Abstract
The vitamin D receptor (VDR) is found in nearly all, if not all, cells in the body. The enzyme that produces the active metabolite of vitamin D and ligand for VDR, namely CYP27B1, likewise is widely expressed in many cells of the body. These observations indicate that the role of vitamin D is not limited to regulation of bone and mineral homeostasis, as important as that is. Rather, the study of its extraskeletal actions has become the major driving force behind the significant increase in research articles on vitamin D published over the past several decades. A great deal of information has accumulated from cell culture studies, in vivo animal studies, and clinical association studies that confirms that extraskeletal effects of vitamin D are truly widespread and substantial. However, randomized, placebo controlled clinical trials, when done, have by and large not produced the benefits anticipated by the in vitro cell culture and in vivo animal studies. In this review, I will examine the role of vitamin D signaling in a number of extraskeletal tissues, and assess the success of translating these findings into treatments of human diseases affecting those extracellular tissues.
Keywords: vitamin D, CYP27B1, cancer, cardiovascular, skin, immune system
Introduction
The original paradigm for vitamin D signaling involved its production in the skin from 7-dehydrocholesterol under the influence of ultraviolet light with spectrum 280–320 (UVB), its removal from the skin carried by the vitamin D binding protein (DBP) to the liver where it was subsequently hydroxylated in the 25 position of the side chain to 25 hydroxyvitamin D (25OHD) by CYP2R1 and CYP27A1 among other 25 hydroxylases, which provided the substrate for the renal 1α hydroxylase (CYP27B1) to form the active metabolite and ligand for the vitamin D receptor (VDR), namely 1,25 dihydroxyvitamin D (1,25(OH)2D). The 1,25(OH)2D/VDR complex acted as a transcription factor in combination with the retinoid X receptor (RXR) to induce the genes that enabled intestinal calcium and phosphate transport, renal reabsorption of calcium, and flow of calcium and phosphate in and out of the skeleton. With the observations that the VDR in found in nearly every cell in the body and that CYP27B1 and 25 hydroxylases are widely expressed, this paradigm has undergone major revisions. Now the concept is that all cells may be targets for 1,25(OH)2D at least at some stage of their differentiation1, that many of these cells make their own 1,25(OH)2D and so are not dependent on the renal production of this metabolite2, that the VDR may act without its classical ligand and/or use alternative ligands,3,4 that RXR is not the only partner for VDR in its transcriptional actions,5,6 and that vitamin D signaling may involve non-genomic as well as genomic mechanisms of action.7–9 These observations underlie the rapid explosion in publications about the mechanism of vitamin D action, not just in regulating bone and mineral homeostasis but in essentially in all biologic processes, with the hope that vitamin D and its metabolites might play an important role in the prevention and treatment of a vast number of diseases––not just rickets, osteomalacia, and osteoporosis, as important as this role is. Pubmed lists 67,463 publications on vitamin D at the time of this writing, with acceleration over the past decade. The number of publications over the past 5 years illustrate this trend: 3154 in 2011, 3613 in 2012, 3890 in 2013, 3995 in 2014, 4236 in 2015, with 1771 so far by May 1, 2016.
Recent studies in which the number of VDR binding sites (putative vitamin D response elements or VDREs) have been determined using Chip-seq have identified anywhere from 1000 to 13,000 in a variety of cells that have been examined, possibly involving 3–4% of the genome.5,10 The majority (67%) of the sites are cell specific.10 Each gene may have multiple VDR binding sites that can be essentially anywhere in the gene, often at great distances from the transcription start site.5,10 Most of the sites are probably not functional (most have not been tested). Using RNA-seq to identify regulated genes, studies have shown anywhere from 100–500 up or down regulated genes in different cells, with limited overlap in the genes regulated among the different cell types.5,10–12 Although it is beyond the scope of this review to discuss the reasons for tissue specificity––likely involving tissue-specific expression of the various coactivator and corepressors that differentially regulate 1,25(OH)2D action,5,13 and epigenetic differences in different tissues that regulate access of the VDR to its VDREs, or even of VDR expression itself14—the tissue-specific effects of vitamin D signaling certainly make it possible to understand the diversity of 1,25(OH)2D effects on different cell types.
In this review, I will explore some of this diversity in a number of cell types and tissues that are not obviously linked to bone and mineral homeostasis (Fig. 1). With each cell/tissue type, I will examine the translational implications of these findings in terms of both the very promising association studies and the less compelling randomized placebo controlled clinical trials (RCTs). I will refer to “vitamin D signaling” or “action” to indicate the impact of the active vitamin D metabolites, 1,25(OH)2D in particular, interacting with its various receptors and cofactors to induce change within the cell by mechanisms to be described. By “vitamin D status” I refer primarily to the levels of circulating 25OHD levels, the most widely used method of assessing vitamin D status. In this review “vitamin D” refers to both vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). Similarly, “25OHD” refers to both 25OHD3 (calcifediol or 25-hydroxycholecalciferol) and 25OHD2 (25-hydroxyergocalciferol), and “1,25(OH)2D” refers to both 1,25(OH)2D3 (calcitriol) and 1,25(OH)2D2 (ercalcitriol).
Figure 1.
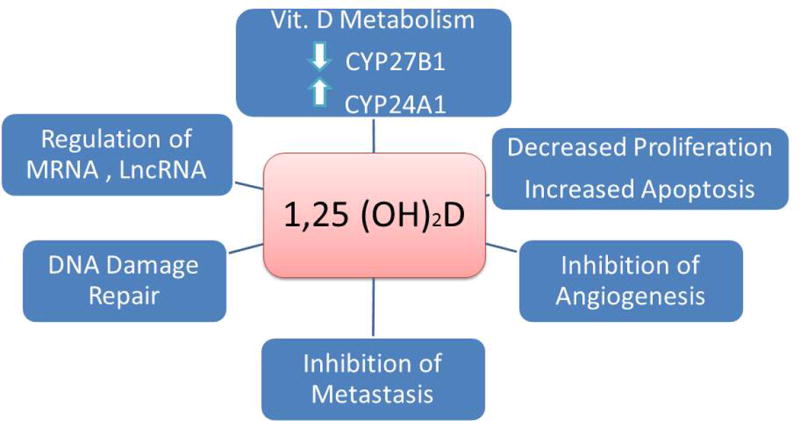
Mechanisms by which 1,25(OH)2D suppresses cancer formation.
Cancer
The antiproliferative, prodifferentiating effects of vitamin D signaling on many, if not most, cell types has raised the hope that vitamin D, 1,25(OH)2D, or one or more of its analogs, would prove useful in the prevention and/or treatment of cancer. Indeed, PubMed lists 8875 articles on the subject at the time of writing. I will briefly review several of the main mechanisms by which vitamin D signaling is thought to prevent/treat cancer, giving examples of some of the cells studied and the animal data for several of the most common and best studied tumors, then examine whether these promising mechanistic and animal studies have been successfully translated to the clinic.
Cellular studies and mechanisms
Alterations in VDR levels and vitamin D metabolism
Most tumors express the VDR, and mutations in the VDR are uncommon.15 However, the expression of the VDR is often lost as a tumor undergoes progressive dedifferentiation, and loss of its expression in a tumor is a bad prognostic sign.16 Similarly, CYP27B1 is expressed in many tumors and mutations are uncommon;15 but like the VDR, expression of CYP27B1 typically declines with progressive dedifferentiation.17,18 On the other hand, CYP24A1 expression is often increased in tumors and is associated with resistance to 1,25(OH)2D.18,19 This increase is secondary to the CYP24A1 gene being part of a region of gene duplication seen in some tumors;20 its overexpression is a poor prognostic sign.21
Regulation of miRNAs and lncRNAs
As discussed previously, VDR binding sites in the genome are numbered in the thousands, and VDR coding transcripts number in the hundreds. However, a substantial level of regulation occurs via microRNAs (miRNAs) and long noncoding RNAs (lncRNAs), a difference being that miRNAs are typically around 20 nucleotides in length, whereas lncRNAs are 200 or more nucleotides. A number of miRNAs have been identified to be regulated by 1,25(OH)2D–VDR and relevant to its antiproliferative actions.22 These include increased expression of miR145, which blocks the expression of E2F3, a key regulator of proliferation,23 or miR-32 that blocks the proapototic protein Bim, which somewhat paradoxically protects the cell (human myeloid leukemia) from AraC induced apoptosis.24 In VDR-null (Vdr−/−) keratinocytes, a number of oncogenic lncRNAs are increased, whereas tumor suppressor lncRNAs are decreased.25
Antiproliferation
1,25(OH)2D typically causes arrest at the Go/G1 and/or G1/S transitions in the cell cycle; this is associated with a decrease in cyclins (varies with cell type) and an increase in the inhibitors of the cyclin-dependent kinases (CDK) such as p21cip1 and p27kip1, again in a cell-specific fashion.26,27 Given the importance of calcium in vitamin D signaling, it was of interest that the antiproliferative actions of 1,25(OH)2D in CRC cell lines are dependent on the expression of the calcium sensing receptor.28 Forkhead box O (FoxO) proteins are transcription factors that suppress proliferation and increase apoptosis. 1,25(OH)2D promotes an increase in the interaction between several of the FoxOs with VDR and FoxO regulators, including Sirt1 and protein phosphatase 1, keeping FoxO dephosphorylated and transcriptionally active, as shown in the SCC25 cell line.29 1,25(OH)2D reduces the levels of other genes linked to proliferation, genes such as MYC, FOS, and JUN as illustrated in colon cancer cell lines.30 1,25(OH)2D stimulates the expression of IGF binding protein 3 (IGFBP3) in prostate and breast cancer cells, thus limiting the ability of IGF I and II to stimulate tumor growth.31,32 The expression of TGF-β2, which is antiproliferative in epithelial cells, is stimulated by 1,25(OH)2D in a number of cell types, including breast and prostate cancer cells.33, 34, 35 Overexpression of components of the Hedgehog (HH) pathway is a major cause of basal cell carcinomas (BCC).36 In the epidermis of VDR-null mice the HH pathway is up regulated; on the other hand 1,25(OH)2D blocks the expression of these components.37 1,25(OH)2D inhibits EGF stimulation of proliferation by inhibiting the expression of EGFR in breast cell lines38 and was shown to target the EGF–EGFR complex to endosomes in the epidermoid cell line A431.39 Mutations in APC, leading to constitutive activation of the Wnt/β-catenin pathway, are the cause of most colorectal cancers (CRC). When activated, β-catenin enters the nucleus where it binds to TCF/LEF sites in genes promoting proliferation (e.g., cyclin D1). 1,25(OH)2D/VDR competes with TCF/LEF for binding to β-catenin and by stimulating the formation of the E-cadherin–catenin complex in the cell membrane limits the amount of β-catenin that can be activated and translocated to the nucleus.40 A similar mechanism exists in the skin, contributing to the ability of vitamin D signaling to protect against skin cancer.41 Moreover, 1,25(OH)2D can increase the expression of the Wnt inhibitor dickkopf (DKK)-1,42 while inhibiting that of the Wnt activator DKK-443 in colon cancer cells. The ability of calcium to enhance the antiproliferative actions of 1,25(OH)2D in these tissues is due, in part, to its role in stimulating E-cadherin–catenin complex formation and reducing the induction of cyclin D1.44
Apoptosis
1,25(OH)2D promotes the apoptosis of a number of cell types, including gastric and colon carcinoma cells,45,46 by stimulating the expression of a number of pro- apoptotic genes such as G0S2 (Go/G1 switch gene 2) in colon cancer,27 BAX in the chronic myeloid leukemia cell line K562,47 DAP (death-associated protein)-3, CFKAR (caspase 8 apoptosis-related cysteine peptidase), FADD (Fas-associated death domain) and a number of caspases (e.g., caspase 3, 4, 6, and 8 genes) in breast cancer cells,34 or by suppressing the expression of a number of pro-apoptotic genes such as Bcl-2 (BCL2) and Bcl-XL (BCL2L1) in K562 cells,47 thus sensitizing cells to apoptosis induced by reactive oxygen species (ROS) and cytokines (e.g., TNF-α), as seen in breast cancer cells.48,49 Moreover, the 1,25(OH)2D–induced increase in intracellular calcium further increases apoptosis by activating the calcium dependent μ-calpain and calcium/calpain-dependent caspase 12, shown in breast cancer cells.50 1,25(OH)2D also promotes autophagy51 in some cancer cells, such as the MCF-7 breast cancer cell line, by inhibiting the anti-autophagy mTOR gene (MTOR) and increasing the levels of the pro-autophagy beclin-1 gene (AMBRA1).
