Abstract
Background
Calcium overload, inflammation, and apoptosis play important roles in myocardial ischemia-reperfusion injury (MIRI). Gastrodin pretreatment can alleviate MIRI. This study observed sarcoplasmic reticulum calcium transport ATPase (Ca2+-ATPase, SERCA) and calcium phosphate (PLB) protein expression in the ventricular remodeling process after myocardial infarction to explore the effect of gastrodin pretreatment on MIRI.
Material/Methods
Healthy 7-week-old male SD rats were randomly divided into a sham group (A), a model group (B), and gastrodin pretreatment groups C, D, and E (100, 200, and 400 mg/kg, respectively) with 20 in each group. Anterior descending coronary artery ligation method was used to establish a rat MIRI model with 30-min ischemia and 120-min reperfusion. Cardiac electrophysiological activity was recorded. Serum IL-6 and IL10 levels were determined by ELISA. SERCA activity was tested by colorimetric phosphorus method. SERCA, PLB, and pSer-PLB protein expression were detected by Western blot.
Results
Compared with the sham group, IL-6 and IL-10 levels were elevated, SERCA2a expression was downregulated, and PLB protein was elevated in the model group (P<0.05). pSer16-PLB showed no significant difference among groups, and the ratio of pSer16-PLB/PLB obviously decreased (P<0.05). IL-6 level gradually declined and IL-10 increased in the gastrodin group following concentration elevation. SERCA 2a expression rose in the gastrodin group in a dose-dependent manner (P<0.05). Elevated PLB protein expression showed no significant difference, while pSer16-PLB protein increased (P<0.05), leading to elevated pSer16 PLB/PLB ratio (P<0.05).
Conclusions
Gastrodin pretreatment alleviates MIRI and inflammation injury by regulating SERCA and PLB expression to decrease calcium overload.
MeSH Keywords: Gastrointestinal Agents; Hypoxia-Ischemia, Brain; Sarcoplasmic Reticulum Calcium-Transporting ATPases
Background
Acute myocardial infarction is an important cause of sudden cardiac death. Early-stage percutaneous coronary intervention or thrombolytic therapy can result in timely recovery of myocardial reperfusion and reduce myocardial infarction range. However, myocardial ischemia reperfusion can aggravate cardiac dysfunction and myocardial cell damage, leading to intracellular calcium overload, apoptosis, and inflammation. Clarifying the mechanism involved is important in the prevention of myocardial ischemia-reperfusion injury. Research shows that calcium overload, neutrophil aggregation, mitochondrial dysfunction, and myocardial apoptosis participate in the myocardial ischemia-reperfusion injury process [1,2], in which intracellular calcium overload plays a leading role [3,4]. Intracellular Ca2+ imbalance results in Ca2+ separation and Ca2+ influx imbalance, causing myocardial intracellular Ca2+ overload. During myocardial ischemia and hypoxia, mitochondria dysfunction results in insufficient production of ATP and the inhibition of calcium pump. The Na+/Ca2+ exchange protein is an ATP-independent transporter, leading to intracellular Na+ elevation and acidosis. PH and ATP recover during myocardial reperfusion. Extra- and intra-cellular PH gradient difference activates Na+/H+ and Na+/Ca2+ exchange, leading to extracellular Ca2+ influx and calcium overload. It causes transient depolarization after myocardial action potential, triggering ventricular arrhythmia [5,6]. Mitochondria regulate intracellular Ca2+ level through a variety of mechanisms. The reperfusion of ischemic myocardium generates oxidative stress, part of the oxygen paradox, in which the reoxygenation of ischemic myocardium generates a degree of myocardial injury that exceeds the injury due to the ischemia alone. Mitochondrial structural damage leads to mitochondrial dysfunction, generating less ATP and causing energy-dependent calcium pump dysfunction on endoplasm omentum, cell membrane, and sarcoplasm. Excessive intracellular Ca2+ cannot be absorbed or eliminated, resulting in intracellular Ca2+ overload [7,8].
