The results found here indicated that phosphatase and tension homolog (PTEN) promoted neuronal differentiation by inhibition of extracellular signal-regulated kinase (ERK) signaling, which in turn induced activation of S6 kinase (S6K). The data suggest that ERK pathways participate in crosstalk with S6K through PTEN signaling during neuronal differentiation of human neural stem cells.
Keywords: Cell signaling, Differentiation, Neural differentiation, Neural stem cell, Proliferation
Abstract
Phosphatase and tension homolog (PTEN) is a widely known negative regulator of insulin/phosphatidylinositol 3-kinase (PI3K) signaling. The PI3K/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) and Ras-extracellular signal-regulated kinase (Ras-ERK) signaling pathways are the chief mechanisms controlling the survival, proliferation, and differentiation of neural stem cells (NSCs). However, the roles of PTEN in Akt/mTOR and ERK signaling during proliferation and neuronal differentiation of human NSCs (hNSCs) are poorly understood. Treatment of proliferating hNSCs with a specific inhibitor of PTEN or overexpression of the PTEN inactive mutant G129E resulted in an increase in the expression levels of Ki67, p-S6 kinase (p-S6K), and p-ERK without affecting p-Akt expression during proliferation of hNSCs. Therefore, we focused on the regulatory effect of PTEN in S6K and ERK signaling during dopaminergic neuronal differentiation of hNSCs. Overexpression of PTEN during neuronal differentiation of hNSCs caused an increase in p-S6K expression and a decrease in p-ERK expression. Conversely, inhibition of PTEN increased p-ERK expression and decreased p-S6K expression. Inhibition of ERK by a specific chemical inhibitor, U0126, promoted neuronal generation, especially of tyrosine hydroxylase-positive neurons. p-S6K expression increased in a time-dependent manner during differentiation, and this effect was enhanced by U0126. These results indicated that PTEN promoted neuronal differentiation by inhibition of ERK signaling, which in turn induced activation of S6K. Our data suggest that ERK pathways participate in crosstalk with S6K through PTEN signaling during neuronal differentiation of hNSCs. These results represent a novel pathway by which PTEN may modulate the interplay between ERK and S6K signaling, leading to increased neuronal differentiation in hNSCs.
Significance
This article adds to the body of knowledge about the mechanism of extracellular signal-regulated kinase (ERK)-mediated differentiation by describing the molecular function of phosphatase and tension homolog (PTEN) during the neuronal differentiation of human neural stem cells (hNSCs). Previous studies showed that S6K signaling promoted neuronal differentiation in hNSCs via the phosphatidylinositol 3-kinase Akt-mammalian target of rapamycin signaling pathway. A further series of studies investigated whether this S6 kinase-induced differentiation in hNSCs involves regulation of ERK signaling by PTEN. The current study identified a novel mechanism by which PTEN regulates neuronal differentiation in hNSCs, suggesting that activating PTEN function promotes dopaminergic neuronal differentiation and providing an important resource for future studies of PTEN function.
Introduction
One extrinsic signaling pathway that is a common mediator of pluripotency in human and mouse embryonic stem cells (ESCs) is the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway [1–4]. PI3K/Akt/mammalian target of rapamycin (mTOR) signaling is a well-defined route by which insulin/insulin-like growth factor (IGF) receptor stimulation can control neuronal differentiation [5]. In response to insulin, Akt is phosphorylated at Ser 473 and Thr 308 and translocates to the plasma membrane. This phosphorylation contributes to the regulation of differentiation in mouse olfactory bulb stem cells (OBSCs) and rat neural stem cells (NSCs) [5, 6]. The function of Akt is mediated through mTOR by activation of p70 ribosomal S6 kinase (S6K). mTOR activity has been linked to cell growth, proliferation, survival, protein translation, and other cellular processes [7, 8]. Among its many functions, mTOR supports the self-renewal capacity of human ESCs (hESCs) and NSCs [9, 10]. In contrast, it has been reported to play an important role in differentiation during embryonic development and in particular the differentiation of several cell types such as oligodendrocytes and rat NSCs [10–13]. mTOR/S6K has also been shown to regulate differentiation in hESCs [14].
Another protein involved in the PI3K/Akt pathway is phosphatase and tension homolog (PTEN). PTEN is a tumor suppressor gene that is frequently mutated in human cancer [15]. Although PTEN is not essential for cell fate determination in the central nervous system (CNS) overall [16, 17], a potential role in differentiation was observed in certain cell types in the brain [5, 18]. Akt activity is negatively regulated by molecules that antagonize PI3K, such as the dual lipid and protein phosphatase PTEN [19]. PTEN has been reported to regulate a variety of cellular functions, such as cell division, survival, apoptosis, migration, and differentiation. Furthermore, there is evidence that PTEN regulates multiple steps in CNS development [20, 21]. In addition, numerous studies have established a role for PI3K/Akt/PTEN in stem cells. In mouse ESCs (mESCs) and adult stem cells, loss of PTEN is correlated with the activation of AkT, increased self-renewal, and proliferation [22–24]. It is well documented that PI3K-dependent signaling is required for maintenance of the mESC pluripotent state in mESCs [25].
Extracellular signal-regulated kinase (ERK) is one of the multiple intracellular effectors downstream of insulin and basic fibroblast growth factor (bFGF) and is known to crosstalk with components of other key signaling pathways. ERK plays a crucial role in the self-renewal of hESCs and hNSCs [4, 26]. However, it has been reported to play diverse roles in early embryogenesis and acts in a cell-context-dependent manner. Recently, the ERK pathway was shown to be involved in the maintenance of self-renewal properties in association with the PI3K/mTOR pathway in cancer stem-like cells [27]. However, the role of intracellular signaling pathways that are activated by extracellular factors in the regulation of hNSC differentiation and the underlying mechanisms remain unknown.
In this study, we investigated the role of PTEN in the control of hNSC proliferation and differentiation. We further examined the potential relationship between PI3K/Akt/mTOR and Ras/ERK signaling pathways in hNSCs. We found that inhibition of PTEN decreased neuronal differentiation by activation of ERK signaling, which in turn induced inhibition of S6K. We also showed that PI3K/Akt/mTOR signaling is essential for hNSC dopaminergic neuronal differentiation through activation of S6K. In contrast, ERK promoted hNSC proliferation through inactivation of S6K. This is the first report of the role of PTEN in crosstalk between S6K and ERK signaling during neuronal differentiation of hNSCs. Our findings provide a new insight into a novel regulatory mechanism of hNSCs and enhance our understanding of the mechanism of hNSC differentiation during the process of neurogenesis.
Materials and Methods
Reagents and Antibodies
LY294002 and U0126 were purchased from Cell Signaling Technologies (Danvers, MA, http://www.cellsignal.com); PF-4708671 was from Calbiochem (Darmstadt, Germany, http://www.merckmillipore.com); and VO-OHpic and rapamycin were from Sigma (St. Louis, MO, https://www.sigmaaldrich.com). For inhibition of a specific signaling pathway, cells were treated with the appropriate inhibitor during proliferation or differentiation. All reagents were dissolved in dimethyl sulfoxide (DMSO; Sigma), and controls were treated with 0.001% DMSO, which was the highest final concentration of DMSO used in the experiments. Addition of DMSO did not affect the viability of control samples. Antibodies to the following proteins were obtained from the indicated commercial sources: p-Akt, p-ERK, p-mTOR, p-S6K, cleaved caspase-3, and PTEN (Cell Signaling Technologies), tyrosine hydroxylase (TH) (Pel-Freez, Rogers, AR, http://www.pelfreez-bio.com), Tuj-1 (Chemicon, Temecula, CA, http://www.emdmillipore.com), and β-actin (Abcam, Cambridge, U.K., http://www.abcam.com).
hNSC Cultures
The hESC culture protocol (HYE-08-03) was approved by the Hanyang University institutional review board. hNSCs were derived from hESCs (H9, University of Wisconsin, Madison, WI, http://www.wisc.edu; CHA13, CHA Stem Cell Institute, http://www.cha.ac.kr) using an in vitro differentiation protocol involving neural induction on feeder layers of MS5 stromal cells followed by consecutive cell passages for selection of NSCs, as previously described [28]. hNSCs that had undergone 2–8 NSC passages were used in this study. hNSCs were cultured in serum-free insulin-transferrin-selenium (ITS) medium [29] in the presence of basic fibroblast growth factor (bFGF; 20 ng/ml; R&D Systems, Minneapolis, MN, https://www.rndsystems.com) and ascorbic acid on poly-L-ornithine/fibronectin-coated culture dishes. Cells were maintained at 37°C in a 5% CO2 incubator, and the ITS medium was changed every other day. For differentiation experiments, after culturing for 1 to 5 days the medium was changed to differentiation medium (ITS-ascorbic acid [ITS-A] without bFGF). Characterization of the cultured monolayer of hNSCs is shown in supplemental online Figure 1.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS), incubated in blocking solution (1% bovine serum albumin/0.03% Triton X-100 in PBS), and then incubated with the following primary antibodies: mouse anti-Ki67 (1:100; Novocastra Laboratories Ltd., Newcastle, U.K., http://www.novocastra.co.uk), rabbit anti-Nestin (1:50; Dr. Ron McKay, National Institutes of Health, Bethesda, MD), mouse anti-GFP (1:1,000; Roche Life Science, https://lifescience.roche.com), mouse anti-Tuj1 (1:2000; Babco, Richmond, CA, http://www.babco.com), and rabbit anti-TH (1:1000; Pelfreez). The following secondary antibodies were used for visualization: Alexa 488 (1:200; Thermo Fisher Scientific, Waltham, MA, http://www.thermofisher.com) and Cy3 (1:200; Jackson Immunoresearch Laboratories, West Grove, PA, https://www.jacksonimmuno.com/). The stained samples were mounted in Vectashield with 4′,6-diamidino-2-phenylindole (DAPI) mounting medium (Vector Laboratories, Burlingame, CA, https://www.vectorlabs.com) and photographed using an epifluorescence microscope (Leica, Wetzlar, Germany, http://www.leica-microsystems.com). Immunoreactive/DAPI-stained cells on culture coverslips were counted in at least 20 randomly selected microscopic fields using an eyepiece grid at a final magnification of ×200 or ×400.
