Abstract
Background: For patients on continuous IV unfractionated heparin (UFH), failing to achieve a therapeutic aPTT by 24 hours can be associated with increased morbidity. A pharmacy clinical surveillance system (PCSS) subtherapeutic aPTT alert was implemented at our institution to improve achievement of therapeutic aPTT goals by 24 hours.
Objective: The primary objective was the time to achieve the minimum goal aPTT before and after the alert implementation. The secondary objectives were to examine the percentage of patients who achieved the minimum goal aPTT by 24 hours and the number of dose changes to achieve the minimum goal aPTT.
Methods: A single-center retrospective study was conducted to include all adult inpatients receiving a continuous UFH infusion during a 3-month period prior to the implementation of a subtherapeutic aPTT alert and a 3-month period after implementation.
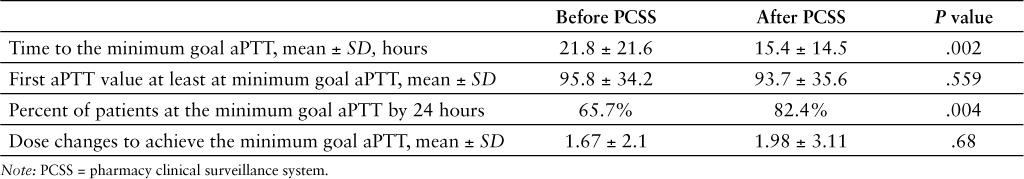
Results: 317 patients were included in the analysis. The average time to achieve the minimum goal aPTT was 21.8 hours prior to alert implementation and 15.4 hours after implementation (p = .002). The percent of patients who achieved the minimum goal aPTT by 24 hours was 65.7% prior to alert implementation and 82.4% after implementation (p = .035). The average number of dose changes necessary to achieve aPTT value to the minimum goal aPTT prior to alert implementation was 1.67 and 1. 98 after implementation (p = .68).
Conclusion: This analysis showed that implementation of a PCSS subtherapeutic aPTT alert for patients on continuous UFH infusions may ensure patients reach goal aPTT faster and facilitate a higher percent of patients who achieve the minimum goal aPTT by 24 hours.
Keywords: alert, aPTT, heparin, monitoring, pharmacy clinical surveillance system
Unfractionated heparin (UFH) is a preferred anticoagulant agent in clinical practice for several indications due to its safety in patients with renal dysfunction, its short half-life, and the availability of a reversal agent. An additional benefit is the ability to monitor the level of anticoagulation with readily accessible laboratory monitoring tools.1
For patients on continuous intravenous (IV) UFH, laboratory monitoring is recommended due to variable pharmacokinetics and pharmacodynamics related to nonspecific binding of heparin to proteins and endothelial cells and patient-specific variables including weight, age, thrombophilias, liver disease, lupus anticoagulants, and disseminated intravascular coagulation.2,3 Although there are known limitations, the most convenient and commonly used laboratory monitoring tool for heparin is the activated partial thromboplastin time (aPTT). The American Heart Association guidelines recommend obtaining an aPTT 6 hours after initiation and after each dose change to assess the effect of the current dose.4 Patients receiving continuous IV UFH are monitored to ensure they reach and maintain therapeutic aPTT values. It has been shown that patients anticoagulated for venous thromboembolism (VTE) who failed to achieve a therapeutic aPTT level by 24 hours can have increased morbidity, such as a higher risk for subsequent recurrent VTE.5–10
Historically, less than 25% of patients at our institution have achieved a therapeutic aPTT by 24 hours regardless of the indication. The target range for aPTT at our institution follows the 2012 CHEST guidelines.11 Implementation of a pharmacy clinical surveillance system (PCSS) with an alert specific to UFH could potentially increase the percent of patients achieving a therapeutic aPTT by 24 hours. PCSS are commonly used by health care facilities to implement a set of rules to identify at-risk patients and facilitate clinician interventions to meet therapeutic goals. One cross-sectional study showed the association between utilizing a PCSS within the hospital and lower mortality rates and lower costs.12 PCSS can be utilized by pharmacists to identify patients who would benefit most from pharmacists' interventions. In the literature, there are limited studies that compare the impact of pharmacists' interventions on clinical outcomes before and after utilization of a PCSS. Examples of these studies include antimicrobial stewardship programs and pharmacy clinical interventions related to cost savings initiatives, such as IV to oral (PO) conversion.13,14
At our institution, a PCSS has been utilized with the goal of improving patient care by integrating data from a variety of hospital clinical information systems to provide alerts in real time. Alerts in the PCSS can be created and modified by the institution. In March 2014, an alert was added to the PCSS at our institution to identify subtherapeutic aPTT values for patients on continuous IV UFH to enable pharmacists to make real-time interventions that maximize IV UFH efficacy while minimizing adverse events. Before implementing the subtherapeutic heparin aPTT alert in the PCSS, there were no system-generated real-time alerts for subtherapeutic aPTT values for patients on continuous UFH infusions.
