Abstract
Background
The management of congenital cystic lung lesions is controversial. Arguments for routine resection during infancy include the possibility of the lesion being Type I pleuropulmonary blastoma (PPB) rather than a cystic congenital pulmonary airway malformation (CPAM). We aimed to identify clinical and radiological features that might distinguish between CPAM and PPB and to develop a diagnostic algorithm based on these features.
Methods
All recorded cases of Type I PPB were retrieved from the International PPB Registry and compared with an institutional cohort of children undergoing resection of CPAM (2002–2013) that was noted at some stage to be at least partially cystic. Regression models were created to identify variables that might differentiate CPAM from PPB. Odds ratio (OR) and positive predictive value (PPV) were calculated for each variable and a decision algorithm developed.
Results
In 112 cases of Type I PPB and 103 of CPAM, factors favoring a diagnosis of CPAM included prenatal detection (OR 89.4), systemic feeding vessel (OR 61.7), asymptomatic (OR 8.0), and hyperinflated lung (OR 6.6). Factors favoring a diagnosis of PPB included bilateral or multisegment involvement (OR 2.4). A decision algorithm that helps to identify lesions requiring resection and those which can be safely observed is presented.
Conclusion
Clinical and radiological features can help to differentiate between CPAM and PPB. Our algorithm allows identification of children at higher risk of PPB in whom we would recommend resection and those at low risk in whom continued close observation is safe.
Keywords: Congenital lung lesion, Pleuropulmonary blastoma, Congenital cystic adenomatoid malformation, Bronchopulmonary sequestration, DICER1, Thoracic surgery
Congenital pulmonary airway malformations (CPAMs) encompass a varied group of disorders that include congenital cystic adenomatoid malformation (CCAM), bronchopulmonary sequestration (BPS), congenital lobar emphysema, and bronchogenic cyst among other entities [1]. Congenital lung abnormalities are increasingly detected in the prenatal period owing to widespread use of imaging [2,3] and improving sonographic resolution. A pathological classification system was proposed by Stocker et al. and later expanded to include types 0 to 4 [4–6]. However, there is often discrepancy between the type of CPAM based on imaging and the ultimate pathological diagnosis after resection [7–9].
There is general agreement that a symptomatic CPAM should be resected, usually soon after birth. However, the appropriate management of asymptomatic CPAMs is controversial. The risks of resection must be weighed against the risks of expectant management. The main argument in favor of routine resection during infancy is the long-term risk of infection, although there is disagreement in the literature about the magnitude of that risk. Some authors also argue that the surgical risk is higher if the lesion has already been infected [3,10,11], although not all studies have documented this difference [12]. Another argument in favor of routine resection is the assumption that early resection results in better compensatory lung growth than resection at a later age; however this remains a hypothetical advantage which is not universally supported in long-term follow-up studies [12,13].
A final argument for routine resection is the possibility that the lesion is not a benign CPAM, but is instead a Type I pleuropulmonary blastoma (PPB). There are three pathologic subtypes of PPB: Type I/Ir, purely cystic; Type II, combined cystic solid; Type III, purely solid. Five-year survival rates for Types I/Ir, II and III PPBs are 91%, 71% and 53%, respectively [14]. Several studies have suggested that these two entities can be indistinguishable based on imaging [7,9], and there is overlap between Type 4 CCAM and Type I PPB on pathology [7]. In our experience, many families of children with an asymptomatic lung lesion opt for surgery owing to the concern that the lesion may be an unrecognized PPB and this appears to be a key factor in surgical decision making. This study was designed to determine if there are clinical or radiological features that might imply higher or lower risk of an individual lesion being CPAM or PPB. Ultimately our goal was to create a decision support tool that could reliably predict that a patient presenting with a CPAM did not have a PPB based on presence or absence of certain clinical and radiological features. We sought a tool that would be of genuine clinical use rather than just providing statistical associations.
1. Methods
The study protocol was approved by the Research Ethics Board of The Hospital for Sick Children, Toronto (Ref: 1000040806) and the Institutional Review Board of Children’s Hospitals and Clinics of Minnesota (Ref: 1312–120).
