Human stem cell-derived preparations can generate insulin-producing implants in immune-incompetent mice. This study proposes the combined use of in vitro, in vivo, and ex vivo markers to evaluate the implants as beta cell replacement therapy. They can be translated to clinical studies after validation in preclinical models.
Keywords: Diabetes, Insulin, Transplantation, Cell therapy, Encapsulation
Abstract
A depleted β-cell mass causes diabetes complications that cannot be avoided by insulin administration. β-Cell replacement can stop their development when restoring insulin’s homeostatic role. This requires a sufficient number and an adequate functional state of the β cells, together defined as “functional β-cell mass.” Intraportal implants of human pancreatic islet cells correct hyperglycemia in patients with type 1 diabetes, but this effect is transient and often incomplete. Studies to improve outcome are hindered by shortage in donor pancreases. Human pluripotent stem cells are a candidate source for mass production of grafts for β-cell replacement. Their in vitro differentiation to pancreatic endoderm (stage 4) and to β-cell-containing preparations (stage 7) provides grafts that generate β-cell implants in mice. In vivo markers indicated a better outcome of device-encapsulated stage 4 cells and microencapsulated stage 7 cells as compared with nonencapsulated grafts. Encapsulation also offers the advantage of representative implant retrieval for direct analysis by ex vivo markers. Combination of in vitro, in vivo, and ex vivo markers allows comparison of different stem cell-derived grafts and implants, with each other and with clinical islet cell preparations that serve as reference. Data in mice provide insights into the biology of stem cell-generated β-cell implants, in particular their capacity to establish and sustain a functional β-cell mass. They can thus be indicative for translation of a graft to similar studies in patients, where metabolic benefit will be an additional marker of primordial importance.
Significance
Human stem cell-derived preparations can generate insulin-producing implants in immune-incompetent mice. Steps are undertaken for translation to patients with type 1 diabetes. Their therapeutic significance will depend on their capacity to establish a functional β-cell mass that provides metabolic benefit. This study proposes the combined use of in vitro, in vivo, and ex vivo markers to assess this potential in preclinical models and in clinical studies.
Need for β-Cell Replacement Therapy in Diabetes
The pancreatic β-cell population is responsible for a tight control of glucose homeostasis so that metabolic needs are adequately met and consequences of abnormally low or high glucose levels avoided. This role requires a sufficient number of β cells and an adequate functional state of the cells, collectively defined as “functional β-cell mass” (FBM) [1]. A deficit of one component can cause diabetes; the resulting hyperglycemic state can subsequently impair the other component and thus aggravate the disease.
Type 1 diabetes is caused by an autoimmune-mediated loss in β-cell number. Insulin administration can compensate the endogenous depletion of the hormone but cannot replace the finely regulated insulin provision by a β-cell population that can adapt its cell number and functions to metabolic needs. It reduces but does not prevent acute and chronic complications of the disease. Type 2 diabetes usually presents as an impaired functional state of the β-cell population, associated to a state of insulin resistance. An insufficient β-cell number can also be implicated if not from the start, then later as a consequence of chronic metabolic disturbances, proceeding to a need for exogenous insulin.
Restoring β-cell number represents the treatment of choice for patients with type 1 diabetes, as well as for a subgroup of patients with type 2 diabetes. It is expected to cure the disease when the replacement β cells exhibit an adequate functional state and thus alleviate its heavy burden on patients and society. Cell therapy for diabetes should thus not only be judged on its ability to replace insulin injections by an endogenous source for the hormone but also, and primarily, on its capacity to restore a rapid and metabolically appropriate insulin delivery in response to acute and chronic glucose variations, a hallmark for a tight glucose control. Strategies for developing such therapy should therefore be guided by markers that assess its ability to generate a functional β-cell mass with adequate and sustained β-cell numbers and functional state.
Benefit and Limitations of Islet Cell Grafts Produced From Human Donor Pancreases
Studies in rodents have demonstrated that diabetes caused by β-cell depletion can be corrected by implants of syngeneic or allogeneic pancreatic islet cells, whereby an intraportal location appeared the most effective [2]. Intraportal transplantation of human islet cell allografts was subsequently shown to restore endogenous glucose control in patients with type 1 diabetes, but this effect is often incomplete and declines during the following years [3]. Several reasons, probably in combination, can explain this shortcoming: an insufficient functional β-cell mass in the graft, unfavorable engraftment conditions, (auto)immune and inflammatory reactivity of recipients, and cytotoxicity of immune-suppressive compounds. The deficit is already detectable from the first months posttransplantation (PT) as shown by the implant’s insulin secretory response during hyperglycemic clamp, an in vivo marker for its FBM [4, 5]. Implants that achieved insulin-independence in initially β-cell-depleted patients exhibited, at PT month 12, a functional capacity less than 60% of that in matched normal controls, with further decline during subsequent years; it was lower in recipients who did not become insulin independent. Despite this shortcoming, implants exerted a metabolic benefit for several years as reflected by reduced HbA1c levels and glycemic variability: this was the case when FBM was restored to minimally 37% of normal control values [5].
