Abstract
Background
It is not uncommon that only mild coronary artery stenosis is grossly revealed after a system autopsy. While coronary artery spasm (CAS) is the suspected mechanism of these deaths, no specific biomarker has been identified to suggest antemortem CAS.
Material/Methods
To evaluate the potential of using phosphorylated myosin light chain 2 (p-MLC2) as a diagnostic marker of antemortem CAS, human vascular smooth muscle cells (VSMCs) were cultured and treated with common vasoconstrictors, including prostaglandins F2α (PGF2α), acetylcholine (ACh), and 5-hydroxy tryptamine (5-HT). The p-MLC2 level was examined in the cultured cells using Western blot analysis and in a rat model of spasm provocation tests using immunohistochemistry (IHC). Effects of increased p-MLC2 level on VSMCs contractile activities were assessed in vitro using confocal immunofluorescence assay. Four fatal cases with known antemortem CAS were collected and subject to p-MLC2 detection.
Results
The p-MLC2 was significantly increased in VSMCs after treatments with vasoconstrictors and in the spasm provocation tests. Myofilament was well-organized and densely stained in VSMCs with high p-MLC2 level, but disarrayed in VSMCs with low p-MLC2 level. Three of the 4 autopsied cases showed strongly positive staining of p-MLC2 at the stenosed coronary segment and the adjacent interstitial small arteries. The fourth case was autopsied at the 6th day after death and showed negative-to-mild positive staining of p-MLC2.
Conclusions
p-MLC2 might be a useful marker for diagnosis of antemortem CAS. Autopsy should be performed as soon as possible to collect coronary arteries for detection of p-MLC2.
MeSH Keywords: Biological Markers, Coronary Artery Disease, Myosin Light Chains, Pathology, Phosphorylation
Background
Patients with coronary artery disease are susceptible to sudden death without any warning or with a short period of premonitory distress [1]. In the forensic autopsy, a finding of diseased coronary artery with over 75% stenosis could lead to a diagnosis of death by coronary atherosclerotic disease. However, a substantial number of sudden deaths have only revealed negative or mild luminal stenosis of coronary arteries after systematic examination of myocardium and epicardial coronary arteries. The cause of death is hence uncertain or probably due to antemortem coronary artery spasm (CAS) if any death trigger is identified [2,3].
The term CAS refers to a pathophysiological phenomenon that may cause myocardial infarction [4] and lead to circulatory collapse and death [5]. In clinical patients, angiographic examinations of the coronary arteries have shown that CAS particularly occurs in cases of variant or unstable angina [4,6,7]. Heart-type fatty acid-binding protein (h-FABP) and myocardial performance index (Tei index) have recently been proposed to be useful for early detection of ischemia coronary vasospasm induced by 5- Fluorouracil [8]. At forensic autopsies, the pathological diagnosis of antemortem CAS is difficult due to limitation of specific markers. From the limited available literature on CAS, only morphological changes within the coronary artery wall have been revealed by pathologists over the last decades. Factor et al. first proposed using the smooth muscle contraction bands in the media of coronary arteries as a postmortem marker of antemortem CAS [9]. Lin et al. further showed that morphological evidence of contraction of smooth muscle cells included typical lengthwise shortening of the cells and squeezing and folding of the nuclei, and after a comparative morphologic study of contraction of smooth muscle cells of hollow viscera, they concluded that these morphological changes could be applied to the diagnosis of CAS [10]. The internal elastic membrane and intimal folds were also shown to be morphologically changed after CAS [11]. The degree of folding was significantly greater in cases of coronary disease compared to those of noncoronary disease [1]. However, identification of these morphological changes is subjective and has interobserver variability. There is no unequivocal marker, to date, that would allow the objective diagnosis of CAS at autopsy.
Although endothelial dysfunction [12], hyperreactivity of vascular smooth muscle cells (VSMCs) [13,14] and adventitial inflammation (e.g., mast cell inflammation) [15] have been proposed to constitute the substrate for susceptibility to CAS, hyperreactivity of VSMCs has been regarded as the most influential one [16]. Various vasoactive stimuli, such as acetylcholine (ACh), 5-hydroxy tryptamine (5-HT), and prostaglandin F2α (PGF2α), can lead to vasoconstriction or CAS through direct stimulation of VSMCs [17–19]. Despite the complex system of intracellular pathways that control the VSMCs contraction, phosphorylation of myosin light chain (p-MLC) is one of the most important steps [20]. Myosin is a hexamer composed of 2 identical heavy chains and 2 pairs of light chains (MLC1 and MLC2). MLC2, also known as myosin regulatory light chain (MRLC or RLC, LC20), is phosphorylated (phosphor-MLC2, or p-MLC2) at Thr18 and Ser19 by myosin light chain kinase (MLCK) in a Ca2+/calmodulin-dependent manner. This phosphorylation is correlated with myosin ATPase activity and smooth muscle contraction [21], and dephosphorylation by protein phosphatases (PPs) decreases muscular tone [22].
The present study aimed to investigate the potential of using p-MLC2 as a molecular marker for diagnosing antemortem CAS at autopsy. To this end, the p-MLC2 level was initially determined in VSMCs exposed to a series of vasoconstrictors with incremental doses and in a rat model of coronary spasm provocation tests. The association between p-MLC2 level and VSMCs contractile activity was then assessed based on the arrangement of myofilament within cytoplasm. The p-MLC2 level was also examined in coronary arteries from forensic cases that had only mild coronary artery stenosis. This is the first report to propose that p-MLC2 could serve as a molecular marker of antemortem CAS.
Material and Methods
Human samples and ethical statements
Four fatal cases that were diagnosed as cardiac deaths due to coronary artery spasm were obtained from the Department of Forensic Medicine, School of Basic Medical Sciences, Fudan University. These cases had only grossly mild coronary artery stenoses. For each case, the coronary artery at the diseased segment and the adjacent heart tissues were collected. Written informed consent to use these samples for research purposes was obtained from each deceased patient’s family members. Samples were collected in accordance with the Helsinki Declaration. Permission to use human samples was obtained from the Institutional Review Board at the School of Basic Medical Sciences, Fudan University. For animal experiments, the protocol was approved by the Ethics Committee of Shanghai Medical College, Fudan University. All efforts were made to minimize suffering.