Western Blot Analysis
To determine levels of protein expression, we prepared protein extracts from hNSCs for Western blotting. Adherent cells were scraped off the culture dishes and lysed by incubation on ice with radio-immunoprecipitation assay lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% sodium deoxycholate, 1% NP-40, 0.1% sodium dodecyl sulfate [SDS]) containing 1 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail, and phosphatase inhibitor cocktail (Roche, Indianapolis, IN, https://www.roche.com). Collected cells were broken by sonication on ice and centrifuged at 10,000g for 20 min at 4°C. Protein concentrations were determined with the Bradford reagent, and 30-µg samples of extracted protein were resolved on SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were incubated in the presence of different primary antibodies at 4°C overnight and then incubated with secondary antibody coupled to horseradish peroxidase. Immunoreactivity was visualized using enhanced chemiluminescence (GE Healthcare Life Sciences, Little Chalfont, U.K., http://www.gelifesciences.com). Protein bands were quantified with a densitometer (VERSAmax; Molecular Devices, Sunnyvale, CA, https://www.moleculardevices.com).
Transduction of hNSCs With Retroviral Vectors
The retroviral vector pCL [30] was modified to give the construct pC5w2-IRES3-green fluorescent proteins (GFP), simultaneously expressing the GFPs. Infection was performed using a Moloney murine leukemia virus (Mo-MLV)-based retroviral vector. This GFP vector was used as a control in transduction experiments. A construct containing the complete PTEN cDNA (1.2 kb) fused to the hemagglutinin (HA) N-terminus was cloned into the pC5w-IRES-GFP vector to generate the vector pC5w-HA-PTEN-IRES-GFP (PTEN-GFP) in which PTEN expression was under control of the viral long terminal repeat (LTR). The PTEN catalytically inactive mutant (PTEN-G129E) that was recently described and characterized was also cloned into the pC5w-IRES-GFP vector. In addition, constructs containing constitutively active S6K1 (S6K1-CA) and S6K1 dominant negative (S6K1-DN) cDNAs fused to the myc N-terminus were subcloned into the pC5w2-IRES3-GFP vector to generate the plasmids, pC5w2-myc-S6K1-CA-IRES3-GFP (S6K1-CA-GFP) and pC5w2-myc-S6K1-DN-IRES3-GFP (S6K1-DN-GFP), in which expression of S6K1-CA and S6K1-DN, respectively, is under the control of the Mo-MLV LTR. The S6K1-CA and S6K1-DN constructs were kindly provided by Dr. Sunghee Um of Sungkyunkwan University (Suwon, Republic of Korea).
The retroviral vectors were transfected into 293 GPG packaging cells (Lipofectamine 2000; Thermo Fisher Scientific) and supernatant containing viral particles (vesicular stomatitis virus glycoprotein G pseudotyped recombinant retrovirus) was harvested 72 hours after incubation. Viral titers were adjusted to 1 × 108 particles per milliliter. Neural precursors were exposed to viral supernatant for 4 hours in the presence of polybrene (1 g/ml), cultured overnight in ITS-A medium plus bFGF, and induced to differentiate the following day by withdrawal of bFGF.
Statistical Analysis
Statistical comparisons were performed with SPSS software (version 17.00, SPSS Inc., Chicago, IL, http://www.ibm.com). One-way analysis of variance followed by Bonferroni’s test was applied for multiple comparisons. Data are expressed as mean ± SEM of at least three independent experiments. We considered p < .05 statistically significant.
Results
Characterization of hNSCs Derived From hESCs
hNSCs derived from H9 ESCs were grown in the presence of bFGF and then differentiated toward predominantly neuronal populations of the central nervous system following withdrawal of mitogens. The cells formed neural rosette structures and abundantly expressed hNSC-specific markers such as nestin or Sox2 and the proliferating cell marker Ki67 (supplemental online Fig. 1A–1C). After 5 days of differentiation, we determined expression of the neuronal marker, TuJ1, and the dopaminergic neuronal marker, TH, by immunostaining (supplemental online Fig. 1D).
Insulin Activates S6K Through PI3K/Akt/mTOR Signaling Pathways in hNSCs
To investigate the insulin effect on PI3K/AKT/mTOR signaling pathways in hESC-derived NSCs, we detected the phosphorylation levels of AKT, Mtor, and S6K 1 day after insulin treatment by Western blotting. Insulin increased the expression levels of p-AKT, p-mTOR, and p-S6K in H9 ESC-derived hNSCs in a concentration-dependent manner (supplemental online Fig. 2A). In addition, we examined the effect of insulin in hNSCs derived from other ESC lines such as CHA13 ESCs and obtained the same results as described above (supplemental online Fig. 2B). The Akt/mTOR/S6K signaling effects of insulin were blocked by the PI3K inhibitor, LY294002 (supplemental online Fig. 2A, 2B). These data show that insulin activated mTOR/S6K via the PI3K-Akt signaling pathway in our culture system.
Induction of Dopaminergic Neuronal Differentiation by Insulin Is Dependent on the mTOR Signaling Pathway
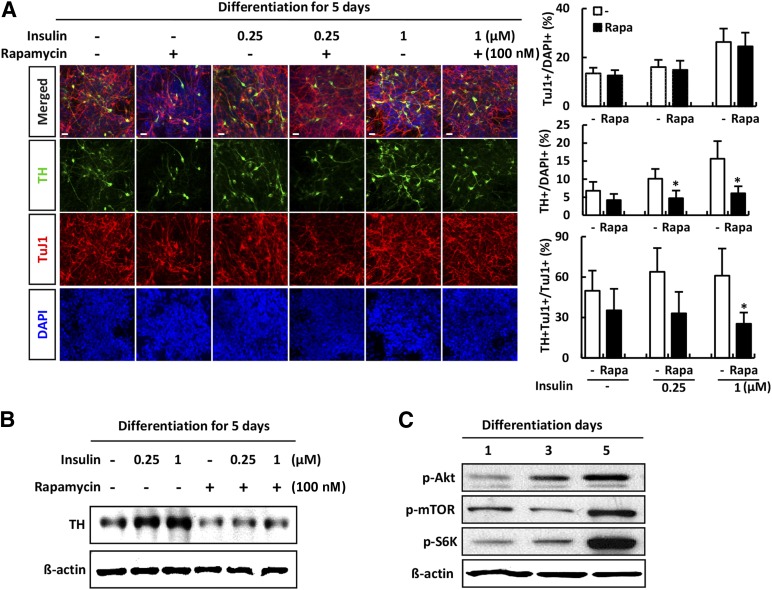
Next, we examined whether the dose-dependent insulin effect was involved in the induction of dopaminergic neurons. Differentiation of hNSCs was induced by culturing for 5 days in the absence of bFGF. hNSCs were exposed to insulin (0 to 1 µM) in the presence or absence of rapamycin. At concentrations from 0 to 1 µM, insulin induced a dose-dependent increase in the number of dopaminergic neurons. As shown in Figure 1A, there was an increase in the percentage of cells positive for the neuronal marker TuJ1 and the percentage of cells positive for the dopaminergic neuronal marker TH. However, relative induction of TuJ1-positive and TH-positive neurons to TuJ1-positive neurons was decreased when rapamycin was added to the medium (Fig. 1A). In the absence of rapamycin, the expression of TH was significantly increased in an insulin dose-dependent manner in comparison with cells in insulin-free medium, as measured by immunoblot analysis. However, rapamycin reduced TH expression even in the absence of insulin (Fig. 1A, 1B). Importantly, levels of Akt, mTOR, and S6K increased during differentiation of hNSCs for 5 days (Fig. 1C). These results showed that the PI3K-Akt pathway is an important mediator of the intracellular signal cascade during insulin-induced neuronal differentiation of hNSCs. Furthermore, mTOR plays a positive role in the insulin-induced differentiation of hNSCs into dopaminergic neurons.
