Summary
Signaling classically involves the secretion of diverse molecules that bind specific cellsurface receptors and engage intracellular transduction cascades. Some exceptions, namely lipophilic agents, can cross plasma membranes to bind intracellular receptors and be carried to the nucleus to regulate transcription. Homeoprotein transcription factors are among the few proteins with such a capacity. Here, we review the signaling activities of homeoproteins in the developing and adult nervous system, with particular emphasis on axon/cell migration and postnatal critical periods of cerebral cortex plasticity. We also describe homeoprotein non-cell autonomous mechanisms and explore how this “novel” signaling pathway impacts emerging research in brain development and physiology. In this context, we explore hypotheses on the evolution of signaling, the role of homeoproteins as early morphogens, and their therapeutic potential for neurological and psychiatric diseases.
Introduction
Homeoprotein transcription factors are the products of developmental genes discovered following the observation of homeotic mutations, primarily in Drosophila. In these homeotic mutants, a segment or part of a segment of the fly is replaced by another segment, or a part normally found on another segment, giving rise to the concept that homeotic genes are endowed with positional information. For example in the Antennapedia mutant, antennas on the head are replaced by legs normally found on the second thoracic segment as if the embryonic cells on the head segment had misread their position (Garber et al., 1983; Lawrence et al., 1983). The signature of homeoprotein transcription factors is a highly conserved 60 amino acid-long DNA binding domain called the homeodomain. The part of the gene encoding the homeodomain is called the homeobox, a 180 nucleotide-long sequence that was used to identify a large number of developmental genes, not all of them having a homeotic function in the sense defined above in the case of Antennapedia (Gehring, 1987). For example, Orthodenticle (Otd) has a homeobox but is considered a gap gene since its mutation leads to the complete loss of the anterior part of the fly and not to a morphological transformation (Finkelstein and Perrimon, 1990). In this review, we shall use the terms of homeogene and homeoprotein (HP) on the basis of the existence of a homeobox or a homeodomain (in the gene or the protein, respectively) independently of a true homeotic function.
Owing to the strong conservation of the homeobox, the cloning of drosophila homeogenes enabled the cloning of mammalian orthologs as well as the discovery of a large number of homeogenes across all vertebrates (Derelle et al., 2007). It must be emphasized that although homeogenes were discovered as early developmental genes that participate in establishing body patterns, they are in fact active at all developmental periods as well as in the adult. Consequently, their functions and mechanisms of action may be rather distinct in these different contexts. In this review we discuss a number of scenarios where alternative roles and modes of action for HPs might be at play. We explore the significance of direct non-cell autonomous activities of several HP transcription factors, which provide novel signaling pathways through HP secretion and internalization.
Homeoproteins and boundary formation
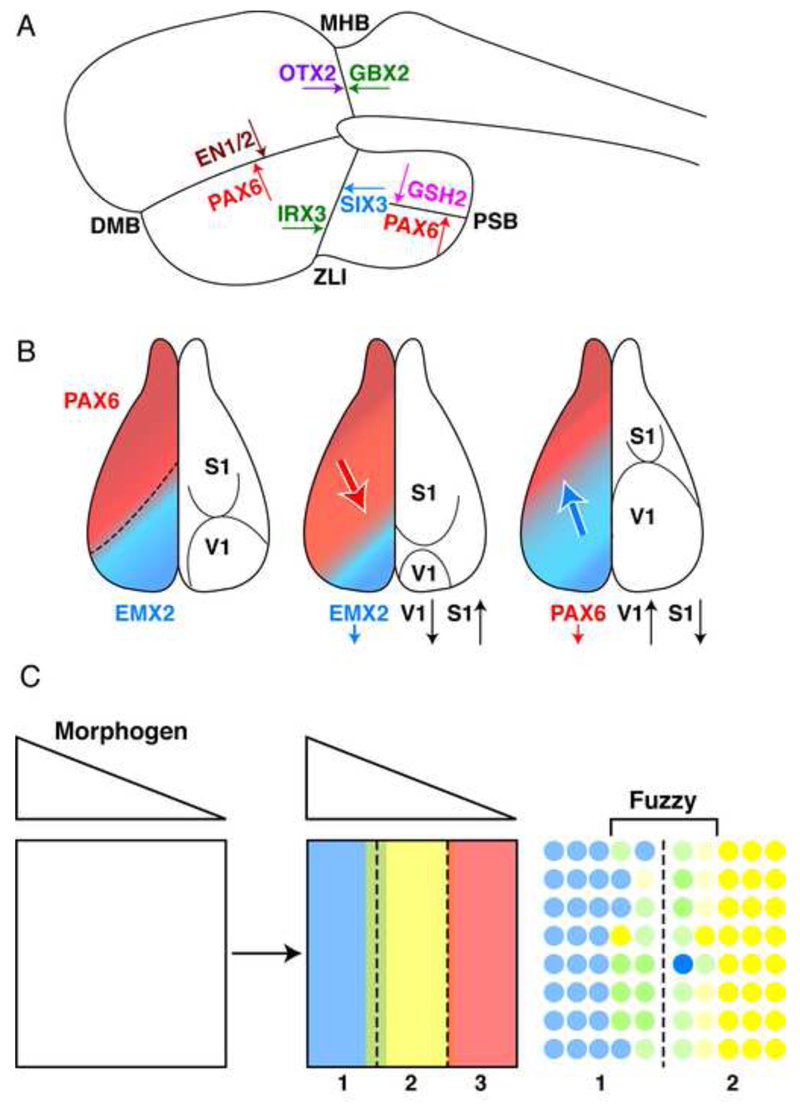
The brain neuroepithelium is compartmentalized very early in embryonic development due to the positioning of boundaries that segment an originally uniform layer of progenitors (Kiecker and Lumsden, 2005). This is of physiological importance since these compartments in the forebrain later differentiate into cortical areas (Brodmann areas) with distinct functions (Zilles and Amunts, 2010). Their formation often involves HPs, with either side of a boundary characterized by the expression of two HPs that are both self-activating and reciprocal inhibitors (Fig. 1A)(Bishop et al., 2000; Broccoli et al., 1999; Di Bonito et al., 2013; Joyner et al., 2000; Katahira et al., 2000; Matsunaga et al., 2000; Millet et al., 1999; O’Leary et al., 2007; O’Leary and Sahara, 2008; Simeone, 2000; Toresson et al., 2000; Yun et al., 2001). Interestingly, the position of some boundaries can be shifted following modifications in the expression levels of one of the two “abutting” HPs, as if each HP was in competition to occupy as much as possible of the neuroectoderm territory. Two classical examples of such “gene expression boundaries” are the midbrain-hindbrain boundary (MHB) and the boundary between the visual (V1) cortex and the somatosensory (S1) cortex. The latter case is schematized in Figure 1B where the S1/V1 boundary is shifted by modifying the balance between Pax6 and Emx2 (O’Leary et al., 2007).
Figure 1.
Homeoproteins and boundary formation. A. Examples of boundaries defined by the expression of abutting homeoproteins with self-activating and reciprocal inhibitory properties. B. The classical example of the competitive activities of Emx2 and Pax6 in the definition of primary somato-sensory and visual cortex areas in the developing mouse cortex. The latter work by the group of Denis O’Leary (O’Leary et al., 2007) refers to the cell autonomous activity of homeoproteins and does not consider their non-cell autonomous activity. C. The graded expression of a morphogen creates several domains within the morphogenetic field, according to the Wolpert’s French flag paradigm (Wolpert, 1969). Each domain is characterized by the expression of a homeoprotein transcription factor (Blue-Yellow-Red) but the boundaries are initially fuzzy.
The involvement of HPs in patterning and boundary formation was first illustrated by Bicoid in drosophila larva development. Following Bicoid mutation, the anterior part of the embryo is transformed into a posterior domain (thus Bicoid). The function of Bicoid is best understood in the context of the French Flag Model (FFM) proposed in 1969 by Lewis Wolpert (Wolpert, 1969). In this model, illustrated in Figure 1C, a morphogen diffusing from a source to a sink can create sharp boundaries (blue-yellow-red) corresponding to the expression of specific genes by distinct cell populations if one introduces expression thresholds. Figure 1C illustrates the fuzziness of gene expression at the level of boundaries and the necessity to introduce sharpening mechanisms (see below). Whatever the case, two seminal papers by Driever and Nusslein-Volhard demonstrated that Bicoid displays a graded expression along the anterior-posterior (A-P) axis of the embryo and that modifying this expression results in a shift in the size of the most anterior (head-thorax) pole (Driever and Nusslein-Volhard, 1988a, b).
Even though the FFM seems still valid in a few specific cases, for example the formation of compartments in the developing neural tube where sonic hedgehog diffusion induces the expression of distinct HPs along the ventral-dorsal axis (Dessaud et al., 2007), the model has evolved considerably, especially given the knowledge that the extracellular milieu does not allow much diffusion (Kerszberg and Wolpert, 2007). In addition, theoretical issues concerning boundary stability and the precision of boundary positioning have to be accounted for (Houchmandzadeh et al., 2002; Yucel and Small, 2006). In fact, these two issues have recently been examined in the case of Bicoid and it is now clear that, contrary to what was initially believed, the graded expression of Bicoid is the result of the transport of its mRNA followed by local translation, rather than due to passive protein diffusion (Lipshitz, 2009). Indeed, the diffusion of Bicoid protein between neighboring nuclei is spatially restricted and this is what provides the robustness of boundary positioning (Gregor et al., 2007a; Gregor et al., 2007b).
Novel homeoprotein functions
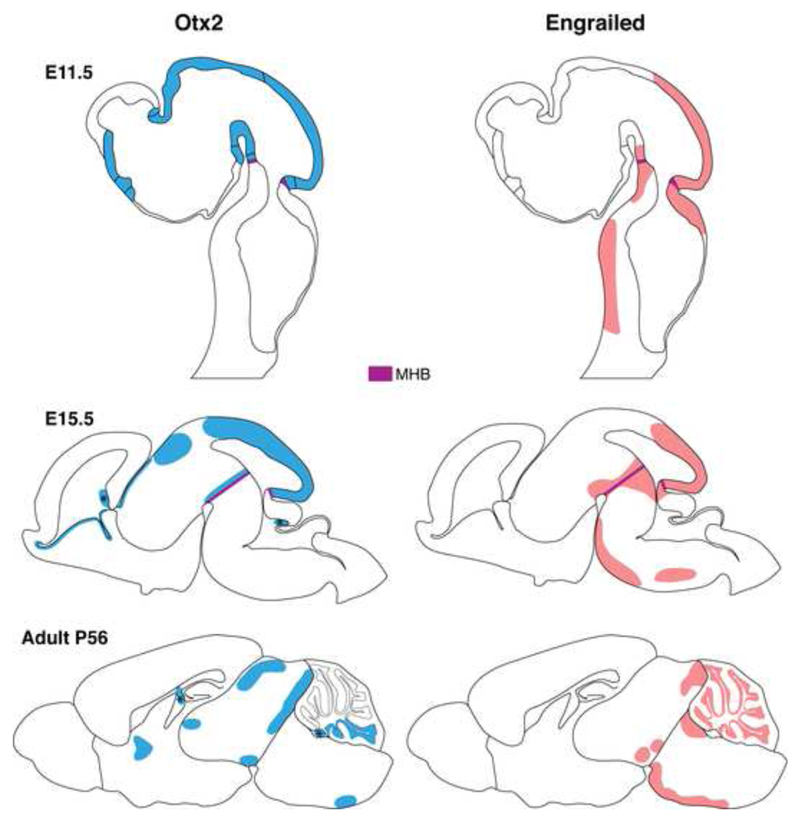
As is the case for many genes, homeogenes can be constantly expressed throughout an animal’s lifespan but with evolving functions. In the nervous system for example, homeogenes are first involved in its patterning and compartmentalization, then in cell and axon migration, and finally in several adult physiological functions. This is well illustrated with the case of the two HPs Engrailed-1 and Engrailed-2 (collectively Engrailed or En1/2) and of Otx2 a homeoprotein of the Bicoid family. During development, En-1 and En-2 brain expression begins at the 1-somite and 5-somite stages respectively (during embryonic age E8) in a patch of cells in the anterior neuroepithelium that correspond to the Mid-Hindbrain Boundary (MHB) region, while Otx2 expression begins earlier in the epiblast ectoderm prior to gastrulation (before E6) and is later expressed throughout the forebrain and midbrain stopping sharply at the MHB. Brain expression of En1/2 and Otx2 is modified in the course of development until adulthood when it is limited to highly restricted midbrain and hindbrain structures (Fig. 2) (Broccoli et al., 1999; Davis and Joyner, 1988; Simeone et al., 1993; Wurst and Bally-Cuif, 2001). En-1, like Otx2, is considered a developmental gap gene since the null mutation results in the loss of mid-hindbrain tissues including part of the cerebellum and inferior colliculi (Wurst et al., 1994). Over the course of development, these proteins are successively involved in the formation of boundaries, in segment polarity, and in axon guidance, yet evolve other functions in the adult. For example En1/2 maintains mesencephalic dopaminergic neurons (Alberi et al., 2004; Alvarez-Fischer et al., 2011; Sonnier et al., 2007) while Otx2 is implicated in brain plasticity (Beurdeley et al., 2012; Spatazza et al., 2013a; Spatazza et al., 2013b; Sugiyama et al., 2008).
