Abstract
Background
Disease management programs based on the chronic care model have achieved successful and long-term improvement in the quality of chronic care delivery and patients’ health behaviors and physical quality of life. However, such programs have not been able to maintain or improve broader self-management abilities or social well-being, which decline over time in chronically ill patients. Disease management efforts, population health management initiatives and innovative primary care solutions are still mainly focused on clinical and functional outcomes and health behaviors (e.g., smoking cessation, exercise, and diet) failing to address individuals’ overall quality of life and well-being. Individuals’ ability to achieve well-being can be assessed with great specificity through the application of social production function (SPF) theory. This theory asserts that people produce their own well-being by trying to optimize the achievement of instrumental goals (stimulation, comfort, status, behavioral confirmation, affection) that provide the means to achieve the larger, universal goals of physical and social well-being.
Discussion
A shift in focus from the management of physical function, disease limitations, and lifestyle behaviors alone to an approach that fosters self-management abilities such as self-efficacy and resource investment as well as overall quality of life, is urgently needed. Disease management interventions should be aimed at adequately addressing all difficulties chronically ill patients face in life, such as the effects of pain and fatigue on the ability to maintain a job and social life and to participate in activities promoting physical and social well-being. Patients’ ability to maintain engagement in stimulating work and social activities with the people who are important to them may be even more important than aspects of disease self-management such as blood pressure or glycemic control. Interventions should aim to make chronically ill patients capable of managing their own well-being and adequately addressing their needs in a broader sense.
Summary
So, is disease management the answer to our problems in the time of aging populations and increased prevalence of unhealthy lifestyles, chronic illnesses, and comorbidity? No! Effective (disease) prevention, disease management, patient-centered care, and high-quality chronic care and/or population health management calls for management of overall well-being.
Keywords: Chronic disease, Disease management, Quality of life, Well-being, Self-management, Health behavior
Background
The complexity of chronic disease profiles, population health management, disease prevention, promotion of healthier lifestyles all demand a patient-centered system of care delivery characterized by long-term coordination among diverse health professionals. Such a patient-centered disease management approach is expected to equip patients with the information and skills necessary to act as capable self-managers and thereby improving patient outcomes [1]. Chronic illnesses, however, continue to be underdiagnosed and undertreated, and approaches to their care rarely combine primary measures (i.e., prevention of disease onset) with preventive secondary measures (i.e., treatment of patients with known risk factors or in the initial stages of disease) [2]. In the United States, increasingly common efforts to transform primary care centers into patient-centered medical facilities have achieved only limited improvements in care quality, implying that further refinement of such interventions is needed [3]. Changes are needed in population health management strategies, the way chronic care is delivered, how diseases are prevented as well as how we assess their outcomes. Research to date suggests strongly that such shifts require multicomponent interventions, such as disease management programs based on the chronic care model [1, 4, 5]. Such programs target patient populations in which significant positive effects of interventions to improve self-care have been demonstrated [4–8].
The chronic care model was developed to guide the redesign and quality improvement of chronic care delivery. Its multidimensional framework outlines the transformation of care from an acute, reactive approach to a planned, population-based strategy rooted in productive interaction between informed, activated patients and proactive health care teams [6–8]. The model defines six interrelated components of the quality of chronic care delivery:
self-management support (empowering patients to self-manage care through planning, goal setting, and problem solving, e.g., with education and guided development of skills, healthy lifestyle, and self-efficacy)
delivery system design (defining health care team members’ roles and delivering evidence-based care that patients understand)
decision support (making care decisions with patients using evidence-based guidelines and specialists’ expertise)
clinical information systems (providing timely reminders for patients and health professionals, planning and coordinating care, monitoring health care team performance)
health care organization (promoting effective strategies at all levels to comprehensively change the care system, developing agreements to coordinate care and address quality issues, providing incentives to improve care quality), and
community linkages (developing partnerships with community organizations to support interventions that complement health services, advocating for policy changes that improve patient care) [4–6].