Figure 1.
Phosphatidylinositol 3-kinase/Akt/mTOR is involved in the induction of dopaminergic neuronal differentiation of human neural stem cells (hNSCs). Differentiation of H9-hNSCs was induced by culturing the cells for 5 days in the absence of basic fibroblast growth factor (bFGF). hNSCs were exposed to insulin (0 to 1 µM) in the presence or absence of rapamycin. (A): Immunofluorescence analyses were performed for TuJ-1 (red) and TH (green); blue: DAPI (magnification ×200). Cells positive for TH were quantified as the percentage of total cells. (B): Whole-cell proteins (30 μg) were analyzed for TH and β-actin expression by Western blotting. Each blot represents three independent experiments. (C): Differentiation of hNSCs was induced by culturing for 5 days in the absence of bFGF, and the intracellular signaling cascade during insulin-induced dopaminergic neuronal differentiation was examined. Whole-cell proteins (30 μg) were analyzed for p-Akt, p-mTOR, p-S6K, and β-actin expression by Western blotting. Error bars are standard errors of the mean (SE). ∗, p < .05, in comparison with withdrawal rapamycin cells. Scale bar = 20 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; mTOR, mammalian target of rapamycin; p, phosphorylated; Rapa, rapamycin; S6K, S6 kinase; TH, tyrosine hydroxylase.
Effects of ERK Signaling Pathway in the Proliferation and Differentiation of hNSCs
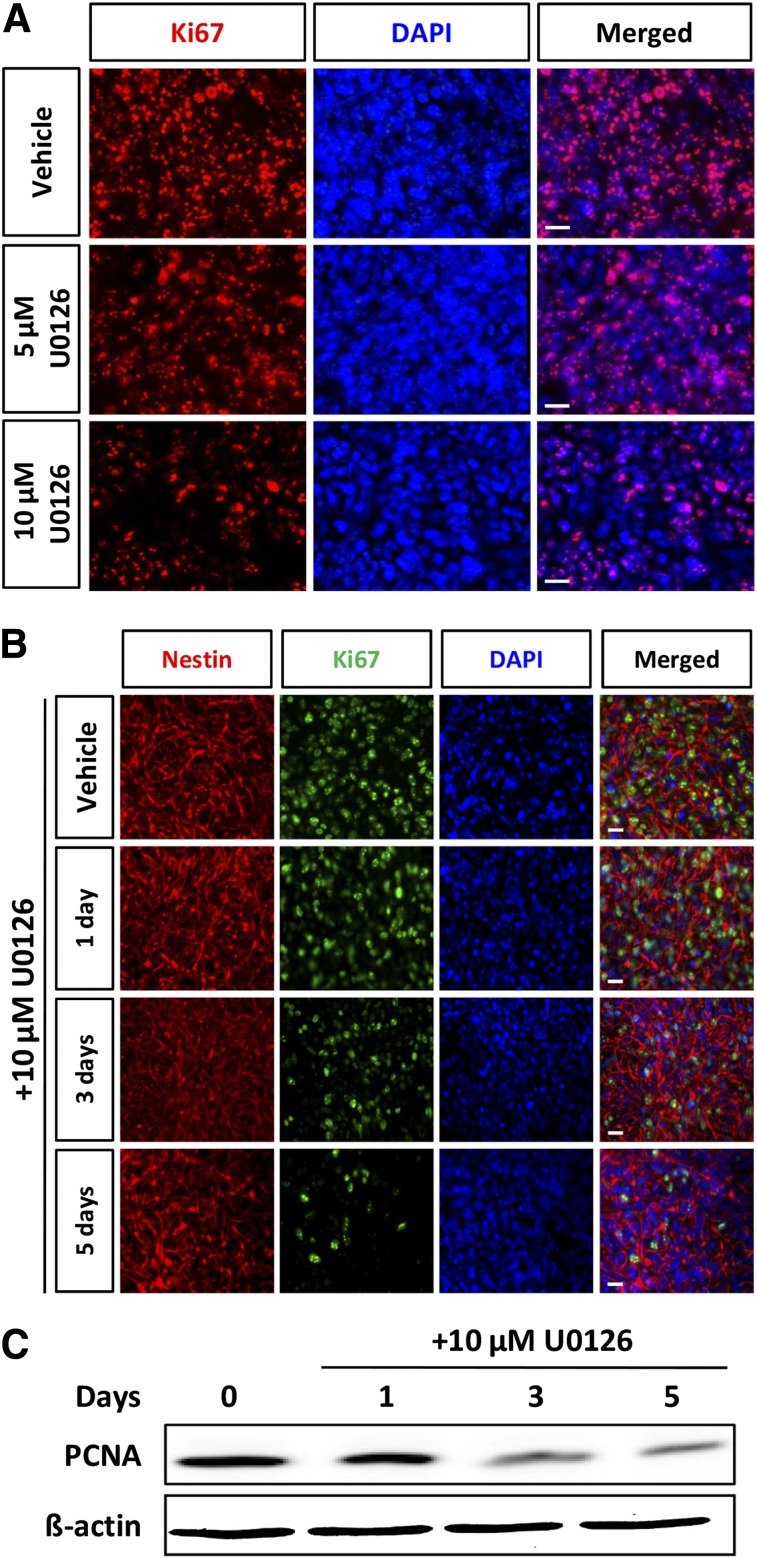
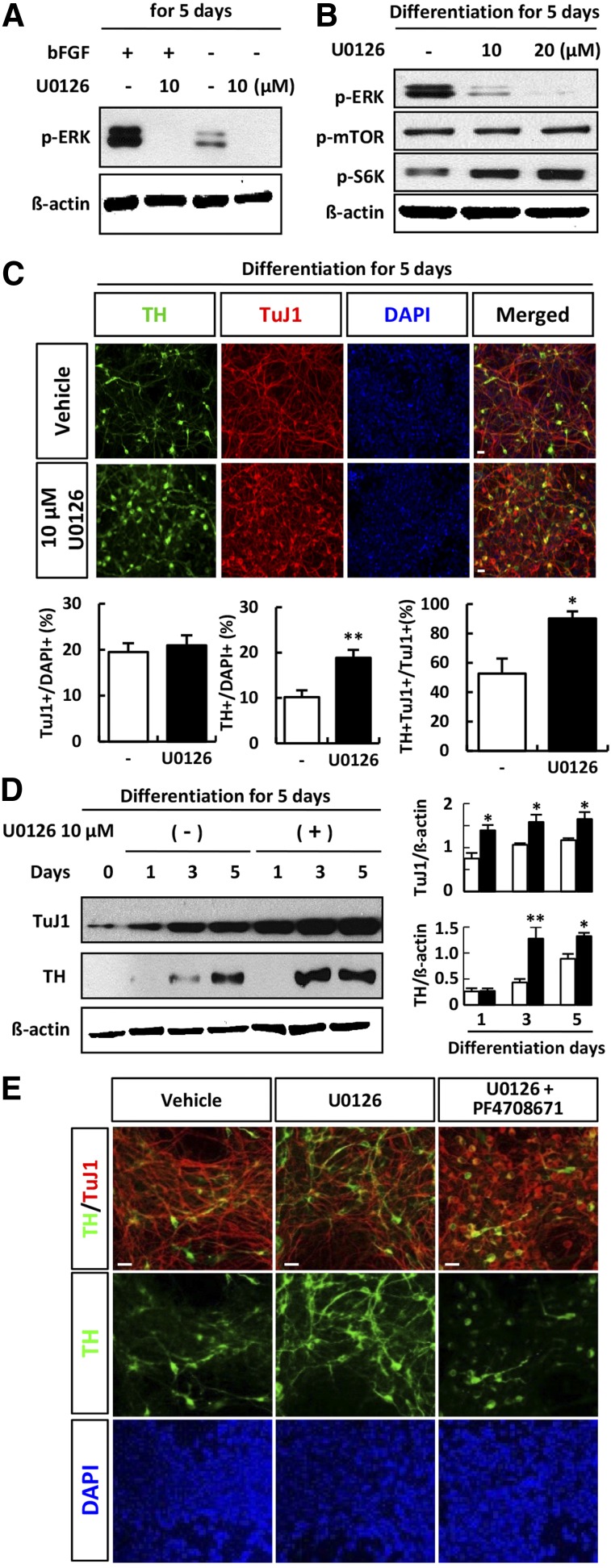
We next investigated whether inhibition of ERK by U0126 affected the proliferation and differentiation of hNSCs. After exposure of hNSCs to inhibitor for 3 days, the number of Ki67-positive cells decreased in a dose-dependent manner (Fig. 2A) and time-dependent manner (Fig. 2B), and the expression of proliferating cell nuclear antigen (PCNA) also decreased (Fig. 2C). bFGF has been shown to promote the proliferation of NSCs thorough activation of ERK, whereas withdrawal of bFGF induces differentiation. During differentiation of hNSCs induced by the removal of bFGF, the expression of ERK was decreased in comparison with that in proliferating hNSCs (Fig. 3A). These results showed that ERK plays an important role in the maintenance of proliferation in these cells. Interestingly, S6K was activated by inhibition of ERK. U0126 treatment abolished the expression of p-ERK (Fig. 3B). Relative induction of TuJ1- and TH-positive neurons to TuJ1-positive neurons was increased when ERK signaling was blocked in differentiating cells (Fig. 3C). Western blot analysis showed that treatment with U0126 increased the expression of TH and TuJ1 in cells derived from H9 ESCs in comparison with controls (Fig. 3D). To clarify the reverse effect of S6K inhibition on dopaminergic neuronal differentiation of hNSCs, we examined the effect of S6K inhibition on ERK inhibition during dopaminergic neuronal differentiation of hNSCs. As was expected, chemical inhibition of S6K in differentiating cells using PF4708671 blocked the increase of TH expression induced by ERK inhibition (Fig. 3E).