1.1. Data sources
Data were retrieved from the International Pleuropulmonary Blastoma Registry (IPPBR) on all recorded cases of Type I (including both Type I and Type Ir) PPB, with central review by the IPPBR pathologists. The IPPBR is a voluntary database established in 1988 that to date has collected diagnosis, treatment, and outcome data on more than 450 PPB cases from physicians and patients worldwide [15]. A comparison cohort comprised all children undergoing resection of a CPAM at The Hospital for Sick Children, Toronto between January 1st 2002 and December 31st 2013 that was noted at some stage to be at least partially cystic. We chose to focus on cystic lesions because these are the ones that can be difficult to distinguish from Type I PPB [8]. The final diagnosis for CPAMs was based on the surgical pathology report from The Hospital for Sick Children. Patients were excluded if they had noncystic malformations, such as congenital lobar emphysema, pure BPS with no cystic component, bronchiectasis or bronchial atresia/focal hyperinflation without cystic change. Only children who had undergone resection were included so as to have a final pathological diagnosis for all cases. The management policy at our institution during the study period did not change significantly over time and was for lesions to undergo resection if they were symptomatic or if there was a joint decision to proceed with resection following an open discussion between parents and surgeon with full disclosure of risks and benefits of the procedure. Data within both groups were collected and analyzed retrospectively.
1.2. Clinical and radiographic features
We selected variables that we felt might help to distinguish between a final diagnosis of CPAM and PPB. Clinical variables of interest included gender, whether the lesion was diagnosed prenatally, age at radiological diagnosis (if postnatal), presence of symptoms, presence of other conditions in the same patient, family history of malignancy, and DICER1 gene mutation status, if available.
Radiology reports from chest X-rays and computed tomography (CT) scans performed either at initial presentation for postnatally diagnosed lesions or during work-up or during a period of observation for prenatally diagnosed lesions were analyzed. The following information was recorded from chest X-rays: presence of cystic lesions, hyperinflation, mediastinal shift, pleural effusion, pneumothorax, involvement of more than one lobe on one side and bilateral abnormalities. Additional information recorded from CT scans included largest cyst size, the nature of the cysts (simple vs complex — septated or containing solid components), presence of pleural nodules, and the presence of a systemic vascular supply.
1.3. Statistical analysis
Continuous variables were compared between groups using Mann–Whitney test. Univariate and multivariate logistic regression models were created to identify variables that might differentiate cases with a final diagnosis of CPAM from those with PPB. For multivariate models all variables were initially included on an exploratory basis; subsequent models were generated using a forward stepwise method with variables entered if p < 0.1 on univariate analysis and remaining in the final model if p < 0.05. The diagnostic accuracy of the multivariate models was assessed by the percentage of cases correctly diagnosed as either CPAM or PPB. Odds ratios (OR) were generated and test parameters (positive predictive value [PPV], sensitivity and specificity) calculated for each variable. Based on the strengths of univariate associations and test parameters (in particular PPV) a decision algorithm was developed to guide future management of these cases. Statistical analysis was performed using InStat v3.10 (GraphPad Software) and SPSS v22 (IBM Corporation). p < 0.05 was considered to be statistically significant. Owing to the retrospective nature of this analysis, there were some missing data points. Missing data were assumed to be missing completely at random and cases with missing data were excluded from univariate and multivariate analyses. Variables with missing data points are indicated in Table 1, with denominators demonstrating how many data points were present.
Table 1.
Results of univariate analyses of features associated with final diagnosis (all cases included).
