Abstract
Background
High-dose chemotherapy(HDC) strategies were developed to avoid unacceptable neurotoxicity associated with craniospinal irradiation in infants with embryonal brain tumors. However, the impact of molecular and pathology characterization in such approaches and long-term outcome have not been widely described in young children.
Methods
We retrospectively collected information from seven North American institutions, on young children with medulloblastoma treated with sequential HDC, as per the CCG 99703 protocol. Data collected included clinical presentation, histology, molecular subgroup, irradiation, ototoxicity, and neurocognitive evaluations.
Results
The cohort included 53 patients diagnosed at a median age of 24 months (2.9–63.2). Seventeen patients(32.1%) had nodular desmoplatic medulloblastoma, all belonging to the Sonic Hedgehog subgroup(SHH), as did 30% of classic medulloblastoma. The 5-year progression free(PFS) and overall survival(OS) was 69.6%(±6·9%) and 76.1%(±6.5%) respectively. Seventeen(32.1%) patients received irradiation(RT), (9 adjuvant RT). Patients with SHH and group 3 medulloblastoma had a 5-year PFS of 86·2%(±7.4%) and 49·1%(±14%) respectively(p=0.03). The 5-year PFS radiation free for group 3 MB was 46.4%. Patients with macroscopic metastasis(M2, M3) had a worst survival. Fifteen(45.5%) patients had significant ototoxicity,. Mean FSIQ for 24 survivors was 91.6(range 52–119).
Conclusion
This HDC strategy led to an encouraging OS while only 20% of the patients received adjuvant RT. SHH medulloblastoma, irrespective of histological subgroup had an excellent outcome. Such intensive therapy may not be needed for this subgroup. Patients with classic histology or group 3, had an encouraging PFS of 58% and 46.4% respectively in absence of adjuvant RT. The neurocognitive profile of the survivors appears to be within the normal range.
Keywords: infant, medulloblastoma, high-dose chemotherapy, molecular subgrouping, neurocognitive outcome
Introduction
The management of brain tumors in young children remains a challenge largely because of the greater vulnerability of the developing brain to treatments. Three decades of infant brain tumors clinical trials have led to significant progress in delineating prognostic factors and also demonstrated gradual improvement in survival.1 Innovative strategies based on high-dose chemotherapy (HDC) were developed to delay or avoid craniospinal irradiation to minimize the risk of deleterious neurocognitive impairment. However, the impact of such approaches on intellectual and functional outcome has not been systematically described and only limited comprehensive reports are currently available. Among these high-dose chemotherapy strategies, the protocol CCG 99703 was a phase I, II study opened from 1998 to 2004 within a limited number of COG institutions, which was testing the feasibility of sequential HDC followed by autologous stem cell rescue in young children newly diagnosed with malignant brain tumors. The primary objective was to delineate the maximum tolerated dose of thiotepa in combination with carboplatin. The secondary objectives were to determine the complete response rate, the overall and event-free survival rate 2. After completion of the chemotherapy protocol, the use of adjuvant radiotherapy was left to the treating physician’s discretion. The evaluation of the use of adjuvant irradiation and neurocognitive outcome was not part of the study objectives. However such information is critical to appraise the long-term toxicity/efficacy profile of this approach. The use of adjuvant irradiation is a major factor to consider in order to delineate the real contribution of HDC in improved outcome associated with this strategy, which currently constitutes the backbone of several protocols in use by different cooperative groups.
The results of CCG 99703 have been recently published 2. However during the multiple interim closures of the study between 1998 and 2004 and following its completion, several North American institutions continued to treat young children, “according to” this approach and these data were not collected. In the current study, we aimed at describing the outcome of these young patients with medulloblastoma treated with sequential HDC, collecting clinical, molecular, radiotherapy data along with long-term outcome including ototoxicity and neurocognitive data.
Patients and methods
Patients eligible for this study were less than six years of age at the time of diagnosis of medulloblastoma and treated according to the CCG 99703 protocol.
Following IRB approval, participating institutions were sent data collection forms to capture demographics, staging, pathology, treatment, toxicity and outcome data. Histological subtype were extracted from institutional report, classified in 3 categories including classic medulloblastoma or medulloblastoma not otherwise specified, large cell anaplastic medulloblastoma (LCA) and nodular desmoplastic medulloblastoma (ND) or medulloblastoma with extensive nodularity (MBEN). For consistency of this report nodular desmoplastic medulloblastoma and medulloblastoma with extensive nodularity are grouped under the same acronym ND/MBEN. Molecular subgrouping was obtained from institutional reports. When the subgroup was not locally available, samples were sent to the Labatt Brain Tumour Research Centre in Toronto for analysis.
The ototoxicity profile was extracted from deidentified audiograms using the raw values from graphic reports. Audiograms were collected at three time points when available (at completion of therapy, at six years of age and on most recent follow-up). The Chang ototoxicity grading scale was used to grade hearing impairment taking into account the impairment of the best ear.3 The use of hearing aids or FM speaker was also recorded. Deidentified neurocognitive evaluations were collected at two different time points when available (at the age 6 years ± 1 year and at most recent follow-up). Because of variations in the timing of neuropsychological assessments, and the absence of fixed battery, attempt was made to interpret the information provided by combining data across domains, converting available scores to standard scores. Neuropsychological reports were reviewed for patients who had at least one assessment at any time point post-treatment. When more than one assessment was available, the evaluation closest to six years of age was chosen.
Treatment protocol
Following the CCG 99703 study guidelines, patients initially underwent maximal safe surgical resection. The extent of resection was evaluated based on surgical report and post surgical imaging. Initial staging was based on pre and postoperative brain and spine MRI report and CSF examination.