Figure 2.
Embryonic and adult expression of Engrailed and Otx2 homeoproteins. At embryonic day E11.5, graded En1/2 expression irradiates from the mid-hindbrain barrier (MHB) into the midbrain. En-1 is also expressed in two lateral stripes along the hindbrain. At E15.5, En-1 expression intensifies around the MHB while En-2 expression extends into the cerebellum. Restricted brain expression of En1/2 continues through to the adult in structures such as the ventral segmental area (VTA), substantia nigra pars compacta, inferior colliculus and the cerebellum. At E11.5, Otx2 expression is restricted to the forebrain and midbrain and stops sharply at the MHB. At E15.5, Otx2 continues to be expressed in restricted midbrain and forebrain structures while patches of expression appear in the cerebellum and hindbrain. Strong Otx2 expression is observed in the choroid plexus (labeled with ‘*’). In the adult, Otx2 is expressed in structures such as the VTA, lateral geniculate nucleus, superior colliculus, medial septum, cerebellum and choroid plexus.
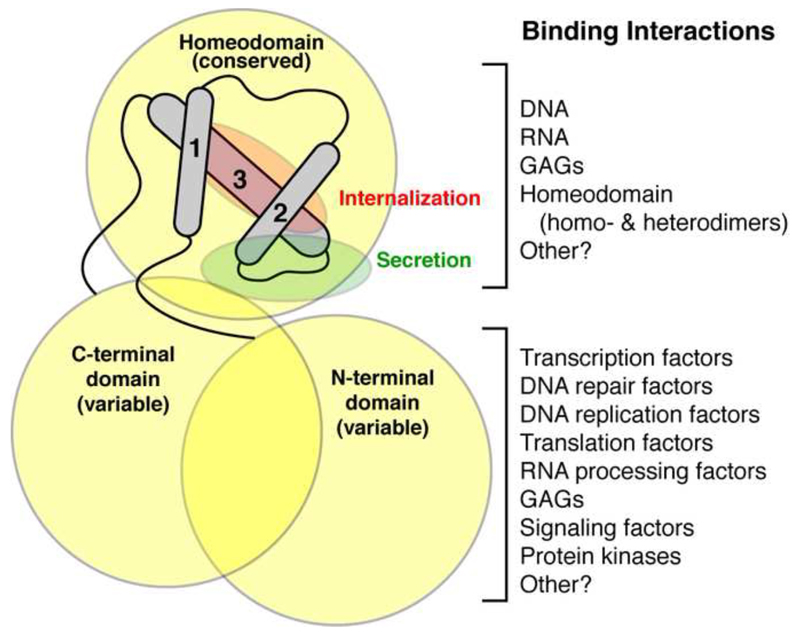
The evolving functions of HPs and the progress in our understanding of these factors have led to some unexpected discoveries (see Fig. 3 for summary of HP structure and function). For example, Bicoid was discovered as a patterning transcription factor during development and later revealed itself as a true locally diffusing morphogen that not only regulates transcription but also translation (Driever and Nusslein-Volhard, 1988b; Dubnau and Struhl, 1996; Rivera-Pomar et al., 1996). It has been shown to regulate the translation of Caudal mRNA during fly development through specific binding to elements within the 3’ untranslated region and by forming a complex with drosophila homologue of eIF4E, which binds capped mRNA (Niessing et al., 1999; Rivera-Pomar et al., 1996). While many HPs contain a consensus eIF4E binding site, direct interaction has been confirmed for only a handful, including HOXA9, PRH, En2, Otx2 and Emx2 (Nedelec et al., 2004; Topisirovic and Borden, 2005). Contrary to Bicoid, none of these HPs are confirmed to interact directly with mRNA. Yet, through interaction with eIF4E, HPs have been shown to regulate mRNA nuclear export, mRNA transport and translation (for review see (Rezsohazy, 2014)). Such cytoplasmic role of HPs takes on even more importance given that they are among the very few proteins shown to transfer into target cells to exert direct non-autonomous activities.
Figure 3.
Homeoproteins bind to a wide range of molecules. The homeodomain contains a conserved 3-helix bundle flanked by variable N- and C-terminal domains that together form the homeoprotein. These domains make a plethora of interactions with DNA, RNA, GAGs and proteins. Consequently, homeoproteins are shown to regulate transcription, RNA processing and translation, DNA replication and damage response and are also implicated in cell signaling. Within the 2nd and 3rd DNA-binding α-helices are the motifs that permit the non-conventional secretion and internalization of the homeodomain.
Homeoprotein intercellular transfer
Leaving aside plants (Lucas et al., 1995; Ruiz-Medrano et al., 2004), it is interesting to note that evidence for direct non-cell autonomous activities of several HP transcription factors actually originated from serendipitous findings. In 1984, we reported that neuronal shape and polarity were regulated through region-specific interactions between neurons and astrocytes (Denis-Donini et al., 1984). This link between shape and position resembled homeosis (ie the transformation of one organ into another) and led us to speculate that HP transcription factors may regulate neuronal shape. To test this hypothesis, the DNA-binding domain (homeodomain, HD) of the Antennapedia HP was scrape-loaded into neurons in culture, a procedure that transiently disrupts the cell membrane. Since the HD is highly conserved among HPs, we hypothesized that upon reaching the nucleus, the Antennapedia HD might chase the endogenous HPs from their cognate genomic binding sites, leading to a change in neuronal shape. This experiment worked (Joliot et al., 1991a), but as a control Antennapedia HD was added directly to the culture medium of intact neurons, anticipating no change in neuronal phenotype. Contrary to expectations, the neurons changed shape, strongly suggesting that we were dealing with an artifact. However, before abandoning this approach, we added FITC-tagged HD to intact neurons and observed, much to our surprise, its capture by live nerve cells and its diffusion through the cytoplasm and into the nucleus (Le Roux et al., 1993).
The sequences responsible for internalization and secretion of HPs lie within the HD itself (Fig. 3) and thus are highly conserved between all HPs (for review, see (Joliot and Prochiantz, 2004)). This important fact has two consequences. First, this means that most of the 200 or so HPs are likely to be capable of being transferred between cells and several were found to be internalized or to pass between cells (Amsellem et al., 2003; Auvray et al., 2012; Chatelin et al., 1996; Di Lullo et al., 2011; Haddad et al., 2008; Joliot and Prochiantz, 2004; Kim et al., 2014; Lesaffre et al., 2007). Of these HPs, five were also successfully tested in vivo (Otx2, Pax6, Engrailed-1 (En1), Engrailed 2 (En2), Vax1 and Hoxd1) (Bardine et al., 2014; Di Lullo et al., 2011; Kim et al., 2014; Layalle et al., 2011; Lesaffre et al., 2007; Sugiyama et al., 2008; Wizenmann et al., 2009). The second consequence is that, because they reside in the HD, these sequences cannot be mutated without modifying the DNA binding and transcriptional regulation activities of HPs. This has rendered the specific disruption of non-cell autonomous HP functions challenging, requiring approaches that are not based on the mutation of the transfer sequences. These strategies will be described below along with examples of HP signaling functions.
Non-cell autonomous functions of homeoproteins
Extracellular Pax6 regulates eye compartment size
Many studies on nervous system boundaries have been accomplished with the idea that HPs only have cell autonomous activities. It is indeed possible that the same cell (close to a future boundary) will express both HPs. In that case, beyond a certain threshold in the difference of HP concentrations, the “winner takes all” due to self-activation and reciprocal inhibition. Thus modifying the concentration (heterozygote or gain of function) of one of the two HPs will modify the position of the boundary. However, in a pure cell autonomous context, this can only happen in cells that co-express the two HPs or, alternatively, requires the activity of morphogens (growth-factors) under HP control that modify, in return, HP expression. In fact the latter bona fide morphogens (meaning non-HP proteins) have been proposed in several models to participate in boundary stabilization (Meinhardt and Gierer, 2000).
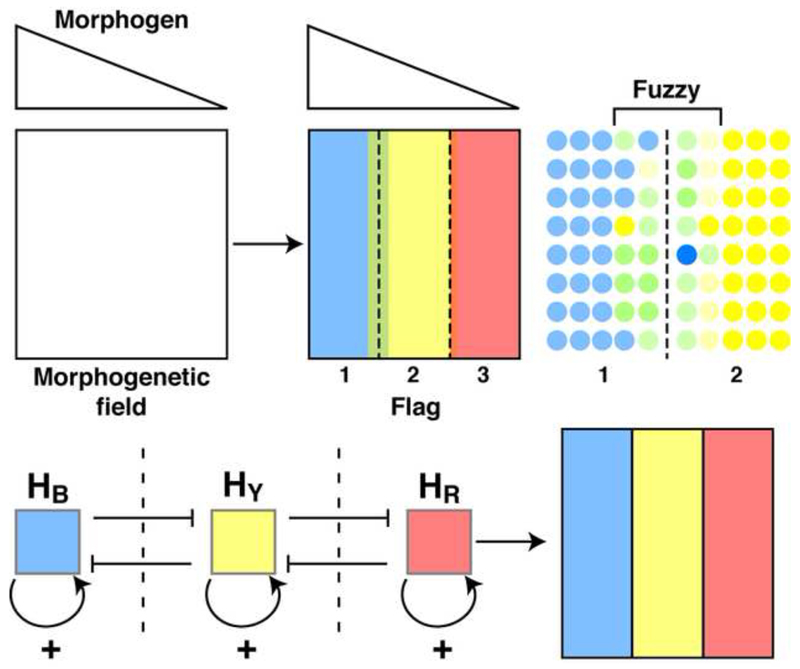
The non-cell autonomous hypothesis illustrated in Figure 4 is more parsimonious since the HPs themselves are considered morphogens, with boundaries being formed or stabilized in response to their diffusion, self-activation, and reciprocal inhibition, which closely corresponds to the definition of morphogens proposed by Alan Turing (Turing, 1952). This scenario posits that the graded expression of a morphogen creates several domains within the morphogenetic field, according to the Wolpert’s FFM. Each domain is characterized by the expression of a HP transcription factor but the boundaries are initially fuzzy while further regularity and positioning of the boundary is permitted by the local diffusion of HPs that self-activate and reciprocally inhibit their counterpart HP (Holcman et al., 2007; Kasatkin et al., 2008).
Figure 4.
The graded expression of a morphogen creates several domains within the morphogenetic field, according to the Wolpert’s French flag paradigm (Wolpert, 1969). Each domain is characterized by the expression of a homeoprotein transcription factor (Blue-Yellow-Red) but the boundaries are initially fuzzy. In this model the further regularity and positioning of the boundary is permitted by the local diffusion of homeoproteins with self-activating and reciprocal inhibitory properties, thus acting as local Turing’s morphogens.