Based on these components, primary care practices implementing the chronic care model deliver care in a manner that activates and involves patients, improves care coordination and evidence-based decision making, and enables monitoring of the effectiveness of care for individual patients, with the overall aim of improving the quality of care. By focusing on clinical and functional outcomes and health behaviors (e.g., smoking cessation, exercise, and diet) [9–11], however, this model largely fails to address patients’ overall quality of life and well-being [12]. Interventions are needed to change chronically ill patients’ behavior and engage them in activities that promote improvement in physical (e.g., functioning, pain management, general health) and mental (e.g., vitality, social functioning, psychological health) quality of life [13, 14]. Care providers should thus prioritize improvement of chronically ill patients’ quality of life while treating or managing illness and impairment [9]. Thus, interventions aiming to maintain or improve chronically ill patients’ well-being by preventing disease onset, promoting healthier lifestyles and social engagement, and aiding the development of self-management abilities are an important complement to population health management [15–18].
Management of overall well-being
Individuals’ ability to achieve well-being can be assessed with great specificity through the application of social production function (SPF) theory. SPF theory, developed by Lindenberg [19], is based on the concept that people make diverse efforts to improve their living conditions, with the overall aim of achieving physical and social well-being. It assumes that as a society, we try to protect well-being by providing care and support to those who depend on it, for example because of functional limitations. The best organisation of such care depends on the manner in which it contributes to well-being. Thus, the determinants of well-being and the best approaches to improving it must be established.
Well-being is a broad concept with physical and social dimensions. Physical well-being is a construct involving optimal comfort and adequate physical and mental stimulation. The state of comfort, which has somatic and emotional components, comprises the presence of a safe, pleasant environment and the absence of physiological needs (i.e. pain, hunger, and thirst). Social well-being can be achieved by obtaining status (social ranking, e.g. based on occupation, lifestyle, or talents), behavioural confirmation (living according to relevant others’ or one’s own norms), and affection (friendship, intimacy, and emotional support, e.g. from a partner, children, or loved ones) [20, 21]. Physical and social well-being are achieved on the way to the ultimate goal of overall subjective well-being (optimal quality of life or mental well-being) [21]. By recognising the hierarchy of well-being goals, we can better understand the impacts of chronic illnesses and the functional limitations that come with it, and thereby determine the types of care and support that individuals require. This theory thus asserts that people produce their own well-being by trying to optimise the achievement of instrumental goals (stimulation, comfort, status, behavioural confirmation, affection) that provide the means to achieve the larger, universal goals of physical and social well-being (Fig. 1) [9]. Nieboer [21] developed a valid, reliable instrument to assess all five instrumental goals (stimulation, comfort, status, behavioural confirmation, and affection) to achieve well-being based on the SPF theory. Those aiming to measure and improve well-being based on the SPF theory can use this instrument.
Fig. 1.
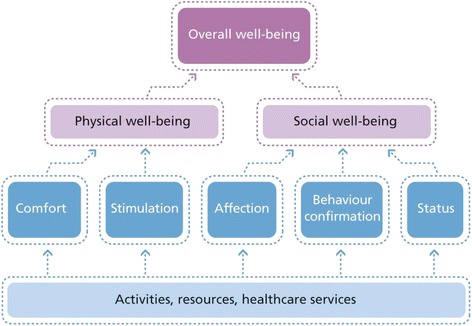
The hierarchy of well-being, according to social production function theory. From: [46] Cramm JM, Nieboer AP: Social cohesion and belonging predict the well-being of community-dwelling older people. BMC Geriatrics 2015. 15:30. DOI:10.1186/s12877-015-0027-y
Illness and functional limitations affect well-being in diverse ways. A person’s resources (e.g. physical condition, social relationships, and income) have been shown to affect well-being in times of illness and of health. For example, social contacts and the myriad forms of support and confirmation received from a partner have important buffering functions [22].
The ability to participate in activities that are important to an individual, an essential component of physical and social well-being, is fostered by social relationships and other resources. Not all activities are equal; withdrawal from multifunctional activities (i.e. those that contribute to multiple goals in the well-being hierarchy) can have a substantial impact on the ability to maintain or achieve well-being. For example, physical exercise can contribute to physical well-being, but engaging in exercise with others (e.g. in a sports club) can also enhance social well-being. Functional limitations leading to withdrawal from important activities thus have major impacts on well-being, unless an individual has the opportunity to substitute for losses. For people with functional limitations, the provision of care and support that facilitate continued engagement in important activities can reduce or avoid loss of well-being [9].
