Abstract
The YAP/TAZ family of transcriptional co‐activators drives cell proliferation in epithelial tissues and cancers. Yet, how YAP and TAZ are physiologically regulated remains unclear. Here we review recent reports that YAP and TAZ act primarily as sensors of epithelial cell polarity, being inhibited when cells differentiate an apical membrane domain, and being activated when cells contact the extracellular matrix via their basal membrane domain. Apical signalling occurs via the canonical Crumbs/CRB‐Hippo/MST‐Warts/LATS kinase cascade to phosphorylate and inhibit YAP/TAZ. Basal signalling occurs via Integrins and Src family kinases to phosphorylate and activate YAP/TAZ. Thus, YAP/TAZ is localised to the nucleus in basal stem/progenitor cells and cytoplasm in differentiated squamous cells or columnar cells. In addition, other signals such as mechanical forces, tissue damage and possibly receptor tyrosine kinases (RTKs) can influence MST‐LATS or Src family kinase activity to modulate YAP/TAZ activity.
Keywords: epithelial polarity, Hippo pathway, mechanosensing, mechanotransduction, TAZ, wound healing, YAP
Abbreviations
- ECM
extracellular matrix
- EGF(R)
epidermal growth factor (receptor)
- RTK
receptor tyrosine kinase
Introduction
Animal tissues, from Drosophila to humans, tend to harbour a population of stem cells that is responsible for maintaining the tissue through cell proliferation and differentiation of daughter cells 1, 2, 3, 4, 5. Stem cells can proliferate to maintain normal tissue homeostasis, but also increase their proliferation in response to mechanical stretching of the tissue or to tissue damage and consequent inflammation. For example, the normal growth of the skin from newborn to adulthood occurs through stretching of the tissue, which promotes proliferation of basal layer stem/progenitor cells. In addition, wounding or infection of the skin also triggers a proliferative response of basal layer cells to replace the damaged skin with new cells. How these events are orchestrated at the molecular level, and whether they become deregulated in human epithelial cancers, is still poorly understood.
Recent discoveries from Drosophila genetics identified the YAP/TAZ family of transcriptional co‐activators (the sole Drosophila homologue is called Yorkie) as being essential regulators of cell proliferation during development and in adult stem cells of the intestine 6, 7, 8, 9. Drosophila Yorkie drives transcription of pro‐proliferative target genes through interaction with the TEAD‐family DNA binding transcription factor Scalloped, as well as additional co‐factors MASK, WBP2 and Brahma 10, 11, 12, 13, 14, 15, 16. Importantly, Yorkie is regulated by the cell polarity machinery in epithelial cells, being activated upon loss of the apical polarity determinant Crumbs, or loss of the planar polarity determinant Fat 17, 18, 19, 20, 21, 22. There is also evidence for Yorkie acting as a sensor of mechanical forces during development, where it promotes cell proliferation in response to epithelial stretch forces acting on the cytoskeleton 23, 24. Furthermore, Yorkie activity is induced upon tissue damage to promote intestinal stem cell proliferation and tissue repair 7, 8, 9, 10.
Here we review the molecular mechanisms responsible for regulation of Yorkie by cell polarity, force and damage in Drosophila. We then examine the regulation of YAP and TAZ in different mammalian epithelial tissues in vivo, which points to the existence of fundamentally conserved mechanisms between Drosophila and mammals. We also examine the regulation of YAP and TAZ during human epithelial cancer progression, where disruption of cell polarity, invasive migration, as well as damage and inflammation all appear to promote the action of YAP and TAZ in the nucleus. Our observations outline a unifying regulatory logic controlling YAP/TAZ co‐activators (summarised in Figs. 1, 2, 3, 4) and also suggest avenues for therapeutic intervention in inflammation and cancer. Finally, we are critical of results in cell culture that are unsupported by related findings in vivo.
Figure 1.
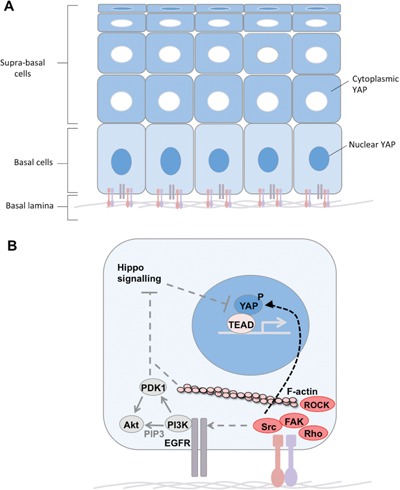
Basal signals promote nuclear YAP localisation. A: In stratified squamous epithelia, YAP/TAZ is nuclear in the basal cell layer which contacts the basal lamina ECM via Integrins. Supra basal cells lose contact with the basal lamina and thus experience reduced Integrin signalling and relocalisation of YAP/TAZ to the cytoplasm. One exception are the extremely flattened terminally differentiated cells, where YAP/TAZ can once again become nuclear, possibly due to mechanical stretching. B: Integrin‐Src‐FAK signalling synergises with EGFR‐PI3K signalling to promote nuclear localisation of YAP. Src can directly tyrosine‐phosphorylate YAP, but may also act indirectly to inhibit Hippo signalling, which inhibits YAP via serine/threonine phosphorylation to promote cytoplasmic retention. PI3K induces PIP3 lipid formation, which may help stabilise Integrin adhesions as well as inducing PDK1 and Akt activation. F‐actin, Rho and ROCK also generate actomyosin contractility to help stabilise Integrin adhesions and thus may contribute to Src activation.
Figure 2.
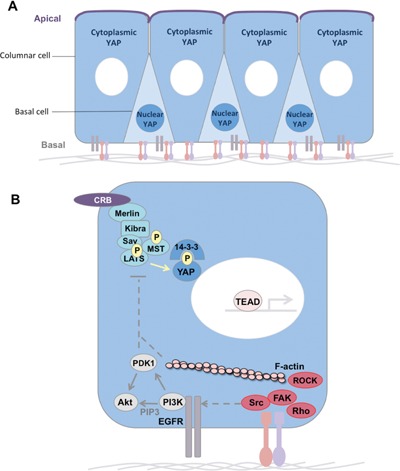
Apical signals inhibit nuclear YAP localisation. A: In columnar epithelia, YAP/TAZ is cytoplasmic in differentiated cells with an apical domain and nuclear in basal layer stem cells which lack an apical domain and contact the basal lamina ECM via Integrins. B: Crumbs‐Merlin‐Kibra‐Salvador‐MST‐LATS signalling (the canonical Hippo pathway) leads to phosphorylation of YAP/TAZ and retention in the cytoplasm (due to binding to 14‐3‐3 proteins) despite contact with the ECM. Thus, strong apical Hippo signalling is able to overcome basal Integrin signalling to maintain YAP/TAZ in the cytoplasm.
Figure 3.
