Abstract
Background: Although evidence suggests that methylxanthines may lower the seizure threshold, the effect of high-dose caffeine on seizure burden in preterm infants is not known. This study reports a secondary post hoc analysis of a randomized controlled trial of early high-dose caffeine citrate therapy in preterm infants, evaluating the effect of caffeine on the seizure burden using amplitude-integrated electroencephalography (aEEG).
Methods: Seventy-four preterm infants (≤30 weeks gestation) were randomized to receive high-dose (n = 37, 80 mg/kg over 36 hours) or standard-dose (n = 37, 30 mg/kg over 36 hours) caffeine citrate over the first 36 hours followed by standard maintenance therapy. Simultaneous recording of two-channel amplitude-integrated EEG was conducted over the first 72 hours of life. The primary outcome of this post hoc analysis was cumulative seizure burden over the first 72 hours of life, measured in seconds.
Results: Fifteen infants were excluded due to short recordings (≤5 hours) or corrupted data files (n = 7 standard dose; n = 8 high dose). The high-dose caffeine group displayed a trend toward an increased incidence of seizures (40% vs. 58%; p = 0.1) and a threefold increase in seizure duration (48.9 vs. 170.9 seconds; p = 0.1).
Conclusion: Early high-dose caffeine therapy was associated with a trend toward an increase in seizure incidence and burden. Future studies of alternative caffeine dosing regimens should include continuous EEG monitoring.
Keywords: : seizures, premature infant, EEG
Introduction
Prolonged mechanical ventilation is a well-established risk factor for the development of bronchopulmonary dysplasia (BPD) in premature infants and has led to the development of a number of strategies to prevent or shorten the duration of mechanical ventilation.1 Chief among those therapies is the use of methylxanthines (including theophylline, aminophylline, and caffeine citrate) to stimulate the respiratory center, increase responsiveness to hypercapnia, and prevent episodes of central apnea.2 As a result, caffeine citrate has gained widespread adoption, owing in part to equivalent enteral/parenteral dosing, better oral absorption, a wider therapeutic index, and longer half-life compared to aminophylline and theophylline. Standard dosing of caffeine citrate was established with a regimen consisting of a loading dose of 20 mg/kg followed by a maintenance dose of 5 mg/(kg·day).3
Meta-analysis of subsequent studies demonstrated a 60% reduction in risk of reintubation due to apnea and a 30% reduction in the risk of BPD with a number needed to treat of 10.4 Furthermore, caffeine exposure is associated with an improvement in neurodevelopmental outcome, with lower rates of cerebral palsy and visual-perceptive problems at 18–22 months, likely mediated through selective improvement of white matter integrity. It remains unclear, however, whether this is a direct effect of caffeine or a secondary effect from reduced episodes of apnea and hypoxia.5,6
Despite the overall success of caffeine treatment, a significant number of infants, nearly 30%, still fail early extubation. To address this shortcoming, higher doses of caffeine have been explored by some researchers and have shown short-term benefit, but with limited long-term neurodevelopmental outcome data.6,7
Although caffeine is generally considered to have a relatively benign safety profile in the dosages typically used in clinical practice, there is a concern, driven by adult literature and animal models, that methylxanthines lower the seizure threshold.8,9 Indeed, there have been case reports of parenteral caffeine citrate, with dosing in the range of 36–136 mg/kg, producing clinical seizures in full-term neonates.10 This inference is of significant concern in the preterm population, one which has routine caffeine exposure and a relatively high (∼40%) incidence of electrographic seizures, the underlying mechanisms of which are not well defined.11–13 It is not currently known how high-dose caffeine may alter the underlying seizure incidence or burden.
In this study, infants were randomized to standard- or high-dose caffeine treatment during the first 3 days of life to evaluate improvements in white matter microstructural development associated with early high-dose caffeine.14 This report represents a secondary analysis of this cohort, assessing the relationship between higher doses of caffeine and the incidence or burden of amplitude-integrated electroencephalography (aEEG)-detected seizures not explained by other clinical variables.
