Abstract
Background
Standard imaging modalities are inaccurate in staging malignant pleural mesothelioma (MPM). Single institution studies suggest that volumetric computed tomography (VolCT) is more accurate but labor intensive. We established a multicenter network to test interobserver variability, accuracy (relative to pathologic stage) and prognostic significance of semi-automated VolCT.
Methods
Six institutions electronically submitted clinical and pathologic data to an established multicenter database on patients with MPM who had surgery. Institutional radiologists reviewed preoperative CT scans for quality then submitted via electronic network (AG mednet) to biostatistical center (BC). Two reference radiologists, blinded to clinical data, performed semi-automated tumor volume calculations using commercially available software (Vitrea Enterprise 6.0), then submitted readings to BC. Study endpoints included: feasibility of network; interobserver variability for VolCT; correlation of tumor volume to pTN stages, and overall survival (OS).
Results
Of 164 cases, 129 were analyzable and read by reference radiologists. Most tumors were <500cm3. A small bias was observed between readers, as one provided consistently larger measurements than the other (mean difference=47.9, p=.0027), but for 80% of cases, the absolute difference was ≤ 200cm3. Spearman correlation between readers was 0.822. Volume correlated with pTN stages and OS, best defined by 3 groups with average volumes of: 91.2, 245.3, 511.3cm3, associated with median OS of 37, 18, 8 months respectively.
Conclusions
For the first time, a multicenter network was established and initial correlations of tumor volume to pTN stages and OS shown. A larger multicenter international study is planned to confirm results and refine correlations.
Keywords: Computed tomography, staging mesothelioma
Accurate clinical staging of malignant pleural mesothelioma (MPM) is known to be problematic. Patients thought to have early stage, potentially resectable tumor by computed tomography (CT) and positron emission tomography (FDG-PET) are frequently found to have more advanced disease at surgical exploration.[1] Discrepancies between clinical and pathological staging are largely related to the diffuse and irregular anatomy of MPM which, unlike many solid tumors, precludes reliable single or bidimensional tumor measurements. In addition, the current primary tumor (T) staging system for MPM[2]is more easily applied to surgical and pathological rather than clinical staging because it defines the local extent of disease according to the depth and extent of involvement of the pleura and adjacent structures, features which are often hard to define on CT, FDG-PET or magnetic resonance imaging (MRI).
Nearly 20 years ago, Pass and colleagues reported the use of three-dimensional CT reconstructions of preresection tumor volume in MPM patients who underwent extrapleural pneumonectomy (EPP) or pleurectomy/decortications (P/D). [3]Tumor volume was found to be representative of T stage and to predict overall and progression-free survival. Since then, several studies have explored the use of manual and semi-automated methods of calculating tumor volume on CT, most frequently for response assessment in MPM patients receiving chemotherapy.[4–11] However, earlier studies required specialized equipment not available at most institutions and were highly labor intensive. The advent of commercially available radiology software and improvements in radiology workflow through the use of hybrid workstations now potentially permit efficient and reproducible calculations of tumor volume in MPM patients.[12]
Better staging of MPM would improve patient selection for treatment and evaluation of outcomes in a notoriously difficult malignancy. The International Association for the Study of Lung Cancer (IASLC) and the International Mesothelioma Interest Group (IMIG) have collaborated on the development of a large international database (“IASLC MPM database”) that is being used to inform revisions of the MPM staging system in the upcoming 8th editions of the AJCC (American Joint Commission on Cancer) and the UICC (International Union Against Cancer) staging manuals.[1,13] This clinical database, coordinated and analyzed by a well established biostatistical center, Cancer Research and Biostatistics (CRAB), offered the opportunity to create a multicenter network to evaluate volumetric CT in the context of TNM staging for MPM. The present feasibility study, a collaboration among six North American institutions, is the prelude to a planned larger international study.