DNA damage repair (DDR)
DNA damage repair mechanisms have probably been best described in the skin. Sunlight induces DNA damage both through its UVB component that damages DNA directly52 and its UVA component that damages DNA through oxidative stress.53 UVB induced DNA damage includes the formation of cyclobutane pyrimidine dimers (CPD) and pyrimidine (6–4) pyrimidone photoproducts (6–4PP) that if not repaired result in C to T or CC to TT mutations.54 VDR-null mouse epidermis is slow to clear CPDs and 6,4PPs following UVB,37,55 but topical 1,25(OH)2D is protective (in VDR-containing skin). In other tissues, chromosomal damage is due to oxidative and other stresses, and is more prevalent in vitamin D deficiency56 or VDR-null mice,57 where it is associated with increased levels of 8-OH-2′-deoxyguanosine (8-OHdG), a marker of oxidative DNA damage.58 800 IU of vitamin D as a daily supplement has been shown to reduce the levels of oxidative DNA damage in human colons.58 1,25(OH)2D induces several genes important for DDR, including XPC (xeroderma pigmentosum complementation group C), DDB2 (damage-specific DNA binding protein 2, also known as XPE), and GADD45 (growth arrest and DNA-damage inducible).59,60 Similarly,1,25(OH)2D induces a number of antioxidant enzymes, including thioredoxin reductase 1,33 superoxide dismutase,33 glucose-6 phosphate dehydrogenase,61 and glutathione peroxidase,27 that protect against oxidative DNA damage.
Inhibition of angiogenesis
Angiogenesis is critical for tumor growth and metastasis. 1,25(OH)2D inhibits the proliferation of endothelial cells, reduces hypoxia-induced expression of VEGF in a variety of cancer cell lines including colon cancer cells,62 and, by inhibiting VEGF-induced endothelial cell sprouting and elongation, reduces neovascularization of tumors such as in breast cancer cells (Fig. 2).63
Figure 2.
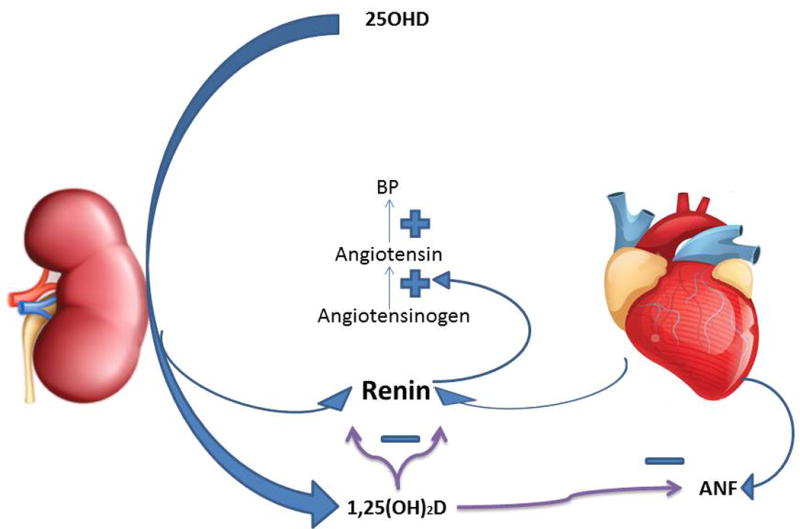
Regulation of cardiovascular function. 1,25(OH)2D inhibits the production of renin by both the kidney and heart as well as ANF by the heart. Renin in turn catalyzes the production of angiotensin, a powerful vasculoconstrictor as well as stimulator of aldosterone production, that results in hypertension.
Inhibition of metastasis
1,25(OH)2D reduces the migration and invasion capacity of tumor cells by several mechanisms.64 It reduces the expression of the matrix protein laminin and its receptors α6 and β4. 1,25(OH)2D also reduces the degradation of the matrix by matrix metalloproteinases and cathepsins produced by cancer cells that otherwise would facilitate their metastasis by inducing inhibitors of these enzymes, as occurs in prostate cancer.65 1,25(OH)2D increases the expression of the PDZ-LIM domain–containing protein 2, a scaffold protein linking different components of the cytoskeleton, enabling the proadhesion, anti-migration, anti-invasion effects of 1,25(OH)2D, as shown in breast cancer cells.66 The increased expression of E-cadherin and decreased expression of CEACAM1 (carcinoembryonic antigen-related cell adhesion molecule 1) by endothelial cells limits binding of cancer cells to endothelial cells, a requirement for their ability to metastasize.67
Animal studies
Although a number of different types of tumors have been examined in animal models, I will focus on just four: colorectal cancer (CRC), breast cancer (BCa), prostate cancer (PCa), and non-melanoma skin cancer (NMSC), as these are common malignancies that have received substantial attention in human studies. I will briefly describe several illustrative examples of vitamin D regulation of tumor development/metastasis for each of these tumor types.
Colorectal cancer
A Western diet low in calcium and vitamin D fed to mice increases their risk of CRC, a risk reversed by supplementing the diet with calcium and vitamin D.68 Chronic inflammation likewise increases CRC in both mice and humans.69 VDR-null mice are particularly sensitive to the inflammatory effects of dextran sulfate sodium (DSS).70 Tumors induced by the combination of azoxymethane (AOM) and DSS can be at least partially prevented with the administration of vitamin D metabolites.71 As previously mentioned, activation of the Wnt/β-catenin pathway is a major cause of CRC. Mice with a mutation in adenomatous polyposis coli (ApcMin), a regulator of the Wnt/β-catenin pathway, develop tumors much faster on a Western diet,72 on a vitamin D–deficient diet,73 or when bred with VDR-null mice.74 1,25(OH)2D and its analogs are protective when animals are fed the vitamin D–deficient diet.73,75
Breast cancer
Similar to CRC, the number of BCa induced, in this case, by dimethylbenzanthracene (DMBA) is increased when the rats are fed a Western diet76 or when DMBA is given to VDR-null mice.77 VDR agonists prevent the growth of breast cancer xenografts regardless of estrogen receptor status.78 The growth of bone metastases is increased with dietary vitamin D deficiency,56 whereas VDR agonists can reduce their growth.79
Prostate cancer
Using xenograft models, studies have demonstrated that vitamin D analogs can inhibit the growth of PCa regardless of androgen receptor status.80 The growth of PC3 prostate cancer cells in bone is increased when mice are fed a vitamin D–deficient diet.81 When the transgenic prostate tumor model LPB-Tag is bred with VDR-null mice, tumors developed more rapidly.82 High doses of 1,25(OH)2D suppress the development of tumors in the TRAMP model (transgenic adenocarcinoma of mouse prostate).83
Non-melanoma skin cancer
Included in this category are squamous cell carcinoma (SCC) and basal cell carcinoma (BCC), the most common of human tumors. In animals, these tumors are induced by chemical induction with DMBA topically or orally, often followed by repeated topical application of phorbol esters or by chronic exposure to UVB. When VDR-null mice are treated with DMBA, nearly all the mice develop skin tumors. Similar results are seen following chronic UVB exposure.84,37 Surprisingly, mice lacking the ability to produce 1,25(OH)2D (CYP27B1 null) do not show increased susceptibility to tumor formation following either DMBA84 or UVB,37 suggesting that the role of VDR in these situations is not 1,25(OH)2D dependent. However, topical 1,25(OH)2D is protective at least of the early effects of UVB,85 and as discussed in the previous section describing mechanisms, 1,25(OH)2D does induce mechanisms that would be expected to protect against tumor development in the skin.
Clinical studies
At this point most, evidence for the role of vitamin D in tumor prevention comes from epidemiologic studies, with few randomized placebo-controlled clinical trials of sufficient size, duration, and compliance of subjects to make conclusive comments regarding its efficacy. The epidemiologic studies are suggestive of benefit, however.
Colorectal cancer
A number of meta-analyses have been performed of studies, totaling thousands of subjects, examining both the relationship of vitamin D intake and/or serum levels of 25OHD on the incidence of CRC.86, 87 One such study found a risk reduction to 0.88 (CI 0.8–0.96) comparing the highest to lowest levels of vitamin D intake86 and 0.67 (CI 0.54–0.80) comparing the highest to the lowest serum 25OHD levels;86 further reduction was found when dietary calcium was taken into account (higher calcium is better). These meta-analyses confirm earlier results from a American Cancer Society cohort study (120,000 men and women)88 and from the National Institutes of Health study (16,000 participants)89 that identified a beneficial association of vitamin D intake or serum 25OHD levels on CRC. That said, from the Women’s Health Initiative 400 IU vitamin D per day did not,90 although this study has been criticized for poor compliance and inadequate vitamin D supplementation. Thus, there is a reasonably consistent set of data supporting the protective effect of vitamin D (and calcium) on CRC development.
Breast cancer
The largest cohort studies91,92 (Nurses Health Study with 88,891 participants and Womens Health Study with 31,487 participants) showed a relative risk (RR) of 0.72 (CI 0.55–0.94) and 0.65 (CI 0.42–1.00), respectively, but only in premenopausal women. Several meta-analyses of both case control and cohort studies have been performed. One such study demonstrated a risk reduction of 0.55 (CI 0.38–0.80) comparing the highest quintile of 25OHD levels to the lowest.93 Another meta-analysis showed a RR of 0.89 (0.82–0.98) for a 10 ng/ml increase in 25OHD when all studies were included, and 0.83 (0.79–0.87) when only case control studies were pooled.94 The Womens Health Initiative (not to be confused with the Womens Health Study mentioned above) did not show a protective role for vitamin D in breast cancer. Thus, the evidence supporting a role for vitamin D in breast cancer prevention is somewhat mixed.
Prostate cancer
In a recent summary of 14 studies examining the association between 25OHD levels and the development of prostate cancer, 11 showed no association.95 Similarly, meta-analyses of studies examining either the association of dietary vitamin D intake to PCa or of serum 25OHD and PCa did not find a benefit for vitamin D.96,94 A RCT examining the effect of high dose 1,25(OH)2D and docetaxol initially showed promise in the treatment of castration-resistance PCa (ASCENT I), but this was not confirmed in a larger trial (ASCENT II).97 Thus, the clinical evidence weighs against vitamin D supplementation being beneficial in the prevention/treatment of prostate cancer.
Non-melanoma skin cancer
Although skin cancer is by far the most common cancer, studies of the epidemiology of BCCs and SCCs are handicapped by the failure of most national registries to list them. Moreover, since UVB is the common etiologic agent for both NMSC and vitamin D production, 25OHD levels can be both a marker of vitamin D sufficiency and UVB exposure, making association studies between vitamin D sufficiency and NMSC problematic. Sudies that have been reported are mixed. In a nested case control study of NMSC incidence in the Osteoporotic Fractures in Men (MrOS) study, those with the highest baseline serum 25OHD levels (30 ng/mL) had a RR of 0.53 (CI 0.3–0.93) compared to those with the lowest baseline 25OHD levels.98 On the other hand, several studies found that higher 25(OH)D levels were associated with an increased risk of BCC.99, 100 Therefore, whether the harm of UVB exposure to the skin outweighs the benefits of UVB exposure with respect to vitamin D production remains controversial.
