Abstract
Introduction
Hospitalization provides an opportunity for smokers to quit, but tobacco-cessation interventions started in hospital must continue after discharge to be effective. This study aimed to improve the scalability of a proven effective post-discharge intervention by incorporating referral to a telephone quitline, a nationally available cessation resource.
Study design
A three-site RCT compared Sustained Care, a post-discharge tobacco-cessation intervention, with Standard Care among hospitalized adult smokers who wanted to quit smoking and received in-hospital tobacco-cessation counseling.
Setting/participants
A total of 1,357 daily smokers admitted to three hospitals were enrolled from December 2012 to July 2014.
Intervention
Sustained Care started at discharge and included automated interactive voice response telephone calls and the patient’s choice of cessation medication for 3 months. Each automated call advised cessation, supported medication adherence, and triaged smokers seeking additional counseling or medication support directly to a telephone quitline. Standard Care provided only medication and counseling recommendations at discharge.
Main outcome measures
Biochemically confirmed past 7–day tobacco abstinence 6 months after discharge (primary outcome); self-reported tobacco abstinence and tobacco-cessation treatment use at 1, 3, and 6 months, and overall (0–6 months). Analyses were done in 2015–2016.
Results
Smokers offered Sustained Care (n=680), versus those offered Standard Care (n=677), did not have greater biochemically confirmed abstinence at 6 months (17% vs 16%, p=0.58). However, the Sustained Care group reported more tobacco-cessation counseling and medication use at each follow-up and higher rates of self-reported past 7–day tobacco abstinence at 1 month (43% vs 32%, p<0.0001) and 3 months (37% vs 30%, p=0.008). At 6 months, the difference narrowed (31% vs 27%, p=0.09). Overall, the intervention increased self-reported 7-day abstinence over the 6-month follow-up (relative risk, 1.25; 95% CI=1.10, 1.40; p=0.0006).
Conclusions
A 3-month post-discharge smoking-cessation intervention for hospitalized smokers who wanted to quit did not increase confirmed tobacco abstinence at 6 months but did increase self-reported abstinence during the treatment period (3 months). Real-time linkage of interactive voice response calls to a quitline, done in this trial to increase scalability of a previously proven cessation intervention, demonstrated short-term promise but did not sustain long-term intervention effectiveness.
Clinical trial registration
Introduction
Cigarette smoking is the leading preventable cause of death in the U.S.1 Clinical guidelines recommend that clinicians offer tobacco-cessation counseling and pharmacotherapy to all adult smokers.2,3 Hospital admission offers smokers a unique opportunity to quit because U.S. hospitals are smoke free, requiring smokers to temporarily abstain from tobacco use while in an environment free of their usual smoking cues. At the same time, the illness requiring hospitalization, especially if tobacco related, may enhance a smoker’s motivation to quit by making the health risks of tobacco more salient.4 Offering tobacco-cessation treatment to hospitalized smokers increases by 40% the proportion of smokers who quit after discharge, but only if treatment started in hospital continues after discharge.4 A hospital quality measure adopted in 2012 by the Joint Commission and endorsed by the National Quality Forum requires hospitals to offer tobacco-cessation counseling and pharmacotherapy to all hospitalized smokers and provide or refer smokers to treatment resources after discharge.4,5
For hospitals, major challenges to providing evidence-based care and satisfying the tobacco quality measure are providing in-hospital cessation services and sustaining tobacco treatment after discharge.6 To address the latter problem, the authors’ previous study developed a system-level intervention to facilitate delivery of tobacco-cessation counseling and medication after hospital discharge. Smokers received a refillable 1-month supply of tobacco-cessation medication at discharge and a series of automated telephone calls using interactive voice response (IVR) technology for 3 months. At each call, a smoker could request a return call from a live tobacco counselor. The previous single-site RCT, Helping HAND (Hospital-initiated Assistance for Nicotine Dependence, HH1), demonstrated the effectiveness of this intervention over standard care for increasing smoking-cessation rates after discharge.7
This model was adapted to improve its scalability for dissemination. In HH1, smokers who requested cessation support at an automated call received a subsequent return call from a hospital-based counselor funded by the research project. In the new model, smokers were transferred directly in a two-step process from the automated call to a telephone quitline provider. Quitlines are an evidence-based resource offering free cessation counseling to U.S. smokers who call a toll-free phone number.8,9 Quitlines’ universal accessibility makes them ideal resources for sustaining tobacco treatment after hospitalization. The new model’s ability to transfer a smoker in real time from an automated call to a quitline service was expected to facilitate treatment use, thereby enhancing tobacco abstinence after discharge.
The new Sustained Care intervention was compared with Standard Care in a multi-site RCT. The hypothesis was that Sustained Care would increase the proportion of individuals who used evidence-based tobacco-cessation treatment and were tobacco abstinent 6 months after hospital discharge. This report also compares these results with those of the previous trial in a post hoc analysis.
Methods
The Helping HAND 2 Trial (HH2), a three-site RCT, was approved by the IRBs of Partners HealthCare and the University of Pittsburgh and registered with the NIH Clinical Trials Registry (#NCT01714323). A detailed study protocol has been published.10
Setting and Subjects
The study was conducted at three hospitals: Massachusetts General Hospital (MGH), a 900-bed teaching hospital in Boston, MA; University of Pittsburgh Medical Center, a 799-bed teaching hospital in Pittsburgh, PA; and North Shore Medical Center, a 411-bed community hospital in Salem, MA. Patients admitted to these hospitals were eligible if they were adults (aged ≥18 years), current smokers (smoked one or more cigarette daily when smoking normally in the month before admission), had >5 minutes of smoking-cessation counseling in the hospital, stated that they planned to try to quit smoking after discharge, and agreed to accept a smoking-cessation medication. Patients were excluded if they had no telephone, were non-English speaking, could not give informed consent or participate in counseling owing to psychiatric or cognitive impairment or communication barrier, were admitted to obstetric or psychiatric units, were admitted for intravenous drug overdose, had medical instability, or had <1 year of estimated life expectancy.