MATERIALS AND METHODS
The multicenter network
Participating investigators represented six North American institutions that treat a high volume of MPM patients, including Memorial Sloan Kettering Cancer Center, New York University, the University of Texas MD Anderson Cancer Center, the University of Pennsylvania, the University of Chicago, and the University of Toronto. Each site had a designated thoracic surgeon or oncologist, and a diagnostic radiologist. Two reference radiologists, one from the Brigham and Women’s Hospital and one from New York University, and both highly experienced in reading CT scans in MPM patients, were responsible for performing the volumetric CT readings. The sites submitting scans and data had Institutional Review Board (IRB) approval from their institutions and appropriate data transfer agreements in place. IRB waiver was obtained at the sites analyzing the volumetric CT scans.
Study Objectives, Patient Eligibility and Data Flow
The primary objective of the study was to determine the feasibility of developing a multicenter network to use volumetric CT as a uniform measurement of clinical T stage in newly diagnosed MPM. Secondary objectives were to determine interobserver variability in estimating tumor volume; to estimate the difference between standard CT and volumetric CT in assessing the clinical T stage; to determine whether tumor volume corresponds to pathologic T and N stages; and to determine whether tumor volume correlates with overall survival (OS) and histological subtype. OS was measured from the date of surgery. The study was not designed to assess the impact of VolCT in prognostic models including known important variables other than tumor stage.
Eligible cases were required to have pathologically proven MPM and to have had a preoperative chest CT scan followed by surgical exploration providing definitive surgical and pathological staging within 30 days of the CT. In order to generate information in a timely manner for this feasibility study, retrospective clinical and imaging data were used. Therefore, operations were performed between January 1, 2005 and December 31, 2013. Previously untreated patients were preferred but for patients who received induction chemotherapy, the CT submitted was required to be the post chemotherapy, immediate preoperative CT. Patients receiving preoperative radiotherapy were excluded. Contrast CT scans with 3mm cuts were preferred but non-contrast scans and cuts up to 5mm thickness were accepted. Allowing up to 5mm thickness cuts took into consideration that during the time frame of this study many Radiology departments did not routinely retain finer cuts in recording CT imaging. Submitted cases were required to have all of the essential clinical information electronically submitted to the IASLC MPM database.
The study schema is shown in Figure 1. The surgeon and radiologist at each institution selected eligible cases. Each CT was reviewed by the participating institutional radiologist to ensure that it was of adequate quality for submission. The institutional radiologist also assigned a clinical T stage after reviewing each scan. After clinical data (including clinical and pathological stage information) were verified to be complete at CRAB, CT scans devoid of patient identifiers were submitted to CRAB via a secure internet connection using AG Mednet (www.agmednet.com). The two reference radiologists were then provided access to the scans by CRAB and independently performed their reviews which were then submitted back to CRAB for assessment of interobserver variability and correlation to clinical data. Volumetric CT assessment was performed by each reference radiologist using Vitrea Enterprise suite 6.0 (Vital Images, Minnesota, USA), a PowerPoint presentation detailing the methodology for performing volumetric assessment was created and shared between the two reference radiologists prior to the volumetric assessment. A demonstration of the technique was performed with a detailed discussion on exclusion of effusion and manual inclusion of any discontinuous areas of tumor and correction of the semi-automatic segmentation (based on Hounsefield units ranging between 20–80 HU) to include all areas of pleural thickening representing tumor. Three WebEx sessions with detailed discussion and demonstration of the methodology, using 8 training cases were organized prior to the volumetric assessments. An independent consensus root cause analyses of the discrepant cases were performed after the final statistical analysis by the 2 reference radiologists.
Figure 1.
