Abstract
Background
Facing rising incidence of basal cell carcinoma (BCC) and increasing pressure to contain healthcare spending, physicians need to contemplate cost-effective paradigms for managing BCC.
Objective
To perform a cost analysis comparing the traditional BCC management scheme to a simplified Detect and Treat (DAT) scheme that eliminates the biopsy prior to initiating definitive treatment.
Methods
A decision analytic model was developed to compare the costs of traditional BCC management with the DAT scheme, under which qualifying lesions diagnosed clinically were either treated with shave removal or referred to Mohs for on-site histologic check. Values for model parameters were based on literature and our institutional data analysis. Costs were based on 2014 Medicare fee schedule.
Results
The average cost per lesion with DAT scheme was $449 for non-Mohs-indicated lesions (vs. $566 with traditional management, $117 in savings) and $819 for Mohs-indicated lesions (vs. $864 with traditional management, $45 in savings). The combined weighted average savings per case was $95 (15% of total average cost). Conclusions were similar under various plausible scenarios.
Limitations
Model parameter values may vary based on individual practices.
Conclusions
A simplified management strategy eliminating routine pre-treatment biopsy can reduce BCC treatment cost without compromising quality of care.
Keywords: Cost analysis, decision analysis model, basal cell carcinoma, shave removal, chemocheck, Detect and Treat scheme
BACKGROUND
Basal cell carcinoma (BCC) is the most common cancer in the United States, accounting for almost 30% of all malignancies. In 2006, the incidence of BCC was about 2.6 million1 and it has continued to rise at an average annual rate of 4–8%2,3. As a result, the cost associated with BCC treatment and morbidity poses a substantial burden on the American healthcare system. Based on Medicare claims, the average cost per episode of care for BCC between 1992 and1995 was almost $6004. Adjusted for inflation, that translates into approximately $800 per case or over $2 billion in total annual spending today.
Traditional BCC management involves biopsy confirmation prior to initiating definitive treatment. This may require several office visits starting with the initial consult and biopsy, followed by a visit for definitive treatment that might include excision/destruction or referral to Mohs micrographic surgery (MMS). This process can lead to protracted periods of waiting for appointments and pathology results before treatment is administered. More importantly, it incurs costs that can potentially be eliminated by a more efficient management scheme.