Discussion
Early studies showed that disease management programs and other interventions based on the chronic care model improved patients’ health behaviors, thereby preventing decline [10, 23–25]. We (Cramm and Nieboer) [23] have documented the challenges within such programs of achieving real gains in terms of patients’ overall well-being. Beyond patients’ self-management of their chronic conditions, interventions have not been able to effectively motivate patients to become proactive participants in care delivery or to self-manage well-being in a broader sense. These challenges reflect the failure of disease management interventions to adequately address the difficulties facing chronically ill patients, such as the effects of pain and fatigue on the ability to maintain a job and social life and to participate in activities promoting physical and social well-being. Patients’ ability to maintain engagement in stimulating work and social activities with the people who are important to them may be even more important than aspects of disease self-management such as blood pressure or glycemic control. Results from a meta-analytic review for example showed a 50 % increased likelihood of survival for people with stronger social relationships [26]. In the larger context of well-being, self-management abilities are known to deteriorate as a consequence of dealing with chronic illness [27], and disease management programs based on the chronic care model have not been able to achieve a shift in this pattern [10, 23]. The implementation of such a program in the Netherlands led to improved physical quality of life, but a decline in mental quality of life [23, 24]. The development of truly patient-centered systems will thus require prioritization of the overall quality of life and well-being of chronically ill patients.
As broader self-management abilities are critical predictors of well-being and mental quality of life [16, 23, 28–30], a shift in focus to include not only traditionally addressed health- and disease-specific aspects (e.g., smoking, physical activity, blood pressure monitoring), but also abilities such as investment behavior (e.g., pursuing interests, keeping busy, maintaining contact with loved ones) and self-efficacy (e.g., belief in one’s ability to achieve goals and express care for others), is urgently needed [31]. Patients living with chronic illness experience not only functional and clinical impacts, but also compromised quality of life due to factors such as anxiety and fear about the impacts of the illness on themselves, their loved ones, and their financial status [32, 33]. Health care professionals’ attention to such worries and concerns and investment in strengthening patients’ ability to cope with them are thus of great importance. Chronically ill patients value personal contact with these professionals highly, as it gives them the opportunity to talk about their concerns; such contact cannot be replaced by e-consultation, the development of patient portals for online information exchange, or similar interventions, which convey the notion that patients must “do everything themselves.” In the implementation of disease management programs, these alternative forms of contact have been found to be insufficient to stop declines in self-management abilities and mental quality of life as consequences of living with chronic illness [23].
Despite achieving improvement in the quality of chronic care delivery, disease management programs in the Netherlands and United States have not been able to successfully address self-management support or mental quality of life issues [23, 34–36]. Approaches to self-management support are the least frequently implemented and most challenging elements of the chronic care model [37], and Elissen and colleagues [38] demonstrated that such support for patients with chronic illness is far from adequate in most European countries. A better understanding of how patients and health care professionals can be encouraged to promote prevention and engage in productive interaction is thus clearly required to improve chronically ill patients’ physical as well as social well-being. We expect that the implementation of interventions to strengthen patients’ investment behavior and self-efficacy would be beneficial, but such an approach requires a different perspective on care delivery. Although there are some examples out there aimed at improving one or two of the instrumental goals to improve well-being, there are no examples of programs aiming to improve all five instrumental goals to improve well-being. Furthermore, evidence about these initiatives is still largely lacking. Using the SPF-IL scale [9] assessing the five instrumental goals to achieve well-being may help to create empirical evidence. Health care professionals must strive to understand each patient’s context [39] and be responsive to his or her needs, values, and preferences, rather than perceiving him or her solely as the object of disease [40]. Berwick [41] conceptualized effective care delivery as that involving health care professionals in the role of “guests” in patients’ lives, rather than “hosts” in the health care system. Such approaches have been shown to improve patient outcomes [42], but they require professionals to interact effectively with patients, gaining and using knowledge of them as complete, unique people [43]. Most health care professionals have not received training in the communication skills and psychological counseling techniques needed to achieve such interaction [44, 45].