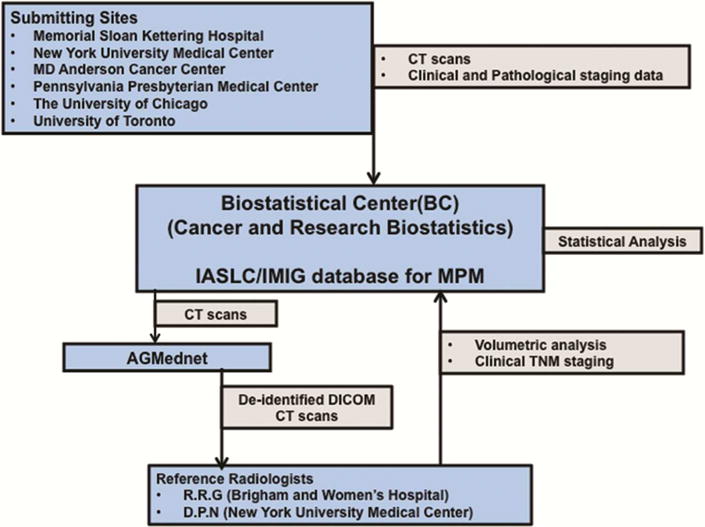
Study schema showing data flow for multicenter study of volumetric CT for staging malignant pleural mesothelioma
Statistical Considerations
A minimum of 120 cases (15 to 30 from each institution) was considered necessary to meet the primary and secondary objectives, in particular the estimation of interobserver variability and the correlations between volumetric CT T stage and surgical/pathological Tstage which would be critical to planning a subsequent larger scale trial. The surgical/pathological T stage was considered the “gold standard” for statistical assessment. The planned sample size estimated the endpoints with reasonable statistical precision as measured by the 95% confidence intervals for the estimates. Spearman correlation was used to compare volume estimates by the two reference radiologists. Histogram analyses of volume distributions were compared between both reference radiologists. The relationship of volumetric CT to other secondary endpoints was considered the subject of exploratory analyses only.
RESULTS
A total of 164 cases were registered to the study. Eight cases were used as training cases and 2 cases were excluded based on CT quality (one due to metal artifact, and one due to poor image quality). Out of the 154 cases with CT quality deemed adequate, both reference radiologists analyzed 130 and one case was excluded from the final analysis as it was an extreme outlier with respect to volume calculations between the reference radiologists. On subsequent consensus review, this difference was found to be secondary to data entry error. Thus, 129 cases were examined by both reference radiologists and form the basis for this report. Clinical stage as defined by the institutional radiologists, final surgical/pathological stage and tumor histology data are shown in Table 1. The majority of patients had either clinical stage II (29%) or III (49%) disease. Considerable tumor upstaging occurred at surgery with most patients having either stage III (42%) or IV (45%) disease. The most common histological subtype was epithelioid (81% of cases). Most submitted scans (n=74, 57%) had a slice thickness of 5 mm with the remainder having slice thicknesses ranging from 2.5 to 3.5mm.
Table 1.
Data for clinical stage (as defined by the institutional radiologists), surgical/pathological stage
| Number of cases (%) | |
|---|---|
| Clinical Stage | |
| I | 4 (3.1%) |
| II | 37 (28.7%) |
| III | 63 (48.8%) |
| IV | 18 (14.0%) |
| Pathological Stage | |
| I | 4 (3.1%) |
| II | 9 (7.0%) |
| III | 54 (41.9%) |
| IV | 58 (45.0%) |
| Histological Subtype | |
| Epithelioid | 105 (81.3%) |
| Biphasic | 12 (9.3%) |
| Sarcomatoid or NOS1 | 12 (9.3%) |
Incomplete staging data from participating sites at the level of T and N descriptor detail required by the IASLC electronic data capture system were available in a small number of cases (4 surgical/pathological stage). For study purposes, clinical T stage was assigned in all cases by the institutional radiologists and was again re-read in a blinded manner by the reference radiologists.
NOS = not otherwise specified.
Radiology-specific results are reported elsewhere and are not discussed in detail here.[14] Briefly, despite their considerable experience in reading CT scans in MPM patients, the reference radiologist frequently differed in their estimate of clinical T stage (using the AJCC, TNM criteria) with one assigning T3 or T4 to 80% and the other to 28% of patients.