Cell culture and treatment
Human vascular smooth muscle cells (VSMCs) were kindly provided by Dr. Ning Sun from the Department of Pathophysiology, School of Basic Medical Sciences, Fudan University and maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Los Angeles, CA, USA) supplemented with 5% fetal bovine serum (FBS) (Gibco). The reagents PGF2α, ACh, and 5-HT were purchased from Sigma Co. (St Louis, MO, USA) and diluted to each working solution based on experimental designs; VSMCs were then treated with PGF2α, ACh, or 5-HT, respectively, in distinct doses for a fixed time or for distinct exposure time for a fixed dose.
Immunohistochemistry (IHC) analysis
IHC staining was performed as previously described [23]. Briefly, coronary arteries and the adjacent heart tissues were formalin-fixed for a week. Samples were then paraffin-embedded and cut into 4-μm slides, which were subsequently subjected to antigen retrieval by heating the slides in a microwave at 100°C for 10 min in 0.1-M citric acid buffer (pH 6.0). Slides were then incubated with the primary antibody against MLC2 (phosphor-Ser 19) (Catalog: 11114, Signalway Antibody Co., College Park, MD, USA) at 4°C overnight. A horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Catalog: ab6721, Abcam Co., Cambridge, UK) was then incubated with the slides at room temperature for 1 h. Immunoreactivity was developed in 0.05% diaminobenzidine (DAB) containing 0.01% hydrogen peroxidase (H2O2).
Spasm provocation tests in rat coronary artery
To assess the p-MLC2 level in spastic coronary artery in vivo, male adult Sprague-Dawley (SD) rats (260 g per rat, n=6) were bred and maintained at the Institute of Laboratory Animal Sciences, Shanghai Medical College, Fudan University. Rats were randomly subgrouped into a vehicle (PBS)-injected control group (n=3) and a 5-HT-injected group (n=3). After rats were anesthetized using 10% chloral hydrate, the heart of each rat was exposed and the left mainstem coronary artery was located. For the experimental group of rats, 1 ml of 5-HT (3 mM/L) was injected into the left mainstem coronary artery, whereas an equal amount of PBS (0.01 mol/L) was injected into the control group of rats. The protocol for provocation of coronary artery spasm was consistent with that of previous reports [24,25]. Two minutes after injection, all rats were sacrificed and the corresponding coronary arteries and adjacent heart tissues were collected and subjected to IHC analysis.
Western blot analysis
After exposure to vasoconstrictors for indicated times, VSMCs in a 100-mm culture dish were lysed at 4°C using lysis buffer (10 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, 1 μg/mL leupeptin, 1 μg/mL pepstatin, 1 μg/mL aprotinin). Concentration of total cell proteins was determined by the bicinchoninic acid (BCA) method. Total proteins were then loaded onto a 12% SDS-PAGE gel and transferred to the nitrocellulose membrane (Millipore, NY, USA) by the electrophoretic transfer system (BioRad, NY, USA). After blocking in 5% fat-free milk for 1 h, the membrane was incubated with specific primary antibody against MLC2 (phosphor-Ser 19) (Catalog: 3675, Cell Signaling Technology, Boston, MA, USA) at 4°C overnight. The bound antibody was visualized using the respective HRP-conjugated secondary antibody (Catalog: ab6789, Abcam) and enhanced chemoluminescent autoradiography (ECL kit, Amersham, Pittsburgh, PA, USA). Glyceraldehyde-phosphate dehydrogenase (GAPDH) was synchronously developed as a loading control. The bands of Western blotting were quantified by densitometry measurement using gene tools from Syngene.
Confocal immunofluorescence (IF) assay
The protocols for IF assay were in accordance with previous descriptions [26]. Briefly, VSMCs with distinct treatments were mounted on cover slips and allowed to grow until 80% confluence. After blocking with normal goat serum (Catalog: AR0009, Boster Biotech. Wuhan, China), cells were incubated with mouse monoclonal MLC2 antibody (Phospho-Ser19) (Catalog: 3675, Cell Signaling Technology, Boston, MA, USA). Slides were then washed with PBS, and incubated with the DyLight 594 AffiniPure Goat anti-Mouse (red) secondary antibody (Catalog: E032410-01, EarthOx Co., San Francisco, CA, USA) at room temperature for 45 min. Finally, cover slips were re-dyed in DAPI solution (1mg/L) containing 50% glycerol. Slides were visualized under a confocal fluorescence microscope (Leica TCS SP5, Germany).
Statistical analysis
Data were calculated as means ± standard deviation (SD). Statistical significance was determined by using the Student’s t-test. A two-tailed value of p<0.05 was considered statistically significant.