Cardiovascular
Cellular and animal studies
VDR- and CYP27B1-null mice have increased levels of renin,101,102 which converts angiotensinogen to angiotensin I, which is further converted to angiotensin II, a powerful vasoconstrictor as well as stimulator of aldosterone production. In these mice blood pressure is increased, with increased cardiac hypertrophy, impaired systolic and diastolic function, and increased arterial stiffness.101–103 Angiotensin inhibitors block cardiac hypertrophy in these mice.101,102 The increased arterial stiffness is associated with a reduction in nitric oxide synthase (NOS) activity,103 and deletion of Vdr specifically in endothelial cells demonstrates impaired relaxation and reduced NOS expression.104 In vitro studies show that 1,25(OH)2D suppresses myocyte hypertrophy,105 and vitamin D deficiency results in smaller myofibrils but increased fibrosis106 in vivo. In global VDR gene knockout mice, renin expression is reduced both in the kidney and the heart, as is the cardiac expression of atrial naturetic factor (ANP).107 However, in the cardiomyocyte-specific VDRKO, increased renin expression is not found, yet cardiac hypertrophy still ensues.108 The cardiac hypertrophy seen in VDRKO mice includes an increase in fibrosis. 1,25(OH)2D suppresses endothelin expression in cardiac fibroblasts,109 a known profibrotic/hypertrophic factor for the heart, whereas the expression of several metalloproteinases are increased and their inhibitors are decreased.110 VDRKO mice are also prone to develop accelerated atherosclerosis,111 whereas 1,25(OH)2D can reduce such lesions in ApoE gene knockout mice, a mouse model of accelerated atherosclerosis, in part by suppressing the immune response in the atherosclerotic plaques.112 Mouse models of hypertension such as the Dahl salt-sensitive rat113 or the spontaneously hypertensive rat114 develop cardiac hypertrophy that can be prevented with the administration of 1,25(OH)2D or one of its analogs. VDRKO mice are also prone to increased thrombosis, with increased platelet aggregations and decreased thrombomodulin.115 1,25(OH)2D, on the other hand, can increase the expression of thrombomodulin and decrease the procoagulant tissue factor.116 Thus, these models suggest an important role for vitamin D signaling in the prevention of cardiovascular disease including heart failure, hypertension, and atherosclerosis.
Clinical studies
Severe vitamin D deficiency is associated with cardiomyopathy and congestive heart failure in children, which are reversible with vitamin D supplementation.117 Epidemiologic studies demonstrate an association between low 25OHD levels and increased risk of cardiac events including death,118,119 whereas other studies have shown this relationship with strokes,120 coronary artery calcification,121 and atherosclerosis.122,123 Not all such studies are positive;124 but recent meta-analyses of a number of these studies have demonstrated an association.125,126 Several large studies of patients on hemodialysis treated with active forms of vitamin D analogs have demonstrated a reduction in cardiovascular mortality,127,128 although these are retrospective reviews not RTCs. Similarly, a number of studies have shown an association between 25OHD levels and blood pressure, with meta-analyses showing a risk reduction of 16%129 to 30%.130 One interesting study employed a Mendelian randomization approach with allelic variants of CYP2R1 and DHCR7 (previously shown in GWAS studies to influence 25OHD levels)131 to demonstrate a positive association between the 25OHD allelic score and lower blood pressure.132 Endothelial function was found to improve in vitamin D–deficient diabetic patients when given vitamin D.133 However, RCTs have generally been disappointing. In one multicenter trial evaluating the use of paricalcitol in renal failure patients, the authors demonstrated a reduction in parathyroid hormone (PTH), but no effect on cardiac function.134 A meta-analysis of 51 RCTs found no significant effect on death, myocardial infarction, stroke, or blood pressure.135 Similar results were found in a separate meta-analysis of four RCTs.126 However, a different meta-analysis of RCTs using vitamin D supplementation with or without calcium did indicate a modest reduction in mortality when vitamin D plus calcium were given.136 In a review of 10 vitamin D trials for hypertension, with vitamin D doses ranging from 400–8751 IU/day, the overall analysis showed no significant effect whether or not calcium was included in the supplement, even when the negative results from the Women’s Health Initiative were excluded.126 Thus, the promise of the animal and human association studies regarding the potential benefit of vitamin D supplementation on prevention and treatment of cardiovascular disease has been difficult to confirm in RCTs.
Metabolism
Pancreas and diabetes mellitus type 2
Cellular and animal studies
The beta cell within pancreatic islets expresses both VDR137 and CYP27B1.138 1,25(OH)2D stimulates insulin secretion in vivo and in vitro,137,139,140 and by stimulating the expression of the insulin receptor promotes glucose uptake by peripheral tissues.141 On the other hand, insulin secretion is reduced in vitamin D deficiency142 and in VDRKO mice.143 However, calcium is important for insulin secretion, and low calcium levels can be suppressive.144 Therefore, the early results with vitamin D deficiency may also have reflected the low calcium levels in this condition, and when VDRKO mice were placed on a rescue diet to maintain normal calcium levels, insulin secretion was not different from wild-type mice.145 The renin/angiotensin system (RAS) may also play a role by impairing beta cell function and insulin sensitivity. As noted previously, 1,25(OH)2D suppresses the RAS in VDRKO mice and has this property in mouse islets, perhaps contributing to the ability of 1,25(OH)2D to stimulate insulin secretion (Fig. 3).146
Figure 3.
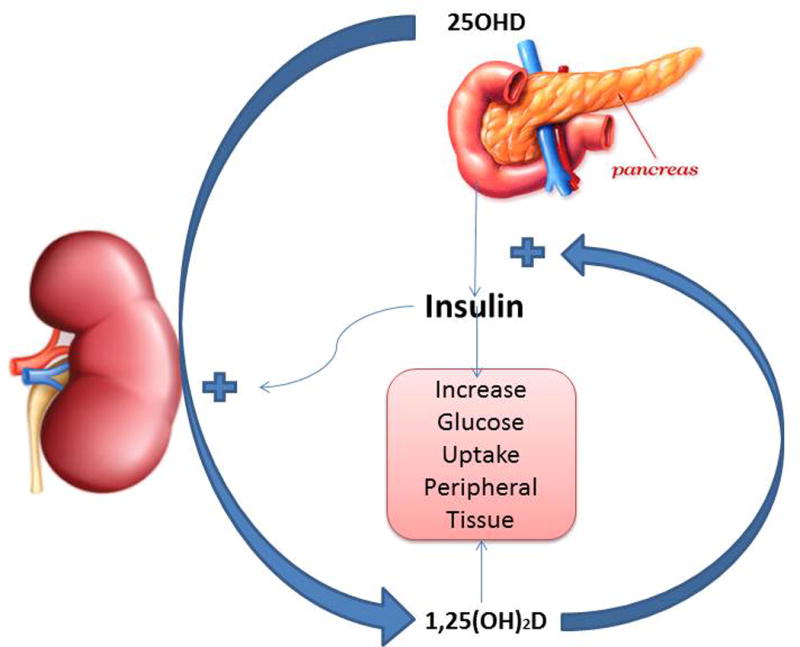
Regulation of insulin secretion and peripheral action. 1,25(OH)2D along with calcium promotes the secretion of insulin and its stimulation of gluose uptake by peripheral tissues. Insulin in turn promotes the production of 1,25(OH)2D by the kidney.
Clinical studies
Most association studies have shown an increased risk of diabetes mellitus type 2 (DM2) with low 25OHD levels.126,147–150 These studies have linked low 25OHD levels to both increased insulin resistance and impaired insulin secretion.150–152 However, not all studies show this relationship,126 and when corrected for BMI and obesity, the association may weaken.153 RCTs have been mixed and generally small. One study in 35 vitamin D–deficient adolescent obese females given 4000 IU vitamin D per day or placebo showed a reduction in fasting insulin levels and HOMA-IR over a 6-month period.154 A study of 89 obese vitamin D–deficient African Americans on a similar protocol showed increased insulin secretion following vitamin D supplementation, but insulin sensitivity was reduced.155 A study using very high doses of vitamin D (mean 88,865 IU/wk for 1 year) versus placebo in 109 prediabetic subjects with 25OHD levels >30 ng/ml failed to demonstrate an effect on insulin secretion, insulin sensitivity, or progression to frank DM2. Serum calcium levels were not reported, but subjects on vitamin D achieved mean 25OHD levels of 70 ng/ml throughout the study. Pittas has recently initiated a large multicenter placebo controlled RCT to study the role of vitamin D in DM2, which hopefully will delineate this role definitively (NIH list of clinical trials).
Adipocyte and obesity
Cellular and animal studies
The adipocyte expresses both the VDR156,157 and CYP27B1.158 The impact of 1,25(OH)2D on adipogenesis is dependent on species and level of differentiation of the cell at the time 1,25(OH)2D is added to the medium—not unlike the situation in bone,159 perhaps not surprisingly since adipocytes and osteoblasts can share a common precursor at least in the bone marrow. In particular, 1,25(OH)2D promotes adipogenesis from human mesenchymal progenitor cells160,161 but is inhibitory in mouse preadipocytes162,163 at least in part by inhibiting the expression of inhibitors of Wnt/β-catenin signaling, thus promoting Wnt/β-catenin stimulation of osteoblastogenesis rather than adipogenesis. Human fibroblasts with VDR mutations are less likely to differentiate into adipocytes in adipogenic medium than normal fibroblasts.164 Mice null for either the VDR or CYP27B1 have less adipose tissue then their normal controls165 even on rescue diets to normalize serum calcium. These results correlate with increased energy expenditure and increased expression of the uncoupling protein UCP1 in their mitochondria. Moreover, these mice are resistant to diet-induced obesity. 1,25(OH)2D inhibits the expression of UCP1 in brown adipose tissue, and overexpression of the VDR in adipocytes decreases energy expenditure leading to increased fat mass.166
Clinical studies
Obesity is associated with reduced 25OHD levels.167 The explanation(s) for this association varies from increased tissue distribution (i.e., increased storage in fat),168 decreased sunlight exposure due to lower outdoor activity levels, to decreased efficiency of vitamin D production in the skin.169 However, clinical trials with vitamin D and calcium have had limited success with respect to reducing obesity or increasing energy expenditure.170,171 Bariatric surgery, although correcting a number of metabolic consequences of obesity such as diabetes mellitus, does not reverse the vitamin D deficiency of obesity. Rather, because of its negative impact on vitamin D absorption, such surgery can make vitamin D deficiency worse, requiring higher than the usually recommended doses of vitamin D to correct the deficiency.172,173
Muscle and falls
Cellular and animal studies
The role of VDR and CYP27B1 in myocytes has been controversial. Although both are expressed in muscle precursor cells, their expression decreases with differentiation into adult muscle fibers174 and was not found in earlier studies.175 That said recent studies indicate that even in adult muscle, VDR is still expressed albeit at very low levels.176 In myoprogenitors both 25OHD and 1,25(OH)2D decrease proliferation and myotube formation, while increasing myotube size by regulating the expression of cell cycle regulators associated with changes in the forkhead box O3 and notch pathways.174,177 The nongenomic actions of 1,25(OH)2D have also received attention in muscle. Calcium, of course, is critical for muscle contraction and relaxation. 1,25(OH)2D stimulates rapid calcium influx in a VDR-dependent fashion through store-operated and voltage-dependent channels, as well as stimulating the release of calcium from intracellular stores (Fig. 5).178,179 These rapid effects involve the translocation of VDR from the nucleus to the membrane, where it is found in caveolae.180–182 In the membrane, the VDR and/or or the non-VDR receptor for 1,25(OH)2D, namely Pdia3 (protein-disulfide isomerase-associated 3, aka MARRS/Erp57/GRp58/ER60), mediate 1,25(OH)2D activation of the cyclic AMP, phospholipase C, and MAP kinase pathways.178,179 However, the physiologic significance of these nongenomic pathways remains controversial. In humans, as well as rodents, vitamin D deficiency leads to a preferential loss of type II muscle fibers associated with fatty infiltration, fibrosis, and loss of strength.183 This results, at least in part, from activation of the ubiquitin proteasome pathway.184 Vitamin D replacement reversed these changes, but supplementation with high dietary calcium alone was only partially protective.184
Figure 5.
