Summary Sentence
Studies employing genetically engineered mouse models indicate that RAF activation is sufficient to induce pancreas intraepithelial neoplasms (PanINs) suggesting that MEK inhibitor-based combination approaches may have clinical utility in patients with pancreatic ductal adenocarcinomas.
Thirty years have passed since missense mutations in RAS were first identified as the transforming factors in the Harvey and Kirsten strains of the Mouse Sarcoma Virus. Somatic mutations of all three RAS genes have since been shown to be among the most prevalent somatic alterations in human cancer. Studies using genetically engineered mouse models (GEMM) of pancreatic and lung cancer, among others, have confirmed that mutant RAS contributes to cancer initiation and maintenance of the transformed phenotype even in the setting of established, metastatic disease. These results have prompted intensive academic- and industry-led efforts to identify direct inhibitors of oncogenic RAS. These efforts have failed to date likely due to the high affinity of the RAS, GTP interaction as have efforts to selectively inhibit the post-translational modifications required for RAS activation. The latter approach was ineffective in KRAS and NRAS mutant tumors as geranylgeranyl modification can substitute for farnesylation in targeting KRAS and NRAS to the plasma membrane.
An alternate approach is to target the effector pathways responsible for RAS mediated transformation. Biochemical studies have identified over 20 distinct RAS effector molecules, the best characterized of which include the RAF proteins, the PI3 kinases and the RAL exchange factors. Our understanding of the contribution of individual RAS effectors to transformation remains incomplete but is likely influenced by the spatial/temporal availability of effectors, the presence or absence of extracellular stimuli and the pattern of co-incident mutational events. In this issue of Cancer Discovery, Collisson and colleagues set out to investigate which of the various RAS effectors are required for tumor initiation and progression in pancreatic ductal adenocarcinoma (PDA)(1). An in depth focus on PDA is justified by the high rate of KRAS mutation in this disease (>90%) and the urgent need to develop effective therapies for this common and almost universally lethal cancer.
Prior studies using GEMMs have shown that expression of mutant KRas leads to the formation of multi-focal pancreas intraepithelial neoplasms (PanINs)(2). Furthermore, co-incident loss of Ink4a/Arf or tp53 function results in the development of invasive pancreatic adenocarcinomas that phenocopy the human disease (3, 4). To determine whether RAF activation is sufficient to initiate pancreatic tumor formation, the authors generated mice with constitutive or conditional expression of BRafV600E in pancreatic cells. The V600E mutation accounts for over 90% of the BRAF mutations found in human tumors and locks the kinase into a constitutively active conformation. In the BRafCA mouse model generated by McMahon and colleagues, the BRafCA allele contains an insert which includes a floxed cassette containing exons 15–18 of human wildtype BRaf cDNA upstream of a modified exon 15 which harbors the V600E mutation (5). The wildtype BRaf allele is expressed prior to Cre-mediated recombination, but upon expression of Cre the wildtype exon 15–18 insert is excised and expression of BRafV600E is initiated under the control of the endogenous BRAF promoter. Targeted expression of BRafV600E using this model in the mouse lung leads to the development of benign lung tumors that progress to adenocarcinoma in the setting of concomitant loss of TP53 or Ink4A/Arf (5). Similarly, conditional melanocyte-specific expression of BRafV600E in mice using this model results in benign melanocytic hyperplasia, which in the setting of coincident Pten loss progresses to invasive melanoma (6).