With respect to CT volume calculations, the majority of tumors were confirmed to be <500cm3 (84 by both readers, 103 by at least one reader). In 80% of cases the absolute difference in volume between the two radiologists was ≤ 200cm3 and in more than 40% of cases the difference was less than ± 60cm3. Approximately 67% agreement was seen between the two readers when measured in the context of the 4 volume categories. The overall correlation between the two radiologists was good (Spearman Corr.=0.822). A small measurement bias was observed between the readers, as one reader tended to provide consistently larger measurements than the other (mean = 47.9, paired t-test for no difference p= .0027). Detailed root cause analyses of the discrepant cases were performed after the final statistical analyses and have been discussed in detail in the Radiology manuscript.
Given the strong predominance of epithelioid tumor histology in this study, it was not feasible to measure the association between tumor volume and histology. However, as shown in Figures 2a and 2b, volume correlated with surgical/pathological T stage (range of median volumes = 23.8cm3 for pT1 to 387.9cm3 for pT4) and with pN stage. Tumor volume also correlated with OS. We did not explore linear or other functional relationships between the continuous volumetric CT measurements and overall survival as we feel this would extend beyond realistic expectations of the data. However, a trend between volumetric CT and survival can be seen in Figure 3 with largest tumors (volumetric CT >511.3 cm3) having the shortest median survival and the smallest tumors (<91.2 cm3) having the longest median survival. Initially this was assessed by dividing tumor volume into quartiles. Survival was very similar for tumors between the first and third quartile, which suggested to us that collapsing these cases into a single group would provide the best fit. The log-rank p-value without the context of Figure 3 would certainly not be enough to support statements about observed differences between groups. However, given the context of Figure 3, it is plain to see where those differences occur. Pairwise p-values confirm the visual observations ne(Table 2). Given the small number of cases, we believe that these results provide reasonable evidence of a trend in overall survival for a 3 group model.
Figures 2.


a and b: Correlation between tumor volume and pathologic T stage (Figure 2a). There are too few T1 cases to provide reliable estimates for this group but otherwise data suggest that tumor volume increases with pathologic T category. Correlation between tumor volume and pathologic N stage (Figure 2b). Only N0, N2 and NX had sufficient numbers to provide reliable estimates but results indicate that volume measurements for N2 tumors are larger than those measured for N0 tumors.
Figure 3.
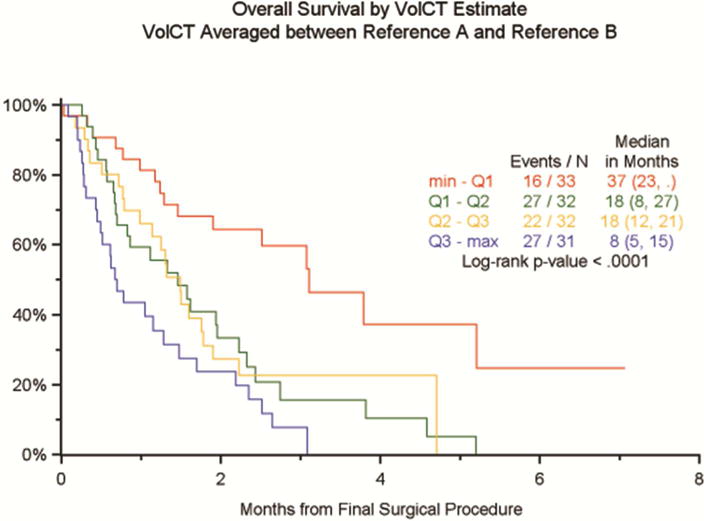
Initial separation of tumor volumes into quartiles show that the best correlation between tumor volume and overall survival is seen with three groups of average volume measurements (91.2, 245.35 and 511.35cm3).
Table 2.