Materials and Methods
Patient selection
Infants born ≤30 completed weeks of gestation, by best obstetrical estimate, and who were admitted to the Neonatal Intensive Care Unit at St. Louis Children's Hospital were prospectively recruited within the first 24 hours of life for a double-blinded randomized control trial of early high-dose caffeine therapy to improve white matter microstructural development. Infants were excluded if they had a known congenital anomaly, respiratory failure (defined as mechanical ventilation with FiO2 > 0.8), or were not expected to survive past the first 72 hours of life. The study was reviewed and approved by the Human Research Protection Office at the Washington University School of Medicine and was registered on ClinicalTrials.gov (NCT00809055). Parents of included infants provided written informed consent before participation.
Procedures
Study drug therapy
Infants were randomized to high- or standard-dose caffeine therapy using parallel 1:1 blocked randomization, generated by the dispensing pharmacist who was not involved in clinical care. The clinical and research teams were blinded to the dosing.
The high-dose group received bolus dosing identical to that used in the trial conducted by Steer et al.,7 while the low-dose group received caffeine bolus dosing consistent with our established institutional practice. In the high-dose caffeine group, a total dose of 80 mg/kg was administered over the first 36 hours of life with a loading dose of 40 mg/kg, a second bolus dose of 20 mg/kg 12 hours later, and then 10 mg/kg at 24 and 36 hours after the initial dose. In the standard-dose caffeine group, a total dose of 30 mg/kg was administered over the first 36 hours of life with a loading dose of 20 mg/kg followed by 10 mg/kg doses at 24 hours after the initial dose. All caffeine citrate was given parenterally at a standard concentration of 20 mg/mL (Cafcit; Bedford Laboratories, Bedford, OH).
Given that the primary aim of the study was focused on the use of caffeine as a neuroprotective agent for white matter, which is thought to be most vulnerable in the first 72 hours after birth,15 only the bolus dosing was different between the groups.
All caffeine therapy was initiated within the first 24 hours of life. Caffeine doses were held for symptoms of caffeine toxicity, including tachycardia, jitteriness, tremors, clinically apparent seizures, and unexplained vomiting. All patients received caffeine citrate 10 mg/kg every 24 hours beginning 48 hours after the initial caffeine citrate dose and continued until resolution of apnea of prematurity as per the attending physician.
EEG recording
All recruited infants underwent continuous limited-channel aEEG monitoring (BRM3; Natus Medical, San Carlos, CA) from the time of enrollment through 72 hours of postnatal life using hydrogel electrodes in the standard C3-P3, C4-P4 configuration. Impedance was checked at least once per day. The recording was downloaded from the monitor and stored for later offline review (AnalyZe; Natus Medical).
Measures
Infant clinical characteristics
Clinical variables were abstracted from the clinical chart. Maternal factors included antenatal steroid administration, method of delivery, maternal age, and placental pathology. Infant characteristics included sex, gestational age, birth weight, race (by maternal report), sedating medications (fentanyl and midazolam) and inotropic medication exposure (dopamine, dobutamine, epinephrine), and the lowest recorded blood glucose during the first week of life. CRIB-II scores were calculated for each infant using the algorithm developed by Parry et al.16
Seizures
Seizures were defined as a series of monotonic form sharp waves, at least 10 seconds in duration, which evolve in frequency, amplitude, and morphology over time and are clearly distinguishable from the background or artifact.17 Status epilepticus was defined as a seizure >5 minutes in length or more than one seizure in a 5-minute period.18
A three-step approach was used to identify seizures. The recording was initially screened by a single investigator (Z.A.V.) for the presence of seizures by identifying a sudden change in the upper and lower margin of the time-compressed tracing19 or for regions marked by the automated seizure detection algorithm integrated into the aEEG monitor. Seizures were confirmed by examining the raw two-channel EEG trace at those time periods to confirm morphology or identify artifact. The remainder of the raw EEG trace was then manually inspected for seizure activity not detected by the automated algorithm or by changes in the baseline. Two additional investigators (A.M.M. and T.E.I.) reviewed overlapping subsets of the recordings to ensure consistent agreement on the presence of rhythmic patterns. Cumulative seizure burden (in seconds) was recorded on an infant-by-infant basis. Although the clinical team was blinded to the aEEG monitor, the diagnosis and treatment of any clinical seizures were recorded in the research record.