Induction chemotherapy consisted of three cycles of cisplatinum (3.5 mg/kg IV day 0), vincristine (0.05mg/kg IV on day 0, 7, 14), cyclophosphamide (60 mg/kg IV day 1, 2) combined with mesna and etoposide (2.5 mg/kg IV on day 0, 1 2) given every 21 days. The consolidation phase included three consecutive cycles of high-dose carboplatin (17 mg/kg IV day 0 and 1) and thiotepa (10 mg/kg IV on day 0, 1 and 2) each followed by autologous stem cell transplantation, delivered 21 to 28 days apart. After completion of the consolidation phase, adjuvant irradiation was left to the treating physician’s discretion. To capture treatment variations between institutions, data on the use of high-dose methotrexate during induction, intrathecal chemotherapy and/or maintenance therapy were recorded.
Subgroup Determination
Molecular subgrouping was performed as previously described from total RNA derived from either FFPE or frozen tissues. 4, 5 Samples were subgrouped using nanoString limited gene expression profiling at all centers but two. The samples from CHLA were evaluated using a 31 genes method TaqMan Low Density Array (TLDA) as previously described. 6 Frozen samples at Colorado Children Hospital were subgrouped using the Human Genome U133plus2 Array (Affymetrix) platform. 7 These 3 methods are equivalent and have been validated against each other. The assignment of SHH versus Group 3 is extremely robust with any of these methods. 4,6,7
Statistical analysis
For descriptive statistics, continuous data were compared using Student-t test. Non-continuous data were compared using Chi-square with a significant p value < 0·05. Both progression free survival (PFS) and overall survival (OS) were right-censored at 5 years and analyzed by the Kaplan-Meier method and p-values reported using the log-rank test (p = 0.05). Statistical analyses were performed in the R statistical environment (v3.1.2), using R packages survival (v2.37–7), and ggplot2(v1.0.0).
Results
The cohort includes 53 young children diagnosed with medulloblastoma between 1998–2012 and treated at seven North American institutions (Alberta Children’s Hospital, Calgary; Hospital for Sick Children, Toronto; Children National Medical Center, Washington; Arnold Palmer Hospital for Children, Orlando; Colorado Children Hospital, Denver; Children’s Hospital of Los Angeles, Los Angeles; Dana Farber Cancer Institute, Boston). Patient’s characteristics are described in table I. The median age at diagnosis was 24 months (range 2.9–63.2). Proper staging was available in 47 patient (86.8%) and incomplete in 6 patients (11.3%) who were missing CSF evaluation. Twenty patients (37.7%) were metastatic at diagnosis. Gross total resection (GTR) was achieved in 35 patients (66%). According to the institution pathology report, 17 tumors (32.1%) were classified as nodular desmoplastic medulloblastomas (ND) or medulloblastoma with extensive nodularity (MBEN), 30 (56.6%) as classic or not otherwise specified medulloblastoma (classic/NOS) and six (11.3%) as large cell anaplastic medulloblastoma (LCA). Molecular subgrouping was available for 43 tumors (81%). Sonic Hedgehog (SHH) subgroup accounted for 55.8%. All ND/MBEN belonged to the SHH subgroup, as did 30 % of the classic/NOS medulloblastomas. Group 3 accounted for 60% of the classic/NOS medulloblastoma and for 60% of the LCA medulloblastoma (Supplemental table I). Metastatic disease was twice more frequent at presentation in group 3 MB (62.5%) than in SHH group (29%).
Table I.
Patient characteristics
| n=53 | |
|---|---|
| Sex ratio | 30M/23F |
| Median age at diagnosis months (range) | 24 (2.9–63.2) |
| Metastatic at diagnosis | 20 (37.7%) |
| M0 | 33 (62.3%) |
| M1 | 7 (13.2) |
| Macroscopic M+ | 13 (22.4%) |
| Gross total resection | 35 (66%) |
| Second look surgery | 6(12.7%) |
| Histological subtypes | |
| Classic/NOS | 30 (56.6%) |
| Nodular Desmoplastic/MBEN | 17 (32.1%) |
| LC/A | 6(11.3%) |
| Molecular subtypes | 43 (81.1%) |
| WNT | 0 |
| SHH | 24 (55.8%) |
| Group 3 | 16 (30.2%) |
| Group 4 | 3(5.7%) |
| Radiotherapy | 17 (32.1%) |
| Adjuvant RT | 9 (16.9%) |
| RT for relapse/progression | 8 (15%) |
| Type of RT* | |
| CSI | 8(50%) |
| Focal RT | 8(50%) |
| High dose Methotrexate during induction | 6 (11.3%) |
| Intrathecal chemotherapy | 3 (5.6%) |
| Maintenance chemotherapy post consolidation | 7 (13.2 %) |
CSI: craniospinal irradiation; F: female; M: male;M0: no metastatic disease; M1: CSF disease; M+ macroscopic metastatic disease; MBEN: medulloblastoma with extensive nodularity; NOS: no otherwise specify; LC/A : large cell anaplastic medulloblastoma; SHH : Sonic HedgeHog; WNT : wtn signaling pathway; RT : radiotherapy;
Treatment
The median time to starting induction chemotherapy was 22 days (range 4–91) from surgery. All but three patients completed three cycles of induction. At completion of induction phase, 39 patients (73·6%) were in complete remission. Eight patients showed partial response and four had stable disease. Two patients had progressive disease, but still proceeded to consolidation. Consolidation HDC was initiated at a median time of 3.4 months from surgery (range 1.9–7.5). Twelve patients had modification or early discontinuation of their consolidation for severe sepsis (3), cardiac arrest (1), severe hearing loss (2) and severe peripheral neuropathy (1). Four patients received fewer than three cycles of consolidation (three patients received one cycle and one patient received two cycles) based on physician preference. The remaining patient did not complete his consolidation as he progressed during that phase. At the end of consolidation, 40 patients (75.4 %) were in complete remission. Six patients showed partial response and four developed progressive disease, one patient had persistent CSF tumor positivity and two patients in CR post induction died of toxicity during consolidation.