Achievement of a metabolic benefit, be it transient and of variable duration, brought islet cell transplantation as option for β cell-depleted patients whose problematic glucose control results in recurrent hypoglycemia. It is in this clinical setting that improvement of current protocols is sought. A major target is the considerable β-cell loss shortly after intraportal injection [6]. Plasma discharge of glutamate decarboxylase (GAD65), a β-cell-specific protein, can serve as marker for this process [7]; comparison of GAD65 levels in the circulation and in the initial grafts suggests that 5% to 40% of the cells are lost during the first PT day [6]. A variable discharge was also detected through plasma microRNA375 levels [8]. It is unclear whether this wide range is related to variability in grafts or in the engraftment in the liver. Implants in a confined extrahepatic site may reduce this variability and thus set conditions to evaluate advantages of associated measures. Anti-inflammatory compounds might suppress the consequences of local damage and of innate reactivity; this is supported by the improved outcome of intraportal islet cell grafts when combined with the CXCR1/2 allosteric inhibitor reparixin [9]. Encapsulation of grafts could protect them against both inflammatory and immune reactivity, as shown in laboratory models [10]. Prevascularization of the implant site is another option to reduce cell losses. However, shortage in human donor pancreases, and variability in their quality, form a serious obstacle to undertaking and comparing different protocols to improve the outcome of human islet cell grafts.
Alternative Sources for β-Cell Replacement in Diabetes
Need for Alternative Sources
The discrepancy between the number of human β-cell grafts that can be prepared from human donor pancreases and the number of patients with diabetes who could benefit from a β-cell replacement therapy have been long recognized. The Belgian organ donation policy and procurement network (Eurotransplant Foundation, Leiden, Netherlands) optimizes the number of organs that become available for clinical transplantation. On average, 100 donor pancreases are retrieved annually in Belgium, with a population of 11 million people. A maximum of 20 clinical islet cell grafts can be produced by a team available 24 hours per day when taking the following into consideration: priority of organs in relation to whole pancreas transplantation, insufficient quality of many organs at arrival, loss of islet cells during isolation and culture procedure, and the need for combining preparations from two to six organs. This number sharply contrasts with the number of patients with type 1 diabetes in this region, totaling an estimated 50,000 cases, with an annual average of 1,155 new-onset cases among patients younger than 40 years (Belgian Diabetes Registry). Although all these patients would benefit from β-cell replacement therapy, not all are considered candidate recipients, at least not in the near future. Inclusion criteria are presently restricted to patients who have been β-cell-depleted for several years and whose unstable metabolic control is associated with hypoglycemic unawareness; their number still markedly exceeds graft availability. These criteria will broaden when protocols demonstrate longer efficacy of implants and no longer require continued administration of immune suppressive drugs; consequently, shortage in clinical grafts will augment. Alternative sources for clinical cell grafts are therefore needed and should be developed for large-scale implementation. In the short term, protocols that result in higher efficacy and better safety than is presently the reality should be the goal.
Alternative Sources in Clinical Development
One Track Uses Primary β-Cell Grafts Isolated From Porcine Pancreases
This approach implements procedures for mass isolation of grafts from late fetal, neonatal, young or adult organs, each found to correct diabetes in small and large animal models. Although these different types of implants have not yet been sufficiently investigated and compared for their biologic properties, porcine islet cell grafts are expected to be superior to currently used human islet cell grafts in terms of their capacity to reproducibly achieve a metabolically adequate functional β-cell mass with physiologic properties. Their production can rely on expertise in industrial pig farming for breeding high-health, medical-grade pigs as organ donors, with the option of using sources that are genetically engineered to facilitate cell survival in humans. Guidelines for clinical testing have been established [11] and measures identified for protecting survival of xenografted porcine β cells in rodents and nonhuman primates [10, 12–14]. It will be necessary to define a combination protocol that is not associated with a higher morbidity than that observed with current human islet cell transplant protocols.