Figure 2.
Extracellular signal-regulated kinase inhibition attenuated proliferation of human neural stem cells (hNSCs). (A): Cells were cultured for 3 days under conditions of proliferation in the presence or absence of U0126 (5 or 10 µM). Representative immunofluorescence for Ki67 (red) and fluorescence of DAPI (blue) is shown. Treatment with U-0126 attenuated Ki67-positive cells in a dose-dependent manner. (B): Representative immunofluorescence for Ki67 (red) and fluorescence of DAPI (blue) is shown. Treatment with 10 µM U-0126-attenuated Ki67-positive cells in a time-dependent manner. (C): hNSCs were treated with U0126 for 3 days, and lysates were subjected to immunoblotting for PCNA and β-actin, as indicated. Each blot represents three independent experiments. Scale bar = 20 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; PCNA, proliferating cell nuclear antigen.
Figure 3.
Extracellular signal-regulated kinase (ERK) inhibition promoted dopaminergic neuronal differentiation of human neural stem cells (hNSCs). (A): hNSCs were treated with or without U0126 during proliferation (with bFGF) and differentiation (in the absence of bFGF). Cell lysates were subjected to immunoblotting with p-ERK and β-actin, as indicated. Each image is representative of three different experiments. (B): Differentiating hNSCs were treated with U0126 for 5 days, and lysates were subjected to immunoblotting for p-ERK, p-mTOR, p-S6K, and β-actin, as indicated. Each blot represents three independent experiments. (C): Differentiating cells with or without U0126 treatment at day 5 were subjected to immunofluorescent staining with anti-TuJ-1 (red) and anti-TH (green); blue: DAPI. Cells positive for TH were quantified as the percentage of total cells. (D): Western blot analysis of TuJ-1, TH, and β-actin in hNPCs after treatment with 10 µM U0126 during differentiation at 1, 3, and 5 days in H9 embryonic stem cell-derived hNSCs. The densities of protein bands were quantified using scanning densitometry, and the ratio of TuJ1 or TH to β-actin is shown in each panel. (E): Differentiating cells treated with 10 µM U0126 only or cotreatment with 10 µM PF-4708671 at day 5 were subjected to immunofluorescence with anti-TuJ-1 (red) and anti-TH (green) antibodies; blue indicates DAPI staining. Error bars are standard errors of the mean (SE). ∗, p < .05; ∗∗, p < .001; in comparison with withdrawal rapamycin cells. Scale bar = 20 μm. Abbreviations: bFGF, basic fibroblast growth factor; DAPI, 4′,6-diamidino-2-phenylindole; ERK, extracellular signal-regulated kinase; mTOR, mammalian target of rapamycin; p, phosphorylated; S6K, S6 kinase; TH, tyrosine hydroxylase.
Promotion of Cell Proliferation by PTEN Inhibition During Proliferation of hNSCs
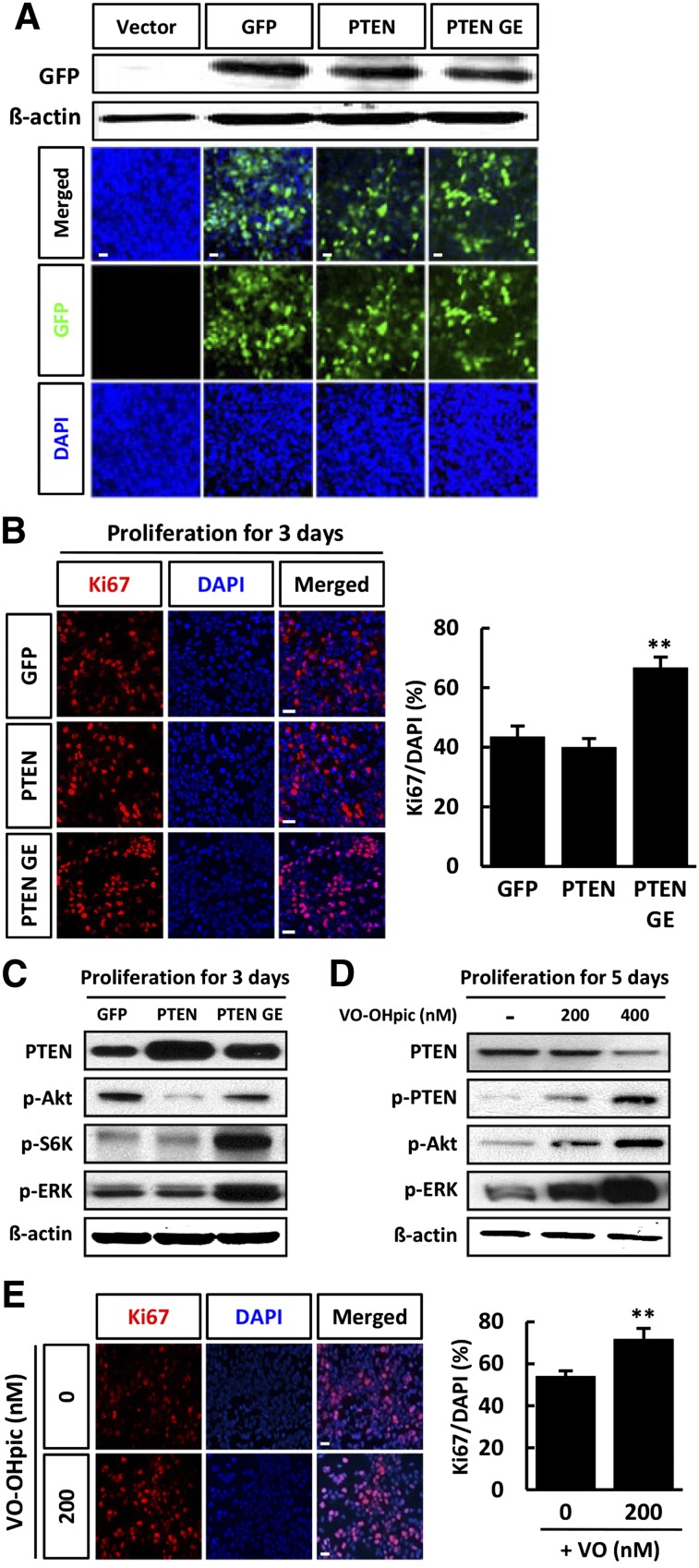
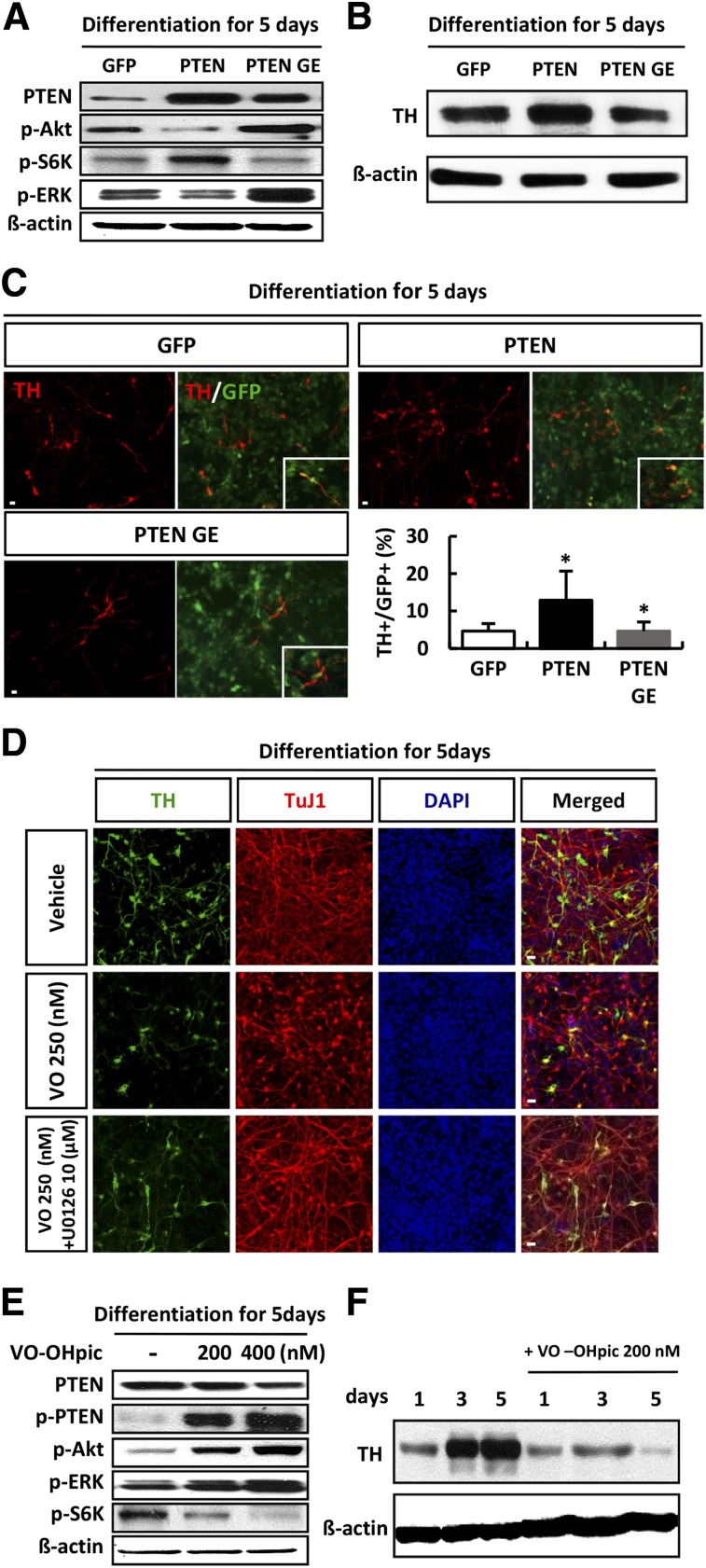
To further study the influence of the PI3K-Akt pathway on the proliferation of hNSCs, we treated cells with retroviral vector expressing GFP only (GFP), PTEN-GFP (PTEN), or a catalytically inactive PTEN mutant (PTN-G129E-GFP [PTEN GE]). Similar infection efficiencies were obtained when cells were infected with the GFP, PTEN, and PTEN GE vectors, as confirmed by immunoblotting with antibody against GFP (Fig. 4A). The total number of Ki67-labeled cells was similar in the PTEN-infected cells and the GFP-infected cells. In contrast, overexpression of mutant PTEN GE caused an increase in the number of Ki67-positive neurons (Fig. 4B). We performed an immunoblotting assay to determine whether overexpression of PTEN or PTEN GE affects Akt and ERK phosphorylation and found that p-Akt levels were reduced in cells infected with the PTEN construct, whereas overexpression of PTEN did not affect p-ERK levels during proliferation (Fig. 4C). PTEN GE overexpression in proliferating hNSCs was not increased p-Akt levels, and levels of both p-ERK and p-S6K were significantly increased in proliferating hNSCs (Fig. 4C).
Figure 4.
PTEN inhibition promoted the proliferation of human neural stem cells (hNSCs). (A): hNSC cultures were infected in parallel with vectors expressing PTEN-GFP, PTEN G129E-GFP, or GFP and cell lysates were subjected to immunoblotting with antibody against GFP. (B): Infected cells were cultured for 3 days in conditions of proliferation. Representative immunofluorescence for Ki67 (red) and fluorescent staining of DAPI (blue), and the proportion of Ki67-positive cells were calculated. (C): Lysates of proliferative cells were subjected to immunoblotting for PTEN, p-Akt, p-S6K, p-ERK, and β-actin, as indicated. Each blot represents three independent experiments. Chemical inhibition of PTEN increases proliferation of hNSCs. hNSCs were treated with or without VO-OHpic during proliferation (in the presence of basic fibroblast