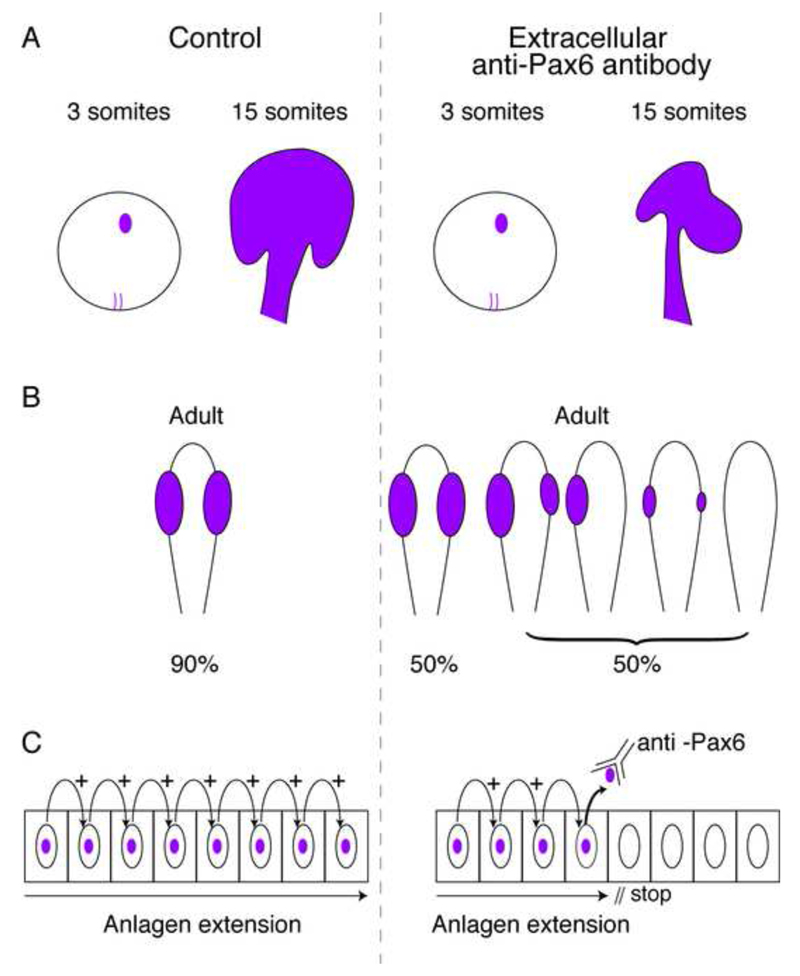
To test whether early HP transfer can regulate the size of a compartment, Pax6 transfer was blocked in the developing zebrafish. Given the limitations in mutating HP transfer sequences without modifying the cell autonomous activities of HPs, a strategy was developed involving the expression of secreted single chain antibodies (scFvs) encoded by mini-genes that could block non-cell autonomous HP transfer functions. Blocking was thus achieved by expressing an extracellular anti-Pax6 scFv through the injection of mRNA encoding the antibody (plus signal peptide) in the egg (Lesaffre et al., 2007). Another approach was to inject a monoclonal antibody in the intercellular space at the blastula stage. In both cases the size of the Pax6 positive eye anlagen was reduced with strong phenotypes including no eye, reduced eye size or asymmetric eyes (Fig. 5). Thus, the non-cell autonomous function of a HP can be crucial for compartmentalization and perhaps boundary formation.
Figure 5.
Pax6 defines eye anlagen. A. An extracellular anti-Pax6 antibody present at early developmental stages in the zebrafish does not modify the initial Pax6 expression analyzed by ISH (3 somite stage) but disrupts the development of the eye anlagen (15 somite stage) (Lesaffre et al., 2007). B. The extracellular anti-Pax6 antibody (as in A) leads to a variety of abnormal eye phenotypes (Lesaffre et al., 2007). C. A working hypothesis for the expansion of the eye anlagen includes the requirement for Pax6 transfer between cells.
The axon guidance hypothesis
Based on the idea that HPs encode “positional information”, it was proposed that HP transfer could participate in axon guidance (Prochiantz and Theodore, 1995). In drosophila development research, it was accepted at the time that these transcription factors are the effectors of position-dependent morphogenetic programs. The mechanisms involved were not known but it was understood that HPs must exert their activity through the regulation of genes participating in most morphogenetic processes, including cell migration and axon growth. A clear example is provided by the axon-guidance factor EphrinA5 and the regulation of its transcription along the A-P axis of the tectum by the two HPs En-1 and En-2 (Flanagan and Vanderhaeghen, 1998; Logan et al., 1996). In this context, it is assumed that EphrinA5 graded expression results from the graded expression of En1/2, which thus plays an indirect role in axon guidance through EphrinA5. The hypothesis of a direct non-cell autonomous guidance activity of En1/2 on incoming axons was initially set aside because the timescale of response seemed incompatible with a transcriptional role for HPs: it would take too long for internalized HPs to travel along axons to their nuclei and for the newly synthesized information to travel back to the growth cone.
The hypothesis was revived (Prochiantz and Joliot, 2003) when, in the early 2000s, Holt and colleagues, building on the observation of local mRNA translation (Brittis et al., 2002; Richter, 2001; Steward and Schuman, 2001) and on the growing evidence that mRNA translation could take place in axons (Steward, 2002), demonstrated that the activity of identified guidance factors such as Sema-3 and Netrin required protein synthesis in growth cones (Campbell and Holt, 2001; Piper and Holt, 2004). At the same time, it was becoming more evident that HPs might regulate translation through interaction with eukaryotic initiation factor eIF4E. These findings suggested that internalized HPs could exert their action locally by regulating protein translation and revived interest in the HP guidance hypothesis as being physiologically relevant.
Engrailed guides axons through local translation
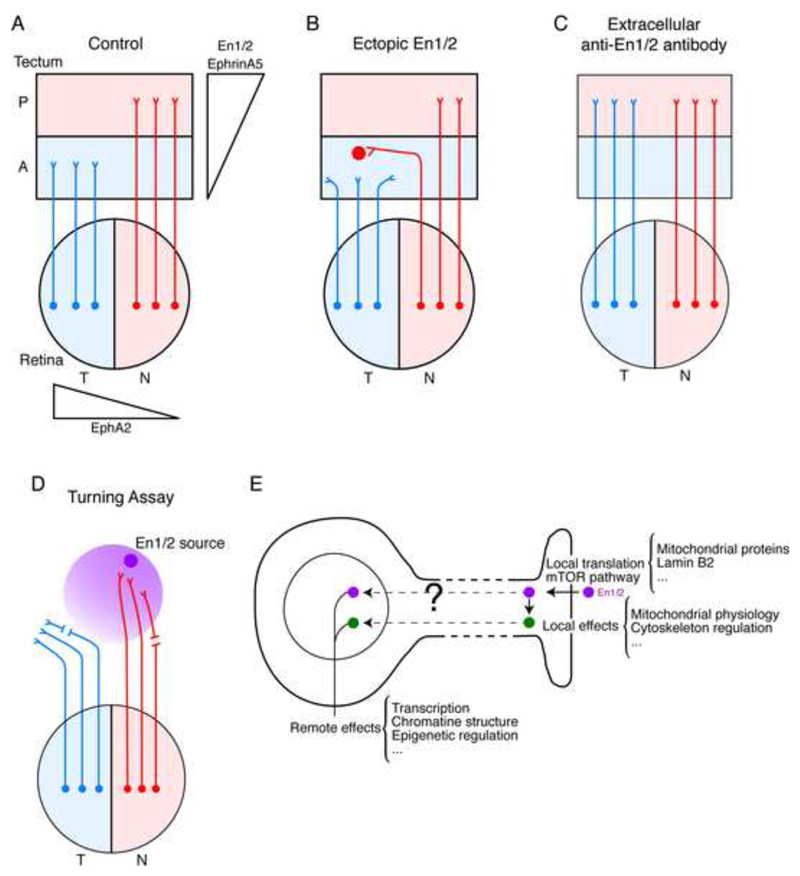
Engrailed expression is graded along the anterior-posterior (A-P) axis of the tectum/superior colliculus (Fig. 6A) (Itasaki et al., 1991; Itasaki and Nakamura, 1992, 1996) and a gain of function by spots of high ectopic Engrailed expression in the anterior part of the tectum results in the repulsion of temporal axons and attraction of nasal axons (Fig. 6B) (Drescher et al., 1997; Friedman and O’Leary, 1996; Itasaki and Nakamura, 1996; Logan et al., 1996; Shigetani et al., 1997). Such an effect of En1/2 on the projection of the retina temporal-nasal axis onto the tectum A-P axis was originally entirely attributed to the regulation of EphrinA5 synthesis by En1/2 (Shigetani et al., 1997). Indeed, the graded expression of EphA2 in the retina and of its EphrinA5 ligand in the tectum plays a key function in the formation of the retino-tectal topography (Cheng et al., 1995; Drescher et al., 1997; Lemke and Reber, 2005). However, En1/2 is present in the extracellular matrix of the tectum of the frog and the chick embryo in a graded expression pattern reflecting intracellular expression (Wizenmann et al., 2009). The in vivo expression of anti-En1/2 scFv in the posterior tectum neutralized extracellular En1/2 leading to an abnormal invasion of the posterior tectum by temporal axons (Fig. 6C) (Wizenmann et al., 2009).
Figure 6.
Graded expression of Engrailed guides retinotectal axons. A. Axons originating from the retina within a temporal-to-nasal EphA2 gradient project onto the tectum within posterior to anterior En1/2 and EphrinA5 gradients. Temporal retinotectal growth cones remain within the anterior tectum while nasal growth cones are attracted to posterior tectum. B. Ectopic expression of En1/2 within anterior tectum results in local repulsion and attraction of temporal and nasal growth cones, respectively (Drescher et al., 1997; Friedman and O’Leary, 1996; Itasaki and Nakamura, 1996; Logan et al., 1996; Shigetani et al., 1997). C. Local expression of secreted single-chain antibody against En1/2 disrupts axon guidance in vivo and results in overgrowth of temporal growth cones that invade the posterior tectum (Wizenmann et al., 2009). D. In vitro guidance assays show that En1/2 attracts nasal growth cones and repels temporal growth cones. This activity is in part local as shown by the attraction and repulsion of nasal and temporal growth cones separated from their cell bodies (Brunet et al., 2005). E. The local activity of En1/2 acts in part through mTOR-dependent translation and results in expression mitochondrial proteins. This activity has local effects on mitochondria, cytoskeleton and other unidentified targets leading to remote effects in the nucleus. En1/2 might also undergo retrograde transport to participate in regulating remote nuclear activity such as transcription and epigenetic events.
This in vivo study was built upon previous in vitro assays that evaluated the trajectory of frog temporal and nasal retinotectal axons within an En2 gradient (Brunet et al., 2005). As illustrated in Figure 6D, in absence of any factor other than En1/2, most nasal axons are attracted and temporal ones are repelled. This activity was specific to En1/2 since Otx2, another internalized HP, had a slight attractive activity on both nasal and temporal axons (Brunet et al., 2005). With this simple model, it was shown that HP internalization was necessary but that activity required domains outside the HD. Indeed, guidance by En1/2 is transcription-independent and involves local translation dependent on the mTOR pathway (Fig. 6E). These in vitro turning assay experiments show that En1/2 guides frog retinotectal axons in the absence of aggregated EphrinA5, suggesting that En1/2 signaling is sufficient for guidance. However, this might be specific to the frog or be due to some cis-interactions between Ephrins and Eph receptors (Suetterlin et al., 2012).
Further studies in the chick clearly demonstrated that Engrailed acts in cooperation with EphrinA5. In vitro experiments using the stripe assay, which consists of growing axons on alternating stripes of anterior and posterior tectal membranes (Walter et al., 1987), showed that the repulsion of temporal retinal axons by posterior membranes was dependent on En1/2 activity (Wizenmann et al., 2009). The same stripe assay had been used to identify EphrinA5 as a repulsive cue (Ciossek et al., 1998), raising the possibility of a physiological interaction between the two signaling pathways. Indeed, while EphrinA5-induced collapse of temporal chick growth cones was only observed at high EphrinA5 concentrations, collapse at sub-threshold EphrinA5 concentrations was restored by the addition of En2 (Wizenmann et al., 2009).
These findings strongly suggest that En1/2 is only necessary at low EphrinA5 concentrations in the range of actual in vivo concentrations. It is fair to say that physiological morphogen concentrations are unknown and that all results found in the literature and obtained in vitro may be non-physiological in the sense that they may involve non-physiological EphrinA5 concentrations. This reasoning leads us to propose the model of Figure 7A where En1/2 is useless in absence of EphrinA5, such as in a knock-out experiment, or at high non-physiological EphrinA5 concentrations typically used in vitro. However at physiological concentrations, En1/2 signaling would become absolutely necessary.
Figure 7.
Synergy exists between EphrinA5 and En1/2 in local growth cones. A. A consequence of EphrinA5/En1/2 synergistic activity is that En1/2 signaling does not operate in absence of EphrinA5 (knock out) or in excess of EphrinA5 (gain of function) but only at EphrinA5 physiological concentrations. B. ATP signaling is driven by En1/2 through local translation and synergizes with EphrinA5 signaling to elicit growth cone responses (Stettler et al., 2012).
