Abstract
The development of biomarkers to predict the progression of Parkinson’s disease (PD) from its earliest stage through its heterogeneous course is critical for research and therapeutic development. The Parkinson’s Progression Markers Initiative (PPMI) study is an ongoing international multicenter, prospective study to validate biomarkers in drug-naïve PD patients and matched healthy controls (HC). We quantified cerebrospinal fluid (CSF) alpha-synuclein (α-syn), amyloid-beta1–42 (Aβ1–42), total tau (t-tau), and tau phosphorylated at Thr181 (p-tau) in 660 PPMI subjects at baseline, and correlated these data with measures of the clinical features of these subjects. We found that CSF α-syn, t-tau and p-tau levels, but not Aβ1–42, were significantly lower in PD compared with HC, while the diagnostic value of the individual CSF biomarkers for PD diagnosis was limited due to large overlap. The level of α-syn, but not other biomarkers, was significantly lower in PD patients with non-tremor-dominant phenotype compared with tremor-dominant phenotype. In addition, in PD patients the lowest Aβ1–42, or highest t-tau/Aβ1–42 and t-tau/α-syn quintile in PD patients were associated with more severe non-motor dysfunction compared with the highest or lowest quintiles, respectively. In a multivariate regression model, lower α-syn was significantly associated with worse cognitive test performance. APOE ε4 genotype was associated with lower levels of Aβ1–42, but neither with PD diagnosis nor cognition. Our data suggest that the measurement of CSF biomarkers in early-stage PD patients may relate to disease heterogeneity seen in PD. Longitudinal observations in PPMI subjects are needed to define their prognostic performance.
Keywords: Parkinson’s disease, Cerebrospinal fluid biomarker, Parkinson’s Progression Markers Initiative, Aβ1–42, Tau, Alpha-synuclein
Introduction
The Parkinson’s Progression Markers Initiative (PPMI), an international multicenter, prospective, longitudinal observational study, was designed to discover and validate biomarkers that predict the heterogeneous progression of Parkinson’s disease (PD) from disease diagnosis onward [27]. PD is a progressive neurodegenerative disease characterized by the relentless accumulation of alpha-synuclein (α-syn) inclusions known as Lewy bodies and neurites (LBs and LNs, respectively) manifested clinically by a broad clinical spectrum of motor and non-motor symptoms, presenting diverse PD subtypes with different clinical features and rates of progression [16]. For example, the DATATOP study explored the heterogeneity of PD clinical features, revealing heterogeneity of PD not only in motor phenotypes (i.e., tremor, rigidity, or postural instability and gait disturbance subtypes), but also in non-motor symptoms (e.g., cognitive decline, autonomic features), age of onset and pathologic features. Several clinicopathologic studies have revealed that LBs, LNs and neuronal loss in different areas in the brain are related to the motor subtypes (tremor vs. akinesia/rigidity) and other clinical features of PD patients [17, 30–32]. These data suggested different pathophysiological mechanisms of the clinical motor subtypes of PD and the disease heterogeneity that have important prognostic implications. Furthermore, the involvement of Alzheimer’s disease (AD) pathology, cortical LBs/LNs, and/or cerebral angiopathy is more prevalent in non-tremor-dominant (non-TD) phenotype than tremor-dominant (TD) phenotype [11, 14, 15, 33, 40].
It is increasingly evident that cognitive impairment, including dementia, is a frequent and highly problematic non-motor manifestation in PD patients, and a risk factor for increased mortality [1, 12, 19], with great variability in progression over time [2, 13, 41]. Clinical markers (e.g., motor phenotype) can be useful to predict disease progression, but, they have limitations. For instance, clinical markers are unstable in early-stage PD and are influenced by the introduction of PD pharmacotherapy. Conversely, biochemical biomarkers may provide insights into the pathogenesis and variable long-term course of PD, and could identify molecular subtypes that may have differential response to treatment.
We previously reported on the association between baseline cerebrospinal fluid (CSF) biomarkers and clinical features in the first 102 PPMI subjects including 63 drug-naïve early PD patients [18]. Although the number of subjects was limited, we described several findings in this qualified cohort: (1) in PD patients, CSF α-syn, amyloid-beta1–42 (Aβ1–42), total tau (t-tau), and tau phosphorylated at Thr181 (p-tau) concentrations were lower than those in healthy controls (HC). (2) The PD-associated lower levels of CSF biomarkers were more prominent in patients with the non-TD phenotype, particularly in patients with postural instability and gait-disturbance dominant motor phenotype (PIGD) compared with TD phenotype. (3) A significant correlation between the concentration of α-syn and t-tau or p-tau, but not Aβ1–42, was observed in both PD and HC. These results suggest the following hypotheses: (1) CSF concentrations of α-syn, Aβ1–42, t-tau, and p-tau in drug-naïve early-stage PD patients are lower than those in HC. (2) In subgroup analysis according to the motor phenotype, and/or CSF biomarker levels, the measures of these CSF biomarkers may distinguish and help to explain brain changes that underlie clinical subgroups of PD, which may influence on progression and response to disease modifying therapies. (3) As multiple and dynamic processes might coexist in PD, the interaction between α-syn and tau or Aβ could play a role in the development or progression of PD complexity and heterogeneity [15].
While our preliminary data were of great interest, they needed to be further explored in a larger cohort since these findings vary across prior studies [4, 22, 23, 28, 29, 34]. Other studies have suggested that lower CSF Aβ1–42 levels predict more rapid cognitive decline in PD [5, 35]. In the large de novo PD cohort of PPMI we are able to investigate cross-sectional associations between CSF biomarkers and cognition which may herald future decline. PPMI is the largest ongoing, prospective, longitudinal, multinational study of drug-naïve early PD and matched HC, and this cohort continues to mature [27]. To describe CSF biomarker levels in PD as compared to HC at baseline, and to test our preliminary study-driven hypotheses in the larger population including very early-stage PD enrolled in 21 qualified clinical sites, we analyzed baseline CSF biomarker levels, and the association with genotypic and clinical features in the full dataset of 660 PPMI subjects (189 HC, 412 PD and 59 scans without evidence of dopamine transporter deficit or SWEDD) in the present study.
Materials and methods
Participants and sample size
As described previously, newly diagnosed, drug-naïve PD patients (N = 423), age- and gender-matched HC subjects (N = 196) and SWEDD individuals (N = 60) were recruited between June 2010 and May 2013, from 21 PD centers in Europe and the United States according to the PPMI protocols (http://ppmi-info.org/study-design). The study was approved by the Institutional Review Board of all participating sites, and written informed consent was obtained from all participants before inclusion in the study. Subjects underwent clinical (motor, neuropsychiatric and cognitive) and imaging assessments and donated biologic samples including CSFs. Of 679 enrolled individuals, 660 subjects (189 HC, 412 PD, and 59 SWEDD) who agreed to donate their CSF samples at baseline visit were included in this study. Detailed standardized protocols for patient selection, clinical assessments, biospecimen analysis, and data acquisition are described elsewhere. A diagnosis of PD in all patients was made within 2 years before the screening visit, and only patients with a Hoehn and Yahr (H&Y) stage of I or II were included, except 2 PD subjects with H&Y III. The disease severity was assessed by use of MDS-UPDRS rating and H&Y stage. As described in our previous study [18], we classified PD by their baseline motor symptoms as manifesting TD, PIGD, or IND using previously published formula [18, 36], but we combined PIGD and IND groups into a non-TD group due to the observation of instability of these subgroups based on our preliminary data analysis. To compare the clinical parameters between groups with the relatively low versus high level of CSF biomarkers, we classified PD groups by quintile levels of CSF biomarker (i.e., biomarker level 0–20, 20–40, 40–60, 60–80 and 80–100 percentile in PD groups) and compare PD groups with the lowest quintile (0–20 percentile) and the highest quintile (80–100 percentile) levels.
