This article describes a highly efficient, fully feeder-free, serum-free method for erythroid differentiation of induced pluripotent stem cells and human embryonic stem cells, including a clinical-grade line, that is amenable to scale-up and as such will be of significant value for basic and translational studies of hematopoiesis and erythropoiesis.
Keywords: Erythropoiesis, Pluripotent stem cells, Hematopoiesis, Small molecule, Cytokines
Abstract
This article describes a good manufacturing practice (GMP)-compatible, feeder-free and serum-free method to produce large numbers of erythroid cells from human pluripotent stem cells (hPSCs), either embryonic or induced. This multistep protocol combines cytokines and small molecules to mimic and surpass the early stages of development. It produces, without any selection or sorting step, a population of cells in which 91.8% ± 5.4% express CD34 at day 7, 98.6% ± 1.3% express CD43 at day 10, and 99.1% ± 0.95% of cells are CD235a positive by day 31 of the differentiation process. Moreover, this differentiation protocol supports extensive expansion, with a single hPSC producing up to 150 hematopoietic progenitor cells by day 10 and 50,000–200,000 erythroid cells by day 31. The erythroid cells produced exhibit a definitive fetal hematopoietic type, with 90%–95% fetal globin and variable proportion of embryonic and adult globin at the protein level. The presence of small molecules during the differentiation protocol has quantitative and qualitative effects; it increases the proportion of adult globin and decreases the proportion of embryonic globin. Given its level of definition, this system provides a powerful tool for investigation of the mechanisms governing early hematopoiesis and erythropoiesis, including globin switching and enucleation. The early stages of the differentiation protocol could also serve as a starting point for the production of endothelial cells and other hematopoietic cells, or to investigate the production of long-term reconstituting hematopoietic stem cells from hPSCs.
Significance
This differentiation protocol allows the production of a large amount of erythroid cells from pluripotent stem cells. Its efficiency is compatible with that of in vitro red blood cell production, and it can be a considerable asset for studying developmental erythropoiesis and red blood cell enucleation, thereby aiding both basic and translational research. In addition to red cells, the early stages of the protocol could also be used as a starting point for the large-scale production of other hematopoietic cell types, including the ultimate goal of generating long-term reconstituting hematopoietic stem cells.
Introduction
Soon after the first derivation of human embryonic stem cells (hESCs) [1], their capacity for multilineage hematopoietic differentiation was demonstrated [2] by using methods based on knowledge acquired from murine embryonic stem cells and developmental studies. More specific and defined methods of differentiation were subsequently developed but were mainly based on coculture with somatic cell lines of various developmental stages [3, 4], most commonly the OP9 murine bone marrow stromal line. Other methods used feeder-free (FF) methods based on the formation of embryoid bodies (EBs) [5, 6]. Together these methods provided a level of erythroid cell production that allowed some developmental studies [7] and concurrently the idea that hESC-derived erythrocytes might be used as an alternative to donated red blood cells (RBCs) in transfusion was emerging. However, these methods only resulted in a small proportion of hESCs being converted to hematopoietic cells [2–6], and the small numbers of erythrocytes displayed predominantly embryonic/yolk sac characteristics [3–6]. As such, these early methods were qualitatively and quantitatively insufficient for a putative therapeutic use.
The production of RBCs from human pluripotent stem cells (hPSCs) is the subject of growing interest because of the increasing global need for clinically safe red blood cells (RBC) to meet transfusion requirements. More efficient and defined methods are needed to progress basic studies of erythropoiesis toward clinical translation either by creating clinical-grade human feeders similar to those routinely used or by devising a completely FF/serum-free (SF) differentiation method. The use of feeders, even xeno-free and current good manufacturing practice (cGMP)-compliant versions, would be prohibitively costly and impractical for eventual large-scale production even if it were possible to generate a line that recapitulates the effects of OP9 and other current systems. However, an efficient FF/SF culture system would offer a more practical solution, particularly in light of the description of several small molecules that might be used to address the issues.
The FF/SF erythroid differentiation of human pluripotent stem cells grown in FF conditions has long been hampered by poor differentiation efficiency, low yield, primitive erythroid character of the cells produced, and frailty of the cells obtained, such that to date no fully FF/SF method has been published. The use of feeders and serum not only hampers the development of putative cGMP protocols but also confounds the findings of basic studies aiming to elucidate the complex biological mechanisms involved in specification and differentiation. The most defined differentiation systems published to date were those of Lu et al. [8] in 2008 and Salvagiotto et al. [9] in 2011. However, in the first study, the hESCs were grown on feeders and the method proved insufficient when they used induced pluripotent stem cells (iPSCs) [10] instead of hESCs; in Salvagiotto and colleagues’ study, the functional erythroid differentiation was performed in a methylcellulose assay, which doesn’t allow for complete evaluation. Several additional studies have been reported, but all used feeders [11] or serum/plasma [12] at some point, whether their focus was to define biological aspects of erythropoiesis or to develop methods for the in vitro production of RBCs for transfusion.
Here, we describe a highly efficient, fully FF/SF method for erythroid differentiation of iPSCs and human embryonic stem cells, including a clinical-grade line, that is amenable to scale-up and as such will be of significant value for basic and translational studies of hematopoiesis and erythropoiesis.
Materials and Methods
Cell Lines
Data are shown for the hESC lines RC9 (Roslin Cells Ltd, Edinburgh, U.K., http://roslincells.com/), H1, and H9 (WiCell, Madison, WI, http://www.wicell.org/), and an iPSC line, 33D6 [13]. In addition, we tested six newly derived iPSC lines from blood group O RhD− donor fibroblasts (University of Edinburgh, Edinburgh, U.K.), five iPSC lines derived from peripheral blood [14], and five other hESC lines, including RC11 (Roslin Cells), all of which differentiated successfully in this protocol. Both RC lines were derived and banked at clinical grade.
Undifferentiated hPSCs were maintained in Stempro medium (Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com) supplemented with 20 ng/ml of basic fibroblast growth factor (FGF) (FGF2; R&D Systems, Abingdon, U.K., https://www.rndsystems.com) on recombinant vitronectin (Thermo Fisher Scientific Life Sciences) and mechanically passaged approximately every 7 days, depending on their level of confluence, using the EZPassage tool (Thermo Fisher Scientific Life Sciences).
For differentiation, hPSC cultures, at 80%–100% confluence, were cut into squares with the EZPassage tool and plated at 200 × 103 to 600 × 103/well in ultra-low-adherence six-well plates (Corning, Corning, NY, https://www.corning.com/) in 3 ml of Stemline II medium per well (Sigma-Aldrich, Gillingham, U.K., https://www.sigmaaldrich.com/) to form EBs.
On day 0, to induce differentiation, the following cytokines (Mix A1) were added: bone morphogenic protein 4 (BMP4) (10 ng/ml) (R&D Systems), Vascular endothelial growth factor 165 (VEGF) (10 ng/ml) Activin A (5 ng/ml) (both from PeproTech EC Ltd., London, U.K., https://www.peprotech.com/), Wnt3A (10 ng/ml) (R&D Systems), and GSK3β inhibitor VIII (2 μM) (N-[4-methoxybenzyl]-Nʹ-[5-nitro-1,3-thiazol-2-yl] urea) or A-A014418 (Calbiochem; Millipore [UK] Ltd., Feltham, U.K., http://www.emdmillipore.com/).
