Abstract
Background
After potentially curative resection of primary colorectal cancer, patients may be monitored by measurement of carcinoembryonic antigen and/or CT to detect asymptomatic metastatic disease earlier.
Methods
A systematic review and meta-analysis was conducted to find evidence for the clinical effectiveness of monitoring in advancing the diagnosis of recurrence and its effect on survival. MEDLINE (Ovid), Embase, the Cochrane Library, Web of Science and other databases were searched for randomized comparisons of increased intensity monitoring compared with a contemporary standard policy after resection of primary colorectal cancer.
Results
There were 16 randomized comparisons, 11 with published survival data. More intensive monitoring advanced the diagnosis of recurrence by a median of 10 (i.q.r. 5–24) months. In ten of 11 studies the authors reported no demonstrable difference in overall survival. Seven RCTs, published from 1995 to 2016, randomly assigned 3325 patients to a monitoring protocol made more intensive by introducing new methods or increasing the frequency of existing follow-up protocols versus less invasive monitoring. No detectable difference in overall survival was associated with more intensive monitoring protocols (hazard ratio 0·98, 95 per cent c.i. 0·87 to 1·11).
Conclusion
Based on pooled data from randomized trials published from 1995 to 2016, the anticipated survival benefit from surgical treatment resulting from earlier detection of metastases has not been achieved.
Does it really help?
Introduction
A variety of monitoring strategies have been used in patients who have had potentially curative surgery for primary colorectal cancer. Their aim has been to detect active disease before it is symptomatic or clinically evident so that further treatment can be instigated. Five randomized trials published from 1995 to 1998 were the subject of a systematic review and meta-analysis published in 20021. Intensive follow-up was associated with significantly earlier detection by a mean of 8·5 months. The combined risk ratio was 0·81 (95 per cent c.i. 0·70 to 0·94) in favour of intensive follow-up. However, the authors found that methods were poorly reported and concluded that ‘large trials are required to identify which components of intensive follow up are most beneficial’. Since then, three large trials2–4 of intensified monitoring have reported. An updated search, systematic review and meta-analysis have been undertaken to examine the effect of these programmes on overall survival including all randomized studies identified.
Methods
A systematic review of literature on follow-up strategies for patients with colorectal cancer was conducted according to the PRISMA guidelines5 and is registered in PROSPERO (CRD42015026835). This study was based on predefined eligibility criteria and conducted according to a predefined methodological approach.
Search strategy
An extensive search for published articles was conducted in collaboration with a medical librarian. The electronic databases of MEDLINE (Ovid), Embase, the Cochrane Library and Web of Science, Scopus, CINAHL (EBSCO), PubMed publisher, Google Scholar, LILACS, SciELO and ProQuest were searched. The searches identified four index terms: large intestinal cancer, surgery, periodical surveillance and mortality or survival. Appropriate thesaurus terms (for MEDLINE, Embase and CINAHL) and keywords in the title and/or abstract were combined by Boolean logical operators, and adapted to the appropriate syntax of each database. The reference lists of reviews and included studies were cross-checked.
Selection of studies
Papers were screened by two independent investigators, arbitrated by a third reviewer. Data were extracted from studies reporting randomly assigned groups of patients in surveillance protocols of differing intensity. Only studies conducted in humans and written in English were included. Studies with inadequate data on survival for meta-analysis were retained for textual summaries of the design, findings and conclusions.
Outcome measures
The primary outcome was the overall survival difference between the existing monitoring strategy compared with a more intensive monitoring strategy.
Quality control
Studies were checked independently for quality using the Cochrane risk of bias tool6. The authors of all studies were approached for further information7.
Data extraction
Data were extracted by one researcher and checked independently by a second reviewer. A third investigator resolved any discrepancies. Patient numbers, baseline characteristics, all-cause mortality, cancer-specific mortality and recurrence rates were retrieved for each study.
Overall survival data were extracted as event rates reported for more versus less intensive monitoring arms of all randomized comparisons. Odds ratios (ORs) and their variances were calculated. Hazard ratios (HRs) were derived from Kaplan–Meier curves. The method described by Williamson and colleagues8 was used to estimate a logarithmic HR with corresponding variance when the number of patients at risk was given at each time point. If these data were not provided, the method of Parmar et al.9 was used. The overall HR with 95 per cent c.i. was estimated using an inverse variance-weighted average10.