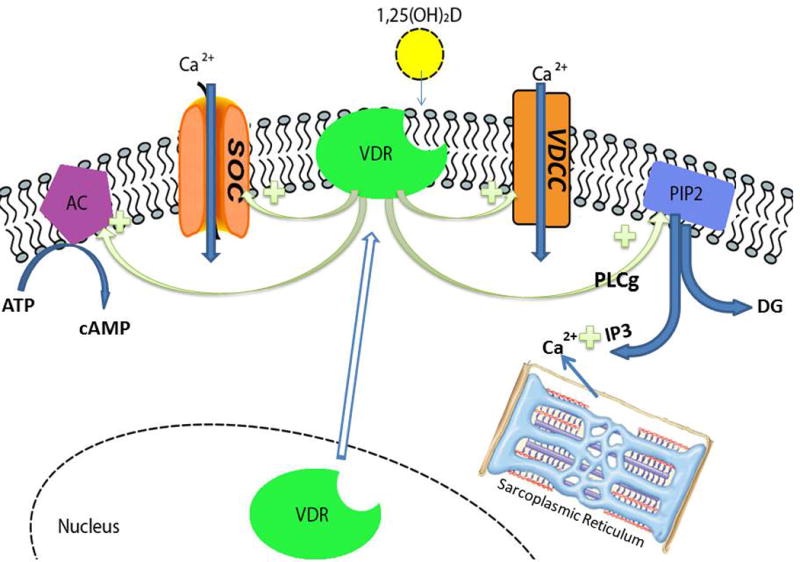
The rapid actions of 1,25(OH)2D–VDR on calcium flux within the muscle cell. VDR is translocated from the nucleus to the membrane where it is activated by 1,25(OH)2D to stimulate calcium influx through store operated (SOC) and voltage dependent (VDCC) calcium channels. 1,25(OH)2D–VDR also activates phospholipase C γ (PLCγ) that in turn hydrolyzes phosphatidylinositol bisphosphate (PIP2) to inositol trisphosphate (IP3) and diacyl glycerol (DG). IP3 stimulates the release of calcium from the sarcoplasmic reticulum; DG activates protein kinase C. 1,25(OH)2D/VDR also activates adenyl cyclase (AC), producing cAMP, and activates the mitogen activated protein kinase (MAPK) pathway (not shown).
Clinical studies
Vitamin D deficiency is associated with increased falls.185 However, trials evaluating the ability of vitamin D to prevent falls have produced mixed.186–189 A large meta-analysis of 26 RTCs in elderly females showed benefit with respect to fall risk reduction especially in vitamin D deficient subjects who were supplemented with both vitamin D and calcium,188 although the effect was generally modest.190 One interesting study evaluating vitamin D supplementation of vitamin D–deficient adults used NMR spectroscopy to measure phosphate labeled metabolites in muscle. These investigators observed faster recovery of phosphocreatine levels in muscle after exercise associated with correction of symptoms of muscle weakness in those receiving the vitamin D.191 However, efforts to provide very large doses of vitamin D (or calcifediol) at infrequent intervals (e.g., 500,000 IU annually or 60,000 IU every mo) have not been successful, and may even increase the fall risk.192,193
Skin and skin diseases
The skin consists of the epidermis to which the hair follicle is attached underlain (epidermis) or surrounded by the dermis with which these structures have strong interactions. Hair follicle cycling, for example, is critically dependent on a specialized group of mesenchymal cells known as the dermal papilla. Both the dermis and epidermis contain specialized immune cells and melanocytes that play important roles in various skin diseases. Both the hair follicle and epidermis are examples of continuously regenerating tissues: the hair follicle undergoing repeated cycles of growth (anagen), collapse (catagen), and rest (telogen) before being reactivated, while the epidermis is continuously renewing itself as stem cells in the stratum basale divide to form transit amplifying cells (TAC) that proliferate then start the differentiation process forming first the stratum spinosum where mature keratins (K1, K10), involucrin (an important component of the cornified envelope), and transglutaminse K (the enzyme cross linking involucrin and other structural components of the cornified envelope), are first expressed. The cells from the stratum spinosum further differentiate into the stratum granulosum, where the bundling protein filaggrin and another critical component of the cornified envelope, loricrin, are made. Furthermore, in the stratum granulosum lipid producing and processing enzymes are expressed that make the specialized ceramides and other lipids that are packaged into lamellar bodies for secretion into the cornified envelope, and that serve to waterproof this terminally differentiated, enucleated layer. 1,25(OH)2D and calcium are critical regulators of these events (Fig. 4).194
Figure 4.
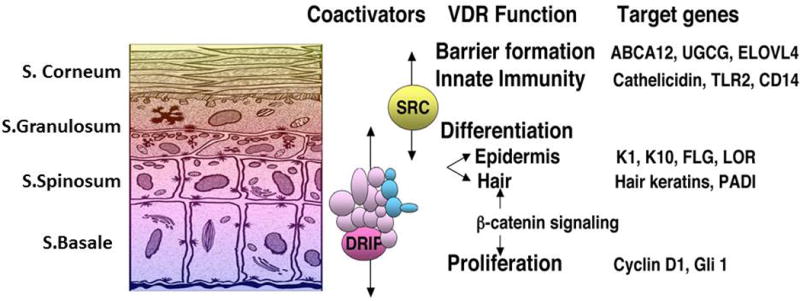
Sequential regulation of epidermal differentiation by 1,25(OH)2D–VDR and its coactivators. The DRIP (Mediator) complex is located primarily in stratum basale/spinosum and modulates 1,25(OH)2D–VDR regulation of proliferation, hair follicle cycling, and the early stages of differentiation, whereas SRC2/3 complexes are preferentially expressed in the upper layers of the epidermis where they modulate 1,25(OH)2D/VDR regulation of barrier function and innate immunity.
Cellular and animal studies
As is well known, the epidermis is the major source of vitamin D3 in the body, produced from 7-dehydrocholesterol in the epidermis under the influence of UVB to form first pre-vitamin D3, which is subsequently isomerized to vitamin D3. The epidermis also expresses the 25-hydroxylase CYP27A1, possibly CYP2R1,195,196 the 1α-hydroxylase CYP27B1,197 and the VDR.198 Thus, the keratinocyte can make its own 1,25(OH)2D from its own substrates, and respond to the 1,25(OH)2D it produces. The highest levels of VDR and CYP27B1 are found in the stratum basale. The VDR is also highly expressed in the stem cells of the hair follicle (bulge region), and deletion or mutations of VDR block post-natal hair follicle regeneration in humans, as well as in mice.199–201 Of particular interest is that hair follicle cycling does not require 1,25(OH)2D, in that mice or humans lacking or having mutations in CYP27B1 do not demonstrate abnormal hair follicle cycling.202,203 Activation of the stem cells in the bulge204,205 and epidermis204,206 involve β-catenin signaling and both require the VDR to do so. However, subsequent differentiation, at least in the epidermis, is blocked by Wnt/β-catenin signaling. What is less clear is the role played by 1,25(OH)2D in this function of the VDR. β-Catenin binds to the VDR in the AF2 domain.207 Like that of the coactivators to be discussed below, this binding is 1,25(OH)2D dependent. Such binding can promote ligand-dependent VDR transcriptional activity, but block that of β-catenin regulated proliferation.207,208 This appears to be central to the ability of 1,25(OH)2D to promote differentiation of keratinocytes (Fig. 4). On the other hand, β-catenin can bind to the VDR in a 1,25(OH)2D-independent fashion,209 perhaps mediating its stem cell–activating role in both epidermal and hair follicle stem cells, promoting differentation. In the keratinocyte, the function of the VDR is also regulated by two major coactivator complexes: mediator (originally known as DRIP or VDR interacting protein) and the steroid hormone receptor complexes (SRC) 2 and 3.13 Mediator 1 (Med1), the main VDR-binding component of the Mediator complex, is expressed primarily in the proliferating cells of the stratum basale and hair follicle, whereas SRC3 is expressed primarily in the more differentiated layers of the epidermis.13 These different coactivator complexes control different aspects of VDR action, with Med 1 more involved in proliferation and hair follicle cycling, whereas SRC2 and SRC3 are more involved in maintenance of the barrier function of the skin including its innate immune function.210–212 That said, these coactivator complexes regulate more than just VDR, and in the case of hair follicle cycling, deletion of Med1 accelerates this process unlike VDR deletion that blocks it.213
Calcium is also critical for epidermal differentiation, acting in concert with 1,25(OH)2D.214 The epidermis and hair follicle express the calcium sensing receptor (CaSR) that is critical for calcium induction of keratinocyte differentiation.215,216 Keratinocyte-specific deletion of CaSR results in impaired epidermal differentiation and barrier function, but hair follicle cycling is not affected.217 Both calcium/CaSR and 1,25(OH)2D/VDR are required for the formation of the E-cadherin–catenin complex. This complex plays a central role during epidermal differentiation through its regulation of phosphoinositide processing in the membrane via phosphatidylinositol 3 kinase (PI3K) and phosphatidyl inositol phosphate 5 kinase 1α (PIP5K1a), the subsequent activation of PLCγ1 by phosphatidylinositol trisphosphate (PIP3) (the product of PIP5K1α acting on phosphatidylinositol bisphosphate (PIP2)), generating the important signaling molecules inositol trisphosphate (IP3) and diacylglycerol (DG) that stimulate intracellular calcium release and protein kinase C activity, respectively.194 Moreover, by binding β-catenin (preventing its activation and nuclear transcription) and α-catenin (the link between the E-cadherin–catenin complex and the cytoskeleton), this complex blocks proliferation while facilitating cell–cell adhesion and further downstream signaling via the cytoskeleton.214 Recent studies have shown the importance of the VDR and the CaSR in both stem cell activation and E-cadherin–catenin complex formation in the response of the skin to wounding218 and skin tumor formation.219
Clinical studies
Psoriasis, a hyperproliferative disease of the skin, is one of the few non skeletal actions of vitamin D to have a well-validated clinical application for 1,25(OH)2D and its analogs. Psoriasis is a complex disease of autoimmunity, so the mechanisms by which 1,25(OH)2D suppresses the immunologic aspects of psoriasis will be discussed in the immune section. That said, the erythematous scaling plaques associated with psoriasis are the result of increased keratinocyte hyperproliferation and decreased differentiation driven by the inflammatory component.220 A number of clinical trials have demonstrated the efficacy and safety of 1,25(OH)2D and its analogs in the treatment of psoriasis, as monotherapy or in combination with topical glucocorticoids.221–224 Successful treatment of other skin diseases with 1,25(OH)2D and its analogs has been less clear. Given that 1,25(OH)2D promotes barrier formation211 and cathelicidin production in keratinocytes,225 the efficacy of 1,25(OH)2D treatment on atopic dermatitis has been examined, but the results, while encouraging, are not conclusive (Fig. 4).226 Ichthyosis is a disorder of keratinization. Individuals with ichthyosis can have very low 25OHD levels and rickets.227 However, it is likely that ichthyosis is the cause of the vitamin D deficiency rather than the other way around, and a trial with the 1,25(OH)2D analog calcipotriol did not improve the disease.228
Immune system and immunologic diseases
The immune system is comprised of two major but interacting forms of immunity: adaptive and innate. Adaptive immunity is initiated by cells specialized in antigen presentation, dendritic cells (DCs) primarily, and the cells responsible for antigen recognition, T and B lymphocytes, by which they are activated to carry out specialized functions including cytokine production, antibody production, and cell killing. The broad classification of T helper and regulatory cells differentiating from parent CD4+ T lymphocytes includes Th1, Th2, Th9, Th17, and Treg cells. This response is adaptive because the cells tailor their response to the antigen presented. The innate immune response involves the activation of Toll-like receptors (TLRs, of which there are 10 in the human genome) that can be expressed by a number of cell types, including polymorphonuclear cells (PMNs), monocytes, macrophages, and a wide variety of epithelial cells, including keratinocytes of the skin, gingiva, intestine, vagina, bladder, and lungs. TLRs are pathogen-recognition receptors that recognize various products of infectious agents, including bacteria and viruses, and trigger the TLR-expressing cell to produce cytokines or various antimicrobial peptides (AMPs), the best studied of which is cathelicidin. This response is innate because it is inherent to a cell from its inception, requiring no adaptation.229 The overall picture is that vitamin D signaling suppresses adaptive immunity, but promotes innate immunity.