Second Track Uses Human β (Progenitor) Cells Derived In Vitro From Pluripotent Stem Cells
This track is based on insights into the biology of stem cells and pancreas development [15, 16], as well as on pioneering work of Novocell/ViaCyte investigators who directed differentiation of human embryonic stem cells (hESCs) to pancreatic endoderm that forms β cells following transplantation in mice [17, 18]. Their methods were adopted, adapted, and extended by other teams, leading to in vitro preparation of different stages in the differentiation of hESCs to β cells and implementation to human-induced pluripotent stem cells [19–23]. The clinical potential of any of these preparations will appear from its ability to form β-cell implants with physiologic properties and, hence, a metabolically adequate functional β-cell mass. The present article discusses markers that can be used in this evaluation.
β-Cell Implants Formed by Human Stem Cell-Derived Grafts
Human stem cell-derived grafts can form β-cell implants in immune-deficient mice. The first demonstration came for grafts of hESC-derived pancreatic endoderm (hPE) that were implanted in the epididymal fat pad [18]. Donor cells were collected at stage 4 of the in vitro differentiation (Fig. 1) and characterized by more than 70% PDX1+NKX6.1+ hormone-negative cells; the small percentages staining for insulin, glucagon, or somatostatin were often double-hormone positive. From PT month 3, implants exhibited a glucose-dependent human C-peptide (hC-peptide) release that protected against chemically induced diabetes; they consisted of more than 50% single-hormone positive cells, staining for insulin, glucagon and somatostatin [18]. This was also the case when implanted in a macroencapsulating device under the skin [24]. In preparation for studies on patients, ViaCyte brought in vitro steps to a scalable, controlled, and regulated cell manufacturing process with standardized quality control procedures [25]. Clinical grafts are produced as combination product (VC-01) consisting of hPE derived from the CyT49 hESC line and a device (Encaptra, ViaCyte, San Diego, CA, http://www.viacyte.com). A prototype of this macrocapsule was shown to protect against alloreactivity in rats [26]. Clinical testing has been launched in a U.S. Food and Drug Administration-approved phase 1 study in patients with type 1 diabetes (ClinicalTrials.gov identifier: NCT02239354).
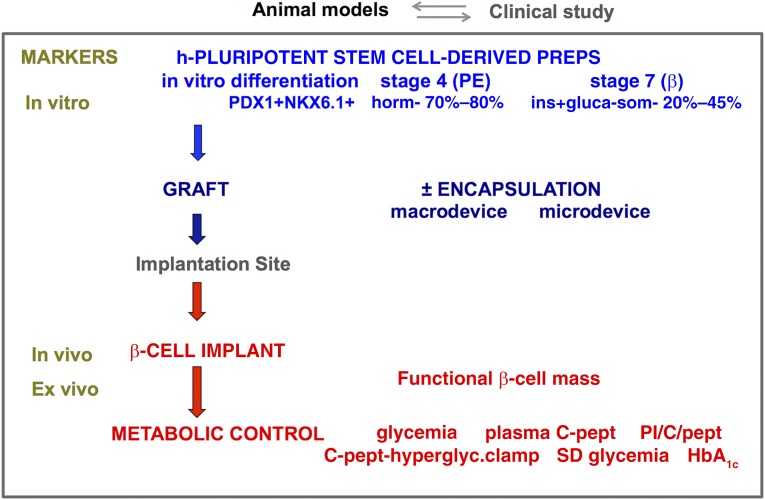
Figure 1.
Combination of markers to assess human stem cell-generated β-cell implants. Human pluripotent stem cell-derived grafts were shown to generate β-cell-containing implants in mice (see text for references). Grafts consisted of differentiation stage 4 or stage 7 cells, characterized by their respective in vitro markers for pancreatic endoderm (PDX1+/NKX6.1+ hormone negative) or for monohormonal β cells (PDX1+/NKX6.1+/INS+). They were implanted without encapsulation in the fat pad, kidney capsule, or subcutis, or they were macroencapsulated in the subcutis or microencapsulated in the peritoneal cavity. β-Cell implants were examined by in vivo and ex vivo markers. Their functional β-cell mass is a key read-out as it determines metabolic control, for which plasma C-peptide, proinsulin/C-peptide ratio, and glycemia serve as markers in mice. A similar assessment can be conducted in patients, where additional data on hyperglycemic clamp, glycemic variability, and HbA1c will indicate whether treatment goals are reached. Abbreviations: PE, pancreatic endoderm; PI/C-pept, proinsulin over C-peptide molar ratio.
