Notch signaling driving the quiescence of progenitors has been shown to be central to progenitor self-renewal. This new molecular definition has tremendous translational consequences because progenitors have been shown to display greater vasculogenic potential. This molecular definition of endothelial progenitor cell (EPC) self-renewal allows assessment of the quality of presumed EPC preparations.
Keywords: Endothelial, Progenitor, Vascular, Angiogenesis
Abstract
Since the discovery of endothelial progenitor cells (EPCs) almost 2 decades ago, there has been great hope in their use in treating chronic ischemic disease. Unfortunately, to date, many of the clinical trials using EPCs have been hampered by the lack of clear definition of this cell population. Attributes of a progenitor population are self-renewal and multipotentiality. Major progress has been achieved moving from a definition of EPCs based on a candidate cell surface molecule to a functional definition based essentially on self-renewal hierarchy of endothelial colony-forming cells (ECFCs). More recent work has seized on this functional characterization to associate gene expression signatures with the self-renewal capacity of ECFCs. In particular, Notch signaling driving the quiescence of progenitors has been shown to be central to progenitor self-renewal. This new molecular definition has tremendous translational consequences, because progenitors have been shown to display greater vasculogenic potential. Also, this molecular definition of EPC self-renewal allows assessment of the quality of presumed EPC preparations. This promises to be the initial stage in progressing EPCs further into mainstream clinical use.
Significance
The development of a therapy using endothelial progenitor cells provides great hope for patients in treating cardiovascular diseases going forward. For continual development of this therapy toward the clinical, further understanding of the fundamental biology of these cells is required. This will enable a greater understanding of their stemness capacity and provide insight into their ability to differentiate and drive tissue regeneration when injected into a host.
Introduction
Cardiovascular diseases remain a leading cause of morbidity and mortality worldwide, accounting for approximately 25% of all deaths. Of these, ischemic heart disease contributed the greatest burden of mortality in the developed and developing worlds [1]. To this end, there have been significant research efforts made to understand the biology of the circulatory system, determining the process of vessel development during embryogenesis to tissue regeneration and repair for maintenance in the adult system. Angiogenesis, the sprouting of blood vessels from pre-existing vessel structures, has been the most widely studied process during situations of tissue regeneration and cancer formation [2]. This paradigm is driven by angiogenic factors secreted by endothelial cells and their surrounding cells, resulting in elongation of the vessel structure and repair. However, it is also well known that mature endothelial cells are terminally differentiated and possess limited proliferative capacity [2, 3].
In 1997, a seminal article was published by Asahara et al., demonstrating the existence of a circulating vascular stem cell. These were termed “endothelial progenitor cells” (EPCs) [4]. EPCs could be isolated directly from blood and reinjected into an ischemic situation in which they formed chimeric vessels in the host, contributing to neovasculogenesis, a developmental process in which a stem cell will form an entirely new vascular structure that then connects to the existing circulatory system [4, 5]. The discovery of EPCs has since initiated a large body of research during the past 2 decades that has brought substantial hope to patients with chronic ischemic disease who have limited medical and surgical therapeutic options.
In these numerous clinical trials, mostly in myocardial infarction and critical limb ischemia, various cell types with presumed progenitor activity were used to promote vascular repair. Improvement was observed after treatment of critical limb ischemia, such as increased pain-free walking distance and reduced leg ulcer size [6, 7], but positive effects were temporary and modest [8]. This has somewhat inhibited the EPC field from its momentum and progression toward a mainstream cell therapy. It is recognized that EPC clinical trials are currently limited by the purity and the quantity of cells to be delivered. Indeed, the “true” biology and identity of an EPC is still clouded. The vast majority of clinical trials have been using EPCs isolated directly from blood using cell surface markers. CD34, VEGFR2, and CD133 staining are conventionally used by flow cytometry to identify circulating EPCs, in addition to classical endothelial markers such as vascular endothelial (VE) cadherin and CD31. However, no combination of markers has been reliably adopted. The circulating CD34 enriched population is almost exclusively (97%) hematopoietic, as colabeled with CD45 [4]. CD34, VEGFR2, and CD133 significantly enrich hematopoietic stem cells and are not specific for EPCs [9]. As a result, surface marker-based definition of EPCs has generated much controversy within the field. Attempts have therefore been made to characterize EPCs at a functional level rather than to base them on their cell surface expression. The characteristics of self-renewal and multipotentiality must be gathered in a single cell type to be called stem cells [10].