Sarcoplasmic reticulum calcium transport ATPase (Ca2+-ATPase, SERCA) and calcium phosphate (PLB) are key factors of Ca2+ intake in sarcoplasmic reticulum. SERCA is mainly responsible for actively transporting Ca2+ from cytoplasm to sarcoplasmic reticulum during myocardial diastole [9,10]. SERCA protein are classified as SERCA1, SERCA2, and SERCA3 based on encoding gene. SERCA2 is divided into 3 subtypes: SERCA2a, SERCA2b, and SERCA2c. SERCA2a highly expresses in skeletal muscle and myocardial tissue. SERCA overexpression can regulate abnormal Ca2+ level in myocardial tissue [11]. PLB mainly exists in the ventricular muscle cells; it can regulate Ca2+ intake together with SERCA2a through phosphorylation and dephosphorylation. PLB phosphorylation enhances SERCA2a affinity with calcium ions, accelerating sarcoplasmic reticulum calcium intake [12]. Gastrodin is effective monomer composition extracted from Gastrodia elata with various pharmacological activities. It was found that gastrodin pretreatment can alleviate ischemia-reperfusion injury, reduce or inhibit extracellular Ca2+ influx, decrease inflammation factor TNF-α and IL-6 release, prevent arrhythmias during ischemia reperfusion, and increase the scavenging ability of free radicals in rats [13,14]. This study observed SERCA and PLB protein expression in the ventricular remodeling process after myocardial infarction to explore the effect of gastrodin pretreatment on MIRI.
Material and Methods
Materials
Experimental animals and grouping
Healthy 7-week-old male SD rats weighting 220~250 g were provided by the Chinese Academy of Medical Sciences Animal Experiment Center (license SYXK-2013-0025). These specific-pathogen-free (SPF) rats were provided with water and food according to experimental animal standards. The rats were randomly divided into a sham group (A), a model group (B), and gastrodin pretreatment groups C, D, and E (100, 200, 400 mg/kg, respectively) with 20 in each group. Gastrodin was intragastrically administrated at 7 days before modeling (twice a day, every 12 h), and continued 3 days after modeling. The dosing volume was 1 ml/100 g, while the rats in group A and B group were given an equal volume of normal saline.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of Linyi City People’s Hospital.
Experimental drugs and reagents
Gastrodin, triphenyl tetrazolium chloride (TTC), and urethane were purchased from Sigma (0.1 g TTC dissolved in 5 ml PBS for use). IL-6, IL-10, and SERCA activity detection kits were from Nanjing Jiancheng Bioengineering Institute, China. SERCA2a, PLB, and pSer16-PLB protein antibodies were provided by ABR, Santa Cruz Biotechnology, USA. Goat Anti-Mouse IgG F(c) secondary antibody was from ZSbio, China. GAPDH antibody was from Kangchen, China.
Experimental methods
Modeling
The rat ischemia reperfusion model was established according to the reference method [15]. The rats received 5% urethane for abdominal cavity anesthesia, and retained a 24-G needle in the caudal vein. The rats were then fixed and connected by ECG (II lead) and animal breathing machine to control breath (tidal volume 8 ml/kg, breathing frequency 70 times/min, inspiration and expiration ratio 1: 2). The left common carotid artery was separated to connect the electrophysiological signal recorder. Analgesia was used during the operation. The root of the left anterior descending coronary artery was ligated, and the rats in the control group received threading but not ligation. After 30-min ischemia, the rats received reperfusion for 2 h. ECG changes was recorded. Ischemia model judgment was: after the left anterior descending coronary artery was ligated for 5 min, II lead showed ST elevation or QRS complex amplitude increase and broadening fusion with T wave; arterial blood pressure lowering >20 mmHg; and cyanosis appeared in vascular ligation of area. Reperfusion model judgment was: cyanosis area disappeared, elevated ST segment decrease >50% at 30 min after reperfusion, eliminating severe atrioventricular block before reperfusion, mean arterial pressure before ligation < 60 mmHg. The rats were euthanized and the heart tissue was collected at 4 days after modeling.