The VDR and CYP27B1 are expressed in most if not all cells of the immune system including the epithelial cells at least when activated (Fig. 6).230–232 Moreover, several of these cells express CYP2R1 and so theoretically can produce 1,25(OH)2D from circulating vitamin D.232 The regulation of CYP27B1 in these cells differs substantially from that in the kidney, being insensitive to hormonal regulators such as PTH and FGF23, its product 1,25(OH)2D, and calcium and phosphate levels. In these immune cells CYP27B1 is stimulated by cytokines such as tumor necrosis (TNF) α and interferon (IFN) γ.233–236 Moreover, transcription of the enzyme that controls 1,25(OH)2D levels within cells, CYP24A1, is absent, defective, or blocked,232,237,238 essentially leaving 1,25(OH)2D with minimum regulation. Thus, activation of these immune cells in diseases such as sarcoidosis or lymphomas can lead to hypercalcemia with elevated 1,25(OH)2D levels.
Figure 6.
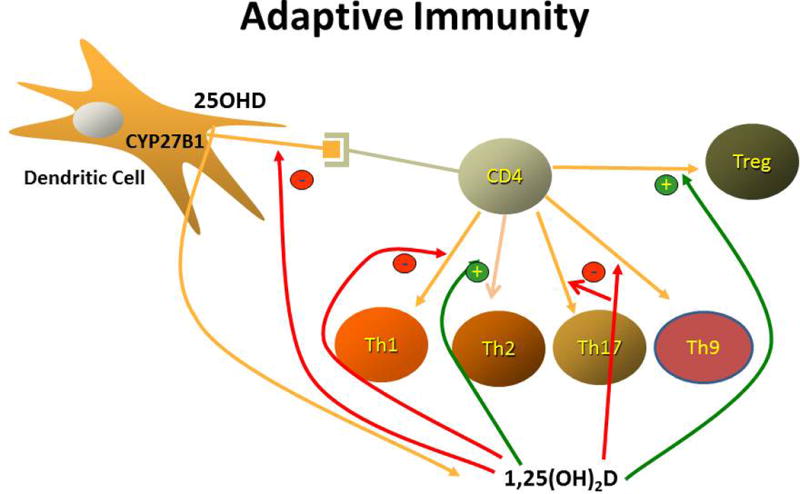
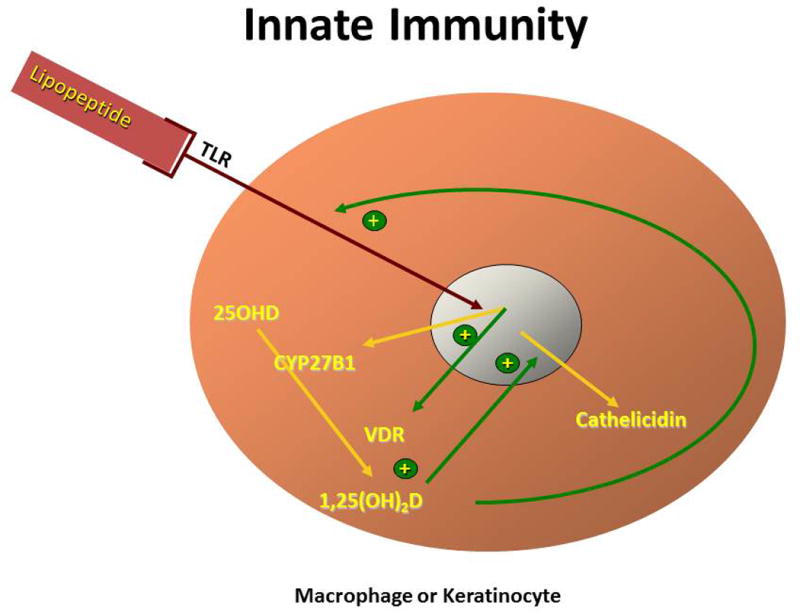
Regulation of the adaptive and innate immune pathways. (A) Adaptive immunity. 1,25(OH)2D, which is produced by dendritic cells, decreases the maturation and antigen presenting ability of dendritic cells and alters the profile of T helper cells that differentiate from the activated CD4 parent cell. In particular, 1,25(OH)2D reduces the formation of Th1, Th17, and Th9 cells, while promoting the differentiation of Th2 and Treg cells. The result is overall suppression of the adaptive immune pathway. (B) Innate immunity. Activation of selective Toll-like receptors (TLR1/2) by products of infectious organisms such as the lipopeptides from M. tuberculosis results in the induction of both the VDR and CYP27B1. In the presence of adequate substrate (25OHD), 1,25(OH)2D is produced that, in combination with the VDR, induces the formation of antimicrobial peptides such as cathelicidin, whichi are capable of killing intracellular organisms like M. tuberculosis.
Adaptive immunity
Cellular studies
1,25(OH)2D decreases the maturation of DCs, as noted by the decreased expression of HLA-DR and costimulatory molecules such as CD40, CD80, and CD86, thus decreasing their ability to present antigen.239 By suppressing IL-12 production (important for Th1 development) and IL-23 and IL-6 production (important for Th17 development), the number of Th1 and Th17 cells is reduced, as is their ability to secrete IFN-γ and IL-2 (from Th1 cells) and IL-17 (from Th17 cells).240–242 These actions limit further recruitment of T cells and their proliferation. The suppression of IL-12 also increases the development of Th2 cells and their production of IL-4, IL-5, and IL-13 further suppressing Th1 and shifting to a Th2 predominance (Fig. 6A). Th9 cells are induced by TGF-β and IL-4. 1,25(OH)2D reduces IL-9 production by these cells.243 1,25(OH)2D induces the differentiation of Treg cells as shown by increased FoxP3 expression.244 Treg cells produce the regulatory cytokine IL-10 that suppresses the development of Th1 and Th17 leading to immune tolerance.245 1,25(OH)2D can also modify the homing properties of T cells, for example by inducing the expression of CCR10, the receptor for CCL27, a keratinocyte specific cytokine, while suppressing CCR9, a gut homing receptor232, potentially accounting for the different effects of 1,25(OH)2D on the inflammatory process within different tissues. The regulation of a number of cytokines involved in the inflammatory process can be both direct and indirect and complex. For example 1,25(OH)2D inhibition of IL-2 expression involves blocking NFAT binding to the IL-2 gene promoter and sequestration of Runx1 by the VDR.241,246 Suppression of IFN-γ expression involves a negative VDRE in the IFN-γ gene promoter.247 The mechanism of IL-17 expression involves blocking NFAT binding to the IL-17 gene promoter, sequestering Runx1 by the VDR, and induction of Foxp3.241 1,25(OH)2D blocks NF-κB by inhibiting its nuclear translocation and binding to its consensus sequences and inhibiting the degradation of IκB (inhibitor of NF-κB).248 Moreover, a negative VDRE has been found in the promoter of the RelB gene, an NF-κB family member249
Animal studies
Animal studies have demonstrated both the good and problematic aspects to the suppression of adaptive immunity by 1,25(OH)2D. Overall myelopoiesis and composition of lymphoid tissue are normal in VDRKO mice, although abnormalities in immune responses to stimuli have been observed, some of which could be reversed with the rescue diet to normalize serum calcium.250 However, a number experimental models of autoimmune diseases including rheumatoid arthritis, psoriasis, type 1 diabetes mellitus (NOD mouse), systemic lupus erythematosus (SLE), experimental allergic encephalitis (EAE, model for multiple sclerosis), and inflammatory bowel disease (IBD) have been prevented/ameliorated with the use of 1,25(OH)2D or one of its analogs.251 The importance of IL-10 in the immunomodulatory actions of 1,25(OH)2D is demonstrated by the increased severity of IBD when IL-10 gene knockout mice are bred with VDRKO mice.69 Moreover, transplantation models involving the aorta, bone, bone marrow, heart, kidney, liver, pancreatic islets, skin, and small bowel have demonstrated a reduction in rejection when the animals are treated with 1,25(OH)2D or one of its analogs.252 On the other hand, the promotion of Th2 numbers and function may have adverse effects on allergic diseases such as asthma and atopic dermatitis. In these diseases, Th2 cells, not Th1 and Th17 cells, dominate the inflammatory response. Calcipotriol, an analog of 1,25(OH)2D, stimulated thymic stromal lymphopoietin (TSLP) in keratinocytes leading to an increased expression of Th2 cytokines and increased inflammatory responses to allergen induced atopic dermatitis and asthma.253 Other studies have been mixed. In normal mice 1,25(OH)2D was shown to be protective against experimentally-induced asthma, including a reduction in IL-4 production and eosinophilic infiltration.254 Part of this may relate to suppression of IL-9, a potent part of the inflammatory response in lungs.243 However, using a very similar protocol, other studies have shown that mice lacking the VDR are also protected from experimentally-induced asthma.255 Although the acute phase of atopic dermatitis is also marked by increased production of Th2 cytokines (IL-4, IL-5, IL-13) that can lead to suppression of antimicrobial peptides such as cathelicidin and increased susceptibility to infection, Th1 cells are predominant in the chronic phase.256 1,25(OH)2D, via its effects on the permeability barrier, increased numbers of Treg cells, and induction of the innate immune system can counter these effects and actually suppress Th2 function.257 The effects of 1,25(OH)2D on infections is also mixed. 1,25(OH)2D inhibition of IFN-γ stimulation of reactive oxygen species and nitric oxide production,258 or suppression of IL-17 (limiting its induction of AMPs and neutrophil recruitment)259 have been shown to reduce resistance to infectious organisms such as Leishmania,258 toxoplasma,260 and Citrobacter.261
Clinical studies
Association studies have found inverse correlations between 25OHD levels and/or vitamin D intake and a number of autoimmune diseases including multiple sclerosis,262 type 1 diabetes,263,264 Crohns disease,265 rheumatoid arthritis,266 lupus,267 and Graves thyroiditis.268 Similarly, an inverse correlation was found between maternal 25OHD levels and the development of eczema and asthma in children269 and, in adults, between 25OHD and asthma in the NHANES survey.270 The majority, although not all, of studies have shown an inverse correlation between 25OHD and atopic dermatitis.271 RTCs have not been plentiful, large, or consistent,272–274 although a meta-analysis of 4 trials with vitamin D supplementation in atopic dermatitis demonstrated benefit.275
Innate immunity
Cellular and animal studies
Stimulation of TLR 2/1 in macrophages276 or TLR2 in keratinocytes225 leads to an increase in CYP27B1 and VDR expression. With adequate substrate (25OHD), these cells produce 1,25(OH)2D that in turn induces AMPs such as cathelicidin and defensins (Fig. 6B). Cathelicidin, in turn, can kill intracellular organisms such as Mycobacterium tuberculosis. In keratinocytes, 1,25(OH)2D also induces the TLR coreceptor CD14. Cathelicidin also promotes the chemotaxis of neutrophils, monocytes, macrophages, and T cells into the skin, and in this way links the adaptive and immune responses in the skin and other tissues.277 It had been appreciated for some time that 1,25(OH)2D was capable of inhibiting the growth of M. tuberculosis in vitro,278 and the elucidation of the TLR–CYP27B1–VDR–cathelicidin pathway demonstrated the mechanism. Moreover, the dependence of the final induction of cathelicidin on the ambient 25OHD concentrations279 explained the susceptibility of vitamin D–deficient subjects to this disease.280 The murine cathelicidin gene lacks a VDRE and so is not responsive to 1,25(OH)2D. However, 1,25(OH)2D stimulates the inducible NOS pathway by which it induces M. tuberculosis killing in macrophages.281 Not all aspects of 1,25(OH)2D induction of the innate immune system are beneficial, however. Chronic activation resulting in overexpression of cathelicidin may lead to the formation of a self–DNA complex that can be detected by plasmacytoid DCs, which become activated and contribute to the psoriatic process.282
Clinical studies
As noted earlier, in atopic dermatitis, production of cathelicidin and other AMPs is reduced by IL-4 and IL-13, and patients with atopic dermatitis are susceptible to microbial superinfections.283 This likely contributes to the improvement of atopic dermatitis in most studies noted earlier. Unfortunately, the ability of vitamin D to treat M. tuberculosis even in vitamin D–deficient populations has not been universally successful.l284–287
Summary and conclusions
The extraskeletal actions of vitamin D are legion as befits a molecule (1,25(OH)2D, the active metabolite of vitamin) whose receptor is found in nearly all cells, many of which also contain the enzyme (CYP27B1) that can produce 1,25(OH)2D in the same cell. Indeed, thousands of cellular and animal studies indicate that vitamin D signaling has a profound effect on most physiologic processes, including cancer prevention, improved cardiovascular function, diabetes prevention, prevention of obesity, improved muscle function, enhanced barrier function of the skin, hair follicle cycling, and prevention of immune related diseases. Indeed, association studies in humans have linked vitamin D deficiency to diseases resulting from disruption of these physiologic processes. However, large RCTs that clearly demonstrate the promising potential of vitamin D in the prevention and treatment of these diseases have either not been done or have produced mixed results. Part of the problem relates to lack of pharmaceutical support for large RCTs involving a drug (i.e., vitamin D) that cannot be patented, or the inability to give high enough doses of the active metabolite or its analogs to get an effect without serious side effects, such as hypercalcemia and hypercalciuria. However, several large government supported RCTs are ongoing and the results from which are eagerly awaited.