Each hospital documented new patients’ smoking status electronically at admission, generating a daily roster of hospitalized smokers. A tobacco-cessation counselor visited each smoker in the hospital to help manage nicotine withdrawal symptoms and offer cessation assistance. For smokers who planned to quit or to try to quit after discharge, counselors provided specific post-discharge medication and counseling recommendations, screened them for study eligibility, and referred them to research staff who confirmed eligibility, obtained informed consent, conducted the baseline assessment, randomly assigned them to study condition, and notified the primary care provider with a note in the electronic health record or sent by fax.
Assignment to Condition
Participants were randomly assigned (1:1) to Sustained Care or Standard Care in permuted blocks of eight, stratified by daily cigarette consumption (ten or fewer versus ten or more) and admitting service (cardiac versus other). Treatment assignment was concealed in sequentially numbered sealed envelopes within each stratum. Research staff opened the next envelope corresponding to the participant’s randomization stratum. The study was not blinded.
Intervention
Smokers in both conditions received the same in-hospital counseling but differed in post-discharge resources. Standard Care provided advice to call a free telephone quitline (1-800-QUIT-NOW) and an individualized post-discharge medication recommendation given to the patient and communicated to hospital staff directly or via chart note.
Sustained Care included two components designed to reduce patient barriers to completing a full course of the tobacco treatment after discharge. First, a 30-day supply of free U.S. Food and Drug Administration–approved tobacco-cessation medication was provided at discharge, refillable twice for up to 90 days of treatment and prescribed by the inpatient physician of record. Medication was chosen by the patient and smoking counselor during the inpatient visit. It could include single agents (nicotine patch, nicotine gum, nicotine lozenge, bupropion, or varenicline) or a combination of these.
Second, five automated IVR telephone calls were initiated at 2, 12, 28, 58, and 88 days after discharge. Each call prompted smokers to quit or stay quit, offered support messages, encouraged adherence to cessation medication, and offered smokers the option of a direct two-step transfer to a telephone quitline. The IVR script encouraged participants to request a transfer if they had resumed smoking but still wanted to quit, needed a medication refill, had problems with medication, or stopped using medication prematurely. The IVR vendor (TelAsk Technologies, Ottawa, Canada) transferred the caller directly to a telephone counseling vendor (Alere Wellbeing, Inc., Seattle, WA), whose registration staff collected basic information and offered to transfer the caller to a Quit Coach to refill medications or start a five-call telephone counseling protocol resembling Alere’s protocols for state quitlines.10 Medication refills were mailed.
Measures/Assessments
Baseline measures collected by in-person interview included demographic factors (age, gender, race/ethnicity, education), smoking history (cigarettes/day, past 30–day use of other tobacco products or electronic cigarette [e-cigarette] use), nicotine dependence (time to first cigarette of the day), prior quit attempts and use of tobacco-cessation treatments, intention to quit after discharge, perceived importance of and confidence in quitting (5-point Likert scales), presence of a smoker at home, alcohol use (Alcohol Use Disorder Identification Test), past-year use of other drugs (marijuana, cocaine, stimulants, or opioids), and depression and anxiety symptoms (Patient Health Questionnaire-4).11,12 Hospital records provided primary discharge diagnosis, health insurance, and length of stay. Medical history was obtained from chart review. Records from the IVR and telephone counseling providers were reviewed to assess the fidelity of intervention delivery.
Research staff conducted follow-up 1, 3, and 6 months after discharge by telephone interview, with mail back-up. They assessed use of tobacco products and tobacco-cessation treatment after discharge. Tobacco-cessation treatment was defined to include any U.S. Food and Drug Administration–approved pharmacotherapy or cessation counseling provided in person or by telephone, Internet, or healthcare provider. Participants received $20 per completed survey.
The primary outcome was biochemically validated past 7–day tobacco abstinence 6 months after discharge. Tobacco abstinence was defined as abstinence from all tobacco products including e-cigarettes. A secondary measure of abstinence that allowed for e-cigarette use was also calculated. To verify self-reported abstinence at 6 months, patients were asked to provide a mailed saliva sample to assay for cotinine, a nicotine metabolite, and compensated $50 for the sample.13 Participants using nicotine-replacement therapy or e-cigarettes were asked to provide an in-person measurement of expired air carbon monoxide. Self-reported abstinence was considered verified if saliva cotinine was ≤10 ng/mL or carbon monoxide <9 ppm.14 Secondary smoking status outcomes were self-reported 7-day point prevalence and continuous abstinence at 1, 3, and 6 months and duration of post-discharge tobacco abstinence. The main measure of post-discharge treatment use was any use of pharmacotherapy or counseling; secondary measures assessed the use of individual drugs and counseling modalities.
Statistical Analysis
A total sample of 1,350 was planned to detect a difference of 4.8% (12.8% vs 8.0%) in the primary outcome measure with 80% power and a two-sided p<0.05. Analyses were done using an intent-to-treat approach. Two-sample t-tests or Wilcoxon rank sums tests were used to compare continuous variables; categorical variables were compared using chi-square tests. Data from all three sites were combined after determining that outcomes did not vary by hospital using Breslow–Day tests. The proportion abstinent by treatment arm at each follow-up was assessed using chi-square tests.15 Secondarily, to assess the overall intervention impact, a longitudinal analysis using generalized estimating equations techniques that included data from all follow-up times was conducted. Patients with missing outcomes at follow-up (including those who died) or whose self-reported abstinence was not biochemically validated were counted as smokers in all of the primary analyses. In a sensitivity analysis, multiple imputation was applied to the missing primary outcome measure, using age, gender, smoking-related disease, and 3-month smoking outcome as predictors in a logistic regression model. The final inference was combined from five sets of imputed samples. A post hoc univariate exploratory analysis compared the rates of study outcomes between the HH1 and HH2 trials. All analyses were conducted using SAS, version 9.4 and a two-sided p<0.05 was considered statistically significant.