Overall 17(32.1%) patients received radiotherapy. Nine patients underwent adjuvant irradiation (six focal irradiation, three CSI at a median dose of 23.4 Gy). All but one, were in complete remission at completion of consolidation and three were metastatic at diagnosis. Eight patients received radiotherapy at time of relapse (two focal irradiation, five CSI, one unknown). The median dose of CSI was 23.4 Gy (23.4–30.6 Gy). Only one patient with ND/MBEN histology received adjuvant radiotherapy. Two patients with SHH subgroup received irradiation (one adjuvant CSI and one focal irradiation at relapse).
Survival
Fourteen (26.4%) patients progressed or relapsed at a median time of 9·7 months from diagnosis (range 3.3–30.5). Five patients did not receive further treatment and died of disease. One patient who showed progressive disease just after induction chemotherapy continued on to consolidation with high dose chemotherapy, craniospinal irradiation when he turned 3 years of age followed by maintenance therapy. He remained alive without evidence of disease 4.4 y after diagnosis.
Eight other patients who relapsed after completion of initial therapy received active salvage therapy: all received radiotherapy, two had repeated surgery and all but one received additional chemotherapy. Four patients were alive at respectively 9.7, 15.7, 30.3 and 122.8 months from relapse (a median time of 5 years from diagnosis (range 3.3–12))
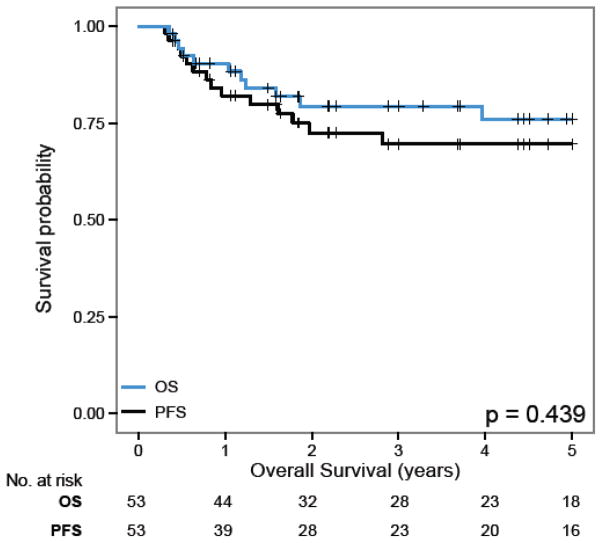
Overall, 42 patients were alive at a median follow-up of 4.5 years (range 0.4–12.6). Among the patients with shorter follow-up time, all except one had recently completed therapy. Nine patients died of disease and two died of treatment related toxicity. The 5 years PFS and OS for the entire cohort were 69.6% (±6·9%) and 76.1% (±6.5%) respectively (Figure 1).
Figure 1.
OS and PFS for the entire cohort
Prognostic factors
Table II describes the prognostic factors for the entire cohort. The PFS differed significantly according to histological subtypes and molecular subgroups. Patients with ND/MBEN had a 5-year PFS of 92.3% (± 7.4%) compared to 59.6% (± 8.9%) for non ND/MBEN patients (p=0.03) (Figure 2 B, E). Patients with SHH medulloblastoma had a significantly better outcome than patients with molecular subgroup 3 or 4. The 5-years PFS and OS were 86.2% (± 7.4%) and 90.3% (± 6.5%), respectively, for the SHH subgroup compared to 49.1% (±14%) and 59.2% (±13.1%) for the group 3 (p=0.03) (Figure 2 A,D). Figure 2 C and F describes the survival curves according to the molecular subgrouping only for the non desmoplastic medulloblastoma. Ten out of the 16 patients with group 3 medulloblastoma were alive at a median follow-up of 4.0 years (range 1.6–9.1 year), five of them being irradiation free (1M0, 3 M1, 1 M3). No significant difference in survival was observed for the eight patients with SHH driven classic MB and the 14 patients with SHH driven ND/MBEN (5y PFS of 87.5% (± 10%) and 88.9% (± 10%), respectively)). Patients with macroscopic metastatic disease (M2, M3) had a worse outcome than M0 and M1 patients (p=0·019) (Supplemental figure 1). The presence of macroscopic metastatic disease was only associated with a worse outcome for the non ND/MBEN and group 3 MB unlike for the ND/MBEN or SHH MB (table III). Five patients with group 3 had M1 disease at diagnosis; all were alive at a median follow-up time of 3.7 years. Patient with GTR had a better PFS and OS (p=0.01). Continuous complete remission (CCR) following induction therapy and after consolidation was associated with better PFS (p=0.006 and 0.000) but only CCR after consolidation remained significant for OS.
Table II.