| CPAM (n = 103) | PPB (n = 112) | OR for CPAM vs PPB | PPV for CPAM | Sensitivity | Specificity | p | |
|---|---|---|---|---|---|---|---|
| Male | 54/103 | 66/112 | 0.8 (0.4–1.3) | 45% | 0.52 | 0.41 | 0.4 |
| Age at presentation in lesions not detected prenatally (months) | Median 48 (IQR 1–72) | Median 11.5 (IQR 4–24) | – | – | – | – | 0.1b |
| Prenatal detection | 86/103 | 6/112 | 89.4 (33.8–236.6) | 93% | 0.83 | 0.95 | <0.0001 |
| Absence of symptoms | 70/103 | 23/110 | 8.0 (4.3–14.9) | 75% | 0.70 | 0.79 | <0.0001 |
| Hyperinflated regiona | 39/98 | 9/111 | 6.6 (3.0–14.5) | 81% | 0.40 | 0.92 | <0.0001 |
| Absence of pneumothoraxa | 8/97 | 27/111 | 3.6 (1.5–8.3) | 51% | 0.92 | 0.24 | 0.003 |
| Unilateral (vs bilateral) abnormalitya | 94/98 | 94/111 | 4.3 (1.4–13.1) | 50% | 0.96 | 0.15 | 0.01 |
| Unilobar (vs multilobar) abnormality on one sidea | 90/98 | 93/111 | 2.2 (0.9–5.3) | 49% | 0.92 | 0.16 | 0.1 |
| Solid component on CT | 30/96 | 5/90 | 7.7 (2.8–21.0) | 86% | 0.31 | 0.94 | <0.0001 |
| Systemic feeding vessel on CT | 24/97 | 0/92 | 61.7 (3.7–1031.8) | 100% | 0.25 | 1.0 | <0.0001 |
| No mediastinal shifta | 69/97 | 77/111 | 1.1 (0.6–2.0) | 47% | 0.71 | 0.31 | 0.9 |
| Multilocular cyst on CT | 67/88 | 73/89 | 0.7 (0.3–1.5) | 48% | 0.76 | 0.18 | 0.4 |
| Simple (vs complex) cyst on CT | 68/82 | 22/89 | 14.8 (7.0–31.3) | 75% | 0.83 | 0.75 | <0.0001 |
| Size of largest cyst (mm) | Median 18 (IQR 12.5–40); n = 63 | Median 50 (IQR 33–70); n = 57 | – | – | – | – | <0.0001b |
| DICER1 gene mutation negative | 1/1 | 8/24 | 5.8 (0.2–158.9) | 11% | 1.0 | 0.66 | 0.36 |
Denominators for each data point are shown to account for missing data; IQR — interquartile range; PPV— positive predictive value.
On X-ray or CT.
Mann–Whitney test.
2. Results
A total of 112 cases of Type I PPB were identified from the IPPBR and all were included. All were confirmed by central pathology review by the IPPBR pathologists. Demographic and radiological features are shown in Table 1. In 6 (5.36%) a pulmonary abnormality was detected prenatally and in 5 of these the provisional prenatal diagnosis was CCAM. The majority therefore presented postnatally with respiratory symptoms at a median age of 11.5 months. There was evidence of a different type of tumor (i.e. not a PPB) at a different site in 16 children who had a PPB. Owing to the nature of this study it was not possible to determine whether the associated tumor was evident prior to or after the diagnosis of PPB. The most frequent of these associated tumors was a cystic nephroma (n = 8) and in all cases these were tumors that have been associated with the PPB familial tumor syndrome [16] and often with DICER1 germline mutations. A CT scan report was available in 92 cases (81.25%); all were abnormal. The most frequent abnormal finding was the presence of a cystic lesion (97.8% of cases). Diameter of the largest cyst was available in 57 cases with a median of 50 mm (range 6–150). Fifteen patients had more than one CT scan with growth over time seen in 8 and no growth in the remaining 7. No cases had a systemic feeding vessel or evidence of pleural nodules. The median duration of follow-up was 42 months during which time 16 patients (10.7%) had a recurrence of their PPB. Ten of these recurrences were local with the remainder being either in the contralateral lung or bilateral. At the time of recurrence 11 tumors showed evidence of pathological progression from Type I PPB at first resection to Type II, Type III, or Type II/III PPB.
There were 114 children who underwent resection of a CPAM at The Hospital for Sick Children between 2002 and 2013. Eleven patients were excluded because they had lesions that were not documented to be cystic at any stage leaving 103 patients in the comparison cohort. Demographic and radiological features are shown in Table 1. None of the patients had a recorded family history of CPAM but it is likely that this information was not solicited in the majority of cases. The majority were detected prenatally and were asymptomatic at the time of resection. For the remaining patients, the median age at diagnosis was 48 months. CT scan reports were available for 97 cases (94%), all of which were abnormal, including cystic lesions in 82 cases, evidence of a systemic arterial supply in 24 cases and no case with pleural nodules. Largest cyst dimension was reported in 63 cases and was median 18 mm (range 2–91). The majority of children had only one CT scan (n = 74); 23 had more than one scan in which growth over time was demonstrated in 7 and no change in 16. The final pathologic diagnosis was CCAM (including hybrid lesions comprising elements of CCAM and sequestration) in 93 patients (90.3%), and the remainder BPS with cystic change (n = 4), bronchogenic cyst (n = 3), bronchial atresia (n = 1) and pulmonary cyst (n = 2). Median duration of follow-up was 4 months after surgery.