Statistical analysis
Review Manager (RevMan) for Windows® version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark) was used for meta-analysis. Funnel plots were used to investigate publication bias. Heterogeneity among the included studies was analysed by means of the I2 measure11. A random-effects meta-analysis was performed after exclusion of trials with a high risk of bias.
ORs were also used to summarize observed effects, and a random-effects logistic regression model was used to provide an overall estimate of an effect for subsets of studies defined by the chosen method of enhanced detection. Subgroup analyses of outcome were performed to account for different diagnostic tests used during follow-up in different randomized trials. Studies were grouped as follows: any site of recurrence; endoscopically detected recurrence; or the clinical setting of follow-up. Sensitivity analyses were performed to identify studies that were estimated to have a high risk of bias.
Meta-analysis was undertaken and forest plots were constructed for all trials that reached the criteria for inclusion. Since the previous meta-analysis calling for large trials1, there have been three large multicentre trials, published in 2006, 2014 and 20162–4 relating to policies of earlier detection of patients suitable for the growing practice of metastasectomy12–14. The analysis was repeated in this subset of trials.
Results
Among 7081 publications, there were 22 relevant articles2–4,15–33 describing 16 randomized comparisons (Fig. 1). Text summaries of all 16 randomized trials are provided in Appendix S1 (supporting information). Five studies were excluded because there were no survival data available for analysis28,31 or they had high risk of bias18,21,23 (Table 1). The remaining 11 studies provided data on overall survival suitable for meta-analysis and, of these, seven2–4,16–17,22,30 included methods that allowed detection of metastases (Table 2, Figs 2 and 3). Two studies20,27 were confined to endoscopic examination following more or less intensive protocols (Table 3). Two studies24,25 followed the same protocol in each arm but were administered in hospital by a specialist or in a general practice setting.
Fig. 1.

Flow chart showing selection of trials for review
Table 1.
Studies excluded from meta-analysis
| Trial | Start | End | Tests | No. of centres | No. of patients randomized | Reason for exclusion |
|---|---|---|---|---|---|---|
| Barillari et al.18 | 1980 | 1990 | Colonoscopy | 1 | 212 | Inadequate survival data |
| Schoemaker et al.21 | 1984 | 1990 | CT | 1 | 325 | Potential lack of allocation concealment |
| Colonoscopy | ||||||
| Secco et al.23 | 1988 | 1996 | CEA | 1 | 337 | Reviewers could not reconcile conclusions with randomized groups |
| Colonoscopy | ||||||
| Ultrasonography | ||||||
| COLOFOL28 | 2006 | 2011 | CEA | 24 | 2571 | Results not yet published |
| CT | ||||||
| CEAwatch31 | 2010 | 2012 | CEA | 11 | 3223 | No outcome data reported |
CEA, carcinoembryonic antigen.
Table 2.
Details of the seven trials included in meta-analysis
| Trial | Start | End | Tests* | No. of centres | No. of patients randomized | Authors' conclusion |
|---|---|---|---|---|---|---|
| CEASL30 | 1982 | 1993 | CEA | 58 | 216† | ‘… highly unlikely that any survival advantage would be demonstrated for patients undergoing second-look surgery’ |
| Ohlsson et al.17 | 1983 | 1986 | Endoscopy | 2 | 107 | ‘Intense follow-up … did not prolong survival in this study’ |
| CT | ||||||
| CEA | ||||||
| Pietra et al.22 | 1987 | 1990 | CEA | 1 | 207 | ‘Our data support use of an intense follow-up plan after primary resection of large-bowel cancer, at least in patients with rectal cancer’ |
| Ultrasonography | ||||||
| CT | ||||||
| Chest X-ray | ||||||
| Colonoscopy | ||||||
| Mäkelä et al.16 | 1988 | 1990 | CEA | 1 | 106 | ‘Earlier detection of recurrent carcinoma by intensified follow-up does not lead to increased re-resectability or improved 5-year survival’ |
| Chest X-ray | ||||||
| CT | ||||||
| Rodriguez -Moranta et al.2 | 1997 | 2001 | CEA | 3 | 259 | ‘there was no difference in the probability of overall survival’ |
| Colonoscopy | ||||||
| CT | ||||||
| Ultrasonography | ||||||
| Chest X-ray | ||||||
| GILDA4 | 1998 | 2006 | CEA | 41 | 1228 | ‘early diagnosis of cancer recurrence is not associated with overall survival benefit’ |
| Colonoscopy | ||||||
| Chest X-ray | ||||||
| Ultrasonography | ||||||
| FACS3 | 2003 | 2009 | CEA | 39 | 1202 | ‘The number of deaths was not significantly different in the combined intensive monitoring groups vs the minimum follow-up group’ |
| CT |
There were more tests, more frequent tests, or both in the group with more intensive monitoring.