Prognostic factors for the progression free survival and the overall survival
| Prognostic factors | 5 y PFS (±SE) | p | 5y OS (±SE) | p |
|---|---|---|---|---|
| Age | ||||
| ≤ 24 months | 72.2% (±9%) | 74.7% (±9·1%) | ||
| > 24 months | 65.5 % (±10.9%) | 0.85 | 77.7% (±8.9%) | 0.91 |
| Metastatic status | ||||
| M0, M1 | 75.5 % (± 7.6%) | 82.1% (± 6.8%) | ||
| Macroscopic M+ | 53.8% (± 13.8%) | 0.02 | 57.7% (± 14.7%) | 003 |
| Extent of resection | ||||
| GTR | 78.0% (± 8%) | 79.0% (± 7.9%) | ||
| Less than GTR | 55.0% (± 11.9%) | 0.01 | 70.5% (±11.2%) | 0.31 |
| Histologic subtype | ||||
| Desmoplastic/MBEN | 92.3% (± 7.4%) | 0.05 | 91.7% (±8%) | 0.24 |
| Classic/NOS | 61.7% (± 9.7%) | 70.3% (± 9.1%) | ||
| Large Cell Anaplastic | 50% (± 20.4%) | 66.7% (±19.2%) | ||
| Molecular subgroup | ||||
| SHH | 86.2% (±7.4%) | 0.03 | 90.3% (±6.5%) | 003 |
| Group 3 | 49.1% (±14%) | 59.2% (±13.1%) | ||
| Group 4 | - | 33·3% (± 27.2%) | ||
| Complete remission post induction | ||||
| CCR | 76.8% (±7.8%) | 0.006 | 80.9% (±7.2%) | 0.07 |
| Less than CCR | 50% (±13.4%) | 61.1 % (±138%) | ||
| Complete remission post consolidation | ||||
| CCR | 77.2% (±7.1%) | 0.000 | 84.8% (±64%) | 0.006 |
| Less than CCR | 40% (±15.5%) | 56% (±17.1%) | ||
| Use of Adjuvant radiotherapy | ||||
| Yes | 77.8% (±13.9%) | 70.7% (±12.9%) | ||
| No | 67.5% (±7.9%) | 0.65 | 79.3% (±7%) | 0.96 |
| Use of HD MTX during induction | ||||
| Yes | 70.6% (±7.3%) | 80% (±17.9%) | ||
| No | 66.7% (±19.2%) | 75.6% (±6.9%) | 0.58 | |
| IT chemotherapy | ||||
| Yes | 100% | 100% | ||
| No | 67.9% (± 7.2%) | 0.33 | 74.7% (±6.8%) | 0.96 |
| Maintenance chemotherapy | ||||
| Yes | 100% | 100% | ||
| No | 64.4% (±7.8%) | 0.09 | 72.3% (±7.3%) | 0.15 |
GTR : gross total resection; CCR complete continuous response; HD MTX : high dose methotrexate; IT: intratechal; MBEN: medulloblastoma with extensive nodularity; NOS: no otherwise specify; SHH : Sonic HedgeHog;
Figure 2.
PFS and OS according to molecular and histology subgroup
Progression free survival (PFS) according to A) molecular subgroup; B) Nodular Desmoplastic versus non Nodular desmoplastic C) by subgroup for Non Nodular Desmoplastic MB only Overall survival (OS) according to D) Molecular subgroups; E) Nodular Desmoplastic/non Nodular Desmoplastic F) by molecular subgroup for Non Nodular Desmoplastic only
Table III.
Prognostic factors according to histological subtype and molecular subgroup
| N | ND/MBEN N=17 5 y PFS |
N | Non ND/MBEN N=36 5y PFS |
N | SHH subgroup N=24 5y PFS |
N | Group 3 N=16 5y PFS |
|
|---|---|---|---|---|---|---|---|---|
| Extend of resection | ||||||||
| GTR | 17 | 92·3 %(±7·4%) | 18 | 66·1% (±12·5%) | 18 | 92·9 %(±6·9%) | 8 | 68·6 %(±18·6%) |
| Less than GTR | 0 | - | 18 | 55% (±11·9%) | 6 | 66·7 %(±19·2%) | 8 | 33·3 %(±18%) |
| p-value | 0.20 | 0.07 | 0.08 | |||||
| Metastatic status | ||||||||
| M0, M1 | 16 | 91·7 %(±8%) | 24 | 66·5 %(±10·4%) | 19 | 87·7 %(±8·2%) | 11 | 65·6 %(±16·4%) |
| M2, M3 | 1 | -* | 12 | 50% (±14·4%) | 5 | 80 %(±1·9%) | 5 | 20 %(±17·9%) |
| p-value | 0·77 | 0.07 | 0.58 | 0.00 | ||||
| Molecular subgroup | ||||||||
| SHH | 14 | 90% (±9·5%) | 10 | 80% (±12·6%) | - | - | ||
| Group3 | 0 | - | 16 | 49·1 %(±14·2%) | - | - | ||
| p-value | 0.21 | |||||||
| Adjuvant RT | ||||||||
| Yes | 1 | -* | 8 | 75% (±15·3%) | 1 | -* | 3 | 66·7 %(±27·2%) |
| No | 16 | 91·7 %(±8%) | 28 | 54·7% (±10·5%) | 23 | 85·6 %(±7·8%) | 13 | 46·4 %(±15·6%) |
| p-value | 0·77 | 0·42 | 0.69 | 0.65 | ||||
| CCR post Induction | ||||||||
| Yes | 16 | 91.7% (±8%) | 22 | 68.1% (±10.8%) | 18 | 92.9%(±6.9%) | 9 | 58.3%(±18.6%) |
| No | 1 | -* | 13 | 46.2 %(±13.8%) | 6 | 66.7%(±19.2%) | 6 | 33.3%(±19.2%) |
| p-value | 0.77 | 0.03 | 0.08 | 0.06 | ||||
| CCR post Consolidation | ||||||||
| Yes | 17 | 92·3 %(±7·4%) | 24 | 68·9% (±9·9%) | 20 | 93·8 %(±6·1%) | 11 | 59·7 %(±15·9%) |
| No | 0 | -* | 10 | 40% (±15·5%) | 4 | 50 %(±25%) | 4 | 25 %(±21·7%) |
| p-value | 0.77 | 0.00 | 0.09 | 0.07 | ||||
Pairwise comparison for each stratum.