Outcomes
Short-term outcome measures potentially modified by an alternative caffeine regimen were evaluated, including total ventilator days, patent ductus arteriosus requiring treatment, BPD (defined as supplemental oxygen requirement at 36 weeks post menstrual age [PMA]), severe retinopathy of prematurity (requiring laser therapy), and death before discharge.
All infants underwent a nonsedated, noncontrast brain magnetic resonance imaging (MRI) at term equivalent age. Scans were reviewed for evidence of brain injury (intraventricular hemorrhage [IVH] and/or white matter injury) by a Neuroradiologist blinded to the caffeine dose. In addition, between 18 and 24 months corrected age, surviving infants returned for a standardized neurodevelopmental test using the Bayley Scales of Infant Development, Third Edition. Testing was performed by a developmental psychologist blinded to caffeine dosing.
Statistical analyses
A univariate comparison of perinatal, clinical, pharmacologic, and outcome factors was made between the high-dose and standard-dose groups using the Mann–Whitney Wilcoxon Test for continuous variables and a two-sided Fisher's Exact Test for categorical variables. Seizure burden and the logarithmic transformation of the seizure burden were compared using the Mann–Whitney Wilcoxon Test. Seizure incidence and status epilepticus incidence were compared between groups using Fisher's Exact Test. All statistical analyses were performed in R version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Sample size was determined by the primary study outcome (white matter diffusion changes measured by MRI at term equivalent age), namely the ability to detect a 5% difference in apparent diffusion coefficient in a single region of interest. Although the incidence of seizures in this population has been previously reported, the effect of caffeine has not previously been evaluated, precluding a priori calculation of statistical power given this sample size.
Results
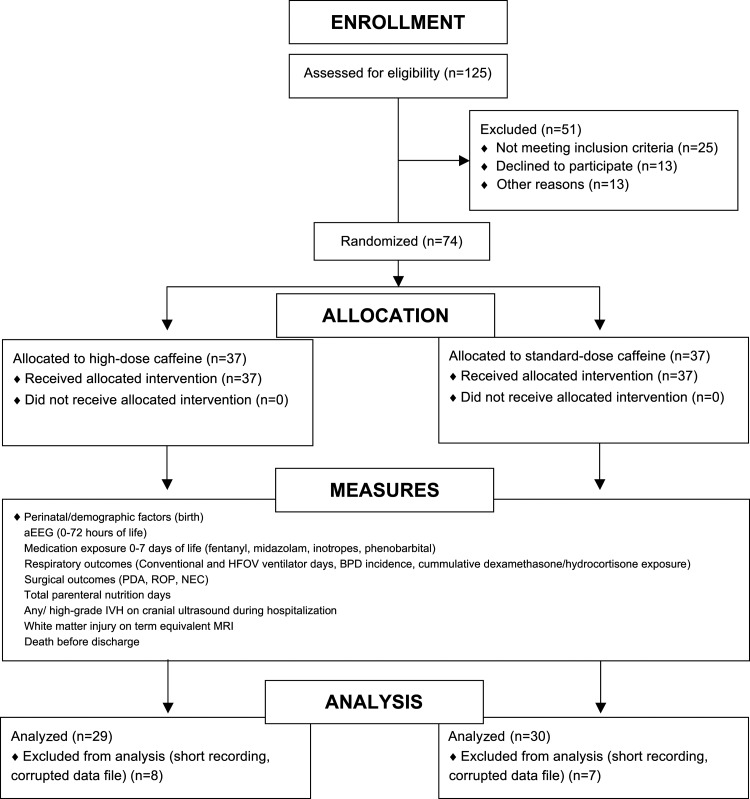
Of the 125 eligible infants admitted during the study window (November 2008 to June 2010), 37 infants were enrolled in each arm of the study for a total of 74 participants. Seven infants from the standard-dose arm and eight infants from the high-dose arm were subsequently excluded due to corrupt data files or recordings ≤5 hours in length. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Figure 1. The mean length of aEEG recording was 66 ± 17 hours and was started at a mean postnatal age of 11 ± 6 hours.