growth factor) (D, E). (D): hNSCs were cultured under conditions of proliferation in the presence or absence of VO-OHpic (200 or 400 nM) for 5 days, and lysates were subjected to immunoblotting with PTEN, p-PTEN, p-Akt, p-ERK, and β-actin, as indicated. Each blot represents three independent experiments. (E): Representative immunofluorescence for Ki67 (red) and fluorescent staining of DAPI (blue) are shown, and the proportion of Ki67-positive cells was calculated. Error bars are standard errors of the mean (SE). ∗∗, p < .001 in comparison with control cells. Scale bar = 20 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ERK, extracellular signal-regulated kinase; GE, PTN-G129E-GFP (a catalytically inactive PTEN mutant); GFP, green fluorescent protein; p, phosphorylated; PTEN, phosphatase and tension homolog; PTEN GE, a catalytically inactive PTEN mutant; S6K, S6 kinase.
Because PTEN is known to be a negative regulator of Akt, we confirmed the effects of PTEN on differentiating hNSCs using VO-OHpic, a specific inhibitor of PTEN. We also evaluated the effects of PTEN inhibition on proliferating hNSCs by immunolabeling of proliferative cells with anti-Ki67 (Fig. 4E). VO-OHpic is an extremely potent inhibitor of PTEN with nanomolar affinity in vitro and in vivo. Treatment of hNSCs with 200 and 400 nM VO-OHPic reduced the level of PTEN and increased the level of p-PTEN, an inhibitory phosphorylated form of PTEN (Fig. 4D). Furthermore, in comparison with untreated cells, VO-OHpic increased the expression of p-Akt and p-ERK, accompanied by an increase in the number of Ki67-positive cells (Fig. 4D, 4E).
PTEN Promotes Dopaminergic Neuronal Differentiation of hNSCs
We next examined the regulatory effect of PTEN on S6K and ERK signaling during dopaminergic neuronal differentiation of hNSCs. Overexpression of PTEN resulted in an increase in the expression level of p-S6K and a decrease in p-Akt and p-ERK expression. Conversely, overexpression of the inactive mutant G129E decreased p-S6K expression and increased expression levels of p-Akt and p-ERK (Fig. 5A). PTEN overexpression in hNSCs that were induced to differentiate in the absence of bFGF caused an increase in the level of TH expression and the number of TH-positive cells; however, overexpression of PTEN G129E resulted in a decrease in TH expression and TH-positive cells (Fig. 5B, 5C). As was expected, chemical inhibition of PTEN in differentiating cells using VO-OHpic caused a reduction in the number of TH- and TuJ1-positive cells, accompanied by activation of Akt and ERK (Fig. 5D, 5E), and blocked TH expression (Fig. 5D, 5F). In addition, chemical inhibition of ERK in differentiating cells using U0126 blocked the decrease of TH expression induced by PTEN inhibition (Fig. 5D). Contrary to our speculation, these results showed that PTEN inhibition resulted in decreased differentiation, even though the level of p-Akt was increased.
Figure 5.
PTEN promoted dopaminergic neuronal differentiation of human neural stem cells (hNSCs). hNSC cultures were infected in parallel with vectors expressing PTEN-GFP, PTEN G129E-GFP, and GFP. After 3 days, the infected cells were cultured for a further 5 days in conditions of differentiation. (A): Lysates from differentiating cells were subjected to immunoblot analysis of PTEN, p-Akt, p-S6K, p-ERK, and β-actin, as indicated. (B, C): Differentiating cells were subjected to immunoblotting (B) and immunofluorescent staining (C) with anti-TH antibody (red). Green: GFP; blue: DAPI (n = 5). (D–F): Chemical inhibition of PTEN decreases dopaminergic neuronal differentiation. hNSCs were treated with or without VO-OHpic during differentiation (in the absence of basic fibroblast growth factor). Cells positive for TH were quantified as the percentage of GFP-positive cells. (D): Differentiating cells treated with VO-OHpic only or cotreatment with 10 µM U0126 at day 5 were subjected to immunofluorescence with anti-TuJ-1 (red) and anti-TH (green) antibodies; blue indicates DAPI staining. (E): Western blot analysis of PTEN, p-PTEN, p-Akt, p-ERK, p-S6K, and β-actin in hNPCs after treatment with 200 or 400 nM VO-OHpic during differentiation for 5 days. Each Western blot image is representative of three different experiments. (F): Differentiating cells with or without VO-OHpic treatment for 5 days were subjected to immunoblotting for TH and β-actin, as indicated. Error bars are standard errors of the mean (SE). ∗, p < .05, in comparison with control cells. Scale bar = 20 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ERK, extracellular signal-regulated kinase; GFP, green fluorescent protein; p, phosphorylated; PTEN, phosphatase and tension homolog PTEN GE, a catalytically inactive PTEN mutant; S6K, S6 kinase; TH, tyrosine hydroxylase.