Conclusions
Disease management programs based on the chronic care model have achieved successful and long-term improvement in the quality of chronic care delivery [34–36] and patients’ health behaviors [10, 23] and physical quality of life [23, 24]. However, such programs have not been able to maintain or improve broader self-management abilities or mental quality of life, which decline over time in chronically ill patients [23, 27, 38]. A shift in focus from the management of physical function, disease limitations, and lifestyle behaviors alone to an approach that fosters self-management abilities such as self-efficacy and resource investment as well as overall quality of life, is urgently needed. Health care professionals must also realize the importance of personal contact with patients to discuss concerns about dealing with chronic illness. However, the implementation of interventions that meet patients’ needs while enhancing their self-management abilities and making them proactive participants in care delivery poses a challenge. The extension of chronically ill patients’ self-care abilities for as long as possible is becoming increasingly important, as better self-management can prevent disease worsening and maintain patients’ independence and physical and mental quality of life. In addition to directly benefitting individual patients, these achievements would reduce demands on overburdened health care systems and improve overall population health. Disease management programs have not yet discovered how to effectively help chronically ill patients become informed, activated self-managers. Patient-centered interventions should aim to make these patients capable of managing their own health and quality of life, thereby improving overall well-being and adequately addressing their needs. So, is disease management the answer to our problems in the time of aging populations and increased prevalence of unhealthy lifestyles, chronic illnesses, and comorbidity? No! Effective (disease) prevention, disease management, patient-centered care, and high-quality chronic care and/or population health management calls for management of overall well-being.
Acknowledgements
None.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Authors’ contributions
JC and AN drafted the manuscript and contributed to refinement equally. Both authors have read and approved its final version.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviation
- SPF
Social production function
Contributor Information
Jane Murray Cramm, Email: cramm@bmg.eur.nl.
Anna Petra Nieboer, Email: nieboer@bmg.eur.nl.
References
- 1.Nolte E, McKee M. Caring for people with chronic conditions: a health system perspective. Maidenhead: Open University Press; 2008. [Google Scholar]
- 2.Roland M, Dusheiko M, Gravelle H, Parker S. Follow-up of people aged 65 and over with a history of emergency admissions. Analysis of routine admission data. BMJ. 2005;330:289–292. doi: 10.1136/bmj.330.7486.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedberg MW, Schneider EC, Rosenthal MB, Volpp KG, Werner RM. Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311(8):815–825. doi: 10.1001/jama.2014.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner EH, Austin BT, Von Korff M. Improving outcomes in chronic illness. Manag Care Q. 1996;4(2):12–25. [PubMed] [Google Scholar]
- 5.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74:511–544. doi: 10.2307/3350391. [DOI] [PubMed] [Google Scholar]
- 6.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff. 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 7.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the chronic care model in the new millennium. Health Aff. 2009;28(1):75–85. doi: 10.1377/hlthaff.28.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norris SL, Glasgow RE, Engelgau MM, O’Connor PJ, McCulloch D. Chronic disease management: a definition and systematic approach to component interventions. Dis Manage Health Outcomes. 2003;11(8):477–488. doi: 10.2165/00115677-200311080-00001. [DOI] [Google Scholar]
- 9.Nieboer AP. Sustainable care in a time of crisis. Inaugural lecture presented at: Erasmus University Rotterdam; 2013 Available at: http://www.bmg.eur.nl/fileadmin/ASSETS/bmg/Onderzoek/Oraties/Nieboer/Oratie_Anna_Nieboer.pdf. Accessed 17 Aug 2015.