The ViaCyte protocol served as basis for modifications and extensions that led to in vitro production of hPE with more homogenous composition (stage 4) and further differentiated preparations containing single-hormone-positive β cells (stage 7) [21–23, 27]. The term “β cell” defines an insulin-positive cell with granule-containing secretory vesicles as described in primary β cells but does not imply exhibition of the same functional properties, or an evolution to such state. In fact, β cells in stage 7 showed little glucose responsiveness of their secretory activity; their purity varied from 20% to 45% with only few other endocrine cells present, several with double-hormone positivity. β-Cell purity could be increased by dissociation of the aggregates and reaggregation [27]. Both stage 4 and stage 7 preparations can form β-cell implants following transplantation (Fig. 1). Stage 4 cells with high NKX6.1+ expression were found to be the source for single-insulin positive cells during further in vitro or in vivo differentiation [20, 23, 27].
Assessment of Human Stem Cell-Generated β-Cell Implants
Stem cell generation of human β-cell implants that control glycemia in mice is a major achievement. It provides proof of principle for this large-scale cell source as candidate therapy for patients with diabetes. Studies in rodents also indicated advantages of macro- and microencapsulation in facilitating and/or preserving β-cell functions [19, 24, 28], opening perspectives for their protective role against the auto-, allo-, and innate reactivity in patients with type 1 diabetes. Experimental conditions did, however, markedly vary in terms of graft characteristics, selected recipients and implant site, read-out criteria, and follow-up with time. They showed similarities between stem cell-generated β cells and primary human β cells, but this reference preparation exhibited poor glucose responsiveness in several studies, raising the need for further evaluation. Researchers also reported differences and attributed them to an immature stage of the newly formed β cells. The standpoint that in vitro-derived human β cells (stage 7) represent a better transplant source than their progenitor preparation (stage 4) [22] is so far only hypothetical as it has not yet been supported by data. This and other key issues of therapeutic relevance are best addressed by comparative analysis before and following transplantation using the same preclinical models and markers. A major read-out is the reproducible establishment of an adequate functional β-cell mass leading to in vivo markers of metabolic control (Fig. 1). Such comparative study has so far not been conducted. In this section, we will discuss the utility of ex vivo markers that analyze composition and function of retrieved stem cell-generated implants. They help interpret in vivo data and directly assess similarities and differences with human primary β cells. Our own data have been collected from implants formed by hESC-derived pancreatic endoderm cells in mice [24]. They serve as a viewpoint on data reported by other laboratories on implants formed by hPE-stage 4 cells [19, 20] or by β-stage 7 cells [21–23, 27, 28] (Fig. 1).
In Vivo Markers for Implants
Plasma hC-peptide levels serve as a circulating marker for the presence of human β cells. Reported data were often collected following intraperitoneal glucose injection, whereby dose and timing can influence measurements (minute 15 in our laboratory [24], and minute 30 and minute 60 in others [18–23, 27]). The peptide appears faster after implanting a β-cell-containing graft (stage 7, within 2 weeks) than one with its progenitors (stage 4, from week 12). It increases during follow-up for both types of implants, which can be attributed to formation of more β cells, activation of more β cells into release, and/or release of more insulin by activated cells; analysis of retrieved implants can identify the underlying mechanism(s). Plasma hC-peptide appearance and level are also influenced by the number of implanted cells and their encapsulated or nonencapsulated form. Subcutaneous implants of the same number of stage 4 cells reached higher levels when macroencapsulated in a device than nonencapsulated, whereas no hC-peptide was detected when microencapsulated [24]. Alginate microencapsulation may thus impair formation of β cells from human progenitor cells; it does not interfere with their hormone secretion as shown by its outcome with human primary β cells as well as with human stem cell-derived β cells (stage 7) following injection in the peritoneal cavity [28, 29]. Studies with human primary β cells showed that hC-peptide levels higher than 3 ng/ml were associated with correction of hyperglycemia and thus indicative for therapeutic insulin effects in mice [29]. Such levels have been reported for both types of stem cell-derived grafts [18, 21, 24]; their course is now to be determined during longer follow-up and interpreted in light of the ex vivo analysis of implants.