Inhibition of ERK Signaling Promoted Dopaminergic Neuronal Differentiation Through S6K Signaling
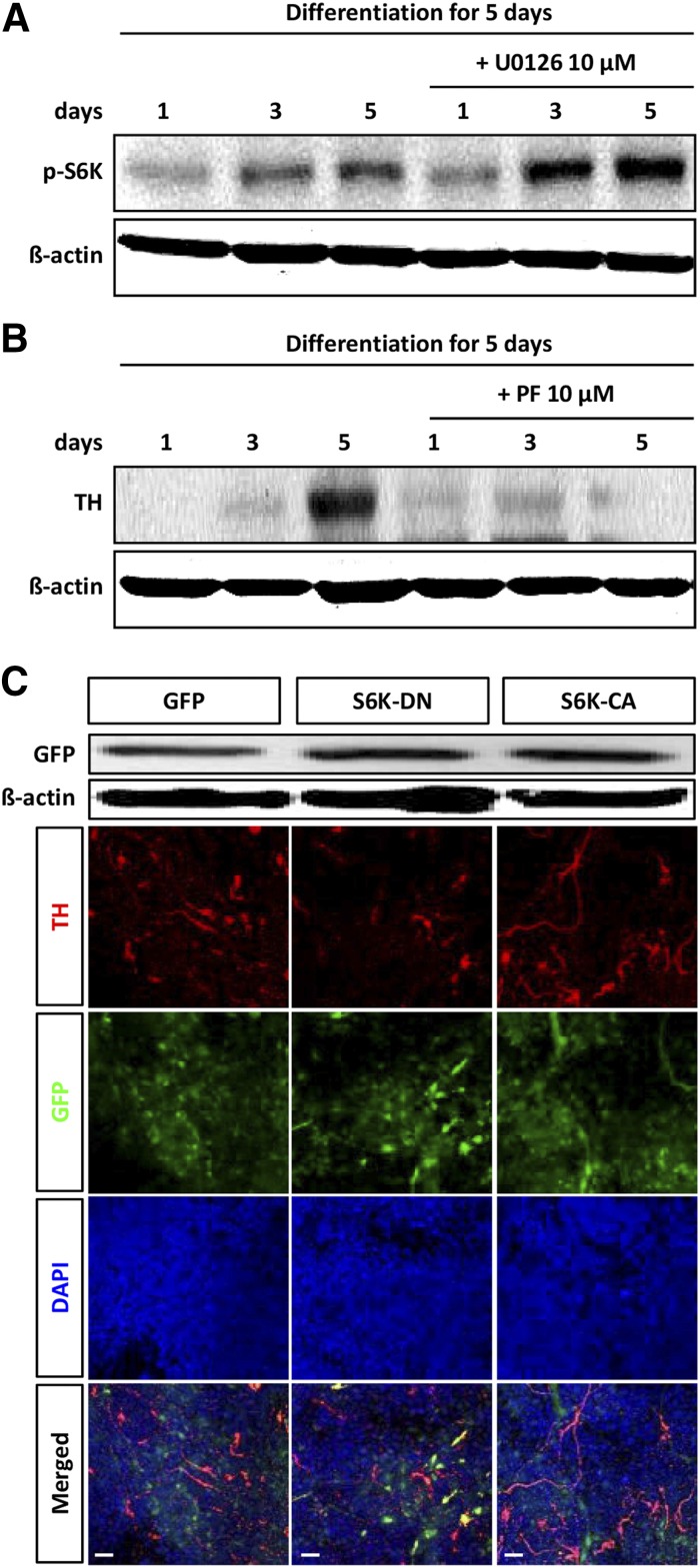
Finally, we examined whether modulation of S6K signaling was directly involved in dopaminergic neuronal differentiation of hNSCs. We observed that S6K signaling is an ultimate signal of the insulin signaling cascade during dopaminergic neuronal differentiation of hNSCs (supplemental online Fig. 2). In addition, the expression of S6K increased in a time-dependent manner during hNSC differentiation (Fig. 6A). Moreover, the level of p-S6K was increased by U0126 treatment (Fig. 6A). The activity of S6K was associated with differentiation of hNSCs. As was expected, inhibition of S6K significantly reduced TH expression (Fig. 6B).
Figure 6.
Suppression of S6K decreases cell number and dopaminergic neuronal differentiation. (A): Differentiating human neural stem cells (hNSCs) were treated with U0126 for 5 days, and lysates were subjected to immunoblotting for p-S6K and β-actin, as indicated. Each blot represents three independent experiments. (B): Western blot analysis of TH and β-actin in hNPCs after treatment with 10 µM PF-4708671 during differentiation at 1, 3, and 5 days in H9 embryonic stem cell (ESC)-derived hNSCs. (C): hNSCs were treated with a retroviral vector expressing constitutively active S6K1 (S6K1-CA-GFP) or dominant negative mutant S6K1 (S6K1-DN-GFP). Similar transduction efficiencies between the two constructs were confirmed by immunoblot analysis of lysates of the transduced cells using an antibody against GFP. Overexpression of S6K1-CA in hNSCs that were induced to differentiate in the absence of basic fibroblast growth factor caused an increase in the number of TH-positive cells; however, overexpression of S6K1-DN resulted in a decrease in the number of TH-positive cells. Scale bar = 20 μm. Abbreviations: CA, constitutively active; DAPI, 4′,6-diamidino-2-phenylindole; DN, dominant negative; GFP, green fluorescent proteins; p, phosphorylated; S6K, S6 kinase; TH, tyrosine hydroxylase.
To further study the influence of the S6K signaling pathway on dopaminergic neuronal differentiation of hNSCs, we treated cells with a retroviral vector expressing constitutively active S6K1 (S6K1-CA-GFP) or dominant negative mutant S6K1 (S6K1-DN-GFP). Similar transduction efficiencies between the two constructs were confirmed by immunoblot analysis of lysates of the transduced cells using an antibody against GFP (Fig. 6C). S6K1-CA overexpression in hNSCs that were induced to differentiate in the absence of bFGF caused an increase in the number of TH-positive cells; however, S6K1-DN overexpression resulted in a decrease in TH-positive cell numbers (Fig. 6C). These results show that S6K1 promotes dopaminergic neuronal differentiation of hNSCs. Our findings indicate the requirement for S6K in hNSC differentiation and suggest that S6K is regulated by ERK signaling pathways.
Discussion
Here, we describe a novel interaction by which the ERK and S6K cascades are modulated by PTEN during proliferation and dopaminergic neuronal differentiation of hNSCs. We found that (a) Akt/mTOR signaling is essential for coordinating dopaminergic neuronal differentiation; (b) mTOR signaling and its downstream effector S6K play a key role in this differentiation; (c) ERK signaling maintains the proliferative capability of hNSCs; (d) PTEN regulates proliferation and differentiation through modulation of the expression level of ERK; (e) inhibition of ERK signaling promotes the dopaminergic neuronal differentiation of hNSCs via activation of S6K; and (f) PTEN regulates the S6K crosstalk of ERK signaling during dopaminergic neuronal differentiation.
Importantly, dopaminergic neuronal differentiation depended on the concentration of insulin in our culture system. In addition, the levels of p-Akt, p-mTOR, and p-S6K—phosphorylated proteins involved in the insulin-mediated signal cascade—were increased during differentiation of hNSCs into dopaminergic neurons. Our previous reports showed the positive function of insulin in the dopaminergic neuronal differentiation of hNSCs [31]. mTOR is a well-known downstream effector of PI3K/Akt. Although mTOR has been reported to regulate cell growth, in our previous study, inhibition of mTOR by rapamycin did not affect proliferation, and proliferating hNSCs maintained the capacity for self-renewal even under conditions of mTOR inhibition. In the present study, the expression of p-mTOR was increased during dopaminergic neuronal differentiation. We found that rapamycin treatment decreased dopaminergic neuronal differentiation even in the absence of insulin. This may be related to previous observations that neuronal differentiation maintained by endogenous insulin is blocked by rapamycin [6]. Our results showed that inhibition of mTOR abrogated the phosphorylation of mTOR and significantly decreased the number of TuJ1- and TH-positive neurons and the expression level of these proteins. In addition, the number of functional dopaminergic neurons also decreased after rapamycin treatment. pS6K is a well-characterized downstream effector of mTOR. Activation of S6K is proposed to be a physiological regulator in the transition from pluripotent hESCs to differentiated cells [32]. We previously reported that S6K is necessary for the differentiation of hNSCs and that S6K activation promotes dopaminergic neuronal differentiation of hNSCs [31].