Functionally Defining EPCs
Self-Renewal Capacity of EPCs
The current gold standard for determining stem cell characteristics is by estimating its ability to self-renew using in vitro single-cell colony-forming assays [11]. This has been attempted before using various populations of EPCs isolated directly from blood that resulted in differences in colony size and timing of appearance after plating [12, 13]. These studies highlighted further the capacity of an EPC to have stem cell characteristics and long culture survivability.
A prominent example using a functional definition of EPCs has stemmed from the description of endothelial colony-forming cells (ECFCs), which are currently defined as the only EPC population distinct from the hematopoietic lineage and entirely devoid of leukocyte or myeloid markers. ECFC assays have reflected a paradigm shift in our understanding of endothelial progenitors.
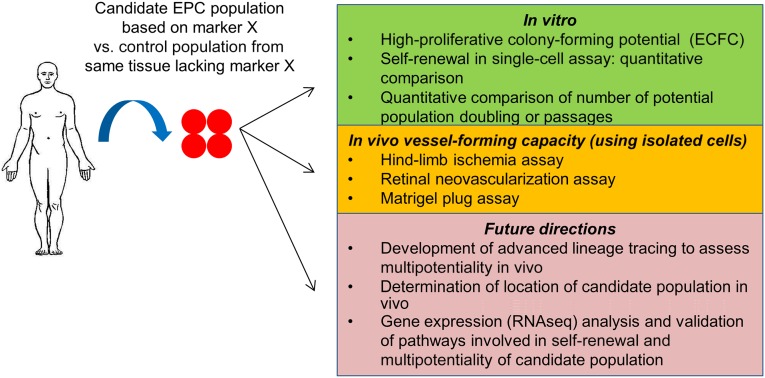
ECFCs were identified and described by Ingram et al., whose in vitro culture method using collagen-coated plates resulted in the capture and long-term culture maintenance of endothelial colonies [14]. Importantly, ECFCs have demonstrated a single-cell self-renewal capacity and form characteristic high-proliferative-potential (HPP) colonies, with each having a significant capacity for long-term culture (>15 passages) and expansion of cells with clear characteristics of endothelial cells [14]. ECFCs can be readily isolated from adult and fetal (umbilical cord blood) circulation but also in greater quantities from vascular structures, particularly those of the placenta, as our group has previously demonstrated [14–19] (Fig. 1).
Figure 1.
Using current and future methods of in vitro and in vivo characterization to assess and compare endothelial progenitor cells and control populations isolated from different tissues of the human body. Abbreviations: ECFC, endothelial colony-forming cell; EPC, endothelial progenitor cell.
Most important, ECFCs have demonstrated a unique hierarchy in self-renewal. This unique description of endothelial heterogeneity allows distinguishing cells with HPP from those with low proliferative potential (LPP) and mature endothelial cells. It was shown quite early on that HPP colonies, when taken in isolation, were able to give rise to new HPP colonies in subsequent passages as well as LPP colonies, whereas LPP colonies never gave rise to HPP. This was the conceptual basis for their self-renewal and thus the hierarchy proposed among ECFCs in vitro [14].
Comparative analysis has also been made between proangiogenic hematopoietic cells (termed “early-outgrowth EPCs”) and ECFCs. It was demonstrated that the early-outgrowth EPCs maintained their monocyte phenotype and, more important, did not constitutively express endothelial nitric oxide synthase isoforms and inducible NOS isoforms, important in pertaining to endothelial properties, when compared with ECFCs [20]. This clearly demonstrates the marked differences between a proangiogenic population and one that is endothelial.
Ultimately however, a candidate EPC population may also be tested in vivo through serial transplantation, as exemplified in the hematopoietic system, in which it is possible to empty the hematopoietic niche through lethal irradiation to further infuse cell populations to be tested [21–23]. It remains debated whether EPCs can be transferred through bone marrow transplantation [24, 25]. In previous attempts, green fluorescent protein-positive bone marrow cells following transplantation did not engage in the repair of the damaged endothelium of mice with chronic vascular disease [26, 27]. Taken together, there is still much to be elucidated around the in vivo self-renewal capacity of an EPC and how this process is initiated during situations of tissue regeneration.