No event but all cases censored CCR: continuous complete remission; GTR: gross total resection; ND/MBEN: nodular desmoplastic medulloblastoma; RT: radiotherapy; SHH: Sonic HedgeHog;
Seventeen patients received irradiation, but adjuvant irradiation was used only for nine patients and was not significantly associated with outcome (p=0.65). Among the 30 patients with classic MB, 23 did not receive adjuvant irradiation. The 5-year PFS in this irradiation free group was 58.5% (±11.5%) and the 5–year PFS for the patients with group 3 MB treated without adjuvant irradiation was 46•4 %(±15•6%)
Ototoxicity
Thirty-three patients had at least one audiometric evaluation. Audiograms were available in 20 patients at completion of treatment (median time of seven months from diagnosis) and in 20 patients on most recent follow-up (median time 4·9 years). Chang final ototoxicity grade for the last available audiograms revealed 11 (33%) grade 0, seven grade 1a (21.2%), five grade 2b (15.1%), nine grade 3 (27.2%), and one grade 4 (3%). Overall 15 (45.5%) children had a hearing loss greater or equal to grade 2 b (>20 and <40 dB at any frequencies below 4kHz), and 13 (39.3%) required hearing support, either hearing aids or FM system.
Neurocognitive outcome
Twenty-four of the 42 surviving patients (57%) had at least one neurocognitive evaluation available Among the surviving patients who did not have NP testing, 12 were less than 5 years of age at time of last follow up, (median age of 3.78 years; range 1.3–13.4).
Six patients had two or more neurocognitive evaluations. Among the 18 patients not tested, nine were under the age of 5 years at last follow-up. The median age at diagnosis of the cohort of patients with neurocognitive evaluation was 29·4 months (range 2.9–63.2). The median age at time of neurocognitive evaluation was 6 years (2.8–9.7). Nine of these tested survivors (37.5%) received irradiation (five focal and four CSI). The demographic and therapeutic characteristics of the tested survivors were not statistically different from those of the entire cohort. Figure 3 provides the results of the neurocognitive evaluations. While seven patients accounted for 70% of the impaired scores, the overall neurocognitive profile was within average range and the mean FSIQ for the entire cohort was 91·6 (SD=16.8).
Figure 3.
Intellectual outcome of 24 survivors
FSIQ: full scale IQ (n=23); VCI verbal comprehensive index (n=19); Perceptual reasoning index (n=20); WMI:working memory index (n=14); PSI: processing speed index (n=18)
Discussion
With a total of 53 patients included in this multi institutional series, we described one of the largest cohort of young children with medulloblastoma treated with sequential HDC. For each of the seven previously reported institutional or cooperative series involving the use of HDC for this population, the total number of patients was less than 40.2, 9–14. Despite the retrospective nature of our study, given the rarity of infant medulloblastoma, our cohort is unique as it captures comprehensive data on a rare group of patients treated with a homogenous HDC strategy.
In addition to the usual demographic and clinical description, we provide histological and more importantly information on molecular characterization which has not been reported so far in infants treated with HDC. We confirm the unique distribution of the ND/MBEN variant in young children, accounting for 32%, a figure consistent with the rate reported in the meta-analysis by Rutkowski and colleagues15 and others institutional or cooperative trials.2, 16 Although our data corroborate the excellent outcome of the ND/MBEN subgroup with a 5-year PFS of 92·3%(± 7.4%), this survival rate obtained with HDC is very similar to the one achieved with conventional chemotherapy. Using the HIT SKK 2000 protocol, Von Bueren and colleagues reported a 5-year PFS of 90%(±7%) in 19 infants with desmoplastic medulloblastoma.17–18 One may argue this particular subgroup does not need such intensive and toxic therapy and the emphasis should be on minimizing treatment related side effects. With the current regimen, the rate of significant hearing loss described and the impact on fertility associated with cyclophosphamide and thiotepa should be taken into consideration.
While the molecular subgrouping of medulloblastoma has emerged as a key prognostic factor in recent literature, most of the current molecular data are generated from large cohorts with limited clinical and therapeutic information of children of all age group. The lack of data on treatment modalities restricts our ability to factor in the potential impact of therapy (i.e use of irradiation, HDC). The method to molecularly characterize was not uniform in our cohort, with 3 different plateforms (Nanostring, TDLA and Affymetrix). However with an 81% rate of molecular characterization, we were able to integrate this molecular information in the analysis of the prognostic factors. The molecular subgrouping stands as a valuable predictive marker for both PFS and OS. We provide for this young age group a correlation between histological subtype and molecular distribution, confirming that 100% of the patients with ND/MBEN belong to the SHH subgroup even with the inclusion of children up to 5 years of age8. More interestingly, the subgroup analysis also indicates that 30% of the classic/NOS medulloblastoma are SHH driven and that these patients have an excellent survival (5-year PFS of 87.5%). While acknowledging the absence of central pathology review, the question of interest is whether HDC regimen significantly improved the outcome of the SHH driven classic medulloblastoma or if the SHH subgroup by itself is a favorable group irrespective of the pathology for children up to 5 years of age. Our data shed new lights on the complex relation between histology and molecular biology in young children and raise furthermore the question as whether the subgrouping should overcome the histology. Upcoming cooperative trials for infants MB should address this important and specific issue.