Pairwise p values confirming visual observations in Figure 3.
| Pairwise Comparison | Pairwise log-rank p-value |
|---|---|
| min – Q1 vs Q1 – Q2 (red vs green) | 0.0018 |
| Q1 – Q2 vs Q2 – Q3 (green vs yellow) | 0.7350 |
| Q2 – Q3 vs Q3 – max (yellow vs blue) | 0.0639 |
COMMENT
To our knowledge, this is the first study to show the feasibility of creating a multicenter network aimed at evaluating the use of volumetric CT in the clinical staging of MPM. Our experience defines factors that will be critical for the design of a larger definitive trial and demonstrates relationships between tumor volume, surgical/pathological stage and OS that have been suggested by previous single institution studies. We show that the known inaccuracies and variability in clinical T staging based on assessment of standard CT may be obviated or supplemented by the use of volumetric evaluation.[1]
The seminal paper by Pass et al. in 1998 included 48 patients who underwent cytoreductive surgery by either EPP (n=25) or P/D (n=23) and had three dimensional CT reconstructions of preresection and postresection tumor volume.[3] Median OS correlated significantly with pre- and postoperative tumor volumes, reflecting the impact of both the initial tumor burden and the extent of cytoreduction. Patients who had a preoperative tumor volume less than 100 cm3 had a median OS of 22 months while those with a larger tumor volume had a median OS of 11 months. The presence of lymph node metastases, the tumor stage and progression-free survival also correlated with preoperative tumor volume. However, calculations of tumor volume required that a single radiologist manually outline tumor borders with a hand-controlled mouse on an individual work station which was then used to calculate three-dimensional volume. The technological limitations at that time meant that these individual, highly labor intensive results could not be extrapolated to a multicenter setting. Nonetheless, they prompted an interest in this area and stimulated efforts to develop more automated and reproducible methods of assessing tumor volume in MPM.
During the past decade, efforts have focused on developing and evaluating less labor intensive, semi-automated methods of volumetric CT analysis.[4,6–11,15] All of these reports are small, single institution studies utilizing for the most part “home grown” software algorithms for volumetric estimation, and frequently focusing on response assessment in patients receiving chemotherapy. A more recent single institution study applied the commercially available software used in this study to volumetric CT assessment of 88 MPM patients who underwent EPP at the Brigham and Women’s Hospital.[12] By univariate and multivariable analyses that included clinical variables (e.g. hemoglobin, platelet count and adjuvant therapy) known to be important, tumor volume was independently associated with OS. The most significant breakpoint in OS appeared to be a tumor volume of 500cm3 or greater. The current study supports a tumor volume greater than 500cm3 as being associated with a poor OS but, somewhat in line with the report from Pass and colleagues, also identifies low and intermediate volume groups that have significantly different outcomes.
Technological advances including commercially available software for volumetric CT evaluation, internet-based systems that allow transmission and sharing of data-dense imaging studies in ways that maintain patient confidentiality, and improvements in radiology work flow now enable this type of multicenter international study. The involvement of a biostatistical center highly experienced in clinical trials and in managing large datasets is also pivotal to study feasibility. The availability of the large, high quality IASLC MPM database utilizing electronic data submission will be essential for accurate clinical correlations. However, the current study highlights areas that should be addressed in the planned, larger international study. Stricter criteria for CT quality with the routine use of intravenous contrast and retention of fine cut CT images (2.5mm or less in thickness) will enhance radiological interpretation.[16] The addition of a third experienced reference radiologist, more extensive group training in software use and further standardization in reporting may reduce variations in final volume calculations.
Refinement of volumetric CT in the clinical staging of MPM and incorporation of this into routine clinical practice has implications that are not merely academic. Large numbers of MPM patients are too elderly or too frail to be considered for major surgical resections. With an increasing range of nonsurgical treatment options for MPM including new chemotherapies, immunotherapy and novel radiotherapy approaches, accurate clinical staging for treatment selection and accurate assessment of response have become important. For better risk patients, more precise methods of clinical staging are needed to select surgical intervention and multimodality therapy. Although other imaging modalities such as magnetic resonance imaging (MRI) and PET have been evaluated for staging and response assessment, CT is less costly and more widely available.[17,18] Therefore, validation of our results in a larger international study will likely have a significant impact on the management of this challenging disease.