If the carcinoembryonic antigen (CEA) level was raised according to study criteria, patients were randomized to have this revealed to the clinical team or not.
Fig. 2.
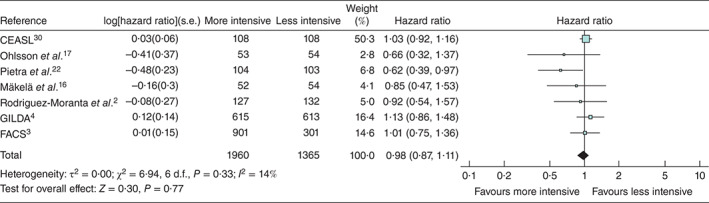
Forest plot showing hazard ratios for death in seven randomized comparisons of more and less intensive follow-up from which hazard ratios could be derived. An inverse-variance random-effects model was used to produce an overall estimated hazards ratio. Hazard ratios are shown with 95 per cent confidence intervals. The studies are ordered according to the year of the start of the inclusion. CEASL is dominant because the weight of the study is dependent on the follow-up time, number of events and number of patients in each treatment arm. The Kaplan–Meier curve in CEASL is plotted up to 25 years. The point estimate in favour of more intensive monitoring in studies by Rodriguez-Moranta et al.2 and Pietra and colleagues22 was attributed by the authors to detection by endoscopy and successful treatment of recurrent rectal carcinoma
Fig. 3.

Forest plot showing odds ratios for death in seven randomized comparisons of more and less intensive follow-up. A Mantel–Haenszel random-effects model was used to produce an overall estimated odds ratio. Odds ratios are shown with 95 per cent confidence intervals
Table 3.
Details of single-centre trials confined to endoscopic methods of monitoring
| Trial | Start | End | Tests | No. of patients | Authors' conclusions |
|---|---|---|---|---|---|
| Kjeldsen et al.20 | 1983 | 1994 | Colonoscopy | 597 | ‘no improvement in overall survival or in cancer-related survival’ |
| Wang et al.27 | 1995 | 2001 | Colonoscopy | 326 | There was higher detection of asymptomatic recurrence and more operations but the authors concluded that more intensive colonoscopy ‘did not improve overall survival’ |
Detailed protocols for investigations used for monitoring and their frequency are summarized in Appendix S2 (supporting information).
Quality of studies: risk of bias
Three studies were found to have a high risk of bias in at least one domain (Table 4). Blinding of participants and personnel (performance bias) was not possible and so there is a remaining risk of bias. Blinding of outcome assessment (detection bias) is not relevant for the main outcome measure, which is death/survival.
Table 4.
Risk of bias
| Reference | Randomization method | Allocation concealment | Incomplete outcome assessment | Selective reporting |
|---|---|---|---|---|
| CEASL30 | Low | Low | Low | Low |
| Ohlsson et al.17 | Unclear | Unclear | Low | Low |
| Pietra et al.22 | Unclear | Unclear | Low | Low |
| Rodriguez-Moranta et al.2 | Low | Low | Low | Low |
| Mäkelä et al.16 | Unclear | Unclear | Low | Low |
| GILDA4 | Unclear | Low | Low | Low |
| FACS3 | Unclear | Unclear | Low | Low |
| Kjeldsen et al.20 | Unclear | Unclear | High* | Low |
| Wang et al.27 | Unclear | Low | Unclear | Low |
| Wattchow et al.24 | Unclear | Low | Low | Low |
| Augestad et al.25 | Low | Low | Low | Low |
| Schoemaker et al.21 | Low | High† | Unclear | Low |
| Secco et al.23 | High‡ | Unclear | High§ | Low |
| CEAwatch31 | Low | Low | Low | Low |
Groups not balanced (290 : 307); drop-outs may not be included in assessment.