At our institution, we have routinely attempted a simplified approach by eliminating the pre-treatment biopsy on a select subset of clinically diagnosed BCCs. While many dermatologists have expressed that they in essence practice the DAT approach in managing select cases of BCCs (personal communications)5, the cost benefit of this practice remains to be established. Using data from our institutional experience and decision analytic methods, our objective was to estimate the costs associated with a “detect and treat” (DAT) strategy compared with the traditional approach for managing BCC.
METHODS
Decision Tree and Strategies
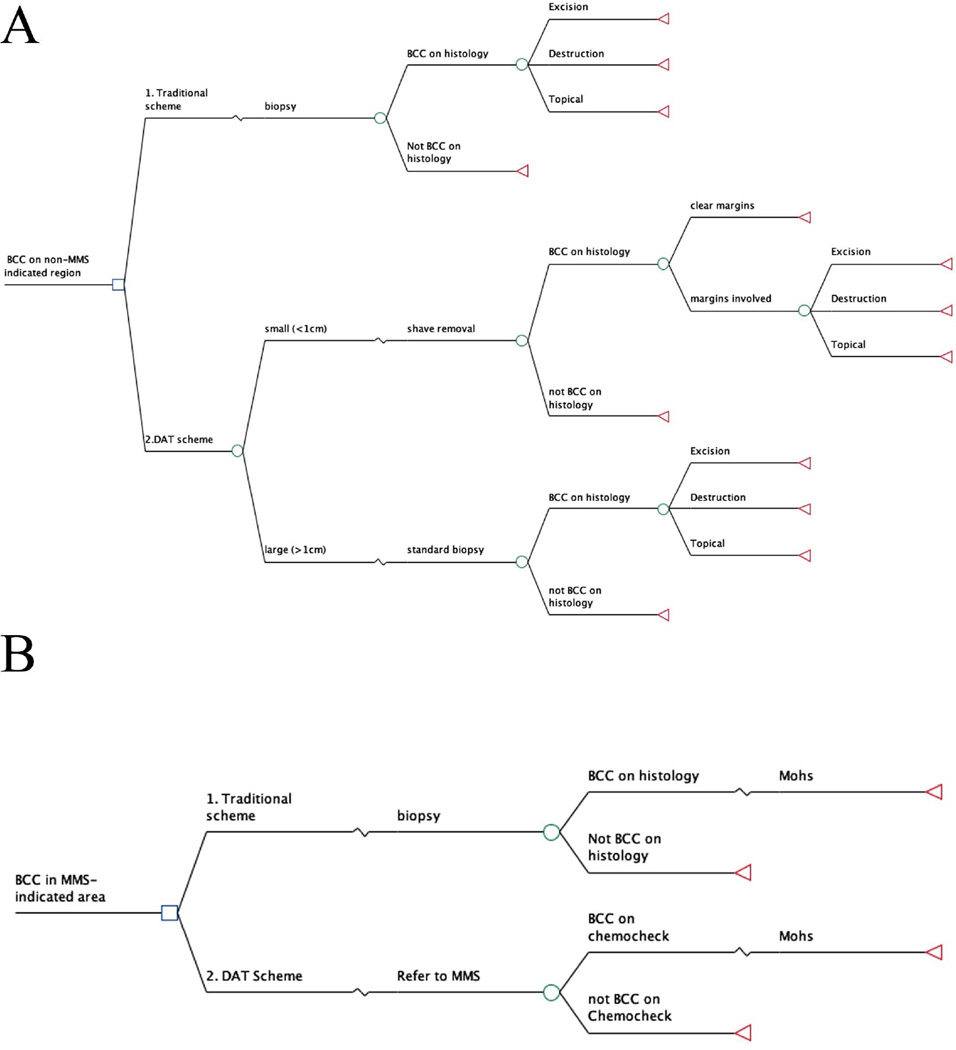
We used decision analysis to estimate the costs associated with competing strategies for BCC management. Decision analysis is a method for simulating and comparing the outcomes of competing strategies under conditions of uncertainty6. The two strategies we compared were a traditional BCC management scheme, which involves pre-treatment biopsy in all cases7, and a “detect and treat” (DAT) scheme (Figure 1). In the DAT scheme, patients presenting with well-delineated and unequivocal BCC lesions ≤1cm diameter on the trunk and extremities are offered shave removals (shave excision, saucerization or tangential shave excision) with histologic confirmation of diagnosis and margin clearance. Patients presenting with BCC in MMS-indicated areas as defined by the 2012 AAD/ACMS/ASDSA/ASMS guidelines8 are referred to MMS directly for on-site histologic confirmation of diagnosis (“chemocheck”) followed by MMS excision in the same visit. MMS-indicated and non-MMS-indicated lesions were modeled separately (Figure 2). The analysis was conducted from a societal perspective, and the primary outcome was the successful removal of the primary lesion. The decision analysis was performed using TreeAge Pro™ 2014 (TreeAge Software, Inc, Williamstown, MA)..
Figure 1.
Decision trees for clinically diagnosed non-MMS-indicated (A) and MMS-indicated (B) lesions. Hypothetical patients move from the initial decision node (square) along branches of the tree according to the probabilities associated with the events represented at each chance node (circles) to the terminal nodes (triangle). The cost at each terminal node is a weighted sum of the probability multiplied by the cost from each branch that leads to that terminal node.
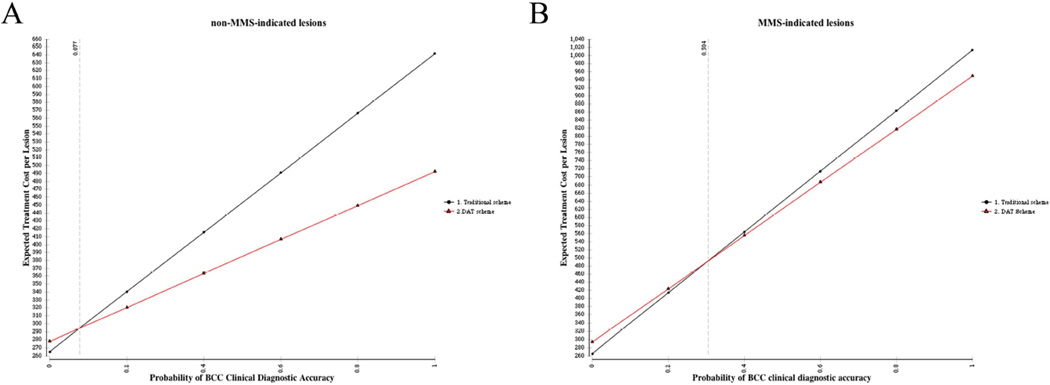
Figure 2.