Results
Treatment of human VSMCs with PGF2α, ACh, and 5-HT increases the p-MLC2 levels
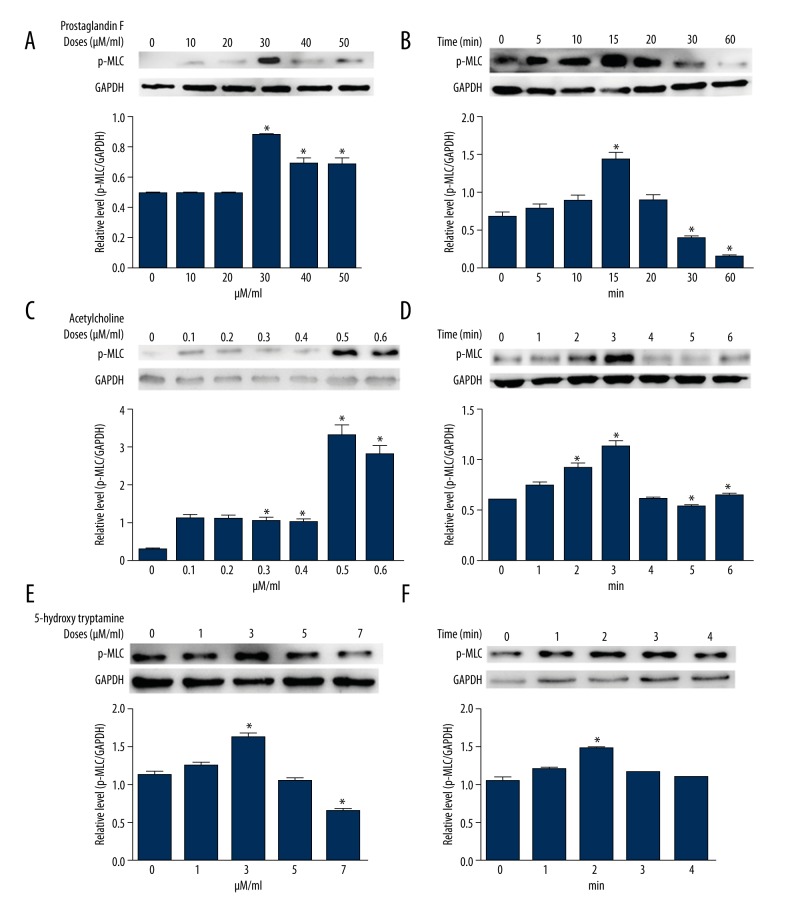
To assess the p-MLC2 level in cultured VSMCs, the common vasoconstrictors PGF2α, ACh, and 5-HT were added to culture medium in distinct doses or for specified times. Upon these vasoconstrictor treatments, VSMCs did not exhibit significant changes in total protein level of MLC2 (data not shown). However, with the treatment of PGF2α, VSMCs showed increases of the phosphorylated MLC2 (p-MLC2) level, with its highest level at the PGF2α dose of 30 μM/ml (Figure 1A). At the fixed dose of 30 μM/ml, MLC2 exhibited a bell-shaped phosphorylation level in a consecutive treatment time, with the highest p-MLC2 level at 15 min after treatment (Figure 1B). During the treatment of ACh, VSMCs showed elevated p-MLC2 levels as the doses increased up to 0.5μM/ml, after which the p-MLC2 began to decrease in VSMCs (Figure 1C). While VSMCs were treated with ACh at a constant dose of 0.5 μM/ml, it was observed that the MLC2 was remarkably phosphorylated after 3-min treatments (Figure 1D). Treatment of VSMCs with 5-HT also increased the p-MLC2 level, with its highest level exhibited by a concentration of 3 μM/ml (Figure 1E). Furthermore, under the constant dose of 3 μM/ml of 5-HT, the MLC2 also exhibited a bell-shaped phosphorylation level, with the highest p-MLC2 level at 2 min after treatment (Figure 1F). These data suggest that vasoconstrictors could potentially increase the p-MLC2 levels in a dose-dependent manner in VSMCs. Phosphorylation of MLC2 is time-sensitive – short-term exposure favors phosphorylation and long-term exposure results in dephosphorylation of MLC2.
Figure 1.
Treatment of human VSMCs with PGF2α, ACh, and 5-HT increases the phosphorylated level of MLC2. Human VSMCs were treated with serial doses of PGF2α2α (A, B), ACh (C, D), and 5-HT (E, F). The p-MLC2 level was detected by Western blotting. Under constant doses of vasoconstrictors, VSMCs were treated with PGF2α (B, dose=30 μM/ml), ACh (D, dose=0.5 μM/ml), and 5-HT (F, dose=3 μM/ml) in incremental time periods, as indicated. The lower bars in each panel are relative p-MLC2 levels after normalization to GAPDH. Data were obtained from 3 independent experiments with each in triplicate. * p<0.05 vs. control (without any reagent treatment in A, C and E, or at the beginning of treatment in B, D, and F) by t-test.
MLC2 is hyper-phosphorylated in spasm provocation tests in rats
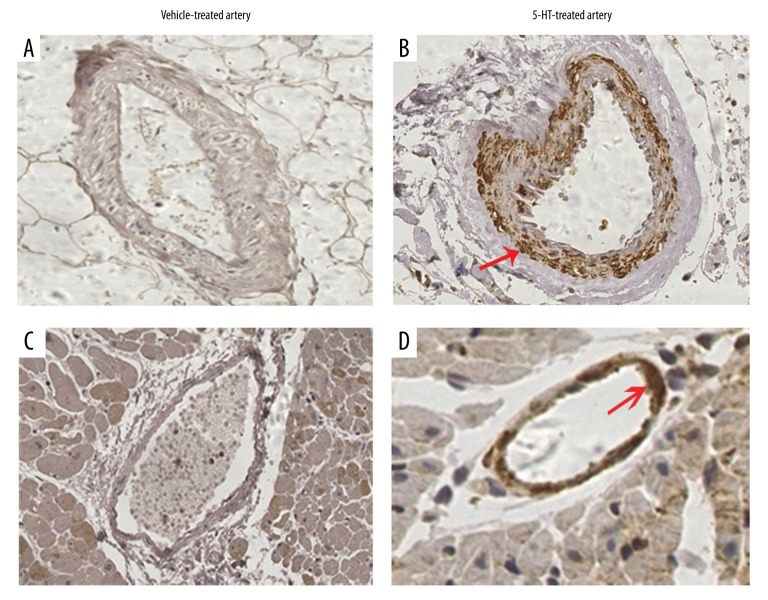
Next, the p-MLC2 level was assessed in spasm provocation tests. Coronary artery spasm (CAS) was initially induced through intracoronary artery injection of 5-HT in experimental rats. IHC analysis revealed that the coronary artery at the PBS-injected segment was negative for p-MLC2 (Figure 2A). In contrast, the coronary artery at the 5-HT-injected segment showed strongly positive staining of p-MLC2. The positive p-MLC2 was mainly concentrated in the tunica media (Figure 2B). In addition, the adjacent interstitial small artery was also absent of p-MLC2 staining in control group (Figure 2C), whereas it showed a rim of positive staining of p-MLC2 around the small artery wall in 5-HT-injected rats (Figure 2D). These data supported the above in vitro observations and suggested that contraction of VSMCs was associated with higher levels of p-MLC2.
Figure 2.