Potency of EPCs
With regard to multipotentiality, unlike the hematopoietic system, there is no obvious assay to evaluate endothelial differentiation. In particular, to date, it is unclear whether all types of endothelial cells (arterial, venous, capillary, and lymphatic) can be obtained from the same progenitor cell or whether each vascular bed has its own progenitors determined during development [28]. Similarly, it is also proposed that endothelial cells from each organ have specific differentiation characteristics, determined by specific angiocrine factors that are released from distinct vascular niches [29–33]. These findings are critically important in the foundation understanding of the in vivo function of an EPC. Whether it is possible to isolate a “generic” EPC progenitor from an organ bed to use in a separate organ for regenerative purposes has yet to be entirely elucidated. It is unclear whether resident progenitors from each vascular bed harbor the potential to give rise to endothelium in a separate organ.
There is also a growing body of evidence in attempting to track presumed progenitor populations using lineage tracing to characterize their fate in vivo upon differentiation. Models used to permanently label cells that express Tie2, VE cadherin, or c-kit have been reported [34–37]. These studies have been conducted to determine the origin and potential fate of presumed EPCs in vascular structures during adult tissue regeneration. These studies have provided significant additional data on endothelial potential of c-kit-expressing cells. A possible limitation, however, remains that the markers used are not exclusive and may not encompass all EPC populations. Furthermore, to date, lineage tracing experiments have remained unable to replicate the hierarchical structure suggested by the ECFC model. This, in our view, is a critical step in determining potency.
In the absence of a consensus definition on the multipotentiality of an EPC, assays have been developed to determine their vessel formation capacity through in vitro and in vivo assays. This includes the development of an in vitro tube formation assay on tissue culture plastic precoated with Matrigel (Corning Life Sciences, Tewksbury, MA, http://www.corning.com). Although this is a widely used functional assay, many cell types independent of endothelial origin can also spontaneously form tubes, and therefore this assay lacks specificity and must be combined with other tangible markers of differentiation [38]. Surface expression of lectins, endocytosis of acetylated low-density lipoprotein, expression of endothelial specific adhesion, and function genes are more or less systematically proposed to fill this shortcoming [39].
The in vivo vessel formation assay is currently a gold standard. The uses of rodent hind-limb or retinal ischemia assays are particularly attractive in testing candidate progenitor populations. Here, cells are injected directly at the site of vascular ligation to ascertain their angiogenic and neovasculogenic capacity [40, 41]. Assessing candidate EPC populations by quantification has also been attempted in pathologies such as diabetes, preeclampsia, and bronchopulmonary dysplasia [42–46]. Since their discovery in 2004, there has been a growing body of evidence to suggest that ECFCs play a role in regenerating vascular structures and can form de novo vessels when injected into an ischemic site [15, 47–49]. To this end, although methods have been put forward in attempting to functionally define EPCs, none of these methods provides a consensus across the field.
Molecular Definition of EPCs
Previous attempts at the molecular definition of EPCs have been based on candidate marker approaches. However, no set of markers proved to be solely linked to progenitors, including a consensus on common strong endothelial markers such as VEGFR2, CD31, and CD144. CD133 is another marker that has also been mentioned in the literature as assessing EPC populations and function; however, as yet there still is not a clear consensus on this target [13, 50]. Given the progress in our understanding of an endothelial self-renewal hierarchy, it is tempting to speculate that a molecular understanding of this specific property may allow a more precise definition of EPCs. Although the in vitro characteristics of ECFCs have been clearly defined, their molecular identity has been lacking. Besides, it has been well reported that ECFCs harbor the entire hierarchy that can be found in vivo in the endothelium from HPP colony-forming cells to fully mature differentiated endothelial cells with highly restricted proliferation capacity. Confirming indications that the expression of CD34 could indicate higher proliferative potential [51], we recently demonstrated that within ECFC cultures, two populations based on CD34 expression existed, CD34+ and CD34− [52], and that only CD34+ cells had the capacity to reproduce HPP colonies in single-cell colony-formation assays. CD34− cells had little or no colony formation capacity. Furthermore, only CD34+ cells had the capacity to reproduce new CD34+ cells and could maintain ECFC cultures for greater than 15 passages; the same could not be seen on CD34− cells. In the absence of CD34+ cells, ECFC cultures collapsed within one or two passages. Therefore, a clear hierarchy in self-renewal could be established on the basis of a single CD34 surface marker in vitro, although this has yet to be established in an in vivo setting (Fig. 1).