On day 2 of differentiation (EBs 48 hours old), a new set of cytokines (Mix A2) was added to the existing culture volume in 0.5 ml of Stemline II per well. Cytokines were added at ×6 concentration to supplement the entire volume such that the final concentrations in the well (assumed volume, 3 ml) were BMP4 (20 ng/ml), VEGF (30 ng/ml), Wnt3A (10 ng/ml), Activin A (5 ng/ml), inhibitor VIII (2 μM), acidic FGF (FGFa) (10 ng/ml) (FGF1; PeproTech), stem cell factor (SCF) (20 ng/ml) (Thermo Fisher Scientific Life Sciences), and β-estradiol (0.4 ng/ml) (Sigma-Aldrich).
On day 3 of differentiation, the EBs were harvested, washed in phosphate-buffered saline (PBS), then dissociated using TrypLESelect ×10 (Thermo Fisher Scientific Life Sciences) for 10 minutes at 37°C. After addition of PBS and centrifugation, the cells were resuspended in fresh Stemline II and plated at 200 × 103/well of a standard six-well tissue culture plate with the following factors (Mix B): BMP4 (20 ng/ml), VEGF (30 ng/ml), FGFa (10 ng/ml), SCF (30 ng/ml), insulin-like growth factor (IGF) 2 (10 ng/ml), thrombopoietin (10 ng/ml) (PeproTech), heparin (5 μg/ml), isobutyl methyl xanthine (IBMX) 50 μM (Sigma-Aldrich) and β-estradiol (0.4 ng/ml).
On day 5 of differentiation, the differentiation factor Mix B was refreshed by adding 0.5 ml Stemline II per well with ×6 concentration of cytokines and the small molecule StemRegenin (SR1) (Cellagen Technology, San Diego, CA, http://www.cellagentech.com/) is added to give a final concentration of 1 μM in the culture medium.
On day 7 of differentiation, the cells underwent a complete medium change and were put back into Stemline II medium supplemented with the Mix B factors. If the number of cells was greater than 500 × 103/ml, the culture was split and the density reset to 200 × 103/ml to support proliferation.
From days 7 to 10 of differentiation, the cell density had to be closely monitored and the cell number was maintained below 106 per ml by adding fully supplemented media and splitting into additional wells as required.
On day 9 of differentiation, a half-dose of Mix B cytokines was added in 0.5 ml of Stemline II per well.
On day 10 of differentiation, after centrifugation, the cells were replated in erythroid liquid culture conditions. That is, the cells were plated at a density of 100 × 103 cells/well in 3 ml of Stemline II per well supplemented with the following factors (Mix C): hydrocortisone 1 μM, SCF (50 ng/ml) (Sigma-Aldrich), Flt3-ligand (16.7 ng/ml) (PeproTech), BMP4 (6.7 ng/ml), interleukin (IL) 3 (6.7 ng/ml) (PeproTech), IL11 (6.7 ng/ml) (PeproTech), IBMX (50 μM), and erythropoietin (EPO) (NeoRecormon, Roche Products Ltd., Welwyn Garden City, U.K., http://www.roche.com) 1.3 U/ml. From days 10 to 17, the preceding cytokines and factors were refreshed every 2 days by adding ×6 Mix C in 0.5 ml of Stemline II.
On days 14 and 16, Pluripotin (SC1) (Stemgent, Reinnervate Ltd., Sedgefield, U.K., http://reinnervate.com/) is also added at a final concentration of 500 nM in the culture medium.
On day 17 of differentiation the cells were centrifuged for 3 minutes at 300g, then replated at a density of 0.5–1 × 106 cells/well in 3 ml of IBIT medium per well (composed of incomplete Iscove’s medium with stable glutamine (Biochrom AG, Millipore Ltd.), 1% bovine serum albumin (BSA; BioXtra; Sigma-Aldrich), 10 μg/ml insulin and 200 μg/ml holo-transferrin (both from Sigma-Aldrich), and xeno-free lipid mixture solution (supplied as ×200; Sigma-Aldrich) supplemented with the following factors and cytokines (Mix D): hydrocortisone (1 μM), SCF (20 ng/ml), IGF 1 (20 ng/ml) (PeproTech), IL3 (6.7 ng/ml), IL11 (6.7 ng/ml), and EPO 2 U/ml. The BSA in the IBIT could be replaced by human serum albumin (1% final vol/vol) for a xeno-free version of the medium.
From days 17 to 24, the medium was refreshed with Mix D every 2 days, added at ×6 concentration in 0.5 ml of IBIT medium per well, as noted earlier.
On day 24 of differentiation, the cells were replated at a density of 0.5–1 × 106/ml in IBIT supplemented with 4 U/ml EPO for 2 days, followed by 5–10 days in IBIT medium alone. The culture medium was refreshed every 2 days by addition of IBIT medium.
Analysis and Characterization
At days 0, 3, 5, 7, and 10, cells were analyzed by flow cytometry for expression of SSEA3, SSEA4, and TRA1-60 (BD Biosciences, Oxford, U.K., http://www.bdbiosciences.com/; R&D Systems; and eBioscience Ltd., Hatfield, U.K., http://www.ebioscience.com/) to track the loss of pluripotency of the hPSCs during the differentiation process.
During differentiation, cells were analyzed by flow cytometry to evaluate their hematopoietic and erythroid characteristics. The antibodies used were directed against CD31, CD34, CD36, CD41a, CD43, CD44, CD45, CD71, and CD235a (BD Biosciences and eBioscience), and the cells were analyzed with a BD FACSCalibur or CantoII flow cytometer (BD Biosciences).
At day 17 onward, the erythroid stage of the cells was also determined by assessment of morphology after Rapid Romanowsky staining of cytospin preparations (TAAB Laboratories Equipment Ltd., Aldermaston, U.K., http://www.taab.co.uk/). Slides were assessed by light microscopy using a Zeiss Axio microscope and an Axio Cam MRc camera (Zeiss, Cambridge, U.K., http://www.zeiss.com/).
At days 0, 10, 17, and 24, the expression of selected genes of interest in the differentiating cells was monitored by quantitative real-time polymerase chain reaction (Taqman 7900; Thermo Fisher Scientific Life Sciences) using a custom low-density array card. The gene panel was selected to comprise genes known to be expressed at various stages of hematopoiesis and erythropoiesis to evaluate the degree of differentiation of the hPSCs.
High-Performance Liquid Chromatography
At day 24 or onward, the globin was analyzed by high-performance liquid chromatography (HPLC) to determine the nature and the proportion of the chains expressed at the protein level. The erythroid cells were washed 3 times in PBS and lysed in water through 3 cycles of freezing/thawing. The cells were then centrifuged at 13,000 g at 4°C for 10 minutes and the supernatant collected for HPLC analysis. Globin chain separation was performed by injecting 10 μl of the supernatant onto a 1.0 × 250-mm C4 column (Phenomenex, Macclesfield, U.K., http://www.phenomenex.com/) with a 42%–56% linear gradient between mixtures of 0.1% trifluoroacetic acid (TFA) in water (Buffer A) and 0.1% TFA in acetonitrile (Buffer B) at flow rate of 0.05 ml/min for 55 minutes on an HPLC Ultimate 3000 system (Dionex, Thermo Fisher Scientific Life Sciences). The column temperature was fixed at 50°C during analysis and the ultraviolet detector was set at 220 nm. Lysates from adult peripheral blood and fetal liver were used as reference controls. Elution times of peaks generated were compared with that of the control peaks for identification. Percentage of total globin was calculated for each chain using the area under the curve function in the Dionex Cromeleon Chromatography Data System.