2.1. Features associated with final diagnosis
All 215 cases were included in the analysis. Univariate analysis was performed to compare features associated with a final diagnosis of CPAM and PPB. The results are shown in Table 1. Features most strongly (OR > 10) associated with a CPAM were prenatal diagnosis, the presence of a systemic feeding vessel on CT and the presence of a simple as opposed to a complex cyst on CT. While size of the largest cyst was significantly larger in cases of PPB, there was no specific cutoff in cyst size that was useful in distinguishing between PPB and CPAM.
In multivariate analysis no model could be generated that significantly improved on the diagnostic accuracy of any univariate model. A model that included prenatal detection alone resulted in an accurate final diagnosis in 89.3% of cases. The best multivariate model (which included prenatal detection, presence of a simple vs complex cyst, presence of a systemic feeding vessel and presence of a hyperinflation region on CXR or CT) resulted in an accurate final diagnosis in 90.5%, an increase of just 1.2%. Of note DICER1 status was not included in multivariate models as DICER1 status was known in only 1 case (<1%) of CPAM and 24 cases of PPB (21%).
Since symptomatic pulmonary lesions are generally resected the decision of whether to resect or not is less relevant for symptomatic lesions. We therefore repeated the analyses for asymptomatic lesions only. The results of univariate analyses for asymptomatic lesions only are shown in Table 2. Once again no multivariate model could improve on the diagnostic accuracy of the best univariate predictor of final diagnosis, i.e. the presence of a prenatal diagnosis.
Table 2.
Results of univariate analyses of features associated with final diagnosis for asymptomatic cases only (n = 93).
| OR for CPAM vs PPB | p | |
|---|---|---|
| Prenatal detection | 149.5 (27.1–824.8) | <0.0001 |
| Hyperinflated regiona | 42.3 (2.5–724.5) | <0.0001 |
| Unilateral (vs bilateral) abnormalitya | 7.1 (1.6–31.3) | 0.009 |
| Unilobar (vs multilobar) abnormality on one sidea | 6.2 (1.8–21.5) | 0.004 |
| Systemic feeding vessel on CT | 18.3 (1.06–317.4) | 0.003 |
| Solid component on CT | 23.3 (1.4–401.9) | 0.001 |
| No mediastinal shifta | 0.6 (0.2–2.3) | 0.54 |
| Multilocular cyst on CT | 1.9 (07–5.7) | 0.26 |
| Simple (vs complex) cyst on CT | 74.4 (8.7–636.9) | <0.0001 |
On either CT or CXR.
2.2. Algorithm creation
Based on the individual statistical parameters for each variable and by combining relevant variables (e.g. high risk imaging findings) we generated a treatment algorithm based on this dataset to inform the decision of which lesions require resection and those which can be safely managed with close clinical and radiological observation. This algorithm is shown in Fig. 1. We have included the numbers of cases used at each stage based on the existing dataset. Some steps included in the algorithm were not of particular benefit in distinguishing between CPAM and PPB in our dataset but have been included owing to their high positive predictive values. These include DICER1 gene mutation status which was only positive in cases of PPB (PPV = 100%) but was only tested in 25 cases overall. Similarly the presence of a systemic feeding vessel on CT scan was never seen in a case of PPB (i.e. PPV = 100% for CPAM) and is therefore included although it was not a strictly necessary step in the algorithm based on our dataset as all cases with a systemic feeding vessel were also detected prenatally. We have included them however in an effort to ‘future proof’ the algorithm. The algorithm correctly identifies 96.7% of cases (208/215), is unable to distinguish between 6 cases and erroneously identifies one case of PPB as a CPAM. This particularly interesting case has previously been reported as a case report [17]. In this case, resection was performed early in life (5 months of age) and it is possible that had a longer period of observation ensued then other features suggestive of a diagnosis of PPB (and in particular, symptoms) may have developed.
Fig. 1.