One-way sensitivity analysis of the impact of clinical diagnostic accuracy on total cost in non-MMS-indicated BCC (A) and MMS-indicated BCC (A). The intersection of the lines in each figure reflects the value at which the two management schemes have equal cost.
In decision analysis, the probability of arriving at any terminal node is estimated by multiplying the probabilities at each preceding chance node along in the decision tree. Key probabilities in our model were based on a review of literature and analysis of our own institutional data, described below. These included the proportion of presenting lesions <1cm in diameter, BCC clinical diagnostic accuracy, shave removal success rate, and the frequency distribution of BCC treatment modalities (Table 1).
Table 1.
Model inputs: probabilities based on literature review and institutional experience
| Parameters | Values |
|---|---|
| Clinical diagnostic accuracy | 80%16,20,21 |
| Presenting BCC <1cm in diameter | 50%6,20,22,23 |
| Frequency distribution of excision in treating BCC |
40%12,13,24,25 |
| Frequency distribution of destruction (electrodessication and curettage) in treating BCC |
30%12,13,24,25 |
| Frequency distribution of topical therapy (Imiquimod) in treating BCC |
30%12,13,24,25 |
| Shave removal success rate for lesions <1cm | 86%a |
Based on our institutional data review of 204 lesions
Model Inputs: from institutional data analysis
Several model parameters were estimated from a retrospective analysis of cases seen at our institution and managed with the DAT approach. The analysis included lesions with unequivocal clinical diagnosis based on clinical and dermoscopic findings. Analysis of shave removal success was restricted to cases that met the following additional criteria: 1) tumor clearly demarcated from normal surrounding skin; 2) less than 1cm in greatest diameter; and 3) lesion site easily amenable to shave removal. Features that excluded BCCs from undergoing shave removal in the DAT scheme despite meeting these criteria were individual patient characteristics and physician clinical judgment such as indications for compromised wound healing or worse scarring, locations that are difficult to access (i.e. flexure/extensor surfaces, skin overlying bony protuberances, skin with superficial varicosities or tendons, etc).
After receiving institutional IRB exemption, we reviewed the consecutive medical procedure logs for the past 5 years for dermatologists (A.M. and C.C.) at a single dermatology office, a tertiary referral center, for information about treatment success, complications, and recurrences. Of the 240 lesions that were managed by the DAT scheme between 2008 and 2013, 36 lesions underwent direct chemocheck verification of diagnosis followed by a MMS procedure, and 204 underwent shave removal with histopathologic confirmation of diagnosis and margin status.
Model Inputs: Costs
Service utilization was estimated from patient billing records, with unit cost estimates from the 2014 Medicare Fee Schedule and retail topical therapy prices (Table 2). The same unit costs were used for the traditional and DAT scheme. For patients managed in the traditional scheme, we assumed that total cost included an initial office visit plus biopsy and pathology, followed by a second office visit plus a definitive treatment procedure (removal or MMS). In the DAT scheme, total cost included the initial office visit plus either shave removal and pathology, or “chemocheck” (biopsy and frozen section) and MMS procedure.
Table 2.
Model inputs: costs
| Fee category | 2014 Medicare CPT code | Average fee ($)a |
|---|---|---|
| Physician office visit | 99212–99215 | 92 |
| Diagnostic biopsy | 11100 | 102 |
| Path | ||
| Surgical pathology | 88305 | 71 |
| Frozen section pathology | 88331 | 99 |
| Primary procedure | ||
| shave removal | 11300–11303 | 127 |
| Excision: trunk and extremities |
11600–11606 | 286 |
| EDC | 17260–17266 | 173 |
| MMS | ||
| 1st stage: head or neck | 17311 | 656 |
| Additional stage: head or neck |
17312 | 385 |
| Topical therapy: Imiquimod |
$300b | |
Average across a range of CPT codes rounded to the nearest $
Average retail price of imiquimod based on 3 boxes (36 packets) as indicated in literature for one course of treatment26
Sensitivity analysis
We used one-way sensitivity analysis to examine the robustness of results to assumptions about model inputs. We tested an interval of +/− 20% for each original input value while holding the other parameters constant. In two-way sensitivity analysis, we simultaneously varied clinical diagnostic accuracy and the probability of shave removal success, as well as other combinations of model parameters.