Sixteen of the 19 non-SHH patients belonged to the group 3. Group 3 was just as frequent in classic/NOS tumors as in LCA (60%). With the limitation of the absence of central pathology review and the small number of LCA, our findings contrast with previously described higher incidence of LCA in group 3 patients all age included19. Pietsch and colleagues8 suggested that all group 3 medulloblastomas in infants should be considered high-risk tumors even in the absence of other high-risk features. Of significant difference, the HIT 2000 protocol did not use intensification with HDC in non-metastatic patients. While our data confirm the significantly lower survival in group 3 (5-year PFS 49%, 5-year OS 59.2%) when compared to SHH subgroup, we observed favorable survival figures for group 3 infant treated with HDC, with half of patients progression free at 5 years even without the systematic use of adjuvant irradiation.
Results of previous studies were only using histological classification. In our cohort, 23 of the 30 patients with classic medulloblastoma were spared from adjuvant radiotherapy and the 5-year PFS was 58.5%, which favorably compares to other irradiation avoidance strategies for classic medulloblastomas, which most series reporting on non metastatic medulloblastomas. Von Bueren and colleagues reported an 5-year EFS of 30% for the group of localized classic MB treated with the HIT SKK 2000.18 The 4-year EFS in the COG P9934 using conventional chemotherapy and focal irradiation was 23% for the group of M0 desmoplastic medulloblastoma, whereas the French group described a 5-year EFS of 64% using high dose busulfan-thiotepa and focal irradiation for children with localized classic medulloblastoma only.11, 20
The single significant impact of the metastatic status was observed when comparing macroscopic disease to M0 and M1 disease. While conflicting data exist on the significance of M1 disease17, 21, our experience may suggest that HDC regimens can successfully control M1 disease in young children, even in group 3 medulloblastomas.
Cohen et al provided information on the use of radiotherapy on the 99703 cohort for 3/4 of their medulloblastoma, describing out of the 23 survivors, 11 who never received radiotherapy, 5 who were irradiated in an adjuvant setting and 2 at the time of relapse. Information was missing for the remaining 2 patients. To further contribute to the description of the landscape of current practice, we report a rather low rate (16.9%) of adjuvant radiotherapy in our cohort. We did not find a significant association between the use of adjuvant irradiation and outcome, but the limited number of patients precludes meaningful conclusions and the exact role of adjuvant irradiation in this population remains unclear. Understanding the pattern of relapse in young children with non-SHH and/or non ND/MBEN medulloblastoma following HDC strategies should help identify patients who may benefit from adjuvant irradiation.
Although neurocognitive preservation is one of the driving justifications of the “baby brain tumors strategies”, information on long-term neurocognitive outcome of young children remains limited.22 A large number of our surviving patients were tested showing a neurocognitive profile within the average range (mean FSIQ of 91.3). However this regimen combining high-dose carboplatin following 300 mg/m2 of cisplatinum, is associated with high rate of significant hearing loss, known to negatively impact academic performance in young children.23
Conclusion
In this large cohort of young children with medulloblastoma, we describe encouraging survival rates using sequential HDC, without systematic delivery of adjuvant radiotherapy, specifically for classic/NOS and group 3 patients. While the described strategy may not be the ultimate answer for all infants with medulloblastoma, it deserves continuous consideration when planning the next generation of infant trials for non desmoplastic and/or non SHH MB, possibly in conjunction with new targeted agents. The potential role and optimal modality of adjuvant irradiation remain unanswered. The evaluation of salvage irradiation is also critically needed to further support the legitimacy of this encouraging irradiation sparing HDC strategy 24.
Supplementary Material
Supplemental figure 1: Kaplan Meier analysis of the progression free survival (PFS) and overall survival according metastatic status
Progression free survival (PFS) according to A) non metastatic (M0) versus Metastatic M + (M1, M2, M3) B) M0 versus M1 (CSF positivity only) versus macroscopic metastasis (M+:M2 and M3). C) combined M0 and M1 versus macroscopic metastasis (M+:M2 and M3).
Overall survival (OS) according to D) non metastatic (M0) versus Metastatic M + (M1, M2, M3) E) M0 versus M1 (CSF positivity only) versus macroscopic metastasis (M+:M2 and M3). F) combined M0 and M1 versus macroscopic metastasis (M+:M2 and M3).
Supplemental table I: Correlation between histology and molecular sub grouping
Acknowledgments
Acknowledgments of research support for the study
V. Ramaswamy supported by a Canadian Institute for Health Research Fellowship, an Alberta-Innovates Health Solutions Clinical Fellowship and a Young Investigator Award from Alex’s Lemonade Stand”.
M.D Taylor supported by operating grants from the Canadian Institute of Health Research operating grant, National Institutes of Health (R01CA159859 and R01CA148699) and the Pediatric Brain Tumor Foundation”
Abbreviations
- CCR
Continous Complete response
- CSI
Craniospinal irradiation
- GTR
Gross total resection
- HDC
High dose chemotherapy
- LCA MB
Large cell anaplastic Medulloblastoma
- ND/MBEN
Nodular desmoplastic/Medulloblastoma with extensive nodularity
- RT
Radiotherapy
- SHH
Sonic Hedgehog signaling pathway medulloblastoma subgroup
- WNT
WNT signaling pathway medulloblastoma subgroup
Footnotes
Study presented at the ISPNO meeting in Singapore June 2014 (poster), SIOP meeting Toronto October 2014 (Best poster award), SNO meeting Miami November 2014 (oral presentation)
Disclaimers: None of the authors have conflict of interest to disclose
Contributor Information
Lucie Lafay-Cousin, Alberta Children’s Hospital, Department of Pediatrics and Oncology, 2888 Shaganappi trail NW T3B 6A8 Calgary, Alberta, Canada.
Amy Smith, Arnold Palmer Hospital, 92 West Miller Street Orlando, FL 32806.
Susan N Chi, Dana-Farber Cancer Institute, Pediatric Neuro-Oncology, 450 Brookline Ave, Boston, MA 02215, USA.