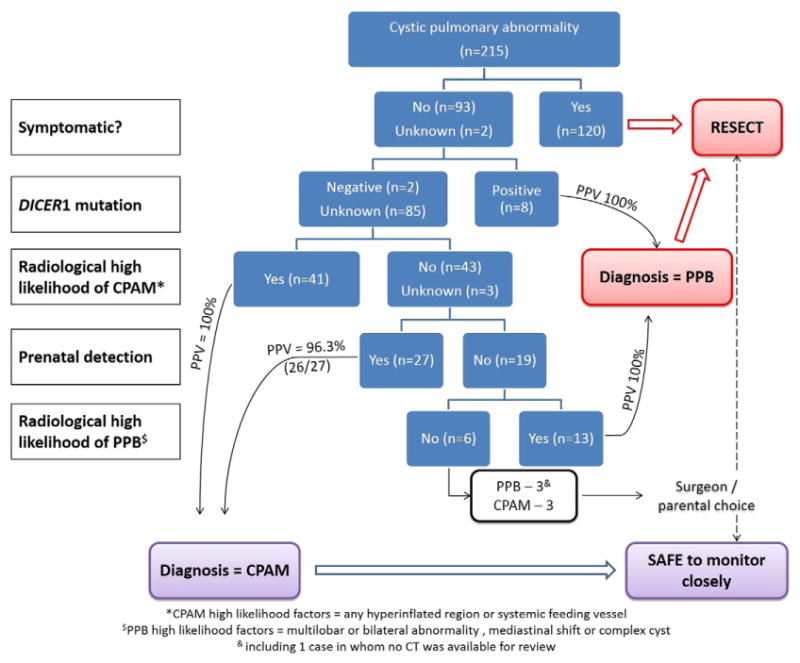
Proposed algorithm for the management of cystic pulmonary abnormalities.
3. Discussion
The decision whether to resect or observe an asymptomatic cystic lung lesion is controversial. One of the main arguments in favor of routine resection is the chance that the lesion may be a PPB rather than a CPAM, and that these two entities may be difficult or impossible to differentiate from each other based on imaging [6,7,9,18]. However, PPB is an extremely rare condition, which occurs with an incidence that is several orders of magnitude lower than CPAM, and previous studies have therefore been limited by the small number of PPBs used for comparison [6]. We addressed this limitation by utilizing the IPPBR database, which contains a large number of cases collected from all over the world. We were able to compare the clinical and radiological features of Type I PPB and CPAM with more than 100 cases in each group, and to develop an algorithm which we hope will provide guidance to clinicians and families when deciding whether to treat an asymptomatic cystic lung lesion with resection or observation.
Our data suggested that there are a number of clinical and radiological features that are predictive for PPB, including: symptoms (particularly pneumothorax), bilateral or multisegment involvement, the presence of a complex cyst and a germline mutation in the DICER1 gene, found in two thirds of PPB [6,14,19]. In contrast, the features that were predictive for CPAM included prenatal diagnosis, systemic feeding vessel, and hyperinflated lung. We made an attempt to use items with a positive or negative predictive value of close to 100% to determine decision points in the algorithm, with the goal being to avoid expectant management for children who did not have a high likelihood of having a CPAM.
Using the algorithm we have generated, patients can be stratified into those that have a very low risk of PPB, those that have a high risk of PPB and those that may have PPB. In our algorithm, only a small proportion (6 out of 215) of patients were in the intermediate group. We acknowledge one prenatally diagnosed, asymptomatic case of PPB in the IPPBR (published as a case report [17]) which does not have other features that make it high risk for PPB. For this reason the algorithm recommends expectant management with frequent careful follow-up of prenatally diagnosed asymptomatic lesions with no other high-risk features. Growth of the lesion or the development of any high-risk features, should prompt resection.
Our algorithm is derived from hard data and provides a more detailed description of patients that may be observed. Our algorithm also includes DICER1 germline gene mutation owing to its close association with PPB [14,16,19,20]. Routine germline DICER1 testing may be useful in all patients with CPAMs who are considering nonoperative management to further inform their risk of having a PPB. Previous reports have also suggested that asymptomatic patients who do not have a family history of PPB/DICER1 familial syndrome and do not have multifocal or bilateral lesions may be safely observed [6,21].