DISCUSSION
170. A MULTICENTER STUDY OF VOLUMETRIC COMPUTED TOMOGRAPHY FOR STAGING MALIGNANT PLEURAL MESOTHELIOMA. Paper presented by Harvey I. Pass, M.D., New York, New York. E-mail: harvey.pass@med.nyu.edu
Discussion by Todd L. Demmy, M.D., New Brunswick, New Jersey.
E-mail: todd.demmy@cinj.rutgers.edu
Dr. T. Demmy (New Brunswick, New Jersey):
Great presentation. One of the cool things about this software is the ability to not only calculate a volume but also the shape, maybe even create an STL file for 3D printing.
Do you plan to take this to another level, not just looking at volume but patterns of growth or regression, shapes of how the tumor spreads that might provide some sort of prognostic information? Thank you.
170. A MULTICENTER STUDY OF VOLUMETRIC COMPUTED TOMOGRAPHY FOR STAGING MALIGNANT PLEURAL MESOTHELIOMA. Response by Harvey I. Pass, M.D., New York, New York. E-mail: harvey.pass@med.nyu.edu
DR. PASS: Yes. I think that would be very interesting to do. It’s a longitudinal study to see how the tumors regress so that you can get an idea with not only surgical patients but chemo patients.
I think that this will also be important to actually serve as a quality control for the operations on mesothelioma because you can also take this and measure it after you’ve operated on the patient and look at those subtle changes and then see how much volume you have left and see if that correlates with survival.
With regard to the reconstructions and being able to figure out where things are, that’s a future hope.
DR. DEMMY: One other quick comment is that one can actually print the 3D rib cage anatomy to plan an adjuvant therapy like PDT. Such preplanning would enhance use of onlay adjuvant therapy after an EPP or radical decortication. DR. PASS: I’m hoping that those three-dimensional images will also give us a greater idea that could compete with MRI with regard to invasion.
170. A MULTICENTER STUDY OF VOLUMETRIC COMPUTED TOMOGRAPHY FOR STAGING MALIGNANT PLEURAL MESOTHELIOMA. Paper presented by Harvey I. Pass, M.D., New York, New York. E-mail: harvey.pass@med.nyu.edu
Discussion by Joseph S. Friedberg, M.D., FACS, Baltimore, Maryland.
E-mail: jfriedberg@smail.umaryland.edu
Dr. J. Friedberg (Baltimore, Maryland):
Harvey, that was fantastic. I was just wondering. As we were discussing the other day, sometimes these tumors are rock hard, some of them are kind of soft. I mean, you started this whole thing with the volumetrics. Have you ever looked at the density of the tumors?
170. A MULTICENTER STUDY OF VOLUMETRIC COMPUTED TOMOGRAPHY FOR STAGING MALIGNANT PLEURAL MESOTHELIOMA. Response by Harvey I. Pass, M.D., New York, New York. E-mail: harvey.pass@med.nyu.edu
DR. PASS: I think that’s a really good point, and I think that CT can do many things in 2015. We can not only measure volume, but in lung cases we can actually segment, and we can see the histologic characteristics by using segmentation techniques like CANARY that you’ll read from the Mayo Clinic that you can actually prognosticate based on a part-solid nodule.
I think you can apply these techniques to this and be able to see histologic, potentially morphologic changes that may characterize a hard bulky versus a soft bulky, which we all know the soft bulkies are what we always want.
DR. FRIEDBERG: Because we all suffer, and I just presented the thing where I think we had an 18% surgical biopsy rate that said it was epithelial, and then you take out the whole thing and you have these sarcomatous elements, which I wouldn’t have operated had I known. And they do tend to be harder, at least has been empirically my experience, and I wonder if this would be the way to get around that.
DR. PASS: I don’t know whether that will actually be that refined with regard to the CT being able to segment. I think that MRI is doing a pretty good job now of trying to do this, distinguishing biphasic from epithelial, and that’s going to continue.