Results
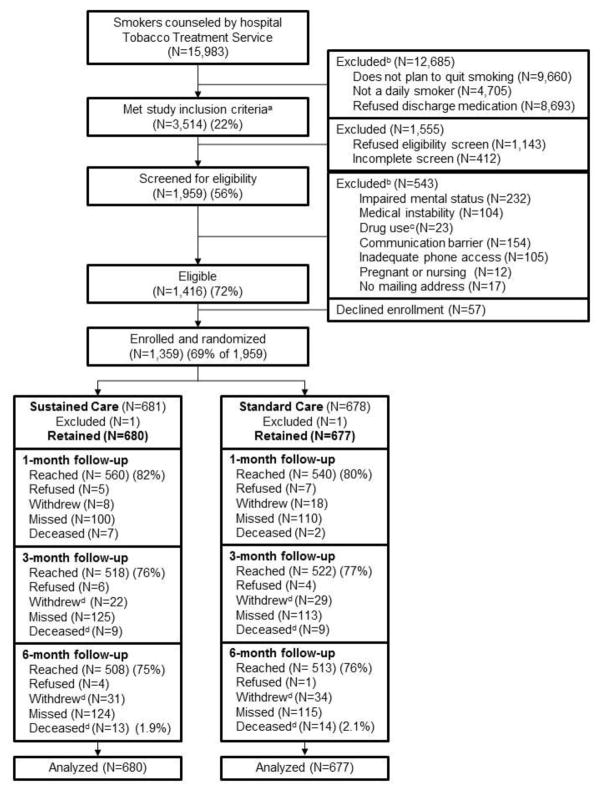
From December 3, 2012, to July 18, 2014, a total of 15,983 smokers were counseled at the three hospitals, and 3,514 (22%) met initial study inclusion criteria (daily smoker, plans to quit smoking, accepts medication) (Figure 1). Of these, 1,959 (56%) could be screened for eligibility and 1,416 (72% of those screened) were eligible. Figure 1 displays the most common reasons for ineligibility. A total of 1,359 smokers (96% of those eligible, 69% of those screened) enrolled and were randomly assigned to receive Sustained Care (n=681) or Standard Care (n=678) after discharge. One participant in each group was excluded post-randomization, leaving 680 and 677 participants for analysis in the Sustained Care and Standard Care groups, respectively. Follow-up survey completion rates were 81% at 1 month, 77% at 3 months, and 75% at 6 months and similar between study groups (Figure 1). Participants lost to follow-up at 6 months were younger (mean age, 46 vs 51 years; p<0.001) and more likely to be male (57% vs 49%, p=0.01) but did not differ in cigarettes/day (16 vs 16). Twenty-seven participants (2%) died, 13 in Sustained Care and 14 in Standard Care. Data were analyzed in 2015–2016.
Figure 1.
Study flow (CONSORT) diagram.
aStudy inclusion criteria: >18 yo, daily smoker, plans to quit and will accept cessation medication after discharge.
bPatients may have had more than one reason for exclusion.
cDrug use refers to intravenous drug overdose as reason for current admission.
dNumbers of patients who withdrew and died are cumulative.
Baseline characteristics and hospital course were comparable between study groups (Table 1). Median hospital stay was 4 days (interquartile range, 3–7 days). The primary discharge diagnoses varied but cardiovascular disease was the largest single category (29%). For 34% of participants, the primary discharge diagnosis was smoking related (Table 1). Groups did not differ in tobacco-cessation medication use in the hospital; 64% of participants reached at 1-month follow-up reported using nicotine-replacement therapy in the hospital.
Table 1.
Baseline Characteristics of Study Participants by Treatment Group
| Sustained care (Intervention) N=680 | Standard care (Control) N=677 | |||
|---|---|---|---|---|
|
| ||||
| Demographics | n | % | n | % |
| Age (mean years, SD) | 49.6 | 12.8 | 49.8 | 12.4 |
| Male sex | 348 | 51.2 | 341 | 50.4 |
| Race/ethnicity | ||||
| White non-Hispanic | 491 | 72.2 | 493 | 72.8 |
| Black non-Hispanic | 103 | 15.1 | 88 | 13.0 |
| Hispanic | 32 | 4.7 | 32 | 4.7 |
| Asian/Pacific Islander | 9 | 1.3 | 5 | 0.7 |
| Native American | 27 | 4.0 | 32 | 4.7 |
| Other/unknown | 18 | 2.6 | 27 | 3.9 |
| Education | ||||
| High school/GED or less | 353 | 51.9 | 326 | 48.2 |
| Some college | 225 | 33.1 | 256 | 37.8 |
| College graduate | 102 | 15.0 | 95 | 14.0 |
| Health insurance | ||||
| Commercial | 184 | 27.1 | 193 | 28.5 |
| Medicare | 207 | 30.4 | 217 | 32.1 |
| Medicaid | 196 | 28.8 | 188 | 27.8 |
| Other | 93 | 13.7 | 79 | 11.7 |
| Medical history | ||||
| Hypertension | 349 | 51.3 | 325 | 48.0 |
| Diabetes | 140 | 20.6 | 134 | 19.8 |
| Hyperlipidemia | 261 | 38.4 | 229 | 33.8 |
| Coronary heart disease | 187 | 27.5 | 182 | 26.9 |
| COPD | 138 | 20.3 | 155 | 22.9 |
| Stroke | 53 | 7.8 | 51 | 7.5 |
| Cancer a | 57 | 8.4 | 53 | 7.8 |
| Tobacco use | ||||
| Cigarettes per day (mean, SD) | 16.0 | 9.5 | 16.0 | 10.3 |
| Years smoked (mean, SD) | 30.5 | 13.8 | 30.0 | 13.6 |
| Time to first cigarette | ||||
| Within 30 minutes of awakening | 509 | 74.9 | 508 | 75.0 |
| >30 minutes | 170 | 25.0 | 167 | 24.7 |
| Past 30 day use of | ||||
| Non-cigarette tobacco product | 72 | 10.6 | 67 | 9.9 |
| Electronic cigarette | 141 | 20.7 | 149 | 22.0 |
| Quitting history and predictors | ||||
| Any past 24-hour quit attempt | 628 | 92.4 | 592 | 87.4 |
| Prior use of | ||||
| Nicotine replacement | 438 | 64.4 | 413 | 61.0 |
| Bupropion | 107 | 15.7 | 118 | 17.4 |
| Varenicline | 197 | 29.0 | 193 | 28.5 |
| Smoking counseling (in person or by telephone) | 103 | 15.1 | 106 | 15.7 |
| Live with smoker | 324 | 47.6 | 330 | 48.7 |
| Plan about smoking after hospital discharge | ||||
| Plan to stay quit | 328 | 48.2 | 327 | 48.3 |
| Plan to try to stay quit | 352 | 51.8 | 350 | 51.7 |
| Importance to quit now (0–4 scale) (mean, SD) | 3.9 | 0.4 | 3.9 | 0.5 |
| Confidence to resist urge to smoke (0–4)(mean, SD) | 3.1 | 1.0 | 3.2 | 1.0 |
| Comorbidities | ||||
| Depression symptoms (PHQ-2) b (mean, SD) | 1.9 | 1.9 | 1.9 | 1.9 |
| Anxiety symptoms (GAD-2) b (mean, SD) | 2.8 | 2.2 | 2.9 | 2.0 |
| Alcohol use (AUDIT-C)c (mean, SD) | 2.8 | 3.2 | 2.7 | 3.1 |
| Past year use of | ||||
| Marijuana | 180 | 26.5 | 158 | 23.3 |
| Drug other than alcohol, tobacco, or marijuana | 43 | 6.3 | 51 | 7.5 |
| Hospital course | ||||
| Length of stay (days) (median, IQR) | 4.0 | 3–7 | 5.0 | 3–7 |
| Primary discharge diagnosis | ||||
| Any smoking related disease d | 237 | 34.9 | 221 | 32.6 |
| ICD-9 groups | ||||
| Circulatory e | 205 | 30.1 | 193 | 28.5 |
| Digestive | 68 | 10.0 | 85 | 12.6 |
| Injury, poisoning | 76 | 11.2 | 76 | 11.2 |
| Respiratory | 64 | 9.4 | 70 | 10.3 |
| Musculoskeletal | 55 | 8.1 | 51 | 7.5 |
| Neoplasm | 30 | 4.4 | 14 | 2.1 |
| Signs, symptoms, ill-defined conditions | 63 | 9.3 | 71 | 10.5 |
| Other, missing | 119 | 17.5 | 117 | 17.3 |
Excludes nonmelanoma skin cancer.