RESULTS
Institutional chart review data
We identified 137 patients (91 men and 46 women) with 204 lesions that received shave removal. The average age of the patients was 66 years (standard deviation 13). 140 (69%) of the lesions were located on the trunk, 59 (29%) on the extremities and 5 (2%) on head and neck. 175 (86%) were successfully removed on initial attempt, defined as clear margins on pathology. The clinical diagnostic accuracy was >99% with 2 misdiagnoses (1 compound nevus, 1 squamous cell carcinoma). The mean lesion size determined by measuring in vivo magnified dermoscopic images was 6.5±2.1mm × 5.0±1.8mm (ranged from 1.5×1.2mm – 11.6×10.7mm) and the mean lateral excision margin determined from pathology reports of excised tissue size adjusted for estimated tissue shrinkage from excision and formalin fixation using 17%9 was 2.9±1.3mm (range 1–7mm). Majority of the lesions were superficial BCCs 97(48%), mixed superficial and nodular 49(24%), and nodular 33(16%). No statistically significant differences were found between successful vs. failed shave removals with respect to patient age and gender or lesion location and histosubtype (superficial vs. nodular) (p>.05). No complications were reported and only 1 recurrence was reported during an average of 3-year follow-up. A total of 36 lesions in 25 patients (18 men and 7 women) underwent chemocheck. The average age was 69 years old (standard deviation15). 30 (83%) of the lesions were located on the head and neck and 6 (17%) on the trunk. The average lesions size was 10.0 ±6.3mm×7.4±4.4 mm (ranged from 3×3mm to 30×20mm). Average number of Mohs stages performed to remove the lesion was 1.6± 0.9. No complications and no recurrence were seen during the average follow-up period of 3 years.
Decision analysis
The average total costs of traditional management non-MMS-indicted and MMS-indicated BCC were $566 and $863 respectively. With the DAT scheme, these costs were $449 and $817, respectively. Thus, the DAT scheme reduced average cost by 21% in non-MMS-indicated BBC, for a savings of $117, and by 5% in MMS-indicated BCC, for a savings of $45. Assuming that 30% of BCCs are treated by MMS10, the weighted average of expected savings per case is $95(15%).
Sensitivity Analysis
One-way sensitivity analysis data are presented in Table 3. The expected savings in the DAT vs. the traditional models are relatively consistent over a range of plausible scenarios. For instance, as clinical diagnostic accuracy decreased, the costs associated with the DAT scheme increased (Figure 3). In MMS-indicated lesions, the two schemes had equivalent costs when clinical diagnostic accuracy was 30%, while in non-MMS-indicated cases this threshold was 8%. Thus, across all lesions, the DAT scheme was less costly as long as clinical diagnostic accuracy exceeded 30%.
Table 3.
Cost savings per lesion associated with DAT vs. traditional scheme with one-way sensitivity analysis of model input probabilities.
| Parameter | Original Input valuea |
Range used for sensitivity analysis (+/− 20% of input value) |
Range of cost savings ($) per non-MMS indicated lesion |
Range of cost savings ($) per MMS- indicated lesion |
Overall range of cost savings ($) as weighted average per lesion |
|---|---|---|---|---|---|
| Clinical diagnostic accuracy |
80% | 60–100% | 85–149 | 27–64 | 68–124 |
| Presenting BCC <1cm in diameter |
50% | 30–70% | 70–164 | 45-45 | 63–128 |
| Frequency distribution of excision in treating BCC |
40% | 20–60% | 113–121 | 45-45 | 93–98 |
| Frequency distribution of destruction (electrodessication and curettage) in treating BCC |
30% | 10–50% | 126-108 | 45-45 | 102-89 |
| Frequency distribution of topical therapy (Imiquimod) in treating BCC |
30% | 10–50%d | 108–126 | 45-45 | 89–102 |
| Shave removal success rate for lesions <1cm |
86% | 66–100%b | 87–138 | 45-45 | 74–110 |
| Proportion of presenting lesions treated with MMSc |
30% | 10–50% | 117-117 | 45-45 | 110-81 |
Original input values taken from table 1, based on literature and institutional data
Maximum value cannot exceed 100%.
Value used to estimate the weighted average saving of one lesion when shave removal and MMS models are combined.