This study has a number of limitations. Firstly, the diagnosis of PPB and CPAM may be dependent on the skill, experience and bias of the pathologist. We have attempted to address this limitation by using cases from the IPPBR, which have all undergone central pathology review by pathologists who are expert in this field, and by using cases from The Hospital for Sick Children, which has a high volume of cystic lung lesions and where the pathological diagnosis of CPAM has been standardized. Secondly data in both groups are limited by the retrospective nature of the study and the referral pattern of the lesions that may bias the data. Thirdly, we relied on radiology reports rather than reviewing the actual images, and therefore we were subject to the possibility of incorrect interpretation of the images by whichever radiologist read it. The effect of these last two limitations should be at least partially ameliorated by the relatively large number of cases reported in each group.
In summary, our data suggest that there are clinical and radiological features that may assist families and clinicians in making the decision to resect or to observe a cystic lung lesion. In the absence of a family history of PPB/DICER1 familial syndrome and/or absence of DICER1 germline mutation our data suggest that close observation in patients with asymptomatic, prenatally diagnosed CPAMs and no high risk features is safe. Resection is recommended for those with significant risk factors for PPB. Future studies may be able to predict with greater sensitivity and specificity the patients who are likely to have PPB with the goal of avoiding unnecessary surgery and morbidity in the remainder.
Appendix A. Discussions
Presented by Nigel Hall, Toronto ON.
Andrea Hayes-Jordan (Houston TX): Thank you for the excellent report. That is information that we really have needed in this disease. What I was curious about is in your patients who had a diagnosis of PPB, what was the average age of diagnosis? Did patients present over six months of age or around the perinatal period?
Nigel Hall: The majority of them presented over six months of age. Some of them in early childhood. The majority were either symptomatic or they presented through screening for either a family member or another tumor elsewhere.
Andrea Hayes-Jordan: That was going to be my second question about the family member and any ovarian cystic disease in the family that may have tipped them off to the DICER1 mutation.
Nigel Hall: Absolutely, so looking at all that together, the DICER1 mutation seems to be a common link and it may be that in fact in the future management of these patients DICER1 testing may become an important part of that pathway.
Mary Fallat (Louisville KY): Is there an end point for when you would say it is time to do something if it’s still there?
Nigel Hall: I think that knowing how to follow up this group of patients and for how long is difficult at present. If we look at the PPBs, then it is true that all of them have developed either high risk features or symptoms fairly early in life so were resected. Therefore I can’t stand here and say categorically that there is an age beyond which no further follow-up is necessary. However, I think it is safe to recommend a period of observation because of that.
Unidentified speaker: I think it is a very nice analysis, Nigel, but when you get down to the bottom of your algorithm and you come to a 96% positive predictive value, that sounds great, but if you just take all of cystic lung disease in an infant, that is what your prediction is going to be because you know that 4% or less are PPB compared to CCAM. It may be useful, yes. Maybe you all should get the DICER mutation if you’re going to observe but if you’re point is to resect them, it really doesn’t change much.
Nigel Hall: I agree. I think our point is to try to avoid resection in those who do not really need it but to do so in a safe way. As you are aware, there are many units who are routinely resecting all of these lesions and so what we hope this can do is to provide some better evidence based on a clinical dataset to actually inform that decision.
Footnotes
Role in the project: JCL and YM, conceived the project; JCL, YM, GW, NJH, DM and AF designed the study; JCL, YM, GW, DAH, LPD, NJH and AF collected data; JCL, NJH, AF, DM, and KAPS analyzed the data; AF, NJH and JCL wrote the first draft of the manuscript which was subsequently revised and approved by all authors.
Level of evidence: Level II.