PHQ-2 (2 depression symptoms, range 0–6). GAD-2 (2 anxiety symptoms, range 0–6). Higher values indicate more symptoms.
AUDIT-C (3 items, range, 0–12). Higher values indicate more alcohol use.
Smoking-related diseases are those specified in the 2014 U.S. Surgeon General’s Report. These include neoplasms (ICD-9 codes 140–151, 157, 161, 162, 180, 188, 189, 204–208), cardiovascular diseases (ICD-9 codes: 410–414, 390–398, 415–417, 420–429, 430–438, 440–448), respiratory diseases (ICD-9 480–492, 496), and perinatal conditions (ICD-9 765, 769, 798.0).
Circulatory includes cardiovascular, peripheral vascular, and cerebrovascular diseases. GED, General Educational Development; COPD, Chronic Obstructive Pulmonary Disease
Participants in the Sustained Care group answered 1,985 (62%) of 3,215 scheduled IVR calls or a median of three (interquartile range, one to four) of five planned calls per person. Transfer to a Quit Coach for additional services was requested at 823 (59%) of the 1,985 answered IVR calls; 50% of requests were for medication only, 24% were for counseling only, and 26% were for both. Overall, 699 (85%) of 823 IVR calls transferred to the quitline for additional service reached a Quit Coach. In 300 cases (36%), this occurred during the transferred call. An additional 399 (48%) participants who were not connected during the original transferred call spoke to an Alere Quit Coach on a return call.
Most participants received the additional services that they requested. Of 266 participants who requested counseling, 258 (97%) spoke with a Quit Coach and 152 (57%) enrolled in the five-call counseling protocol. Of 400 individuals who requested a medication refill, 369 (92%) reached a Quit Coach but only 298 (75%) requested and received medication.
Table 2 displays participants’ self-reported use of tobacco-cessation treatment after discharge. Patients with missing data were counted as having received no treatment. Similar findings were obtained when the analysis excluded patients with missing data. Participants in the Sustained Care group, compared with the Standard Care group, were more likely to report that they had used any tobacco-cessation treatment in the month after hospital discharge (72% vs 45%, p<0.0001), including both counseling (18% vs 12%, p=0.002) and pharmacotherapy (70% vs 41%, p<0.0001). The cumulative use of all treatments increased over 6 months, and remained significantly higher in the Sustained Care group through 6 months (Table 2). Current use of medication at the time of every follow-up assessment was also higher in the Sustained Care group, compared with the Standard Care group (Table 2). The most common post-discharge medication used by smokers in both groups was nicotine-replacement therapy (Table 2). Bupropion and varenicline were each used by <6% of participants, with no difference in use by study group (data not shown).
Table 2.
Use of Smoking Cessation Treatment After Hospital Discharge by Treatment Groupa
| Outcome measure | Sustained care N=680 |
Standard care N=677 |
Relative risk (95% CI) |
|---|---|---|---|
|
| |||
| % | % | ||
| Any use of smoking cessation treatment b (%) | |||
| 1 month | 72.2 | 45.2 | 1.60 (1.45–1.76)** |
| 3 months (cumulative) | 81.5 | 59.7 | 1.37 (1.27–1.47)** |
| 6 months (cumulative) | 85.3 | 66.2 | 1.29 (1.21–1.37)** |
| Any use of smoking cessation counseling b (%) | |||
| 1 month | 18.1 | 12.1 | 1.49 (1.15–1.93)* |
| 3 months (cumulative) | 30.1 | 21.0 | 1.44 (1.19–1.73)** |
| 6 months (cumulative) | 36.5 | 28.7 | 1.27 (1.09–1.49)* |
| Any use of smoking cessation medication b (%) | |||
| 1 month | 70.4 | 40.9 | 1.72 (1.55–1.91)** |
| 3 months (cumulative) | 80.3 | 54.8 | 1.47 (1.36–1.58)** |
| 6 months (cumulative) | 83.7 | 60.6 | 1.38 (1.29–1.48)** |
| Current use of smoking cessation medication c (%) | |||
| 1 month follow-up | 56.3 | 30.7 | 1.83 (1.61–2.09)** |
| 3 month follow-up | 42.4 | 25.8 | 1.64 (1.40–1.91)** |
| 6 month follow-up | 26.8 | 18.8 | 1.43 (1.17–1.74)** |
| Any use of nicotine replacement b,d (%) | |||
| 1 month | 68.4 | 39.4 | 1.73 (1.56–1.93)** |
| 3 months (cumulative) | 77.9 | 52.3 | 1.49 (1.37–1.62)** |
| 6 months (cumulative) | 81.6 | 57.5 | 1.42 (1.32–1.53)** |
| Any use of specific counseling resources e (%) | |||
| Quit Coach through study quitline vendor | 20.3 | ||
| Other telephone counseling (e.g., state quitline) | 1.6 | 4.3 | 0.38 (0.19–0.75)* |
| Healthcare provider | 12.1 | 12.6 | 0.96 (0.72–1.28) |
| Web-based counseling | 6.5 | 8.6 | 0.76 (0.52–1.10) |
| In-person counseling | 3.2 | 4.0 | 0.81 (0.47–1.41) |
Note: Boldface indicates statistical significance
p<0.005;
p<0.001.