Comparison of Plasma hC-Peptide Levels in Fasting and Glucose-Injected Recipients Is an Index of the Glucose Responsiveness of the β-Cell Population in the Implant
We selected measurements at minute 15 following intraperitoneal glucose bolus instead of minutes 30 and 60, as they are closer to the acute responsiveness of β cells. Stage 4 recipients showed a threefold increase reaching levels higher than 3 ng/ml [24]. This was also the case for minutes 30 and 60 measurements in stage 4 [18] and stage 7 [21] recipients, but levels at these time points can be influenced by a time-dependent recruitment of β cells into secretory activity [30]. The latter process and the occurrence of glucose-unresponsive cells need to be investigated on retrieved implants. Such studies may also explain the poor glucose responsiveness in other studies on phase 4 and phase 7 implants [19, 22, 23].
The plasma proinsulin to hC-peptide ratio is an index for the conversion activity in β cells that are secretory active. When elevated, it can reflect a conversion immaturity or abnormality but can also result from low insulin reserves in responding cells; its significance requires ex vivo analysis. At the time of “therapeutic” plasma C-peptide levels in stage 4 recipients, we did not observe an increase in this ratio [24].
Ex Vivo Markers for Implants
A direct and longitudinal analysis of the β-cell population in implants should provide relevant information for development of a β-cell replacement therapy. Encapsulated implants in a confined site offer the advantage of their quantitative retrieval for ex vivo studies. We will discuss markers that help assess isolated implants as β-cell replacement. Particular attention is given to the functional β-cell mass and the extent to which it exhibits properties of clinical grade human primary β-cell preparations (Fig. 2). Our data on hPE-generated implants at PT week 20 serve as frame in this review; they were collected at “therapeutic” plasma C-peptide values. They are not meant as evidence for the best therapeutic product but as illustration for the information that can be gained by ex vivo markers. Data from other studies will be discussed, although experimental conditions greatly varied and therefore interfere with conclusions. Data can vary with different derivations from the same stem cell line, or with different lines and protocols. Variability is also expected for graft preparations that differ in pretreatment, in composition and viability, and in encapsulation and implant conditions, as well for differences in local microenvironment and metabolic state of recipients. A uniform quantitative and qualitative comparison of grafts and implants could provide valuable information for further development. Quality controlled human pancreatic β-cell preparations should serve as reference. Such investigation is feasible and needed before claims are made on the best therapeutic to be further developed for patients.
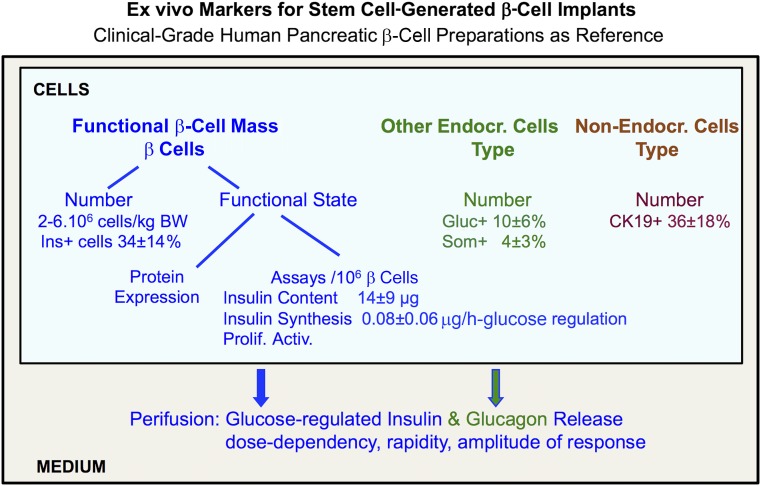
Figure 2.
Ex vivo markers for stem cell-generated β-cell implants. Ex vivo markers analyze retrieved implants for their cellular composition and functions (see text for references). Data are compared with those collected for clinical grafts prepared from human pancreatic β-cell isolates. Figure shows data for this reference preparation, which requires minimally 2.106 β cells per kilogram of BW to achieve a metabolic effect in recipients with type 1 diabetes. Abbreviations: BW, body weight; Endocr., endocrine; Ins., insulin; Prolif. Activ., proliferation activities.
Endocrine Cell Composition
Stem cell-generated hPE grafts resulted, in our study, in implants with more than 90% single-hormone-positive cells (PT week 20), half of them positive for insulin (46 ± 4%), the other half for glucagon (23 ± 1%) or somatostatin (20 ± 5%), percentages that are similar to those in islets within the human neonatal pancreas [31]. Their endocrine purity was twofold higher than that in clinically used human islet cell grafts, which contain significantly higher proportions of CK19+ duct cells (Fig. 2). In rodent studies with rat and human islet cell grafts, we consistently found a better outcome of preparations with high endocrine purity, comprising both β and α cells [29, 32–34]. This observation might reflect positive interactions between neighboring endocrine cells, as found in vitro for β cells aggregated to β cells or α cells [35]. It could also express reduced negative interference by duct cells, via mechanisms we previously discussed [34]. The nonendocrine fraction was larger in nonencapsulated implants [24]. A mixed endocrine composition was also reported by other studies on hPE-generated implants [18, 19]. Further studies are needed to assess whether a high and mixed endocrine purity is consistently achieved, and, more important, whether it positively influences long-term metabolic outcome.