To better understand the interaction between the two signaling pathways, the mechanisms underlying En1/2 local activity need to be clarified. A recent search for local translational targets upon En1/2 addition to growth cones found an extremely surprising 60% of targets consisting of nuclear-encoded mitochondrial proteins, in particular proteins that, like Ndufs1 and Ndufs3, are part of mitochondrial complex I (Stettler et al., 2012). It was subsequently shown that growth cones synthesize and secrete ATP less than 100 sec following En1/2 addition. Extracellular ATP is degraded into Adenosine which activates AR1 receptors in synergy with EphrinA5 signaling; an AR1 agonist can fully replace En1/2, whereas an AR1 antagonist annihilates local En1/2 activity (Fig. 7B) (Stettler et al., 2012).
A consequence of this “interaction model” is that HP signaling does not work alone but requires physiological, though not necessarily physical, interactions with bona fide signaling molecules. This is further illustrated by experiments in the fly wing disk showing that Engrailed and Decapentaplegic (DPP) interact in the formation of the anterior cross vain (ACV) (Layalle et al., 2011). Genetic interaction between Engrailed and DPP had been demonstrated by the absence of ACV formation in a double En/DPP hypomorph but not in DPP or En hypomorph (Blair, 2007). Impeding Engrailed after secretion by the Patched-expressing region of the wing disk also led to disrupted ACV formation. Furthermore, quantification of Mother Against DPP (MAD) phosphorylation showed that DPP transduction in vivo requires Engrailed signaling. Given that Engrailed cooperates with Ephrin signaling in the tectum and with DPP signaling in the fly wing disk, it is evident that the same HP may have distinct partners in different systems.
Non-autonomous Vax1, Pax6, and Hox proteins in development
Engrailed has an effect on axon guidance but does not seem to modify axon growth (Brunet et al., 2005). This is in contrast with a recent report on Vax1, a homeoprotein expressed in the ventro-medial hypothalamic area (vHT) (Hallonet et al., 1999; Hallonet et al., 1998) shown to stimulate the growth of retinal axons. Kim and colleagues (Kim et al., 2014) demonstrate that Vax1 is secreted by cells from the vHT at the level of the optic chiasma and transferred into retinal ganglion cells. A series of in vivo and in vitro experiments strongly suggest that the stimulation of axon elongation requires the internalization of Vax1 and local protein translation. Moreover, internalization is enhanced by the specific binding of this HP to heparan sulfates, giving weight to the idea of a sugar code for HP recognition (see below). Another point in common with Engrailed, in addition to the regulation of local translation, is a possible interaction with bona fide signaling. Indeed, based on the suggestion that Slit has a repulsive function for retinal axons (Bertuzzi et al., 1999), the authors have shown that neutralizing Slit with soluble Robo (Robo-Fc) cooperates with Vax1 to stimulate axon growth in the vHT area.
Another example of a direct non-cell autonomous HP activity is the role of Pax6 on the migration of oligodendrocyte precursor cells (OPCs) (Di Lullo et al., 2011). In the chick ventral neural tube at E.4, OPCs (expressing Olig2) were found to migrate in the close vicinity of Pax6-expressing cells and, similar to Engrailed in the tectum, 5% of Pax6 is in the extracellular milieu. In vivo electroporation of a plasmid encoding Pax6 with an added signal peptide for secretion (to avoid an increased intracellular cell autonomous concentration) resulted in enhanced OPC dispersal while extracellular expression of an anti-Pax6 scFv (Lesaffre et al., 2007) decreased cell dispersal. In addition, in vitro flat-mount preparations treated with antibodies and HPs confirmed Pax6 influence on OPC migration and showed that it requires both Pax6 internalization and the presence of Netrin. This latter point provides another example of a physiological interaction between classical and HP signaling pathways.
Early patterning along the A-P axis of developing vertebrates involves interactions between the Spemann organizer (SO) and surrounding tissues during gastrulation. The precise expression of Hox HPs within the mesoderm plays a role in body plan organization, while signaling from SO and non-organizing mesoderm copies this positional information to surrounding ectoderm (Ruiz i Altaba, 1993). Durston and colleagues recently showed that non-cell autonomous signaling of Hox proteins is responsible for inducing their own expression in neurectoderm (Bardine et al., 2014). They demonstrated that Hoxd1 is transferred from mesoderm to neuroectoderm and that misexpression of several Hox genes in the mesoderm leads to similar misexpression in the neuroectoderm. These findings once again show how intercellular transfer is likely a primitive mechanism, which allows simple and direct sharing of information between tissues in this context.
A role for non-autonomous Engrailed signaling in the stabilization of axon terminals
Recent work by Holt and colleagues on frog tadpole brain takes advantage of En1 internalization properties (Yoon et al., 2012). In a search for mRNAs translated in RGC growth cones following En1 internalization, these authors developed a non-canonical amino acid tagging strategy associating the use of amino acid analogs with two dimensional gel electrophoresis and mass spectrometry. Among several targets of En1, the most robust was LaminB2, a very unexpected result since lamins belong to a class of intermediate filaments present on the internal side of the nuclear envelope and in direct contact with the heterochromatin (Burke and Stewart, 2013). It came as a surprise that a nuclear protein could be locally translated in nerve terminals, meaning that its mRNA is specifically transported into the axon (also into dendrites as shown by in situ hybridization on frog eyes). Locally translated LaminB2 is targeted to mitochondria and when down-regulated with morpholinos, mitochondrial morphology and membrane potential is modified, which is a likely cause of the axon degeneration observed in stage 45 morpholino-treated frogs. Given that En1 is not made by retinal ganglion cells but in the target area where it is present in the extracellular matrix (Wizenmann et al., 2009), it is tempting to speculate that En1 trans-synaptic transport stabilizes retinal ganglion cell terminals within their target territory.
The above observations raise the question of whether HP signaling is involved in the well-established phenomenon of adult local translation, in particular at post-synaptic sites. Several groups have focused their attention on the transport of mRNAs into dendrites and the physiological importance of local translation (Darnell and Richter, 2012; Holt and Schuman, 2013; Richter and Klann, 2009; Tom Dieck et al., 2014). We speculate that HP transfer at the synapse may participate in the regulation of local translation. If so, it would mean that the post-synaptic side of the synapse would receive information brought by HPs, in particular information concerning the origin of the firing axon. For example it would not only receive a hyperpolarizing or a depolarization signal, but would also know the origin of the firing axons making it possible to respond differently to axons liberating the same mediator but coming from distinct brain nuclei. In support of this hypothesis, one must consider that most HPs are expressed in the adult, have the sequences necessary for intercellular transport and may also have a sequence allowing their direct binding to eIF4E, as previously mentioned (Figs. 2 and 3). It will indeed be paramount to know which locally distributed mRNAs are translated upon HP internalization and how they can participate in the physiological response. An exciting possibility is the implication of local production of ATP and liberation of Ca++ to serve local cellular and molecular events, such as vesicle fusion, endocytosis, cytoskeleton modification or protein phosphorylation. Indeed, adult synaptoneurosomes (vesicles derived from adult dendrites) prepared from the ventral midbrain, contain mRNAs encoding mitochondrial mRNAs that, in growth cones, are activated upon internalization of En1/2 leading to a local, rapid and transient synthesis of ATP (Stettler et al., 2012).
Otx2 function in cerebral cortex plasticity
Another HP for which postnatal and adult functions have been defined is Otx2, which regulates critical periods of plasticity in the cerebral cortex. In its post-natal development, the cerebral cortex has to adapt to the environment through a learning process that is most efficient during restricted periods of time called critical periods (CPs) (Hensch, 2004). CPs vary in time and duration across the cerebral cortex, in relation to the local cortical functions. Since the pioneering studies by Hubel and Wiesel (Wiesel and Hubel, 1965), the learning of binocular vision in mammals has served as a major CP paradigm. Suturing an eye before the opening or after the closure of CP for binocular vision has little consequence on the visual acuity of the deprived eye, whereas closing the eye during CP leads to amblyopia. This loss of visual acuity is mostly cortical and corresponds to the retraction of thalamic terminals corresponding to the deprived eye and the spreading of the terminals corresponding to the active eye. It is thus a morphological event that parallels a physiological modification. Strikingly, this period of plasticity seems to be triggered by a shift in the Excitatory/Inhibitory balance associated with the maturation of a specific class of fast-spiking GABAergic inhibitory neurons that synthesize parvalbumin (hereafter FSPV-cells) and are localized in layers III/IV of the cerebral cortex, in particular (but not only) the visual cortex (Fagiolini et al., 2004; Fagiolini and Hensch, 2000; Hensch, 2005).
CP opening in rodents is triggered shortly after the first visual experience, when the eyes open, and is paralleled by FSPV-cell maturation with the enhanced expression of several proteins, including parvalbumin, and the assembly of a complex extracellular matrix structure, the perineuronal nets (PNNs) identified by Camillo Golgi 100 years ago (Celio et al., 1998). Keeping mice in the dark retards FSPV-cell maturation, strongly suggesting that it involves an activity-dependent phenomenon. In the course of CP, Otx2 HP also accumulates inside FSPV-cells. As this increase does not take place in the dark, it must also be activity-dependent (Sugiyama et al., 2008). Intriguingly, Otx2 is not expressed by FSPV-cells but imported from one or several external sources and, in dark-reared animals or before CP normal opening (P16), the infusion of Otx2 protein in the cortex leads to its specific capture by FSPV-cells and to their maturation (Sugiyama et al., 2008). It was thus proposed that, in the mouse, Otx2 accumulation by FSPV-cells is necessary and sufficient for binocular CP opening at P20 and closure at P40.
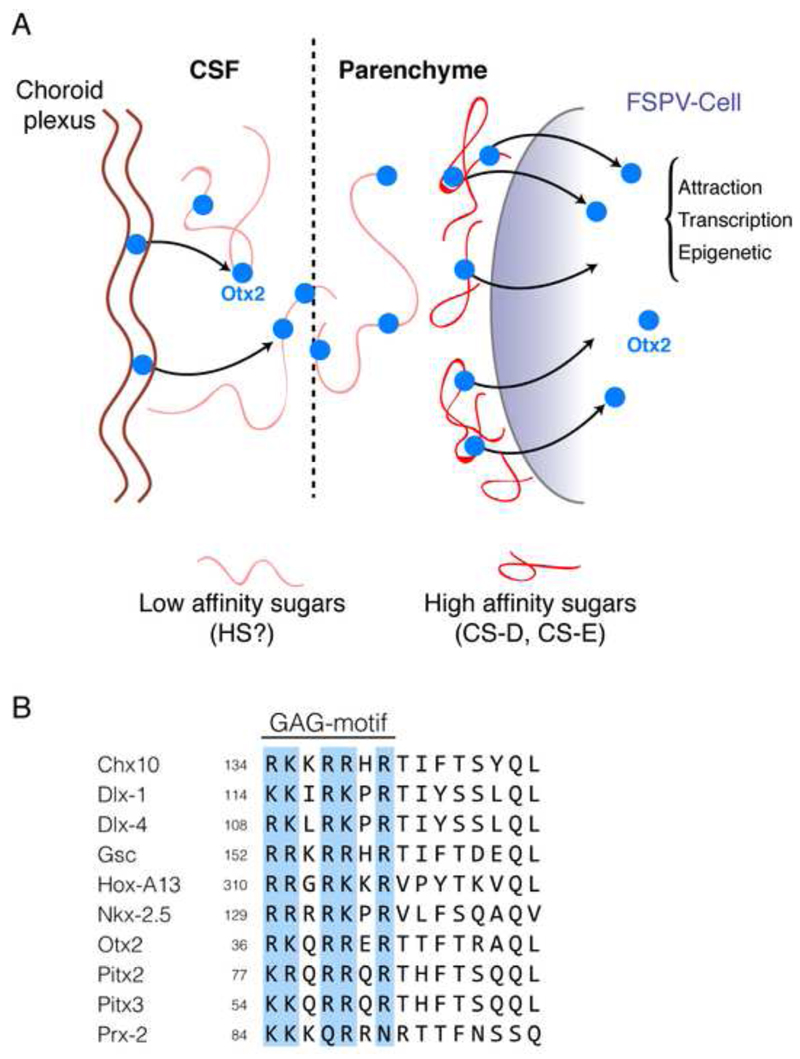
This finding raised a large number of questions, most of which are still unresolved. Only two of them will be discussed in depth: (i) the reason why Otx2 is primarily captured by FSPV-cells; and (ii) the anatomical source of Otx2. Otx2 capture by FSPV-cells primarily was very surprising given that HPs in vitro are internalized indiscriminately by all cell types. In fact, to prevent their immediate capture by cells surrounding the site of infusion, HPs are pre-incubated with polysialic acid (PSA), a sugar that shows low affinity for HP DNA-binding sites (Joliot et al., 1991b). This allows HP diffusion such that internalization only takes place when HPs meet with a “binding moiety” of higher affinity (Fig. 8A). The presence of complex sugars in FSPV-associated PNNs evokes the possibility that glycosaminoglycans (GAGs) might act as specific binding site for Otx2. This was confirmed by the identification of a GAG binding domain (RKQRRERTTFTRAQL, thus RK-peptide) within Otx2, immediately upstream of its HD (Beurdeley et al., 2012). This motif has high affinity for chondroitin sulfates D and E (CS-D and CS-E), which have sulfates in disaccharide unit positions 2 and 4 or 2 and 6, respectively. This finding has been strengthened by Kitagawa and colleagues who showed that when the cortical 4-sulfation/6-sulfation ratio is modified in vivo, Otx2 accumulation by FSPV-cells is perturbed (Miyata et al., 2012).