MLC2 is hyper-phosphorylated in spasm provocation tests. Provocation of coronary artery spasm was performed with an intracoronary injection of 5-HT in rats. (A) IHC analysis of the p-MLC2 in the coronary artery at the PBS-injected segment. (B) IHC staining of p-MLC2 in the spastic coronary segment in the 5-HT-injected group of rats. (C) In the vehicle (PBS)-treated heart, the interstitial small artery adjacent to the injection site was stained with a specific antibody against p-MLC2 using IHC analysis. (D) In the 5-HT-treated coronary arteries, the interstitial small artery which was adjacent to the injection site was stained with p-MLC2 antibody using IHC. Red arrows highlight the positive staining of p-MLC2. Magnification: 400×.
Increased p-MLC2 level promotes VSMC contractile activities in vitro
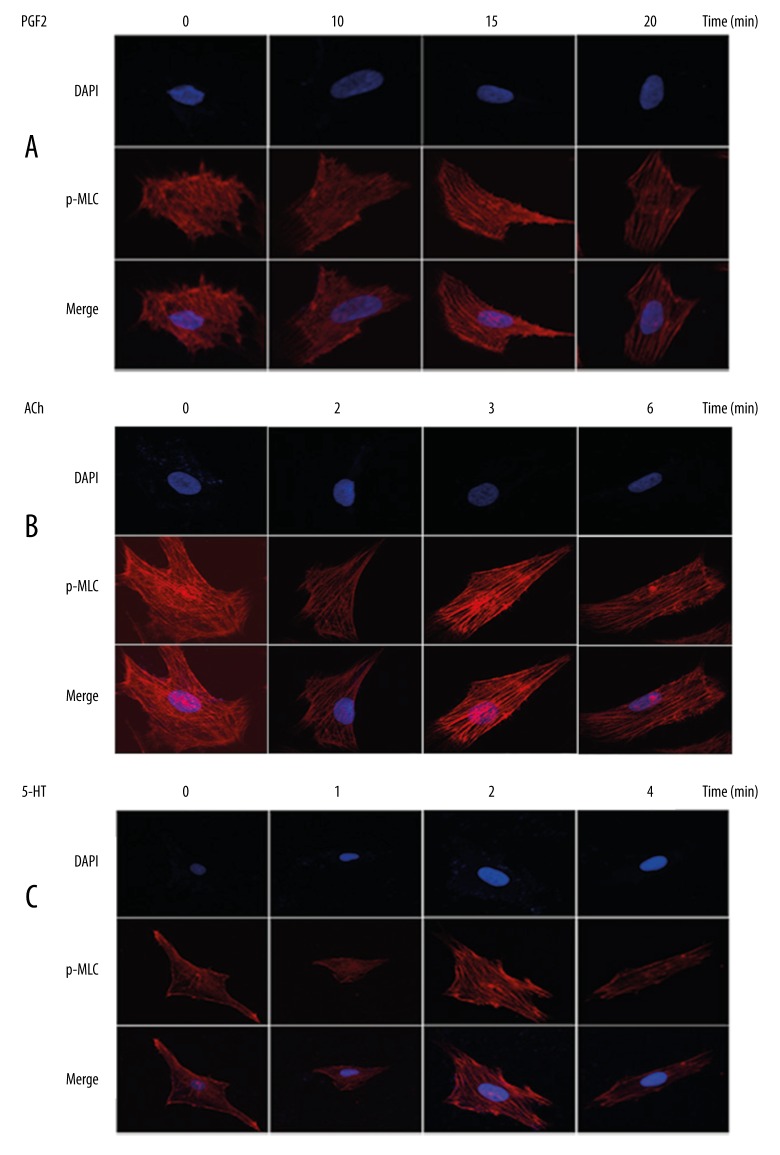
Subsequently, effects of MLC2 phosphorylation on VSMCs contraction were assessed in vitro. As MLC2 is a component of cytoskeleton (myofilament), the arrangement of myofilament could be revealed by staining of p-MLC2 using confocal immunofluorescence assays. When VSMCs were exposed to PGF2α (30 μM/ml), myofilament (represented by p-MLC2) was gradually lined up in the same direction with the exposure time increasing up to 15 min (Figure 3A). The synclastic orientation of myofilament suggested a strong contractile activity; therefore, this observation suggested that contraction of VSMCs was promoted soon after phosphorylation of MLC2. As evidenced by Figure 1, longer exposure to vasoconstrictors could lead to decreased p-MLC2 levels (dephosphorylation process). Hence, extending exposure time was utilized here to mimic the dephosphorylation of MLC2. We observed that exposure to PGF2α for 20 min led to less-organized myofilament as compared with 15-min exposure (Figure 3A), indicating that after 15 min, dephosphorylation of MLC2 occurred and might blunt p-MLC2-mediated cell contraction. Similarly, the best organization of myofilament was observed by exposure of VSMCs to ACh for 3 min (Figure 3B) or to 5-HT for 2 min (Figure 3C) when myofilament was arranged in an ordered direction and was brightly revealed. With extended exposure time, the fluorescence dimmed and the arrangement of myofilament became disarrayed (Figure 3B, 3C). These observations suggest that increased p-MLC2 level promotes the contractile activities of VSMCs, while dephosphorylation of MLC2 (by increasing exposure time) blunts its phosphorylation-mediated VSMCs hyperreactivity.
Figure 3.
Increased p-MLC2 level promotes VSMCs contractile activities in vitro. Staining of p-MLC2 using immunofluorescence assay was utilized to reveal myofilaments within the cytoplasm of VSMCs. VSMCs were exposed to PGF2α (A), ACh (B), and 5-HT (C) for indicated times. The best-organized myofilament was observed by exposure to PGF2α for 15 min, to ACh for 3 min, and to 5-HT for 2 min. Extension of exposure time led to dephosphorylation and, hence, less organized myofilament and dimmed fluorescence.