Elizabeth Wells, Pediatrics, Neurology & Integrative Systems Biology Brain Tumor Institute, Children’s National Health System, Washington, DC.
Jennifer Madden, Children’s Hospital Colorado, Associate Professor, Pediatrics, University of Colorado, 13123 E. 16th Ave, Aurora, CO 80045.
Ashley Margol, Children’s Hospital Los Angeles, Keck School of Medicine of University of Southern California, 4650 Sunset Boulevard, MS #54, Los Angeles, CA 90027-6016.
Vijay Ramaswamy, Division of Pediatric Hematology Oncology, Hospital for Sick Children, 555 university avenue, Toronto, ON, M5G 1X8.
Jonathan Finlay, Nationwide Children’s Hospital and the Ohio State University, 700 Children’s Drive, Columbus, Ohio 43205, USA.
Michael D Taylor, Division of Neurosurgery, Hospital for Sick Children, 555 university avenue, Toronto, ON, M5G 1X8.
Girish Dhall, Children’s Hospital Los Angeles, Keck School of Medicine of University of Southern California, 4650 Sunset Boulevard, MS #54, Los Angeles, CA 90027-6016.
Douglas Strother, Alberta Children’s Hospital, Departments of Pediatrics and Oncology, 2888 Shaganappi trail NW T3B 6A8 Calgary, Alberta, Canada.
Mark W Kieran, Dana-Farber Cancer Institute, Pediatric Neuro-Oncology, 450 Brookline Ave, Boston, MA 02215, USA.
Nicholas K Foreman, University of Colorado, 13123 East 16th avenue B115, Aurora, CO, 80045.
Roger J Packer, Center for Neuroscience and Behavioral Medicine, Brain Tumor Institute Children’s National Health System, 111 Michigan Ave, NW, Washington, DC. 20010.
E Bouffet, Division of Pediatric Hematology Oncology, Hospital for Sick Children, 555 university avenue, Toronto, ON, M5G 1X8.
References
- 1.Lafay-Cousin L, Strother D. Current treatment approaches for infants with malignant central nervous system tumors. Oncologist. 2009;14:433–44. doi: 10.1634/theoncologist.2008-0193. [DOI] [PubMed] [Google Scholar]
- 2.Cohen BH, Geyer JR, Miller DC, Curran JG, Zhou T, Holmes E, Ingles SA, Dunkel IJ, Hilden J, Packer RJ, Pollack IF, Gajjar A, Finlay JL Children’s Oncology Group. A Pilot study of intensive chemotherapy with peripheral hematopoietic cell support for children less than 3 years of age with malignant brain tumors, the CCG-99703 Phase I/II Study. A report from the Children’s Oncology Group. Pediatr Neurol. 2015;53:31–46. doi: 10.1016/j.pediatrneurol.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28:1788–95. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 4.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–72. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramaswamy V, Remke M, Bouffet E, Faria CC, Perreault S, Cho YJ, Shih DJ, Luu B, Dubuc AM, Northcott PA, Schüller U, Gururangan S, McLendon R, Bigner D, Fouladi M, Ligon KL, Pomeroy SL, Dunn S, Triscott J, Jabado N, Fontebasso A, Jones DT, Kool M, Karajannis MA, Gardner SL, Zagzag D, Nunes S, Pimentel J, Mora J, Lipp E, Walter AW, Ryzhova M, Zheludkova O, Kumirova E, Alshami J, Croul SE, Rutka JT, Hawkins C, Tabori U, Codispoti KE, Packer RJ, Pfister SM, Korshunov A, Taylor MD. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14:1200–7. doi: 10.1016/S1470-2045(13)70449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margol AS, Robison NJ, Gnanachandran J, Hung LT, Kennedy RJ, Vali M, Dhall G, Finlay JL, Erdreich-Epstein A, Krieger MD, Drissi R, Fouladi M, Gilles FH, Judkins AR, Sposto R, Asgharzadeh S. Tumor-associated macrophages in SHH subgroup of medulloblastomas. Clin Cancer Res. 2015;21:1457–65. doi: 10.1158/1078-0432.CCR-14-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman LM, Donson AM, Nakachi I, Griesinger AM, Birks DK, Amani V, Hemenway MS, Liu AK, Wang M, Hankinson TC, Handler MH, Foreman NK. Molecular sub-group-specific immunophenotypic changes are associated with outcome in recurrent posterior fossa ependymoma. Acta Neuropathol. 2014;12:731–45. doi: 10.1007/s00401-013-1212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietsch T, Schmidt R, Remke M, Korshunov A, Hovestadt V, Jones DT, Felsberg J, Kaulich K, Goschzik T, Kool M, Northcott PA, von Hoff K, von Bueren AO, Friedrich C, Mynarek M, Skladny H, Fleischhack G, Taylor MD, Cremer F, Lichter P, Faldum A, Reifenberger G, Rutkowski S, Pfister SM. Prognostic significance of clinical, histopathological, and molecular characteristics of medulloblastomas in the prospective HIT2000 multicenter clinical trial cohort. Acta Neuropathol. 2014;128:137–49. doi: 10.1007/s00401-014-1276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi SN, Gardner SL, Levy AS, Knopp EA, Miller DC, Wisoff JH, Weiner HL, Finlay JL. Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol. 2004;22:4881–7. doi: 10.1200/JCO.2004.12.126. [DOI] [PubMed] [Google Scholar]
- 10.Dhall G, Grodman H, Ji L, Sands S, Gardner S, Dunkel IJ, McCowage GB, Diez B, Allen JC, Gopalan A, Cornelius AS, Termuhlen A, Abromowitch M, Sposto R, Finlay JL. Outcome of children less than three years old at diagnosis with non- metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer. 2008;50:1169–75. doi: 10.1002/pbc.21525. [DOI] [PubMed] [Google Scholar]