With this in mind, it became possible to conduct gene expression studies between cells with or without self-renewal capacity. This molecular characterization was therefore based on a functional attribute of progenitors and more likely to be functionally useful. It was determined that CD34+ cells were enriched for genes related to cell quiescence such as cdkn1c (coding for p57, a cell cycle inhibitor) and il33, an endothelial cytokine known as a negative regulator of cell cycle [53]. Indeed, DNA content and cell cycle analysis clearly confirmed the reduced proliferation of CD34+ cells. CD34+ cells were mostly in G0/G1 phase, whereas CD34− cells were in S/G2M phase of cycling. This quiescence was secondary to high levels of Notch signaling, as witnessed by increased expression of its target genes (hey1, hes1), which is known to regulate cell cycling with stem/progenitor populations [54, 55]. Inhibition of Notch signaling with a γ-secretase inhibitor caused the reduction of cdkn1c and il33 expression, resulting in CD34+ cells rapidly moving out of quiescence, whereas Notch signaling induction using the DLL4 ligand triggered ECFC quiescence. Of significant interest, loss of Notch signaling and quiescence in CD34+ cells resulted in a loss of their self-renewal potential and colony-forming capacity.
Although CD34 cannot be trusted to have equal importance in determining EPC function in various situations, it can be argued that the gene expression signature reported here might reflect the progenitor function regardless of context. Furthermore, we do acknowledge that the high expression of Notch in in vitro CD34+ cells may represent an arterial phenotype, because in vivo venous endothelial cells, for example, have low Notch expression. Nonetheless, we have used an in vitro setting to initially provide this molecular signature that can now be seized upon to further define in vivo EPC populations (Fig. 1).
Translational Implications of the Functional Definition of EPCs
Identifying EPCs functionally could have major implications for their use as a cellular therapy for preclinical scenarios. Vessel formation capacities were the best reflection of the cells’ multipotency, as discussed above. First, tube formation in Matrigel culture was delayed in progenitor (CD34+) cells but was sustained over time in comparison with differentiated (CD34−) cells [52]. This delay was probably reflective of the need for CD34+ progenitors to terminally differentiate. Second, comparison of both populations in vivo in a murine hind-limb ischemia model revealed significantly improved reperfusion for progenitor cells; however, both populations had significant reperfusion capacity when compared with saline control populations. Furthermore, there were significant differences in the types of vascular structures that had been formed during this time by the two populations. For differentiated endothelial cells, only sporadic small capillary-like structures were observed within the ischemic musculature of the host, with reduced level of engraftment observed. Conversely, when progenitor cells defined by their self-renewal potential were injected, twice as many cells engrafted in the host, resulting in large functional vascular structures that spanned across the entire musculature. This clearly highlights the importance of functionally defining the vascular progenitor cell population.
Importantly, it has been further clarified that the current gold-standard endothelial culture media only allow significant expansion of the CD34− fraction of ECFCs, whereas the CD34+ remains relatively unchanged in absolute numbers and is strongly diluted upon passaging [51, 52]. This might explain the observed differences in efficiency of cell preparations at different passages in preclinical assays. Defining progenitors at the molecular level can therefore provide a way to standardize the therapeutic potential of a given ECFC preparation, providing a significant step forward in developing ECFCs into a cell therapy for chronic ischemic disease.
Conclusion
In summary, it has been almost 2 decades since EPCs first came to light, and in this time there have been significant attempts to seize upon their use as a clinical therapy for ischemic disease. Major progress has been achieved in the field in defining EPCs functionally rather than through cell surface markers. Although attempts have been made, a similar in vivo functional definition based on self-renewal and potency remains to be completed. Seizing upon this functional definition, it has been possible to explore the expression signature of cells that harbor self-renewal potential and therefore progenitor function. Linking the molecular and functional definitions of EPCs is an essential step toward better and more robust cell isolation, preparation, and standardization. This, we believe, is the initial stage in progressing EPCs further to mainstream clinical therapy.
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (APP1023368), Rebecca L. Cooper Research Foundation and the University of Queensland Minor Equipment Grant. J.P. was supported by the National Heart Foundation of Australia Postdoctoral Research Fellowship. K.K. was supported by an NHMRC Career Development Fellowship.