MLC2 is highly phosphorylated in cardiac deaths due to coronary artery spasm
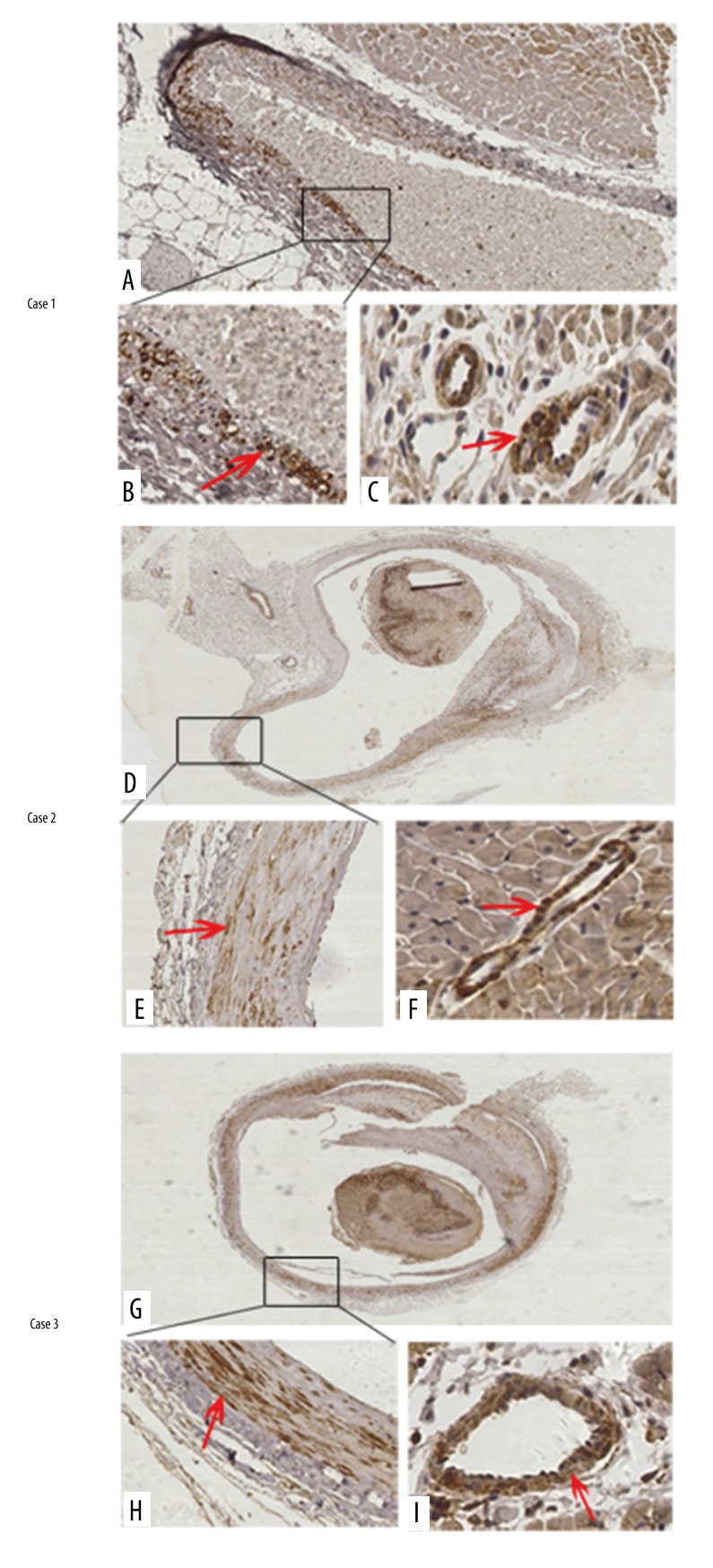
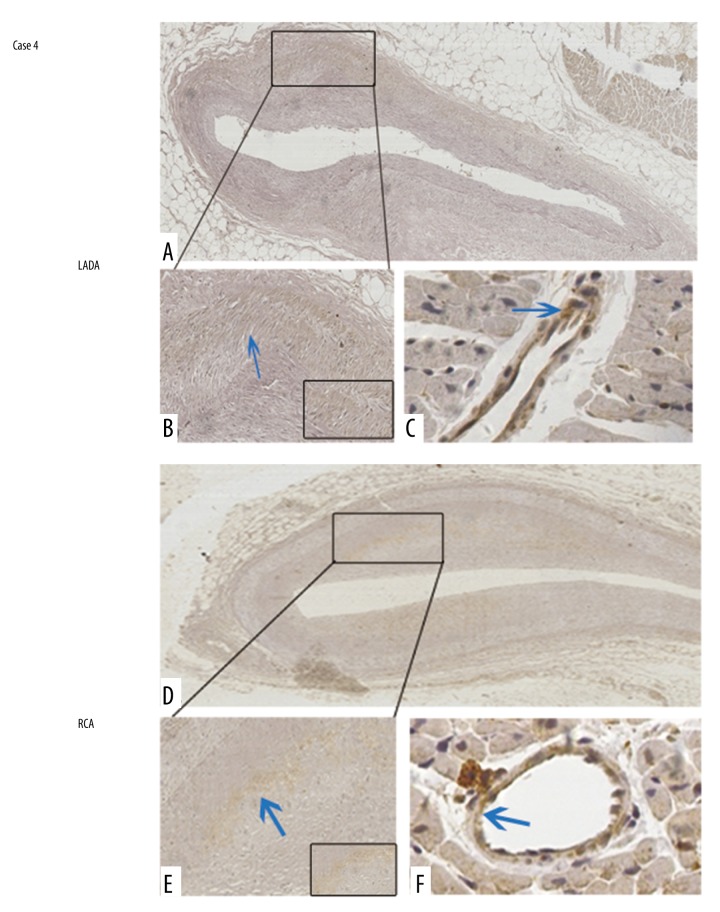
Thereafter, the p-MLC2 level was assessed in human coronary arteries from autopsied cases with the cause of deaths as cardiac deaths due to known antemortem CAS. Mild coronary luminal stenosis was grossly observed in these cases. Characteristics of the 4 cases are summarized in Table 1. The first 3 cases were all mildly occluded by atherosclerotic plaques in the left anterior descending artery (LADA). In Case 1, it was observed that p-MLC2 was positively stained around the coronary artery wall (Figure 4A, 4B). Adjacent to the stenosed LADA, the interstitial small artery also showed a rim of strong staining of p-MLC2 (Figure 4C). In Cases 2 and 3, a thrombus was observed within the lumina of LADA and showed positive staining of p-MLC2. Most importantly, p-MLC2 was strongly positively stained within the tunica media of LADA in Case 2 (Figure 4D, 4E) and Case 3 (Figure 4G, 4H). The interstitial small artery, which is located adjacent to the diseased coronary artery, consistently showed strongly positive staining of p-MLC2 in Case 2 (Figure 4F) and Case 3 (Figure 4I), reinforcing the picture of the high phosphorylated level of MLC2 in mildly stenosed coronary arteries. However, Case 4 which was stenosed at both the LADA (Figure 5A, 5B) and right coronary artery (RCA) (Figure 5D, 5E), showing negative-to-mildly-positive staining of p-MLC2. The corresponding interstitial small artery was consistently revealed to be barely positive for p-MLC2 (Figure 5C for LADA, and Figure 5F for RCA). The absence of p-MLC2 at the stenosed coronary segment and adjacent interstitial small artery wall might be due to the late autopsy time after death (6 days), which can result in dephosphorylation of MLC2 if delayed. Taken together, these data suggest that 75% of cases (3 out of the 4) showed significantly positive staining of p-MLC2 in autopsied cases with no significant pathologic findings other than mild coronary artery stenosis.