Analysis CSF biomarkers
CSF was collected by standardized lumbar puncture procedures. Shipment and storage were performed as described in the PPMI biologics manual (http://ppmi-info.org) and in our previous study [18]. The coded frozen aliquots of CSF were transferred from the PPMI Biorepository Core laboratories to the University of Pennsylvania and to Covance for analyses. CSF Aβ1–42, t-tau and p-tau were measured using the xMAP-Luminex platform with INNOBIA AlzBio3 immunoassay kit-based reagents (Fujirebio-Innogenetics, Ghent, Belgium), as described previously. Following the standardized operating procedure (SOP) of the University of Pennsylvania Biomarker Core laboratory, duplicate 75 µL aliquots of standards, aqueous controls and CSF samples (including two CSF pools for quality control) were analyzed, and a total of 38 runs showed mean variability in the concentration (%CV) of Aβ1–42, t-tau and p-tau in CSF pools were 9.0, 6.7, and 8.2 %, respectively. The results for t-tau in 6 baseline samples (2 HC and 4 PD) and p-tau in 2 baseline samples of PD did not meet the SOP criterion requiring a bead count of at least 50; therefore, these values were excluded from statistical analysis. CSF α-syn and CSF hemoglobin levels were analyzed using appropriate commercially available sandwich type ELISA kits (Covance, Dedham, MA), as previously described. A total of 81 runs for α-syn according to the SOP at Covance including 2 independent QC samples were conducted, and the mean variability of α-syn measurement over 81 runs was 17 %.
Genotyping
At screening visit, genomic DNA was extracted from whole blood of subject. APOE genotypes were determined with the use of allele-specific oligonucleotide probes labeled with fluorogenic reporter (TaqMan method). Two non-synonymous single nucleotide polymorphisms (SNP), rs429358 (APOE-C112R) and rs7412 (APOE-R158C) were genotyped in order to distinguish between ε2, ε3, and ε4 alleles. TaqMan assays were used according to the manufacturer’s instruction to genotype these SNPs on a 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). PCR amplification, plate reading and allelic discrimination were performed on SDS instrumentation using the Computer Software SDS V2.4 2010. We classified subjects by their APOE genotypes; the presence or absence of APOE ε4 genotypes (C allele in both SNP sites).
SNPs of SNCA and MAPT genes were determined using Illumina NeuroX array on whole-blood extracted DNA per manufacturer’s protocol (Illumina Inc., San Diego, CA). The NeuroX array is an Illumina Infinium iSelect HD Custom Genotyping array containing Illumina standard content exonic variants and additional custom variants designed for neurological disease studies. Of the custom variants, approximately 12,000 are designed to study PD and are applicable to both large population studies of risk factors and to investigations of familial disease and known mutations. The Genotyping Analysis Module within Genome Studio version 1.9.4 was used to analyze data. The threshold call rate for sample inclusion was 95 %, and quality control of sample handling was determined by comparing the subject’s gender with the genotypic gender estimated from X chromosome heterogeneity. The resulting cluster plot for all selected SNPs showed good cluster separation and a high degree of confidence in genotype calling.
Statistical analysis
All clinical, genetic, and CSF biomarkers data included in this study were simultaneously downloaded from the PPMI database on June 23, 2014 and analyzed by 2 independent laboratories (University of Pennsylvania and University of Iowa) according to an agreed upon statistical plan. These laboratories agreed on all analyzed results reported here. Statistical analysis was performed using SAS 9.3 (SAS Institute, Cary, NC), and values with p < 0.05 were regarded as statistically significant. CSF biomarker levels, demographic data and clinical variables were compared between groups using Chi-square tests for categorical variables and Mann–Whitney U test or Kruskal–Wallis test for continuous variables, as appropriate. The comparison of various clinical parameters or CSF biomarkers between groups is exploratory in this observational study; therefore, analyses were done without correction for multiple comparison. The correlation between CSF biomarker levels were evaluated using Spearman’s rank correlation. To examine the effects of the level of CSF biomarkers on the clinical variables, we divided PD patients by quintile of each CSF biomarkers or ratios, and compared the clinical parameters between the highest quintile and the lowest quintile (e.g., ≤20 vs. >80 percentile of α-syn). The effect of clinical variables on specific CSF biomarker levels were examined in univariate and multivariate linear regression models with adjustment for confounding factors. Any variables that had univariate associations with p values less than 0.15 were included in a multivariate model. A backward stepwise-selection model was used to develop a final multivariate linear regression model to evaluate the association of levels of CSF biomarkers or ratios with individual clinical variables in PD patients after controlling for possible confounding factors; i.e., age, gender, and age at onset.
Results
Demographics and clinical characteristics
CSF α-syn, Aβ1–42, t-tau, and p-tau concentrations were measured in 97 % of the 683 total subjects enrolled in the PPMI study (N = 660; 189 HC, 412 PD and 59 SWEDD). The baseline age, gender distribution or education level of HC was not significantly different compared to the PD cohort (Table 1). The severity and disability assessed by the Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) total score, cognitive performance [i.e., verbal memory assessed by Hopkins Verbal Learning Test-revised (HVLT-R), processing speed/attention assessed by Symbol Digit Modality Test (SDMT), executive function/working memory assessed by WMS-III Letter-Number Sequencing Test (LNS), visuospatial abilities assessed by Benton Judgment of Line Orientation test (BJLO), and global cognitive function assessed by Montreal Cognitive Assessment (MoCA)], and neuropsychiatric symptoms in HC, PD and SWEDD are summarized in Table 1. As expected, the test scores for motor symptoms (higher MDS-UPDRS III score), global cognitive function (lower MoCA score), neuropsychiatric symptoms (higher SCOPA-AUT and STAI score), verbal memory (lower HVLT-R score), semantic fluency (lower semantic fluency score), processing speed/attention (low SDMT score) and dopamine transporter ligand (DaT scan; DaTSCAN™ or [123I]β-CIT) uptake in the PD group were significantly different from those in the HC group. Clinical variables in the SWEDD group were similar to those of the PD subjects; however, the UPSIT score and DaT uptake in SWEDD subjects were significantly higher than in the PD group and more comparable to the HC group. The UPDRS-I (non-motor symptoms) and UPDRS-III (motor symptoms) scores of the SWEDD group were significantly higher and lower, respectively, than those scores of PD subjects.
Table 1.
Median and range of demographic information and clinical characteristics at baseline of 660 PPMI subjects
| Clinical variables Median (range) |
HC (N = 189) | PD (N = 412) | SWEDD (N = 59) | p value by Mann–Whitney test | |
|---|---|---|---|---|---|
| HC vs. PD | PD vs. SWEDD | ||||
| Age (years) | 62.05 (30.62–83.68) | 62.37 (33.5–84.88) | 63.25 (38.34–78.82) | 0.5002 | 0.4597 |
| Gender, F/M (% of male) | 69/120 (63 %) | 142/270 (66 %) | 23/36 (61 %) | 0.6263# | 0.4964# |
| Education (years) | 16 (8–24) | 16 (5–26) | 16 (8–24) | 0.0737 | 0.3263 |
| Age at onset (years) | – | 60.56 (25.37–83.01) | 60.74 (35.31–77.28) | – | 0.3911 |
| Duration of disease*, (months) | – | 4.22 (0.03–35.83) | 3.87 (0.53–37.00) | – | 0.9409 |
| CSF Hb >200 ng/mL, N (%) | 35 (19 %) | 84 (20 %) | 11 (19 %) | 0.5933# | 0.7548# |
| H&Y stage (N, %) | |||||
| Stage 0 | – | 0 | 0 | – | 0.1794# |
| Stage 1 | 179 (43.4 %) | 33 (55.9 %) | |||
| Stage 2 | 231 (56.1 %) | 26 (44.1 %)) | |||
| Stage 3–5 | 2 (0.5 %) | 0 | |||
| Missing | 0 (0 %) | 0 | |||
| MDS-UPDRS score | |||||
| Part I | 2 (0–17) | 5 (0–24) | 7 (0–27) | <0.001 | 0.0017 |
| Part II | 0 (0–6) | 5 (0–22) | 3 (0–25) | <0.001 | 0.2002 |
| Part III | 0 (0–13) | 20 (4–51) | 13 (2–42) | <0.001 | <0.0001 |
| Total | 3 (0–20) | 31 (7–72) | 26 (4–91) | <0.001 | 0.0093 |
| UPSIT score | 35 (11–40) | 22 (1–40) | 34 (12–39) | <0.001 | <0.0001 |
| HVLT-R score | |||||
| Total recall | 26 (15–35) | 25 (9–36) | 25 (13–31) | 0.0003 | 0.7998 |
| Delayed recall | 10 (2–12) | 9 (0–12) | 9 (0–12) | <0.001 | 0.8146 |
| Discr. recognition | 11 (−4 to 12) | 10(−4 to 12) | 10 (−2 to 12) | 0.0001 | 0.0408 |
| WMS-III LNS score | 11 (2–20) | 11(2–20) | 10 (4–15) | 0.2173 | 0.1192 |
| BJLO score | 14 (4–15) | 13 (5–15) | 13 (5–15) | 0.0602 | 0.9900 |
| Semantic fluency | 52 (22–80) | 48 (20–103) | 43 (23–81) | 0.0009 | 0.0226 |
| SDMT score | 47 (20–83) | 42 (7–82) | 43 (19–71) | <0.001 | 0.6167 |
| SCOPA-AUT score | 5 (0–20) | 8 (0–39) | 12 (3–44) | <0.001 | 0.0003 |
| MoCA | 28 (26–30) | 28 (17–30) | 28 (17–30) | <0.001 | 0.6791 |
| [MoCA <26, N] | [0, 0.0 %] | [91, 22.1 %] | [9, 15.3 %] | ||
| MSEADL | – | 90 (70–100) | 95 (75–100) | – | 0.0515 |
| STAI score | 54 (40–105) | 62 (40–137) | 65 (40–113) | <0.001 | 0.0875 |
| DAT scan (median uptake) | |||||
| Caudate median | 2.88 (1.32–5.2) | 1.95 (0.39–3.71) | 2.86 (1.38–4.01) | <0.001 | <0.0001 |
| Putamen median | 2.09 (0.64–3.89) | 0.79 (0.24–2.17) | 2.11 (0.78–3.01) | <0.001 | <0.0001 |
Disease duration was defined by (enrollment date–diagnosis date)
p values by Chi-square test
When PD subjects were classified by their motor phenotypes as previously described [18, 36], 293 had a TD phenotype and the others were either PIGD (N = 73) or indeterminate (IND; N = 45). In this study, we combined PIGD and IND groups to create a non-TD group (N = 118), previously reported [20]. The disease severity and disability (higher MDS-UPDRS total score, p = 0.0418), anxiety (higher State-Trait Anxiety Inventory score; STAI, p = 0.0029), activities of daily living (lower Modified Schwab and England Activities of Daily Living score; MSEADL, p = 0.0109) and DAT binding in the putamen (lower score, p = 0.0184) in non-TD group were significantly different from TD patients, while other clinical parameters showed no significant difference (Table S1).