Figure 8.
The association between Otx2 and proteoglycans results in cell-specific accumulation. A. Otx2 is attracted to fast-spiking parvalbumin (FSPV) cells through high affinity interactions with chondroitin sulfate (CS) proteoglycans in the perineuronal nets (PNNs) that ensheath these cells (Beurdeley et al., 2012). As polysialic acid is required for infused recombinant Otx2 to diffuse within the parenchyme, we hypothesize that endogenous Otx2 associates with low affinity sugars (heparin sulfate (HS) or HS-proteoglycans) upon secretion from the choroid plexus into the cerebrospinal fluid (CSF). This association could help stabilize Otx2 in the CSF and might facilitate its diffusion in the parenchyme and its specific capture by cells expressing complex sugars with very high Otx2 affinity. B. Alignment of a subset of homeoproteins that contain an RK-, RR-, KR- or KK-doublet within a glycosaminoglycan (GAG) binding motif just upstream of the homeodomain. This motif is implicated in the specificity of Otx2 for the PNNs of FSPV cells and may help establish a sugar code for homeoprotein internalization specificity in vivo.
The GAG-binding motif in Otx2 appears to impart high specificity: mutating the RK doublet compromised specific Otx2 accumulation in FSPV-cells (Beurdeley et al., 2012). Interestingly, when En-2, which also contains potential GAG-binding sequences, was infused in the cortex, it did not show any specificity for PV-cells. This observation raises the possibility of a sugar code for HP recognition (Fig. 8B). In view of the role of En1/2 in growth cone decisions (Brunet et al., 2007), the demonstration that complex sugars are involved in guidance (Holt and Dickson, 2005; Irie et al., 2002; Kim et al., 2014; Poulain and Chien, 2013; Silver and Silver, 2014) deserves to be revisited from the perspective of En1/2 capture.
A fundamental issue concerns the source of cortical non-cell autonomous Otx2. An obvious source is the eye itself. Otx2 is expressed by bipolar cells and can pass to RGCs (Sugiyama et al., 2008). Antagonizing this passage prevents CP opening suggesting that Otx2 signaling in the eye itself is important (Sugiyama et al., 2008). However this effect could be due to compromised intra-retinal local activity and does not demonstrate that the source of cortical Otx2 is the retina. In favor of a retinal source is the fact that recombinant Otx2 injected in the eye reaches FSPV-cells in the cortex (Sugiyama et al., 2008). The protein is thus able to make the journey from the retina through the dLGN to the cortex, crossing several synapses along the way. Although this may be the case, a more general Otx2 source was sought, given the fact that Otx2 is present in FSPV-cells outside of the visual cortex (discussed below). The choroid plexus was thus considered as a putative Otx2 source for the entire cortex. Indeed Otx2 is expressed by this structure early in development and throughout adulthood (Johansson et al., 2013). In agreement with this hypothesis, deletion of Otx2 specifically in the adult choroid plexus decreased the amount of protein detected in FSPV-cells (Spatazza et al., 2013b), with important physiological consequences.
A two-threshold model for cerebral cortex plasticity
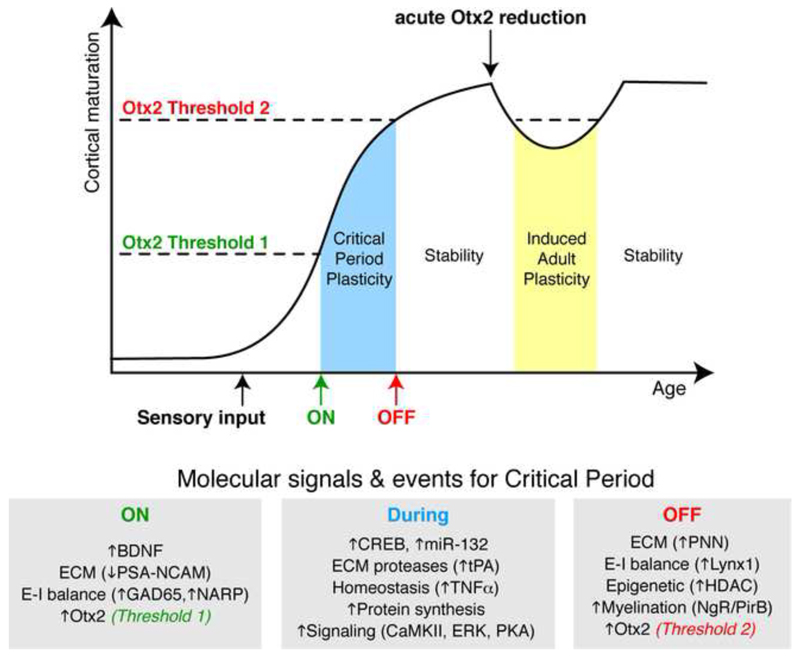
On the face of it, there is an apparent contradiction in that Otx2 arrival opens plasticity at P20 and yet also closes it by P40. Since the amount of Otx2 in the cells increases between P20 and P40 and then remains stable (Spatazza et al., 2013b), the most parsimonious explanation is the existence of two thresholds, one to open and one to close plasticity (Fig. 9). Indeed, cortical infusion of Otx2 in pre-critical period mice results in the anticipation of both the opening and closing (by P30) of their ocular dominance critical period. Interestingly, crossing the second threshold in the reverse direction (in the adult) by blocking Otx2 internalization with the RK-peptide or by down-regulating Otx2 synthesis in the choroid plexus gives access to the “plastic zone”. An 80% down-regulation of Otx2 synthesis in the choroid plexus led to decrease in FSPV-cell Otx2 content, to decreased PV expression and PNN assembly, and to the reopening of plasticity (Spatazza et al., 2013b). This two-threshold model (Spatazza et al., 2013b) can be seen as a temporal version of the French flag model proposed by Lewis Wolpert in 1969 (Wolpert, 1969). It reinforces the idea that Otx2, and probably other HPs, can act as morphogens in the course of development and, possibly, in the adult.
Figure 9.
A two-threshold model for Otx2 in critical period timing. Otx2 accumulates in FSPV-cells after eye opening in the mouse and opens a critical period of heightened plasticity once a first concentration threshold is reached. Continued accumulation leads to a second threshold after which the critical period closes. Several molecules, such as growth factors and receptors, and events, such as excitatory-inhibitory balance and epigenetics, work in concert with Otx2 to “turn” plasticity either on or off. During the critical period, changes in extracellular matrix and cell homeostasis provide a permissive state for the remodeling of connectivity. Afterwards and throughout adulthood, Otx2 continues to bathe the cortex and maintains the cortical network in a mature non-plastic state. By reducing Otx2 accumulation in FSPV cells to levels below the second threshold in the adult, cortical plasticity is induced and opens the possibility of new adult therapies.
It is also noteworthy that Otx2 is imported into FSPV-cells throughout the cortex and not just in the visual cortex (Spatazza et al., 2013b). This is consistent with the choroid plexus being a general source and suggests that Otx2 might be a general regulator of critical period opening and closure in other cortical areas. The idea is appealing but seemingly at odds with the fact that CPs are not synchronous throughout the cerebral cortex. A possible explanation is that, although present in the extracellular space throughout the cortex, Otx2 is only internalized after activity-dependent PNN assembly, thus the expression of Otx2-binding sites at the surface of FSPV-cells. This hypothesis is supported by the fact that in absence of visual input (dark-reared mice) FSPV-cells do not mature and do not accumulate Otx2 (Sugiyama et al., 2008). The infusion of high amounts of Otx2 in the visual cerebral cortex before CP opening accelerates FSPV-cell maturation (Sugiyama et al., 2008) possibly because the initial and “premature” Otx2 internalization promotes PNN assembly by FSPV-cells and initiates PNN-dependent Otx2 uptake. If so, Otx2-dependent CP would open in response to the first stimulation of the system in question (auditory, visual, etc.), and the first and second thresholds would be specific to each system.
The latter model suggests that while Otx2 signaling would open and close CPs at different times in different regions of the cerebral cortex, at adult stages it would maintain a non-plastic state in all regions. This aspect is of interest in the field of psychiatric diseases subsumed under the generic term of schizophrenia. Indeed, several studies suggest that they may be related to a CP taking place in the Dorso-Lateral Prefrontal Cortex during adolescence and are also marked by adult cognitive dysfunctions (Uhlhaas et al., 2009a; Uhlhaas et al., 2010; Uhlhaas et al., 2009b; Uhlhaas and Singer, 2010, 2012). This hypothesis is also supported by many studies showing an association between schizophrenia and FSPV-cell physiology (Curley et al., 2011; Curley et al., 2013; Curley and Lewis, 2012; Hashimoto et al., 2003; Insel, 2010; Lewis et al., 2012; Lewis et al., 2005).
Therapeutic potentials of homeoproteins
Although cerebral cortex plasticity is very much reduced after CP closure, some plasticity remains, suggesting that it could be enhanced in the adult. This might be of interest when considering novel therapeutic approaches for neurodevelopmental diseases and from a physiopathological perspective, in particular after events such as stroke that may benefit from reactivation of plasticity. In fact, several studies have been devoted to these questions and a variety of strategies to reopen plasticity in the adult have been proposed (Fawcett, 2009; Levelt and Hubener, 2012; Spolidoro et al., 2009). This diversity probably indicates that there are several ways to modify the Excitatory/Inhibitory balance in the adult. However, the work by Pizzorusso and colleagues showing that degrading the GAGs within PNNs reopens plasticity in the adult rat (Pizzorusso et al., 2002) seems particularly relevant in the context of this review. The infusion of the CS-D/E-binding RK-peptide into visual cortex competes for endogenous Otx2 capture by FSPV-cells, leading to a decrease in both parvalbumin expression and PNN assembly in the adult mouse (Beurdeley et al., 2012). Most importantly RK-peptide infusion reopens plasticity, allowing one to install amblyopia in the adult or, even more strikingly, to cure adult mice rendered amblyopic through monocular deprivation during CP. Although the direct degradation of GAGs within PNNs, as performed by Pizzorusso and colleagues, may act through more than one mechanism, it is tempting to propose the decrease in Otx2 uptake observed following GAG hydrolysis (Beurdeley et al., 2012) participates in the reopening of plasticity.
The RK-peptide study suggested that plasticity is a default state of the system and that the permanent capture of Otx2 maintains the system in a non-plastic state. This was readily confirmed by experiments in which Otx2 was specifically deleted in the adult choroid plexus (Spatazza et al., 2013b). Not only was it shown that Otx2 recombination in the choroid plexus allows one to install amblyopia in the adult but also that it was able to cure amblyopic adult mice. From the point of view of therapeutic strategies, it is of note that the choroid plexus is easily accessible from the circulatory system. As a proof of principle, Otx2 concentration could be modified by a temporally reversible strategy; anti-Otx2 morpholinos were injected in the venous circulation leading to a rapid decrease in the amount of Otx2 present in the choroid plexus (Spatazza et al., 2013b).