Results
To improve the yield and maturity of hPSC-derived erythroid cells, we have developed a multistep, cGMP-compatible differentiation protocol that combines cytokines and small molecules to mimic the early stages of development while enhancing the intermediate populations. We have successfully used this method on more than 10 different hPSC lines that had also been grown in FF/SF conditions before differentiation.
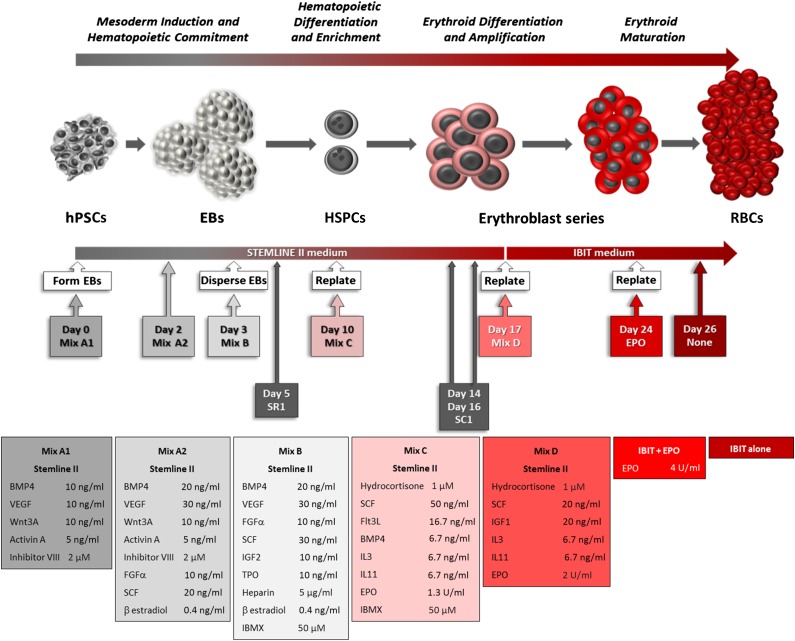
The method comprises five major steps, illustrated in Figure 1 and detailed in the Materials and Methods section. The small molecules were tested at various stages of the differentiation protocol for various lengths of time and were found to be most effective in inducing maximum amplification when added to culture medium following the timing indicated.
Figure 1.
Diagram representing the feeder-free and serum-free erythroid differentiation of hPSCs augmented by the addition of small molecules. Abbreviations: BMP, bone morphogenic protein; EBs, embryoid bodies; EPO, erythropoietin; FGF, fibroblast growth factor; Flt3L, Flt3-ligand; hPSCs, human pluripotent stem cells; HSPCs, hematopoietic stem and progenitor cells; IBIT, IMDM + bovine serum albumin, insulin, transferrin; IBMX, isobutyl methyl xanthine; IGF, insulin-like growth factor; IL, interleukin; RBCs, red blood cells; SCF, stem cell factor; TPO, thrombopoietin; VEGF, vascular endothelial growth factor 165.
The undifferentiated hPSC colonies were initially allowed to form EBs after being mechanically cut, which minimized the size of the EBs, in order to optimize exposure to the cytokine and small molecule mix A1 that was added on day 0 of the differentiation. On the second day of differentiation (48-hour EBs), the cytokines and GSK3β inhibitor VIII are renewed (Mix A2) with an increase of BMP4 and VEGF concentration plus the introduction of FGFα, SCF, and β-estradiol to enforce the differentiation toward mesoderm while priming for the hemato-endothelial compartment [15–17].
Because canonical Wnt signaling triggered by Wnt3A has been reported to be mimicked by GSK3β inhibitors [18] that prevent phosphorylation of β catenin, causing its release from the axin complex, we tested whether small molecule GSK3β inhibitors could be used instead of cytokines to deliver a more potent signal at a lower cost. We initially tested BIO (2′Z,3′E)-6-bromo-indirubin-3′-oxime), the first archetypal GSK3β inhibitor, with poor results, likely because of the wide range of off-target kinases it inhibits (supplemental online Table 1). When we replaced BIO with inhibitor VIII, a highly specific GSK3β inhibitor, we observed that it was not only an apparent replacement for Wnt3A but when used in combination it increased the Wnt effect and improved the quality of the cells produced later in the differentiation process. This was demonstrated by the persistence of intact cells after day 24 in contrast to cultures lacking inhibitor VIII where cell numbers fell as the cells become fragile and broke up (supplemental online Fig. 4). Subsequently, we found that GSK3β inhibitor CHIR99021 could be used in place of inhibitor VIII; however, the concentration had to be closely controlled because this more potent agent could favor the formation of mesenchymal stem/stroma cells over the hemato-endothelial compartment in our protocol (data not shown).
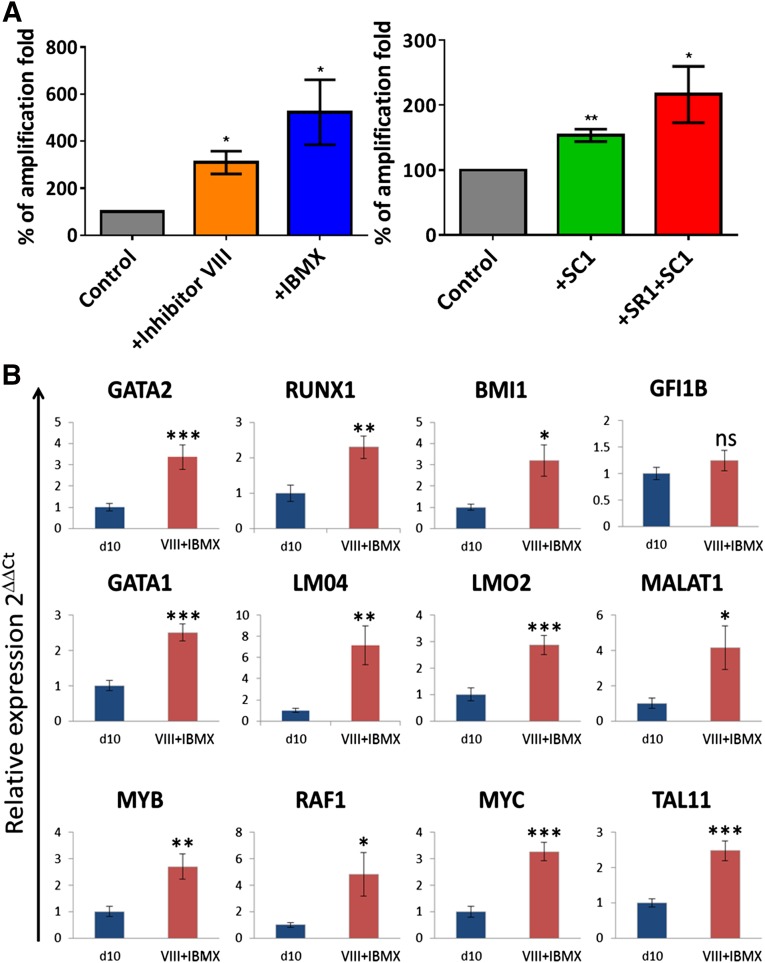
As shown in Figure 2A the addition of inhibitor VIII alone could have a significant effect on the overall yield of cells produced, but this effect varied depending on the hPSC line used (supplemental online Fig. 1A and 1B, left panels). The main effect of inhibitor VIII, when used alone or with Wnt3A, was the qualitative improvement observed in the late-stage cells.