FIG. 1.
CONSORT diagram depicting sample size at each stage of the study. aEEG, amplitude-integrated electroencephalography; BPD, bronchopulmonary dysplasia; CONSORT, Consolidated Standards of Reporting Trials; HFOV, high frequency oscillatory ventilation; MRI, magnetic resonance imaging; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; ROP, retinopathy of prematurity.
Of the 59 infants analyzed, antenatal factors and baseline characteristics were generally similar between groups. Infants in the high-dose group were somewhat less mature and of lower birth weight, but this difference was not statistically significant. Usage of medications was similar between groups with the exception of greater fentanyl exposure in the high-dose caffeine group (Table 1). Outcomes were similar in both groups, in particular there was no difference in brain injury (IVH/white matter injury [WMI]), respiratory outcomes (ventilator days/BPD), or neurodevelopmental outcomes at 2 years (Table 2).
Table 1.
Sample Characteristics
| Standard-dose caffeine N = 30 | High-dose caffeine N = 29 | p | |
|---|---|---|---|
| Perinatal factors | |||
| Gestational age, mean (SD), weeks | 26.5 (1.9) | 25.8 (2.0) | 0.15 |
| Birth weight, mean (SD), g | 946 (248) | 840 (241) | 0.07 |
| Male sex, n (%) | 16 (53) | 16 (55) | 1.00 |
| Race | |||
| Caucasian, n (%) | 9 (30) | 14 (49) | 0.54 |
| African American, n (%) | 18 (60) | 12 (41) | |
| Hispanic, n (%) | 1 (3) | 1 (3) | |
| Asian, n (%) | 2 (7) | 2 (7) | |
| Maternal age, mean (SD), years | 24.7 (6.1) | 28.4 (7.7) | 0.07 |
| Antenatal steroids | |||
| None, n (%) | 4 (13) | 2 (7) | 0.67 |
| Complete, n (%) | 16 (53) | 13 (45) | 0.61 |
| Chorioamnionitis,an (%) | 12 (40) | 9 (31) | 0.59 |
| Vaginal delivery, n (%) | 10 (33) | 7 (24) | 0.57 |
| CRIB-II score, median (range) | 10 (3–19) | 12 (4–17) | 0.64 |
| Umbilical cord blood pH, mean (SD) | 7.29 (0.07) | 7.29 (0.11) | 0.59 |
| Clinical factors | |||
| Lowest recorded glucose, mean (SD), g/dL | 72.1 (28.4) | 66.7 (19.4) | 0.29 |
| 5-minute Apgar score, median (range) | 7 (3–9) | 6 (2–9) | 0.31 |
| Midazolam,b mean (SD), mg/kg | 0.01 (0.02) | 0.02 (0.05) | 0.35 |
| Fentanyl,b mean (SD), μg/kg | 12.9 (37.6) | 49.4 (93.5) | <0.01c |
| Inotropes,d median (range), hours | 1.2 (4.2) | 24.5 (63.9) | 0.16 |
| Surfactant administration, n (%) | 30 (100) | 29 (100) | 1.00 |
Histologic diagnosis.
Total medication exposure during the first week of life.
Denotes significance at p ≤ 0.05.
Combined inotrope exposure during the first week of life, including dopamine, dobutamine, and epinephrine. Comparisons made using Mann–Whitney U test for continuous variables or Fisher's Exact Test (two-sided) for categorical variables.
SD, standard deviation.
Table 2.