METHODS
An observational single-center retrospective chart review was conducted using an electronic reporting system to identify all adult (18 years and older) inpatients at our institution receiving a continuous infusion of UFH. The study evaluated patients on continuous IV UFH for a 3-month period after the implementation of a subtherapeutic aPTT alert, allowing for a 5-month washout period (September–November 2014). The results were compared to the same 3-month period in the year prior to the implementation (September–November 2013).
The study excluded patients with UFH orders that did not include an aPTT goal, patients who were started on continuous UFH at an outside hospital, patients with an aPTT goal that included a value of less than 50 seconds, patients who received continuous UFH for less than 24 hours, and patients who received continuous UFH in the emergency department (ED) for more than 8 hours.
The primary endpoint was the time to achieve the minimum goal aPTT before and after the implementation of the subtherapeutic aPTT alert. The secondary objectives were to examine the percent of patients who achieved the minimum goal aPTT by 24 hours as well as the number of dose changes to achieve the minimum goal aPTT.
Approval from the institutional review board was obtained for all information gathered. Information collected from patients' electronic charts included patient demographics, indications for UFH, aPTT results, initial dose of continuous IV UFH (units/h), and number of dose changes to achieve a minimum goal aPTT.
Data are presented as mean ± standard deviation. A p value less than .05 was considered statistically significant. Chi-square test was used to compare categorical data and Student's t test was used to compare continuous data.
When the subtherapeutic heparin aPTT alert was added to the PCSS, aPTT values were transmitted to PCSS immediately after the laboratory result was made available in the hospital electronic system. Any aPTT value below 50 seconds for patients on a continuous UFH infusion were identified in the PCSS as subtherapeutic. Pharmacists analyzed the alerts and acted on them by assessing the heparin dose and aPTT goal with the purpose of making an intervention, if needed, in real time.
RESULTS
Overall, 1,045 patients were evaluated for inclusion. Of those, 728 patients did not meet the inclusion criteria and were excluded from the study (Figure 1). The majority of patients were excluded for not having an aPTT goal documented within the order (n = 603). Other reasons for exclusion included patients started on continuous UFH at an outside hospital (n = 60), patients who received continuous UFH for less than 24 hours (n = 41), patients who had an aPTT goal that included a value of less than 50 seconds (n = 17), and patients who received continuous UFH in the ED for more than 8 hours (n = 7). A total of 317 patients were included in the analysis, 156 patients were evaluated in the before implementation group, and 161 patients were evaluated in the after implementation group.
Figure 1.
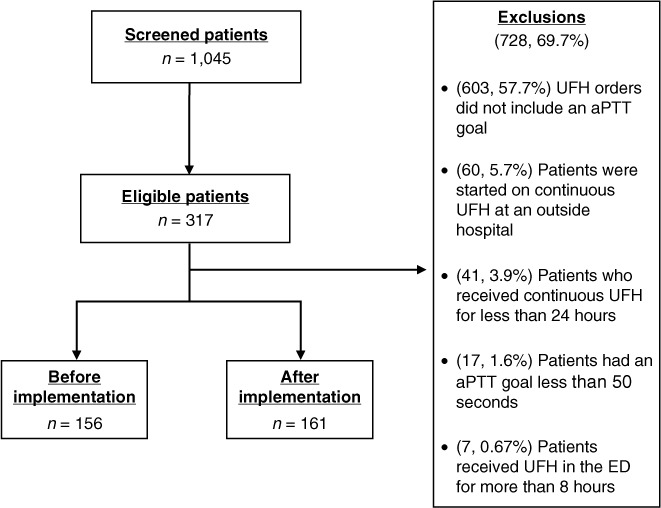
Screening and exclusion criteria. ED = emergency department; UFH = unfractionated heparin.