Participants lost to follow-up or with missing data are counted as having received no treatment.
Use is defined here as starting any treatment since hospital discharge. It does not assess duration of use or use at the time when the follow-up survey was completed. Smoking cessation treatment includes counseling or any FDA-approved pharmacotherapy. Smoking cessation medication includes nicotine replacement products, bupropion, or varenicline. Smoking cessation counseling could be provided by the Alere quit coach (intervention group only) or by a doctor or other healthcare provider, hospital or community counselor, or state telephone quitline.
There were no between-group differences for use of bupropion or varenicline, which were each used by <6% of study participants.
Current use is defined as use at the time of the follow-up survey. It differs from cumulative use that is reported above.
Percent of participants who reported ever using specific smoking cessation counseling resources at 1, 3, or 6 month assessments. Participants could endorse more than one counseling resource. The Quit Coach was only available to the Sustained Care group.
Sustained Care specifically increased the use of telephone-based counseling after hospital discharge. The Sustained Care condition aimed to facilitate access to a telephone-based Quit Coach. This was the counseling resource most often used by this group; 56% of the 248 participants who reported receiving any counseling during 6 months of follow-up used a Quit Coach (Table 2).
Self-reported past 7–day tobacco abstinence rates were higher in the Sustained Care group than in the Standard Care group at 1 month (43% vs 32%, relative risk [RR]=1.35, 95% CI=1.18, 1.56, p<0.0001) and 3 months (37% vs 30%, RR=1.22, 95% CI=1.05, 1.42, p=0.008) but the difference narrowed at 6 months and was no longer statistically significant (31% vs 27%, RR=1.16, 95% CI=0.98, 1.37, p=0.09). An alternative analysis that counted e-cigarette use but not conventional tobacco product use as abstinence did not alter these results (Table 3). Outcomes did not differ in subgroups defined by age, gender, race, cigarettes/day, length of hospital stay, or discharge diagnosis. A longitudinal generalized estimating equations analysis, which measured the overall impact of the Sustained Care intervention over the 6-month follow-up, found a 25% increase in self-reported past 7–day tobacco abstinence (RR=1.25, 95% CI=1.10, 1.40, p=0.0006).
Table 3.
Tobacco Abstinence After Hospital Discharge, by Groupa
| Outcome measure | Sustained care N=680 |
Standard care N=677 |
Relative risk (95% Cl) | ||
|---|---|---|---|---|---|
|
| |||||
| n | % | n | % | ||
| Self-reported tobacco abstinence | |||||
| Abstinent for the past 7 days (%)-no e-cigs allowed b | |||||
| 1 month | 295 | 43.4 | 217 | 32.1 | 1.35 (1.18–1.56)**** |
| 3 months | 253 | 37.2 | 206 | 30.4 | 1.22 (1.05–1.42)*** |
| 6 months | 209 | 30.7 | 180 | 26.6 | 1.16 (0.98–1.37)* |
| Abstinent for the past 7 days (%)-e-cigs allowed c | |||||
| 1 month | 317 | 46.6 | 245 | 36.2 | 1.29 (1.13–1.46)**** |
| 3 months | 270 | 39.7 | 233 | 34.4 | 1.15 (1.01–1.33)** |
| 6 months | 228 | 33.5 | 201 | 29.7 | 1.13 (0.97–1.32) |
| Abstinent since hospital discharge b (%) | |||||
| 1 month | 211 | 31.0 | 179 | 26.4 | 1.17 (0.99–1.39)* |
| 3 months | 147 | 21.6 | 122 | 18.0 | 1.20 (0.97–1.49)* |
| 6 months | 121 | 17.8 | 101 | 14.9 | 1.19 (0.94–1.52) |
| Days abstinent after discharge | |||||
| Median (IQR) | 14.0 | 3–90 | 7.0 | 2–75*** | |
| Biochemically confirmed tobacco abstinence d | |||||
| Abstinent for the past 7 days at 6 mo (%) | 113 | 16.6 | 105 | 15.5 | 1.07 (0.84–1.37) |
Note: Boldface indicates statistical significance;
p<0.10;
p<0.05;
p<0.01;
p<0.0001.
Participants with missing outcome data are counted as smokers in these analyses.
Includes no reported use of cigarettes, other tobacco products, or electronic cigarettes.
Includes no reported use of cigarettes or other tobacco products but allows electronic cigarettes.
Pre-specified primary outcome measure: self-reported past 7 day tobacco abstinence (includes cigarettes, other tobacco products, and electronic cigarettes) at 6 month follow-up confirmed by saliva cotinine <=10 ng/ml or CO <9ppm. Participants are counted as smokers if they do not provide a biological sample or exceed cutoffs.
Among 429 participants who reported nonsmoking at 6 months, 297 (69%) provided a biological sample for confirmation of self-report (Sustained Care, 68%; Standard Care, 70%). Abstinence was confirmed in 218 (73%) of received samples (Sustained Care, 72%; Standard Care, 74%). For the entire study sample, the rate of biochemically validated past 7–day tobacco abstinence, the pre-specified primary outcome, did not differ between groups at 6 months (17% vs 16%, p=0.58). This result did not change when multiple imputation was applied to missing biochemical outcome data.16 There was no statistically significant difference in any of these outcome measures by study site (data not shown).
The median duration of self-reported continuous tobacco abstinence after hospital discharge was longer in the Sustained Care arm than in the Standard Care arm (14 vs 7 days, p=0.004). At 1-month follow-up, the proportion of Sustained Care versus Standard Care participants who were continuously abstinent was 30% versus 26% (p=0.06) (Table 3).