Comparison of CSF biomarkers between HC and PD
The levels of CSF α-syn, t-tau and p-tau as well as the ratios of these measures including the t-tau/Aβ1–42 ratio, p-tau/Aβ1–42 ratio were lower, and Aβ1–42/α-syn ratio was higher in the PD subjects, compared with HC group, while levels of CSF Aβ1–42 as well as the p-tau/t-tau, t-tau/α-syn and p-tau/α-syn ratios were not different between groups (Table 2). The significantly lower level of α-syn in PD patients relative to HC subjects was still observed (p = 0.0005) when we excluded subjects with high CSF hemoglobin (Hb) levels (>200 ng/mL) to avoid potential impact of contaminant plasma Hb [18]. When we excluded the 102 subjects (63 PD and 39 HC) reported in our preliminary study [18] and tested this subset of the full PPMI population (349 PD and 150 HC) there was little change in the statistical significance of the results. Therefore, we report the findings in the full population that includes these 102 subjects in this manuscript. The CSF biomarker levels of SWEDD subjects indicated that they had values between the HC and PD groups, and the median concentrations of CSF α-syn and Aβ1–42 in SWEDD subjects were significantly higher than in the PD group.
Table 2.
Comparison of baseline CSF biomarker levels in 660 PPMI subjects
| CSF biomarkers | HC (N = 189) | PD (N = 412) | SWEDD (N = 59) |
p value by Mann–Whitney test |
|
|---|---|---|---|---|---|
| HC vs. PD | PD vs. SWEDD | ||||
| α-Syn (pg/mL), N Median (range) [95 % CI] |
189 1981.83 (592.56–8608.91) [2048–2361] |
412 1715.06 (332.93–6694.55) [1769–1921] |
59 1959.82 (743.07–7201.49) [1873–2408] |
0.0002 | 0.0259 |
| α-Syn (pg/mL), N Median (range) [95 % CI]* |
154 1975.95 (592.56–8608.91) [2033–2395] |
328 1708.76 (332.93–5110.77) [1732–1886] |
48 1955.99 (743.07–3954.43) [1837–2306] |
0.0005 | 0.0383 |
| Aβ1–42 (pg/mL), N Median (range) [95 % CI] |
189 378.5 (88.8–879.5) [361.5–394.1] |
412 367.9 (129.2–796.5) [360.8–380.3] |
59 403.1 (155.8–628.4) [376.5–432.2] |
0.3858 | 0.013 |
| t-tau (pg/mL), N Median (range) [95 % CI] |
187 44.8 (18.4–223.1) [48.63–56.46] |
408 41 (14.4–121) [42.92–46.47] |
59 40.9 (22.6–141) [42.45–54.43] |
0.001 | 0.3784 |
| p-tau181 (pg/mL), N Median (range) [95 % CI] |
189 14.1 (5.1–73.3) [16.59–19.95] |
410 12.25 (4.7–94.1) [14.66–16.61] |
59 12.8 (6.1–70.8) [14.13–20.30] |
0.0004 | 0.3388 |
| t-tau/Aβ1–42, N Median (range) [95 % CI] |
187 0.11 (0.05–2.12) [0.1355–0.1896] |
408 0.11 (0.04–0.52) [0.1198–0.1323] |
59 0.11 (0.05–0.5) [0.1085–0.1526] |
0.0242 | 0.4398 |
| p-tau181/Aβ1–42, N Median (range) [95 % CI] |
189 0.04 (0.02–0.66) [0.0462–0.0645] |
410 0.03 (0.01–0.51) [0.0404–0.0470] |
59 0.03 (0.02–0.18) [0.0361–0.0540] |
0.0096 | 0.5957 |
| p-tau181/t-tau, N Median (range) [95 % CI] |
187 0.31 (0.13–1.4) [0.3418–0.3963] |
406 0.3 (0.08–2.14) [0.3493–0.3932] |
59 0.3 (0.13–1.23) [0.3154–0.4383] |
0.5234 | 0.9715 |
| Aβ1–42/α-syn, N Median (range) [95 % CI] |
189 0.19 (0.02–0.64) [0.1908–0.2176] |
412 0.21 (0.06–1.03) [0.2189–0.2380] |
59 0.21 (0.06–0.57) [0.1944–0.2448] |
0.0045 | 0.5351 |
| t-tau/α-syn, N Median (range) [95 % CI] |
187 0.02 (0.01–0.06) [0.0239–0.0260] |
408 0.02 (0.01–0.06) [0.0249–0.0266] |
59 0.02 (0.01–0.04) [0.0221–0.0263] |
0.5378 | 0.2254 |
| p-tau181/α-syn, N Median (range) [95 % CI] |
189 0.01 (0–0.04) [0.0084–0.0100] |
410 0.01 (0–0.11) [0.0088–0.0103] |
59 0.01 (0–0.03) [0.0072–0.0101] |
0.8567 | 0.585 |
Data from subjects with CSF Hgb <200 ng/mL
To test the hypothesis from our previous study that lower level CSF concentrations of α-syn, t-tau, and p-tau in PD patients may be significantly associated with the non-TD phenotype, we compared the levels of CSF biomarkers between TD and non-TD patients (Table 3). The level of CSF α-syn in the non-TD group was significantly lower than that in the TD group (p = 0.0271), and this was still observed after exclusion of subjects with high CSF Hb levels (p = 0.0376). However, none of the other CSF biomarkers were significantly different between the TD and non-TD groups.
Table 3.