- 11.Bergthold G, El Kababri M, Varlet P, Dhermain F, Sainte-Rose C, Raquin MA, Kieffer V, Goma G, Grill J, Valteau-Couanet D, Dufour C. High-dose busulfan-thiotepa with autologous stem cell transplantation followed by posterior fossa irradiation in young children with classical or incompletely resected medulloblastoma. Pediatr Blood Cancer. 2014;61:907–12. doi: 10.1002/pbc.24954. [DOI] [PubMed] [Google Scholar]
- 12.Thorarinsdottir HK, Rood B, Kamani N, Lafond D, Perez-Albuerne E, Loechelt B, Packer RJ, MacDonald TJ. Outcome for children <4 years of age with malignant central nervous system tumors treated with high-dose chemotherapy and autologous stem cell rescue. Pediatr Blood Cancer. 2007;48:278–84. doi: 10.1002/pbc.20781. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez RB, Sethi R, Depauw N, Pulsifer MB, Adams J, McBride SM, Ebb D, Fullerton BC, Tarbell NJ, Yock TI, Macdonald SM. Proton radiation therapy for pediatric medulloblastoma and supratentorial primitive neuroectodermal tumors: outcomes for very young children treated with upfront chemotherapy. Int J Radiat Oncol Biol Phys. 2013;87:120–6. doi: 10.1016/j.ijrobp.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Martínez A, Lassaletta A, González-Vicent M, Sevilla J, Díaz MA, Madero L. High-dose chemotherapy with autologous stem cell rescue for children with high risk and recurrent medulloblastoma and supratentorial primitive neuroectodermal tumors. J Neurooncol. 2005;71:33–8. doi: 10.1007/s11060-004-4527-4. [DOI] [PubMed] [Google Scholar]
- 15.Rutkowski S, von Hoff K, Emser A, Zwiener I, Pietsch T, Figarella-Branger D, Giangaspero F, Ellison DW, Garre ML, Biassoni V, Grundy RG, Finlay JL, Dhall G, Raquin MA, Grill J. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol. 2010;28:4961–8. doi: 10.1200/JCO.2010.30.2299. [DOI] [PubMed] [Google Scholar]
- 16.Leary SE, Zhou T, Holmes E, Geyer JR, Miller DC. Histology predicts a favorable outcome in young children with desmoplastic medulloblastoma: a report from the children’s oncology group. Cancer. 2011;117:3262–7. doi: 10.1002/cncr.25856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, Graf N, Emser A, Pietsch T, Wolff JE, Kortmann RD, Kuehl J. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–86. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 18.von Bueren AO, von Hoff K, Pietsch T, Gerber NU, Warmuth-Metz M, Deinlein F, Zwiener I, Faldum A, Fleischhack G, Benesch M, Krauss J, Kuehl J, Kortmann RD, Rutkowski S. Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol. 2011;13:669–79. doi: 10.1093/neuonc/nor025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–14. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashley DM, Merchant TE, Strother D, Zhou T, Duffner P, Burger PC, Miller DC, Lyon N, Bonner MJ, Msall M, Buxton A, Geyer R, Kun LE, Coleman L, Pollack IF. Induction chemotherapy and conformal radiation therapy for very young children with nonmetastatic medulloblastoma: Children’s Oncology Group study P9934. J Clin Oncol. 2012;30:3181–6. doi: 10.1200/JCO.2010.34.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders RP, Onar A, Boyett JM, Broniscer A, Morris EB, Qaddoumi I, Armstrong GT, Boop FA, Sanford RA, Kun LE, Merchant TE, Gajjar A. M1 Medulloblastoma: high risk at any age. J Neurooncol. 2008;90:351–5. doi: 10.1007/s11060-008-9671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sands SA, Oberg JA, Gardner SL, Whiteley JA, Glade-Bender JL, Finlay JL. Neuropsychological functioning of children treated with intensive chemotherapy followed by myeloablative consolidation chemotherapy and autologous hematopoietic cell rescue for newly diagnosed CNS tumors: an analysis of the Head Start II survivors. Pediatr Blood Cancer. 2010;54:429–36. doi: 10.1002/pbc.22318. [DOI] [PubMed] [Google Scholar]
- 23.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy:underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–96. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 24.Müller K, Mynarek M, Zwiener I, Siegler N, Zimmermann M, Christiansen H, Budach W, Henke G, Warmuth-Metz M, Pietsch T, von Hoff K, von Bueren A, Bode U, Rutkowski S, Kortmann RD, Fleischhack G, Tippelt S. Postponed is not canceled: role of craniospinal radiation therapy in the management of recurrent infant medulloblastoma--an experience from the HIT-REZ 1997 & 2005 studies. Int J Radiat Oncol Biol Phys. 2014;88:1019–24. doi: 10.1016/j.ijrobp.2014.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1: Kaplan Meier analysis of the progression free survival (PFS) and overall survival according metastatic status
Progression free survival (PFS) according to A) non metastatic (M0) versus Metastatic M + (M1, M2, M3) B) M0 versus M1 (CSF positivity only) versus macroscopic metastasis (M+:M2 and M3). C) combined M0 and M1 versus macroscopic metastasis (M+:M2 and M3).
Overall survival (OS) according to D) non metastatic (M0) versus Metastatic M + (M1, M2, M3) E) M0 versus M1 (CSF positivity only) versus macroscopic metastasis (M+:M2 and M3). F) combined M0 and M1 versus macroscopic metastasis (M+:M2 and M3).
Supplemental table I: Correlation between histology and molecular sub grouping