This trial (HH2) adapted an intervention proven effective in the prior HH1 trial.7 To explore differences between trial outcomes, HH1 patients (who were all from MGH) were compared with HH2 patients enrolled at MGH. To maximize comparability of the samples, this analysis excluded 50 (9%) of the 528 HH2 patients enrolled at MGH who did not meet HH1 enrollment criteria owing to homelessness, past-year drug use other than alcohol or marijuana, or admission for drug overdose. Table 4 compares the study outcomes of the 397 patients enrolled in HH1 to the 478 MGH patients enrolled in HH2 who met HH1 inclusion criteria.7 At baseline, HH1 and HH2 patients enrolled at MGH differed in gender (male, 49% vs 58%; p=0.007) and race/ethnicity (non-Hispanic white, 81% vs 75%; p<0.001), but not in age (mean=53 years), education (high school diploma or less, 51% vs 47%), or cigarettes/day (mean=17, both groups).
Table 4.
Comparison of Outcomes from Two RCTs: Helping Hand 1 vs. Helping Hand 2 (Massachusetts General Hospital data only) a
| Sustained care (Intervention) | Standard care (Control) | |||
|---|---|---|---|---|
|
| ||||
| Outcome measure | HH1 N=198 |
HH2 N=244 |
HH1 N=199 |
HH2 N=234 |
|
| ||||
| Self-reported tobacco abstinence | % | % | % | % |
| Abstinent for the past 7 days b (%) | ||||
| 1 month | 52.0 | 48.8 | 39.2 | 37.2 |
| 3 months | 44.9 | 40.2 | 36.7 | 37.6 |
| 6 months | 40.9 | 32.8* | 28.1 | 32.1 |
| Biochemically confirmed tobacco abstinence c | ||||
| Abstinent for the past 7 days (%) – 6 months | 25.8 | 18.0** | 15.1 | 19.2 |
| Use of tobacco cessation treatment after discharge | ||||
| Any smoking cessation treatment d (%) | ||||
| 1 month | 82.8 | 74.6** | 62.8 | 52.1** |
| 3 months (cumulative) | 86.9 | 82.8 | 76.4 | 67.5** |
| 6 months (cumulative) | 89.9 | 86.1 | 80.4 | 71.4** |
| Any smoking cessation medication d (%) | ||||
| 1 month | 78.8 | 73.0 | 58.8 | 48.7** |
| 3 months (cumulative) | 82.8 | 81.6 | 66.3 | 64.1 |
| 6 months (cumulative) | 85.9 | 84.0 | 70.4 | 68.4 |
| Any smoking cessation counseling e (%) | ||||
| 1 month | 41.0 | 20.8**** | 25.6 | 13.4*** |
| 3 months (cumulative) | 62.3 | 36.7**** | 44.3 | 21.2**** |
| 6 months (cumulative) | 73.5 | 41.0**** | 55.1 | 27.5**** |
| Process measures (Intervention group only) f | ||||
| Answered any IVR call (%) | 90.9 | 91.4 | -- | -- |
| Ever requested a medication refill | 58.1 | 59.0 | -- | -- |
| Ever received a medication refill | 51.5 | 41.8*** | -- | -- |
| Ever requested counseling | 51.0 | 40.2*** | -- | -- |
| Ever spoke to a counselor | 50.0 | 38.5*** | -- | -- |
| Ever enrolled in the 5-call protocol | -- | 25.4 | -- | -- |
Note: Boldface indicates statistical significance;
p<0.10;
p<0.05;
p<0.01;
p<0.0001.
To maximize comparability to HH1, HH2 data presented in this table are limited to smokers who were admitted to MGH, were not homeless and did not report past year use of a drug other than tobacco, alcohol, or marijuana. Of 528 patients enrolled at MGH in HH2, 50 were excluded in this analysis.
Includes no reported use of cigarettes, other tobacco products, or electronic cigarettes.
Pre-specified primary outcome measure: self-reported past 7 day tobacco abstinence at 6 month follow-up confirmed by saliva cotinine <=10 ng/ml or CO <9ppm. Participants are counted as smokers if they do not provide a biological sample or exceed cutoffs.
Smoking cessation treatment includes counseling or any FDA-approved pharmacotherapy. Smoking cessation medication includes nicotine replacement products, bupropion, or varenicline.
Smoking cessation counseling resources included a Quit Coach provided by the study (intervention group only), doctor or other healthcare provider, hospital or community counselor, state telephone quitline. The question asked to assess counseling use differed between studies. HH1: “Since leaving the hospital, did you talk to a counselor or doctor about your smoking? Who did you talk to?” HH2:“Since you left [HOSPITAL] on [DATE], have you used any program or resource to help you quit smoking, such as the internet, telephone counseling, a group program, or your healthcare provider?”
Data are derived from records of TelAsk Technologies, Inc. (IVR provider for HH1 and HH2); Alere Wellbeing, Inc. (quitline and medication refill provider forHH2); and research study records (counseling and refill provider for HH1). IVR, interactive voice response
At 6 months, the Sustained Care rates of biochemically confirmed abstinence (18% vs 26%, p=0.049) and self-reported abstinence (33% vs 41%, p=0.078) were lower in HH2 than in HH1, whereas abstinence rates in the Standard Care groups did not differ significantly between studies (Table 4). Fewer HH2 patients in either study arm reported receiving smoking-cessation counseling after discharge; however, a firm conclusion is limited by differences between the two studies in the wording of the question asked (Table 4, footnote). Records of intervention delivery revealed two differences between studies. First, fewer participants in HH2 than HH1 requested counseling support, which corroborates the finding from patient self-report. Second, although similar proportions of HH1 and HH2 participants indicated at IVR calls that they wanted medication refills, fewer participants in HH2 than HH1 actually received medication refills that they had indicated needing (Table 4).
Discussion
This large multisite RCT tested the effectiveness of a program to promote long-term tobacco cessation for hospitalized cigarette smokers who received smoking counseling as inpatients and wanted to quit smoking. The strategy to help smokers quit was to facilitate continuation of tobacco-cessation treatment begun in the hospital. The treatment model was adapted from an intervention with proven effectiveness in a previous single-site randomized trial.7 In the current trial (HH2) as in the earlier trial (HH1), the intervention improved smokers’ use of both tobacco-cessation counseling and pharmacotherapy after discharge and increased the proportion of patients who reported being abstinent from tobacco for the duration of treatment (3 months post-discharge). However, the intervention effect in HH2 waned after treatment ended, and at 6 months it was not statistically significant as it had been in HH1.7 Specifically, the intervention group’s cessation rate at 6 months was lower in HH2 than in HH1, whereas control groups’ cessation rates were similar (Table 4).