Cards not in envelopes; groups not balanced (167 : 158).
Method unclear; groups not balanced (108 : 84 and 84 : 61).
Patients excluded from survival rather than censored if lost to follow-up. COLOFOL has not reported so cannot be assessed; the methods of assignment were not described by Barillari et al.18, and this did not appear to be a true randomized comparison.
Effectiveness of more intensive monitoring in advancing detection
For studies in which the time difference in detection was given (9 of 16) the advance in diagnosis was 2–30 months, with a median of 10 (i.q.r. 5–24) months (Table 5).
Table 5.
Cancer recurrence rates and difference in time to detection in RCTs of monitoring strategies following potentially curative resection of colorectal cancer
| Recurrence (%) | Detection advance (months) | ||||
|---|---|---|---|---|---|
| Reference | Recruitment | Methods tested* | Intensive | Control | |
| CEASL30 | 1982–1993 | CEA | –† | –† | 11 |
| Ohlsson et al.17 | 1983–1986 | CEA, CT, endoscopy | 4 | ||
| Kjeldsen et al.20 | 1983–1984 | Endoscopy | 26 | 26 | 9 |
| Mäkelä et al.16 | 1988–1990 | CT | 42 | 39 | 5 |
| Wang et al.27 | 1995–2001 | Endoscopy | 8 | 11 | 13 |
| GILDA4 | 1998–2006 | CT, endoscopy, liver ultrasonography | 15 | 13 | 6 |
| Wattchow et al.24 | 1998–2001 | Setting: surgeon- or GP-led | 2 | ||
| FACS3 | |||||
| CEA | 2003–2009 | CEA | 22 | 14 | 24 |
| CT | 2004–2009 | CT | 20 | 14 | 30 |
| CEA and CT | 2005–2009 | CEA, CT | 17·5 | 14 | 24 |
Only tests that were not the same in the two groups.
Only a minority of patients meeting the stringent carcinoembryonic antigen (CEA) criteria were randomized, so detection was similar in both randomized groups and effectively 100 per cent.
Main outcome measure: effectiveness in improving survival
The numbers of randomized patients and the numbers of all detection events and deaths are given in Tables 6–8. The principal result derives from the meta-analysis of HRs based on trials from which these could be estimated. The summary HR estimate was 0·98 (95 per cent c.i. 0·87 to 1·11), with no evidence of significant heterogeneity (I2 = 14 per cent) (Fig. 2).
Table 6.
All-cause mortality rates in randomized trials
| All-cause mortality | ||
|---|---|---|
| Intensive follow-up | Less intensive follow-up | |
| CEASL30 | 91 of 108 (84·3) | 88 of 108 (81·5) |
| Mäkelä et al.16 | 23 of 52 (44) | 27 of 54 (50) |
| Ohlsson et al.17 | 15 of 53 (28) | 22 of 54 (41) |
| Kjeldsen et al.20 | 88 of 290 (30·3) | 100 of 307 (32·6) |
| Pietra et al.22 | 28 of 104 (26·9) | 43 of 103 (41·7) |
| Schoemaker et al.21 | 43 of 167 (25·7) | 55 of 158 (34·8) |
| Secco et al.23 | 73 of 192 (38·0) | 81 of 145 (55·9) |
| GILDA4 | 113 of 615 (18·4) | 105 of 613 (17·1) |
| Rodriguez-Moranta et al.2 | 21 of 127 (16·5) | 27 of 132 (20·5) |
| Wattchow et al.24 | 32 of 76 (42) | 25 of 81 (31) |
| Wang et al.27 | 42 of 165 (25·5) | 50 of 161 (31·1) |
| Augestad et al.25 | 1 of 55 (2) | 4 of 55 (7) |
| FACS3,34 | 164 of 901 (18·2) | 48 of 301 (15·9) |
| CEAwatch31 | n.r. | n.r. |
| COLOFOL28 | n.r. | n.r. |
| Total | 734 of 2905 (25·3) | 675 of 2272 (29·7) |
Values in parentheses are percentages. n.r., Not reported.
Table 7.