TTC staining for infarction area determination
After washing with PBS, the left ventricle was frozen at −80°C and cut into slices 5-mm thick. Then the section was incubated in 1% TTC solution at pH8.5 and 37°C for 30 min. The infarction area showed no staining while normal tissue showed reddish brown. Image pro plus 6.0 software was used to calculate infarction area and IS (infarct size)/LV (left ventricular), AAR (area at risk)/LV (left ventricular), and IS (infarct size)AAR (area at risk).
ELISA
Serum IL-6 and IL-10 levels were tested at 450 nm on a microplate reader according to the manual.
SERCA activity detection
SERCA activity was tested by colorimetric phosphorus method according to the kit instructions.
Western blot
Cardiac tissue was cracked by RIPA to extract protein. Protein concentration was determined by Bradford method. The protein was separated by SDS-PAGE and transferred to PVDF membrane. After blocking by skim milk for 2 h, the membrane was incubated in SERCA2a, PLB, and pSer16-PLB monoclonal antibodies (1:1000) at 4ºC overnight, followed by secondary antibody (1:2500) incubation for 1 h. The membrane was developed by chemiluminescent agent and exposed on X-ray. Protein imaging system software and Quantity One imaging analysis software were used for data analysis.
Statistical analysis
SPSS 20.0 (IBM, USA) was used for statistical analysis. Measurement data were first tested by normality test and presented as mean ± standard deviation (χ̄±S). One-way ANOVA or LSD test were used for mean value comparison. P<0.05 was considered as statistical significance.
Results
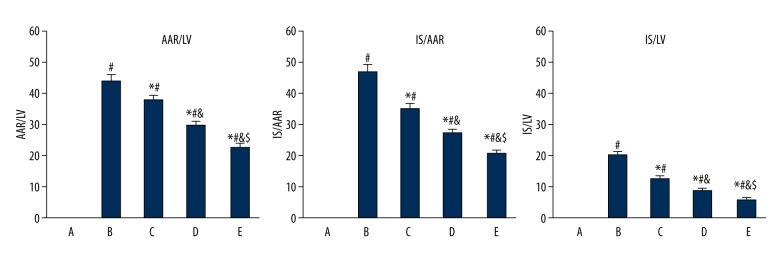
Gastrodin pretreatment impact on IS/LV, IS/AAR, and AAR/LV in ischemia reperfusion rats
The IS and AAR in the groups were B>C>D>E<A (P<0.05), while LV in the groups showed no significant difference (Table 1). IS/LV, IS/AAR, and AAR/LV in the gastrodin pretreatment group were obviously lower than in the model group, and the difference was dose-dependent (P<0.05). Group E showed the most significant effects (Figure 1).
Table 1.
The IS, LV, AAR of rats in different groups.
| Group | Dose (mg/kg) | IS (cm2) | AAR (cm2) | LV (cm2) |
|---|---|---|---|---|
| A | — | 0.00 | 0.00 | 3.56±0.39 |
| B | — | 0.73±0.23# | 1.55±0.27# | 3.51±0.38 |
| C | 100 | 0.48±0.16#* | 1.35±0.29#* | 3.55±0.36 |
| D | 200 | 0.30±0.12#*& | 1.10±0.26#*& | 3.68±0.32 |
| E | 400 | 0.18±0.07#*&$ | 0.85±0.25#*&$ | 3.73±0.41 |
A – control; B – model group; C–E – gastrodin pretreatment group.
P<0.05, compared with group A;
P<0.05, compared with group B;
P<0.05, compared with group C;
P<0.05, compared with group D.
Figure 1.
Gastrodin pretreatment effect on IS/LV, IS/AAR, and AAR/LV in ischemia reperfusion rats. A, control; B, model group; C–E, gastrodin pretreatment group. # P<0.05, compared with group A; * P<0.05, compared with group B; & P<0.05, compared with group C; $ P<0.05, compared with group D.