- 10.Cramm JM, Adams SA, Walters BH, Tsiachristas A, Bal R, Huijsman R, Rutten-Van Mölken MP, Nieboer AP. The role of disease management programs in the health behavior of chronically ill patients. Patient Educ Couns. 2014;95(1):137–142. doi: 10.1016/j.pec.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Lemmens KM, Nieboer AP, van Schayck CP, Asin JD, Huijsman R. A model to evaluate quality and effectiveness of disease management. Qual Saf Health Care. 2008;17(6):447–453. doi: 10.1136/qshc.2006.021865. [DOI] [PubMed] [Google Scholar]
- 12.Barr VJ, Robinson S, Marin-Link B, Underhill L, Dotts A, Ravensdale D, Salivaras S. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Q. 2003;7:73–82. doi: 10.12927/hcq.2003.16763. [DOI] [PubMed] [Google Scholar]
- 13.Jarvis J, Skinner TC, Carey ME, Davies MJ. How can structured selfmanagement patient education improve outcomes in people with type 2 diabetes? Diabetes Obes Metab. 2009;12:12–19. doi: 10.1111/j.1463-1326.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RM, Funnell MM. Patient empowerment: myths and misconceptions. Patient Educ Couns. 2010;79:277–282. doi: 10.1016/j.pec.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cramm JM, Nieboer AP. The effects of social and physical functioning and self-management abilities on well-being among patients with cardiovascular diseases, chronic obstructive pulmonary disease, and diabetes. Appl Res Qual Life. 2014;9:113–121. doi: 10.1007/s11482-013-9216-z. [DOI] [Google Scholar]
- 16.Cramm JM, Nieboer AP. Self-management abilities, physical health and depressive symptoms among patients with cardiovascular diseases, chronic obstructive pulmonary disease, and diabetes. Patient Educ Couns. 2012;87(3):411–415. doi: 10.1016/j.pec.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Marino P, Sirey JA, Raue PJ, Alexopoulos GS. Impact of social support and self-efficacy on functioning in depressed older adults with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3(4):713–718. doi: 10.2147/copd.s2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu LM, Austin J, Hamilton JG, Valdimarsdottir H, Isola L, Rowley S, Warbet R, Winkel G, Redd WH, Rini C. Self‐efficacy beliefs mediate the relationship between subjective cognitive functioning and physical and mental well‐being after hematopoietic stem cell transplant. Psychooncology. 2012;21(11):1175–1184. doi: 10.1002/pon.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindenberg SM. Continuities in the theory of social production functions. In: Lindenberg SM, Ganzeboom HBG, editors. Verklarende sociologie: opstellen voor Reinhard Wippler. Amsterdam: Thesis Publishers; 1996. pp. 169–184. [Google Scholar]
- 20.Ormel J, Lindenberg SM, Steverink N, Von Korff M. Quality of life and social production functions: a framework for understanding health effects. Soc Sci Med. 1997;45:1051–1063. doi: 10.1016/S0277-9536(97)00032-4. [DOI] [PubMed] [Google Scholar]
- 21.Nieboer A, Lindenberg S, Boomsma A, Van Bruggen AC. Dimensions of well-being and their measurement: the SPF-IL scale. Soc Indic Res. 2005;73:313–353. doi: 10.1007/s11205-004-0988-2. [DOI] [Google Scholar]
- 22.Nieboer A, Lindenberg S. Substitution, buffers and subjective well-being: a hierarchical approach. In: Gullone E, Cummins RA, editors. The universality of subjective well-being indicators. Dordrecht, the Netherlands: Kluwer Academic Publishers; 2002. pp. 175–189. [Google Scholar]
- 23.Cramm JM, Nieboer AP. Disease management: the need for a focus on broader self-management abilities and quality of life. Popul Health Manag. 2015 doi: 10.1089/pop.2014.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cramm JM, Tsiachristas A, Adams SA, Hipple-Walters BH, Bal R, Huijsman R, Rutten-van Mölken MPMH, Nieboer AP. Evaluating Disease Management Programmes in the Netherlands. Rotterdam: Sociaal-Medische Wetenschappen (SMW), 2014.