Acknowledgments
I wish to acknowledge the number of collaborators who have worked with me over the years in our investigations of vitamin D mechanisms of action in non-skeletal tissues. This work has been funded by NIH grants RO1 AR050023 and AR 051930 and Veterans Affairs grant IBX001066.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Bikle DD. Clinical counterpoint: vitamin D: new actions, new analogs, new therapeutic potential. Endocrine reviews. 1992;13:765–84. doi: 10.1210/edrv-13-4-765. [DOI] [PubMed] [Google Scholar]
- 2.Bikle DD. Extra renal synthesis of 1,25 dihydroxyvitamin D and its Health Implications. Clin Rev in Bone and Min Metab. 2009;7:114–25. [Google Scholar]
- 3.Skorija K, Cox M, Sisk JM, et al. Ligand-independent actions of the vitamin D receptor maintain hair follicle homeostasis. Molecular endocrinology (Baltimore, Md) 2005;19:855–62. doi: 10.1210/me.2004-0415. [DOI] [PubMed] [Google Scholar]
- 4.Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. The Journal of steroid biochemistry and molecular biology. 2014;144(Pt A):28–39. doi: 10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pike JW. Genome-wide principles of gene regulation by the vitamin D receptor and its activating ligand. Molecular and cellular endocrinology. 2011;347:3–10. doi: 10.1016/j.mce.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Z, Bikle DD. Cloning of the human phospholipase C-gamma1 promoter and identification of a DR6-type vitamin D-responsive element. The Journal of biological chemistry. 1997;272:6573–7. doi: 10.1074/jbc.272.10.6573. [DOI] [PubMed] [Google Scholar]
- 7.Nemere I, Garbi N, Hammerling GJ, Khanal RC. Intestinal cell calcium uptake and the targeted knockout of the 1,25D3-MARRS (membrane-associated, rapid response steroid-binding) receptor/PDIA3/Erp57. The Journal of biological chemistry. 2010;285:31859–66. doi: 10.1074/jbc.M110.116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemere I, Yoshimoto Y, Norman AW. Calcium transport in perfused duodena from normal chicks: enhancement within fourteen minutes of exposure to 1,25-dihydroxyvitamin D3. Endocrinology. 1984;115:1476–83. doi: 10.1210/endo-115-4-1476. [DOI] [PubMed] [Google Scholar]
- 9.Bravo S, Paredes R, Izaurieta P, et al. The classic receptor for 1alpha,25-dihydroxy vitamin D3 is required for non-genomic actions of 1alpha,25-dihydroxy vitamin D3 in osteosarcoma cells. Journal of cellular biochemistry. 2006;99:995–1000. doi: 10.1002/jcb.21031. [DOI] [PubMed] [Google Scholar]
- 10.Carlberg C. Genome-wide (over)view on the actions of vitamin D. Front Physiol. 2014;5:167. doi: 10.3389/fphys.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlberg C, Seuter S, Heikkinen S. The first genome-wide view of vitamin D receptor locations and their mechanistic implications. Anticancer research. 2012;32:271–82. [PubMed] [Google Scholar]
- 12.Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352–60. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oda Y, Sihlbom C, Chalkley RJ, et al. Two distinct coactivators, DRIP/mediator and SRC/p160, are differentially involved in vitamin D receptor transactivation during keratinocyte differentiation. Molecular endocrinology (Baltimore, Md) 2003;17:2329–39. doi: 10.1210/me.2003-0063. [DOI] [PubMed] [Google Scholar]
- 14.Saccone D, Asani F, Bornman L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene. 2015;561:171–80. doi: 10.1016/j.gene.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Narvaez CJ, Matthews D, LaPorta E, Simmons KM, Beaudin S, Welsh J. The impact of vitamin D in breast cancer: genomics, pathways, metabolism. Front Physiol. 2014;5:213. doi: 10.3389/fphys.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santagata S, Thakkar A, Ergonul A, et al. Taxonomy of breast cancer based on normal cell phenotype predicts outcome. The Journal of clinical investigation. 2014;124:859–70. doi: 10.1172/JCI70941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matusiak D, Benya RV. CYP27A1 and CYP24 expression as a function of malignant transformation in the colon. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2007;55:1257–64. doi: 10.1369/jhc.7A7286.2007. [DOI] [PubMed] [Google Scholar]
- 18.Brozek W, Manhardt T, Kallay E, Peterlik M, Cross HS. Relative Expression of Vitamin D Hydroxylases, CYP27B1 and CYP24A1, and of Cyclooxygenase-2 and Heterogeneity of Human Colorectal Cancer in Relation to Age, Gender, Tumor Location, and Malignancy: Results from Factor and Cluster Analysis. Cancers. 2012;4:763–76. doi: 10.3390/cancers4030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tannour-Louet M, Lewis SK, Louet JF, et al. Increased expression of CYP24A1 correlates with advanced stages of prostate cancer and can cause resistance to vitamin D3-based therapies. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:364–72. doi: 10.1096/fj.13-236109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albertson DG, Ylstra B, Segraves R, et al. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nature genetics. 2000;25:144–6. doi: 10.1038/75985. [DOI] [PubMed] [Google Scholar]
- 21.Borkowski R, Du L, Zhao Z, et al. Genetic mutation of p53 and suppression of the miR-17 approximately 92 cluster are synthetic lethal in non-small cell lung cancer due to upregulation of vitamin D Signaling. Cancer research. 2015;75:666–75. doi: 10.1158/0008-5472.CAN-14-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang S, Gao L, Yang Y, et al. miR-145 mediates the antiproliferative and gene regulatory effects of vitamin D3 by directly targeting E2F3 in gastric cancer cells. Oncotarget. 2015;6:7675–85. doi: 10.18632/oncotarget.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gocek E, Wang X, Liu X, Liu CG, Studzinski GP. MicroRNA-32 upregulation by 1,25-dihydroxyvitamin D3 in human myeloid leukemia cells leads to Bim targeting and inhibition of AraC-induced apoptosis. Cancer research. 2011;71:6230–9. doi: 10.1158/0008-5472.CAN-11-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang YJ, Bikle DD. LncRNA: a new player in 1alpha, 25(OH)(2) vitamin D(3) /VDR protection against skin cancer formation. Experimental dermatology. 2014;23:147–50. doi: 10.1111/exd.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hager G, Formanek M, Gedlicka C, Thurnher D, Knerer B, Kornfehl J. 1,25(OH)2 vitamin D3 induces elevated expression of the cell cycle-regulating genes P21 and P27 in squamous carcinoma cell lines of the head and neck. Acta Otolaryngol. 2001;121:103–9. doi: 10.1080/000164801300006353. [DOI] [PubMed] [Google Scholar]
- 27.Palmer HG, Sanchez-Carbayo M, Ordonez-Moran P, Larriba MJ, Cordon-Cardo C, Munoz A. Genetic signatures of differentiation induced by 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer research. 2003;63:7799–806. [PubMed] [Google Scholar]
- 28.Liu G, Hu X, Chakrabarty S. Vitamin D mediates its action in human colon carcinoma cells in a calcium-sensing receptor-dependent manner: downregulates malignant cell behavior and the expression of thymidylate synthase and survivin and promotes cellular sensitivity to 5-FU. International journal of cancer. 2010;126:631–9. doi: 10.1002/ijc.24762. [DOI] [PubMed] [Google Scholar]
- 29.An BS, Tavera-Mendoza LE, Dimitrov V, et al. Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Molecular and cellular biology. 2010;30:4890–900. doi: 10.1128/MCB.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer MB, Goetsch PD, Pike JW. VDR/RXR and TCF4/beta-catenin cistromes in colonic cells of colorectal tumor origin: impact on c-FOS and c-MYC gene expression. Molecular endocrinology (Baltimore, Md) 2012;26:37–51. doi: 10.1210/me.2011-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colston KW, Perks CM, Xie SP, Holly JM. Growth inhibition of both MCF-7 and Hs578T human breast cancer cell lines by vitamin D analogues is associated with increased expression of insulin-like growth factor binding protein-3. J Mol Endocrinol. 1998;20:157–62. doi: 10.1677/jme.0.0200157. [DOI] [PubMed] [Google Scholar]
- 32.Huynh H, Pollak M, Zhang JC. Regulation of insulin-like growth factor (IGF) II and IGF binding protein 3 autocrine loop in human PC-3 prostate cancer cells by vitamin D metabolite 1,25(OH)2D3 and its analog EB1089. Int J Oncol. 1998;13:137–43. doi: 10.3892/ijo.13.1.137. [DOI] [PubMed] [Google Scholar]
- 33.Peehl DM, Shinghal R, Nonn L, et al. Molecular activity of 1,25-dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. The Journal of steroid biochemistry and molecular biology. 2004;92:131–41. doi: 10.1016/j.jsbmb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Swami S, Raghavachari N, Muller UR, Bao YP, Feldman D. Vitamin D growth inhibition of breast cancer cells: gene expression patterns assessed by cDNA microarray. Breast cancer research and treatment. 2003;80:49–62. doi: 10.1023/A:1024487118457. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Yang J, Venkateswarlu S, Ko T, Brattain MG. Autocrine TGFbeta signaling mediates vitamin D3 analog-induced growth inhibition in breast cells. Journal of cellular physiology. 2001;188:383–93. doi: 10.1002/jcp.1125. [DOI] [PubMed] [Google Scholar]
- 36.Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. The Journal of investigative dermatology. 1998;110:885–8. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 37.Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. The Journal of investigative dermatology. 2011;131:2289–97. doi: 10.1038/jid.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGaffin KR, Chrysogelos SA. Identification and characterization of a response element in the EGFR promoter that mediates transcriptional repression by 1,25-dihydroxyvitamin D3 in breast cancer cells. J Mol Endocrinol. 2005;35:117–33. doi: 10.1677/jme.1.01813. [DOI] [PubMed] [Google Scholar]
- 39.Cordero JB, Cozzolino M, Lu Y, et al. 1,25-Dihydroxyvitamin D down-regulates cell membrane growth- and nuclear growth-promoting signals by the epidermal growth factor receptor. The Journal of biological chemistry. 2002;277:38965–71. doi: 10.1074/jbc.M203736200. [DOI] [PubMed] [Google Scholar]
- 40.Byers SW, Rowlands T, Beildeck M, Bong YS. Mechanism of action of vitamin D and the vitamin D receptor in colorectal cancer prevention and treatment. Reviews in endocrine & metabolic disorders. 2011;13:31–8. doi: 10.1007/s11154-011-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bikle DD. The vitamin D receptor: a tumor suppressor in skin. Discovery medicine. 2011;11:7–17. [PMC free article] [PubMed] [Google Scholar]
- 42.Aguilera O, Pena C, Garcia JM, et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28:1877–84. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
- 43.Pendas-Franco N, Garcia JM, Pena C, et al. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene. 2008;27:4467–77. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 44.Ordonez-Moran P, Alvarez-Diaz S, Valle N, Larriba MJ, Bonilla F, Munoz A. The effects of 1,25-dihydroxyvitamin D3 on colon cancer cells depend on RhoA-ROCK-p38MAPK-MSK signaling. The Journal of steroid biochemistry and molecular biology. 2010;121:355–61. doi: 10.1016/j.jsbmb.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 45.Diaz GD, Paraskeva C, Thomas MG, Binderup L, Hague A. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer research. 2000;60:2304–12. [PubMed] [Google Scholar]
- 46.Pan L, Matloob AF, Du J, et al. Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A /sodium butyrate-induced and 5-aza-2′-deoxycytidine-induced PTEN upregulation. The FEBS journal. 2010;277:989–99. doi: 10.1111/j.1742-4658.2009.07542.x. [DOI] [PubMed] [Google Scholar]
- 47.Kizildag S, Ates H, Kizildag S. Treatment of K562 cells with 1,25-dihydroxyvitamin D3 induces distinct alterations in the expression of apoptosis-related genes BCL2, BAX, BCLXL, and p21. Ann Hematol. 2009;89:1–7. doi: 10.1007/s00277-009-0766-y. [DOI] [PubMed] [Google Scholar]