Local recurrence rates in randomized trials
| Local recurrence | ||
|---|---|---|
| Intensive follow-up | Less intensive follow-up | |
| CEASL30 | n.r. | n.r. |
| Mäkelä et al.16 | 12 of 52 (23) | 11 of 54 (20) |
| Ohlsson et al.17 | 11 of 53 (21) | 8 of 54 (15) |
| Kjeldsen et al.19,20 | 49 of 290 (16·9) | 42 of 307 (13·7) |
| Pietra et al.22 | 20 of 104 (19·2) | 26 of 103 (25·2) |
| Schoemaker et al.21 | 7 of 167 (4·2) | 11 of 158 (7·0) |
| Secco et al.23 | 41 of 192 (21·4) | 35 of 145 (24·1) |
| GILDA4 | 36 of 615 (5·9) | 32 of 613 (5·2) |
| Rodriguez-Moranta et al.2 | 11 of 127 (8·7) | 13 of 132 (10·1) |
| Wattchow et al.24 | n.r. | n.r. |
| Wang et al.27 | 10 of 165 (6·1) | 12 of 161 (7·5) |
| Augestad et al.25 | 6 of 55 (11) | 8 of 55 (15) |
| FACS3,34 | 35 of 901 (3·9) | 6 of 301 (2·0) |
| CEAwatch31 | 31 of 316 (9·8) | 13 of 1182 (1·1) |
| COLOFOL28 | n.r | n.r. |
| Total | 269 of 3037 (8·9) | 217 of 3265 (6·6) |
Values in parentheses are percentages. n.r., Not reported.
Table 8.
Distant recurrence rates in randomized trials
| Distant recurrence | ||
|---|---|---|
| Intensive follow-up | Less intensive follow-up | |
| CEASL30 | 32 of 108 (29·6) | n.r. |
| Mäkelä et al.16 | 10 of 52 (19) | 10 of 54 (19) |
| Ohlsson et al.17 | 9 of 53 (17) | 12 of 54 (22) |
| Kjeldsen et al.19,20 | 34 of 290 (11·7) | 48 of 307 (15·6) |
| Pietra et al.22 | 15 of 104 (14·4) | 21 of 103 (20·4) |
| Schoemaker et al.21 | n.r. | n.r. |
| Secco et al.23 | 38 of 192 (19·8) | 47 of 145 (32·4) |
| GILDA4 | 59 of 615 (9·6) | 42 of 613 (6·9) |
| Rodriguez-Moranta et al.2 | 20 of 127 (15·7) | 19 of 132 (14·4) |
| Wattchow et al.24 | n.r. | n.r. |
| Wang et al.27 | n.r. | n.r. |
| Augestad et al.25 | 3 of 55 (5) | 4 of 55 (7) |
| FACS3,34 | 39 of 901 (4·3) | 18 of 301 (6·0) |
| CEAwatch31 | n.r. | n.r. |
| COLOFOL28 | n.r. | n.r. |
| Total | 259 of 2497 (10·4) | 221 of 1764 (12·5) |
Values in parentheses are percentages. n.r., Not reported.
The meta-analysis of simple ORs for death based on the same seven studies is shown in Fig. 3; the summary OR for death was 0·91 (95 per cent c.i. 0·71 to 1·16). ORs were derived from the percentage of deaths in each arm at the time of reporting, whereas the HR gives an estimate of the overall relative survival, which is more relevant when considering a time-to-event endpoint.
A meta-analysis of ORs for death is also shown for two studies in which the difference in monitoring was confined to endoscopy, and two studies for which the difference was between a hospital/specialist setting and a general practice setting (Fig. 4).
Fig. 4.