I think that the ability of CT, besides volume, will be able to segment and see characteristics that are different that may add to the volumetric measurement with regard to prognosis.
DR. FRIEDBERG: This was great. Thank you.
170. A MULTICENTER STUDY OF VOLUMETRIC COMPUTED TOMOGRAPHY FOR STAGING MALIGNANT PLEURAL MESOTHELIOMA. Paper presented by Harvey I. Pass, M.D., New York, New York. E-mail: harvey.pass@med.nyu.edu
Discussion by Wickii T. Vigneswaran, M.D., M.B.A., Maywood, Illinois.
E-mail: wickii.vigneswaran@lumc.edu
Dr. W. Vigneswaran (Maywood, Illinois):
We are currently looking at the density of tumor using Hounsfield units on the CT scan. There seems to be some correlation between the histology and the Hounsfield units in the CT scan. We hope to present the data soon.
170. A MULTICENTER STUDY OF VOLUMETRIC COMPUTED TOMOGRAPHY FOR STAGING MALIGNANT PLEURAL MESOTHELIOMA. Response by Harvey I. Pass, M.D., New York, New York. E-mail: harvey.pass@med.nyu.edu
DR. PASS: I am glad that the IASLC is every year now. We’ll be able to hear that soon.
Acknowledgments
The authors thank Lynn Shemanski, Ph.D. at Cancer Research and Biostatistics for her oversight of data analyses and review of the manuscript. The authors also thank Melody Owens, Ph.D. for her assistance in manuscript preparation. This article was authored on behalf of Malignant Mesothelioma Volumetric CT Study Group:
Memorial Sloan-Kettering Cancer Center: Dr. Valerie Rusch, Dr. Michelle Ginsberg; University of Texas and MD Anderson Cancer Center: Dr. David Rice, Dr. Jeremy Erasmus; New York University Medical Center: Dr. Harvey Pass, Dr. David Naidich; The University of Chicago: Dr. Hedy L, Kindler, Dr. Samuel Armato, Dr. Christopher Strauss, Dr. Wickii Vigneshwaran; Penn Presbyterian Medical Center: Dr. Joseph Friedberg, Dr. Sharyn Katz; University of Toronto: Dr. Marc de Perrot, Dr. Demetrios Pastios; Cancer Research and Biostatistics: Dori Giroux, M.S., Lynn Shemanski, Ph.D., Alan Mitchell, M.S. This study was supported in part by the Mesothelioma Applied Research Foundation. Dr. Valerie Rusch’s work is supported in part by National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Fifty-second Annual Meeting of The Society of Thoracic Surgeons, Phoenix, AZ, Jan 23–27, 2016.
Set conflict box: Dr Rice discloses a financial relationship with the Olympus Corporation.
Query author on spelling of Dr Hedi L Kindler’s first name – Hedi or Hedy.