Value is the remainder after excision and destruction because all 3 have to add up to 1. (Probability= 1-probability of destruction-probability of excision)
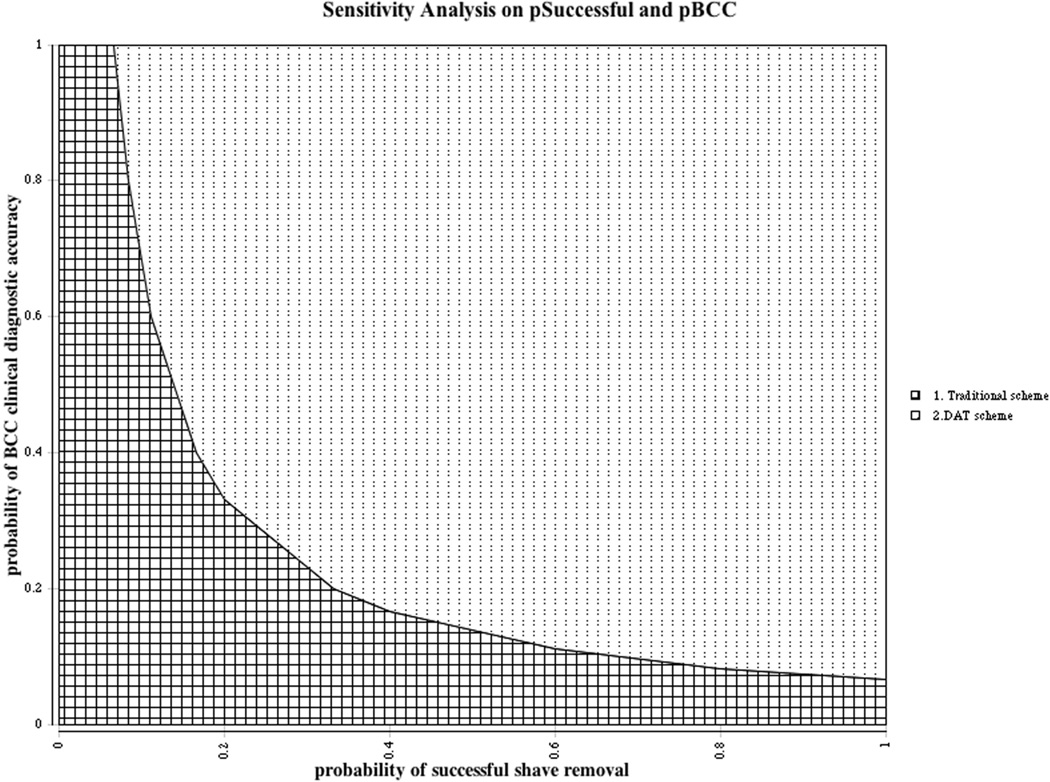
Figure 3.
Two-way sensitivity analysis varying clinical diagnostic accuracy and rate of shave removal success for non-MMS-indicated BCC management. The dotted area indicates all combinations of values for which the cost associated with the DAT scheme is less than the Traditional scheme. The border between the dotted and checkered regions is the line of cost equivalence between the two management schemes.
In non-MMS-indicated BCC, the DAT scheme was less costly than traditional management as long as clinical diagnostic accuracy and probability of successful shave removal both exceeded 30%, holding other model parameters constant (Figure 3). In this and other sensitivity analyses we conducted, the threshold parameter values at which the two strategies had equivalent costs were well below published estimates and those based on our institutional experience.
The magnitude of expected savings achieved by using the DAT scheme is dependent on the percent of lesions that may benefit, namely the percent of all presenting BCCs that are MMS-indicated lesions and percent of non-MMS-indicated lesions that are suitable for shave removal. To assess this, we combined the two decision models and varied the distribution of MMS-indicated and non-MMS-indicated lesions, as well as the probability of shave removal for the non-MMS-indicated lesions. Across all possible values of these parameters, holding other model inputs constant, the DAT scheme was equal or less costly than the traditional scheme, with expected savings between $0 and $234. For instance, if 100% of BCCs were MMS-indicated, the expected saving per case is $45 dollars because only the MMS portion of the DAT scheme would be utilized. Conversely, if no lesions were MMS-indicated, the expected savings is solely a function of the probability of the shave removal and increases to a maximum of $234, if all lesions are assumed to be suitable for shave removal.
DISCUSSION
We performed a cost analysis comparing the traditional management scheme for BCC with a simpler DAT approach. Our results suggest that the DAT scheme is less costly under a wide range of parameter values derived from literature and our own clinical experience.