Author Contributions
J.P., P.D., and K.K.: manuscript writing, final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
K.K. has an uncompensated patent around isolation of endothelial progenitors. The other authors indicated no potential conflicts of interest.
References
- 1.Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: A review of global methodologies of mortality measurement. Circulation. 2013;127:749–756. doi: 10.1161/CIRCULATIONAHA.112.128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 5.Chavakis E, Koyanagi M, Dimmeler S. Enhancing the outcome of cell therapy for cardiac repair: Progress from bench to bedside and back. Circulation. 2010;121:325–335. doi: 10.1161/CIRCULATIONAHA.109.901405. [DOI] [PubMed] [Google Scholar]
- 6.Kawamoto A, Katayama M, Handa N, et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: A phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. 2009;27:2857–2864. doi: 10.1002/stem.207. [DOI] [PubMed] [Google Scholar]
- 7.Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 8.Walter DH, Krankenberg H, Balzer JO, et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: A randomized-start, placebo-controlled pilot trial (PROVASA) Circ Cardiovasc Interv. 2011;4:26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348. [DOI] [PubMed] [Google Scholar]
- 9.Case J, Mead LE, Bessler WK, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.He S, Nakada D, Morrison SJ. Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol. 2009;25:377–406. doi: 10.1146/annurev.cellbio.042308.113248. [DOI] [PubMed] [Google Scholar]
- 11.Beerman I, Bhattacharya D, Zandi S, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci USA. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masuda H, Alev C, Akimaru H, et al. Methodological development of a clonogenic assay to determine endothelial progenitor cell potential. Circ Res. 2011;109:20–37. doi: 10.1161/CIRCRESAHA.110.231837. [DOI] [PubMed] [Google Scholar]
- 13.Campioni D, Zauli G, Gambetti S, et al. In vitro characterization of circulating endothelial progenitor cells isolated from patients with acute coronary syndrome. PLoS One. 2013;8:e56377. doi: 10.1371/journal.pone.0056377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 15.Patel J, Seppanen E, Chong MS, et al. Prospective surface marker-based isolation and expansion of fetal endothelial colony-forming cells from human term placenta. Stem Cells Translational Medicine. 2013;2:839–847. doi: 10.5966/sctm.2013-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingram DA, Mead LE, Moore DB, et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 17.Nijmeh H, Balasubramaniam V, Burns N, et al. High proliferative potential endothelial colony-forming cells contribute to hypoxia-induced pulmonary artery vasa vasorum neovascularization. Am J Physiol Lung Cell Mol Physiol. 2014;306:L661–L671. doi: 10.1152/ajplung.00244.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duong HT, Comhair SA, Aldred MA, et al. Pulmonary artery endothelium resident endothelial colony-forming cells in pulmonary arterial hypertension. Pulm Circ. 2011;1:475–486. doi: 10.4103/2045-8932.93547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafiee A, Fisk NM, Hutmacher DW, et al. Fetal endothelial and mesenchymal progenitors from the human term placenta: potency and clinical potential. Stem Cells Translational Medicine. 2015;4:419–423. doi: 10.5966/sctm.2014-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheshier SH, Morrison SJ, Liao X, et al. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiel MJ, He S, Ashkenazi R, et al. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 24.Aicher A, Rentsch M, Sasaki K, et al. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- 25.Hillebrands JL, Klatter FA, van Dijk WD, et al. Bone marrow does not contribute substantially to endothelial-cell replacement in transplant arteriosclerosis. Nat Med. 2002;8:194–195. doi: 10.1038/nm0302-194. [DOI] [PubMed] [Google Scholar]
- 26.Perry TE, Song M, Despres DJ, et al. Bone marrow-derived cells do not repair endothelium in a mouse model of chronic endothelial cell dysfunction. Cardiovasc Res. 2009;84:317–325. doi: 10.1093/cvr/cvp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuno Y, Nakamura-Ishizu A, Kishi K, et al. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood. 2011;117:5264–5272. doi: 10.1182/blood-2011-01-330720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 29.Nolan DJ, Ginsberg M, Israely E, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler JM, Nolan DJ, Vertes EL, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding BS, Nolan DJ, Butler JM, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kao DI, Lacko LA, Ding BS, et al. Endothelial cells control pancreatic cell fate at defined stages through EGFL7 signaling. Stem Cell Rep. 2015;4:181–189. doi: 10.1016/j.stemcr.