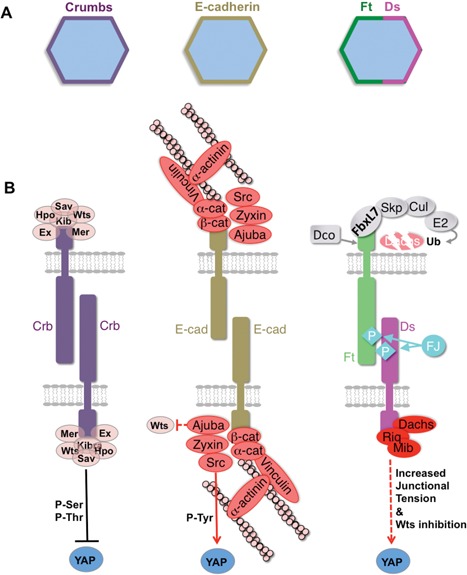
Regulation of YAP by Crumbs and Cadherin signalling. A: Crumbs and E‐cadherin distribute around the entire circumference of the epithelial cell's apical surface. In contrast, Fat and Dachsous cadherins planar polarise to opposite ends of the cell. B: Crumbs signals via canonical Merlin‐Ex‐Kibra‐Sav‐Hpo‐Warts/LATS signalling to inhibit YAP by direct ser/thr phosphorylation and cytoplasmic retention. E‐cadherin recruits Ajuba/Zyxin proteins, which may directly inhibit Warts/LATS kinases and Src family kinases, which tyrosine phosphorylate and activate YAP. Dachsous recruits the Dachs myosin, which increases junctional tension, as well as Riq and Mib, which may directly inhibit Warts/LATS kinases.
Figure 4.
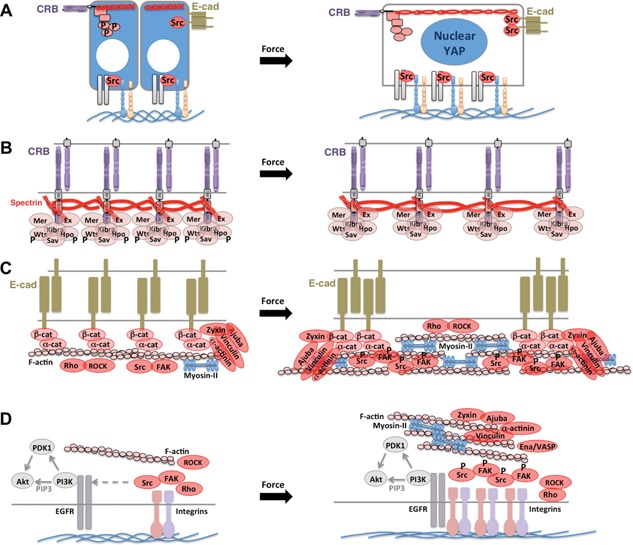
Models of mechano‐sensing that may control YAP localisation. A: Columnar epithelial cells exhibit cytoplasmic YAP at high density, but nuclear YAP at low density (which induces spreading out of cells). B: Model for inhibition of apical Crumbs‐Hippo signalling upon cellular stretching (due to de‐clustering of Crumbs complexes). C: Model for activation of Src at adherens junctions upon cellular stretching (due to induction of actomyosin contractility to resist stretching, clustering of adherens junctions, and recruitment of Ajuba/Zyxin family proteins as well as alpha‐actinin and Vinculin). D: Model for activation of basal Integrin‐Src signalling upon cellular stretching (due to formation of focal adhesions which cluster Integrins and recruit Ajuba/Zyxin, alpha‐actinin and Vinculin).
Yorkie as polarity‐sensor, mechano‐sensor and damage‐sensor in vivo
Apical Crumbs signalling represses Yorkie
The apical polarity determinant Crumbs was long thought to be essential for cells to maintain an apical domain, so it was surprising when loss of Crumbs was discovered to cause tissue overgrowth in Drosophila adult tissues, such as the wing or eye 17, 18. The overgrown crumbs‐mutant tissues were found to have normal apical‐basal polarity, due to the presence of the redundant factor Bazooka/Par3, and also exhibited upregulation of Yorkie‐target genes 17, 18, 24, 25, 26, 27. Crumbs was found to bind directly to Expanded, via its FERM‐binding domain, and thus to activate the canonical Hippo‐Warts kinase cascade to repress Yorkie activity 17, 18, 28, 29. Recent work has confirmed that phosphorylated Warts kinase can be detected precisely where Crumbs is localised in the developing wing 30. Thus, Crumbs is not only a key apical domain determinant, but also has a second function in activating Hippo signalling to repress Yorkie (Fig. 2).
Junctional Ft‐Ds cadherins and E‐cadherin associated signals regulate Yorkie
The planar polarity determinants Fat (Ft) and Dachsous (Ds) are atypical cadherins that localise to adherens junctions with E‐cadherin, but in an asymmetric fashion 31, 32 (Fig. 3). Ft‐Ds interactions are well known to cause the planar polarisation of the atypical myosin Dachs (D), which acts as an F‐actin motor protein to increase tension at adherens junctions to promote tissue elongation via biasing the orientation of cell divisions and cell‐cell rearrangements 33, 34, 35, 36 (Fig. 3). Interestingly, loss of Fat produces not only a failure of tissue elongation, but also tissue overgrowth due to activation of Yorkie‐target genes 19, 20, 21, 22, 34, 37 (Fig. 3). This activation of Yorkie‐driven growth was found to depend strictly on the accumulation of the Dachs myosin at adherens junctions, and stabilisation of Dachs at junctions is sufficient to drive tissue overgrowth 34, 38, 39. Dachs appears to activate Yorkie by antagonising Warts 40, but the kinases Minibrain (Mnb) and Riquiqui (Riq) also appear to be involved and these can directly phosphorylate Warts to repress its activity 41 (Fig. 3).
The F‐actin associated proteins Ajuba, Zyxin, and Src localise to adherens junctions and promote activation of Yorkie‐target genes and tissue growth in the fly wing and eye 23, 42, 43, 44, 45, 46, 47, 48. Since mammalian Src family kinases (Src, Fyn, Yes) are known to phosphorylate and activate mammalian homologues of Yorkie (YAP, or Yes‐associated protein, and TAZ) 49, 50, it is plausible that Ajuba and Zyxin act to promote Src activity at adherens junctions and thereby activate Yorkie to drive tissue growth. Alternatively, Ajuba and Zyxin may directly inhibit the Warts kinase, to reduce the inhibitory phosphorylation of Yorkie by this kinase 23.
Basal Integrin signalling may activate Yorkie in intestinal stem cells
Integrins are localised to the basal side of epithelial cells, where they play a key role in cell adhesion to the extracellular matrix 51. In the Drosophila intestine, proliferation of stem cells depends critically on Integrins and their intracellular signal transducers such as Talin 52, 53. How Integrin signalling promotes stem cell proliferation remains unclear, but both Src and Yorkie are of pivotal importance for proliferation of these cells, suggesting a potential regulatory connection 54. Notably, intestinal stem cells lack an apical domain, so are likely to have no Crumbs‐Hippo‐Warts signalling and thus strongly active Yorkie that requires input from basal Integrin‐Src signalling to maintain stem cell proliferation. Thus, Yorkie appears to act as a sensor of cell polarity to promote proliferation in stem cell populations.
Mechanical stretching activates Yorkie
In addition to acting as a sensor of cell polarity, another possible physiological function for Yorkie is as a mechanosensor – originally proposed in mammalian cell culture for YAP/TAZ 55, 56. In the developing fly wing, peripheral epithelial cells become circumferentially stretched by the morphogen‐driven growth of the central wing pouch, and the stretched cells respond by proliferating more to produce a near‐uniform level of proliferation across the entire tissue 57, 58, 59, 60, 61. How these cells sense mechanical forces was unclear, until it was revealed that the degree of stretching correlated with increased Yorkie‐target gene activity 24.