Our institutional data suggest that the DAT strategy is associated with complication and recurrence rates comparable to those reported with traditionally-managed BCCs. In an average of 3 years of follow-up, we observed only 1 recurrence in 204 lesions treated by shave removal and no reoccurrences in the 36 lesions treated by chemocheck/MMS. These rates are well below the 3–5% five-year recurrence rate reported for surgical excision and 15–20% for topical therapies6,11–14.
Our institutional data are consistent with prior studies. In 1994, Harrison et al found less than 1% 5-year reoccurrence rate in 60 patients who underwent shave excision of non-perinasal BCCs less than 2.5cm in diameter15. In another more recent cohort study, Abramson et al followed the outcome of 184 clinically diagnosed BCCs under 1.5cm in diameter that were treated by shave removal, for an average of 5.2 years. Of these lesions, 2 were misdiagnosed, 3 had initial treatment failure and 1 had reoccurred5. These studies and our data collectively show that shave removal can be an effective means to treat small BCCs on trunk and extremities. Other concerns of shave removal include margin selection and cosmesis of lesion site. The average margin size of our shaved lesions was estimated to be 2.9±1.3mm, comparable to prior study by Wolf and Zitelli16, who demonstrated via MMS that the optimal clinical margin for excising BCCs <2cm was 4mm in order to obtain clear en-face margins. Our margin size was potentially smaller because Wolf and Zitelli looked at all BCCs including complex and infiltrative lesions; whereas we only looked at clinically small and well defined BCCs. Regarding cosmesis of the lesion site, the study by Abramson et al rated the healed lesion site based on cosmetic outcome and found that 78.5% of the lesions healed with smooth, oval, flat, and white scars, which was comparable or superior to that seen with electrodessication and curettage5. In addition, we are only advocating shave removal for small lesions on trunk and extremities that are not MMS-indicated thus are generally not in cosmetically sensitive areas.
For the MMS-indicated lesions, there is no discrepancy in treatment procedure under the DAT scheme except for performing an onsite biopsy with frozen section in the same visit as the surgery instead of advance biopsy. Therefore, there is no expected difference in cosmetic or disease outcome, as was observed in our institutional experience.
We took a relatively conservative approach when applying estimated parameters to our decision analysis model. For example, while our diagnostic accuracy was >99%, we decided to use 80% from literature to better reflect the average dermatology practice. This number is expected to rise with the increasing using of imaging tools such as dermoscopy15,17, 18. In addition, we set the percent of shave removal applicability to 50% of non-MMS-indicated lesions factoring in presenting size <1cm and clinical judgment, but we anticipate this number to be higher as other studies on shave removal have set the size threshold at 1.5–2.5cm without compromising success rate17,19.
This study only counted direct medical cost savings. Potential indirect savings from the DAT scheme could be even more significant, including reduced number of trips to the doctor’s office and the hours missed from work for patients and/or their caretakers. Similarly, under the DAT scheme, patient wait time for appointments and time between diagnosis and receiving treatment is greatly reduced. Furthermore, patients are only subjected to one invasive procedure. This is particularly important for patients undergoing MMS, whose lesions are generally in cosmetically sensitive areas. Performing the onsite biopsy and the procedure in the same visit eliminates the social embarrassment associated with exposing biopsy sites in public and work settings while waiting to schedule surgery. In addition, the DAT scheme allows MMS surgeons to see the candidate lesions in their original morphology instead of the previously biopsied lesions under the traditional scheme, which may not be clearly identifiable after weeks of healing between original biopsy and the scheduled MMS procedure. The risks of needing repeat biopsy for site confirmation or even wrong site surgery may happen. From quality of care point of view, the DAT model definitely has the advantage on correct site recognition for MMS-indicated lesions20.
Several limitations should be noted. First, the data collected are not based on a multicenter study or a validated method. Since our sample size was relatively small, more larger scale studies could help to further support the efficacy of the DAT approach. Second, cost estimates in our model were based on average national Medicare fees and thus do not address regional variation in resource costs and billing practices. Third, as a tertiary institutional referral center, estimates based on patients at our institution may not be generalizable to all provider settings. In particular, physician clinical judgment and characteristics of patient population influence the proportion of candidate lesions for the DAT scheme. Also, physician practice variability such as determining margin size and physician experience can affect clearance success and reoccurrence rates. We did try to address this through sensitivity analyses. Finally, the duration of follow-up was an average of three years, and it is possible more recurrences would be observed with longer follow-up.