Comparison of baseline CSF biomarker levels in 411 PD patients with different motor phenotypes
| CSF biomarkers | Motor phenotypes | p value by Mann–Whitney test | |
|---|---|---|---|
| TD (N = 293) | Non-TD (N = 118) | ||
| α-Syn (pg/mL), N Median (range) [95 % CI] |
293 1791.99 (332.93–6694.55) [1798–1982] |
118 1562.99 (581.17–4709.78) [1602–1870] |
0.0271 |
| α-Syn (pg/mL), N Median (range) [95 % CI]* |
231 1772.08 (332.93–5110.77) [1765–1956] |
96 1567.87 (581.17–4197.42) [1562–1819] |
0.0376 |
| Aβ1–42 (pg/mL), N Median (range) [95 % CI] |
293 371.3 (129.2–796.5) [360.6–383.4] |
118 354.9 (160.6–688) [347.6–385.6] |
0.2901 |
| t-tau (pg/mL), N Median (range) [95 % CI] |
290 41.3 (15.4–121) [43.09–47.36] |
117 39.9 (14.4–110.5) [40.05–46.56] |
0.308 |
| p-tau181 (pg/mL), N Median (range) [95 % CI] |
291 12.7 (5.7–94.1) [14.87–17.29] |
118 11.3 (4.7–51.3) [12.97–16.21] |
0.0674 |
| t-tau/Aβ1–42, N Median (range) [95 % CI] |
290 0.11 (0.04–0.52) [0.12–0.13] |
117 0.11 (0.06–0.49) [0.11–0.13] |
0.4394 |
| p-tau181/Aβ1–42, N Median (range) [95 % CI] |
291 0.03 (0.01–0.51) [0.04–0.05] |
118 0.03 (0.02–0.14) [0.04–0.04] |
0.2669 |
| p-tau181/t-tau, N Median (range) [95 % CI] |
288 0.3 (0.08–2.14) [0.35–0.40] |
117 0.29 (0.12–1.06) [0.32–0.40] |
0.2765 |
| Aβ1–42/α-syn, N Median (range) [95 % CI] |
293 0.21 (0.06–1.03) [0.21–0.24] |
118 0.23 (0.06–0.6) [0.22–0.25] |
0.0931 |
| t-tau/α-syn, N Median (range) [95 % CI] |
290 0.02 (0.01–0.06) [0.024–0.026] |
117 0.03 (0.01–0.06) [0.025–0.028] |
0.2443 |
| p-tau181/α-syn, N Median (range) [95 % CI] |
291 0.01 (0–0.11) [0.009–0.011] |
118 0.01 (0–0.03) [0.008–0.010] |
0.7448 |
Data from subjects with CSF Hgb <200 ng/mL
Consistent with our preliminary study report [18], as well as results from other studies, the current results from the full baseline cohort of PPMI confirmed the low diagnostic sensitivity and specificity, with significant between-group overlap (Supplementary Fig. 1). When we used stepwise selection with adjustment for confounders (age, gender and education level) to best fit the model of predictor for PD diagnosis using ranked biomarker levels, a lower α-syn (p = 0.0169), p-tau (p = 0.0277) and t-tau/Aβ1–42 ratio (p = 0.0006), and a higher Aβ1–42/α-syn ratio (p = 0.0297) was significantly associated with PD diagnosis.
Comparison of clinical parameters according to the quintile levels of CSF biomarkers in PD patients
We compared clinical variables between PD groups according to the quintile levels of each CSF biomarker or their ratios (Table 4, and detailed median values in Table S2 to S4). For the p-tau, p-tau/α-syn, and p-tau/Aβ1–42 values, we did not observe significantly different scores for clinical variables between the groups with the levels of highest (>80 %, Q5) and lowest (≤20 %, Q1) quintile (Table S3, S4). However, the group with the lowest level of CSF Aβ1–42 showed more severe olfactory dysfunction (lower UPSIT score; p = 0.0081), lower (worse) semantic fluency (p = 0.0186), SDMT (p = 0.0010) and MSEADL (p = 0.0363) scores, and lower DAT ligand uptake in caudate (p = 0.0005) and putamen (p = 0.0166), as compared to the respective PD group with highest quintile level of CSF Aβ1–42. On the other hand, PD patients with the highest quintile t-tau/Aβ1–42 ratio showed higher disease severity (UPDRS total score; p = 0.0270), and lower (worse) HVLT-R total recall (p = 0.0155), delayed recall (p = 0.0330), LNS score (p = 0.0192), semantic fluency (p = 0.0026) and SDMT score (p < 0.0001) as compared with the respective PD group with the lowest quintile t-tau/Aβ1–42 ratio values; however, the DAT ligand uptake values for the highest and lowest quintiles for the t-tau/Aβ1–42 ratio were similar. The PD group with the highest quintile p-tau/t-tau ratio values had less severe motor dysfunction (MDS-UPDRS-III, p = 0.0429) and disease severity (MDS-UPDRS total, p = 0.0047), and higher UPSIT (p = 0.0415) and SDMT scores (p = 0.0279) as compared to the lowest group. The PD patients with the highest quintile t-tau/α-syn ratio values showed lower (worse) semantic fluency (p = 0.0338), SDMT (p < 0.0001), and BJLO scores (p = 0.0164) as compared to the lowest group. The PD patients with the lowest quintile of Aβ1–42/α-syn ratio or with the lowest quintile of α-syn and t-tau showed greater autonomic dysfunction (higher SCOPA-AUT score; p = 0.0042) or greater (worse) STAI score (p = 0.0081 for α-syn and p = 0.0267 for t-tau), respectively, compared with PD groups with the highest quintile. It should be noted that age and age at onset in the groups with highest quintile level of biomarkers were significantly different from those in the groups with lowest levels (Table 4). The PD patients with the highest quintile of α-syn, t-tau, t-tau/Aβ1–42 and t-tau/α-syn or those with the lowest quintile of Aβ1–42 and p-tau/t-tau showed older age and later disease onset. In addition, we observed a difference in gender distribution between the groups of highest and lowest level of Aβ1–42. Therefore, we included age, gender and age at onset as confounders in the multivariate regression analysis.
Table 4.
Comparison of clinical variables between groups of PD with the highest and lowest quintile levels of CSF biomarkers or their ratios
| Clinical variables, p values* |
α-Syn (N = 412) |
Aβ1–42 (N = 412) |
t-tau (N = 408) |
t-tau/Aβ1–42 (N = 408) |
p-tau/t-tau (N = 406) |
t-tau/α-syn (N = 408) |
Aβ1–42/α-syn (N = 412) |
|---|---|---|---|---|---|---|---|
| Age | 0.0180 | 0.0244 | <0.0001 | <0.0001 | 0.0003 | 0.0040 | <0.0001 |