Because PTEN negatively regulates the PI3K/Akt pathway, we performed experiments to study the influence of the PI3K-Akt pathway on dopaminergic neuronal differentiation using hNSCs that were retrovirally infected with wild-type PTEN or an inactive PTEN mutant. The PTEN tumor suppressor gene was the first phosphatase gene shown to frequently exhibit somatic mutation in various human cancers, including glioma. PTEN and PI3K have opposing effects on phosphatidylinositol phosphate levels and, consequently, opposing effects on cell proliferation and survival [33, 34]. Besides carcinogenesis, PTEN may play a critical role in neurogenesis, as suggested by the increased proliferation observed in mice and mouse NSCs lacking PTEN [16]. We found that the proliferation of hNSCs was similar in the PTEN-infected cells and the GFP-infected cells. In contrast, overexpression of mutant PTEN GE caused an increase in the number of Ki67-positive neurons. Under our experimental condition, the moderate increase in the levels of PTEN did not affect the proliferation of hNSCs in the presence of bFGF. These mitogens activate the ERK1/2 pathways [35] and could then counteract the putative effects of PTEN phosphatase activity on proliferation. A previous study reported that PTEN overexpression did not affect the proliferation of OBSCs in the present bFGF and EGF [5]. Accordingly, we cannot rule out a role for PTEN in maintaining the homeostasis of the cell proliferation in hNSCs. PTEN expression is induced during neuronal differentiation, and suppression of PTEN levels decreases the yield of neuronal cells [36, 37]. In addition, loss of PTEN leads to the exhaustion of adult hematopoietic stem cells [38, 39], and conditional deletion of PTEN in both embryonic and adult NSCs promotes sustained self-renewal and stem/progenitor expansion [16, 24]. Similarly, we observed that inhibition of PTEN by a PTEN inactive mutant or specific chemical inhibitor blocked the appearance of TH-positive neurons accompanied by enhancement of proliferation. In contrast to our results, a previous study showed that loss of PTEN promoted differentiation of mouse OBSCs into neurons and astrocytes and that PTEN overexpression decreased the differentiation of these cells only in the absence of insulin/IGF-1 [5]. In addition, PTEN knockout mice do not show any changes in the number of neurons or astrocytes [16]. Inhibition of PTEN leads to death of the resulting immature neurons, but PTEN is not required for astrocytic differentiation [36]. The nuclear localization of PTEN in PC12 cells is associated with cell-cycle arrest in G1 [36]. These discordant results probably reflect differences in the influence of PTEN depending on cell type, its subcellular localization, developmental stage, and extracellular components such as insulin. In this study, inhibition of PTEN by treatment with a specific inhibitor stimulated levels of both p-Akt and p-ERK in hNSCs. However, we could not observe the increase of p-AKT level by overexpression of PTEN GE mutant. These observations were not expected, and we did not know why this happened. However, we consistently obtained the increase of p-S6K and p-ERK upon the overexpression of PTEN GE. In contrast, the number of TH-positive neurons was increased in hNSCs overexpressing PTEN in comparison with those that overexpressed the PTEN inactive mutant or were treated with specific PTEN inhibitor. Moreover, the expression of ERK was not increased in hNSCs overexpressing PTEN. PTEN overexpression in PC12 cells inhibits neuronal differentiation with a reduction in both p-Akt and p-ERK [40]. Although p-Akt expression was increased by inhibition of PTEN in our experimental conditions, the differentiation of hNSCs was decreased. We expected to observe an increase in p-Akt levels in hNSCs that inhibits the PTEN signaling concomitant with the increase in hNSC differentiation; however, we obtained the opposite result. The major finding of the present study is that the effects of PTEN on S6K phosphorylation lead to dopaminergic neuronal differentiation via the ERK signaling pathway. We found that the expression of ERK was increased in cells expressing a PTEN inactive mutant and in cells treated with a PTEN-specific inhibitor in both the presence and absence of bFGF. Interestingly, the increase in p-ERK was accompanied by a reduction in p-S6K, despite the induction of Akt by PTEN inhibition. These results show that PTEN modulates both proliferation and differentiation through the regulation of ERK. We therefore examined the role of ERK during the proliferation and differentiation of hNSCs.
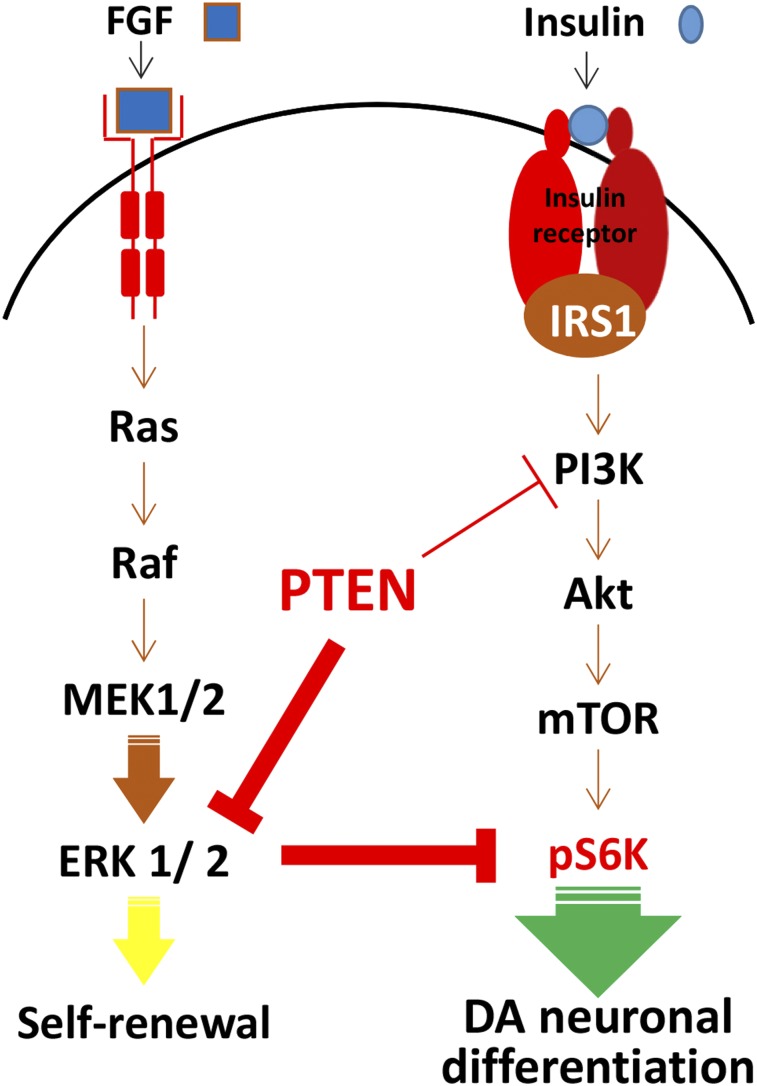
Fibroblast growth factors are major factors involved in the self-renewal of hESCs and have been shown to activate the ERK cascade [26, 41]. ERK is known to have cell survival effects; however, in neurons, ERK has been reported to be involved in both positive and negative regulation of differentiation, depending on extracellular stimuli [42–44]. Of interest, a recent finding suggests cross-inhibitory regulation between the PI3K/Akt and Ras/ERK signaling pathways, by which each contributes to the self-renewal and differentiation of glioma stem-like cells [27]. Therefore, increased ERK activity might be masking the effect of p-Akt on the activation of mTOR or S6K. In this study, we identified a role of the ERK pathway during proliferation and dopaminergic neuronal differentiation of hNSCs. Inhibition of ERK reduced the number of proliferating NSCs and enhanced the expression of TuJ1- and TH-positive neurons and their immunoreactivity in differentiating neurons. We also found that the expression of ERK was reduced during differentiation. Importantly, inhibiting ERK significantly increased the levels of p-S6K without activation of Akt and mTOR. These results indicated that ERK might directly inhibit S6K. This phenomenon was confirmed by Western blot analysis of hNSCs with or without U0126 and rapamycin treatment during differentiation. We showed that levels of S6K gradually increased during differentiation from days 1 to 5 and that the expression of S6K was higher in U0126-treated cells. Furthermore, the mTOR inhibitor rapamycin completely abolished S6K signaling. Treatment with rapamycin did not impair proliferation, although we observed decreased dopaminergic neuronal differentiation. Activation of S6K is proposed to be a physiological regulator in the cascade from pluripotent hESCs to differentiation [32]. Many developmental models have shown that the onset of protein synthesis is vital for generating and maintaining a differentiated state. S6K acts on many substrates in addition to ribosomal protein S6, including transcription factors, RNA helicases, and other protein substrates involved in translation initiation and elongation [45]. To determine whether S6K is dispensable for the maintenance of proliferation of hNSCs, we selectively inhibited S6K using a specific inhibitor and found that inhibition of S6K inhibits dopaminergic neuronal differentiation without affecting hNSC proliferation. These results imply that constant regulation of mTOR/S6K signaling is required for dopaminergic neuronal differentiation. Finally, ERK signaling is one of the effectors downstream of PTEN signaling. As shown in this study, PTEN blocks ERK signaling and ERK promotes neuronal generation, especially dopaminergic neuronal differentiation through the S6K signaling pathway in hNSCs (Fig. 7).
Figure 7.