Table 1.
Characteristics of the 4 sudden unexpected deaths.
| Case No. | Age (y) | Sex | Circumstances surrounding deaths | PMI (d) | Gross findings of the heart | Microscopic examination of the heart |
|---|---|---|---|---|---|---|
| 1 | 41 | M | Physically exercising | 3 | LADA mild stenosis | Mild atherosclerosis of LADA |
| 2 | 67 | F | Had an altercation | 2 | LADA mild stenosis, increased heart weight | Mild atherosclerosis of LADA, focal interstitial fibrosis |
| 3 | 56 | M | Had an altercation | 1 | LADA mild stenosis, mottled appearance at left anterior ventricle | Mild atherosclerosis of LADA, focal interstitial fibrosis |
| 4 | 71 | M | Had an altercation | 6 | LADA and RCA stenosis | Mild atherosclerosis of LADA and RCA |
M – male; F – female; PMI – postmortem interval; LADA – left anterior descending artery; RCA – right coronary artery.
Figure 4.
MLC2 is highly phosphorylated in sudden cardiac deaths with mild coronary artery stenosis. In Case 1, the p-MLC2 was positively stained around LADA wall (A) with a focally magnified view in (B). Adjacent to the stenosed LADA segment, the interstitial small artery was also positively stained of p-MLC2 (C). In Case 2, the p-MLC2 was strong-positively stained around the LADA wall (D) with a focally magnified view in (E). Adjacent to the stenosed LADA segment, interstitial small artery was also positively stained p-MLC2 (F). In Case 3, the p-MLC2 was strong-positively stained around the LADA wall (G) with a focally magnified view in (H). Adjacent to the stenosed LADA segment, the interstitial small artery also had positively stained p-MLC2 (I). Magnification: 100× for upper images and 400× for bottom images. Red arrows indicate strongly positive staining.
Figure 5.
MLC2 is barely phosphorylated in Case 4. In the LADA, although the lumen was mildly stenosed, p-MLC2 was barely detected under low (A) and high (B) magnification views. Adjacent to the LADA with lesions, the interstitial small artery was also barely stained with p-MLC2 (C). In the RCA, p-MLC2 was barely detected under low (D) and high (E) magnification views. Adjacent to the LADA with lesions, the interstitial small artery was also barely stained with p-MLC2 (F). Magnification: 100× for upper images and 400× for bottom images. Blue arrows indicate mildly positive staining. Magnified insets at right bottoms of (B) and (E) highlight the almost negative staining of p-MLC2 within coronary artery walls.
Discussion
Despite the predisposition of coronary artery disease to circulatory collapse and death by coronary artery spasm (CAS), no specific molecular marker has been identified to suggest an antemortem CAS at autopsy [9]. The diagnosis of antemortem CAS in patients with coronary artery diseases is made mainly based on comprehensive evaluation of information, including clinical angiographies (if any), premonitory distress, spastic triggers (e.g., catecholamine rapid rise by physical or mental stress), and morphological changes of the coronary artery wall. The present study investigated the potential of using p-MLC2 as a molecular marker of antemortem CAS.
Our data initially showed that common vasoconstrictors such as PGF2α, ACh, and 5-HT increased the p-MLC2 levels in human VSMCs. Spasm provocation tests also showed that the p-MLC2 level was consistently elevated in spastic coronary arteries of rats. These data indicated that hyper-phosphorylated MLC2 was accompanied with smooth muscle contraction induced by vasoconstrictors. We further assessed the effects of MLC2 phosphorylation (via short-term exposure to vasoactive stimuli) and dephosphorylation (via long-term exposure) on contraction of VSMCs by assuming the cause-effect relationship between increased p-MLC2 level and smooth muscle contractile activity. The function of MLC2 depends largely on its phosphorylation by myosin light chain kinases (MLCK) [27] at both Ser 19 and Thr 18 sites [28,29], but fades after dephosphorylation by myosin light chain phosphatase (MLCP) [30]. Phosphorylation of MLC2 at either site leads to activation of intracellular cytoskeletal contraction-relaxation cycles and favors contractile activity [31,32], whereas dephosphorylation of MLC2 slows relaxation of arterial smooth muscle [28] and improves vascular endothelial dysfunction [32]. Based on our observations in Figure 1, MLC2 in VSMCs with short-term exposure to vasoconstrictors was phosphorylation-dominant, whereas it was more likely dephosphorylated if exposure time was extended. Hence, short-term and long-term exposures were used to mimic endogenous phosphorylation and dephosphorylation, respectively, of MLC2 in VSMCs. Our results show that short-term exposure to vasoconstrictors (phosphorylation-dominant) led to increasingly arranged myofilament (as developed by p-MLC2), whereas long-term exposure (dephosphorylation of MLC2) resulted in disarrayed myofilaments and dimmed fluorescence. These observations support our hypothesis that phosphorylation of MLC2 accounts for the hypercontractile activity of VSMCs, consistent with previous reports that attributed smooth muscle contraction to phosphorylation of MLC2 [21,28].