A rescuing role of HPs and their putative use as therapeutic proteins, thanks to their transduction domain, is further supported by the demonstration that exogenous Otx2, a HP expressed in adult bipolar cells in the retina from where it transported to RGCs (Sugiyama et al., 2008), protects neurons in a mouse model of glaucoma (Torero Ibad et al., 2011). RGCs, the projection neurons from eye to brain, degenerate in glaucoma, the main cause of blindness in the human. Since it was shown that Otx2 is transported from bipolar cells to RGCs in the retina and based on the studies described for mDA neurons, it was speculated that this HP might protect RGCs from degeneration. In absence of good rodent models for glaucoma, two models were developed, one in vitro and another one in vivo. The in vitro model consisted in dissociating adult retina and quantifying the death of the cells either in mixed cultures (mouse) or purified RGC cultures (rat). In both cases, the addition of Otx2 increased the number of surviving RGCs by 3 to 4 fold. The in vivo model was based on insult by injection of NMDA and it was shown that injection of recombinant Otx2 protein internalized in RGC saves 100% of the cells and preserves visual acuity. Although the two models are not true glaucoma models, these experiments suggest that Otx2 has a protective effect against RGC degeneration.
It is noteworthy that Engrailed in the adult is expressed by the granule cells (En-2) of the cerebellum and by the mesencephalic dopaminergic (mDA) neurons (En1/2) in the ventral midbrain (Eells, 2003; Simon et al., 2001). En-2 function in the adult cerebellum is not known although several reports have associated mutations in EN2 with autistic syndromes (Brielmaier et al., 2012; Choi et al., 2012; Gourion et al., 2004). More data are available on the function of En-1 in adult mDA neurons. Mice heterozygous for En1 progressively lose their mDA neurons through retrograde degeneration, leading to motor and non-motor defects, reflecting several aspects of the human disease (Alberi et al., 2004; Nordstrom et al., 2015; Sonnier et al., 2007). The in vivo internalization of recombinant En1/2 by mDA neurons blocks their degeneration in the mutant and increases the amount of dopamine per neuron. Interestingly, En1 infused in the midbrain also protected mDA neurons against the toxicity of A30P alpha-synuclein, MPTP and 6-OHDA (Alvarez-Fischer et al., 2011).
The mechanism(s) of action of En-1 are complex, but presumably involve the specific translation of nuclear mRNAs encoding mitochondrial proteins, including Ndufs1 and Ndufs3, similarly to what was found in RGC growth cones. The levels of the two proteins are reduced in mDA neurons of adult En-1 heterozygote mice, while the rescuing activity of En1/2 is lost when Ndufs1 translation is inhibited using specific siRNAs (Alvarez-Fischer et al., 2011). This is interesting in view of the many studies that converge toward mitochondrial complex I as a Parkinson disease “hub”. However, the most intriguing aspect of this observation is the parallel it makes with axon guidance (Stettler et al., 2012) and maintenance (Yoon et al., 2012) and the regulation of mitochondrial protein synthesis by En-1. It is not known whether this finding can be generalized, but - as mentioned above - it is of note that most HPs have both, transfer sequences and different protein interaction sequences outside of the HP (Fig. 3).
With these examples involving Otx2 and En-1, it is tempting to consider HPs in general as putative therapeutic proteins. Indeed, the internalization sequence, also called “Penetratin”, has already been used as a peptide vector for the internalization of several hydrophilic compounds with pharmacological activities (Bechara and Sagan, 2013; Cleal et al., 2013; Joliot and Prochiantz, 2004; Prochiantz, 2000). The use of the full length HPs is more of a challenge but has also been considered outside of the nervous system. For example, it was shown that exogenous HOXB4 and HOXC4 could be used to amplify hematopoietic stem cells in vitro (Auvray et al., 2012; Haddad et al., 2007; Haddad et al., 2008). Furthermore, HPs have diagnostic potential, as several groups have recently proposed to use HPs detected in the body fluids as markers of cancer aggressiveness and to develop potential therapeutic tools based on HP role in cell transformation (Bell et al., 2012; Beltran et al., 2013; Javed and Langley, 2014; McGrath et al., 2013a; McGrath et al., 2013b; Morgan et al., 2011).
Perspectives
HPs are expressed in all eukaryotes, not just multicellular organisms but also in unicellular systems such as yeast or unicellular green algae (Derelle et al., 2007). The signaling functions of HPs were established in plants well before the hypothesis for such a role was raised for animals. The case of Knotted-1 (KN1) in maize and Arabidopsis has been particularly well studied. It was shown, as in animals, that the HD of KN1 is necessary and sufficient for intercellular transport (Kim et al., 2005; Lucas et al., 1995). Importantly, intercellular diffusion of proteins (including HPs) in plants, is simplified in comparison with animals, by the existence of intercellular channels, called plasmodesmata, which are necessary to mediate cell-to-cell communication, despite the presence of cellulose walls. As HP existence precedes the separation between metaphytes and metazoans and because the HD is necessary and sufficient for intercellular transport in the two “kingdoms”, Joliot and colleagues looked at the transport of KN1 HD between animal cells (Tassetto et al., 2005). Not only did they demonstrate such transport, they verified that a mutation that blocks transport in plants also blocks it between animal cells and that a rescue mutant, transported between cells in plants, is also transferred in the animal model. These important findings strongly suggested that HP signaling shares common characteristics in plants and animals that must have preceded the separation of plant and animal kingdoms and may thus have been present even in the first multicellular organisms.
The latter reasoning raises a large number of unresolved issues. A first issue is the generality of the phenomenon. Most data obtained in vivo only concern a handful of HPs (Otx2, En1/2, Pax6, Hoxd1, and Vax1). Due to the conservation of the internalization and secretion sequences it is possible that most HPs can travel between cells, although this remains to be proven in vitro and in vivo and, even so, each case may have different underlying functions yet to be understood. A second important issue is the physiological interaction with classical signaling pathways. Based on the few examples described above, we have proposed that HP and bona fide signaling interact. The same protein, as shown for Engrailed, might interact with a variety of signaling pathways, depending on the species, organ and developmental period. It is also possible that the scope of various interactions is limited for a given HP. Regardless, it will be important to search for such interactions with each HP in different paradigms. In the same context, it might be of interest to verify if ATP synthesis regulation is specific for Engrailed or is also valid for other HPs. This is indeed of particular interest for the nervous system, which has high metabolic demand. In the context of signaling and receptors, one must consider the possibility of direct HP receptors or binding sites expressed at the surface of specific cell types. Otx2 recognizes PNN sugars through a short GAG-binding domain and many HPs have similar GAG-binding sequences (Fig. 8B). However, this does not mean that they share the same cellular specificity. For example, Engrailed infused in the cerebral cortex is not specifically captured by FSPV-cells and behaves similarly to mutated Otx2. This raises the hypothesis of a sugar code for HP recognition and internalization, a program of research per se.
Finally, and possibly not exhaustively, the issue of identifying direct HP targets must be tackled. A first remark is that cell autonomous and non-cell autonomous targets do not necessarily fully overlap, owing to post-translational modifications and differing cellular compartments. Furthermore, these targets are likely to include several classes of macromolecules. As mentioned above, some HPs not only regulate gene transcription but also mRNA translation. Non-cell autonomous translation can take place not only in the cell body but also in dendrites and in growing axons. Thus, HP transduction might be of physiological value when considering the regulation of local translation at the level of the synapse. Another remark pertains to epigenetics. It is fair to say that epigenetic changes following HP internalization have not been formally demonstrated. Yet the report that internalized Engrailed provokes the translation of LaminB2 in the frog tadpole alludes to this direction (Yoon et al., 2012). While a local and mitochondrial effect is demonstrated for LaminB2, it cannot be forgotten that this protein is typically nuclear and impacts on the structure of the heterochromatin, and thus on global gene regulation, development and aging (Burke and Stewart, 2013; Coffinier et al., 2010; O’Sullivan and Karlseder, 2012). There may also be a correlation between the requirement of Otx2 for the opening at P20 of a critical period for binocular vision and the fact that epigenetic changes are required for this transition (Fagiolini et al., 2009; Medini and Pizzorusso, 2008; Putignano et al., 2007).
All in all, there is growing effort to associate identified functions with HP transduction. However, there is much work ahead to understand the molecular and cellular mechanisms permitting HP transfer and to decipher the complete array of physiological functions regulated by these novel signaling pathways from the embryo through to adulthood.
Acknowledgments
This work was supported by Collège de France, Centre National de la Recherche Scientifique (CNRS), Institut National de la Santé et de la Recherche Médicale (INSERM) and Fondation Bettencourt Schueller. Support from European Research Council (Advanced Grant N°339379) is acknowledged. We thank Dr. Julia Fuchs and Prof. Edith Heard for critical reading and France Maloumian for figure design.
References
- Alberi L, Sgado P, Simon HH. Engrailed genes are cell-autonomously required to prevent apoptosis in mesencephalic dopaminergic neurons. Development. 2004;131:3229–3236. doi: 10.1242/dev.01128. [DOI] [PubMed] [Google Scholar]
- Alvarez-Fischer D, Fuchs J, Castagner F, Stettler O, Massiani-Beaudoin O, Moya KL, Bouillot C, Oertel WH, Lombes A, Faigle W, Joshi RL, et al. Engrailed protects mouse midbrain dopaminergic neurons against mitochondrial complex I insults. Nat Neurosci. 2011;14:1260–1266. doi: 10.1038/nn.2916. [DOI] [PubMed] [Google Scholar]
- Amsellem S, Pflumio F, Bardinet D, Izac B, Charneau P, Romeo PH, Dubart-Kupperschmitt A, Fichelson S. Ex vivo expansion of human hematopoietic stem cells by direct delivery of the HOXB4 homeoprotein. Nat Med. 2003;9:1423–1427. doi: 10.1038/nm953. [DOI] [PubMed] [Google Scholar]
- Auvray C, Delahaye A, Pflumio F, Haddad R, Amsellem S, Miri-Nezhad A, Broix L, Yacia A, Bulle F, Fichelson S, Vigon I. HOXC4 homeoprotein efficiently expands human hematopoietic stem cells and triggers similar molecular alterations as HOXB4. Haematologica. 2012;97:168–178. doi: 10.3324/haematol.2011.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardine N, Lamers G, Wacker S, Donow C, Knoechel W, Durston A. Vertical signalling involves transmission of hox information from gastrula mesoderm to neurectoderm. PLoS One. 2014;9:e115208. doi: 10.1371/journal.pone.0115208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara C, Sagan S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013;587:1693–1702. doi: 10.1016/j.febslet.2013.04.031. [DOI] [PubMed] [Google Scholar]
- Bell D, Bell A, Roberts D, Weber RS, El-Naggar AK. Developmental transcription factor EN1--a novel biomarker in human salivary gland adenoid cystic carcinoma. Cancer. 2012;118:1288–1292. doi: 10.1002/cncr.26412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran AS, Graves LM, Blancafort P. Novel role of Engrailed 1 as a prosurvival transcription factor in basal-like breast cancer and engineering of interference peptides block its oncogenic function. Oncogene. 2013 doi: 10.1038/onc.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuzzi S, Hindges R, Mui SH, O’Leary DD, Lemke G. The homeodomain protein vax1 is required for axon guidance and major tract formation in the developing forebrain. Genes Dev. 1999;13:3092–3105. doi: 10.1101/gad.13.23.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurdeley M, Spatazza J, Lee H, Sugiyama S, Bernard C, Di Nardo AA, Hensch TK, Prochiantz A. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci. 2012;32:9429–9437. doi: 10.1523/JNEUROSCI.0394-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Blair SS. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol. 2007;23:293–319. doi: 10.1146/annurev.cellbio.23.090506.123606. [DOI] [PubMed] [Google Scholar]