| Gender | 0.1366 | 0.0033 | 0.0940 | 1.0000 | 0.2455 | 0.6243 | 0.6164 |
| Education | 0.1302 | 0.3627 | 0.3028 | 0.4524 | 0.9959 | 0.2536 | 0.0449 |
| Age at onset | 0.0219 | 0.0478 | <0.0001 | <0.0001 | 0.0002 | 0.0239 | 0.0001 |
| Duration of disease | 0.1214 | 0.4109 | 0.4423 | 0.4420 | 0.6730 | 0.4109 | 0.9188 |
| MDS-UPDRS III | 0.6262 | 0.2548 | 0.3923 | 0.0669 | 0.0429 | 0.4804 | 0.4750 |
| MDS-UPDRS total | 0.3960 | 0.1576 | 0.4093 | 0.0270 | 0.0047 | 0.3225 | 0.1300 |
| UPSIT | 0.9410 | 0.0081 | 0.4896 | 0.0523 | 0.0415 | 0.1678 | 0.0774 |
| MoCA | 0.9668 | 0.2895 | 0.6433 | 0.1829 | 0.5667 | 0.3429 | 0.6083 |
| HVLT-R total recall | 0.6679 | 0.4910 | 0.7495 | 0.0155 | 0.4235 | 0.2149 | 0.2520 |
| HVLT-R delayed recall |
0.8450 | 0.5656 | 0.8008 | 0.0330 | 0.6232 | 0.1470 | 0.8371 |
| HVLT-R disc. recog. | 0.6652 | 0.0524 | 0.1825 | 0.1661 | 0.7271 | 0.9986 | 0.4217 |
| LNS total | 0.7480 | 0.6036 | 0.0909 | 0.0192 | 0.2873 | 0.0776 | 0.2294 |
| Semantic fluency | 0.3664 | 0.0186 | 0.6587 | 0.0026 | 0.3637 | 0.0338 | 0.3664 |
| SDMT | 0.5096 | 0.0010 | 0.3142 | <0.0001 | 0.0279 | 0.0001 | 0.0783 |
| STAI | 0.0081 | 0.0727 | 0.0267 | 0.1669 | 0.9205 | 0.1712 | 0.4363 |
| BJLO | 0.2443 | 0.3274 | 0.6009 | 0.0963 | 0.2960 | 0.0164 | 0.6341 |
| SCOPA-AUT | 0.2583 | 0.1663 | 0.4615 | 0.0608 | 0.2791 | 0.1586 | 0.0042 |
| MSEADL | 0.4027 | 0.0363 | 0.3628 | 0.5223 | 0.2378 | 0.6689 | 0.7588 |
| DaT scan | |||||||
| Caudate mean | 1.0000 | 0.0005 | 0.0879 | 0.5334 | 0.7855 | 0.1754 | 0.2042 |
| Putamen mean | 0.1746 | 0.0166 | 0.2135 | 0.8244 | 0.2718 | 0.4981 | 0.5645 |
All p values <0.05 are in bold font
Data from subjects with CSF Hgb <200 ng/mL
p values by Mann–Whitney U test. The results of CSF biomarkers or ratios that showed p values >0.05 for all clinical variables (p-tau, p-tau/Aβ1–42 and p-tau/α-syn) are not presented, but the mean (SD) values of clinical variables for all CSF biomarkers or ratios are presented in supplementary materials
Association of CSF biomarkers with clinical variables in PD patients
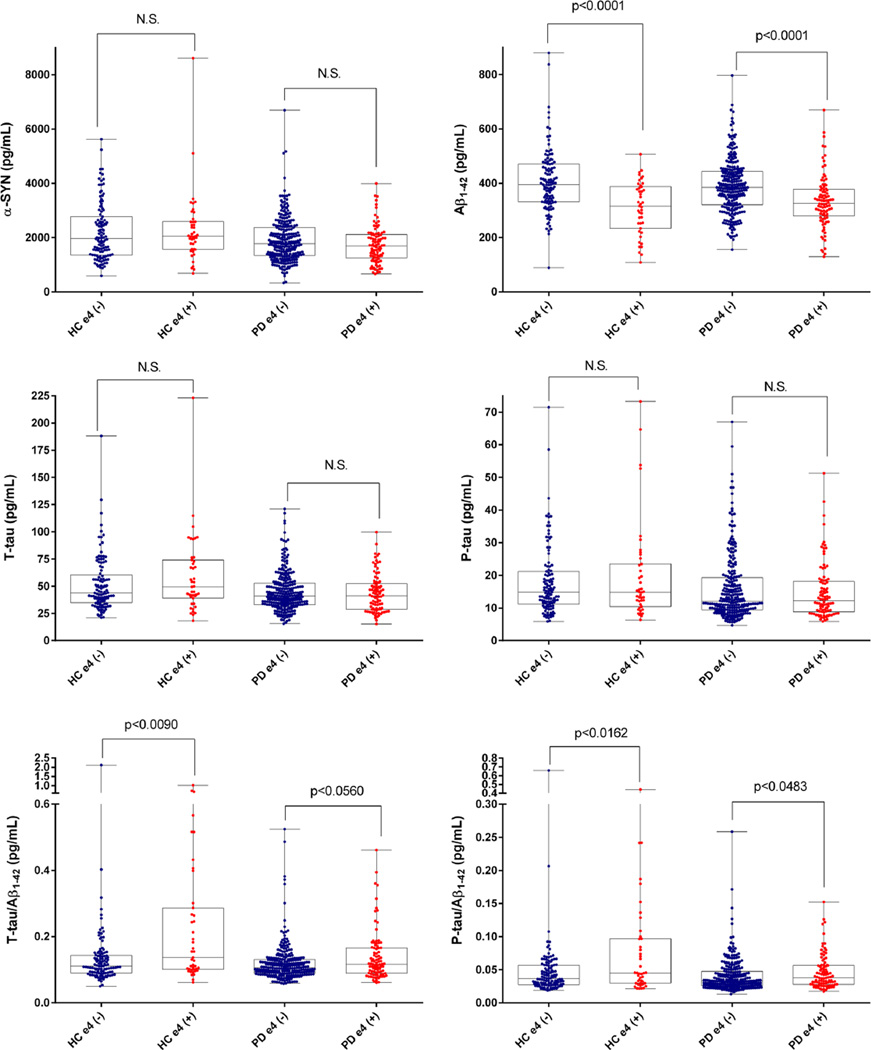
A lower CSF α-syn level was significantly associated with a lower score (more severe symptoms) on a range of neuropsychological tests including semantic fluency [β = 0.0101 (SE = 0.0047); p = 0.0318), BJLO score [β = 0.0036 (SE = 0.0012); p = 0.0021) and SDMT score [β = 0.0135 (SE = 0.0047); p = 0.0046], and high STAI score [worse anxiety, β = −0.0267 (SE = 0.0078); p = 0.0007]. MSEADL score [β = 0.0062 (SE = 0.0025); p = 0.0121], SDMT [β = 0.0087 (SE = 0.0040); p = 0.0302] and uptake of DAT ligand in caudate [β = 0.0006 (SE = 0.0002); p = 0.0101] showed a positive correlation with Aβ1–42 level (Table S5). Given the association of lower CSF Aβ1–42 level with APOE ε4 allele (Fig. 1), we evaluated whether APOE genotypes was associated with clinical parameters. There was no significant difference in all clinical parameters studied here between APOE ε4 negative and positive PD patients. In addition, the APOE genotype (presence or absence of ε4 allele) was not significantly associated with any clinical variable with or without adjustment for CSF Aβ1–42 level (Table S6). The high level of CSF t-tau was associated with lower SDMT score [β = −0.0113 (SE = 0.0051); p = 0.0271] and higher uptake of DAT ligand in putamen [β = 0.0003 (SE = 0.0001); p = 0.0361]. The higher CSF t-tau/α-syn ratio was significantly associated with lower SDMT [β = −0.0105 (SE = 0.0037); p = 0.0042] and BJLO score [β = −0.0028 (SE = 0.0009); p = 0.0023], and high STAI anxiety score [β = 0.0158 (SE = 0.0080); p = 0.0480]. In addition, the lower p-tau/α-syn ratio was associated with higher semantic fluency [β = −0.0101 (SE = 0.0046); p = 0.0307], and the higher Aβ1–42/α-syn ratio was associated with worse delayed recall (lower HVLT-R delayed recall score), [β = −0.0020 (SE = 0.0010); p = 0.0485)]. The lower p-tau level was associated with higher UPDRS total score [β = −0.0106 (SE = 0.0054); p = 0.0498].
Fig. 1.
CSF biomarker levels according to APOE genotypes in 547 HC and PD
Correlation between the levels of CSF α-syn and tau or Aβ1–42
Consistent with our previous findings that reported a significant correlation between tau species and α-syn for the first time in human CSFs in a relatively small number of PD and HC subjects [18], in the current study we observed a strong correlation between CSF α-syn and t-tau in all subjects (Spearman r = 0.7074, p < 0.0001). The correlation between the levels of CSF α-syn and t-tau was observed in both HC (r = 0.8221, p < 0.0001) and PD (r = 0.6594, p < 0.0001) subjects. The levels of CSF α-syn also showed a moderate correlation with the p-tau levels (r = 0.4178, p < 0.0001), and a weak but significant correlation with Aβ1–42 levels (r = 0.3523, p < 0.0001). These significant correlations were observed in both the HC and PD groups (Fig. 2). In addition, there were significant correlations between other biomarker pairs (Table S7).
Fig. 2.
Correlation between the levels of CSF α-syn and Aβ1–42, t-tau or p-tau in 601 HC and PD. *p values by Mann–Whitney U test
Association of CSF biomarkers with genotypes of SNCA, APOE and MAPT
The genotype frequencies for several alleles in SNCA, APOE or MAPT genes are listed in Table S8. The genotype frequencies for APOE ε4 allele, SNCA allele rs390105 and MAPT haplotype frequencies in PD patients were not significantly different from those in HC subjects. However, genotype frequencies for SNCA allele rs356181 in PD patients were significantly different from those in HC subjects (p = 0.0075) consistent with previous GWAS findings [24].