Figure 2.
Analysis of the quantitative effects of the small molecules used on erythroid differentiation of H1 embryonic stem cells (ESCs). (A): Additional use of small molecules increased the yield of erythroid cells. Histograms representative of the small molecule effect on the yield of erythroid cells from the H1 hESC line, expressed as percentage of total amplification relative to the control. Left panel, effect of inhibitor VIII and IBMX assessed at day 24 of the differentiation. n = 3; mean ± SD. Unpaired t test; ∗, p < .05. Right panel, effect of SR1 and SR1 + SC1 assessed on day 31 of the differentiation, when used in addition of Inhibitor VIII and IBMX. n ≥ 4; mean ± SD. Unpaired t test; ∗, p < .05, ∗∗, p < .01. (B): Inhibitor VIII and IBMX addition increase the transcription of key genes involved in hematopoiesis. Quantitative polymerase chain reaction analysis of the effect of inhibitor VIII and IBMX at day 10 of the differentiation on genes known to be expressed during hematopoietic and erythroid development. n = 4 performed in duplicate; mean ± SD. Unpaired t test; ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001. Abbreviations: IBMX, isobutyl methyl xanthine; ns, not significantly different.
On the third day of differentiation, the EBs were enzymatically dissociated and the cells were plated at low density in fresh Stemline II with Mix B, including IBMX.
IBMX is a nonspecific inhibitor of cAMP and cGMP phosphodiesterases that is commonly used to increase the pool of intracellular cAMP [19] and to reduce the effect of tumor necrosis factor (TNF) α. The role of cAMP in HSC specification has recently been reported by Diaz et al. [20], and increased cAMP has been shown to drive erythroid proliferation [21]. TNFα is a recognized inhibitor of hematopoietic progenitor development and of erythropoiesis [22]. Therefore, we tested whether IBMX could improve the yield and quality of cells generated during differentiation. As shown in Figure 2A (and supplemental online Fig. 3), IBMX significantly increased the number of cells produced per differentiating hPSC without any effect on the cell phenotype.
Mix B was renewed every 2 days with the inclusion of 1 μM SR1 on day 5 where required. A complete medium change on day 7 was done to remove debris and dead cells, as well as to provide sufficient nutrients for the proliferative burst that occurs from days 7 to 10. At this point, the cell density had to be closely monitored to prevent the loss of hematopoietic potential and proliferation.
The cytokine formulation used from days 3 to 10 was based on our previous work and that of others [23–25]. It was intended to favor and maintain the formation and amplification of the multipotent hematopoietic progenitor cells (HPCs), as shown in the marker profile and gene expression (Fig. 3; supplemental online Fig. 2). Moreover, the quantitative polymerase chain reaction analyses (Fig. 2B) demonstrated a positive effect of the inclusion of inhibitor VIII and IBMX on the expression of a set of key hematopoietic mRNAs. GATA1 and 2, RUNX1, BMI1, GFI1B, LMO2 and 4, MYB, RAF1, MYC, and TAL1 (SCL) were chosen as transcription factors with an established role in hematopoietic stem cells and/or erythroid lineage commitment and which we expected to be expressed within this specific time frame. The long noncoding RNA MALAT1 was added to the panel because it shows an interesting correlation of expression with some genes involved in erythroid differentiation [26]. The addition of inhibitor VIII and IBMX significantly increased the relative expression of all of these genes at day 10 with the exception of GFI1B, consistent with expression data showing its expression increasing at the later megakaryocyte-erythroid progenitor stage [26].
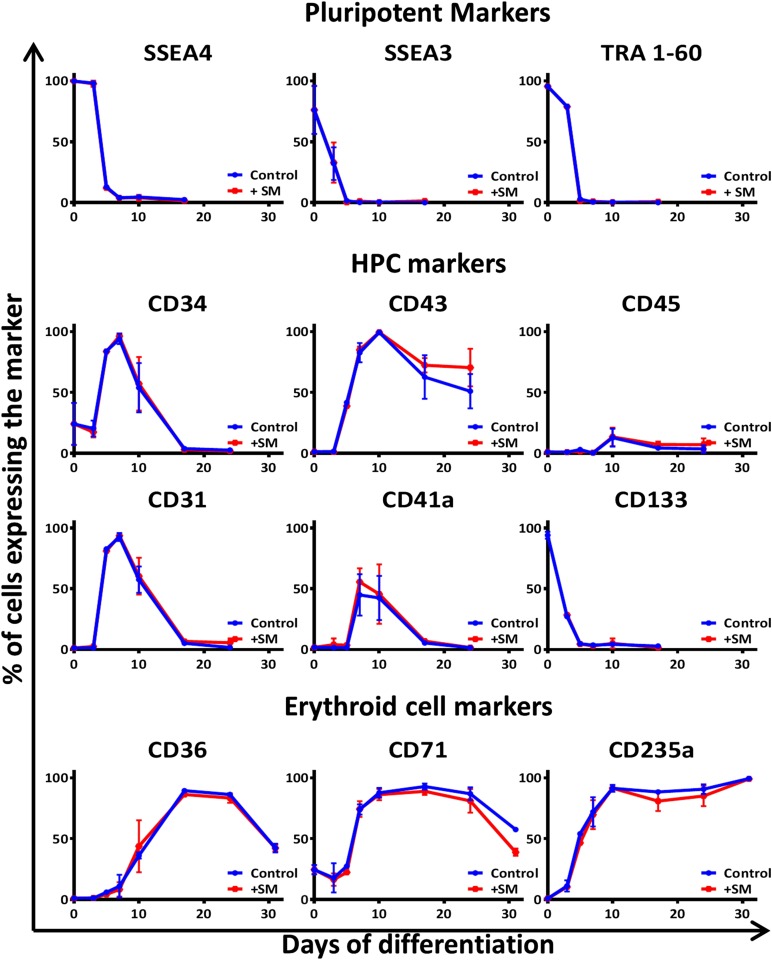
Figure 3.
Analysis of the qualitative effects of the small molecules used on erythroid differentiation of H1 embryonic stem cells (ESCs). Addition of small molecules does not modify the surface marker profile of differentiating cells. Antigenic profile of the culture during the erythroid differentiation of H1 human ESCs with (red lines) or without (Control; blue lines) small molecules (+SM; inhibitor VIII + IBMX + SR1 + SC1). The pluripotent markers show the loss of pluripotency during the first 5 days. The HPC markers show the emergence of the hematopoietic cells with a peak of hemangioblastic markers CD34, CD31, and CD41a at day 7. The erythroid cell markers CD36 (denoting the commitment toward erythroid lineage) and CD235a (also known as glycophorin A) confirm the evolution of the culture toward an almost pure erythroid population between days 17 and 31. Abbreviations: HPC, hematopoietic progenitor cell; SM, small molecules. An example of individual flow cytometry plots is shown in supplemental online Fig. 7.
In light of previous reports that antagonists of the aryl hydrocarbon receptor (AhR) increase proliferation of HPC [27], we added SR1 to extend the period over which CD34-positive hematopoietic cells emerged. HPCs are characterized in part by expression of the CD34 antigen and the maximum proportion of cells expressing CD34+ was observed at day 7, as shown in Figure 3, where CD34 can be detected on 91.8% ± 5.4% of cells analyzed.