Outcomes
| Standard-dose caffeine N = 30 | High-dose caffeine N = 29 | pa | |
|---|---|---|---|
| Total ventilator days, mean (SD) | 11.7 (17.4) | 15.2 (22.4) | 0.49 |
| BPD, n (%) | 16 (53) | 14 (50) | 1.0 |
| Any IVH, n (%) | 7 (23) | 7 (24) | 1.0 |
| Grade III/IV IVH, n (%) | 2 (7) | 3 (10) | 0.67 |
| White matter injury, n (%) | 3 (10) | 5 (17) | 0.47 |
| PDA requiring treatment, n (%) | 17 (57) | 16 (55) | 1.0 |
| ROP, n (%) | 3 (10) | 0 (0) | 0.23 |
| Death before discharge, n (%) | 3 (10) | 7 (24) | 0.18 |
| BSID-III, age 2 years | |||
| Cognition, mean (SD) | 86.8 (8.4) | 86.1 (10.1) | 0.67 |
| Language, mean (SD) | 87.0 (10.5) | 85.3 (22.7) | 1.0 |
| Motor, mean (SD) | 86.7 (8.9) | 84.9 (11.4) | 0.32 |
Comparisons made using Mann–Whitney U test for continuous variables or Fisher's Exact Test (two sided) for categorical variables.
Denotes significance at p ≤ 0.05.
BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage; PDA, patent ductus arteriosus; ROP, retinopathy of prematurity.
Infants in the high-dose caffeine group had a trend toward a higher incidence of seizures (58% vs. 40%) and a threefold increase in seizure burden (48.9 vs. 170.9 seconds, p = 0.10 in both cases). Seizure burden in the low-dose group ranged between 0 and 240 seconds, compared to 0 and 2174 seconds in the high-dose group. There was no difference in the number of clinical seizures requiring treatment or status epilepticus (Table 3).
Table 3.
Seizure Outcomes by Caffeine Dose
| Standard-dose caffeine N = 30 | High-dose caffeine N = 29 | p | |
|---|---|---|---|
| Seizure incidence, n (%) | 12 (40) | 17 (58) | 0.19 |
| Seizure burden, mean (SEM), seconds | 48.9 (17.4) | 170.9 (75.4) | 0.10 |
| Log seizure burden, mean (SEM) | 1.70 (0.40) | 2.78 (0.48) | 0.10 |
| Clinical seizure requiring treatment, n (%) | 3 (10) | 2 (7) | 1.0 |
| Status epilepticus,an (%) | 0 (0) | 1 (3) | 0.49 |
Defined as a seizure greater than 5 minutes in length or more than one seizure in a 5-minute period.
SEM, standard error of the mean.
Discussion
The results of this secondary analysis of a double-blinded randomized control trial demonstrate that early high-dose caffeine treatment is associated with a greater incidence of electrographic seizures and greater total seizure burden, nearly a threefold increase, although this association did not achieve statistical significance. This association is of note, however, as there were no differences whatsoever in the outcome measures that might be expected to be improved by early high-dose caffeine therapy, namely the need for invasive ventilation or diagnosis of BPD.
The etiology of seizures in term infants is often identifiable and includes hypoxic–ischemic injury, intracranial hemorrhage, infection, or hypoglycemia.20 In contrast, although preterm infants display a higher incidence of electrographic seizures,11,12,21 there is a less clear etiology. IVH has been clearly associated with seizures in the preterm infant,22–24 and it is likely that hypoxic–ischemic injury is common and underrecognized. In this study, there was no difference in rates of IVH, hypoglycemia, or perinatal hypoxic ischemia (using the 5-minute Apgar score as proxy), leaving the difference in caffeine dose as the most likely explanation for the between-group difference in seizure burden. It is also important to note that the pathophysiology of the preterm infant brain may be primed for seizures by virtue of asynchronous development of excitatory (GABAA, NMDA, AMPA) and inhibitory (GABAB) channels leading to a greater likelihood of “kindling,” turning brief repetitive electrical discharges into electrographic seizures.25 It is plausible that high-dose caffeine may thus have greater impact in the immature brain on seizure threshold.