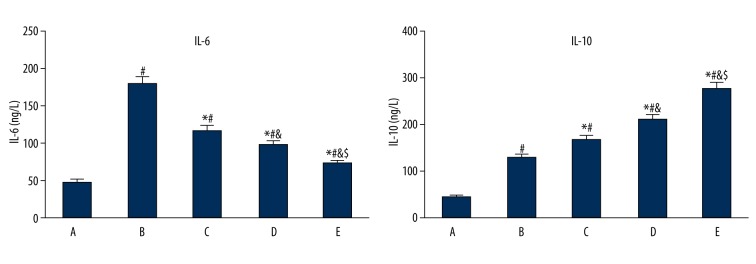
Gastrodin pretreatment impact on serum IL-6 and IL-10 in ischemia reperfusion rats
Serum IL-6 and IL-10 were increased significantly in group B compared with group A (P<0.05). IL-6 decreased, while IL-10 increased in group C, D, and E compared with group B, and the difference was dose-dependent (P<0.05). Group E presented the most remarkable effect (Figure 2).
Figure 2.
Gastrodin pretreatment effect on serum IL-6 and IL-10 in ischemia reperfusion rats. A, control; B, model group; C–E, gastrodin pretreatment group. # P<0.05, compared with group A; * P<0.05, compared with group B; & P<0.05, compared with group C; $ P<0.05, compared with group D.
Gastrodin pretreatment impact on SERCA activity in ischemia reperfusion rats
SERCA activity decreased in group B compared with group A (P<0.05), whereas it increased in groups C, D, E compared with group B, and the difference was dose-dependent (P<0.05). Group E presented the most obvious effect (Figure 3).
Figure 3.
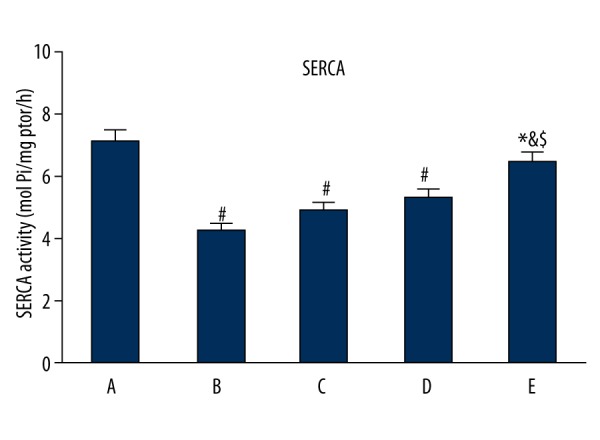
Gastrodin pretreatment impact on SERCA activity in ischemia reperfusion rats. A, control; B, model group; C–E, gastrodin pretreatment group. # P<0.05, compared with group A; * P<0.05, compared with group B; & P<0.05, compared with group C; $ P<0.05, compared with group D.
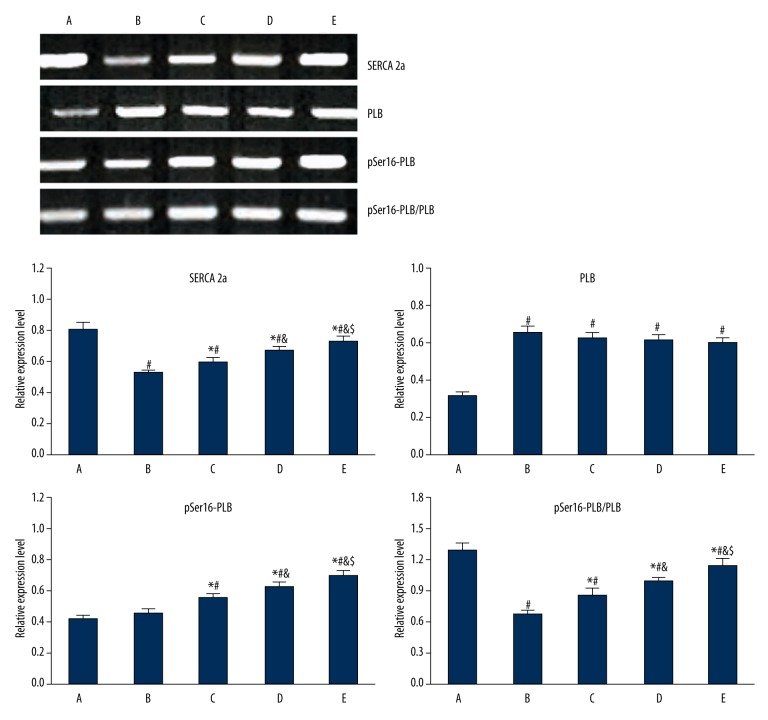
Gastrodin pretreatment impact on SERCA 2a, PLB, and pSer16-PLB protein expression in myocardial tissue
Compared with group A, myocardial tissue in group B showed SERCA 2a downregulation (P<0.05) and PLB elevation (P<0.05). pSer16-PLB showed no significant change, and the ratio of pSer16-PLB/PLB was decreased (P<0.05). SERCA 2a expression was upregulated in the gastrodin group compared with group B (P<0.05). Elevated PLB protein expression showed no significant difference, while pSer16-PLB protein increased (P<0.05), leading to pSer16 PLB/PLB ratio elevation (P<0.05) (Figure 4).