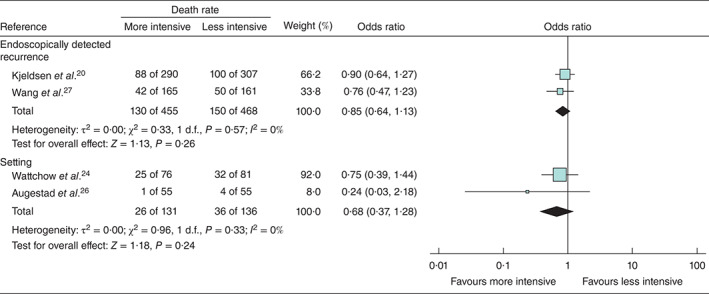
Forest plot for death in two studies in which the difference in monitoring was confined to endoscopy, and two studies for which the difference was between a hospital/specialist setting and a general practice setting. A Mantel–Haenszel random-effects model was used to produce an overall estimated odds ratio. Odds ratios are shown with 95 per cent confidence intervals
There is a residual possibility of publication bias as demonstrated in the asymmetry of the forest plot for the main analysis (Fig. 2) and the funnel plot (Fig. 5). Because of the proportion of the weight accredited by RevMan to CEASL (Carcino-Embryonic Antigen Second Look), and because in this study the monitoring was solely by CEA and not CT, a sensitivity analysis was performed after exclusion of CEASL. This did not alter the conclusion (HR 0·92, 0·76 to 1·36)
Fig. 5.

Funnel plot of studies included in meta-analysis. Reference numbers are shown
The three trials reporting from 2006 to 2016 were larger, multicentre, better quality studies and contributed 2689 (80·9 per cent) of the 3325 patients included in the overall meta-analysis (Fig. 2). The summary estimated HR from a meta-analysis of these trials was 1·05 (0·87 to 1·27) (Fig. 6).
Fig. 6.
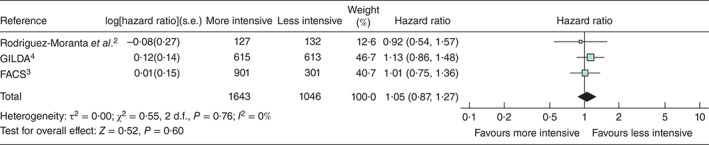
Subset analysis of three large multicentre RCTs published in 2006, 2014 and 2016, which included 80·9 per cent of all patients in the full systematic review. A random-effects inverse-variance model was used to produce an overall estimated hazards ratio. Hazard ratios are shown with 95 per cent confidence intervals
Discussion
The question addressed by this systematic review is whether follow-up strategies involving more intensive monitoring, with more frequent investigation and/or additional methods of detection, lead to an improvement in overall survival. Meta-analyses of the ORs and derived HRs from seven RCTs including 3325 patients showed no survival benefit from successively intensified monitoring policies. The authors of ten2–4,16–17,20,24–25,27,30 of the 11 trials reported no survival benefit from more intensive monitoring. This could have been due to lack of power, but the meta-analysis is consistent with the findings of individual studies. Three studies4,24,26 that reported outcomes on quality of life found no differences with respect to this outcome.
A random-effects model and, where possible, HRs were used to quantify outcomes, as the outcomes of interest occur over time. Owing to data limitations, ORs were used for some comparisons. Where it was possible to do both analyses, there was no difference in the conclusion. A limitation of this systematic review is that publication bias may have affected the observed outcomes as unpublished data, abstracts and presentations were not included. However, consideration of this possible bias would likely make a survival benefit even less plausible.
Intensive follow-up was reported to show significantly improved survival by the authors of only one study22. It was attributed to reappearance of treatable residual disease after resection of rectal (as opposed to colonic) cancer. In exploratory subset analyses, other studies2,4 showed a survival difference in favour of more intensive monitoring benefit where local recurrence of rectal cancer was found endoscopically.
Earlier meta-analyses1,35 suggested a favourable effect on survival. This was not found in the present meta-analysis, which included larger and methodologically more robust trials reported in the past couple of years. These showed poorer survival in the more intensively screened groups, although the results were not significant individually. The survival results of this updated meta-analysis show no benefit. Although not statistically significant, the point estimates consistently suggest an adverse effect on survival. What these later RCTs have in common is that they were multicentre studies run from trial centres. It is possible that the commitment of physicians involved in follow-up has made a difference to outcomes in smaller institutional studies. Many of these patients will have had individualized treatment including systemic chemotherapy, but no overall benefit from monitoring and earlier detection has been shown in the meta-analysis. This analysis gives a coherent and trustworthy, but disappointingly negative, message about the hoped-for survival benefit of intensification of active monitoring after primary resection of colorectal cancer.