To evaluate the effects of genotypes for alleles of APOE, MAPT or SNCA gene on the levels of CSF biomarkers, we compared the CSF biomarker levels according to the genotypes in the HC and PD groups. As the numbers of SWEDD subjects according to genotypes were limited, we excluded this group from these analyses. The level of CSF Aβ1–42 in ε4 carriers was significantly lower and the p-tau/Aβ1–42 ratio of ε4 carriers was higher than those of ε4 non-carriers in both HC and PD groups. The t-tau/Aβ1–42 ratio in ε4 carriers in the HC group was significantly higher than that of ε4 non-carrier in the HC group. However, this relationship was not observed in the PD group (Fig. 1, Table S9). APOE genotype was not associated with α-syn, t-tau or p-tau level. Aβ1–42 levels were lower in the HC (241.0 ± 106.9, N = 4) and PD (297.3 ± 77.63, N = 9) subjects who were homozygous for the ε4 allele (i.e., ε4/ε4) as compared to the other genotypes, although the numbers of subjects were very limited. When we compared the CSF biomarker levels between H1/H1 haplotype (H1/H1) and H2 haplotype in a recessive model (H1/H2 or H2/H2) of the MAPT gene, there were no differences in CSF biomarker levels or their ratios in both HC and PD subjects, except for the higher level of α-syn in the HC subjects who were in the H2-positive group compared to HC in the H2-negative group (p = 0.0236) (Table S10). For SNCA genotypes, there were no differences in all measured CSF biomarker levels or their ratios between genotypes for rs356181 allele or rs3910105 allele (Table S11, S12).
Discussion
Our previous report of a subset of PPMI baseline subjects found that the levels of CSF α-syn, t-tau and p-tau in early-stage, untreated PD patients were significantly lower than levels in HC subjects [18]. Mostly consistent with these initial findings, analysis of our qualified full baseline dataset of 660 PPMI subjects here enable us to report several key findings: (1) CSF concentrations of α-syn, t-tau and p-tau, but not Aβ1–42, were lower in PD compared with HC, but with significant overlap between the groups; (2) the concentration of CSF α-syn in non-TD phenotype PD patients was lower than in TD PD patients; (3) the relatively lower level of CSF Aβ1–42 or the higher CSF t-tau/Aβ1–42 ratio was associated with more severe baseline cognition and motor symptoms of PD when we stratified PD patients into quintiles based on CSF biomarker levels; (4) there was a strong, significant correlation between the level of CSF α-syn and t-tau, less so for p-tau and Aβ1–42, and between Aβ1–42 and tau species in both HC and PD. (5) APOE ε4 genotype was associated with low levels of CSF Aβ1–42 in both the HC and PD subjects, but not with diagnosis of PD or clinical features.
Several previous reports support our finding of lower levels of CSF tau proteins (t-tau and p-tau) in PD compared with HC [23, 34], although other studies have not observed this finding [4, 22, 28, 29]. This discrepancy may be caused by one or more of several factors, including different characteristics of the control group (e.g., control with other neurologic symptoms but without neurodegeneration vs. healthy control), different time interval from initial diagnosis in the PD groups, or the different analytical methods used to measure the CSF tau species. It is likely that the levels of CSF α-syn and tau species in early-stage PD patients are lower, analogous to the finding of lower CSF Aβ1–42 levels in patients with probable AD compared with HC. However, these biomarker findings in PD patients appear to have little diagnostic value. They may help to explain mechanisms of neurodegeneration that underlie disease heterogeneity in PD, including variation of clinical characteristics and prognosis.
Conceptually, α-syn aggregation in the CNS of PD patients may lower the release of α-syn into CSF similar to how amyloid plaque formation leads to lowered Aβ1–42 release into the CSF in AD. Basic research and neuropathology studies have suggested interaction between α-syn and tau in the brain [7, 8, 10, 43], which are further supported by our findings of a significant correlation between CSF α-syn and tau. However, the interactions between α-syn and tau proteins, if any, are not specific to PD and do not contribute to the pathogenesis of PD, since the strong correlation was also observed in the HC group (Fig. 2). Rather, differences in the interaction between α-syn and tau species or Aβ1–42 may contribute to the variable progression and/or differential clinical and pathological features of PD [38]. In some autopsy studies of PD, topographical distribution of amyloid pathology and tau pathology were significantly correlated with rapid development of PD dementia [6, 19, 38]. In fact, when we classified PD patients by quintile levels of CSF biomarkers, PD patients with low Aβ1–42 and a high t-tau/Aβ1–42 ratio, but not α-syn levels, showed more severe clinical symptoms compared with patients with high CSF Aβ1–42 concentrations and low t-tau/Aβ1–42 ratio values (Table 4). This suggested that the more severe non-motor symptoms may in part be driven by concurrent amyloid and tau rather than Lewy body pathology. The positive association between α-syn and tau species was observed not only in PPMI subjects but also in other disease cohorts, such as AD in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) subjects [37], although the directions of alteration of CSF α-syn and tau species in PD are opposite to those in AD. However, in some cognitively impaired ADNI subjects with high CSF tau levels, there was a reduction in CSF α-syn raising the possibility that these individuals may have CNS LBs/LNs in addition to AD pathology, but more longitudinal studies of the ADNI cohort are needed to establish this with certainty.
Future long-term longitudinal observations in the PPMI cohort will be required to test the predictive performance of these biomarkers. The presence of AD pathology and interaction with LB pathology in the brain of PD patients (high CSF t-tau and low Aβ1–42, but low α-syn) may be a contributor to rapid progression of cognitive decline in PD patients as compared to PD patients who do not have AD pathology. In the PPMI dataset, we are able to explore whether these contributing pathological processes may already be contributing to early clinical phenotypes, even given the narrow range of variability in cognitive and motor measures in de novo PD. In the multivariate regression analysis with adjustment for confounders, α-syn, Aβ1–42, t-tau or t-tau/α-syn ratio was significantly associated with multiple clinical measures, while t-tau, p-tau, p-tau/α-syn or Aβ1–42/α-syn was associated with single measure. The lower levels of Aβ1–42 were significantly associated with poor daily activity (low MSEADL score), executive functioning (low SDMT score) and lower DAT score in caudate, and lower levels of α-syn were significantly associated with poor semantic fluency, visuospatial cognition (low BJLO score), executive functioning and worse anxiety (high STAI score), while high level of t-tau/α-syn ratio was significantly associated with low SDMT and BJLO scores and high STAI score. It has been reported that scales for activity of daily living (e.g., UPDRS II or MSEADL score) were predictors of earlier need for symptomatic treatment (i.e., rapid progression) of PD [25, 26]. In addition, recent studies reported that lower levels of CSF Aβ1–42 [5, 35] or higher amyloid plaque burden on PET imaging [9] in PD is a predictor of rapid cognitive decline. We anticipate that these cross-sectional changes in cognitive and functional measures, together with CSF biomarkers suggesting underlying AD pathology, will identify those PD patients likely to show more rapid decline in these non-motor measures. We observed a significant difference in CSF α-syn levels between TD and non-TD motor phenotype of PD, but not in CSF Aβ1–42, t-tau or p-tau levels. However, when we evaluated this in a subset population (TD = 250, non-TD = 98) that had removed subjects (N = 102) reported in our preliminary study [18], the significant difference of α-SYN level between TD and non-TD disappeared, implying that the significant finding of α-SYN is mostly specific to the subset of patients included in our preliminary study. Several studies suggest that motor phenotype of PD could be a good predictor of PD progression [16, 41]. However, the motor phenotype in PD, particularly at a very early stage, might not be stable, and many patients with TD or IND phenotype can change to PIGD phenotype before the development of dementia [3]. Therefore, the motor phenotype at early-stage PD may have limited ability to predict disease progression. Although longitudinal data for the whole PPMI cohort is essential to evaluate the predictive performance of CSF biomarkers for PD progression, the baseline data in this large cohort suggest that CSF biomarkers in early PD subjects already distinguish subtle baseline differences and therefore may have predictive value for disease progression.
A significant association of APOE genotypes with CSF level of Aβ1–42, but not other biomarkers was observed. Because the ε4 allele is well known to promote Aβ pathology and plaque burden, but is not directly related to PD [39], we would predict that amyloid pathology may be a mediator of cognitive performance in PD. In support of this, a recent longitudinal study in a large cohort reported that APOE ε4 allele was associated with lower performance over time of memory, attention, executive functioning and language processing in PD patients, whereas the MAPT and SNCA genetic variants were not [21]. In addition, a meta-analysis published in 2009 reported a significant association of APOE ε4 allele with dementia in PD patients [42]. However, it should be noted that the association of APOE genotypes with cognitive function in PD has to be carefully interpreted, since we found a significant association of APOE ε4 allele with lower levels of CSF Aβ1–42. The association of APOE genotype with cognitive, motor and other clinical indices in our PD patients was not significant. Therefore, our results suggest that lower level of CSF Aβ1–42 is more directly mediating cognitive dysfunction in PD than APOE ε4 genotype. The frequency of SNPs in the SNCA rs356181 allele of the PD subjects was significantly different from that observed in HC, consistent with previous GWAS results [24]. However, we did not observe significant effects of individual SNPs or haplotypes of SNCA and MAPT genes on the CSF levels of α-syn and tau species, respectively.