References
- 1.Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the International Association for the Study of Lung Cancer Mesothelioma Database. J Thorac Oncol. 2012;7:1631–9. doi: 10.1097/JTO.0b013e31826915f1. [DOI] [PubMed] [Google Scholar]
- 2.Rusch VW. Pleural mesothelioma. In: Edge SB, Byrd DR, Compton CC, et al., editors. Cancer Staging Manual. New York: American Joint Commission on Cancer; 2010. pp. 271–7. [Google Scholar]
- 3.Pass HI, Temeck BK, Kranda K, et al. Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1998;115:310–8. doi: 10.1016/S0022-5223(98)70274-0. [DOI] [PubMed] [Google Scholar]
- 4.Sensakovic WF, Armato SG, III, Straus C, et al. Computerized segmentation and measurement of malignant pleural mesothelioma. Med Phys. 2011;38(1):238–44. doi: 10.1118/1.3525836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pass HI, Brewer GJ, Dick R, et al. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: Final results. Ann Thorac Surg. 2008;86:383–90. doi: 10.1016/j.athoracsur.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Liu F, Zhao B, Krug LM, et al. Assessment of therapy responses and prediction of survival in malignant pleural mesothelioma through computer-aided volumetric measurement on computed tomography scans. J Thorac Oncol. 2010;5:879–84. doi: 10.1097/JTO.0b013e3181dd0ef1. [DOI] [PubMed] [Google Scholar]
- 7.Mollberg NM, Parsad NM, Armato SG, III, et al. Three-dimensional stereoscopic volume rendering of malignant pleural mesothelioma. Int Surg. 2012;97:65–70. doi: 10.9738/CC66.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frauenfelder T, Tutic M, Weder W, et al. Volumetry: An alternative to assess therapy response for malignant pleural mesothelioma? Eur Respir J. 2011;38(1):162–8. doi: 10.1183/09031936.00146110. [DOI] [PubMed] [Google Scholar]
- 9.Ak G, Metintas M, Metintas S, et al. Three-dimensional evaluation of chemotherapy response in malignant pleural mesothelioma. Eur J Radiol. 2010;74(1):130–5. doi: 10.1016/j.ejrad.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Armato SG, III, Oxnard GR, Kocherginsky M, et al. Evaluation of semiautomated measurements of mesothelioma tumor thickness on CT scans. Acad Radiol. 2005;12:1301–9. doi: 10.1016/j.acra.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Armato SG, III, Oxnard GR, MacMahon H, et al. Measurement of mesothelioma on thoracic CT scans: A comparison of manual and compuer-assisted techniques. Med Phys. 2004;31(5):1105–15. doi: 10.1118/1.1688211. [DOI] [PubMed] [Google Scholar]
- 12.Gill RR, Richards WG, Yeap BY, et al. Epithelial malignant pleural mesothelioma after extrapleural pneumonectomy: stratification of survival with CT derived tumor volume. Am J Roentgenol. 2012;198:359–63. doi: 10.2214/AJR.11.7015. [DOI] [PubMed] [Google Scholar]
- 13.Rusch VW, Chansky K, Nowak A, et al. Initial analysis of the IASLC malignant pleural mesothelioma database:Implications for the 8th editions of the AJCC and UICC staging manuals. Journal of Thoracic Oncology. 2015;10(9 Suppl 2):S223. (Abstract) [Google Scholar]
- 14.Gill RR. North American multicenter volumetric CT study for clinical staging of malignant pleural mesothelioma: Feasibility and logistics of setting up a quantitative imaging study. J Thorac Oncol. 2016 doi: 10.1016/j.jtho.2016.04.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plathow C, Klopp M, Thieke C, et al. Therapy response in malignant pleural mesothelioma – role of MRI using RECIST, modified RECIST and volumetric approaches in comparison with CT. Eur Radiol. 2008;18(8):1635–43. doi: 10.1007/s00330-008-0918-9. [DOI] [PubMed] [Google Scholar]
- 16.Winer-Muram HT, Jennings SG, Meyer CA, et al. Effect of varying CT section width on volumetric measurement of lung tumors and application of compensatory equations. Radiology. 2003;229:184–94. doi: 10.1148/radiol.2291020859. [DOI] [PubMed] [Google Scholar]
- 17.Nowak AK, Francis RJ, Phillips MJ, et al. A novel prognostic model for malignant mesothelioma incorporating quantitative FDG-PET imaging with clinical parameters. Clin Cancer Res. 2010;16(8):2409–17. doi: 10.1158/1078-0432.CCR-09-2313. [DOI] [PubMed] [Google Scholar]
- 18.Giesel FL, Bischoff H, von Tengg-Kobligk H, et al. Dynamic contrast-enhanced MRI of malignant pleural mesothelioma. A feasibility study of noninvasive assessment, therapeutic follow-up, and possible predictor of improved outcome. Chest. 2006;129:1570–6. doi: 10.1378/chest.129.6.1570. [DOI] [PubMed] [Google Scholar]