Supplementary Material
Appendix S1. Text summaries of 16 randomized trials
Appendix S2. Spreadsheet of all monitoring methods (Excel spreadsheet)
Acknowledgements
The authors thank W. M. Bramer (Erasmus Medical Centre) for performing the search; and C. Russell (Chair of the Pulmonary Metastasectomy in Colorectal Cancer Trial Steering Committee) and C. Brew-Graves (leader of the Surgical and Interventional Trials Unit (SITU), University College London), who actively supported this work, some of which was carried out within the SITU. The following authors of papers reporting RCTs have corresponded with the review authors and agreed to be acknowledged: A. Castells (Rodriguez-Moranta et al.), C. Verberne (CEAwatch), D. Schoemaker (Schoemaker et al.), G. Rosati, R. Fossati (GILDA), K. M. Augestad (Augestad et al.), L. Roncorini (Pietra et al.) and P. A. Wille-Jørgensen (COLOFOL).
This study received no specific funding. F.F. is partly funded by the British Heart Foundation.
Disclosure: The authors declare no conflict of interest.
Supporting information
Additional supporting information may be found in the online version of this article:
Appendix S1 Text summaries of 16 randomized trials (Word document)
Appendix S2 Spreadsheet of all monitoring methods (Excel spreadsheet)
Editor's comment


References
- 1. Renehan AG, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 2002; 324: 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodriguez-Moranta F, Saló J, Arcusa A, Boadas J, Piñol V, Bessa Xet al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol 2006; 24: 386–393. [DOI] [PubMed] [Google Scholar]
- 3. Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill Aet al. FACS Trial Investigators. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA 2014; 311: 263–270. [DOI] [PubMed] [Google Scholar]
- 4. Rosati G, Ambrosini G, Barni S, Andreoni B, Corradini G, Luchena Get al. ; GILDA working group. A randomized trial of intensive versus minimal surveillance of patients with resected Dukes B2-C colorectal carcinoma. Ann Oncol 2016; 27: 274–280. [DOI] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman ADet al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roberts I, Ker K, Edwards P, Beecher D, Manno D, Sydenham E. The knowledge system underpinning healthcare is not fit for purpose and must change. BMJ 2015; 350: h2463. [DOI] [PubMed] [Google Scholar]
- 8. Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002; 21: 3337–3351. [DOI] [PubMed] [Google Scholar]
- 9. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 10. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barton MK. Metastasectomy on the rise across several cancer types. CA Cancer J Clin 2015; 65: 163–164. [DOI] [PubMed] [Google Scholar]
- 13. Bartlett EK, Simmons KD, Wachtel H, Roses RE, Fraker DL, Kelz RRet al. The rise in metastasectomy across cancer types over the past decade. Cancer 2015; 121: 747–757. [DOI] [PubMed] [Google Scholar]
- 14. Jawed I, Wilkerson J, Prasad V, Duffy AG, Fojo T. Colorectal cancer survival gains and novel treatment regimens: a systematic review and analysis. JAMA Oncol 2015; 1: 787–795. [DOI] [PubMed] [Google Scholar]
- 15. Makela J, Laitinen S, Kairaluoma MI. Early results of follow-up after radical resection for colorectal cancer. Preliminary results of a prospective randomized trial. Surg Oncol 1992; 1: 157–161. [DOI] [PubMed] [Google Scholar]
- 16. Mäkelä JT, Laitinen SO, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer. Results of a prospective randomized trial. Arch Surg 1995; 130: 1062–1067. [DOI] [PubMed] [Google Scholar]
- 17. Ohlsson B, Breland U, Ekberg H, Graffner H, Tranberg KG. Follow-up after curative surgery for colorectal carcinoma. Randomized comparison with no follow-up. Dis Colon Rectum 1995; 38: 619–626. [DOI] [PubMed] [Google Scholar]
- 18. Barillari P, Ramacciato G, Manetti G, Bovino A, Sammartino P, Stipa V. Surveillance of colorectal cancer: effectiveness of early detection of intraluminal recurrences on prognosis and survival of patients treated for cure. Dis Colon Rectum 1996; 39: 388–393. [DOI] [PubMed] [Google Scholar]