In the current study, Collisson et al. express BRafV600E in the mouse pancreas by crossing BRafCA mice with mice that express Cre recombinase under the control of the p48/PTF1 gene (p48Cre; BRafCA/+), a pancreas-specific transcription factor expressed at E9.5. None of the progeny survived past weaning indicating that expression of BRafV600E is toxic for normal pancreatic development, a result which contrasts with the viability of the p48Cre; KRasLSL-G12D mice. To bypass the embryonic lethality observed in the p48Cre; BRafCA/+ model, the authors generated mice (Pdx1::CreERT2; BRafCA/+) in which conditional expression of BRafV600E is activated postnatally via tamoxifen-induced Cre recombinase activity driven by the Pdx-1/IPF1 promoter. In parallel, the authors used this system to generate mice expressing physiological levels of activated KRasG12D in the pancreas (Pdx1::CreERT2; KRasLSL-G12D). Notably, constitutive expression of activated BRAFV600E in the adult pancreas phenocopied oncogenic activation of KRasG12D, including the development of PanIN lesions lacking primary cilia in the exocrine pancreas, upregulation of the ductal marker cytokeratin 19, increased proliferation and increased expression of nuclear phosphorylated-ERK1/2. Moreover, concomitant expression of mutant p53 (Pdx1::CreERT2;BRafCA/+; Trp53LSL-R270H/+) resulted in the development of PDA with abundant stroma and desmoplasia similar to that seen in KRas/tp53 mutant PDA mouse models and human PDA.
In contrast, mice expressing mutant PI3 kinase alpha in the mouse pancreas (Pdx1::CreERT2; Pik3calat-H1047R) had no apparent phenotype. These results suggest that RAF but not PI3 kinase activation is sufficient to induce PanIN development. Limitations of this study include the possibility that oncogenic RAS does not exclusively activate p110α PI3 kinase and that the spectrum of downstream effectors activated by the kinase domain mutant may differ from that regulated by an activated wild-type allele. Furthermore, in light of recent findings that the Class IB PI3K p110γ isoform is overexpressed in PDA (7) and that deletion of Pten in mouse pancreatic centroacinar cells leads to ductal malignancy (8), the current data do not fully exclude the possibility that activation of PI3 kinase signaling by other mechanisms may be sufficient to induce PanIN formation.
Overall, the results imply that inhibition of RAF signaling may be an effective therapeutic approach in patients with KRAS mutant pancreatic tumors. Highly selective RAF inhibitors were recently shown to prolong the survival of patients with BRAFV600E melanoma. These agents, however, inhibit RAF activation in a mutant selective manner and are thus ineffective in tumors that expression activated RAS (9). Highly selective, allosteric inhibitors of MEK have also shown promising activity in BRAF mutant melanoma and provide an alternative approach to inhibiting ERK pathway activity in KRAS mutant tumors (10). To determine whether MEK inhibitors could inhibit ERK signaling at a non-toxic dose, Collisson et al. treated KRasLSL-G12D, Trp53LSL-R270H/+, p48Cre mice with PD0325901, an allosteric inhibitor of MEK1 and MEK2. Treatment with PD032901 potently downregulated ERK activity, as measured by a decline in phosphorylated ERK expression, indicating that sufficient intratumoral levels of the MEK inhibitor could be achieved at non-toxic doses to potently inhibit ERK pathway activation. This result is notable, as resistance of pancreatic tumors to systemic cytotoxic therapies has been attributed to limited drug exposure resulting from poor intratumoral perfusion. To determine whether sufficient ERK pathway inhibition could be maintained to induce meaningful antitumor effects, the authors turned to an orthotopic, syngeneic model of PDA. In this model, treatment with the MEK inhibitor was associated with downregulation of phosphorylated ERK expression and an improvement in survival. In sum, the results provide strong rationale for clinical trials of MEK inhibitors in patients with advanced pancreatic cancer, but also highlight the logistical challenges associated with the use of GEMMs for preclinical drug development.