Stem cell-derived β-cell grafts did not, as judged from PT week 2 and 10 analyses, result in implants with mixed endocrine purity [21–23]. The endocrine part corresponded almost entirely to single hormone-positive β cells. There is little information on its purity and on the identity of nonendocrine cells. It is unknown whether virtual absence of α cells has consequences for the functional β-cell mass and its responses to glucose.
Studies have so far not followed cell numbers in the implants. This will be necessary to evaluate the β-cell population and identify conditions for its preservation, growth, or decline. It is not known whether in vitro-formed β cells survive equally well in an implant as those formed in vivo. Although such information is not necessarily applicable to implants in patients, it represents a basis for preparing clinical studies and provides markers and data for analyzing and interpreting their outcome in humans.
Functional State of β Cells
The replacement β-cell population is expected to achieve and maintain glucose control. Rapid and adequate insulin secretory responses to changes in glucose concentration represent a key mechanism. This property can be assessed in vitro by perifusing retrieved cells at rapidly increasing and decreasing glucose levels. At PT week 20, the hPE-generated β cells responded by rapidly elevating or suppressing their insulin release, with similar kinetics as human primary β cells [24]. The amount of glucose-induced release per 103 β cells was, however, 60% smaller; it was amplified by glucagon but this did not reduce the difference with the reference cells. The lower secretory responses were attributable, at least in part, to a smaller size of the cellular hormone store (8.3 ± 1.6 vs. 14.4 ± 1.4 ng per 103 β cells). It is unknown whether stem cell-generated α cells acted positively on their neighboring β cells, as was the case for isolated rat pancreatic cells [35]. They also exhibited rapid responses to changing glucose concentrations, similarly to human primary α cells [24], which raises the possibility that they can compensate for dysfunctional pancreatic α cells in diabetic subjects. The glucose-responsiveness of retrieved hPE-derived β cells appeared also from their (pro)insulin synthetic activity, however, again, 50% lower than in primary β cells.
Further studies are now needed to examine whether these lower amplitudes reflect a lower percentage of activated β cells or lower glucose-induced signals in all cells, and whether this is caused by an immature stage in their functional differentiation. There are so far no functional studies reported on implants from other conditions and laboratories. In view of in vivo data, it is likely that they will also show insulin synthesis and release activities with lower amplitudes than primary human β cells, supporting the views that stem cell-generated β-cell implants studied so far are still functionally immature [21–23, 27].
There is not at present a clear and generally accepted definition of a “functionally mature human β cell,” as there is also not a definition for a “genuine” or “bona fide” β cell. Defining a fixed-marker set might be particularly difficult for believers in intercellular heterogeneity within the normal pancreatic β-cell population [30]. Absence of such set does not omit the significance of a longitudinal follow-up of stem cell-generated implants; ex vivo markers can examine whether β cells reach a stable number and functional state that achieves and maintains in vivo markers of metabolic control in recipients (Fig. 1). This effect should be evaluated versus the in vitro characteristics of the grafts, in particular the minimal initial cell number needed, as this is an index for their therapeutic efficacy. Such studies can be conducted in preclinical mouse models. Its clinical relevance faces the influence of species differences and the limitation of the short life span of the mice. The information can nevertheless clarify the biology of stem cell-generated human β cells, with comparison with pancreatic primary human β cells. Functional markers as collected ex vivo and in vivo can then also be related to the expression of protein markers that have been reported to characterize mature [36, 37] and differentiated β cells, be it as β-cell specific or β-cell “forbidden” [38, 39].