One great novelty of this study was that we evaluated the potential of using p-MLC2 as a diagnostic marker for antemortem CAS at autopsy. Four autopsied cases that had known antemortem CAS and death triggers were obtained. Mild coronary stenosis was grossly observed in these cases. Using the IHC analysis, we found that 3 out of the 4 cases showed strongly positive staining of p-MLC2 at both the occluded coronary segment and adjacent interstitial small arteries. Positive staining of p-MLC2 was mainly accumulated in the tunica media around artery wall, which is of great supportive value since VSMCs are histologically lined up predominantly in the tunica media. Of note, the fourth case, with coronary artery stenosis at both RCA and LADA, showed barely positive staining of p-MLC2. Delayed autopsy in this case (6 days after death) might be assumed. Phosphorylation itself is time-sensitive and reversible [33]. It has been shown that kinases may be altered and thus imbalance the phosphorylation/dephosphorylation processes after death [34]. Our in vitro assays also supported that long-term exposure to vasoconstrictors favored dephosphorylation of MLC2 in VSMCs. Hence, delayed autopsy might be a factor involved in the negative identification of phosphoproteins. Autopsy should be initiated as soon as possible in deaths suspected of CAS. In all, the high positive rate of p-MLC2 (75%) in these patients with nonlethal coronary artery disease shows the importance of additional analysis such as p-MLC2 detection in cases that would be otherwise diagnosed as sudden infant death syndrome (SIDS) or sudden unexpected nocturnal death syndrome (SUNDS) [35]. Routine detection of p-MLC2 using IHC might be beneficial in the diagnosis of antemortem CAS.
Interestingly, thrombi in the lumina of the coronary artery also showed strongly positive staining of p-MLC2, as seen in Case 2 and Case 3. Thrombus is mainly composed of red blood cells, platelets, and fibrin, with the absence of VSMCs. The strong positive staining of p-MLC2 found in thrombi after coronary artery stenosis merits further investigation.
There are several limitations to the present study. We only collected 4 autopsy cases. The time-based detection of p-MLC2 should be conducted in a larger human sample size with different autopsy times after death. MLC2 could be monophosphorylated (at Ser 19 or Thr 18) and diphosphorylated (at both Ser 19 and Thr 18) [28,29], but our study only detected monophosphorylation at Ser 19. Whether phosphorylation at Thr 18 has any synergistic or antagonistic effect on p-MLC2 (at Ser 19)-mediated contractile activity remains to be elucidated. This limitation also raises great interest into future investigation of whether p-MLC2 at Thr 18 could serve as a diagnostic marker of antemortem CAS.
Conclusions
The present study showed p-MLC2 levels were increased in vasoconstrictors-treated VSMCs and in spasm provocation tests. The strongly positive staining of p-MLC2 in 75% of cases with antemortem CAS strongly suggests the usefulness of p-MLC2 as a postmortem diagnostic marker of antemortem CAS at autopsy. Unlike the morphological changes occurring after CAS [1,9–11], p-MLC2 could serve as a molecular marker that avoids interobserver variability and hence confers objectivity. Autopsy should be initiated as soon as possible in deaths suspected of CAS.
Acknowledgements
We heartily thank Dr. Jennifer Bynum from the Department of Pathology, Johns Hopkins Hospital, Baltimore, MD, USA for her help in polishing our manuscript.
Abbreviations
- MLC2
myosin light chain 2
- CAS
coronary artery spasm
- VSMCs
vascular smooth muscle cells
- PGF2α
prostaglandins F2 alpha
- ACh
acetylcholine
- 5-HT
5-hydroxy tryptamine
- IHC
immunohistochemistry
- IF
immunofluorescence
- MLCK
myosin light chain kinase
- BCA
bicinchoninic acid
- GAPDH
glyceraldehydes phosphate dehydrogenase
- LADA
left anterior descending artery
- RCA
right coronary artery
- SIDS
sudden infant death syndrome
- SUNDS
sudden unexpected nocturnal death syndrome
Footnotes
Statement
The authors declare that they have no competing interests
Source of support: This work was supported by the Open Project Program of Shanghai Key Laboratory of Forensic Medicine (No. KF1306)
References
- 1.Mortensen ES, Rognurn TO, Straume B, Jorgensen L. Evidence at autopsy of spasm in the distal right coronary artery in persons with coronary heart disease dying suddenly. Cardiovasc Pathol. 2007;16:336–43. doi: 10.1016/j.carpath.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Basso C, Burke M, Fornes P, et al. Guidelines for autopsy investigation of sudden cardiac death. Virchows Archiv. 2008;452:11–18. doi: 10.1007/s00428-007-0505-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang HY, Yao QS, Zhu SH, et al. The autopsy study of 553 cases of sudden cardiac death in Chinese adults. Heart Vessels. 2014;29:486–95. doi: 10.1007/s00380-013-0388-0. [DOI] [PubMed] [Google Scholar]
- 4.Maseri A, Labbate A, Baroldi G, et al. Coronary vasospasm as a possible cause of myocardial-infarction – conclusion derived from study of preinfarction angina. New Engl J Med. 1978;299:1271–77. doi: 10.1056/NEJM197812072992303. [DOI] [PubMed] [Google Scholar]
- 5.Buxton AE, Goldberg S, Harken A, et al. Coronary-artery spasm immediately after myocardial revascularization – recognition and management. New Engl J Med. 1981;304:1249–53. doi: 10.1056/NEJM198105213042101. [DOI] [PubMed] [Google Scholar]
- 6.Fukai T, Koyanagi S, Takeshita A. Role of coronary vasospasm in the pathogenesis of myocardial-infarction – study in patients with no significant coronary stenosis. Am Heart J. 1993;126:1305–11. doi: 10.1016/0002-8703(93)90527-g. [DOI] [PubMed] [Google Scholar]
- 7.Bogaty P, Hackett D, Davies G, Maseri A. Vasoreactivity of the culprit lesion in unstable angina. Circulation. 1994;90:5–11. doi: 10.1161/01.cir.90.1.5. [DOI] [PubMed] [Google Scholar]