Although there is concern that seizures in preterm infants may be associated with adverse neurodevelopmental outcomes,12 current treatment guidelines do not exist and there is preliminary evidence that antiepileptic treatments may, in fact, cause depolarization of the cell membrane in immature neurons, potentially worsening seizures rather than treating them.26 To preserve clinical equipoise, the results of the aEEG recording were not available to the clinical team.
In this study, we utilized a multistep review of limited-channel aEEG recording for the detection of seizures. This approach yielded a seizure incidence in the control group similar to that reported in other studies using a similar methodology.11,12 While this differs from the seizure incidence reported in studies utilizing conventional EEG,27,28 we anticipate that the shift in toward an increased seizure burden would be consistent, regardless of the exact EEG technique utilized.
It is worthy of note that in this small study comparing high- and low-dose caffeine, we did not find any benefit for respiratory outcomes. Indeed, infants in the high-dose group had a greater mean number of ventilator days, likely also explaining the greater sedation exposure in the high-dose group. All other short-term outcomes were nearly identical between the groups.
An obvious limitation of this study is the sample size. Although there was a difference in seizure incidence and burden between the groups, there were too few subjects to achieve statistical significance. aEEG monitoring was performed prospectively in this study, but was the secondary outcome measure. Given the effect size of about 0.4 demonstrated in this current cohort, a study with 80% power and α = 0.05 would require ∼100 infants per study arm.
The outcome of this study should inform future studies of caffeine citrate therapy in preterm infants. Although this particular regimen did not demonstrate a difference in respiratory outcomes, an alternative approach may be successful, particularly in conjunction with new noninvasive ventilation strategies such as neurally activated ventilator assist (NAVA) and nasal interfaces specifically designed for infants <1000 g, which were not yet commercially available when this study was conducted. In addition to monitoring respiratory and neurodevelopmental outcomes, our findings would suggest that future studies of high-dose caffeine should consider the use of continuous EEG or aEEG monitoring and be powered appropriately to assess for any difference in seizure burden.
Acknowledgments
Financial support from Intellectual and Developmental Disabilities Research Center (IDDRC) at Washington University (NIH/NICHD P30 HD062171), Washington University Institute of Clinical and Translational Sciences KL2 Training Program (NIH/NCATS KL2 TR000450), Doris Duke Charitable Foundation, and National Institute of Child Health and Development (R01 HD057098; K12 HD055931-06).
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Martin RJ, Fanaroff AA, Walsh MC. Fanaroff and Martin's Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. Philadelphia, PA: Saunders/Elsevier; 2011 [Google Scholar]
- 2.Aranda JV, Turmen T. Methylxanthines in apnea of prematurity. Clin Perinatol. 1979;6:87–108 [PubMed] [Google Scholar]
- 3.Comer AM, Perry CM, Figgitt DP. Caffeine citrate: A review of its use in apnoea of prematurity. Paediatr Drugs. 2001;3:61–79 [DOI] [PubMed] [Google Scholar]
- 4.Henderson-Smart DJ, De Paoli AG. Methylxanthine treatment for apnoea in preterm infants. In: Cochrane Database of Systematic Reviews. The Cochrane Collaboration (Ed). Chichester, UK: John Wiley & Sons, Ltd; 2010: pp. 15–20 [DOI] [PubMed] [Google Scholar]
- 5.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. . Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902 [DOI] [PubMed] [Google Scholar]
- 6.Gray PH, Flenady VJ, Charles BG, Steer PA, Caffeine Collaborative Study Group. Caffeine citrate for very preterm infants: Effects on development, temperament and behaviour. J Paediatr Child Health. 2011;47:167–172 [DOI] [PubMed] [Google Scholar]