There are a number of study limitations. Although the PPMI cohort is the largest ongoing, prospective, longitudinal, qualified cohort, the number of subjects in subgroup analysis (e.g., subgroup of motor phenotypes or genotypes) may be a limitation for the analysis. In addition, we tried to compare the CSF biomarker levels in PD patients according to their motor phenotypes. A major limitation of this analysis, however, is that the motor phenotypes of many patients are not yet fully determined (i.e., IND phenotype or possible change of phenotype over time) and the clinical assessments of non-motor symptoms were limited. More importantly, the current results are cross-sectional, and not longitudinal data analyses. Thus, we are limited at this time in our attempts to directly test for the predictive performance of CSF biomarkers for heterogeneous PD progression. However, in spite of the limited variability in cognitive, motor and clinical measures, our data suggest that CSF biomarkers could help dissect disease heterogeneity that may already be developing at this stage of early motor stages of PD. We anticipate that maturation of the PPMI study with long-term longitudinal follow-up observation will expand on these findings and resolve these limitations.
Acknowledgments
The Parkinson’s Progression Markers Initiative Group includes Shirley Lasch; Emily Flagg; Werner Poewe, MD; Todd Sherer, PhD; Claire Meunier; Alice Rudolph, PhD; Cindy Casaceli, MBA; John Seibyl, MD; Susan Mendick, MPH; Norbert Schuff, PhD; Liz Uribe, MS; Jon Yankey, MS; Karen Crawford; Tatiana Foroud, PhD; Paola Casalin, MD, PhD, FRCP; Giulia Malferrari, PhD; Keith Hawkins, PsyD; David Russell, MD, PhD; Laura Leary, BS; Stewart Factor, DO; Barbara Sommerfeld, RN, MSN; Penelope Hogarth, MD; Emily Pighetti; Karen Williams; David Standaert, MD, PhD; Stephanie Guthrie, MSN; Robert Hauser, MD, MBA; Joseph Jankovic, MD; Christine Hunter, RN; Matthew Stern, MD; Abigail Darin; Jim Leverenz, MD; Marne Baca; Sam Frank, MD; Cathi-Ann Thomas, RN, MS; Irene Richard, MD; Cheryl Deeley, MSN; Linda Rees, MPH; Fabienne Sprenger, MD; Wolfgang Oertel, MD; Diana Willeke; Holly Shill, MD; Hubert Fernandez, MD; Jennifer Mule; Daniela Berg, MD; Katharina Gauss; Douglas Galasko, MD; Deborah Fontaine, BSN, MS; Zoltan Mari, MD; Arita McCoy, RN; David Brooks, MD; Bina Shah, BSc; Paolo Barone, MD, PhD; Stuart Isaacson, MD; Angela James; Alberto Espay, MD, MSc; Kristy Espay; Dominic Rowe, MD, PhD; Madelaine Ranola, BN.
The Parkinson’s Progression Markers Initiative Group Affiliations Institute for Neurodegenerative Disorders, New Haven, Connecticut (Lasch, Seibyl, Mendick, Russell, Leary); Clinical Trials Coordination Center, University of Rochester, Rochester, New York (Flagg, Casaceli); Innsbruck Medical University, Innsbruck, Austria (Poewe, Sprenger); The Michael J. Fox Foundation for Parkinson’s Research, New York, New York (Sherer, Meunier); Institute on Aging, Center for Neurodegenerative Disease Research, Perelman School of Medicine, University of Pennsylvania, Philadelphia (Rudolph); University of California, San Francisco (Schuff); University of Iowa, Iowa City (Uribe, Yankey); Laboratory of Neuro Imaging, Department of Neurology, David Geffen School of Medicine, University of California, Los Angeles (Crawford); Coriell Institute for Medical Research, Camden, New Jersey (Scutti); BioRep, Milan, Italy (Casalin, Malferrari); Yale University, New Haven, Connecticut (Hawkins); Emory University School of Medicine, Atlanta, Georgia (Factor, Sommerfeld); Oregon Health and Science University, Portland (Hogarth, Pighetti); Northwestern University, Chicago, Illinois (Williams); University of Alabama at Birmingham (Standaert, Guthrie); Baylor College of Medicine, Houston, Texas (Hauser, Jankovic, Hunter); University of Pennsylvania, Philadelphia (Stern, Darin); University of Washington, Seattle (Leverenz, Baca); Boston University, Boston, Massachusetts (Frank, Thomas); University of Rochester, Rochester, New York (Richard, Deeley); Parkinson’s Institute, Sunnyvale, California (Rees); Paracelsus-Elena-Klinik, Kassel, Germany (Oertel, Willeke); Cleveland Clinic, Cleveland, Ohio (Shill, Fernandez, Mule); University of Tuebingen, Tuebingen, Germany (Berg, Gauss); University of California, San Diego (Galasko, Fontaine); Johns Hopkins University, Baltimore, Maryland (Mari, McCoy); Imperial College London, London, England (Brooks, Shah); University of Salerno, Salerno, Italy (Barone); Parkinson’s Disease and Movement Disorders Center of Boca Raton, Boca Raton, Florida (Isaacson, James); University of Cincinnati, Cincinnati, Ohio (A. Espay, K. Espay); Macquarie University, Sydney, Australia (Rowe, Ranola).
Frasier is an employee of The Michael J. Fox Foundation for Parkinson’s Research. Marek has been a consultant for Pfizer Inc, GE Healthcare, Merck and Co, Eli Lilly and Co, Bristol-Myers Squibb, Piramal, Neotope Biosciences, and Neuro Phage Pharmaceuticals and has equity interest in Molecular NeuroImaging. Kieburtz has been a consultant for the National Institute of Neurological Disorders and Stroke, National Institutes of Health, US Food and Drug Administration, Veterans Affairs, Abbott, Acorda, Aptiv, Astra-Zeneca, Auspex, BiogenIdec, Biotie, Biovail, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Ceregene, CHDI, Civitas, Clintrex, Cynapsus, Eli Lilly and Co, Endo, Impax, Intec, Ipsen, Isis, Knopp, Lundbeck, LZ Therapeutics, Medivation, Merck and Co, Merz, Neo-tope/Elan, Novartis, Orion, Otsuka, Pharma2B, Phytopharm, Roche, Siena Biotech, Sofinnova, Synagile, Synosia, Teva, UCB Pharma, Upsher-Smith, US World Meds, Vaccinex, Vectura, and Xenoport; and has performed legal consulting for Thompson Hine. Tanner has served on the scientific advisory boards of The Michael J. Fox Foundation for Parkinson’s Research and the National Spasmodic Dystonia Association; has been a consultant for Impax Pharmaceuticals and Adamas Pharmaceuticals; and is an employee of the Parkinson’s Institute and Clinical Center. Chowdhury is an employee of The Michael J. Fox Foundation for Parkinson’s Research. Shaw was previously a consultant for Fujirebio and collaborates on quality assessment activities as part of the Parkinson’s Progression Markers Initiative and as part of the Alzheimer’s Disease Neuroimaging Initiative. Leverenz has received compensation for consultation from Bayer Pharmaceuticals, Navidea Biopharmaceuticals, and Piramal Healthcare. Galasko serves as editor of Alzheimer’s Disease Research and Treatment; serves on data safety monitoring boards for Elan, Janssen, and Balance Pharmaceuticals; and is a consultant for Elan Pharmaceuticals;
Funding/Support This work was supported by The Michael J. Fox Foundation for Parkinson’s Research, Abbott, Avid Radiopharmaceuticals, BiogenIdec, Covance, Elan, Eli Lilly and Co, F. Hoffman–LaRoche Ltd, GE Healthcare, Genentech, Glaxo Smith Kline, Merck and Co, Pfizer Inc, and UCB Pharma SA. Trojanowski and Chen-Plotkin were supported by core grant P50NS053488-05 from the Morris K. Udall Center of Excellence for Parkinson’s Disease Research. Irwin was supported by Training in Age-Related Neurodegenerative Diseases grant T32-AG000255 from the National Institute on Aging, National Institutes of Health. Singleton was supported by Grant Z01AG000949-06 from the Intramural Research Program, National Institute on Aging, National Institutes of Health. Ju-Hee was supported by Grant MRC 2014009392 from the National Research Foundation of Korea, Ministry of Science, ICT and Future Planning. Kieburtz has received grants and/or research support from the National Eye Institute, National Institute of Neurological Disorders and Stroke, National Institute on Aging, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Neurosearch, and Medication. Tanner has received grants and/or research support from Brin Foundation, James and Sharron Clark, Parkinson’s Institute and Clinical Center, Parkinson’s Disease Foundation, US Army Medical Research Acquisition Activity (Telemedicine and Advanced Technology Research Center—managed Neurotoxin Exposure Treatment Research Program), National Institute of Neurological Disorders and Stroke, Agency for Healthcare and Research Quality, and National Institute of Environmental Health Sciences. Galasko receives research support from the National Institutes of Health, and the Alzheimer’s Drug Discovery Foundation.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00401-016-1552-2) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest No other disclosures were reported.