- 48.Weitsman GE, Ravid A, Liberman UA, Koren R. Vitamin D enhances caspase-dependent and independent TNF-induced breast cancer cell death: the role of reactive oxygen species. Annals of the New York Academy of Sciences. 2003;1010:437–40. doi: 10.1196/annals.1299.079. [DOI] [PubMed] [Google Scholar]
- 49.Weitsman GE, Koren R, Zuck E, Rotem C, Liberman UA, Ravid A. Vitamin D sensitizes breast cancer cells to the action of H2O2: mitochondria as a convergence point in the death pathway. Free Radic Biol Med. 2005;39:266–78. doi: 10.1016/j.freeradbiomed.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Sergeev IN. Vitamin D and cellular Ca2+ signaling in breast cancer. Anticancer research. 2012;32:299–302. [PubMed] [Google Scholar]
- 51.Hoyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jaattela M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. 2005;12:1297–309. doi: 10.1038/sj.cdd.4401651. [DOI] [PubMed] [Google Scholar]
- 52.Freeman SE, Hacham H, Gange RW, Maytum DJ, Sutherland JC, Sutherland BM. Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:5605–9. doi: 10.1073/pnas.86.14.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10058–63. doi: 10.1073/pnas.0502311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hussein MR. Ultraviolet radiation and skin cancer: molecular mechanisms. Journal of cutaneous pathology. 2005;32:191–205. doi: 10.1111/j.0303-6987.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 55.Demetriou SK, Ona-Vu K, Teichert AE, Cleaver JE, Bikle DD, Oh DH. Vitamin D Receptor Mediates DNA Repair and Is UV Inducible in Intact Epidermis but Not in Cultured Keratinocytes. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ooi LL, Zhou H, Kalak R, et al. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer research. 2010;70:1835–44. doi: 10.1158/0008-5472.CAN-09-3194. [DOI] [PubMed] [Google Scholar]
- 57.Kallay E, Pietschmann P, Toyokuni S, et al. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429–35. doi: 10.1093/carcin/22.9.1429. [DOI] [PubMed] [Google Scholar]
- 58.Fedirko V, Bostick RM, Long Q, et al. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2010;19:280–91. doi: 10.1158/1055-9965.EPI-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moll PR, Sander V, Frischauf AM, Richter K. Expression profiling of vitamin D treated primary human keratinocytes. Journal of cellular biochemistry. 2007;100:574–92. doi: 10.1002/jcb.21061. [DOI] [PubMed] [Google Scholar]
- 60.Akutsu N, Lin R, Bastien Y, et al. Regulation of gene Expression by 1alpha,25-dihydroxyvitamin D3 and Its analog EB1089 under growth-inhibitory conditions in squamous carcinoma Cells. Molecular endocrinology (Baltimore, Md) 2001;15:1127–39. doi: 10.1210/mend.15.7.0655. [DOI] [PubMed] [Google Scholar]
- 61.Bao BY, Ting HJ, Hsu JW, Lee YF. Protective role of 1 alpha, 25-dihydroxyvitamin D3 against oxidative stress in nonmalignant human prostate epithelial cells. International journal of cancer. 2008;122:2699–706. doi: 10.1002/ijc.23460. [DOI] [PubMed] [Google Scholar]
- 62.Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. 2007;6:1433–9. doi: 10.1158/1535-7163.MCT-06-0677. [DOI] [PubMed] [Google Scholar]
- 63.Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–20. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- 64.Sung V, Feldman D. 1,25-Dihydroxyvitamin D3 decreases human prostate cancer cell adhesion and migration. Molecular and cellular endocrinology. 2000;164:133–43. doi: 10.1016/s0303-7207(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 65.Bao BY, Yao J, Lee YF. 1alpha, 25-dihydroxyvitamin D3 suppresses interleukin-8-mediated prostate cancer cell angiogenesis. Carcinogenesis. 2006;27:1883–93. doi: 10.1093/carcin/bgl041. [DOI] [PubMed] [Google Scholar]
- 66.Vanoirbeek E, Eelen G, Verlinden L, et al. PDLIM2 expression is driven by vitamin D and is involved in the pro-adhesion, and anti-migration and -invasion activity of vitamin D. Oncogene. 2014;33:1904–11. doi: 10.1038/onc.2013.123. [DOI] [PubMed] [Google Scholar]
- 67.Liu W, Guo M, Ezzat S, Asa SL. Vitamin D inhibits CEACAM1 to promote insulin/IGF-I receptor signaling without compromising anti-proliferative action. Laboratory investigation; a journal of technical methods and pathology. 2011;91:147–56. doi: 10.1038/labinvest.2010.144. [DOI] [PubMed] [Google Scholar]
- 68.Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88–92. doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Molecular endocrinology (Baltimore, Md) 2003;17:2386–92. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 70.Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. American journal of physiology Gastrointestinal and liver physiology. 2008;294:G208–16. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 71.Murillo G, Nagpal V, Tiwari N, Benya RV, Mehta RG. Actions of vitamin D are mediated by the TLR4 pathway in inflammation-induced colon cancer. The Journal of steroid biochemistry and molecular biology. 2003;121:403–7. doi: 10.1016/j.jsbmb.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang K, Lamprecht SA, Shinozaki H, et al. Dietary calcium and cholecalciferol modulate cyclin D1 expression, apoptosis, and tumorigenesis in intestine of adenomatous polyposis coli1638N/+ mice. The Journal of nutrition. 2008;138:1658–63. doi: 10.1093/jn/138.9.1658. [DOI] [PubMed] [Google Scholar]
- 73.Xu H, Posner GH, Stevenson M, Campbell FC. Apc(MIN) modulation of vitamin D secosteroid growth control. Carcinogenesis. 2010;31:1434–41. doi: 10.1093/carcin/bgq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng W, Wong KE, Zhang Z, et al. Inactivation of the vitamin D receptor in APC(min/+) mice reveals a critical role for the vitamin D receptor in intestinal tumor growth. International journal of cancer. 2011;130:10–9. doi: 10.1002/ijc.25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huerta S, Irwin RW, Heber D, et al. 1alpha,25-(OH)(2)-D(3) and its synthetic analogue decrease tumor load in the Apc(min) Mouse. Cancer research. 2002;62:741–6. [PubMed] [Google Scholar]
- 76.Lipkin M, Newmark HL. Vitamin D, calcium and prevention of breast cancer: a review. Journal of the American College of Nutrition. 1999;18:392S–7S. doi: 10.1080/07315724.1999.10718903. [DOI] [PubMed] [Google Scholar]
- 77.Zinser GM, Welsh J. Effect of Vitamin D3 receptor ablation on murine mammary gland development and tumorigenesis. The Journal of steroid biochemistry and molecular biology. 2004;89–90:433–6. doi: 10.1016/j.jsbmb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 78.VanWeelden K, Flanagan L, Binderup L, Tenniswood M, Welsh J. Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089. Endocrinology. 1998;139:2102–10. doi: 10.1210/endo.139.4.5892. [DOI] [PubMed] [Google Scholar]
- 79.El Abdaimi K, Dion N, Papavasiliou V, et al. The vitamin D analogue EB 1089 prevents skeletal metastasis and prolongs survival time in nude mice transplanted with human breast cancer cells. Cancer research. 2000;60:4412–8. [PubMed] [Google Scholar]
- 80.Bhatia V, Saini MK, Shen X, et al. EB1089 inhibits the parathyroid hormone-related protein-enhanced bone metastasis and xenograft growth of human prostate cancer cells. Mol Cancer Ther. 2009;8:1787–98. doi: 10.1158/1535-7163.MCT-09-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng Y, Zhou H, Ooi LL, Snir AD, Dunstan CR, Seibel MJ. Vitamin D deficiency promotes prostate cancer growth in bone. Prostate. 2011;71:1012–21. doi: 10.1002/pros.21316. [DOI] [PubMed] [Google Scholar]
- 82.Mordan-McCombs S, Brown T, Wang WL, Gaupel AC, Welsh J, Tenniswood M. Tumor progression in the LPB-Tag transgenic model of prostate cancer is altered by vitamin D receptor and serum testosterone status. The Journal of steroid biochemistry and molecular biology. 2010;121:368–71. doi: 10.1016/j.jsbmb.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krishnan AV, Trump DL, Johnson CS, Feldman D. The role of vitamin D in cancer prevention and treatment. Endocrinol Metab Clin North Am. 2010;39:401–18. doi: 10.1016/j.ecl.2010.02.011. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ellison TI, Smith MK, Gilliam AC, Macdonald PN. Inactivation of the Vitamin D Receptor Enhances Susceptibility of Murine Skin to UV-Induced Tumorigenesis. The Journal of investigative dermatology. 2008;128:2508–17. doi: 10.1038/jid.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gupta R, Dixon KM, Deo SS, et al. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. The Journal of investigative dermatology. 2007;127:707–15. doi: 10.1038/sj.jid.5700597. [DOI] [PubMed] [Google Scholar]
- 86.Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29:3775–82. doi: 10.1200/JCO.2011.35.7566. [DOI] [PubMed] [Google Scholar]
- 87.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: Serum vitamin D and colorectal adenoma risk. Prev Med. 2011;53:10–6. doi: 10.1016/j.ypmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 88.Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. Journal of the National Cancer Institute. 2004;96:1015–22. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 89.Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32:789–803. doi: 10.1016/j.clinthera.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 90.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. The New England journal of medicine. 2006;354:684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 91.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin d and risk of breast cancer. Journal of the National Cancer Institute. 2002;94:1301–11. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 92.Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;167:1050–9. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- 93.Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast cancer research and treatment. 2010;121:469–77. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 94.Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. International journal of cancer. 2011;128:1414–24. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 95.van der Rhee HJ, de Vries E, Coebergh JW. Does sunlight prevent cancer? A systematic review. European journal of cancer. 2006;42:2222–32. doi: 10.1016/j.ejca.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 96.Gilbert R, Martin RM, Beynon R, et al. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer causes & control : CCC. 2011;22:319–40. doi: 10.1007/s10552-010-9706-3. [DOI] [PubMed] [Google Scholar]
- 97.Scher HI, Jia X, Chi K, et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol. 2011;29:2191–8. doi: 10.1200/JCO.2010.32.8815. [DOI] [PubMed] [Google Scholar]
- 98.Tang JY, Parimi N, Wu A, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer causes & control : CCC. 2010;21:387–91. doi: 10.1007/s10552-009-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asgari MM, Tang J, Warton ME, et al. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. The Journal of investigative dermatology. 2010;130:1438–43. doi: 10.1038/jid.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eide MJ, Johnson DA, Jacobsen GR, et al. Vitamin D and nonmelanoma skin cancer in a health maintenance organization cohort. Archives of dermatology. 2011;147:1379–84. doi: 10.1001/archdermatol.2011.231. [DOI] [PubMed] [Google Scholar]