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sultana N, Zhang L, Yan J, et al. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun. 2015;6:8701. doi: 10.1038/ncomms9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Berlo JH, Kanisicak O, Maillet M, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivasan RS, Dillard ME, Lagutin OV, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zovein AC, Hofmann JJ, Lynch M, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmeisser A, Garlichs CD, Zhang H, et al. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 39.Alphonse RS, Vadivel A, Zhong S, et al. The isolation and culture of endothelial colony-forming cells from human and rat lungs. Nat Protoc. 2015;10:1697–1708. doi: 10.1038/nprot.2015.107. [DOI] [PubMed] [Google Scholar]
- 40.Brenes RA, Jadlowiec CC, Bear M, et al. Toward a mouse model of hind limb ischemia to test therapeutic angiogenesis. J Vasc Surg. 2012;56:1669–1679; discussion 1679. doi: 10.1016/j.jvs.2012.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw LC, Neu MB, Grant MB. Cell-based therapies for diabetic retinopathy. Curr Diab Rep. 2011;11:265–274. doi: 10.1007/s11892-011-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hazra S, Rasheed A, Bhatwadekar A, et al. Liver X receptor modulates diabetic retinopathy outcome in a mouse model of streptozotocin-induced diabetes. Diabetes. 2012;61:3270–3279. doi: 10.2337/db11-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakravarthy H, Beli E, Navitskaya S, et al. Imbalances in mobilization and activation of pro-inflammatory and vascular reparative bone marrow-derived cells in diabetic retinopathy. PLoS One. 2016;11:e0146829. doi: 10.1371/journal.pone.0146829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muñoz-Hernandez R, Miranda ML, Stiefel P, et al. Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension. 2014;64:165–171. doi: 10.1161/HYPERTENSIONAHA.113.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker CD, Seedorf GJ, Wisniewski BL, et al. Endothelial colony-forming cell conditioned media promote angiogenesis in vitro and prevent pulmonary hypertension in experimental bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2013;305:L73–L81. doi: 10.1152/ajplung.00400.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker CD, Balasubramaniam V, Mourani PM, et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J. 2012;40:1516–1522. doi: 10.1183/09031936.00017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarz TM, Leicht SF, Radic T, et al. Vascular incorporation of endothelial colony-forming cells is essential for functional recovery of murine ischemic tissue following cell therapy. Arterioscler Thromb Vasc Biol. 2012;32:e13–e21. doi: 10.1161/ATVBAHA.111.239822. [DOI] [PubMed] [Google Scholar]
- 48.Kang KT, Coggins M, Xiao C, et al. Human vasculogenic cells form functional blood vessels and mitigate adverse remodeling after ischemia reperfusion injury in rats. Angiogenesis. 2013;16:773–784. doi: 10.1007/s10456-013-9354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood JA, Colletti E, Mead LE, et al. Distinct contribution of human cord blood-derived endothelial colony forming cells to liver and gut in a fetal sheep model. Hepatology. 2012;56:1086–1096. doi: 10.1002/hep.25753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker CD, Ryan SL, Ingram DA, et al. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med. 2009;180:454–461. doi: 10.1164/rccm.200901-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferreras C, Cole CL, Urban K, et al. Segregation of late outgrowth endothelial cells into functional endothelial CD34- and progenitor-like CD34+ cell populations. Angiogenesis. 2015;18:47–68. doi: 10.1007/s10456-014-9446-1. [DOI] [PubMed] [Google Scholar]
- 52.Patel J, Wong HY, Wang W, et al. Self-renewal and high proliferative colony forming capacity of late-outgrowth endothelial progenitors is regulated by cyclin-dependent kinase inhibitors driven by notch signaling. Stem Cells. 2016;34:902–912. doi: 10.1002/stem.2262. [DOI] [PubMed] [Google Scholar]
- 53.Sundlisaeter E, Edelmann RJ, Hol J, et al. The alarmin IL-33 is a notch target in quiescent endothelial cells. Am J Pathol. 2012;181:1099–1111. doi: 10.1016/j.ajpath.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Wen Y, Bi P, Liu W, et al. Constitutive notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol Cell Biol. 2012;32:2300–2311. doi: 10.1128/MCB.06753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohtsuka T, Ishibashi M, Gradwohl G, et al. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]