Clinical Implications
Data in preclinical models have stimulated translation of human stem cell-derived preparations to clinical studies and hence raised expectations in a society that is has been eagerly awaiting a cure for type 1 diabetes. Preparing and conducting clinical research projects is a major step toward this objective but has several more steps ahead, best taken without shortcuts. They open a window to critical information and insights that cannot be gained in laboratory models. The promising outcome of implants in mice is, however, not necessarily predictive for that in patients with type 1 diabetes. Local and systemic conditions are different, with consequences for survival and differentiation of implanted cells. The patients’ reactivity to the graft will have to be investigated, distinguishing innate, auto-, and allo-immune processes. Encapsulation has shown advantages for stem cell-generated implants in mice, but extrapolation to humans is still to be demonstrated. Its advantage in offering access to implants for ex vivo analysis will, however, be as valuable as in rodents, providing biologic information when circulating markers are not yet, or no longer, detectable. Such direct analysis is not possible for intraportal implants and will be difficult and fragmentary for nonencapsulated implants that integrate into host tissue. It becomes feasible and representative for encapsulated implants from which donor cells can be quantitatively retrieved. Their cellular composition and functions can be followed with time (Fig. 1) and related to characteristics at start and in vivo markers. Ex vivo markers allow assessment of differentiation process to β cells, their functional maturation, and changes in mass. They also provide information on the type and function of non-β cells in the implant and in interstitial tissue. Tissue surrounding the resected device gives histologic insight into its microenvironment, with possible signs of host reactivity. During a first phase of clinical studies, ex vivo analysis can be considered as the prime source of information on the path to clinical trials.
The therapeutic significance of stem cell-generated β-cell implants depends on their capacity to establish a functional β-cell mass that provides metabolic benefit to diabetes patients. A second phase of clinical studies is thus expected to implement information from ex vivo markers to achieve in vivo signs of β-cell functions, as indicated by the markers used in the preclinical models and those in clinical use to indirectly measure functional β-cell mass of implants and its metabolic significance in patients with type 1 diabetes. Achieving an outcome that is comparable to that of intraportally injected human pancreatic β-cell grafts would represent another major step.
Acknowledgments
Research was supported by grants from the European Commission (FP7 241883 and H2020 681070), Juvenile Diabetes Research Foundation (17-2013-296), and the Flemish Government (IWT130138). T.R. is Ph.D. fellow of Research Foundation Flanders.
Author Contributions
D.P.: manuscript writing, collection and/or assembly of data, manuscript revisions, final approval of manuscript; T.R., I.D.M., and Z.L.: collection and/or assembly of data, manuscript revisions, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
D.P. is member of the scientific and clinical advisory board of ViaCyte-Inc. and is a compensated consultant for Nestlé Institute Health Sciences, Lausanne, Switzerland, and Beta-Cell N.V., Belgium. The other authors indicated no potential conflicts of interest.
References
- 1.Pipeleers D, Chintinne M, Denys B, et al. Restoring a functional beta-cell mass in diabetes. Diabetes Obes Metab. 2008;10(suppl 4):54–62. doi: 10.1111/j.1463-1326.2008.00941.x. [DOI] [PubMed] [Google Scholar]
- 2.Lacy PE, Davie JM. Transplantation of pancreatic islets. Annu Rev Immunol. 1984;2:183–198. doi: 10.1146/annurev.iy.02.040184.001151. [DOI] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 4.Keymeulen B, Gillard P, Mathieu C, et al. Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc Natl Acad Sci USA. 2006;103:17444–17449. doi: 10.1073/pnas.0608141103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillard P, Hilbrands R, Van de Velde U, et al. Minimal functional β-cell mass in intraportal implants that reduces glycemic variability in type 1 diabetic recipients. Diabetes Care. 2013;36:3483–3488. doi: 10.2337/dc13-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksson O, Eich T, Sundin A, et al. Positron emission tomography in clinical islet transplantation. Am J Transplant. 2009;9:2816–2824. doi: 10.1111/j.1600-6143.2009.02844.x. [DOI] [PubMed] [Google Scholar]