- 25.Hung DY, Rundall TG, Tallia AF, Cohen DJ, Halpin HA, Crabtree BF. Rethinking prevention in primary care: applying the chronic care model to address health risk behaviors. Milbank Q. 2007;85(1):69–91. doi: 10.1111/j.1468-0009.2007.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cramm JM, Nieboer AP. Chronically ill patients’ self-management abilities to maintain overall well-being: what is needed to take the next step in the primary care setting? BMC Fam Pract. 2015;16:123. doi:10.1186/s12875-015-0340-8. [DOI] [PMC free article] [PubMed]
- 28.Steverink N, Lindenberg S, Slaets JPJ. How to understand and improve older people’s self-management of wellbeing. Eur J Ageing. 2005;2:235–244. doi: 10.1007/s10433-005-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cramm JM, Hartgerink JM, Steyerberg EW, Bakker TJ, Mackenbach JP, Nieboer AP. Understanding older patients’ self-management abilities: functional loss, self-management, and well-being. Qual Life Res. 2013;22(1):85–92. doi: 10.1007/s11136-012-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramm JM, Hartgerink JM, de Vreede PL, Bakker TJ, Steyerberg EW, Mackenbach JP, Nieboer AP. The role of self-management abilities in the achievement and maintenance of well-being, prevention of depression and successful ageing. Eur J Ageing. 2012;9(4):353–360. doi: 10.1007/s10433-012-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cramm JM, Strating MMH, de Vreede PL, Steverink N, Nieboer AP. Development and validation of a short version of the Self-Management Ability Scale (SMAS) Health Qual Life Outcomes. 2012;10:9. doi: 10.1186/1477-7525-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerteis M, Edgman-Levitan S, Daley J, Delbanco TL. Through the patients’ eyes: understanding and promoting patient-centered care. San Francisco: Jossey-Bass; 1993. [Google Scholar]
- 33.Gerteis M, Edgman-Levitan S, Walker JD, Stokes DM, Cleary PD, Delbanco TL. What patients really want. Health Manage Q. 1993;15(3):2–6. [PubMed] [Google Scholar]
- 34.Cramm JM, Nieboer AP. Short and long term improvements in quality of chronic care delivery predict program sustainability. Soc Sci Med. 2014;101:148–154. doi: 10.1016/j.socscimed.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 35.Cramm JM, Nieboer AP. High-quality chronic care delivery improves experiences of chronically ill patients receiving care. Int J Qual Health Care. 2013;25(6):689–695. doi: 10.1093/intqhc/mzt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson ML, Wu S, Schaefer J, Bonomi AE, Shortell SM, Mendel PJ, Marsteller JA, Louis TA, Rosen M, Keeler EB. Assessing the implementation of the chronic care model in quality improvement collaboratives. Health Serv Res. 2005;40:978–996. doi: 10.1111/j.1475-6773.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glasgow RE, Davis CL, Funnell MM, Beck A. Implementing practical interventions to support chronic illness self-management. Jt Comm J Qual Patient Saf. 2003;29:563–574. doi: 10.1016/s1549-3741(03)29067-5. [DOI] [PubMed] [Google Scholar]
- 38.Elissen A, Nolte E, Knai C, Brunn M, Chevreul K, Conklin A, Durand-Zaleski I, Erler A, Flamm M, Frølich A, Fullerton B, Jacobsen R, Saz-Parkinson Z, Sarria-Santamera A, Sönnichsen A, Vrijhoef H. Is Europe putting theory into practice? A qualitative study of the level of self-management support in chronic care management approaches. BMC Health Serv Res. 2013;13:117. doi: 10.1186/1472-6963-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med. 2000;51:1087–1110. doi: 10.1016/S0277-9536(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 40.Institute of Medicine: Crossing the Quality Chasm . A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 41.Berwick DM. What patient-centered should mean: confessions of an extremist. Health Aff. 2009;28:555–565. doi: 10.1377/hlthaff.28.4.w555. [DOI] [PubMed] [Google Scholar]
- 42.Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70(4):351–379. doi: 10.1177/1077558712465774. [DOI] [PubMed] [Google Scholar]
- 43.Greene SM, Tuzzio L, Cherkin D. A framework for making patient-centered care front and center. Perm J. 2012;16(3):49–53. doi: 10.7812/tpp/12-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skochelak S. A decade of reports calling for change in medical education: what do they say? Acad Med. 2010;85:26–33. doi: 10.1097/ACM.0b013e3181f1323f. [DOI] [PubMed] [Google Scholar]
- 45.Towle A, Godolphin W. The neglect of chronic disease self-management in medical education: involving patients as educators. Acad Med. 2011;86:1350. doi: 10.1097/ACM.0b013e3182308d25. [DOI] [PubMed] [Google Scholar]
- 46.Cramm JM, Nieboer AP. Social cohesion and belonging predict the well-being of community-dwelling older people. BMC Geriatr. 2015;15:30. doi: 10.1186/s12877-015-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