Further confirmation of hematopoietic identity is provided by analysis of the CD43 antigen [28]; at day 10, flow cytometry analysis showed that 98.6% ± 1.3% of cells expressed this important marker (Fig. 3; supplemental online Fig. 2A). From the panel of antigens detected on the cells from days 7 to 10, and notably the simultaneous presence of CD31, CD34, CD41a, CD43, and CD235a, it appears that most cells were at the hemangioblastic or posthemangioblastic stage [5]. Between days 7 and 10, when using regular, adherent cell culture plastics, we observed a great proliferation of cells budding from a web of loosely attached cells (supplemental online Fig. 6), which could be likened to a hemogenic endothelium [29]. This was consistent with the expression of the markers observed at the cell surface, and further cultivation of the attached cell fraction in appropriate conditions resulted in the generation of CD144+/CD31+ endothelial cells (data not shown). However, we also achieved similar expansion and erythroid differentiation when the cells were maintained in nonadherent plastic from day 3 to day 10 (data not shown); this would be important for scale-up as the entire protocol can be performed in suspension for example in bags or stirred tank reactors.
From days 10 to 17, the cells were placed in erythroid-specific liquid culture starting from low-seeding density. The small molecule SC1 [30], a dual RasGAP and ERK1/2 inhibitor, was added on days 14 and 16 to enhance the multiplication of the erythroid progenitors by slowing down progression toward a more mature phenotype. This resulted in the longer persistence of the CD43 and CD44 [31, 32] markers (Fig. 3; supplemental online Figs. 2A and 5B) and a higher yield of erythroid cells (Fig. 2A; supplemental online Figs. 1 and 5A). The addition of these small molecules did not otherwise modify the antigenic profile of the differentiating cells (Fig. 3).
At day 17, the antigen expression profile, as well as rapid Romanowsky staining of cytospins, showed that most cells were of the erythroid lineage, with most of them pro- or basophilic normoblasts (Figs. 3 and 4). At this stage, the basal medium was switched to IBIT because Stemline II did not support further erythroblast maturation. The corresponding cytokine cocktail Mix D of hydrocortisone, SCF, IGF1, IL3, IL11, and EPO was refined [24] to produce cells that display the highest levels of erythrocytic markers and was again refreshed every 2 days.
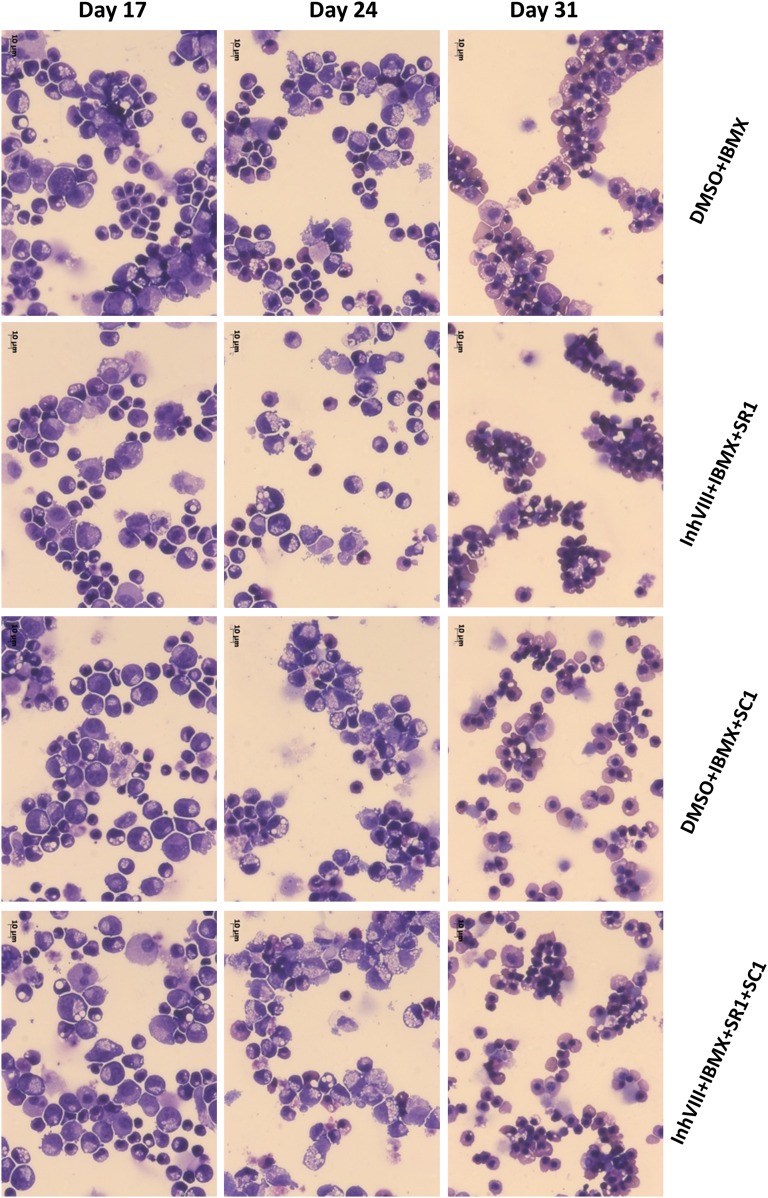
Figure 4.
Erythroid cell morphology progresses over time in the differentiating cultures and in the late stages is substantially improved by the addition of different combinations of SMs as labeled. Photographs of rapid Romanowsky staining of cytospin preparations at different stages of the differentiation that show the effects of different combination of small molecules on the morphology of the differentiating and differentiated cells at days 17, 24, and 31 of the protocol. Original magnification ×400; scale bars = 10 μm. For reference, images of the morphologically distinct stages of erythroid differentiation are within Figures 4 and 5 of Hu et al. [44]. Abbreviations: DMSO, dimethyl sulfoxide; IBMX, isobutyl methyl xanthine; SM, small molecules.
At day 24 of the differentiation, most cells were erythroid, as shown by the flow cytometry analysis, with expression of CD235a on 84.8% ± 8.7% of the cells and CD36 on 85.3% ± 3.3% of the cells (Fig. 3; supplemental online Fig. 2A). Importantly, no sorting or other purification step was required to achieve these predominantly erythroid cultures. The corresponding cytospins show a variable distribution among the basophilic, polychromatic, and orthochromatic subclasses of erythroblasts, depending on the differentiation condition tested and the origin of the hPSCs used (Fig. 4). From this point, when maintained in IBIT supplemented with EPO for 2 days, followed by 5–10 days in IBIT medium alone, the differentiating cells evolved toward an almost homogenous population of orthochromatic normoblasts with 99.1% ± 0.95% expressing CD235a and up to 10% of cells undergoing spontaneous enucleation (Fig. 4). Despite a longer period of amplification, the cells treated with all 4 small molecules also exhibited more mature morphology at day 31, with more enucleated cells visible. In contrast, cells that had not received inhibitor VIII and IBMX rapidly died off and fragmented in culture after day 24, demonstrating the relative fragility of cells grown under those conditions (supplemental online Fig. 4).