- 7.Steer P, Flenady V, Shearman A, Charles B, Gray PH, Henderson-Smart D, et al. . High dose caffeine citrate for extubation of preterm infants: A randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2004;89:F499–F503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu NS. Caffeine- and aminophylline-induced seizures. Epilepsia. 1981;22:85–94 [DOI] [PubMed] [Google Scholar]
- 9.Chrościńska-Krawczyk M, Jargiełło-Baszak M, Wałek M, Tylus B, Czuczwar SJ. Caffeine and the anticonvulsant potency of antiepileptic drugs: Experimental and clinical data. Pharmacol Rep. 2011;63:12–18 [DOI] [PubMed] [Google Scholar]
- 10.Banner W, Czajka PA. Acute caffeine overdose in the neonate. Am J Dis Child. 1980;134:495–498 [DOI] [PubMed] [Google Scholar]
- 11.Wikström S, Pupp IH, Rosén I, Norman E, Fellman V, Ley D, et al. . Early single-channel aEEG/EEG predicts outcome in very preterm infants. Acta Paediatr. 2012;101:719–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vesoulis ZA, Inder TE, Woodward LJ, Buse B, Vavasseur C, Mathur AM. Early electrographic seizures, brain injury, and neurodevelopmental risk in the very preterm infant. Pediatr Res. 2014;75:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis AS, Hintz SR, Van Meurs KP, Li L, Das A, Stoll BJ, et al. . Seizures in extremely low birth weight infants are associated with adverse outcome. J Pediatr. 2010;157:720–725.e1–e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McPherson C, Neil JJ, Tjoeng TH, Pineda R, Inder TE. A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr Res. 2015;78:198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle LW, Cheong J, Hunt RW, Lee KJ, Thompson DK, Davis PG, et al. . Caffeine and brain development in very preterm infants. Ann Neurol. 2010;68:734–742 [DOI] [PubMed] [Google Scholar]
- 16.Parry G, Tucker J, Tarnow-Mordi W. CRIB II: An update of the clinical risk index for babies score. Lancet. 2003;361:1789–1791 [DOI] [PubMed] [Google Scholar]
- 17.Scher MS. Controversies regarding neonatal seizure recognition. Epileptic Disord. 2002;4:139–158 [PubMed] [Google Scholar]
- 18.Lowenstein DH, Bleck T, Macdonald RL. It's time to revise the definition of status epilepticus. Epilepsia. 1999;40:120–122 [DOI] [PubMed] [Google Scholar]
- 19.Hellström-Westas L, Rosén I, Swenningsen NW. Silent seizures in sick infants in early life. Diagnosis by continuous cerebral function monitoring. Acta Paediatr Scand. 1985;74:741–748 [DOI] [PubMed] [Google Scholar]
- 20.Volpe JJ. Neurology of the Newborn. 5th ed. Philadelphia, PA: Saunders/Elsevier; 2008 [Google Scholar]
- 21.Scher MS, Aso K, Beggarly ME, Hamid MY, Steppe DA, Painter MJ. Electrographic seizures in preterm and full-term neonates: Clinical correlates, associated brain lesions, and risk for neurologic sequelae. Pediatrics. 1993;91:128–134 [PubMed] [Google Scholar]
- 22.Strober JB, Bienkowski RS, Maytal J. The incidence of acute and remote seizures in children with intraventricular hemorrhage. Clin Pediatr (Phila). 1997;36:643–647 [DOI] [PubMed] [Google Scholar]
- 23.Seay AR, Bray PF. Significance of seizures in infants weighing less than 2,500 grams. Arch Neurol. 1977;34:381–382 [DOI] [PubMed] [Google Scholar]
- 24.Shah DK, Zempel J, Barton T, Lukas K, Inder TE. Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr Res. 2010;67:102–106 [DOI] [PubMed] [Google Scholar]
- 25.Holmes GL, Ben-Ari Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr Res. 2001;49:320–325 [DOI] [PubMed] [Google Scholar]
- 26.Pressler RM, Mangum B. Newly emerging therapies for neonatal seizures. Semin Fetal Neonatal Med. 2013;18:216–223 [DOI] [PubMed] [Google Scholar]
- 27.Shellhaas RA, Clancy RR. Characterization of neonatal seizures by conventional EEG and single-channel EEG. Clin Neurophysiol. 2007;118:2156–2161 [DOI] [PubMed] [Google Scholar]
- 28.Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120:770–777 [DOI] [PubMed] [Google Scholar]