One possible mechanosensor is Crumbs itself, which binds to the apical Spectrin cytoskeleton – a mechanically deformable network that is required for Crumbs to activate Hippo signalling 24, 62. Stretching of the apical domain of the cell correlates with a decrease in the local density of Crumbs molecules, which may then decrease the ability of Crumbs to activate Hippo‐Warts signalling and repress Yorkie 24. In support of this model, either forcing the clustering of Crumbs with an extracellular ligand (Crumbs itself expressed on neighbouring cells) or increasing the local concentration of Hippo kinase can strongly activate Hippo‐Warts signalling and prevent stretch‐induced Yorkie activation 24. Interestingly, mechanical stretching of wing cells also leads to increased recruitment of phosphorylated myosin‐II 60, and the same phenomenon occurs upon disruption of the apical Spectrin cytoskeleton 62, again supporting a mechanosensory role for Spectrins.
Another possible mechanosensor is the adherens junction, whose associated proteins Ajuba and Zyxin promote Yorkie activation in response to force upon the actomyosin cytoskeleton 23, 44. It will be interesting to test whether the physiological stretch forces that occur during development are sufficient to affect recruitment of Ajuba and Zyxin and their ability to activate Src and/or inhibit Warts. Presently, there is evidence that non‐physiological reduction of acto‐myosin contractility at adherens junctions leads to reduced Ajuba association 23, but this may simply be due to the requirement for actomyosin in maintaining the adherens junctions themselves. One plausible mechanism for mechanosensing is via the alpha‐catenin protein, which can unfold under force to reveal a Vinculin binding site, but further work is necessary to test the role of Vinculin in regulation of Yorkie 63.
Tissue damage activates Yorkie
Another physiological function for Yorkie is sensing tissue damage 64. This role is best understood in the Drosophila intestine, where damaging agents such as pathogenic bacteria or chemical treatment with the insecticide Paraquat produce a massive stem cell proliferation response to regenerate the tissue that depends upon Yorkie activity 7, 8, 9. Interestingly, Yorkie is required both in the stem cells for proliferation and in the differentiated epithelial cells to sense damage. Yorkie induces expression of JAK‐STAT pathway ligands (Upds) which signal to stem cells to further promote their proliferation 7, 8, 9. Precisely, how Yorkie senses tissue damage remains unclear, and this is a fundamentally important question to answer to fully understand the physiological roles of Yorkie in vivo. Recent work suggests that Yorkie inhibits the infection‐sensing Toll receptor – Dorsal/NF‐kappaB pathway, which in turn inhibits Yorkie activation 65. Thus, tissue damage sensing by Yorkie is likely to act in a parallel and antagonistic manner to infection sensing, perhaps to promote a sterile‐inflammation response rather than an infection response. Further work is necessary to explore this possible role of Yorkie.
YAP/TAZ as polarity sensor in vivo
Repression of YAP/TAZ by apical signals
The mammalian Yorkie homologs YAP and TAZ are clearly regulated by the presence or absence of an apical domain in mammalian epithelial cells 49. In columnar epithelia, YAP and TAZ remain cytoplasmic, while in basal layer epithelial stem cells that lack an apical domain, YAP and TAZ localise to the nucleus 49. The organisation of the bronchial epithelium is a good example of this phenomenon, and it has been shown that apical signalling requires CRB3, a Crumbs homolog 66 (Fig. 2).
Stimulation of YAP/TAZ by basal signals
Nuclear localisation of YAP and TAZ also appears to be promoted by Integrin signalling upon attachment of cells to the basement membrane 49, 67, 68. In squamous epithelia, YAP and TAZ are nuclear in basal layer stem/progenitor cells but cytoplasmic in most suprabasal differentiating cells, despite the fact that squamous epithelial cells never differentiate an apical domain 49 (Fig. 1). This finding suggests that loss of Integrin‐mediated contact with the basement membrane extracellular matrix triggers relocalisation of YAP and TAZ. The organisation of the skin epithelium is a good example of this mode of regulation, and regulation of YAP has been shown to depend on Integrin‐Src signalling in this tissue to drive proliferation of basal layer stem/progenitor cells 49, 67, 68 (Fig. 1). It will be interesting to test whether Integrin‐Src signalling regulates YAP/TAZ in other tissues where Integrins, Src or YAP are known to drive cell proliferation such as during liver regeneration 69, 70.
A role for signalling from adherens junctions?
Whether junctionally localised factors directly regulate YAP and TAZ remains controversial. The atypical cadherins Fat and Dachsous have multiple homologs in mammals, but knockouts tend to affect tissue shape rather than tissue growth in epithelia 71 (but they reveal a role in both neural and nephron proliferation 72, 73, 74, 75). Furthermore, disruption of adherens junctions in alpha‐catenin knockout skin does not lead to a reduction in YAP/TAZ activity or reduced cell proliferation, but rather leads to overproliferation – suggesting a possibly indirect activation of YAP/TAZ via increased Integrin‐Src signalling in alpha‐catenin knockout skin 50, 76, 77. Further work is necessary to investigate whether junctionally associated Zyxin/TRIP6, Ajuba/WTIP or Src family members contribute to YAP/TAZ activation in vivo, as they do for Drosophila Yorkie (Fig. 3).
YAP/TAZ as mechano‐sensor in vivo
YAP and TAZ were first proposed to be mechanosensors based on results in cell culture 55, 56. The key regulatory mechanism in cell culture is the attachment of cells to their basal substratum via Integrins 49, 67, 68, 78, whose ‘outside‐in’ signalling is firmly implicated in mechanosensation 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91 (Fig. 4). In addition, apical signals from Crumbs‐Hippo‐Warts may be reduced upon stretching of the apical Spectrin cytoskeleton 24 while adherens junction or Integrin signals via Zyxin‐Ajuba‐Vinculin‐Src‐FAK may be increased due to the actomyosin contractile response to tension or stretch 60, 92, which then induces clustering and activation of E‐cadherin or Integrins and thus Src and FAK to drive YAP/TAZ to the nucleus 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105 (Fig. 4). Importantly, there is not yet conclusive evidence that YAP or TAZ (unlike Yorkie) can respond to mechanical force in vivo. Since YAP and TAZ promote normal cell proliferation in the skin, it may be that the stretching of the skin during post‐natal growth or adult obesity induce YAP and TAZ activity to enable the skin to grow to cover the entire surface area of the body. Further work is necessary to develop mechanical stretching systems for epithelia to measure the requirement for YAP and TAZ in stretch‐dependent growth in vivo.
YAP/TAZ as damage‐sensor in vivo
In the mammalian intestinal epithelium, overexpression of YAP was found to be sufficient to promote increased stem cell proliferation 106. Notably, YAP and TAZ double conditional knockout mice appear not to affect normal gut homeostasis, but even YAP single‐knockouts reduce the tissue damage‐induced or APC‐mutant induced proliferation response of this tissue 107, 108, 109, 110. Note that there is much controversy over how APC loss leads to YAP/TAZ nuclear localisation 107, 108, 109, 110, and that YAP can in fact remain cytoplasmic in human or mouse adenomas that retain an apical domain and normal columnar organisation 49. Importantly, both tissue damage and APC loss lead to a strong increase in YAP levels, which may include increased transcription of YAP as well as stabilisation of the YAP protein 107, 108. Mechanistically, cytokine receptor signalling, particularly the gp130 co‐receptor, has been implicated in activating Src family kinases and YAP in the mouse intestine in response to mucosal injury to promote proliferative wound healing in the mouse gut 111. Independent work confirms a key role for Src kinase in mouse intestinal proliferation and tumour formation 54. Whether cytokine receptors are the sole signal regulating Src and/or YAP/TAZ in damaged tissues remains to be clarified.