Recently, the MEK inhibitor trametinib (GSK1120212) was shown to improve survival as compared to chemotherapy in a randomized trial of patients with metastatic melanoma whose tumors harbored V600E/K BRAF mutation (10). On the basis of these results, FDA approval for the use of trametinib in patients with BRAF mutant melanoma is anticipated. Notably, twenty-two patients with pancreatic cancer were treated with trametinib within the context of the phase 1 trial of this agent (11). One patient achieved a partial response although several additional patients were noted to have minor responses or stable disease. Although these results are disappointing in light of the GEMM studies reported by Collisson and colleagues, they are consistent with studies of human cancer cell lines performed by this group and others which show that in contrast to BRAF mutant cell lines which are with rare exception, sensitive to MEK inhibition, KRAS mutant cell lines exhibit variable sensitivity to MEK inhibitors. The basis for this heterogeneity of MEK-dependence in KRAS mutant cell lines has been explored in colorectal cancer cell lines and in this context can be attributed in part by the presence of PIK3CA co-mutation in some models (12). While PIK3CA mutations are rarely observed in pancreatic cancers, Collisson et al. show that MEK inhibition in KRAS mutant PDA cell lines is associated with a reciprocal increase in the expression of phosphorylated AKT and that co-treatment with a selective inhibitor of AKT is associated with synergy in many, but not all, models.
In sum, the results reported by Collisson et al. in concert with the clinical experience to date indicate that despite the sufficiency of RAF activation for PanIN development, MEK inhibitor based combination approaches will be needed to induce durable tumor regressions in most patients with KRAS mutant PDA. Future laboratory studies will be needed to define the molecular basis for the variable response of KRAS mutant PDA tumors to MEK inhibition, as such studies would aid in the development of rational MEK inhibitor based combination strategies.
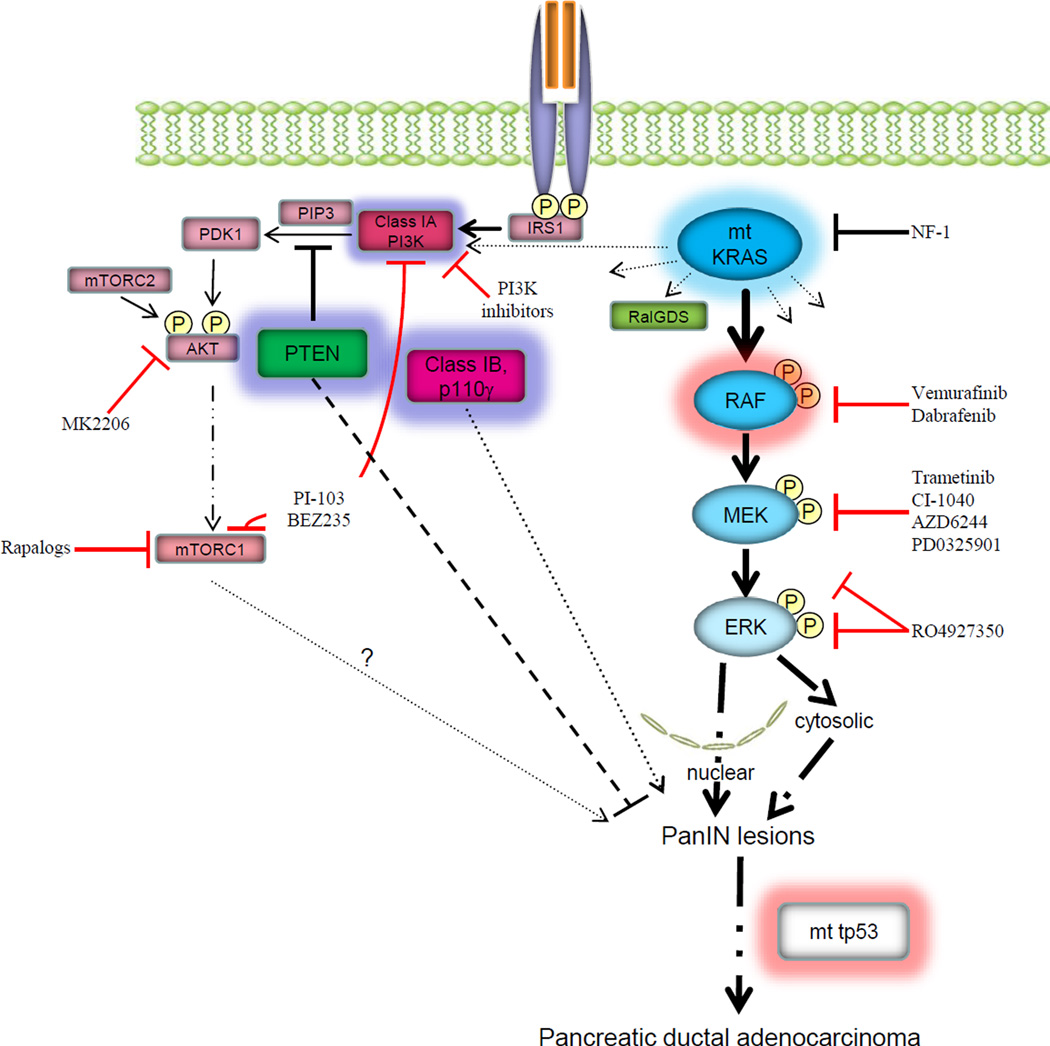
Figure 1. Targeting RAS effectors in pancreatic ductal adenocarcinoma (PDA.