Baseline characteristics are represented in Table 1. The majority of patients, in both groups, were Caucasian (83% and 84%) and male (65% and 60%) in the before and after implementation groups, respectively, with no statistically significant difference. The mean age was similar in both groups, 67.9 years in the before implementation and 64.9 years in the after implementation group (p= .34). The mean actual body weight was 72.8 kg in the before implementation group and 84.3 kg in the after implementation group (p = .0001). The mean initial continuous UFH infusion dose was 986.7 units/h in the before implementation group and 1082.7 units/h in the after implementation group (p = .02). The most common indications for continuous UFH infusion therapy were atrial fibrillation and VTE.
Table 1.
Baseline characteristics
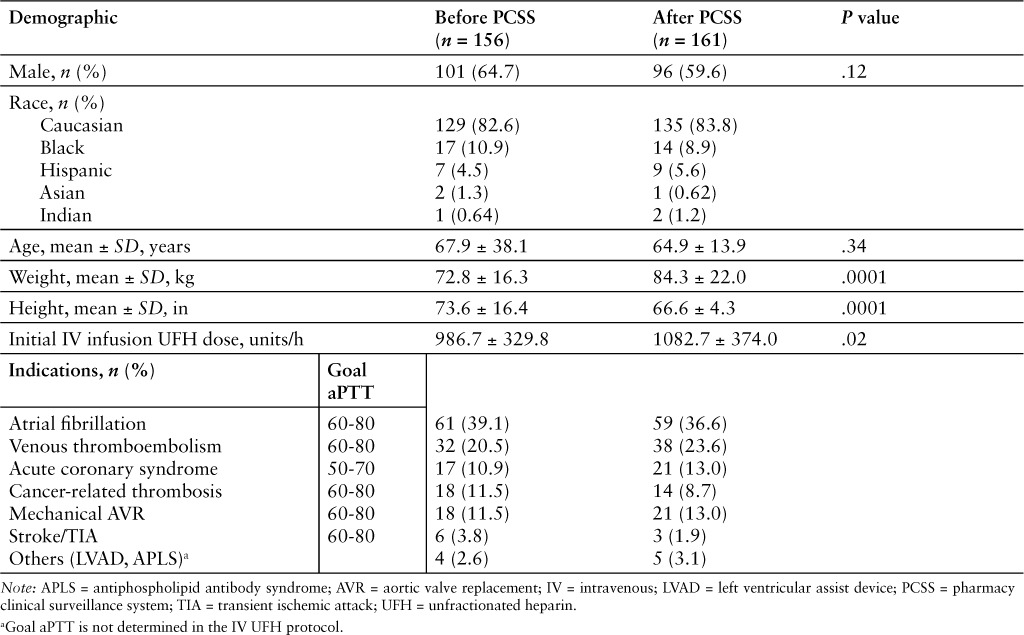
Table 2.
Effect of PCSS alert on aPTT
DISCUSSION
At our institution, heparin is dosed using the traditional approach of an ordering provider using a paper-based nomogram to adjust the infusion dose every 6 hours based on the aPTT. Previous quality improvement data from our institution have shown a delay in achieving therapeutic aPTT goals in patients receiving IV UFH. One reason for this may be that clinicians have to actively look for the aPTT result and adjust the dose, which may be delayed due to demanding clinician workload. As previous studies have shown, achieving a therapeutic aPTT by 24 hours is important to improve patient outcomes for patients receiving continuous infusions of UFH.5–10
In the literature, studies have suggested that some methods for achieving therapeutic aPTT values faster for patients on continuous UFH infusions include pharmacy-based, computer-assisted dosing of heparin; anti-Xa based protocols for UFH monitoring; weight-based nomograms; and nurse-driven heparin dosing nomograms.15–21 At the time of this study, our institution did not have these other mechanisms in place for dosing and monitoring UFH infusions.