In the mammalian skin epithelium, overexpression of YAP is also sufficient to promote increased basal layer proliferation 76, 77, 112. Double conditional knockouts for YAP and TAZ reduce skin proliferation and also reduce the ability of skin wounds to heal 49. As in the gut, damage‐induced upregulation of YAP and TAZ can be observed around the wound site, suggesting that these factors may directly respond to tissue damage to promote the proliferative response 49. The elevation of YAP/TAZ levels in response to skin damage requires Src family kinase signalling, although the signals acting upstream of Src remain unclear 49. It will also be interesting to test whether YAP and TAZ contribute to the inflammatory response that often accompanies different types of tissue damage.
In the mammalian liver, overexpression of YAP, or loss of upstream components of the Hippo pathway such as Merlin/Sav, MST1/2, or Mob1a/1b drive tissue overgrowth 69, 113, 114, 115, 116, 117. YAP knockout livers are relatively normal sized but lose some hepatocytes and biliary cells 113. It will be interesting to see whether the YAP/TAZ double knockout livers are also normally sized, and whether they have difficulty in regenerating after partial hepatectomy 118, 119.
YAP/TAZ in human epithelial cancers
Most human cancers are epithelial in origin and progression towards malignant carcinoma involves a disruption of apical‐basal polarisation, invasive migration and damage/inflammation. All three of these malignant changes would be expected to induce YAP/TAZ nuclear localisation. Loss of the apical domain would be predicted to disrupt Crumbs‐Hippo signalling to activate YAP/TAZ 49, 66, 68. Increased contact with the extracellular matrix would be predicted to increase Integrin‐Src signalling to activate YAP/TAZ 49, 67, 68. Invasive migration involves force‐generation that may further activate Integrin‐Src signalling and YAP/TAZ 49, 67, 68. Damage and/or inflammatory responses may also contribute to stimulation of YAP/TAZ activity in cancer 49, 54, 68, 111. Notably, these responses may not be limited only to the proliferating cancer cells themselves but also occur in the cancer‐associated fibroblasts that promote tumour invasion 78. Thus, YAP/TAZ may be the missing link that explains why cancers appear to behave as ‘the wound that never heals’, why inflammation promotes malignancy or why disruption of epithelial polarity and morphology is such a universal and predictive hallmark of malignant carcinomas.
Are other functions proposed for YAP/TAZ in vitro operative in vivo?
Since the localisation and activity of YAP and TAZ can be readily examined in cell culture, a veritable myriad of interventions have been proposed to affect their activity in these assays. For the most part, there is no evidence that any of these cell culture discoveries actually reflect a physiologically relevant mechanism of YAP/TAZ regulation in vivo. Here we focus on just a few examples.
Does Wnt signalling activate YAP/TAZ, or vice versa, in vivo?
YAP/TAZ was reported to inhibit Wnt signalling via interactions between YAP/TAZ and either beta‐catenin 120, dishevelled 121, or the axin/beta‐TrCP destruction complex 109. These reports predict that loss of YAP/TAZ should result in activation of Wnt‐beta‐catenin signalling in vivo, and this does not appear to be the case in the YAP/TAZ double knockout mouse intestine 108, 109 or in Drosophila yorkie or mask mutants or RNAi 6, 10, 29. Furthermore, these reports also predict that overexpression of YAP or Yorkie should inhibit Wnt‐beta‐catenin signalling in vivo, and once again there is no convincing evidence for this effect in mice or flies 6, 10, 29.
More plausible is the notion that YAP/TAZ‐TEAD and beta‐catenin‐TCF complexes may cooperate, or antagonise, on the promoters of particular target genes 122, 123. Promoters are indeed where most cross‐talk between signalling pathways to the nucleus takes place in multicellular organisms, because this mechanism allows for the combinatorial regulation of gene expression necessary for multicellular development 124. It is also plausible that Wnt‐beta‐catenin signalling may simply transcriptionally induce YAP in certain tissues such as the intestinal crypt.
A more recent report proposed that Wnt signalling activates YAP/TAZ via the non‐canonical ‘alternative’ Wnt‐Frizzled pathway 125. The Frizzled receptor family is conserved between Drosophila and mammals, yet in Drosophila loss of Frizzled signalling causes defects in planar cell polarity and/or beta‐catenin activation but not widespread tissue undergrowth or loss of Yorkie activity 126, 127. Instead, it seems that strong activation of Frizzled in cell culture can artefactually induce Rho GTPase activation and acto‐myosin contractility, which then indirectly activates YAP/TAZ, possibly via mechanical force effects 125. Analysis of Frizzled knockout mice is necessary to determine whether YAP/TAZ activation is physiologically involved in Frizzled signalling in mammals.
Does BMP/Smad signalling activate YAP/TAZ, or vice versa, in vivo?
YAP was initially reported to bind to the inhibitory Smad7 to antagonise Smad3/4 signalling 128. Later work proposed that YAP cooperates with Smad1 in nuclear transcription 129 and that TAZ promotes nuclear localisation and transcriptional activity of Smad2/3‐4 complexes 130. Next, YAP/TAZ was proposed to bind to Smad2/3 to retain them in the cytoplasm in cells cultured at high density, such that both signal transducers become nuclear at low density 131. This latter work was challenged by a recent report that cell density regulates Smad activation via its effects on the subcellular localisation of TGF‐beta/BMP receptors, rather than via YAP/TAZ 132, and forced a response from the first group 133. An independent group reported that the interaction of YAP/TAZ with Smad2/3 was cell‐type specific 134. Notably, the BMP/Smad (Drosophila Dpp/Mad) pathway is conserved in Drosophila but genetic analysis has revealed no evidence of direct crosstalk with Hippo‐Yorkie signalling (although there may be indirect effects via Ds‐Ft‐Fj gradients) 34. Further work is necessary to test whether the TGFbeta/Smad2 (Drosophila Activin/Smad2) pathway might affect Yorkie in Drosophila 135. It will also be interesting to genetically test whether crosstalk between YAP/TAZ and Smad signalling operates in mouse tissues.
Does GPCR signalling activate YAP/TAZ in vivo?
The G‐protein‐coupled receptor (GPCR) agonist ligands lysophosphatidic acid (LPA) and sphingosine 1‐phosphate (S1P) were reported to activate YAP/TAZ via the G12/13 or Gq/11 protein in cell culture, which activates Rho GTPase to alter actomyosin contractility 136, 137, 138. Other GPCR agonist ligands glucagon and epinephrine were found to inhibit YAP/TAZ activity via GS, cAMP and protein kinase A (PKA) 136, 139. Whether any of these signals physiologically regulate YAP/TAZ in vivo remains unclear. The only apparently supporting evidence from Drosophila is that pka mutant tissue overproliferates (though this may be due to ectopic Hedgehog signalling), while overexpression of PKA causes apoptosis 139. On the contrary, the effect of pka silencing by RNAi on Yorkie target genes cyclinE or expanded is very mild 139. Further work is needed to clarify whether GPCRs or PKA are truly physiologically involved in regulation of Yorkie or YAP/TAZ in vivo.
Does the Mevalonate pathway activate YAP/TAZ in vivo?
Two reports suggested that YAP/TAZ nuclear localisation was dependent on the SREBP/Mevalonate pathway, which turns acetyl‐CoA via mevalonate into lipid precursors such as Farnesyl‐PP (a cholesterol and other sterol precursor) and GeranylGeranyl‐PP (a precursor for prenylation of proteins such as small GTPases) 140, 141. The proposed mechanism was that inhibition of mevalonate biosynthesis by Statins (which inhibit HMG‐CoA reductase) impairs prenylation of the Rho GTPase. However, patients taking Statin drugs do not report massive side effects on stem cell proliferation and tissue homeostasis, nor are Statins known to have potent anti‐cancer effects in many solid tumour types, suggesting that Statins cannot completely inhibit the action of YAP and TAZ in vivo. In addition, the SREBP/Mevalonate pathway is conserved in Drosophila but appears to specifically affect lipid synthesis and cell growth rather than produce Yorkie‐like proliferation phenotypes 142, 143. Finally, there is no evidence that regulation of Rho GTPase prenylation is a physiological mechanism of YAP/TAZ regulation in vivo.