- 7.Ling Z, De Pauw P, Jacobs-Tulleneers-Thevissen D, et al. Plasma GAD65, a marker for early β-cell loss after intraportal islet cell transplantation in diabetic patients. J Clin Endocrinol Metab. 2015;100:2314–2321. doi: 10.1210/jc.2015-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanak MA, Takita M, Shahbazov R, et al. Evaluation of microRNA375 as a novel biomarker for graft damage in clinical islet transplantation. Transplantation. 2015;99:1568–1573. doi: 10.1097/TP.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 9.Citro A, Cantarelli E, Maffi P, et al. CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest. 2012;122:3647–3651. doi: 10.1172/JCI63089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution. Adv Drug Deliv Rev. 2014;67-68:35–73. doi: 10.1016/j.addr.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 12.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 13.Cozzi E, Bosio E, Seveso M, et al. Xenotransplantation as a model of integrated, multidisciplinary research. Organogenesis. 2009;5:288–296. doi: 10.4161/org.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samy KP, Martin BM, Turgeon NA, et al. Islet cell xenotransplantation: A serious look toward the clinic. Xenotransplantation. 2014;21:221–229. doi: 10.1111/xen.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Scharfmann R. Pancreatic development as a basis for the definition of new therapies for diabetes. Endocr Dev. 2007;12:1–11. doi: 10.1159/000109599. [DOI] [PubMed] [Google Scholar]
- 17.D’Amour KA, Agulnick AD, Eliazer S, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 18.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 19.Bruin JE, Rezania A, Xu J, et al. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia. 2013;56:1987–1998. doi: 10.1007/s00125-013-2955-4. [DOI] [PubMed] [Google Scholar]
- 20.Rezania A, Bruin JE, Xu J, et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31:2432–2442. doi: 10.1002/stem.1489. [DOI] [PubMed] [Google Scholar]
- 21.Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 22.Pagliuca FW, Millman JR, Gürtler M, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russ HA, Parent AV, Ringler JJ, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34:1759–1772. doi: 10.15252/embj.201591058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motté E, Szepessy E, Suenens K, et al. Beta Cell Therapy Consortium EU-FP7 Composition and function of macroencapsulated human embryonic stem cell-derived implants: Comparison with clinical human islet cell grafts. Am J Physiol Endocrinol Metab. 2014;307:E838–E846. doi: 10.1152/ajpendo.00219.2014. [DOI] [PubMed] [Google Scholar]
- 25.Schulz TC, Young HY, Agulnick AD, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7:e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumagai-Braesch M, Jacobson S, Mori H, et al. The TheraCyte device protects against islet allograft rejection in immunized hosts. Cell Transplant. 2013;22:1137–1146. doi: 10.3727/096368912X657486. [DOI] [PubMed] [Google Scholar]
- 27.Agulnick AD, Ambruzs DM, Moorman MA, et al. Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Translational Medicine. 2015;4:1214–1222. doi: 10.5966/sctm.2015-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vegas AJ, Veiseh O, Gürtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016;22:306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs-Tulleneers-Thevissen D, Chintinne M, Ling Z, et al. Beta Cell Therapy Consortium EU-FP7 Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia. 2013;56:1605–1614. doi: 10.1007/s00125-013-2906-0. [DOI] [PubMed] [Google Scholar]
- 30.Pipeleers DG. Heterogeneity in pancreatic β-cell population. Diabetes. 1992;41:777–781. doi: 10.2337/diab.41.7.777. [DOI] [PubMed] [Google Scholar]
- 31.Rahier J, Wallon J, Henquin JC. Cell populations in the endocrine pancreas of human neonates and infants. Diabetologia. 1981;20:540–546. doi: 10.1007/BF00252762. [DOI] [PubMed] [Google Scholar]
- 32.Pipeleers DG, Pipeleers-Marichal M, Vanbrabandt B, et al. Transplantation of purified islet cells in diabetic rats. II. Immunogenicity of allografted islet β-cells. Diabetes. 1991;40:920–930. doi: 10.2337/diab.40.7.920. [DOI] [PubMed] [Google Scholar]
- 33.Keymeulen B, Anselmo J, Pipeleers D. Length of metabolic normalization after rat islet cell transplantation depends on endocrine cell composition of graft and on donor age. Diabetologia. 1997;40:1152–1158. doi: 10.1007/s001250050800. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs-Tulleneers-Thevissen D, Bartholomeus K, Suenens K, et al. Human islet cell implants in a nude rat model of diabetes survive better in omentum than in liver with a positive influence of beta cell number and purity. Diabetologia. 2010;53:1690–1699. doi: 10.1007/s00125-010-1721-0. [DOI] [PubMed] [Google Scholar]
- 35.Pipeleers D. The biosociology of pancreatic B cells. Diabetologia. 1987;30:277–291. doi: 10.1007/BF00299019. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura W, Kondo T, Salameh T, et al. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol. 2006;293:526–539. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Artner I, Hang Y, Mazur M, et al. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martens GA, Jiang L, Hellemans KH, et al. Clusters of conserved beta cell marker genes for assessment of beta cell phenotype. PLoS One. 2011;6:e24134. doi: 10.1371/journal.pone.0024134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuit F, Van Lommel L, Granvik M, et al. β-cell-specific gene repression: A mechanism to protect against inappropriate or maladjusted insulin secretion? Diabetes. 2012;61:969–975. doi: 10.2337/db11-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]