BCC is already the most common malignancy in the United States and is the 5th most expensive cancer to treat in Medicare2. The incidence of BCC is expected to continue to rise as the elderly population in the United States grows. Facing the pressure to control healthcare spending in the current political and economic environment, it is critical to manage BCC treatment in a cost-effective manner. Under the DAT scheme, we can save an estimated $95 per case of BCC. Extrapolating based on an estimated annual incidence of 3–4 million, the savings can be as much as $3–$400 million dollars a year in direct medical cost alone.
In conclusion, from a societal perspective, our simplified DAT management scheme is cost advantageous while maintaining the quality of care. It generates savings both directly through treatment costs and indirectly through conserved labor productivity. In addition, it reduces patient wait time and enhances patient convenience. Ultimately, the decision of the optimal treatment of BCC is between the patient and their physician, but we believe the DAT scheme is both an economically beneficial and patient convenient alternative to the traditional approach for the treatment of BCCs.
Capsule summary.
Escalating basal cell carcinoma incidence and treatment costs heighten the need for cost-effective management.
A simplified “Detect and Treat” scheme that eliminates a routine pre-treatment biopsy can lead to an average cost savings of 15% per treated lesion.
The simplified scheme can potentially reduce cost without compromising care.
Acknowledgments
IRB Exemption granted by Memorial Sloan Kettering Cancer Center
Funding sources: None
None
Abbreviations used
- BCC
basal cell carcinoma
- DAT
Detect and Treat
- MMS
Mohs micrographic surgery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no potential conflicts of or competing interests to disclose.
Statement on Prior Presentation: None
REFERENCES
- 1.Rogers HW, Weinstock MA, Harris AR, et al. Incidence Estimate of Nonmelanoma Skin Cancer in the United States, 2006. Arch Dermatol. 2010 Mar 10;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 2.Mudigonda T, Pearce DJ, Yentzer BA, Williford P, Feldman SR. The Economic Impact of Non- Melanoma Skin Cancer: A Review. J Natl Compr Canc. Ne. 2010 Aug;8(8):888–896. doi: 10.6004/jnccn.2010.0066. [DOI] [PubMed] [Google Scholar]
- 3.Bath-Hextall F, Leonardi-Bee J, Smith C, Meal A, Hubbard R. Trends in incidence of skin basal cell carcinoma. Additional evidence from a UK primary care database study. Int J Cancer. 2007 Nov 1;121(9):2105–2108. doi: 10.1002/ijc.22952. [DOI] [PubMed] [Google Scholar]
- 4.Chen JG, Fleischer AB, Jr, Smith ED, et al. Cost of nonmelanoma skin cancer treatment in the United States. Dermatol Surg. 2001 Dec;27(12):1035–1038. doi: 10.1046/j.1524-4725.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- 5.Kimball AB, Resneck JS., Jr The US dermatology workforce: a specialty remains in shortage. Journal of the American Academy of Dermatology. 2008 Nov;59(5):741–745. doi: 10.1016/j.jaad.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Malatack JJ, Schaid DJ, Urbach AH, et al. Choosing a pediatric recipient for orthotopic liver transplantation. The Journal of pediatrics. 1987 Oct;111(4):479–489. doi: 10.1016/s0022-3476(87)80105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCN. National Comprehensive Cancer Network clinical practice guidelines in oncology: Basal cell and squamous cell skin cancers, version 1. 2015 doi: 10.6004/jnccn.2004.0001. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: A report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Journal of the American Academy of Dermatology. 2012 Oct;67(4):531–550. doi: 10.1016/j.jaad.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Zitelli JA. Burow's grafts. Journal of the American Academy of Dermatology. 1987 Aug;17(2 Pt 1):271–279. doi: 10.1016/s0190-9622(87)70203-5. [DOI] [PubMed] [Google Scholar]