References
- 1.Kotecha S, Barbato A, Bush A, et al. Antenatal and postnatal management of congenital cystic adenomatoid malformation. Paediatr Respir Rev. 2012;13:162–71. doi: 10.1016/j.prrv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgerald DA. Congenital cyst adenomatoid malformations: resect some and observe all. Paediatr Respir Rev. 2007;8:67–76. doi: 10.1016/j.prrv.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Laberge JM, Puligandla P, Flageole H. Asymptomatic congenital lung malformations. Semin Pediatr Surg. 2005;14:16–33. doi: 10.1053/j.sempedsurg.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Stocker JT, Madewell JE, Drake RM. Congenital cystic adenomatoid malformation of the lung. Classification and morphologic spectrum. Hum Pathol. 1977;8:155–71. doi: 10.1016/s0046-8177(77)80078-6. [DOI] [PubMed] [Google Scholar]
- 5.Stocker JT. Cystic lung disease in infants and children. Fetal Pediatr Pathol. 2009;28:155–84. doi: 10.1080/15513810902984095. [DOI] [PubMed] [Google Scholar]
- 6.Priest JR, Williams GM, Hill DA, et al. Pulmonary cysts in early childhood and the risk of malignancy. Pediatr Pulmonol. 2009;44:14–30. doi: 10.1002/ppul.20917. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira C, Himidan S, Pastor AC, et al. Discriminating preoperative features of pleuropulmonary blastomas (PPB) from congenital cystic adenomatoid malformations (CCAM): a retrospective, age-matched study. Eur J Pediatr Surg. 2011;21:2–7. doi: 10.1055/s-0030-1267923. [DOI] [PubMed] [Google Scholar]
- 8.Lezmi G, Verkarre V, Khen-Dunlop N, et al. FGF10 Signaling differences between type I pleuropulmonary blastoma and congenital cystic adenomatoid malformation. Orphanet J Rare Dis. 2013;8:130. doi: 10.1186/1750-1172-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill DA, Jarzembowski JA, Priest JR, et al. Type I pleuropulmonary blastoma: pathology and biology study of 51 cases from the international pleuropulmonary blastoma registry. Am J Surg Pathol. 2008;32:282–95. doi: 10.1097/PAS.0b013e3181484165. [DOI] [PubMed] [Google Scholar]
- 10.Papagiannopoulos K, Hughes S, Nicholson AG, Goldstraw P. Cystic lung lesions in the pediatric and adult population: surgical experience at the Brompton hospital. Ann Thorac Surg. 2002;73:1594–8. doi: 10.1016/s0003-4975(02)03469-0. [DOI] [PubMed] [Google Scholar]
- 11.Laberge JM, Flageole H, Pugash D, et al. Outcome of the prenatally diagnosed congenital cystic adenomatoid lung malformation: a Canadian experience. Fetal Diagn Ther. 2001;16:178–86. doi: 10.1159/000053905. [DOI] [PubMed] [Google Scholar]
- 12.Komori K, Kamagata S, Hirobe S, et al. Radionuclide imaging study of long-term pulmonary function after lobectomy in children with congenital cystic lung disease. J Pediatr Surg. 2009;44:2096–100. doi: 10.1016/j.jpedsurg.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 13.Kunisaki SM, Powelson IA, Haydar B, et al. Thoracoscopic vs open lobectomy in infants and young children with congenital lung malformations. J Am Coll Surg. 2014;218:261–70. doi: 10.1016/j.jamcollsurg.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Messinger YH, Stewart DR, Priest JR, et al. Pleuropulmonary blastoma: a report on 350 central pathology-confirmed pleuropulmonary blastoma cases by the International Pleuropulmonary Blastoma Registry. Cancer. 2015;121:276–85. doi: 10.1002/cncr.29032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The International Pleuropulmonary Blastoma Registry http://www.ppbregistry.org.
- 16.Schultz KA, Yang J, Doros L, et al. DICER1-pleuropulmonary blastoma familial tumor predisposition syndrome: a unique constellation of neoplastic conditions. Pathol Case Rev. 2014;19:90–100. doi: 10.1097/PCR.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mechoulan A, Leclair MD, Yvinec M, et al. Pleuropulmonary blastoma: a case of early neonatal diagnosis through antenatal scan screening. Gynecol Obstet Fertil. 2007;35:437–41. doi: 10.1016/j.gyobfe.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Nasr A, Himidan S, Pastor AC, et al. Is congenital cystic adenomatoid malformation a premalignant lesion for pleuropulmonary blastoma? J Pediatr Surg. 2010;45:1086–9. doi: 10.1016/j.jpedsurg.2010.02.067. [DOI] [PubMed] [Google Scholar]
- 19.Hill DA, Ivanovich J, Priest JR, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doros L, Schultz KA, Stewart DR, et al. DICER1-related disorders. In: PRA, AMP, AHH, et al., editors. GeneReviews. Seattle, Washington: University of Washington; 2014. [Google Scholar]
- 21.Ng C, Stanwell J, Burge DM, et al. Conservative management of antenatally diagnosed cystic lung malformations. Arch Dis Child. 2014;99:432–7. doi: 10.1136/archdischild-2013-304048. [DOI] [PubMed] [Google Scholar]