Do growth factors such as EGF receptor ligands or other RTK ligands activate YAP/TAZ in vivo?
The receptor tyrosine kinase (RTK) family of plasma membrane receptors is defined by a variable extracellular domain and a common intracellular tyrosine kinase domain and includes EGFRs, InsulinR, IGF1R, PDGFRs, CSF1R, Kit, Flk2, FGFRs, TrkA/B/C, AXL, Ret, ALK, DDR1/2, Ros and Eph receptors. Binding of ligands such as EGF to the EGFR leads to Tyrosine Kinase activation and trans‐phosphorylation, which then recruits signal transducers to the multiple phospho‐Tyrosine motifs in the intracellular domain. The most commonly activated signal transduction pathways downstream of RTK activation include Ras‐MAPK, Src family kinase, PI3K‐Akt‐TOR, PLCgamma, and Vav signalling – with different RTKs activating specific subsets of these pathways. In cell culture, it was reported that addition of EGF to cells was able to induce nuclear localisation of YAP/TAZ in a PI3K‐dependent fashion 67, 144. Results in Drosophila support the notion that PI3K signalling can activate Yorkie 145. There is also a requirement for minimal TOR activation to maintain Yorkie activity in Drosophila 146. In mice, there is a good correlation between EGF ligand and receptor expression and YAP/TAZ nuclear localisation in skin 49, and EGF ligands such as amphiregulin (AREG) are also transcriptional targets of YAP/TAZ in several tissues, forming a possible positive feedback loop in vivo 74, 110, 147, 148. Overall, these promising results support the notion that certain forms of RTK signalling might physiologically regulate YAP/TAZ activity in vivo. Further work in vertebrate models will be necessary to establish which RTKs are genetically required to regulate YAP/TAZ in different tissues, and how RTKs might cross‐talk with Integrin‐Src or E‐cadherin‐Src signals in vivo.
Conclusions
Results from Drosophila and mouse genetics firmly establish the Yorkie/YAP/TAZ family as a sensor of cell polarity, mechanical forces and tissue damage in vivo. These three inputs are frequently misregulated in cancer, providing a possible explanation for the frequent nuclear localisation of YAP/TAZ in malignant tumour cells, where YAP/TAZ appear to contribute to malignant behaviour. Future work will need to examine the mechanism by which YAP/TAZ can respond to these physiological signals in both normal tissues and cancers. Although other signals have been proposed to regulate YAP/TAZ in cultured cells, it remains unclear whether any of these alternative signals are truly of physiological relevance in both flies and mice. Perhaps the most promising of the newly proposed signals are the RTKs, although which of these receptors is necessary to regulate YAP/TAZ in mouse tissues or tumours requires further genetic analysis. Overall, the prospects remain bright for a crucial role for YAP/TAZ signalling in both normal tissue homeostasis and cancer, making this pathway an attractive biomarker and target for therapy.
The authors have declared no conflicts of interest.
References
- 1. Arwert EN, Hoste E, Watt FM. 2012. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer 12: 170–80. [DOI] [PubMed] [Google Scholar]
- 2. Blanpain C, Fuchs E. 2014. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344: 1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Flier LG, Clevers H. 2009. Stem cells, self‐renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71: 241–60. [DOI] [PubMed] [Google Scholar]
- 4. Losick VP, Morris LX, Fox DT, Spradling A. 2011. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell 21: 159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang H, Edgar BA. 2012. Intestinal stem cell function in Drosophila and mice. Curr Opin Genet Dev 22: 354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang J, Wu S, Barrera J, Matthews K, et al. 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122: 421–34. [DOI] [PubMed] [Google Scholar]
- 7. Shaw RL, Kohlmaier A, Polesello C, Veelken C, et al. 2010. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development 137: 4147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Staley BK, Irvine KD. 2010. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol 20: 1580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karpowicz P, Perez J, Perrimon N. 2010. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development 137: 4135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sidor CM, Brain R, Thompson BJ. 2013. Mask proteins are cofactors of Yorkie/YAP in the Hippo pathway. Curr Biol 23: 223–8. [DOI] [PubMed] [Google Scholar]
- 11. Sansores‐Garcia L, Atkins M, Moya IM, Shahmoradgoli M, et al. 2013. Mask is required for the activity of the Hippo pathway effector Yki/YAP. Curr Biol 23: 229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang X, Milton CC, Poon CL, Hong W, et al. 2011. Wbp2 cooperates with Yorkie to drive tissue growth downstream of the Salvador‐Warts‐Hippo pathway. Cell Death Differ 18: 1346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan SW, Lim CJ, Huang C, Chong YF, et al. 2011. WW domain‐mediated interaction with Wbp2 is important for the oncogenic property of TAZ. Oncogene 30: 600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin Y, Xu J, Yin MX, Lu Y, et al. 2013. Brahma is essential for Drosophila intestinal stem cell proliferation and regulated by Hippo signaling. Elife 2: e00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L, Ren FF, Zhang Q, Chen YB, et al. 2008. The TEAD/TEF family of transcription factor scalloped mediates hippo signaling in organ size control. Dev Cell 14: 377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu S, Liu Y, Zheng YG, Dong JX, et al. 2008. The TEAD/TEF family protein scalloped mediates transcriptional output of the hippo growth‐regulatory pathway. Dev Cell 14: 388–98. [DOI] [PubMed] [Google Scholar]
- 17. Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, et al. 2010. The apical‐basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci USA 107: 15810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ling C, Zheng Y, Yin F, Yu J, et al. 2010. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci USA 107: 10532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silva E, Tsatskis Y, Gardano L, Tapon N, et al. 2006. The tumor‐suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol 16: 2081–9. [DOI] [PubMed] [Google Scholar]
- 20. Cho E, Feng Y, Rauskolb C, Maitra S, et al. 2006. Delineation of a fat tumor suppressor pathway. Nat Genet 38: 1142–50. [DOI] [PubMed] [Google Scholar]
- 21. Bennett FC, Harvey KF. 2006. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol 16: 2101–10. [DOI] [PubMed] [Google Scholar]
- 22. Willecke M, Hamaratoglu F, Kango‐Singh M, Udan R, et al. 2006. The fat cadherin acts through the hippo tumor‐suppressor pathway to regulate tissue size. Curr Biol 16: 2090–100. [DOI] [PubMed] [Google Scholar]
- 23. Rauskolb C, Sun S, Sun G, Pan Y, et al. 2014. Cytoskeletal tension inhibits Hippo signaling through an Ajuba‐Warts complex. Cell 158: 143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fletcher GC, Elbediwy A, Khanal I, Ribeiro PS, et al. 2015. The spectrin cytoskeleton regulates the Hippo signalling pathway. EMBO J 34: 940–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grusche FA, Richardson HE, Harvey KF. 2010. Upstream regulation of the hippo size control pathway. Curr Biol 20: R574–82. [DOI] [PubMed] [Google Scholar]
- 26. Hafezi Y, Bosch JA, Hariharan IK. 2012. Differences in levels of the transmembrane protein Crumbs can influence cell survival at clonal boundaries. Dev Biol 368: 358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grzeschik NA, Parsons LM, Allott ML, Harvey KF, et al. 2010. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol 20: 573–81. [DOI] [PubMed] [Google Scholar]