Diagram showing the regulation of dopaminergic neuronal differentiation of human neural stem cells (hNSCs). ERK signaling is one of the effectors downstream of PTEN signaling. We showed that PTEN blocks ERK signaling and that ERK promotes dopaminergic neuronal differentiation through S6K signaling pathway in hNSCs. Abbreviations: DA, dopaminergic; ERK, extracellular signal-regulated kinase; FGF, fibroblast growth factor; mTOR, mammalian target of rapamycin; p, phosphorylated; PTEN, phosphatase and tension homolog; S6K, S6 kinase.
This study identified a novel regulatory system operating in hNSCs and provided new evidence for the mechanisms by which PI3K/Akt/mTOR and ERK are linked to the regulation of S6K. PI3K/Akt signaling is required for cell survival and differentiation of hNSCs. During differentiation of hNSCs into dopaminergic neurons, the insulin-mediated signal cascade involving p-Akt, p-mTOR, and p-S6K is activated. We also found that mTOR/S6K plays a key role in the differentiation of hNSCs. Significantly, PTEN regulates S6K via ERK signaling. Because ERK has an inhibitory effect on S6K, this may help to maintain the proliferative state of hNSCs. This is the first report of the link between PI3K/Akt/mTOR and ERK and the regulation of S6K through PTEN in hNSCs. Discovery of the key mechanisms involved in maintaining proliferation and differentiation of hNSCs is necessary before cell therapy can achieve a high degree of efficiency. Our findings might help to improve stem cell therapy quality control and minimize the risks.
Supplementary Material
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (NRF-2008-0062287) through the Medical Research Center at Hanyang University College of Medicine, Republic of Korea, and by a grant from the Korean Health Technology Research and Development Project, Ministry of Health and Welfare, Republic of Korea (A120524).
Author Contributions
J.E.L. and M.S.L.: conception and design, collection and/or assembly of data, manuscript writing; J.H.P.: collection and/or assembly of data; C.H.P. and H.C.K.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Armstrong L, Hughes O, Yung S, et al. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum Mol Genet. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 2.Paling NR, Wheadon H, Bone HK, et al. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48,063–48,070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 3.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24:344–350. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 4.Wang B, Gao Y, Xiao Z, et al. Erk1/2 promotes proliferation and inhibits neuronal differentiation of neural stem cells. Neurosci Lett. 2009;461:252–257. doi: 10.1016/j.neulet.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Otaegi G, Yusta-Boyo MJ, Vergaño-Vera E, et al. Modulation of the PI 3-kinase-Akt signalling pathway by IGF-I and PTEN regulates the differentiation of neural stem/precursor cells. J Cell Sci. 2006;119:2739–2748. doi: 10.1242/jcs.03012. [DOI] [PubMed] [Google Scholar]
- 6.Han J, Wang B, Xiao Z, et al. Mammalian target of rapamycin (mTOR) is involved in the neuronal differentiation of neural progenitors induced by insulin. Mol Cell Neurosci. 2008;39:118–124. doi: 10.1016/j.mcn.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proc Natl Acad Sci USA. 2001;98:7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gingras AC, Raught B, Sonenberg N. Control of translation by the target of rapamycin proteins. Prog Mol Subcell Biol. 2001;27:143–174. doi: 10.1007/978-3-662-09889-9_6. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Su P, Wang L, et al. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc Natl Acad Sci USA. 2009;106:7840–7845. doi: 10.1073/pnas.0901854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato A, Sunayama J, Matsuda K, et al. Regulation of neural stem/progenitor cell maintenance by PI3K and mTOR. Neurosci Lett. 2010;470:115–120. doi: 10.1016/j.neulet.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 11.Scott PH, Brunn GJ, Kohn AD, et al. Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1998;95:7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekulić A, Hudson CC, Homme JL, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 13.Fishwick KJ, Li RA, Halley P, et al. Initiation of neuronal differentiation requires PI3-kinase/TOR signalling in the vertebrate neural tube. Dev Biol. 2010;338:215–225. doi: 10.1016/j.ydbio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Easton RM, Cho H, Roovers K, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 16.Groszer M, Erickson R, Scripture-Adams DD, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 17.Marino S, Krimpenfort P, Leung C, et al. PTEN is essential for cell migration but not for fate determination and tumourigenesis in the cerebellum. Development. 2002;129:3513–3522. doi: 10.1242/dev.129.14.3513. [DOI] [PubMed] [Google Scholar]
- 18.Yue Q, Groszer M, Gil JS, et al. PTEN deletion in Bergmann glia leads to premature differentiation and affects laminar organization. Development. 2005;132:3281–3291. doi: 10.1242/dev.01891. [DOI] [PubMed] [Google Scholar]
- 19.Simpson L, Parsons R. PTEN: life as a tumor suppressor. Exp Cell Res. 2001;264:29–41. doi: 10.1006/excr.2000.5130. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Liu F, Ross AH. PTEN regulation of neural development and CNS stem cells. J Cell Biochem. 2003;88:24–28. doi: 10.1002/jcb.10312. [DOI] [PubMed] [Google Scholar]
- 21.Stiles B, Groszer M, Wang S, et al. PTENless means more. Dev Biol. 2004;273:175–184. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 22.He XC, Yin T, Grindley JC, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korkaya H, Paulson A, Charafe-Jauffret E, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groszer M, Erickson R, Scripture-Adams DD, et al. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci USA. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welham MJ, Kingham E, Sanchez-Ripoll Y, et al. Controlling embryonic stem cell proliferation and pluripotency: the role of PI3K- and GSK-3-dependent signalling. Biochem Soc Trans. 2011;39:674–678. doi: 10.1042/BST0390674. [DOI] [PubMed] [Google Scholar]
- 26.Greber B, Coulon P, Zhang M, et al. FGF signalling inhibits neural induction in human embryonic stem cells. EMBO J. 2011;30:4874–4884. doi: 10.1038/emboj.2011.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunayama J, Matsuda K, Sato A, et al. Crosstalk between the PI3K/mTOR and MEK/ERK pathways involved in the maintenance of self-renewal and tumorigenicity of glioblastoma stem-like cells. Stem Cells. 2010;28:1930–1939. doi: 10.1002/stem.521. [DOI] [PubMed] [Google Scholar]
- 28.Rhee YH, Ko JY, Chang MY, et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J Clin Invest. 2011;121:2326–2335. doi: 10.1172/JCI45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okabe S, Forsberg-Nilsson K, Spiro AC, et al. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech Dev. 1996;59:89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- 30.Park CH, Kang JS, Yoon EH, et al. Proneural bHLH neurogenin 2 differentially regulates Nurr1-induced dopamine neuron differentiation in rat and mouse neural precursor cells in vitro. FEBS Lett. 2008;582:537–542. doi: 10.1016/j.febslet.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Lee JE, Lim MS, Park JH, et al. S6K promotes dopaminergic neuronal differentiation through PI3K/Akt/mTOR-dependent signaling pathways in human neural stem cells. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9325-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Easley CA, 4th, Ben-Yehudah A, Redinger CJ, et al. mTOR-mediated activation of p70 S6K induces differentiation of pluripotent human embryonic stem cells. Cell Reprogram. 2010;12:263–273. doi: 10.1089/cell.2010.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 34.Myers MP, Pass I, Batty IH, et al. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci USA. 1998;95:13,513–13,518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kessaris N, Jamen F, Rubin LL, et al. Cooperation between sonic hedgehog and fibroblast growth factor/MAPK signalling pathways in neocortical precursors. Development. 2004;131:1289–1298. doi: 10.1242/dev.01027. [DOI] [PubMed] [Google Scholar]
- 36.Lachyankar MB, Sultana N, Schonhoff CM, et al. A role for nuclear PTEN in neuronal differentiation. J Neurosci. 2000;20:1404–1413. doi: 10.1523/JNEUROSCI.20-04-01404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Cristofano A, Pesce B, Cordon-Cardo C, et al. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Grindley JC, Yin T, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 40.Musatov S, Roberts J, Brooks AI, et al. Inhibition of neuronal phenotype by PTEN in PC12 cells. Proc Natl Acad Sci USA. 2004;101:3627–3631. doi: 10.1073/pnas.0308289101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang HB, Kim JS, Kwon HJ, et al. Basic fibroblast growth factor activates ERK and induces c-fos in human embryonic stem cell line MizhES1. Stem Cells Dev. 2005;14:395–401. doi: 10.1089/scd.2005.14.395. [DOI] [PubMed] [Google Scholar]
- 42.Milosevic J, Brandt A, Roemuss U, et al. Uracil nucleotides stimulate human neural precursor cell proliferation and dopaminergic differentiation: involvement of MEK/ERK signalling. J Neurochem. 2006;99:913–923. doi: 10.1111/j.1471-4159.2006.04132.x. [DOI] [PubMed] [Google Scholar]
- 43.Woessmann W, Zwanzger D, Borkhardt A. ERK signaling pathway is differentially involved in erythroid differentiation of K562 cells depending on time and the inducing agent. Cell Biol Int. 2004;28:403–410. doi: 10.1016/j.cellbi.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Kunath T, Saba-El-Leil MK, Almousailleakh M, et al. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- 45.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.