Figure 4.
Gastrodin pretreatment impact on SERCA 2a, PLB, and pSer16-PLB protein expression in myocardial tissue. A, control; B, model group; C, D, E, gastrodin pretreatment group. # P<0.05, compared with group A; * P<0.05, compared with group B; & P<0.05, compared with group C; $ P<0.05, compared with group D.
Discussion
Myocardial ischemia-reperfusion injury is a common pathological change in clinical practice. Coronary artery bypass surgery, thrombolytic therapy, and percutaneous coronary angioplasty significantly improve the prognosis of patients with acute myocardial infarction. However, MIRI, such as arrhythmia and cell apoptosis, seriously affects cardiac function recovery. Alleviating MIRI has a critical role in the treatment of acute myocardial infarction. Gastrodin has a protective effect on cerebral and myocardial ischemia reperfusion. It can enhance coronary blood flow, reduce endothelin-1 expression in MIRI, and alleviate inflammatory factor TNF-α and IL-6 levels. It also can alleviate oxidative stress, prevent thrombosis, decrease peripheral vascular resistance, inhibit myocardial hypertrophy, and suppress myocardial apoptosis [13,14]. This study showed that IS/LV, IS/AAR, and AAR/LV in the gastrodin pretreatment group were lower than in the model group, and the difference was dose-dependent, suggesting that gastrodin pretreatment can protect myocardium by reducing the myocardial infarction area after ischemia reperfusion.
Recent research revealed that inflammatory lesions have an important role in the MIRI process [9,10]. IL-6 is mainly derived from mononuclear macrophages, endothelial cells, vascular smooth muscle cells, and T/B lymphocytes. It can induce ICAM-1 gene expression in myocardial cells and promote granulocytes to enter the myocardial ischemia area. ICAM-1 mRNA overexpression leads to LFA-1 signaling transduction on the surface of neutrophils to promote adhesion of neutrophils and endothelial cells. It also mediates neutrophil infiltration and has a negative inotropic action in proinflammatory factors [11,12]. IL-10 is a type of multi-function negative regulatory factor, and is mainly derived from Th2 cells, activated B cells, monocytes, and macrophages. It is a protective cytokine that participates in a variety of cell biological regulations. It reactively increases after myocardial infarction to downregulate inflammatory reaction. Our results show that serum IL-6 and IL-10 was elevated in the MIRI model group, while IL-6 declined and IL-10 increased in the gastrodin group, and the difference was dose-dependent, indicating that gastrodin can alleviate inflammatory injury and protect myocardial tissue.