- 28. Robinson BS, Huang J, Hong Y, Moberg KH. 2010. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM‐domain protein Expanded. Curr Biol 20: 582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oh H, Irvine KD. 2009. In vivo analysis of Yorkie phosphorylation sites. Oncogene 28: 1916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun S, Reddy BV, Irvine KD. 2015. Localization of Hippo signalling complexes and Warts activation in vivo. Nat Commun 6: 8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brittle A, Thomas C, Strutt D. 2012. Planar polarity specification through asymmetric subcellular localization of Fat and Dachsous. Curr Biol 22: 907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hale R, Strutt D. 2015. Conservation of planar polarity pathway function across the animal kingdom. Annu Rev Genet 49: 529–51. [DOI] [PubMed] [Google Scholar]
- 33. Mao Y, Rauskolb C, Cho E, Hu WL, et al. 2006. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development 133: 2539–51. [DOI] [PubMed] [Google Scholar]
- 34. Rogulja D, Rauskolb C, Irvine KD. 2008. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell 15: 309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mao Y, Tournier AL, Bates PA, Gale JE, et al. 2011. Planar polarization of the atypical myosin Dachs orients cell divisions in Drosophila. Genes Dev 25: 131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bosveld F, Bonnet I, Guirao B, Tlili S, et al. 2012. Mechanical control of morphogenesis by Fat/Dachsous/Four‐jointed planar cell polarity pathway. Science 336: 724–7. [DOI] [PubMed] [Google Scholar]
- 37. Tyler DM, Baker NE. 2007. Expanded and fat regulate growth and differentiation in the Drosophila eye through multiple signaling pathways. Dev Biol 305: 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodrigues‐Campos M, Thompson BJ. 2014. The ubiquitin ligase FbxL7 regulates the Dachsous‐Fat‐Dachs system in Drosophila. Development 141: 4098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bosch JA, Sumabat TM, Hafezi Y, Pellock BJ, et al. 2014. The Drosophila F‐box protein Fbxl7 binds to the protocadherin fat and regulates Dachs localization and Hippo signaling. Elife 3: e03383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vrabioiu AM, Struhl G. 2015. Fat/Dachsous signaling promotes Drosophila wing growth by regulating the conformational state of the NDR kinase Warts. Dev Cell 35: 737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Degoutin JL, Milton CC, Yu E, Tipping M, et al. 2013. Riquiqui and minibrain are regulators of the hippo pathway downstream of Dachsous. Nat Cell Biol 15: 1176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Das Thakur M, Feng Y, Jagannathan R, Seppa MJ, et al. 2010. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr Biol 20: 657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rauskolb C, Pan G, Reddy BV, Oh H, et al. 2011. Zyxin links fat signaling to the hippo pathway. PLoS Biol 9: e1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gaspar P, Holder MV, Aerne BL, Janody F, et al. 2015. Zyxin antagonizes the FERM protein expanded to couple F‐actin and Yorkie‐dependent organ growth. Curr Biol 25: 679–89. [DOI] [PubMed] [Google Scholar]
- 45. Kwon HJ, Waghmare I, Verghese S, Singh A, et al. 2015. Drosophila C‐terminal Src kinase regulates growth via the Hippo signaling pathway. Dev Biol 397: 67–76. [DOI] [PubMed] [Google Scholar]
- 46. Fernandez BG, Jezowska B, Janody F. 2014. Drosophila actin‐capping protein limits JNK activation by the Src proto‐oncogene. Oncogene 33: 2027–39. [DOI] [PubMed] [Google Scholar]
- 47. Enomoto M, Igaki T. 2013. Src controls tumorigenesis via JNK‐dependent regulation of the Hippo pathway in Drosophila. EMBO Rep 14: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sun G, Irvine KD. 2013. Ajuba family proteins link JNK to Hippo signaling. Sci Signal 6: ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Elbediwy A, Vincent‐Mistiaen ZI, Spencer‐Dene B, Stone RK, et al. 2016. Integrin signalling regulates YAP/TAZ to control skin homeostasis. Development, in press, doi 10.1242/dev.133728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li P, Silvis MR, Honaker Y, Lien WH, et al. 2016. alphaE‐catenin inhibits a Src‐YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway. Genes Dev 30: 798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bulgakova NA, Klapholz B, Brown NH. 2012. Cell adhesion in Drosophila: versatility of cadherin and integrin complexes during development. Curr Opin Cell Biol 24: 702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lin G, Zhang X, Ren J, Pang Z, et al. 2013. Integrin signaling is required for maintenance and proliferation of intestinal stem cells in Drosophila. Dev Biol 377: 177–87. [DOI] [PubMed] [Google Scholar]
- 53. You J, Zhang Y, Li Z, Lou Z, et al. 2014. Drosophila perlecan regulates intestinal stem cell activity via cell‐matrix attachment. Stem Cell Rep 2: 761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cordero JB, Ridgway RA, Valeri N, Nixon C, et al. 2014. c‐Src drives intestinal regeneration and transformation. EMBO J 33: 1474–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhao B, Wei X, Li W, Udan RS, et al. 2007. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21: 2747–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dupont S, Morsut L, Aragona M, Enzo E, et al. 2011. Role of YAP/TAZ in mechanotransduction. Nature 474: 179–83. [DOI] [PubMed] [Google Scholar]
- 57. Hufnagel L, Teleman AA, Rouault H, Cohen SM, et al. 2007. On the mechanism of wing size determination in fly development. Proc Natl Acad Sci USA 104: 3835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aegerter‐Wilmsen T, Aegerter CM, Hafen E, Basler K. 2007. Model for the regulation of size in the wing imaginal disc of Drosophila. Mech Dev 124: 318–26. [DOI] [PubMed] [Google Scholar]
- 59. Aegerter‐Wilmsen T, Heimlicher MB, Smith AC, de Reuille PB, et al. 2012. Integrating force‐sensing and signaling pathways in a model for the regulation of wing imaginal disc size. Development 139: 3221–31. [DOI] [PubMed] [Google Scholar]
- 60. Mao Y, Tournier AL, Hoppe A, Kester L, et al. 2013. Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. EMBO J 32: 2790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Legoff L, Rouault H, Lecuit T. 2013. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development 140: 4051–9. [DOI] [PubMed] [Google Scholar]
- 62. Deng H, Wang W, Yu J, Zheng Y, et al. 2015. Spectrin regulates Hippo signaling by modulating cortical actomyosin activity. Elife 4: e06567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yao MX, Qiu W, Liu RC, Efremov AK, et al. 2014. Force‐dependent conformational switch of alpha‐catenin controls vinculin binding. Nature Commun 5: 4525. [DOI] [PubMed] [Google Scholar]
- 64. Grusche FA, Degoutin JL, Richardson HE, Harvey KF. 2011. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev Biol 350: 255–66. [DOI] [PubMed] [Google Scholar]
- 65. Liu B, Zheng Y, Yin F, Yu J, et al. 2016. Toll receptor‐mediated hippo signaling controls innate immunity in Drosophila. Cell 164: 406–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X. 2015. Crumbs3‐mediated polarity directs airway epithelial cell fate through the Hippo pathway effector Yap. Dev Cell 34: 283–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim NG, Gumbiner BM. 2015. Adhesion to fibronectin regulates Hippo signaling via the FAK‐Src‐PI3 K pathway. J Cell Biol 210: 503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Elbediwy A, Vincent‐Mistiaen ZI, Spencer‐Dene B, Stone RK, et al. 2016. Integrin signalling regulates YAP/TAZ to control skin homeostasis Development, in press, doi: 10.1242/dev.133728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dong J, Feldmann G, Huang J, Wu S, et al. 2007. Elucidation of a universal size‐control mechanism in Drosophila and mammals. Cell 130: 1120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Speicher T, Siegenthaler B, Bogorad RL, Ruppert R, et al. 2014. Knockdown and knockout of beta1‐integrin in hepatocytes impairs liver regeneration through inhibition of growth factor signalling. Nat Commun 5: 3862. [DOI] [PubMed] [Google Scholar]