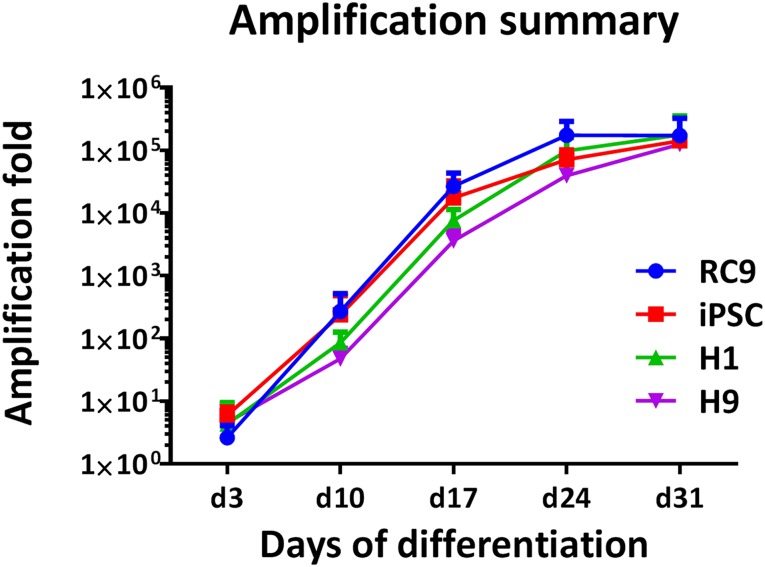
Having tested the small molecule and cytokine mixes individually and together to assess the extent of their effect (exemplified in supplemental online Fig. 5), the cumulative effect of the factors tested support the production of up to 200,000 erythroid cells from a single hPSC (Fig. 5) over 31 days. As such, a full transfusion unit of 2 × 1012 cells could be generated from just 10 million hPSCs, which can easily be harvested from a single 6-well plate or T75 flask.
Figure 5.
Illustration of the yield achieved with different cell lines. Fold amplification of the initial input of human pluripotent stem cells (hPSCs) to the erythroid stage obtained with four different hPSC lines: RC9, n = 5; H1, n = 4; H9, n = 3; iPSC 33D6, n = 5. Abbreviation: iPSC, induced pluripotent stem cell.
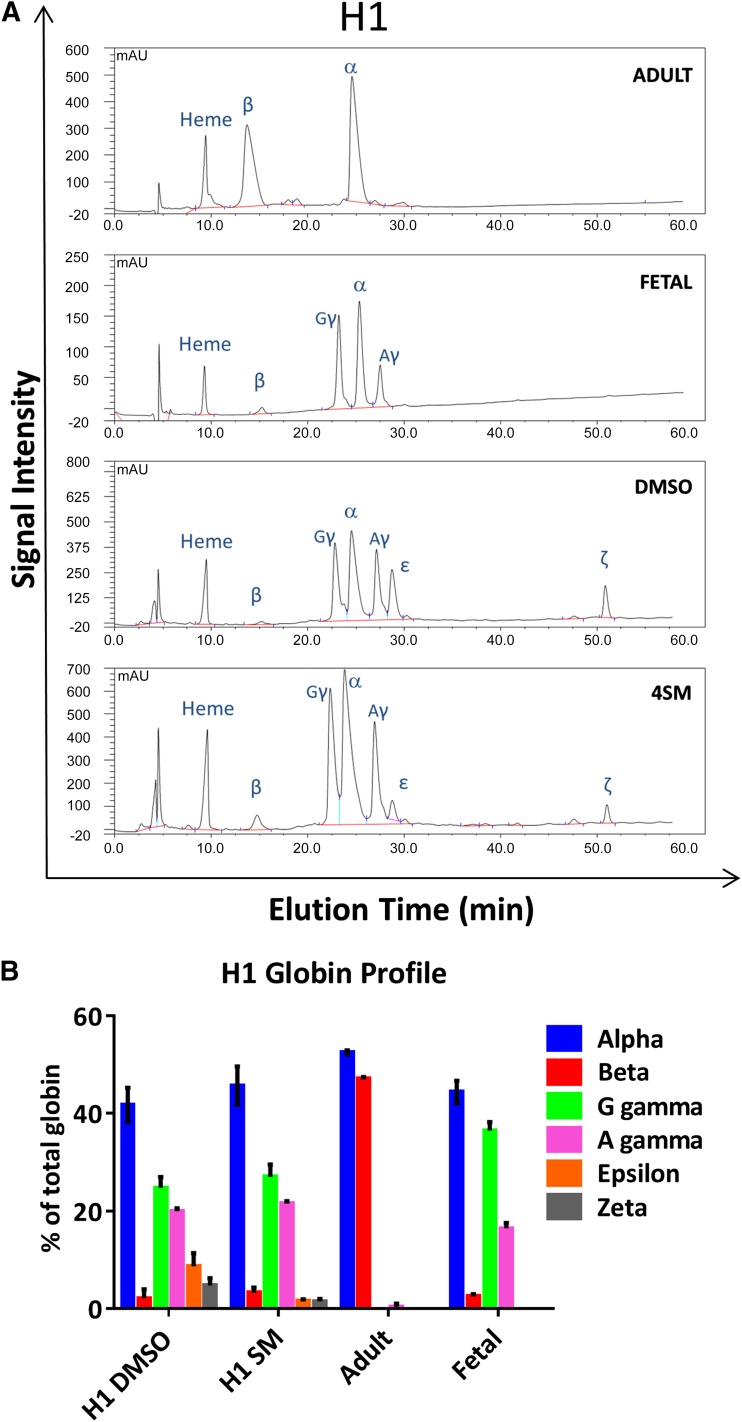
Furthermore, at day 31, HPLC analysis of globin chain expression confirmed the definitive character of the cells produced [7] (Fig. 6B). Fetal globin chains (α and γ) predominated with low, residual expression of embryonic globin (ε and ζ chains) and a small amount of the adult β globin chain at the protein level. The small molecules used decreased the proportion of embryonic globin (ε chain, 11.3% ± 2.4% with dimethyl sulfoxide [DMSO] alone vs. 3.1% ± 1.1%, with small molecules, and ζ chain 5.4% ± 0.8% vs. 1.8% ± 0.3%) and increased the proportion of adult β-globin chain (3.4% ± 0.4% vs. 2.2% ± 0.6%) (Fig. 6A and 6B; supplemental online Figs. 2B and 5C; mean values from H1 and H9 lines). The expression of more developmentally mature globin in response to the addition of the small molecules may be due to increased survival or amplification of definitive progenitors over the precursors that produce erythroid cells with embryonic globin. This predominantly fetal globin expression is compatible with transfusion use and is similar to umbilical cord blood; the detection of β-globin protein indicates that the locus is not fully silenced, and so switching to adult globin might be achieved in these cells with additional stimuli.
Figure 6.
High-performance liquid chromatography (HPLC) analysis of the globin chains expressed at day 31 of H1 human embryonic stem cell (hESC) differentiation. (A): Example of elution profiles obtained by HPLC of H1 hESC-derived erythroid cells with (SM) and without (DMSO) small molecules and of the adult and fetal control used to determine the elution time of the different globin chains. (B): Globin chains were assessed at the protein level after erythroid differentiation with (SM) or without (DMSO) the 4 small molecules added (inhibitor VIII + isobutyl methyl xanthine + SR1 + SC1). Lysates from adult peripheral blood and fetal liver red blood cells were used as reference controls to establish the elution time of each peak. Abbreviations: DMSO, dimethyl sulfoxide; SM, small molecules.