To explore why long-term cessation outcomes differed between the two studies, the authors compared study records of intervention delivery and participants’ reports of cessation treatment. These showed that the IVR intervention component performed well in both studies. A similarly large proportion of participants in each study answered at least one IVR call and used it to request further treatment. The HH2 study confirmed prior work showing that IVR technology is an efficient way to systematically maintain contact with smokers after hospital discharge.7,17–19 Intensifying the frequency of IVR calls or extending them beyond 3 months is an option that might increase or prolong the effectiveness of a Sustained Care intervention.
The design of the HH1 and HH2 trials had three major differences that could have contributed to the discrepancy in their outcomes. First, HH2 was conducted in three sites whereas HH1 was conducted in one site. However, the outcomes in HH2 did not differ by site, suggesting that expansion to additional hospitals is not the explanation. Second, HH2 expanded eligibility criteria to include smokers with serious psychiatric comorbidity and other substance abuse, who have lower quit rates with cessation interventions.20,21 However, only 9% of HH2 patients would be excluded by HH1 criteria, and the difference in 6-month cessation outcomes persisted if they were excluded (Table 4).
Third, the HH2 study altered the HH1 intervention with the goal of increasing its scalability. In HH2, smokers who requested additional support at a post-discharge IVR call were transferred directly to the quitline provider rather than having a hospital-based smoking counselor call them back, as occurred in HH1. Smokers seeking treatment were hypothesized to prefer an immediate connection from an IVR call to a treatment resource. Providing a direct link to a quitline, which can be accessed in real time, was expected to enhance the use of cessation support after discharge. However, this does not appear to have occurred. Program records, corroborated by patient self-reports, suggest that fewer HH2 patients than HH1 patients ever spoke to a counselor and fewer HH2 patients who requested a medication refill received it (Table 4). Thus, the Sustained Care group appears to have received a somewhat lower intensity of treatment in the HH2 study than in the HH1 study.
One potential reason is the multistep process required for a patient to reach a live Quit Coach. The IVR-to-quitline transfer worked well in HH2 but once a patient reached the quitline, linking to treatment service was not as seamless as anticipated. Patients had to speak to a separate registration agent to collect baseline information before they could reach a Quit Coach for counseling or a medication refill. This added time to the transferred call. Because the patient had no advance knowledge of when IVR calls were made, the call could come when extended conversation was not possible. In more than half of cases, the caller did not remain on the phone long enough to reach a Quit Coach. Instead, Quitline staff had to call the patient back to deliver treatment. By contrast, HH1 patients requesting additional service were simply told to expect a return call from a study-based counselor with access to their records; these patients were reached at high rates. Overall, the HH1 system appears to have been a less cumbersome way to sustain tobacco treatment delivery after discharge. Providing the option for a return call, either from hospital staff or quitline staff, might be a better way for an IVR system to engage smokers in counseling after hospital discharge. Alternately, quitlines might bypass or streamline the registration step when accepting transfers from IVR systems. Additional research is needed to identify ways to streamline the delivery of tobacco treatment after discharge when linking IVR services to quitlines.
Limitations
This study has several limitations. First, cessation rates at 1 and 3 months were not validated because the study protocol called for biochemical confirmation only at 6 months. The confirmation rate of self-reported abstinence at 6 months did not differ by study arm, suggesting that validation might have confirmed the difference in self-reported abstinence at 1 and 3 months. Second, the independent contributions of free medication and interactive voice response support to the treatment effect cannot be separated. Third, the results apply only to hospitalized smokers who plan to quit after discharge and are willing to consider using medication. Finally, 25% of participants were lost to follow-up by the 6-month assessment, 31% of those not smoking did not provide a saliva sample for verification, and 27% of samples did not confirm cessation. These rates compare favorably to those of other low-contact hospital-based trials.6 Furthermore, because the rates did not differ by study group, they should not have introduced bias into the study results.
Conclusions
This trial aimed to enhance the scalability of a previously proven smoking-cessation intervention for hospital patients by linking post-discharge automated IVR calls to a telephone quitline in real time. The new model was feasible and replicated the original intervention’s effectiveness in increasing the delivery of smoking-cessation counseling and medication for 3 months after hospital discharge compared with standard care. It produced higher self-reported smoking-cessation rates at all measurement points; however, the difference was statistically significant only for 3 months after hospital discharge, and the findings but did not replicate the original intervention’s long-term effectiveness after treatment ended. To sustain the tobacco abstinence stimulated by a hospital admission, future research should explore ways to improve patient engagement with scalable treatment services such as quitlines and consider intensifying the frequency and extending the duration of IVR contacts.
Acknowledgments
TelAsk Technologies of Ottawa, Canada, developed and provided the interactive voice response services. Alere Wellbeing, Inc. provided the telephone quitline services. We are grateful to the smoking-cessation counselors and research staff at all of the study sites for helping to conduct of the study. In particular, we acknowledge the contribution of Esa Davis, MD, of University of Pittsburgh, who replaced Dr. Tindle as Principal Investigator of the University of Pittsburgh Medical Center site in 2014, and Izabela Wisniewska, BA, who conducted data collection at the North Shore Medical Center site.
This study is part of the Consortium of Hospitals Advancing Research on Tobacco (CHART) initiative, jointly sponsored by the National Heart, Lung, and Blood Institute (NHLBI), National Cancer Institute (NCI), National Institute on Drug Abuse, and NIH Office of Behavioral and Social Science Research.22
NIH/NHLBI grant #R01-HL11821. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Dr. Rigotti had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Drs. Rigotti and Park receive royalties from UpToDate. Dr. Rigotti has been an unpaid consultant for Pfizer, Inc. and Alere Wellbeing, Inc., regarding smoking cessation. She has received travel expenses from Pfizer to attend a consultant meeting for which she received no honorarium. Dr. Park has a grant from Pfizer to provide free varenicline for use in a trial funded by NCI. Dr. Levy has been a paid consultant to CVS, Inc., to provide expertise on tobacco policy. Dr. Singer has been a paid consultant for Pfizer, Inc., but on matters separate from smoking cessation. Dr. Carpenter is an employee of Alere Wellbeing, Inc. No other authors have any conflicts of interest to disclose.