- 71. Mao YP, Mulvaney J, Zakaria S, Yu TA, et al. 2011. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1‐Fat4 signaling during mammalian development. Development 138: 947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Saburi S, Hester I, Fischer E, Pontoglio M, et al. 2008. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–5. [DOI] [PubMed] [Google Scholar]
- 73. Cappello S, Gray MJ, Badouel C, Lange S, et al. 2013. Mutations in genes encoding the cadherin receptor‐ligand pair DCHS1 and FAT4 disrupt cerebral cortical development. Nat Genet 45: 1300+. [DOI] [PubMed] [Google Scholar]
- 74. Badouel C, Zander MA, Liscio N, Bagherie‐Lachidan M, et al. 2015. Fat1 interacts with Fat4 to regulate neural tube closure, neural progenitor proliferation and apical constriction during mouse brain development. Development 142: 2781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bagherie‐Lachidan M, Reginensi A, Pan Q, Zaveri HP, et al. 2015. Stromal Fat4 acts non‐autonomously with Dchs1/2 to restrict the nephron progenitor pool. Development 142: 2564– U50. [DOI] [PubMed] [Google Scholar]
- 76. Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, et al. 2011. Yap1 acts downstream of alpha‐catenin to control epidermal proliferation. Cell 144: 782–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Silvis MR, Kreger BT, Lien WH, Klezovitch O, et al. 2011. Alpha‐catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal 4: ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Calvo F, Ege N, Grande‐Garcia A, Hooper S, et al. 2013. Mechanotransduction and YAP‐dependent matrix remodelling is required for the generation and maintenance of cancer‐associated fibroblasts. Nat Cell Biol 15: 637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Miyamoto S, Akiyama SK, Yamada KM. 1995. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 267: 883–5. [DOI] [PubMed] [Google Scholar]
- 80. Kornberg L, Earp HS, Parsons JT, Schaller M, et al. 1992. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion‐associated tyrosine kinase. J Biol Chem 267: 23439–42. [PubMed] [Google Scholar]
- 81. Coyer SR, Singh A, Dumbauld DW, Calderwood DA, et al. 2012. Nanopatterning reveals an ECM area threshold for focal adhesion assembly and force transmission that is regulated by integrin activation and cytoskeleton tension. J Cell Sci 125: 5110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Humphrey JD, Dufresne ER, Schwartz MA. 2014. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15: 802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Legate KR, Wickstrom SA, Fassler R. 2009. Genetic and cell biological analysis of integrin outside‐in signaling. Genes Dev 23: 397–418. [DOI] [PubMed] [Google Scholar]
- 84. Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–89. [DOI] [PubMed] [Google Scholar]
- 85. Even‐Ram S, Artym V, Yamada KM. 2006. Matrix control of stem cell fate. Cell 126: 645–7. [DOI] [PubMed] [Google Scholar]
- 86. Ingber DE. 2006. Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol 50: 255–66. [DOI] [PubMed] [Google Scholar]
- 87. Horton ER, Astudillo P, Humphries MJ, Humphries JD. 2015. Mechanosensitivity of integrin adhesion complexes: role of the consensus adhesome. Exp Cell Res, in press, doi: 10.1016/j.yexcr.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 88. Horton ER, Byron A, Askari JA, Ng DH, et al. 2015. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol 17: 1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bottcher RT, Fassler R. 2014. Membrane tension drives ligand‐independent integrin signaling. EMBO J 33: 2439–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schiller HB, Fassler R. 2013. Mechanosensitivity and compositional dynamics of cell‐matrix adhesions. EMBO Rep 14: 509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schiller HB, Hermann MR, Polleux J, Vignaud T, et al. 2013. Beta1‐ and alphav‐class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin‐based microenvironments. Nat Cell Biol 15: 625–36. [DOI] [PubMed] [Google Scholar]
- 92. Fernandez‐Gonzalez R, Simoes Sde M, Roper JC, Eaton S, et al. 2009. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell 17: 736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wada KI, Itoga K, Okano T, Yonemura S, et al. 2011. Hippo pathway regulation by cell morphology and stress fibers. Development 138: 3907–14. [DOI] [PubMed] [Google Scholar]
- 94. Benham‐Pyle BW, Pruitt BL, Nelson WJ. 2015. Mechanical strain induces E‐cadherin‐dependent Yap1 and beta‐catenin activation to drive cell cycle entry. Science 348: 1024–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Das A, Fischer RS, Pan D, Waterman CM. 2016. YAP nuclear localization in the absence of cell‐cell contact is mediated by a filamentous actin‐dependent, myosin II‐ and phospho‐YAP‐independent pathway during extracellular matrix mechanosensing. J Biol Chem 291: 6096–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. McLachlan RW, Kraemer A, Helwani FM, Kovacs EM, et al. 2007. E‐cadherin adhesion activates c‐Src signaling at cell‐cell contacts. Mol Biol Cell 18: 3214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Samak G, Gangwar R, Crosby LM, Desai LP, et al. 2014. Cyclic stretch disrupts apical junctional complexes in Caco‐2 cell monolayers by a JNK‐2‐, c‐Src‐, and MLCK‐dependent mechanism. Am J Physiol‐Gastr L 306: G947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Giannone G, Sheetz MP. 2006. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol 16: 213–23. [DOI] [PubMed] [Google Scholar]
- 99. Wu Y, Kanchanawong P, Zaidel‐Bar R. 2015. Actin‐delimited adhesion‐independent clustering of E‐cadherin forms the nanoscale building blocks of adherens junctions. Dev Cell 32: 139–54. [DOI] [PubMed] [Google Scholar]
- 100. Guo Z, Neilson LJ, Zhong H, Murray PS, et al. 2014. E‐cadherin interactome complexity and robustness resolved by quantitative proteomics Sci Signal 7: rs7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yeatman TJ. 2004. A renaissance for SRC. Nat Rev Cancer 4: 470–80. [DOI] [PubMed] [Google Scholar]
- 102. Cui Y, Hameed FM, Yang B, Lee K, et al. 2015. Cyclic stretching of soft substrates induces spreading and growth. Nat Commun 6: 6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang JG, Miyazu M, Matsushita E, Sokabe M, et al. 2001. Uniaxial cyclic stretch induces focal adhesion kinase (FAK) tyrosine phosphorylation followed by mitogen‐activated protein kinase (MAPK) activation. Biochem Biophys Res Commun 288: 356–61. [DOI] [PubMed] [Google Scholar]
- 104. Margadant F, Chew LL, Hu X, Yu H, et al. 2011. Mechanotransduction in vivo by repeated talin stretch‐relaxation events depends upon vinculin. PLoS Biol 9: e1001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Delanoe‐Ayari H, Al Kurdi R, Vallade M, Gulino‐Debrac D, et al. 2004. Membrane and acto‐myosin tension promote clustering of adhesion proteins. Proc Natl Acad Sci USA 101: 2229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Camargo FD, Gokhale S, Johnnidis JB, Fu D, et al. 2007. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17: 2054–60. [DOI] [PubMed] [Google Scholar]