Discussion
Methods of differentiation that rely on feeder cells and/or serum introduce uncontrolled variables that may confound the findings of mechanistic studies, particularly those investigating the effect of growth factors and cytokines on hPSC differentiation. There are still challenges that translate on a practical level into difficulty in switching from complex commercial medium (e.g., Stemline II) to completely chemically defined conditions (e.g., APEL medium [33] or using recombinant human serum albumin) while maintaining similar efficiency. Nonetheless, more highly defined culture conditions advance progress toward the goal to translate basic science into biotechnology and clinical utility, and many applications could benefit from an improved method for the hematopoietic differentiation of hPSCs. Among the most obvious is the capability to provide large amounts of reproducible material, which will allow more precise investigation of the mechanisms governing hematopoiesis, including globin switching and enucleation during erythropoiesis, by enabling multiomics and functional studies or pharmacological screening. The use of liquid culture conditions simplifies the testing of soluble factors as well as the scale up into bioreactors.
This method also forms the basis of a cGMP-compatible system that could be scaled up to derive sufficient cells for in vivo and first-in-human studies of cultured red blood cells once efficient enucleation is attained.
The use of small molecules has expanded from mechanistic signaling studies to use for reprogramming of somatic cells and maintenance of PSCs [30, 34]. More recently they have increasingly been used in methods to differentiate hPSCs in various cell types from all germ layers [35]. Several methods using small molecules for the endothelial differentiation of hPSCs have already been published [36, 37], in which the use of GSK3β inhibitors resulted in enhanced induction of mesodermal lineages as similar to that reported here. Also, the recent publication of Patsch et al. [38] described an endothelial differentiation method, very similar to the one of Sahara et al. [36], using forskolin to activate adenyl cyclase and resulting in increased intracellular cAMP and enhanced differentiation.
The use of small molecule effectors specifically to promote erythroid differentiation of hPSCs has not been reported previously, although activin inhibitors such as SB 431542 have been used at early time points to try to enhance the production of a more definitive hematopoietic stem and progenitor cell and thereby alter the balance of primitive (embryonic globin expressing) and definitive (fetal/adult globin-expressing) erythroid cells [39]. Similarly, Smith et al. [40] reported that aryl hydrocarbon receptor activation with 6-formylindolo[3,2-b]carbazole (FICZ) increased HPC with the capacity to undergo erythroid and megakaryocytic differentiation. Somewhat surprisingly, we saw long-lasting effects of small molecule use such that exposure to GSK3β inhibitors between days 0 and 3, transient use of SR1 at day 5, and SC1 at days 14–16 have strong effects on the quality of the cells produced up to 2–3 weeks later.
Before the introduction of these small molecules in the differentiation process, we observed a strong decline of the erythroid culture after day 24, consistent with the observation of Feng et al. [10] that terminal differentiation of hPSC-derived erythroid cells is impaired, leading to cell death. This method still uses EBs and is not yet compatible with two-dimensional monolayer induction; however, compared with the monolayer system reported by Salvagiotto et al. [9], we generated up to 150 times the number of CD43+ cells over 10 days instead of 14 days. The most similar published method with regard to cell yield was reported by Dias et al. [11]. However, that method used xenogenic feeder cells and took more than 65 days to reach a similar stage of maturation.
Perhaps the most remarkable property of the differentiation system reported here is the relative purity of erythroid cell production in the absence of any sorting or selection steps. The cytokine/small molecule combinations are sufficient to induce extensive hematopoietic, and subsequently erythroid, differentiation and expansion while not supporting other lineages. The few (<5%) nonerythroid cells that are observed at late time points are monocytic in morphology and phenotype. Therefore, we believe that this protocol surpasses previously published methods with respect to the level of definition of the components, the yield of erythroid cells obtained and the time period during which this was achieved.
As with other published methods, the level of enucleation is limited; however, if the total yield of cells is used along with the percentage enucleation to calculate the absolute number of enucleated cells generated per hPSC, this method produces a higher total number of reticulocytes than methods with higher percentage enucleation but much lower cell expansion. The recent report of Rouzbeh et al. [41] demonstrated that it might be possible to increase the percentage of cells undergoing enucleation by reducing the expression of micro RNA30a (MIR30a); however, there was very little, if any, cell expansion in their system. Furthermore, knockdown of an MIR is a difficult strategy to use routinely, whereas identification of the MIR30a targets and development of small molecules effectors would increase the utility of the method and could be easily tested in the high-yield, highly defined differentiation system reported here.
This study highlights several interesting leads for future investigation. We observed an additive activity of Wnt3A signaling and GSK3β inhibitors, which could be due to the role of noncanonical Wnt signaling or additional action of GSK3β, such as the maintenance of DNA methylation [42], but shows that it would be erroneous to assume that a small molecule GSK3β inhibitor can simply replace a bioactive Wnt glycoprotein without full analysis of downstream effects. The timing and duration of small molecule supplementation were essential; for example, we found that the use of SC1 (Pluripotin) during the first week of differentiation, or for a more prolonged period, had a negative effect on cell proliferation, although by analogy to its reported effects on PSCs [30], it might have been expected to have maximal beneficial effect at early time points on the first arising hematopoietic progenitors.
We also found that the published phenotypic action of some small molecules may be dependent on the combination of all the factors involved. For example, we did not observe any effect when using the AhR agonist FICZ [40] or with stauprimide [43] in our method. This may because the target pathways are active only in a more primitive differentiation stage (in the case of FICZ) or because we had already stimulated these pathways with other components of our system as we observed lower levels of apoptosis and higher erythroid specification and expansion in the absence of FICZ than those reported by Smith et al. [40] when it was added. We are performing detailed analyses to determine the molecular mechanisms underlying the high efficiency generation of erythroid cells that we report here.
Conclusion
The combination of cytokines and small molecules in a feeder-free, serum-free protocol has proven to be an effective solution to most of the issues that have hampered researchers trying to produce large numbers of erythroid cells from hPSCs. This protocol results in high differentiation efficiency, high yield, and the generation of robust cells that have definitive erythroid characteristics, as demonstrated by the predominant expression of nonembryonic globin. These cells can readily be used in studies to model normal or diseased erythropoiesis, and the large cell numbers achievable would allow extensive analysis, including multiomics, metabolic, and chromatin-protein interaction assays (such as chromatin immunoprecipitation [ChIP or ChIP sequencing]) over sequential time points from the same culture. We have demonstrated this potential by using the method to differentiate iPSC from normal donors and those with pyruvate kinase deficiency (PKD) before and after gene correction, and we were able to determine the effect of gene correction in erythroid cells differentiated from PKD-iPSCs [14].
Given the level of definition, robustness, simplicity, and absence of genetic modification, the differentiation method presented here also offers a versatile tool to study most aspects of hPSC-derived hematopoiesis. Notably, it could be a stepping stone in the quest to produce transplantable, long-term reconstituting hematopoietic stem cells from hPSCs that might be used in the future to replace donated stem cells and/or combined with genome engineering technologies to correct disease-causing mutations.
Supplementary Material
Acknowledgments
This work was funded by the Wellcome Trust (Grants 087430/Z/08 and 102610), the Scottish Funding Council Horizon Fund, and by the Scottish National Blood Transfusion Service.