Myocardial intracellular calcium homeostasis plays an important role in maintaining heart function. SERCA is a key factor in sarcoplasmic reticulum calcium intake, and SERCA2a is the main form of SERCA expressed in myocardial tissue [16,17]. Myocardial tissue calcium influx after ischemia leads to intracellular calcium overload, calcium pump dysfunction, sarcoplasmic reticulum calcium intake reduction, and SERCA activity decline [18,19]. As the main SERCA regulatory protein, PLB acts through phosphorylation and dephosphorylation [20,21]. Ser16 phosphorylation occurs prior to Thr17 phosphorylation in PLB, and this study tested pSer16-PLB protein expression level. This study showed that, compared with the sham group, myocardial tissue in the model group had decreased SERCA activity, SERCA 2a downregulation, PLB elevation, no significant change in pSer16-PLB, and the ratio of pSer16-PLB/PLB was reduced. SERCA activity was enhanced and SERCA 2a expression was upregulated in the gastrodin group compared with the model group. Elevated PLB protein expression showed no significant difference, while pSer16-PLB protein increased, leading to pSer16-PLB/PLB ratio elevation. Our results indicated that SERCA 2a expression, SERCA activity, and pSer16-PLB/PLB ratio were reduced in MIRI. PLB suppression of SERCA was enhanced, resulting in impaired rat myocardial systolic/diastolic function. Gastrodin pretreatment can regulate calmodulin expression and alleviate ischemia-reperfusion injury.
Conclusions
Gastrodin pretreatment can reduce ischemia-reperfusion injury and inflammation by mediating SERCA and PLB expression, and alleviating intracellular calcium overload.
Abbreviations
- MIRI
myocardial ischemia-reperfusion injury
- SERCA
sarcoplasmic reticulum calcium transport ATPase
- PLB
calcium phosphate
- TTC
triphenyl tetrazolium chloride
- IS
infarct size
- LV
left ventricular
- AAR
area at risk
Footnotes
Source of support: Departmental sources
References
- 1.Correa F, Buelna-Chontal M, Chagoya V, et al. Inhibition of the nitric oxide/cyclic guanosine monophosphate pathway limited the cardioprotective effect of post-conditioning in hearts with apical myocardial infarction. Eur J Pharmacol. 2015;765:472–81. doi: 10.1016/j.ejphar.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Kambara T, Shibata R, Ohashi K, et al. C1q/tumor necrosis factor-related protein 9 protects against acute myocardial injury through an adiponectin receptor I-AMPK-dependent mechanism. Mol Cell Biol. 2015;35:2173–85. doi: 10.1128/MCB.01518-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonca E, Kurt C. Cardioprotective effect of thymoquinone: A constituent of Nigella sativa L., against myocardial ischemia/reperfusion injury and ventricular arrhythmias in anaesthetized rats. Pak J Pharm Sci. 2015;28:1267–73. [PubMed] [Google Scholar]
- 4.Patil KD, Halperin HR, Becker LB. Cardiac arrest: Resuscitation and reperfusion. Circ Res. 2015;116:2041–49. doi: 10.1161/CIRCRESAHA.116.304495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu HJ, Yang JY, Jin M, et al. Glycyrrhetinic Acid protects the heart from ischemia/reperfusion injury by attenuating the susceptibility and incidence of fatal ventricular arrhythmia during the reperfusion period in the rat hearts. Cell Physiol Biochem. 2015;36:741–52. doi: 10.1159/000430134. [DOI] [PubMed] [Google Scholar]
- 6.Miskolczi G, Gonczi M, Kovacs M, et al. Further evidence for the role of gap junctions in the delayed antiarrhythmic effect of cardiac pacing. Can J Physiol Pharmacol. 2015;93:545–53. doi: 10.1139/cjpp-2014-0518. [DOI] [PubMed] [Google Scholar]
- 7.Barrabes JA, Inserte J, Agullo L, et al. Effects of the selective stretch-activated channel blocker GsMtx4 on stretch-induced changes in refractoriness in isolated rat hearts and on ventricular premature beats and arrhythmias after coronary occlusion in swine. PLoS One. 2015;10:e0125753. doi: 10.1371/journal.pone.0125753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szepesi J, Acsai K, Sebok Z, et al. Comparison of the efficiency of Na+/Ca2+ exchanger or Na+/H+ exchanger inhibition and their combination in reducing coronary reperfusion-induced arrhythmias. J Physiol Pharmacol. 2015;66:215–26. [PubMed] [Google Scholar]