- 8.Turan T, Agac MT, Aykan AC, et al. Usefulness of heart-type fatty acid-binding protein and myocardial performance index for early detection of 5-fluorouracil cardiotoxicity. Angiology. 2016 doi: 10.1177/0003319716637516. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Factor SM, Cho S. Smooth-muscle contraction bands in the media of coronary-arteries – a postmortem marker of antemortem coronary spasm. J Am Coll Cardiol. 1985;6:1329–37. doi: 10.1016/s0735-1097(85)80221-7. [DOI] [PubMed] [Google Scholar]
- 10.Lin CS, Goldfischer M, Sicular A, et al. Morphodynamics and pathology of blood vessels III – comparative morphologic study of contraction of smooth muscle cells of hollow viscera and its application to vasoconstriction and vasospasm. Angiology. 1998;49:503–22. doi: 10.1177/000331979804900701. [DOI] [PubMed] [Google Scholar]
- 11.Svendsen E, Tindall AR. The internal elastic membrane and intimal folds in arteries – important but neglected structures? Acta Physiol Scand Suppl. 1988;572:1–71. [PubMed] [Google Scholar]
- 12.Vanhoutte PM, Shimokawa H. Endothelium-derived relaxing factor and coronary vasospasm. Circulation. 1989;80:1–9. doi: 10.1161/01.cir.80.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Kaski JC, Maseri A, Vejar M, et al. Spontaneous coronary-artery spasm in variant angina is caused by a local hyperreactivity to a generalized constrictor stimulus. J Am Coll Cardiol. 1989;14:1456–63. doi: 10.1016/0735-1097(89)90382-3. [DOI] [PubMed] [Google Scholar]
- 14.Bertrand ME, Lablanche JM, Tilmant PY, et al. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary arteriography. Circulation. 1982;65:1299–306. doi: 10.1161/01.cir.65.7.1299. [DOI] [PubMed] [Google Scholar]
- 15.Forman MB, Oates JA, Robertson D, et al. Increased adventitial mast-cells in a patient with coronary spasm. New Engl J Med. 1985;313:1138–41. doi: 10.1056/NEJM198510313131807. [DOI] [PubMed] [Google Scholar]
- 16.Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–82. doi: 10.1161/CIRCULATIONAHA.111.037283. [DOI] [PubMed] [Google Scholar]
- 17.Furchgott RF, Zawadzki JV. The obligatory role of endothelial-cells in the relaxation of arterial smooth-muscle by acetylcholine. Nature. 1980;288:373–76. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 18.Mcfadden EP, Clarke JG, Davies GJ, et al. Effect of intracoronary serotonin on coronary vessels in patients with stable angina and patients with variant angina. New Engl J Med. 1991;324:648–54. doi: 10.1056/NEJM199103073241002. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Niu L, Cui L, et al. Hesperetin inhibits rat coronary constriction by inhibiting Ca(2+) influx and enhancing voltage-gated K(+) channel currents of the myocytes. Eur J Pharmacol. 2014;735:193–201. doi: 10.1016/j.ejphar.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 20.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–36. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 21.Tan JL, Ravid S, Spudich JA. Control of nonmuscle myosins by phosphorylation. Annu Rev Biochem. 1992;61:721–59. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]
- 22.van der Voort IR, Knapp J, Konturek JW, et al. Expression and functional role of serine/threonine phosphatases in rat esophagus. Med Sci Monit. 2004;10(5):BR123–29. [PubMed] [Google Scholar]
- 23.Li LL, Xue AM, Li BX, et al. JMJD2A contributes to breast cancer progression through transcriptional repression of the tumor suppressor ARHI. Breast Cancer Res. 2014;16(3):R56. doi: 10.1186/bcr3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sueda S, Kohno H, Miyoshi T, et al. Maximal acetylcholine dose of 200 mug into the left coronary artery as a spasm provocation test: Comparison with 100 mug of acetylcholine. Heart Vessels. 2015;30:771–78. doi: 10.1007/s00380-014-0563-y. [DOI] [PubMed] [Google Scholar]
- 25.Sueda S, Kohno H, Fukuda H, et al. Clinical impact of selective spasm provocation tests: Comparisons between acetylcholine and ergonovine in 1508 examinations. Coron Artery Dis. 2004;15:491–97. doi: 10.1097/00019501-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Gao P, Wei Y, Zhang Z, et al. Synergistic effects of c-Jun and SP1 in the promotion of TGFbeta1-mediated diabetic nephropathy progression. Exp Mol Pathol. 2016;100(3):441–50. doi: 10.1016/j.yexmp.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Walsh MP. Calcium-dependent mechanisms of regulation of smooth-muscle contraction. Biochem Cell Biol. 1991;69(12):771–800. doi: 10.1139/o91-119. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland C, Walsh MP. Myosin regulatory light chain diphosphorylation slows relaxation of arterial smooth muscle. J Biol Chem. 2012;287:24064–76. doi: 10.1074/jbc.M112.371609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scruggs SB, Solaro RJ. The significance of regulatory light chain phosphorylation in cardiac physiology. Arch Biochem Biophys. 2011;510:129–34. doi: 10.1016/j.abb.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartshorne DJ, Ito M, Erdodi F. Role of protein phosphatase type 1 in contractile functions: Myosin phosphatase. J Biol Chem. 2004;279:37211–14. doi: 10.1074/jbc.R400018200. [DOI] [PubMed] [Google Scholar]
- 31.Davis JS, Hassanzadeh S, Winitsky S, et al. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107:631–41. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 32.Cheng XW, Wang XB, Wan YF, et al. Myosin light chain kinase inhibitor ML7 improves vascular endothelial dysfunction via tight junction regulation in a rabbit model of atherosclerosis. Mol Med Rep. 2015;12:4109–16. doi: 10.3892/mmr.2015.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pesaresi P, Pribil M, Wunder T, Leister D. Dynamics of reversible protein phosphorylation in thylakoids of flowering plants: the roles of STN7, STN8 and TAP38. Biochim Biophys Acta. 2011;1807:887–96. doi: 10.1016/j.bbabio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Sato M, Yamanaka H, Iwasaki M, et al. Altered phosphatidylinositol 3-kinase and calcium signaling in cardiac dysfunction after brain death in rats. Ann Thorac Surg. 2016;102(2):556–63. doi: 10.1016/j.athoracsur.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Diaz FJ, Loewe C, Jackson A. Death caused by myocarditis in Wayne County, Michigan: A 9-year retrospective study. Am J Forensic Med Pathol. 2006;27:300–3. doi: 10.1097/01.paf.0000221045.67949.6e. [DOI] [PubMed] [Google Scholar]