. Berner SH, Willis FB, Martinez J. Treatment of carpal tunnel syndrome with Dynasplint: a randomized, controlled trial. J Med. 2008;1(1):90-94. [Google Scholar]
- 4. Berner SH, Willis FB, Shanmugam R. Pain from carpal tunnel syndrome reduced with dynamic splinting: a retrospective study of 156 patients. J Clin Med Res. 2009;1(2):22-25. [Google Scholar]
- 5. Burke FD, Wilgis EF, Dubin NH, Bradley MJ, Sinha S. Relationship between the duration and severity of symptoms and the outcome of carpal tunnel surgery. J Hand Surg Am. 2006;31(9):1478-1482. [DOI] [PubMed] [Google Scholar]
- 6. CDC.gov Occupational Safety. http://www.cdc.gov/mmwr/preview/mmwrhtml/00001423.htm
- 7. Ettema AM, Amadio PC, Cha SS, Harrington JR, Harris AM, Offord KP. Surgery versus conservative therapy in carpal tunnel syndrome in people aged 70 years and older. Plast Reconstr Surg. 2006;118(4):947-958;discussion 959-960. [DOI] [PubMed] [Google Scholar]
- 8. Furia JP, Willis FB, Shanmugam R, Curran SA. Systematic review of contracture reduction in the lower extremity with dynamic splinting. Adv Ther. 2013;30(8):763-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guyette TM, Wilgis EF. Timing of improvement after carpal tunnel release. J Surg Orthop Adv. 2004;13(4):206-209. [PubMed] [Google Scholar]
- 10. Hansen TB, Dalsgaard J, Meldgaard A, Larsen K. A prospective study of prognostic factors for duration of sick leave after endoscopic carpal tunnel release. BMC Musculoskelet Disord. 2009;10:Article 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris-Adamson C, Eisen EA, Kapellusch J, et al. Biomechanical risk factors for carpal tunnel syndrome: a pooled study of 2474 workers. Occup Environ Med. 2015;72:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huisstede BM, Hoogvliet P, Randsdorp MS, Glerum S, van Middelkoop M, Koes BW. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91(7):981-1004. [DOI] [PubMed] [Google Scholar]
- 13. Katz JN, Amick BC, III, Keller R, et al. Determinants of work absence following surgery for carpal tunnel syndrome. Am J Ind Med. 2005;47(2):120-130. [DOI] [PubMed] [Google Scholar]
- 14. Katz JN, Simmons BP. Clinical practice. Carpal tunnel syndrome. N Engl J Med. 2002;346(23):1807-1812. [DOI] [PubMed] [Google Scholar]
- 15. Keir PJ, Rempel DM. Pathomechanics of peripheral nerve loading. Evidence in carpal tunnel syndrome. J Hand Ther. 2005;18(2):259-269. [DOI] [PubMed] [Google Scholar]
- 16. Kerwin G, Williams CS, Seiler JG., III The pathophysiology of carpal tunnel syndrome. Hand Clin. 1996;12:243-251. [PubMed] [Google Scholar]
- 17. Levine DW, Simmons BP, Koris MJ, et al. A Self-Administered Questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75(11):1585-1592. [DOI] [PubMed] [Google Scholar]
- 18. Mishra S, Prabhakar S, Lal V, Modi M, Das CP, Khurana D. Efficacy of splinting and oral steroids in the treatment of carpal tunnel syndrome: a prospective randomized clinical and electrophysiological study. Neurol India. 2006;54(3):286-290. [DOI] [PubMed] [Google Scholar]
- 19. Nobuta S, Sato K, Nakagawa T, Hatori M, Itoi E. Effects of wrist splinting for carpal tunnel syndrome and motor nerve conduction measurements. Ups J Med Sci. 2008;113(2):181-192. [DOI] [PubMed] [Google Scholar]
- 20. Palmer KT, Harris EC, Coggon D. Carpal tunnel syndrome and its relation to occupation: a systematic literature review. Occup Med (Lond). 2007;57(1):57-66. [DOI] [PubMed] [Google Scholar]
- 21. Pomerance J, Fine I. Outcomes of carpal tunnel surgery with and without supervised postoperative therapy. J Hand Surg Am. 2007;32(8):1159-1163. [DOI] [PubMed] [Google Scholar]
- 22. Roquelaure Y, Ha C, Fouquet N, et al. Attributable risk of carpal tunnel syndrome in the general population: implications for intervention programs in the workplace. Scand J Work Environ Health. 2009;35(5):342-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rotman MB, Enkvetchakul BV, Megerian JT, Gozani SN. Time course and predictors of median nerve conduction after carpal tunnel release. J Hand Surg Am. 2004;29(3):367-372. [DOI] [PubMed] [Google Scholar]
- 24. Storey P, Armstrong D, Dear H, Bradley M, Burke F. Pilot randomised controlled trial comparing C-Trac splints with Beta Wrist Braces for the management of carpal tunnel syndrome. Hand Therapy. 2013;18(2):35-41. [Google Scholar]
- 25. Strickland JW, Gozani SN. Accuracy of in-office nerve conduction studies for median neuropathy: a meta-analysis. J Hand Surg Am. 2011;36(1):52-60. [DOI] [PubMed] [Google Scholar]
- 26. Wasiak R, Pransky G. The impact of procedure type, jurisdiction and other factors in workers’ compensation on work-disability outcomes following carpal tunnel surgery. Work. 2007;28(2):103-110. [PubMed] [Google Scholar]
- 27. Werner RA, Andary M. Electrodiagnostic evaluation of carpal tunnel syndrome. Muscle Nerve. 2011;44(4):597-607. [DOI] [PubMed] [Google Scholar]
- 28. Wilder-Smith EP, Seet RC, Lim EC. Diagnosing carpal tunnel syndrome—clinical criteria and ancillary tests. Nat Clin Pract Neurol. 2006;2(7):366-374. [DOI] [PubMed] [Google Scholar]
- 29. Wilgis EF, Burke FD, Dubin NH, Sinha S, Bradley MJ. A prospective assessment of carpal tunnel surgery with respect to age. J Hand Surg Br. 2006;31(4):401-406. [DOI] [PubMed] [Google Scholar]