References
- 1.Aarsland D, Andersen K, Larsen JP, et al. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289:18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 3.Alves G, Larsen JP, Emre M, et al. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord. 2006;21:1123–1130. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- 4.Alves G, Brønnick K, Aarsland D, et al. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson’s disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2010;81:1080–1086. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- 5.Alves G, Lange J, Blennow K, et al. CSF Aβ42 predicts early-onset dementia in Parkinson disease. Neurology. 2014;82:1784–1790. doi: 10.1212/WNL.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 6.Compta Y, Parkkinen L, O’Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain. 2011;134:1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duka T, Duka V, Joyce JN, Sidhu A. α-Synuclein contributes to GSK-3β-catalyzed tau phosphorylation in Parkinson’s disease model. FASEB J. 2009;23:2820–2830. doi: 10.1096/fj.08-120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300:636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 9.Gomperts SN, Locascio JJ, Rentz D, et al. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013;80:85–91. doi: 10.1212/WNL.0b013e31827b1a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo JL, Covell DJ, Daniels JP, et al. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154:103–117. doi: 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halliday GM, Holton JL, Revesz T, Dickson DW. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122:187–204. doi: 10.1007/s00401-011-0852-9. [DOI] [PubMed] [Google Scholar]
- 12.Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson’s disease in the United Kingdom. Mov Disord. 2004;19:1043–1049. doi: 10.1002/mds.20216. [DOI] [PubMed] [Google Scholar]
- 13.Hughes TA, Ross HF, Musa S, et al. A 10-year study of the incidence of and factors predicting dementia in Parkinson’s disease. Neurology. 2000;54:1596–1602. doi: 10.1212/wnl.54.8.1596. [DOI] [PubMed] [Google Scholar]
- 14.Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72:587–598. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin DJ, Lee VM, Trojanowski JQ. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14:626–636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. Neurology. 1990;40:1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 17.Jellinger KA. Post mortem studies in Parkinson’s disease-is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl. 1999;56:1–29. doi: 10.1007/978-3-7091-6360-3_1. [DOI] [PubMed] [Google Scholar]
- 18.Kang JH, Irwin DJ, Chen-Plotkin AS, et al. Association of cerebrospinal fluid β-amyloid 1–42, t-tau, p-tau181, and α-synuclein levels with clinical features of drug-naïve patients with early Parkinson disease. JAMA Neurol. 2013;70:1277–1287. doi: 10.1001/jamaneurol.2013.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology. 2002;59:1708–1713. doi: 10.1212/01.wnl.0000036610.36834.e0. [DOI] [PubMed] [Google Scholar]
- 20.Lewis SJG, Foltynie T, Blackwell AD, et al. Heterogeneity of Parkinson’s disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry. 2005;76:343–348. doi: 10.1136/jnnp.2003.033530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mata IF, Leverenz JB, Weintraub D, et al. APOE, MAPT, and SNCA genes and cognitive performance in Parkinson disease. JAMA Neurol. 2014;71:1405–1412. doi: 10.1001/jamaneurol.2014.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, et al. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011;10:230–240. doi: 10.1016/S1474-4422(11)70014-X. [DOI] [PubMed] [Google Scholar]
- 23.Montine TJ, Shi M, Quinn JF, et al. CSF Aβ42 and tau in Parkinson’s disease with cognitive impairment. Mov Disord. 2010;25:2682–2685. doi: 10.1002/mds.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nalls MA, Pankratz N, Lill CM, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parashos SA, Swearingen CJ, Biglan KM, et al. Determinants of the timing of symptomatic treatment in early Parkinson disease: the National Institutes of Health Exploratory Trials in Parkinson Disease (NET-PD) Experience. Arch Neurol. 2009;66:1099–1104. doi: 10.1001/archneurol.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parashos SA, Luo S, Biglan KM, et al. Measuring disease progression in early Parkinson disease: the National Institutes of Health Exploratory Trials in Parkinson Disease (NET-PD) Experience. JAMA Neurol. 2014;71:710–716. doi: 10.1001/jamaneurol.2014.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Initiative Parkinson Progression Marker. The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parnetti L, Tiraboschi P, Lanari A, et al. Cerebrospinal fluid biomarkers in Parkinson’s disease with dementia and dementia with Lewy bodies. Biol Psychiatry. 2008;64:850–855. doi: 10.1016/j.biopsych.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Parnetti L, Chiasserini D, Bellomo G, et al. Cerebrospinal fluid tau/α-synuclein ratio in Parkinson’s disease and degenerative dementia. Mov Disord. 2011;8:1428–1435. doi: 10.1002/mds.23670. [DOI] [PubMed] [Google Scholar]
- 30.Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol. 1991;50:743–755. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Rajput AH, Sitte HH, Rajput A, et al. Globus pallidus dopamine and Parkinson motor subtype: clinical and brain biochemical correlation. Neurology. 2008;70:1403–1410. doi: 10.1212/01.wnl.0000285082.18969.3a. [DOI] [PubMed] [Google Scholar]
- 32.Rajput AH, Voll A, Rajput ML, et al. Course in Parkinson disease subtypes: a 39-year clinicopathologic study. Neurology. 2009;73:206–212. doi: 10.1212/WNL.0b013e3181ae7af1. [DOI] [PubMed] [Google Scholar]
- 33.Selikhova M, Williams DR, Kempster PA, et al. A clinicopathological study of subtypes in Parkinson’s disease. Brain. 2009;132:2947–2957. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 34.Shi M, Bradner J, Hancock AM, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69:570–580. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid β1–42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75:1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stebbins GT, Goetz CG, Burn DJ, et al. How to identify tremor dominant and postural instability/gait difficulty groups with the Movement Disorder Society Unified Parkinson’s Disease Rating Scale: comparison with the Unified Parkinson’s Disease Rating Scale. Mov Disord. 2013;28:668–670. doi: 10.1002/mds.25383. [DOI] [PubMed] [Google Scholar]
- 37.Toledo JB, Korff A, Shaw LM, et al. CSF α-synuclein improves diagnostic and prognostic performance of CSF tau and Aβ in Alzheimer’s disease. Acta Neuropathol. 2013;126:683–697. doi: 10.1007/s00401-013-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toledo JB, Gopal P, Raible K, et al. Pathological a-synuclein distribution in subjects with coincident Alzheimer’s and Lewy body pathology. Acta Neuropathol. 2015 doi: 10.1007/s00401-015-1526-9. (E-pub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuang D, Leverenz JB, Lopez OL, et al. APOE e4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70:223–228. doi: 10.1001/jamaneurol.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van de Berg WD, Hepp DH, Dijkstra AA, et al. Patterns of α-synuclein pathology in incidental cases and clinical subtypes of Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S28–S30. doi: 10.1016/S1353-8020(11)70011-6. [DOI] [PubMed] [Google Scholar]
- 41.Williams-Gray CH, Foltynie T, Brayne CEG, et al. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 42.Williams-Gray CH, Goris A, Saiki M, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson’s disease. J Neurol. 2009;256:493–498. doi: 10.1007/s00415-009-0119-8. [DOI] [PubMed] [Google Scholar]
- 43.Wills J, Jones J, Haggerty T, et al. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp Neurol. 2010;225:210–218. doi: 10.1016/j.expneurol.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]