Other data have shown that adding a PCSS positively impacts patient outcomes and creates opportunity for cost savings; however, this has mostly been focused on antimicrobial stewardship and cost savings initiatives.12–14 To our knowledge, implementation of a PCSS has not been directly assessed for monitoring patients on continuous UFH infusions. In this study, a PCSS alert for subtherapeutic aPTT results was evaluated as a method to facilitate achieving therapeutic targets faster by providing aPTT results to clinicians in real time, so that adjustments could be made immediately following the availability of laboratory results. Results of this study show that implementing a subtherapeutic heparin aPTT alert using a PCSS can decrease the time to reach a therapeutic aPTT. The impact was notable as the percent of patients who achieved an aPTT at goal by 24 hours was significantly higher after the implementation.
The limitations of our study merit some consideration. First, the study design includes a single center and was a retrospective design. Additionally, the study only analyzed a surrogate marker, aPTT goal, rather than safety and efficacy outcomes such as rates of thrombosis and bleeding. Lastly, our decision to exclude patients with UFH orders that did not include an aPTT goal resulted in excluding a large number of patients (57.7%). This was a retrospective study and the aPTT goal that was not included in the order may have been discussed during medical rounds, written in patient paper chart, or verbally communicated with the pharmacist. This issue has been resolved after implementing an order set for IV UFH that requires the clinician to define the aPTT goal for all IV UFH orders.
This study demonstrates the value of implementing a subtherapeutic aPTT alert at our institution. Results of this study are reflective of our institution and its specific heparin-related protocols. A future multicenter study is needed to confirm the benefit of adding a subtherapeutic heparin alert in a PCSS. This study should include clinical endpoints such as bleeding and thromboses. In addition to the subtherapeutic aPTT alert, another similar alert specific to supratherapeutic aPTT results could be implemented to ensure that dosing is not placing patients at risk of bleeding.
CONCLUSION
Our study suggests that implementation of a PCSS alert for subtherapeutic aPTT results is another strategy to ensure patients on continuous IV UFH reach their goal aPTT faster and facilitates a higher percent of patients reaching the minimum goal aPTT by 24 hours.
ACKNOWLEDGMENTS
The authors declare no conflicts of interest. No funding or financial support was received.
Footnotes
*Department of Pharmacy, Brigham and Women's Hospital, Boston, Massachusetts.
REFERENCES
- 1.Eikelboom J, Hirsh J. Monitoring unfractionated heparin with the aPTT: Time for a fresh look. Thromb Haemost. 2006;96:547–552. [PubMed] [Google Scholar]
- 2.Hirsh J. Low-molecular-weight heparin: A review of the results of recent studies of the treatment of venous thromboembolism and unstable angina. Circulation. 1998;98(15):1575–1582. doi: 10.1161/01.cir.98.15.1575. [DOI] [PubMed] [Google Scholar]
- 3.Olson J, Arkin C, Brandt J et al. College of American Pathologists Conference XXXI on laboratory monitoring of anticoagulant therapy: Laboratory monitoring of unfractionated heparin therapy. Arch Pathol Lab Med. 1998;122(9):782–798. [PubMed] [Google Scholar]
- 4.Hirsh J, Anand SS, Halperin JL et al. Guide to anticoagulant therapy: Heparin: A statement for healthcare professionals from the American Heart Association. Circulation. 2001;103(24):2994–3018. doi: 10.1161/01.cir.103.24.2994. [DOI] [PubMed] [Google Scholar]