- Brielmaier J, Matteson PG, Silverman JL, Senerth JM, Kelly S, Genestine M, Millonig JH, DiCicco-Bloom E, Crawley JN. Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS One. 2012;7:e40914. doi: 10.1371/journal.pone.0040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- Broccoli V, Boncinelli E, Wurst W. The caudal limit of Otx2 expression positions the isthmic organizer. Nature. 1999;401:164–168. doi: 10.1038/43670. [DOI] [PubMed] [Google Scholar]
- Brunet I, Di Nardo AA, Sonnier L, Beurdeley M, Prochiantz A. The topological role of homeoproteins in the developing central nervous system. Trends Neurosci. 2007;30:260–267. doi: 10.1016/j.tins.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438:94–98. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol. 2013;14:13–24. doi: 10.1038/nrm3488. [DOI] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–515. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- Chatelin L, Volovitch M, Joliot AH, Perez F, Prochiantz A. Transcription factor hoxa-5 is taken up by cells in culture and conveyed to their nuclei. Mech Dev. 1996;55:111–117. doi: 10.1016/0925-4773(95)00478-5. [DOI] [PubMed] [Google Scholar]
- Cheng HJ, Nakamoto M, Bergemann AD, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- Choi J, Ababon MR, Matteson PG, Millonig JH. Cut-like homeobox 1 and nuclear factor I/B mediate ENGRAILED2 autism spectrum disorder-associated haplotype function. Hum Mol Genet. 2012;21:1566–1580. doi: 10.1093/hmg/ddr594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciossek T, Monschau B, Kremoser C, Loschinger J, Lang S, Muller BK, Bonhoeffer F, Drescher U. Eph receptor-ligand interactions are necessary for guidance of retinal ganglion cell axons in vitro. Eur J Neurosci. 1998;10:1574–1580. doi: 10.1046/j.1460-9568.1998.00180.x. [DOI] [PubMed] [Google Scholar]
- Cleal K, He L, Watson PD, Jones AT. Endocytosis, intracellular traffic and fate of cell penetrating peptide based conjugates and nanoparticles. Curr Pharm Des. 2013;19:2878–2894. doi: 10.2174/13816128113199990297. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Chang SY, Nobumori C, Tu Y, Farber EA, Toth JI, Fong LG, Young SG. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc Natl Acad Sci U S A. 2010;107:5076–5081. doi: 10.1073/pnas.0908790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Eggan SM, Lazarus MS, Huang ZJ, Volk DW, Lewis DA. Role of glutamic acid decarboxylase 67 in regulating cortical parvalbumin and GABA membrane transporter 1 expression: implications for schizophrenia. Neurobiol Dis. 2013;50:179–186. doi: 10.1016/j.nbd.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. J Physiol. 2012;590:715–724. doi: 10.1113/jphysiol.2011.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Richter JD. Cytoplasmic RNA-binding proteins and the control of complex brain function. Cold Spring Harb Perspect Biol. 2012;4:a012344. doi: 10.1101/cshperspect.a012344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CA, Joyner AL. Expression patterns of the homeo box-containing genes En-1 and En-2 and the proto-oncogene int-1 diverge during mouse development. Genes Dev. 1988;2:1736–1744. doi: 10.1101/gad.2.12b.1736. [DOI] [PubMed] [Google Scholar]
- Denis-Donini S, Glowinski J, Prochiantz A. Glial heterogeneity may define the three-dimensional shape of mouse mesencephalic dopaminergic neurones. Nature. 1984;307:641–643. doi: 10.1038/307641a0. [DOI] [PubMed] [Google Scholar]
- Derelle R, Lopez P, Le Guyader H, Manuel M. Homeodomain proteins belong to the ancestral molecular toolkit of eukaryotes. Evol Dev. 2007;9:212–219. doi: 10.1111/j.1525-142X.2007.00153.x. [DOI] [PubMed] [Google Scholar]
- Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- Di Bonito M, Narita Y, Avallone B, Sequino L, Mancuso M, Andolfi G, Franze AM, Puelles L, Rijli FM, Studer M. Assembly of the auditory circuitry by a Hox genetic network in the mouse brainstem. PLoS Genet. 2013;9:e1003249. doi: 10.1371/journal.pgen.1003249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lullo E, Haton C, Le Poupon C, Volovitch M, Joliot A, Thomas JL, Prochiantz A. Paracrine Pax6 activity regulates oligodendrocyte precursor cell migration in the chick embryonic neural tube. Development. 2011;138:4991–5001. doi: 10.1242/dev.066282. [DOI] [PubMed] [Google Scholar]
- Drescher U, Bonhoeffer F, Muller BK. The Eph family in retinal axon guidance. Curr Opin Neurobiol. 1997;7:75–80. doi: 10.1016/s0959-4388(97)80123-7. [DOI] [PubMed] [Google Scholar]
- Driever W, Nusslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988a;54:83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Driever W, Nusslein-Volhard C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988b;54:95–104. doi: 10.1016/0092-8674(88)90183-3. [DOI] [PubMed] [Google Scholar]
- Dubnau J, Struhl G. RNA recognition and translational regulation by a homeodomain protein. Nature. 1996;379:694–699. doi: 10.1038/379694a0. [DOI] [PubMed] [Google Scholar]
- Eells JB. The control of dopamine neuron development, function and survival: insights from transgenic mice and the relevance to human disease. Curr Med Chem. 2003;10:857–870. doi: 10.2174/0929867033457700. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol. 2009;19:207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J. Molecular control of brain plasticity and repair. Prog Brain Res. 2009;175:501–509. doi: 10.1016/S0079-6123(09)17534-9. [DOI] [PubMed] [Google Scholar]
- Finkelstein R, Perrimon N. The orthodenticle gene is regulated by bicoid and torso and specifies Drosophila head development. Nature. 1990;346:485–488. doi: 10.1038/346485a0. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- Friedman GC, O’Leary DD. Retroviral misexpression of engrailed genes in the chick optic tectum perturbs the topographic targeting of retinal axons. J Neurosci. 1996;16:5498–5509. doi: 10.1523/JNEUROSCI.16-17-05498.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber RL, Kuroiwa A, Gehring WJ. Genomic and cDNA clones of the homeotic locus Antennapedia in Drosophila. Embo J. 1983;2:2027–2036. doi: 10.1002/j.1460-2075.1983.tb01696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ. Homeo Boxes in the study of development. Science. 1987;236:1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- Gourion D, Leroy S, Bourdel MC, Goldberger C, Poirier MF, Olie JP, Krebs MO. Cerebellum development and schizophrenia: an association study of the human homeogene Engrailed 2. Psychiatry Res. 2004;126:93–98. doi: 10.1016/j.psychres.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Gregor T, Tank DW, Wieschaus EF, Bialek W. Probing the limits to positional information. Cell. 2007a;130:153–164. doi: 10.1016/j.cell.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor T, Wieschaus EF, McGregor AP, Bialek W, Tank DW. Stability and nuclear dynamics of the bicoid morphogen gradient. Cell. 2007b;130:141–152. doi: 10.1016/j.cell.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Caignard A, Visentin G, Vigon I, Fichelson S, Amsellem S. The HOXB4 homeoprotein improves ex vivo generation of functional human NK-cell progenitors. Leukemia. 2007;21:1836–1839. doi: 10.1038/sj.leu.2404725. [DOI] [PubMed] [Google Scholar]
- Haddad R, Pflumio F, Vigon I, Visentin G, Auvray C, Fichelson S, Amsellem S. The HOXB4 homeoprotein differentially promotes ex vivo expansion of early human lymphoid progenitors. Stem Cells. 2008;26:312–322. doi: 10.1634/stemcells.2007-0721. [DOI] [PubMed] [Google Scholar]
- Hallonet M, Hollemann T, Pieler T, Gruss P. Vax1, a novel homeobox-containing gene, directs development of the basal forebrain and visual system. Genes Dev. 1999;13:3106–3114. doi: 10.1101/gad.13.23.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallonet M, Hollemann T, Wehr R, Jenkins NA, Copeland NG, Pieler T, Gruss P. Vax1 is a novel homeobox-containing gene expressed in the developing anterior ventral forebrain. Development. 1998;125:2599–2610. doi: 10.1242/dev.125.14.2599. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch T. Critical period plasticity in local cortical circuits. Nature Reviews Neuroscience. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Holcman D, Kasatkin V, Prochiantz A. Modeling homeoprotein intercellular transfer unveils a parsimonious mechanism for gradient and boundary formation in early brain development. J Theor Biol. 2007;249:503–517. doi: 10.1016/j.jtbi.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Holt CE, Dickson BJ. Sugar codes for axons? Neuron. 2005;46:169–172. doi: 10.1016/j.neuron.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Schuman EM. The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron. 2013;80:648–657. doi: 10.1016/j.neuron.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchmandzadeh B, Wieschaus E, Leibler S. Establishment of developmental precision and proportions in the early Drosophila embryo. Nature. 2002;415:798–802. doi: 10.1038/415798a. [DOI] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Irie A, Yates EA, Turnbull JE, Holt CE. Specific heparan sulfate structures involved in retinal axon targeting. Development. 2002;129:61–70. doi: 10.1242/dev.129.1.61. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Ichijo H, Hama C, Matsuno T, Nakamura H. Establishment of rostrocaudal polarity in tectal primordium: engrailed expression and subsequent tectal polarity. Development. 1991;113:1133–1144. doi: 10.1242/dev.113.4.1133. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Nakamura H. Rostrocaudal polarity of the tectum in birds: correlation of en gradient and topographic order in retinotectal projection. Neuron. 1992;8:787–798. doi: 10.1016/0896-6273(92)90099-y. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Nakamura H. A role for gradient en expression in positional specification on the optic tectum. Neuron. 1996;16:55–62. doi: 10.1016/s0896-6273(00)80023-9. [DOI] [PubMed] [Google Scholar]
- Javed S, Langley SE. Importance of HOX genes in normal prostate gland formation, prostate cancer development and its early detection. BJU Int. 2014;113:535–540. doi: 10.1111/bju.12269. [DOI] [PubMed] [Google Scholar]
- Johansson PA, Irmler M, Acampora D, Beckers J, Simeone A, Gotz M. The transcription factor Otx2 regulates choroid plexus development and function. Development. 2013;140:1055–1066. doi: 10.1242/dev.090860. [DOI] [PubMed] [Google Scholar]
- Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci USA. 1991a;88:1864–1868. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004;6:189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- Joliot AH, Triller A, Volovitch M, Pernelle C, Prochiantz A. alpha-2,8-Polysialic acid is the neuronal surface receptor of antennapedia homeobox peptide. New Biol. 1991b;3:1121–1134. [PubMed] [Google Scholar]
- Joyner AL, Liu A, Millet S. Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr Opin Cell Biol. 2000;12:736–741. doi: 10.1016/s0955-0674(00)00161-7. [DOI] [PubMed] [Google Scholar]
- Kasatkin V, Prochiantz A, Holcman D. Morphogenetic gradients and the stability of boundaries between neighboring morphogenetic regions. Bull Math Biol. 2008;70:156–178. doi: 10.1007/s11538-007-9246-5. [DOI] [PubMed] [Google Scholar]
- Katahira T, Sato T, Sugiyama S, Okafuji T, Araki I, Funahashi J, Nakamura H. Interaction between Otx2 and Gbx2 defines the organizing center for the optic tectum. Mech Dev. 2000;91:43–52. doi: 10.1016/s0925-4773(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Kerszberg M, Wolpert L. Specifying positional information in the embryo: looking beyond morphogens. Cell. 2007;130:205–209. doi: 10.1016/j.cell.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6:553–564. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- Kim JY, Rim Y, Wang J, Jackson D. A novel cell-to-cell trafficking assay indicates that the KNOX homeodomain is necessary and sufficient for intercellular protein and mRNA trafficking. Genes Dev. 2005;19:788–793. doi: 10.1101/gad.332805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Min KW, Kang KH, Lee EJ, Kim HT, Moon K, Choi J, Le D, Lee SH, Kim JW. Regulation of retinal axon growth by secreted Vax1 homeodomain protein. Elife. 2014;3 doi: 10.7554/eLife.02671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Johnston P, Struhl G. Different requirements for homeotic genes in the soma and germ line of Drosophila. Cell. 1983;35:27–34. doi: 10.1016/0092-8674(83)90204-0. [DOI] [PubMed] [Google Scholar]