Mutational activation of KRAS is found in >90% of PDA and contributes to tumor initiation and progression of the disease. PDA is almost universally fatal due to late-stage at diagnosis and intrinsic resistance to conventional chemotherapy and radiation. Data from Collisson and colleagues suggests that activation of BRAF (highlighted in red) is sufficient to recapitulate the tumor initiation and progression seen with activated KRAS in mouse and human PDA. These data imply that targeting RAF/MEK/ERK signaling downstream of KRAS may be of clinical utility in PDA. Treatment with MEK inhibitors is associated with a reciprocal increase in AKT activity and MEK inhibitor based combinatorial approaches will be needed to induce durable tumor regressions. Selective inhibitors of RAF (Vemurafinib, Dabrafenib) and MEK (Trametinib) kinases have recently been shown to prolong survival in patients with BRAFV600E mutant melanomas. Selective inhibitors of RAF induce a paradoxical activation of ERK signaling in KRAS mutant tumors whereas MEK inhibitors downregulate ERK pathway activity irrespective of tumor genotype.
References
- 1.Collisson EA, Trejo CL, Silva JM, Gu S, Korkola JE, Heiser LM, et al. A Central Role for RAF->MEK->ERK Signaling in the Genesis of Pancreatic Ductal Adenocarcinoma. Cancer discovery. 2012 doi: 10.1158/2159-8290.CD-11-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 3.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes & development. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes & development. 2007;21:379–384. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nature genetics. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edling CE, Selvaggi F, Buus R, Maffucci T, Di Sebastiano P, Friess H, et al. Key role of phosphoinositide 3-kinase class IB in pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:4928–4937. doi: 10.1158/1078-0432.CCR-10-1210. [DOI] [PubMed] [Google Scholar]
- 8.Stanger BZ, Stiles B, Lauwers GY, Bardeesy N, Mendoza M, Wang Y, et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Joseph EW, Pratilas CA, Poulikakos PI, Tadi M, Wang W, Taylor BS, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14903–14918. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. The New England journal of medicine. 2012 doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 11.Infante JR, Fecher LA, Nallapareddy S, Gordon MS, Flaherty K, Cox DC, et al. Safety and Efficacy Results from the First-Time-in-HUman Study of the Oral MEK 1/2 Inhibitor GSK1120212. Journal of Clinical Oncology, 2010 ASCO Annual Meeting Proceedings. 2010;28 [Google Scholar]
- 12.Halilovic E, She QB, Ye Q, Pagliarini R, Sellers WR, Solit DB, et al. PIK3CA mutation uncouples tumor growth and cyclin D1 regulation from MEK/ERK and mutant KRAS signaling. Cancer research. 2010;70:6804–6814. doi: 10.1158/0008-5472.CAN-10-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]