- 107. Cai J, Zhang N, Zheng Y, de Wilde RF, et al. 2010. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev 24: 2383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cai J, Maitra A, Anders RA, Taketo MM, et al. 2015. beta‐Catenin destruction complex‐independent regulation of Hippo‐YAP signaling by APC in intestinal tumorigenesis. Genes Dev 29: 1493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Azzolin L, Panciera T, Soligo S, Enzo E, et al. 2014. YAP/TAZ incorporation in the beta‐catenin destruction complex orchestrates the Wnt response. Cell 158: 157–70. [DOI] [PubMed] [Google Scholar]
- 110. Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, et al. 2015. Yap‐dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature 526: 715–8. [DOI] [PubMed] [Google Scholar]
- 111. Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, et al. 2015. A gp130‐Src‐YAP module links inflammation to epithelial regeneration. Nature 519: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang H, Pasolli HA, Fuchs E. 2011. Yes‐associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci USA 108: 2270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang N, Bai H, David KK, Dong J, et al. 2010. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell 19: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lee KP, Lee JH, Kim TS, Kim TH, et al. 2010. The Hippo‐Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA 107: 8248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yin F, Yu J, Zheng Y, Chen Q, et al. 2013. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 154: 1342–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhou D, Conrad C, Xia F, Park JS, et al. 2009. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16: 425–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Nishio M, Hamada K, Kawahara K, Sasaki M, et al. 2012. Cancer susceptibility and embryonic lethality in Mob1a/1b double‐mutant mice. J Clin Invest 122: 4505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Grijalva JL, Huizenga M, Mueller K, Rodriguez S, et al. 2014. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol 307: G196–204. [DOI] [PubMed] [Google Scholar]
- 119. Wang C, Zhang L, He Q, Feng X, et al. 2012. Differences in Yes‐associated protein and mRNA levels in regenerating liver and hepatocellular carcinoma. Mol Med Rep 5: 410–4. [DOI] [PubMed] [Google Scholar]
- 120. Imajo M, Miyatake K, Iimura A, Miyamoto A, et al. 2012. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta‐catenin signalling. EMBO J 31: 1109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Varelas X, Miller BW, Sopko R, Song S, et al. 2010. The Hippo pathway regulates Wnt/beta‐catenin signaling. Dev Cell 18: 579–91. [DOI] [PubMed] [Google Scholar]
- 122. Heallen T, Zhang M, Wang J, Bonilla‐Claudio M, et al. 2011. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332: 458–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tao J, Calvisi DF, Ranganathan S, Cigliano A, et al. 2014. Activation of beta‐catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology 147: 690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Barolo S, Posakony JW. 2002. Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev 16: 1167–81. [DOI] [PubMed] [Google Scholar]
- 125. Park HW, Kim YC, Yu B, Moroishi T, et al. 2015. Alternative Wnt signaling activates YAP/TAZ. Cell 162: 780–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Struhl G, Casal J, Lawrence PA. 2012. Dissecting the molecular bridges that mediate the function of Frizzled in planar cell polarity. Development 139: 3665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chen CM, Struhl G. 1999. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development 126: 5441–52. [DOI] [PubMed] [Google Scholar]
- 128. Ferrigno O, Lallemand F, Verrecchia F, L'Hoste S, et al.2002. Yes‐associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF‐beta/Smad signaling. Oncogene 21: 4879–84. [DOI] [PubMed] [Google Scholar]
- 129. Alarcon C, Zaromytidou AI, Xi Q, Gao S, et al. 2009. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF‐beta pathways. Cell 139: 757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Varelas X, Sakuma R, Samavarchi‐Tehrani P, Peerani R, et al. 2008. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem‐cell self‐renewal. Nat Cell Biol 10: 837–48. [DOI] [PubMed] [Google Scholar]
- 131. Varelas X, Samavarchi‐Tehrani P, Narimatsu M, Weiss A, et al. 2010. The Crumbs complex couples cell density sensing to Hippo‐dependent control of the TGF‐beta‐SMAD pathway. Dev Cell 19: 831–44. [DOI] [PubMed] [Google Scholar]
- 132. Nallet‐Staub F, Yin X, Gilbert C, Marsaud V, et al. 2015. Cell density sensing alters TGF‐beta signaling in a cell‐type‐specific manner, independent from Hippo pathway activation. Dev Cell 32: 640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Narimatsu M, Samavarchi‐Tehrani P, Varelas X, Wrana JL. 2015. Distinct polarity cues direct Taz/Yap and TGFbeta receptor localization to differentially control TGFbeta‐induced Smad signaling. Dev Cell 32: 652–6. [DOI] [PubMed] [Google Scholar]
- 134. Grannas K, Arngarden L, Lonn P, Mazurkiewicz M, et al. 2015. Crosstalk between Hippo and TGFbeta: subcellular localization of YAP/TAZ/Smad complexes. J Mol Biol 427: 3407–15. [DOI] [PubMed] [Google Scholar]
- 135. Hevia CF, de Celis JF. 2013. Activation and function of TGFbeta signalling during Drosophila wing development and its interactions with the BMP pathway. Dev Biol 377: 138–53. [DOI] [PubMed] [Google Scholar]
- 136. Yu FX, Zhao B, Panupinthu N, Jewell JL, et al. 2012. Regulation of the Hippo‐YAP pathway by G‐protein‐coupled receptor signaling. Cell 150: 780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Regue L, Mou F, Avruch J. 2013. G protein‐coupled receptors engage the mammalian Hippo pathway through F‐actin: F‐Actin, assembled in response to Galpha12/13 induced RhoA‐GTP, promotes dephosphorylation and activation of the YAP oncogene. BioEssays 35: 430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mo JS, Yu FX, Gong R, Brown JH, et al. 2012. Regulation of the Hippo‐YAP pathway by protease‐activated receptors (PARs). Genes Dev 26: 2138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yu FX, Zhang Y, Park HW, Jewell JL, et al. 2013. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev 27: 1223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, et al. 2014. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol 16: 357–66. [DOI] [PubMed] [Google Scholar]
- 141. Wang Z, Wu Y, Wang H, Zhang Y, et al. 2014. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci USA 111: E89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Porstmann T, Santos CR, Griffiths B, Cully M, et al. 2008. SREBP activity is regulated by mTORC1 and contributes to Akt‐dependent cell growth. Cell Metab 8: 224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kunte AS, Matthews KA, Rawson RB. 2006. Fatty acid auxotrophy in Drosophila larvae lacking SREBP. Cell Metab 3: 439–48. [DOI] [PubMed] [Google Scholar]
- 144. Fan R, Kim NG, Gumbiner BM. 2013. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3‐kinase and phosphoinositide‐dependent kinase‐1. Proc Natl Acad Sci USA 110: 2569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Strassburger K, Tiebe M, Pinna F, Breuhahn K, et al. 2012. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol 367: 187–96. [DOI] [PubMed] [Google Scholar]
- 146. Parker J, Struhl G. 2015. Scaling the Drosophila wing: TOR‐dependent target gene access by the Hippo pathway transducer Yorkie. PLoS Biol 13: e1002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhang J, Ji JY, Yu M, Overholtzer M, et al. 2009. YAP‐dependent induction of amphiregulin identifies a non‐cell‐autonomous component of the Hippo pathway. Nat Cell Biol 11: 1444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Han SX, Bai E, Jin GH, He CC, et al. 2014. Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J Immunol Res 2014: 261365. [DOI] [PMC free article] [PubMed] [Google Scholar]