- 5.Basu D, Gallus A, Hirsh J, Cade J. A prospective study of the value of monitoring heparin treatment with the activated partial thromboplastin time. N Engl J Med. 1972;287:324–327. doi: 10.1056/NEJM197208172870703. [DOI] [PubMed] [Google Scholar]
- 6.Hull RD, Raskob GE, Brant RF et al. Relation between the time to achieve the lower limit of the aPTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis. Arch Intern Med. 1997;157(22):2562–2568. [PubMed] [Google Scholar]
- 7.Raskob GE, Carter CJ, Hull RD. Heparin therapy for venous thrombosis and pulmonary embolism. Blood Rev. 1988;2(4):251–258. doi: 10.1016/0268-960x(88)90014-8. [DOI] [PubMed] [Google Scholar]
- 8.Turpie AG, Robinson JG, Doyle DJ et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med. 1989;320:352–357. doi: 10.1056/NEJM198902093200604. [DOI] [PubMed] [Google Scholar]
- 9.Hull RD, Raskob GE, Hirsh J et al. Continuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1986;315:1109–1114. doi: 10.1056/NEJM198610303151801. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri JF, Bonnet JL, Bouvier JL et al. Intravenous thrombolysis in myocardial infarction. Influence of the quality of the anticoagulation on the early recurrence rate of angina or infarction. Arch Mal Coeur Vaiss. 1988;81:1037–1041. [PubMed] [Google Scholar]
- 11.Garcia DA, Baglin TP, Weitz JI et al. Antithrombotic therapy and prevention of thrombosis (9th ed.) CHEST. 2012;141(2):e24S–e43S. doi: 10.1378/chest.11-2291. (suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amarasingham R, Plantinga L, Diener-West M et al. Clinical information technologies and inpatient outcomes. Arch Intern Med. 2009;169:108–114. doi: 10.1001/archinternmed.2008.520. [DOI] [PubMed] [Google Scholar]
- 13.Cannella C. Importance and impact of antimicrobial stewardship. Hosp Pharm. 2010;45(11 suppl 1):S1–S5. [Google Scholar]
- 14.Calloway S, Akilo H, Bierman K. Impact of a clinical decision support system on pharmacy clinical interventions, documentation efforts, and costs. Hosp Pharm. 2013;48(9):744–752. doi: 10.1310/hpj4809-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mungall D, Lord M, Cason S et al. Developing and testing a system to improve the quality of heparin anticoagulation in patients with acute cardiac syndromes. Am J Cardiol. 1998;82(5):574–579. doi: 10.1016/s0002-9149(98)00405-6. [DOI] [PubMed] [Google Scholar]
- 16.Kershaw B, White RH, Mungall D et al. Computer-assisted dosing of heparin. Management with a pharmacy-based anticoagulation service. Arch Intern Med. 1994;154(9):1005–1011. [PubMed] [Google Scholar]
- 17.Guervil D, Rosenberg A, Winterstein A et al. Activated partial thromboplastin time versus antifactor Xa heparin assay in monitoring unfractionated heparin by continuous intravenous infusion. Ann Pharmacother. 2011;45(7–8):861–868. doi: 10.1345/aph.1Q161. [DOI] [PubMed] [Google Scholar]
- 18.Oyen L, Nishimura R, Ou N et al. Effectiveness of a computerized system for intravenous heparin administration: Using information technology to improve patient care and patient safety. Am Heart Hosp J. 2005;3(2):75–81. doi: 10.1111/j.1541-9215.2005.04394.x. [DOI] [PubMed] [Google Scholar]
- 19.Toth C, Voll C. Validation of a weight-based nomogram for the use of intravenous heparin in transient ischemic attack or stroke. Stroke. 2002;33(3):670–674. doi: 10.1161/hs0302.104168. [DOI] [PubMed] [Google Scholar]
- 20.Brown G, Dodek P. An evaluation of empiric vs. nomogram-based dosing of heparin in an intensive care unit. Crit Care Med. 1997;25(9):1534–1538. doi: 10.1097/00003246-199709000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Williams TD, Sullivan K, Lacey C et al. Nurse-driven intravenous heparin protocol: Quality improvement initiative. AACN Adv Crit Care. 2010;21(2):152–161. doi: 10.1097/NCI.0b013e3181d2f2c6. [DOI] [PubMed] [Google Scholar]