Contributions are as follows: Design and conduct of the study, Drs. Rigotti, Regan, Tindle, Levy, Chang, Park, Carpenter, Singer, Ms. Reyen, Ms. Kelley; Data collection, Ms. Kelley, Ms. Reyen, Ms. Streck, Mr. Reid, Mr. Ylioja; Data management, Dr. Regan; Data analysis, Drs. Chang, Regan, Levy; Manuscript drafting, Dr. Rigotti; Interpretation of the data and manuscript review and approval, Drs. Rigotti, Tindle, Regan, Levy, Chang, Park, Carpenter, Singer, Ms. Reyen, Ms. Kelley, Mr. Reid, Ms. Streck, Mr. Ylioja.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. DHHS. The health consequences of Smoking—50 years of progress: A report of the surgeon general. Atlanta, GA: CDC, National Center for Chronic Disease Prevention and Health Promotion (U.S.) Office on Smoking and Health; 2014. [Accessed March 7, 2016]. www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. [Google Scholar]
- 2.Fiore MC, Jaen CR, Baker TB, et al. clinical practice guideline. Rockville, MD: U.S. DHHS. Public Health Service; [Accessed March 7, 2016]. Treating tobacco use and dependence: 2008 update. http://www.ncbi.nlm.nih.gov/books/NBK63952/ [Google Scholar]
- 3.Siu AL. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. preventive services task force recommendation statement. Ann Intern Med. 2015;163(8):622–634. doi: 10.7326/M15-2023. http://dx.doi.org/10.7326/M15-2023. [DOI] [PubMed] [Google Scholar]
- 4.Fiore MC, Goplerud E, Schroeder SA. The joint commission’s new tobacco-cessation measures--will hospitals do the right thing? N Engl J Med. 2012;366(13):1172–1174. doi: 10.1056/NEJMp1115176. http://dx.doi.org/10.1056/NEJMp1115176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Quality Forum. [Accessed March 7, 2016];NQF endorses behavioral health measures. 2014 Mar 7; www.qualityforum.org/news_and_resources/Press_Releases/2014/NQF_endorses_behavioral_health_Measures.aspx.
- 6.Rigotti NA, Clair C, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012;5:001837. doi: 10.1002/14651858.CD001837.pub3. http://dx.doi.org/10.1002/14651858.cd001837.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigotti NA, Regan S, Levy DE, et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: A randomized clinical trial. JAMA. 2014;312(7):719–728. doi: 10.1001/jama.2014.9237. http://dx.doi.org/10.1001/jama.2014.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stead LF, Perera R, Lancaster T. A systematic review of interventions for smokers who contact quitlines. Tob Control. 2007;16(Suppl 1):i3–8. doi: 10.1136/tc.2006.019737. http://dx.doi.org/10.1136/tc.2006.019737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob Control. 2007;16(Suppl 1):i53–i59. doi: 10.1136/tc.2006.019794. http://dx.doi.org/10.1136/tc.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid ZZ, Regan S, Kelley JH, et al. Comparative effectiveness of post-discharge strategies for hospitalized smokers: Study protocol for the helping HAND 2 randomized controlled trial. BMC Public Health. 2015;15:109. doi: 10.1186/s12889-015-1484-0. http://dx.doi.org/10.1186/s12889-015-1484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. ambulatory care quality improvement project (ACQUIP). Alcohol use disorders identification test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. http://dx.doi.org/10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 12.Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas. 1993;53(4):1117–1126. http://dx.doi.org/10.1177/0013164493053004024. [Google Scholar]
- 13.SRNT Subcommittee on Biochemical V. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. http://dx.doi.org/10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 14.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. http://dx.doi.org/10.1080/1462220031000070552. [PubMed] [Google Scholar]
- 15.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318(26):1728–1733. doi: 10.1056/NEJM198806303182605. http://dx.doi.org/10.1056/NEJM198806303182605. [DOI] [PubMed] [Google Scholar]
- 16.Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: Missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102(10):1564–1573. doi: 10.1111/j.1360-0443.2007.01946.x. http://dx.doi.org/10.1111/j.1360-0443.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- 17.Reid RD, Pipe AL, Quinlan B, Oda J. Interactive voice response telephony to promote smoking cessation in patients with heart disease: A pilot study. Patient Educ Couns. 2007;66(3):319–326. doi: 10.1016/j.pec.2007.01.005. http://dx.doi.org/10.1016/j.pec.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Reid RD, Mullen KA, Slovinec D’Angelo ME, et al. Smoking cessation for hospitalized smokers: An evaluation of the "Ottawa Model". Nicotine Tob Res. 2010;12(1):11–18. doi: 10.1093/ntr/ntp165. http://dx.doi.org/10.1093/ntr/ntp165. [DOI] [PubMed] [Google Scholar]
- 19.Regan S, Reyen M, Lockhart AC, Richards AE, Rigotti NA. An interactive voice response system to continue a hospital-based smoking cessation intervention after discharge. Nicotine Tob Res. 2011;13(4):255–260. doi: 10.1093/ntr/ntq248. http://dx.doi.org/10.1093/ntr/ntq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberger AH, Pilver CE, Hoff RA, Mazure CM, McKee SA. Changes in smoking for adults with and without alcohol and drug use disorders: Longitudinal evaluation in the U.S. population. Am J Drug Alcohol Abuse. 2013;39(3):186–193. doi: 10.3109/00952990.2013.785557. http://dx.doi.org/10.3109/00952990.2013.785557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackowick KM, Lynch MJ, Weinberger AH, George TP. Treatment of tobacco dependence in people with mental health and addictive disorders. Curr Psychiatry Rep. 2012;14(5):478–485. doi: 10.1007/s11920-012-0299-2. http://dx.doi.org/10.1007/s11920-012-0299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley WT, Stevens VJ, Zhu SH, Morgan G, Grossman D. Overview of the consortium of hospitals advancing research on tobacco (CHART) Trials. 2012;13:122. doi: 10.1186/1745-6215-13-122. http://dx.doi.org/10.1186/1745-6215-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]