- 9.Smith IC, Vigna C, Levy AS, et al. The effects of buthionine sulfoximine treatment on diaphragm contractility and SERCA pump function in adult and middle aged rats. Physiol Rep. 2015;3:e12547. doi: 10.14814/phy2.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komin N, Moein M, Ellisman MH, Skupin A. Multiscale modeling indicates that temperature dependent [Ca2+]i spiking in astrocytes is quantitatively consistent with modulated SERCA activity. Neural Plast. 2015;2015:683490. doi: 10.1155/2015/683490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan J, Schmid E, Hosseinzadeh Z, et al. Impact of Janus kinase 3 on cellular Ca release, store operated Ca(2+) entry and Na(+)/Ca(2+) exchanger activity in dendritic cells. Cell Physiol Biochem. 2015;36:2287–98. doi: 10.1159/000430192. [DOI] [PubMed] [Google Scholar]
- 12.Wiernicki B, Rozwadowska N, Malcher A, et al. Human myoblast transplantation in mice infarcted heart alters the expression profile of cardiac genes associated with left ventricle remodeling. Int J Cardiol. 2015;202:710–21. doi: 10.1016/j.ijcard.2015.09.115. [DOI] [PubMed] [Google Scholar]
- 13.Peng Z, Wang S, Chen G, et al. Gastrodin alleviates cerebral ischemic damage in mice by improving anti-oxidant and anti-inflammation activities and inhibiting apoptosis pathway. Neurochem Res. 2015;40:661–73. doi: 10.1007/s11064-015-1513-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Zhang R, Qiao Y, et al. Gastrodin ameliorates depression-like behaviors and up-regulates proliferation of hippocampal-derived neural stem cells in rats: involvement of its anti-inflammatory action. Behav Brain Res. 2014;266:153–60. doi: 10.1016/j.bbr.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 15.Lungkaphin A, Pongchaidecha A, Palee S, et al. Pinocembrin reduces cardiac arrhythmia and infarct size in rats subjected to acute myocardial ischemia/reperfusion. Appl Physiol Nutr Metab. 2015;40:1031–37. doi: 10.1139/apnm-2015-0108. [DOI] [PubMed] [Google Scholar]
- 16.Soller KJ, Verardi R, Jing M, et al. Rheostatic regulation of the SERCA/phospholamban membrane protein complex using non-coding RNA and single-stranded DNA oligonucleotides. Sci Rep. 2015;5:13000. doi: 10.1038/srep13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vutthasathien P, Wattanapermpool J. Regular exercise improves cardiac contractile activation by modulating MHC isoforms and SERCA activity in orchidectomized rats. J Appl Physiol (1985) 2015;119:831–39. doi: 10.1152/japplphysiol.00224.2015. [DOI] [PubMed] [Google Scholar]
- 18.Tran K, Loiselle DS, Crampin EJ. Regulation of cardiac cellular bioenergetics: Mechanisms and consequences. Physiol Rep. 2015;3:e12464. doi: 10.14814/phy2.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devenyi RA, Sobie EA. There and back again: Iterating between population-based modeling and experiments reveals surprising regulation of calcium transients in rat cardiac myocytes. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.07.016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu W, Jiang WB. Pitavastatin-attenuated cardiac dysfunction in mice with dilated cardiomyopathy via regulation of myocardial calcium handling proteins. Acta Pharm. 2014;64:105–15. doi: 10.2478/acph-2014-0004. [DOI] [PubMed] [Google Scholar]
- 21.Sirenko S, Maltsev VA, Maltseva LA, et al. Sarcoplasmic reticulum Ca2+ cycling protein phosphorylation in a physiologic Ca2+ milieu unleashes a high-power, rhythmic Ca2+ clock in ventricular myocytes: Relevance to arrhythmias and bio-pacemaker design. J Mol Cell Cardiol. 2014;66:106–15. doi: 10.1016/j.yjmcc.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]