- Layalle S, Volovitch M, Mugat B, Bonneaud N, Parmentier ML, Prochiantz A, Joliot A, Maschat F. Engrailed homeoprotein acts as a signaling molecule in the developing fly. Development. 2011;138:2315–2323. doi: 10.1242/dev.057059. [DOI] [PubMed] [Google Scholar]
- Le Roux I, Joliot AH, Bloch-Gallego E, Prochiantz A, Volovitch M. Neurotrophic activity of the Antennapedia homeodomain depends on its specific DNA-binding properties. Proc Natl Acad Sci U S A. 1993;90:9120–9124. doi: 10.1073/pnas.90.19.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, Reber M. Retinotectal mapping: new insights from molecular genetics. Annu Rev Cell Dev Biol. 2005;21:551–580. doi: 10.1146/annurev.cellbio.20.022403.093702. [DOI] [PubMed] [Google Scholar]
- Lesaffre B, Joliot A, Prochiantz A, Volovitch M. Direct non-cell autonomous Pax6 activity regulates eye development in the zebrafish. Neural Develop. 2007;2 doi: 10.1186/1749-8104-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt CN, Hubener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lipshitz HD. Follow the mRNA: a new model for Bicoid gradient formation. Nat Rev Mol Cell Biol. 2009;10:509–512. doi: 10.1038/nrm2730. [DOI] [PubMed] [Google Scholar]
- Logan C, Wizenmann A, Drescher U, Monschau B, Bonhoeffer F, Lumsden A. Rostral optic tectum acquires caudal characteristics following ectopic engrailed expression. Curr Biol. 1996;6:1006–1014. doi: 10.1016/s0960-9822(02)00645-0. [DOI] [PubMed] [Google Scholar]
- Lucas WJ, Bouche-Pillon S, Jackson DP, Nguyen L, Baker L, Ding B, Hake S. Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science. 1995;270:1980–1983. doi: 10.1126/science.270.5244.1980. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Araki I, Nakamura H. Pax6 defines the di-mesencephalic boundary by repressing En1 and Pax2. Development. 2000;127:2357–2365. doi: 10.1242/dev.127.11.2357. [DOI] [PubMed] [Google Scholar]
- McGrath SE, Michael A, Morgan R, Pandha H. EN2: a novel prostate cancer biomarker. Biomark Med. 2013a;7:893–901. doi: 10.2217/bmm.13.115. [DOI] [PubMed] [Google Scholar]
- McGrath SE, Michael A, Pandha H, Morgan R. Engrailed homeobox transcription factors as potential markers and targets in cancer. FEBS Lett. 2013b;587:549–554. doi: 10.1016/j.febslet.2013.01.054. [DOI] [PubMed] [Google Scholar]
- Medini P, Pizzorusso T. Visual experience and plasticity of the visual cortex: a role for epigenetic mechanisms. Front Biosci. 2008;13:3000–3007. doi: 10.2741/2905. [DOI] [PubMed] [Google Scholar]
- Meinhardt H, Gierer A. Pattern formation by local self-activation and lateral inhibition. Bioessays. 2000;22:753–760. doi: 10.1002/1521-1878(200008)22:8<753::AID-BIES9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Millet S, Campbell K, Epstein DJ, Losos K, Harris E, Joyner AL. A role for Gbx2 in repression of Otx2 and positioning the mid/hindbrain organizer. Nature. 1999;401:161–164. doi: 10.1038/43664. [DOI] [PubMed] [Google Scholar]
- Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci. 2012;15:414–422. doi: 10.1038/nn.3023. [DOI] [PubMed] [Google Scholar]
- Morgan R, Boxall A, Bhatt A, Bailey M, Hindley R, Langley S, Whitaker HC, Neal DE, Ismail M, Whitaker H, Annels N. Engrailed-2 (EN2): a tumor specific urinary biomarker for the early diagnosis of prostate cancer. Clin Cancer Res. 2011;17:1090–1098. doi: 10.1158/1078-0432.CCR-10-2410. [DOI] [PubMed] [Google Scholar]
- Nedelec S, Foucher I, Brunet I, Bouillot C, Prochiantz A, Trembleau A. Emx2 homeodomain transcription factor interacts with eukaryotic translation initiation factor 4E (eIF4E) in the axons of olfactory sensory neurons. Proc Natl Acad Sci U S A. 2004;101:10815–10820. doi: 10.1073/pnas.0403824101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing D, Dostatni N, Jackle H, Rivera-Pomar R. Sequence interval within the PEST motif of Bicoid is important for translational repression of caudal mRNA in the anterior region of the Drosophila embryo. Embo J. 1999;18:1966–1973. doi: 10.1093/emboj/18.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom U, Beauvais G, Ghosh A, Pulikkaparambil Sasidharan BC, Lundblad M, Fuchs J, Joshi RL, Lipton JW, Roholt A, Medicetty S, Feinstein TN, et al. Progressive nigrostriatal terminal dysfunction and degeneration in the engrailed1 heterozygous mouse model of Parkinson’s disease. Neurobiol Dis. 2015;73:70–82. doi: 10.1016/j.nbd.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan RJ, Karlseder J. The great unravelling: chromatin as a modulator of the aging process. Trends Biochem Sci. 2012;37:466–476. doi: 10.1016/j.tibs.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Holt C. RNA translation in axons. Annu Rev Cell Dev Biol. 2004;20:505–523. doi: 10.1146/annurev.cellbio.20.010403.111746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Poulain FE, Chien CB. Proteoglycan-mediated axon degeneration corrects pretarget topographic sorting errors. Neuron. 2013;78:49–56. doi: 10.1016/j.neuron.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochiantz A. Messenger proteins: homeoproteins, TAT and others. Curr Opin Cell Biol. 2000;12:400–406. doi: 10.1016/s0955-0674(00)00108-3. [DOI] [PubMed] [Google Scholar]
- Prochiantz A, Joliot A. Can transcription factors function as cell-cell signalling molecules? Nat Rev Mol Cell Biol. 2003;4:814–819. doi: 10.1038/nrm1227. [DOI] [PubMed] [Google Scholar]
- Prochiantz A, Theodore L. Nuclear/growth factors. Bioessays. 1995;17:39–44. doi: 10.1002/bies.950170109. [DOI] [PubMed] [Google Scholar]
- Putignano E, Lonetti G, Cancedda L, Ratto G, Costa M, Maffei L, Pizzorusso T. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron. 2007;53:747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Rezsohazy R. Non-transcriptional interactions of Hox proteins: inventory, facts, and future directions. Dev Dyn. 2014;243:117–131. doi: 10.1002/dvdy.24060. [DOI] [PubMed] [Google Scholar]
- Richter JD. Think globally, translate locally: what mitotic spindles and neuronal synapses have in common. Proc Natl Acad Sci U S A. 2001;98:7069–7071. doi: 10.1073/pnas.111146498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD, Klann E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev. 2009;23:1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- Rivera-Pomar R, Niessing D, Schmidt-Ott U, Gehring WJ, Jackle H. RNA binding and translational suppression by bicoid. Nature. 1996;379:746–749. doi: 10.1038/379746a0. [DOI] [PubMed] [Google Scholar]
- Ruiz i, Altaba A. Induction and axial patterning of the neural plate: planar and vertical signals. J Neurobiol. 1993;24:1276–1304. doi: 10.1002/neu.480241004. [DOI] [PubMed] [Google Scholar]
- Ruiz-Medrano R, Xoconostle-Cazares B, Kragler F. The plasmodesmatal transport pathway for homeotic proteins, silencing signals and viruses. Curr Opin Plant Biol. 2004;7:641–650. doi: 10.1016/j.pbi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Shigetani Y, Funahashi JI, Nakamura H. En-2 regulates the expression of the ligands for Eph type tyrosine kinases in chick embryonic tectum. Neurosci Res. 1997;27:211–217. doi: 10.1016/s0168-0102(96)01151-0. [DOI] [PubMed] [Google Scholar]
- Silver DJ, Silver J. Contributions of chondroitin sulfate proteoglycans to neurodevelopment, injury, and cancer. Curr Opin Neurobiol. 2014;27:171–178. doi: 10.1016/j.conb.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A. Positioning the isthmic organizer where Otx2 and Gbx2 meet. Trends Genet. 2000;16:237–240. doi: 10.1016/s0168-9525(00)02000-x. [DOI] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Mallamaci A, Stornaiuolo A, D’Apice MR, Nigro V, Boncinelli E. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO J. 1993;12:2735–2747. doi: 10.1002/j.1460-2075.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon HH, Saueressig H, Wurst W, Goulding MD, O’Leary DD. Fate of midbrain dopaminergic neurons controlled by the engrailed genes. J Neurosci. 2001;21:3126–3134. doi: 10.1523/JNEUROSCI.21-09-03126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnier L, Le Pen G, Hartmann A, Bizot JC, Trovero F, Krebs MO, Prochiantz A. Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1. J Neurosci. 2007;27:1063–1071. doi: 10.1523/JNEUROSCI.4583-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatazza J, Di Lullo E, Joliot A, Dupont E, Moya KL, Prochiantz A. Homeoprotein signaling in development, health, and disease: a shaking of dogmas offers challenges and promises from bench to bed. Pharmacol Rev. 2013a;65:90–104. doi: 10.1124/pr.112.006577. [DOI] [PubMed] [Google Scholar]
- Spatazza J, Lee HH, Di Nardo AA, Tibaldi L, Joliot A, Hensch TK, Prochiantz A. Choroid-Plexus-Derived Otx2 Homeoprotein Constrains Adult Cortical Plasticity. Cell Rep. 2013b;3:1815–1823. doi: 10.1016/j.celrep.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolidoro M, Sale A, Berardi N, Maffei L. Plasticity in the adult brain: lessons from the visual system. Exp Brain Res. 2009;192:335–341. doi: 10.1007/s00221-008-1509-3. [DOI] [PubMed] [Google Scholar]
- Stettler O, Joshi RL, Wizenmann A, Reingruber J, Holcman D, Bouillot C, Castagner F, Prochiantz A, Moya KL. Engrailed homeoprotein recruits the adenosine A1 receptor to potentiate ephrin A5 function in retinal growth cones. Development. 2012;139:215–224. doi: 10.1242/dev.063875. [DOI] [PubMed] [Google Scholar]
- Steward O. Translating axon guidance cues. Cell. 2002;110:537–540. doi: 10.1016/s0092-8674(02)00934-0. [DOI] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- Suetterlin P, Marler KM, Drescher U. Axonal ephrinA/EphA interactions, and the emergence of order in topographic projections. Semin Cell Dev Biol. 2012;23:1–6. doi: 10.1016/j.semcdb.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Tassetto M, Maizel A, Osorio J, Joliot A. Plant and animal homeodomains use convergent mechanisms for intercellular transfer. EMBO Rep. 2005;6:885–890. doi: 10.1038/sj.embor.7400487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom Dieck S, Hanus C, Schuman EM. SnapShot: local protein translation in dendrites. Neuron. 2014;81:958–958 e951. doi: 10.1016/j.neuron.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Topisirovic I, Borden KL. Homeodomain proteins and eukaryotic translation initiation factor 4E (eIF4E): an unexpected relationship. Histol Histopathol. 2005;20:1275–1284. doi: 10.14670/HH-20.1275. [DOI] [PubMed] [Google Scholar]
- Torero Ibad R, Rheey J, Mrejen S, Forster V, Picaud S, Prochiantz A, Moya KL. Otx2 promotes the survival of damaged adult retinal ganglion cells and protects against excitotoxic loss of visual acuity in vivo. J Neurosci. 2011;31:5495–5503. doi: 10.1523/JNEUROSCI.0187-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- Turing AM. The chemical basis of morphogenesis. Phil trans B. 1952;237:37–72. [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolic D, Singer W. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009a;3:17. doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci. 2010;14:72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci U S A. 2009b;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Walter J, Kern-Veits B, Huf J, Stolze B, Bonhoeffer F. Recognition of position-specific properties of tectal cell membranes by retinal axons in vitro. Development. 1987;101:685–696. doi: 10.1242/dev.101.4.685. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wizenmann A, Brunet I, Lam JSY, Sonnier L, Beurdeley M, Zarbalis K, Weisenhorn-Vogt D, Weiln C, Dwivedy A, Joliot A, Wurst W. Extracellular Engrailed participates in the topographic guidance of retinal axons in vivo. Neuron. 2009;64:355–366. doi: 10.1016/j.neuron.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- Wurst W, Auerbach AB, Joyner AL. Multiple developmental defects in Engrailed-1 mutant mice: an early mid- hindbrain deletion and patterning defects in forelimbs and sternum. Development. 1994;120:2065–2075. doi: 10.1242/dev.120.7.2065. [DOI] [PubMed] [Google Scholar]
- Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat Rev Neurosci. 2001;2:99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- Yoon BC, Jung H, Dwivedy A, O’Hare CM, Zivraj KH, Holt CE. Local translation of extranuclear lamin B promotes axon maintenance. Cell. 2012;148:752–764. doi: 10.1016/j.cell.2011.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel G, Small S. Morphogens: precise outputs from a variable gradient. Curr Biol. 2006;16:R29–31. doi: 10.1016/j.cub.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- Zilles K, Amunts K. Centenary of Brodmann’s map--conception and fate. Nat Rev Neurosci. 2010;11:139–145. doi: 10.1038/nrn2776. [DOI] [PubMed] [Google Scholar]