Author Contributions
E.N.O.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; L.M.: conception and design, collection and assembly of data; A.M.: collection and assembly of data, data analysis, manuscript writing; A.C.: collection of data, administrative support; S.C.: collection of data; J.C.M.: conception and design, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
E.N.O. and J.C.M. are named as inventors on patent publication no. WO/2014/013255 and international application no. PCT/GB2013/051917 (“Erythroid Production”), filed by the University of Glasgow. The other authors indicated no potential conflicts of interest.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vodyanik MA, Bork JA, Thomson JA, et al. Human embryonic stem cell-derived CD34+ cells: Efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 4.Olivier EN, Qiu C, Velho M, et al. Large-scale production of embryonic red blood cells from human embryonic stem cells. Exp Hematol. 2006;34:1635–1642. doi: 10.1016/j.exphem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Zambidis ET, Peault B, Park TS, et al. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang KH, Nelson AM, Cao H, et al. Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood. 2006;108:1515–1523. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu C, Olivier EN, Velho M, et al. Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood. 2008;111:2400–2408. doi: 10.1182/blood-2007-07-102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu SJ, Feng Q, Park JS, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salvagiotto G, Burton S, Daigh CA, et al. A defined, feeder-free, serum-free system to generate in vitro hematopoietic progenitors and differentiated blood cells from hESCs and hiPSCs. PLoS One. 2011;6:e17829. doi: 10.1371/journal.pone.0017829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Q, Lu SJ, Klimanskaya I, et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 2010;28:704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- 11.Dias J, Gumenyuk M, Kang H, et al. Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev. 2011;20:1639–1647. doi: 10.1089/scd.2011.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobari L, Yates F, Oudrhiri N, et al. Human induced pluripotent stem cells can reach complete terminal maturation: In vivo and in vitro evidence in the erythropoietic differentiation model. Haematologica. 2012;97:1795–1803. doi: 10.3324/haematol.2011.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan GJ, Hay DC, Park IH, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51:329–335. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garate Z, Quintana-Bustamante O, Crane AM, et al. Generation of a high number of healthy erythroid cells from gene-edited pyruvate kinase deficiency patient-specific induced pluripotent stem cells. Stem Cell Reports. 2015;5:1053–1066. doi: 10.1016/j.stemcr.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lengerke C, Schmitt S, Bowman TV, et al. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Pearson S, Sroczynska P, Lacaud G, et al. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development. 2008;135:1525–1535. doi: 10.1242/dev.011767. [DOI] [PubMed] [Google Scholar]
- 17.Pick M, Azzola L, Mossman A, et al. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells. 2007;25:2206–2214. doi: 10.1634/stemcells.2006-0713. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature. 2008;453:338–344. doi: 10.1038/nature07042. [DOI] [PubMed] [Google Scholar]
- 19.Collins DM, Murdoch H, Dunlop AJ, et al. Ndel1 alters its conformation by sequestering cAMP-specific phosphodiesterase-4D3 (PDE4D3) in a manner that is dynamically regulated through Protein Kinase A (PKA) Cell Signal. 2008;20:2356–2369. doi: 10.1016/j.cellsig.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Diaz MF, Li N, Lee HJ, et al. Biomechanical forces promote blood development through prostaglandin E2 and the cAMP-PKA signaling axis. J Exp Med. 2015;212:665–680. doi: 10.1084/jem.20142235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wannatung T, Lithanatudom P, Leecharoenkiat A, et al. Increased erythropoiesis of beta-thalassaemia/Hb E proerythroblasts is mediated by high basal levels of ERK1/2 activation. Br J Haematol. 2009;146:557–568. doi: 10.1111/j.1365-2141.2009.07794.x. [DOI] [PubMed] [Google Scholar]
- 22.Buck I, Morceau F, Cristofanon S, et al. Tumor necrosis factor alpha inhibits erythroid differentiation in human erythropoietin-dependent cells involving p38 MAPK pathway, GATA-1 and FOG-1 downregulation and GATA-2 upregulation. Biochem Pharmacol. 2008;76:1229–1239. doi: 10.1016/j.bcp.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Perlingeiro RC, Kyba M, Bodie S, et al. A role for thrombopoietin in hemangioblast development. Stem Cells. 2003;21:272–280. doi: 10.1634/stemcells.21-3-272. [DOI] [PubMed] [Google Scholar]
- 24.Olivier E, Qiu C, Bouhassira EE. Novel, high-yield red blood cell production methods from CD34-positive cells derived from human embryonic stem, yolk sac, fetal liver, cord blood, and peripheral blood. Stem Cells Translational Medicine. 2012;1:604–614. doi: 10.5966/sctm.2012-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang CC, Kaba M, Ge G, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, Orozco C, Boyer J, et al. BioGPS: An extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108:2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelosi E, Castelli G, Martin-Padura I, et al. Human haemato-endothelial precursors: cord blood CD34+ cells produce haemogenic endothelium. PLoS One. 2012;7:e51109. doi: 10.1371/journal.pone.0051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S, Do JT, Zhang Q, et al. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci USA. 2006;103:17266–17271. doi: 10.1073/pnas.0608156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kansas GS, Muirhead MJ, Dailey MO. Expression of the CD11/CD18, leukocyte adhesion molecule 1, and CD44 adhesion molecules during normal myeloid and erythroid differentiation in humans. Blood. 1990;76:2483–2492. [PubMed] [Google Scholar]
- 32.Chen K, Liu J, Heck S, et al. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci USA. 2009;106:17413–17418. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng ES, Davis R, Stanley EG, et al. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat Protoc. 2008;3:768–776. doi: 10.1038/nprot.2008.42. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Jiang K, Ding S. Concise review: A chemical approach to control cell fate and function. Stem Cells. 2012;30:61–68. doi: 10.1002/stem.768. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Li K, Wei W, et al. Chemical approaches to stem cell biology and therapeutics. Cell Stem Cell. 2013;13:270–283. doi: 10.1016/j.stem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahara M, Hansson EM, Wernet O, et al. Manipulation of a VEGF-Notch signaling circuit drives formation of functional vascular endothelial progenitors from human pluripotent stem cells. Cell Res. 2014;24:820–841. doi: 10.1038/cr.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lian X, Bao X, Al-Ahmad A, et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Rep. 2014;3:804–816. doi: 10.1016/j.stemcr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patsch C, Challet-Meylan L, Thoma EC, et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol. 2015;17:994–1003. doi: 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy M, Awong G, Sturgeon CM, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Reports. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Smith BW, Rozelle SS, Leung A, et al. The aryl hydrocarbon receptor directs hematopoietic progenitor cell expansion and differentiation. Blood. 2013;122:376–385. doi: 10.1182/blood-2012-11-466722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rouzbeh S, Kobari L, Cambot M, et al. Molecular signature of erythroblast enucleation in human embryonic stem cells. Stem Cells. 2015;33:2431–2441. doi: 10.1002/stem.2027. [DOI] [PubMed] [Google Scholar]
- 42.Meredith GD, D’Ippolito A, Dudas M, et al. Glycogen synthase kinase-3 (Gsk-3) plays a fundamental role in maintaining DNA methylation at imprinted loci in mouse embryonic stem cells. Mol Biol Cell. 2015;26:2139–2150. doi: 10.1091/mbc.E15-01-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu S, Wurdak H, Wang J, et al. A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell. 2009;4:416–426. doi: 10.1016/j.stem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Hu J, Liu J, Xue F, et al. Isolation and functional characterization of human erythroblasts at distinct stages: Implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013;121:3246–3253. doi: 10.1182/blood-2013-01-476390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.