- 10.Essers BAB, Dirksen CD, Nieman FHM, et al. Cost-effectiveness of Mohs micrographic surgery vs surgical excision for basal cell carcinoma of the face. Arch Dermatol. 2006 Feb;142(2):187–194. doi: 10.1001/archderm.142.2.187. [DOI] [PubMed] [Google Scholar]
- 11.Roozeboom MH, Arits AHHM, Nelemans PJ, Kelleners-Smeets NWJ. Overall treatment success after treatment of primary superficial basal cell carcinoma: a systematic review and meta-analysis of randomized and nonrandomized trials. Brit J Dermatol. 2012 Oct;167(4):733–756. doi: 10.1111/j.1365-2133.2012.11061.x. [DOI] [PubMed] [Google Scholar]
- 12.Joseph AK, Mark TL, Mueller C. The period prevalence and costs of treating nonmelanoma skin cancers in patients over 65 years of age covered by medicare. Dermatol Surg. 2001 Nov;27(11):955–959. doi: 10.1046/j.1524-4725.2001.01106.x. [DOI] [PubMed] [Google Scholar]
- 13.Ceilley RI, Del Rosso JQ. Current modalities and new advances in the treatment of basal cell carcinoma. International journal of dermatology. 2006 May;45(5):489–498. doi: 10.1111/j.1365-4632.2006.02673.x. [DOI] [PubMed] [Google Scholar]
- 14.Love WE, Bernhard JD, Bordeaux JS. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review. Arch Dermatol. 2009 Dec;145(12):1431–1438. doi: 10.1001/archdermatol.2009.291. [DOI] [PubMed] [Google Scholar]
- 15.Giacomel J, Zalaudek I. Dermoscopy of superficial basal cell carcinoma. Dermatol Surg. 2005 Dec;31(12):1710–1713. doi: 10.2310/6350.2005.31314. [DOI] [PubMed] [Google Scholar]
- 16.Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987 Mar;123(3):340–344. [PubMed] [Google Scholar]
- 17.Abramson AK, Krasny MJ, Goldman GD. Tangential shave removal of basal cell carcinoma. Dermatol Surg. 2013 Mar;39(3 Pt 1):387–392. doi: 10.1111/dsu.12106. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Martin J, Vazquez-Lopez F, Perez-Oliva N, Argenziano G. Dermoscopy of small basal cell carcinoma: study of 100 lesions 5 mm or less in diameter. Dermatol Surg. 2012 Jun;38(6):947–950. doi: 10.1111/j.1524-4725.2012.02358.x. [DOI] [PubMed] [Google Scholar]
- 19.Harrison PV. Shave excision for basal cell carcinoma. The Journal of dermatologic surgery and oncology. 1994 May;20(5):350. doi: 10.1111/j.1524-4725.1994.tb01637.x. [DOI] [PubMed] [Google Scholar]
- 20.Urbach AH, Zitelli BJ, Blatt J, Gartner JC, Malatack JJ. Elevated alpha- fetoprotein in a neonate with a benign hemangioendothelioma of the liver. Pediatrics. 1987 Oct;80(4):596–597. [PubMed] [Google Scholar]
- 21.Thomas DJ, King AR, Peat BG. Excision margins for nonmelanotic skin cancer. Plastic and reconstructive surgery. 2003 Jul;112(1):57–63. doi: 10.1097/01.PRS.0000067479.77859.31. [DOI] [PubMed] [Google Scholar]
- 22.Haws AL, Rojano R, Tahan SR, Phung TL. Accuracy of biopsy sampling for subtyping basal cell carcinoma. Journal of the American Academy of Dermatology. 2012 Jan;66(1):106–111. doi: 10.1016/j.jaad.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 23.Perkins JL, Liu Y, Mitby PA, et al. Nonmelanoma skin cancer in survivors of childhood and adolescent cancer: a report from the childhood cancer survivor study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Jun 1;23(16):3733–3741. doi: 10.1200/JCO.2005.06.237. [DOI] [PubMed] [Google Scholar]
- 24.Koyuncuer A. Histopathological evaluation of non-melanoma skin cancer. World journal of surgical oncology. 2014;12:159. doi: 10.1186/1477-7819-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.John Chen G, Yelverton CB, Polisetty SS, et al. Treatment patterns and cost of nonmelanoma skin cancer management. Dermatol Surg. 2006 Oct;32(10):1266–1271. doi: 10.1111/j.1524-4725.2006.32288.x. [DOI] [PubMed] [Google Scholar]
- 26.Bath-Hextall F, Bong J, Perkins W, Williams H. Interventions for basal cell carcinoma of the skin: systematic review. Bmj. 2004 Sep 25;329(7468):705. doi: 10.1136/bmj.38219.515266.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanaclocha F, Dauden E, Badia X, et al. Cost-effectiveness of treatment of superficial basal cell carcinoma: surgical excision vs. imiquimod 5% cream. Brit J Dermatol. 2007 Apr;156(4):769–771. doi: 10.1111/j.1365-2133.2006.07726.x. [DOI] [PubMed] [Google Scholar]