- 19. Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. The pattern of recurrent colorectal cancer in a prospective randomised study and the characteristics of diagnostic tests. Int J Colorectal Dis 1997; 12: 329–334. [DOI] [PubMed] [Google Scholar]
- 20. Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg 1997; 84: 666–669. [PubMed] [Google Scholar]
- 21. Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology 1998; 114: 7–14. [DOI] [PubMed] [Google Scholar]
- 22. Pietra N, Sarli L, Costi R, Ouchemi C, Grattarola M, Peracchia A. Role of follow-up in management of local recurrences of colorectal cancer: a prospective, randomized study. Dis Colon Rectum 1998; 41: 1127–1133. [DOI] [PubMed] [Google Scholar]
- 23. Secco GB, Fardelli R, Gianquinto D, Bonfante P, Baldi E, Ravera Get al. Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. Eur J Surg Oncol 2002; 28: 418–423. [DOI] [PubMed] [Google Scholar]
- 24. Wattchow DA, Weller DP, Esterman A, Pilotto LS, McGorm K, Hammett Z. General practice vs surgical-based follow-up for patients with colon cancer: randomised controlled trial. Br J Cancer 2006; 94: 1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Augestad KM, Vonen B, Aspevik R, Nestvold T, Ringberg U, Johnsen R. Should the surgeon or the general practitioner (GP) follow up patients after surgery for colon cancer? A randomized controlled trial protocol focusing on quality of life, cost-effectiveness and serious clinical events. BMC Health Serv Res 2008; 8: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Augestad KM, Norum J, Dehof S, Aspevik R, Ringberg U, Nestvold Tet al. Cost-effectiveness and quality of life in surgeon versus general practitioner-organised colon cancer surveillance: a randomised controlled trial. BMJ Open 2013; 3: pii: e002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang T, Cui Y, Huang WS, Deng YH, Gong W, Li CJ. The role of postoperative colonoscopic surveillance after radical surgery for colorectal cancer: a prospective, randomized clinical study. Gastrointest Endosc 2009; 69: 609–615. [DOI] [PubMed] [Google Scholar]
- 28. Wille-Jørgensen P, Laurberg S, Påhlman L, Carriquiry L, Lundqvist N, Smedh Ket al. An interim analysis of recruitment to the COLOFOL trial. Colorectal Dis 2009; 11: 756–758. [DOI] [PubMed] [Google Scholar]
- 29. Hansdotter Andersson P, Wille-Jørgensen P, Horváth-Puhó E, Petersen SH, Martling A, Sørensen HTet al. The COLOFOL trial: study design and comparison of the study population with the source cancer population. Clin Epidemiol 2016; 8: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Treasure T, Monson K, Fiorentino F, Russell C. The CEA Second-Look Trial: a randomised controlled trial of carcinoembryonic antigen prompted reoperation for recurrent colorectal cancer. BMJ Open 2014; 4: e004385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verberne CJ, Zhan Z, van den Heuvel E, Grossmann I, Doornbos PM, Havenga Ket al. Intensified follow-up in colorectal cancer patients using frequent carcino-embryonic antigen (CEA) measurements and CEA-triggered imaging: results of the randomized ‘CEAwatch’ trial. Eur J Surg Oncol 2015; 41: 1188–1196. [DOI] [PubMed] [Google Scholar]
- 32. Verberne CJ, Wiggers T, Grossmann I, de Bock GH, Vermeulen KM. Cost-effectiveness of a carcinoembryonic antigen (CEA)-based follow-up programme for colorectal cancer (the CEA Watch trial). Colorectal Dis 2016; 18: O91–O96. [DOI] [PubMed] [Google Scholar]
- 33. Grossmann EM, Johnson FE, Virgo KS, Longo WE, Fossati R. Follow-up of colorectal cancer patients after resection with curative intent – the GILDA trial. Surg Oncol 2004; 13: 119–124. [DOI] [PubMed] [Google Scholar]
- 34. Pugh SA, Shinkins B, Fuller A, Mellor J, Mant D, Primrose JN. Site and stage of colorectal cancer influence the likelihood and distribution of disease recurrence and postrecurrence survival: data from the FACS randomized controlled trial. Ann Surg 2016; 263: 1143–1147. [DOI] [PubMed] [Google Scholar]
- 35. Jeffery GM, Hickey BE, Hider P. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev 2002; (1)CD002200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Text summaries of 16 randomized trials
Appendix S2. Spreadsheet of all monitoring methods (Excel spreadsheet)