- 101.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. The Journal of clinical investigation. 2002;110:229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney international. 2008;74:170–9. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- 103.Andrukhova O, Slavic S, Zeitz U, et al. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Molecular endocrinology (Baltimore, Md) 2014;28:53–64. doi: 10.1210/me.2013-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ni W, Watts SW, Ng M, Chen S, Glenn DJ, Gardner DG. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension. 2014;64:1290–8. doi: 10.1161/HYPERTENSIONAHA.114.03971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. The Journal of clinical investigation. 1996;97:1577–88. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weishaar RE, Kim SN, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. The American journal of physiology. 1990;258:E134–42. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- 107.Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. American journal of physiology Endocrinology and metabolism. 2005;288:E125–32. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 108.Chen S, Law CS, Grigsby CL, et al. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838–47. doi: 10.1161/CIRCULATIONAHA.111.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gardner DG, Chen S, Glenn DJ. Vitamin D and the heart. Am J Physiol Regul Integr Comp Physiol. 2013;305:R969–77. doi: 10.1152/ajpregu.00322.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. The Journal of steroid biochemistry and molecular biology. 2007;103:416–9. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- 111.Szeto FL, Reardon CA, Yoon D, et al. Vitamin D receptor signaling inhibits atherosclerosis in mice. Molecular endocrinology (Baltimore, Md) 2012;26:1091–101. doi: 10.1210/me.2011-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takeda M, Yamashita T, Sasaki N, et al. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler Thromb Vasc Biol. 2010;30:2495–503. doi: 10.1161/ATVBAHA.110.215459. [DOI] [PubMed] [Google Scholar]
- 113.Choi JH, Ke Q, Bae S, et al. Doxercalciferol, a pro-hormone of vitamin D, prevents the development of cardiac hypertrophy in rats. J Card Fail. 2011;17:1051–8. doi: 10.1016/j.cardfail.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kong J, Kim GH, Wei M, et al. Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. The American journal of pathology. 2010;177:622–31. doi: 10.2353/ajpath.2010.091292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aihara K, Azuma H, Akaike M, et al. Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. The Journal of biological chemistry. 2004;279:35798–802. doi: 10.1074/jbc.M404865200. [DOI] [PubMed] [Google Scholar]
- 116.Ohsawa M, Koyama T, Yamamoto K, Hirosawa S, Kamei S, Kamiyama R. 1alpha,25-dihydroxyvitamin D(3) and its potent synthetic analogs downregulate tissue factor and upregulate thrombomodulin expression in monocytic cells, counteracting the effects of tumor necrosis factor and oxidized LDL. Circulation. 2000;102:2867–72. doi: 10.1161/01.cir.102.23.2867. [DOI] [PubMed] [Google Scholar]
- 117.Uysal S, Kalayci AG, Baysal K. Cardiac functions in children with vitamin D deficiency rickets. Pediatr Cardiol. 1999;20:283–6. doi: 10.1007/s002469900464. [DOI] [PubMed] [Google Scholar]
- 118.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marniemi J, Alanen E, Impivaara O, et al. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005;15:188–97. doi: 10.1016/j.numecd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 121.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. Journal of the American Society of Nephrology : JASN. 2009;20:1805–12. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carrelli AL, Walker MD, Lowe H, et al. Vitamin D deficiency is associated with subclinical carotid atherosclerosis: the Northern Manhattan study. Stroke; a journal of cerebral circulation. 2011;42:2240–5. doi: 10.1161/STROKEAHA.110.608539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Melamed ML, Muntner P, Michos ED, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:1179–85. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Messenger W, Nielson CM, Li H, et al. Serum and dietary vitamin D and cardiovascular disease risk in elderly men: a prospective cohort study. Nutr Metab Cardiovasc Dis. 2012;22:856–63. doi: 10.1016/j.numecd.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–29. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pittas AG, Chung M, Trikalinos T, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Annals of internal medicine. 2010;152:307–14. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. Journal of the American Society of Nephrology : JASN. 2005;16:1115–25. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 128.Naves-Diaz M, Alvarez-Hernandez D, Passlick-Deetjen J, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney international. 2008;74:1070–8. doi: 10.1038/ki.2008.343. [DOI] [PubMed] [Google Scholar]
- 129.Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2011;29:636–45. doi: 10.1097/HJH.0b013e32834320f9. [DOI] [PubMed] [Google Scholar]
- 130.Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. 2013;28:205–21. doi: 10.1007/s10654-013-9790-2. [DOI] [PubMed] [Google Scholar]
- 131.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2:719–29. doi: 10.1016/S2213-8587(14)70113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–5. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 134.Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. Jama. 2012;307:674–84. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 135.Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. The Journal of clinical endocrinology and metabolism. 2011;96:1931–42. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 136.Rejnmark L, Avenell A, Masud T, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. The Journal of clinical endocrinology and metabolism. 2012;97:2670–81. doi: 10.1210/jc.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee S, Clark SA, Gill RK, Christakos S. 1,25-Dihydroxyvitamin D3 and pancreatic beta-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology. 1994;134:1602–10. doi: 10.1210/endo.134.4.8137721. [DOI] [PubMed] [Google Scholar]
- 138.Bland R, Markovic D, Hills CE, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. The Journal of steroid biochemistry and molecular biology. 2004;89–90:121–5. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- 139.Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84–90. doi: 10.1210/endo-119-1-84. [DOI] [PubMed] [Google Scholar]
- 140.Sandler S, Buschard K, Bendtzen K. Effects of 1,25-dihydroxyvitamin D3 and the analogues MC903 and KH1060 on interleukin-1 beta-induced inhibition of rat pancreatic islet beta-cell function in vitro. Immunol Lett. 1994;41:73–7. doi: 10.1016/0165-2478(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 141.Wolden-Kirk H, Overbergh L, Christesen HT, Brusgaard K, Mathieu C. Vitamin D and diabetes: its importance for beta cell and immune function. Molecular and cellular endocrinology. 2011;347:106–20. doi: 10.1016/j.mce.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 142.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–5. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 143.Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:509–11. doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
- 144.Hagstrom E, Hellman P, Lundgren E, Lind L, Arnlov J. Serum calcium is independently associated with insulin sensitivity measured with euglycaemic-hyperinsulinaemic clamp in a community-based cohort. Diabetologia. 2007;50:317–24. doi: 10.1007/s00125-006-0532-9. [DOI] [PubMed] [Google Scholar]
- 145.Gysemans C, van Etten E, Overbergh L, et al. Unaltered diabetes presentation in NOD mice lacking the vitamin D receptor. Diabetes. 2008;57:269–75. doi: 10.2337/db07-1095. [DOI] [PubMed] [Google Scholar]
- 146.Cheng Q, Li YC, Boucher BJ, Leung PS. A novel role for vitamin D: modulation of expression and function of the local renin-angiotensin system in mouse pancreatic islets. Diabetologia. 2011;54:2077–81. doi: 10.1007/s00125-011-2100-1. [DOI] [PubMed] [Google Scholar]
- 147.Mezza T, Muscogiuri G, Sorice GP, et al. Vitamin D deficiency: a new risk factor for type 2 diabetes? Ann Nutr Metab. 2012;61:337–48. doi: 10.1159/000342771. [DOI] [PubMed] [Google Scholar]
- 148.Forouhi NG, Ye Z, Rickard AP, et al. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia. 2012;55:2173–82. doi: 10.1007/s00125-012-2544-y. [DOI] [PubMed] [Google Scholar]
- 149.Song Y, Wang L, Pittas AG, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes care. 2013;36:1422–8. doi: 10.2337/dc12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Khan H, Kunutsor S, Franco OH, Chowdhury R. Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proc Nutr Soc. 2013;72:89–97. doi: 10.1017/S0029665112002765. [DOI] [PubMed] [Google Scholar]
- 151.Lim S, Kim MJ, Choi SH, et al. Association of vitamin D deficiency with incidence of type 2 diabetes in high-risk Asian subjects. The American journal of clinical nutrition. 2013;97:524–30. doi: 10.3945/ajcn.112.048496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Scragg R, Sowers M, Bell C, Third National H, Nutrition Examination S Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes care. 2004;27:2813–8. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 153.Grimnes G, Emaus N, Joakimsen RM, et al. Baseline serum 25-hydroxyvitamin D concentrations in the Tromso Study 1994–95 and risk of developing type 2 diabetes mellitus during 11 years of follow-up. Diabet Med. 2010;27:1107–15. doi: 10.1111/j.1464-5491.2010.03092.x. [DOI] [PubMed] [Google Scholar]
- 154.Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. The American journal of clinical nutrition. 2013;97:774–81. doi: 10.3945/ajcn.112.050013. [DOI] [PubMed] [Google Scholar]
- 155.Harris SS, Pittas AG, Palermo NJ. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes Metab. 2012;14:789–94. doi: 10.1111/j.1463-1326.2012.01605.x. [DOI] [PubMed] [Google Scholar]
- 156.Kamei Y, Kawada T, Kazuki R, Ono T, Kato S, Sugimoto E. Vitamin D receptor gene expression is up-regulated by 1, 25-dihydroxyvitamin D3 in 3T3-L1 preadipocytes. Biochemical and biophysical research communications. 1993;193:948–55. doi: 10.1006/bbrc.1993.1717. [DOI] [PubMed] [Google Scholar]
- 157.Querfeld U, Hoffmann MM, Klaus G, et al. Antagonistic effects of vitamin D and parathyroid hormone on lipoprotein lipase in cultured adipocytes. Journal of the American Society of Nephrology : JASN. 1999;10:2158–64. doi: 10.1681/ASN.V10102158. [DOI] [PubMed] [Google Scholar]
- 158.Li J, Byrne ME, Chang E, et al. 1alpha,25-Dihydroxyvitamin D hydroxylase in adipocytes. The Journal of steroid biochemistry and molecular biology. 2008;112:122–6. doi: 10.1016/j.jsbmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bikle DD. Vitamin D and bone. Current osteoporosis reports. 2012;10:151–9. doi: 10.1007/s11914-012-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Narvaez CJ, Simmons KM, Brunton J, Salinero A, Chittur SV, Welsh JE. Induction of STEAP4 correlates with 1,25-dihydroxyvitamin D3 stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. Journal of cellular physiology. 2013;228:2024–36. doi: 10.1002/jcp.24371. [DOI] [PubMed] [Google Scholar]
- 161.Nimitphong H, Holick MF, Fried SK, Lee MJ. 25-hydroxyvitamin D(3) and 1,25-dihydroxyvitamin D(3) promote the differentiation of human subcutaneous preadipocytes. PloS one. 2012;7:e52171. doi: 10.1371/journal.pone.0052171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee H, Bae S, Yoon Y. Anti-adipogenic effects of 1,25-dihydroxyvitamin D3 are mediated by the maintenance of the wingless-type MMTV integration site/beta-catenin pathway. Int J Mol Med. 2012;30:1219–24. doi: 10.3892/ijmm.2012.1101. [DOI] [PubMed] [Google Scholar]
- 163.Blumberg JM, Tzameli I, Astapova I, Lam FS, Flier JS, Hollenberg AN. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. The Journal of biological chemistry. 2006;281:11205–13. doi: 10.1074/jbc.M510343200. [DOI] [PubMed] [Google Scholar]
- 164.Malloy PJ, Feldman BJ. Cell-autonomous regulation of brown fat identity gene UCP1 by unliganded vitamin D receptor. Molecular endocrinology (Baltimore, Md) 2013;27:1632–42. doi: 10.1210/me.2013-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology. 2009;150:651–61. doi: 10.1210/en.2008-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wong KE, Kong J, Zhang W, et al. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. The Journal of biological chemistry. 2011;286:33804–10. doi: 10.1074/jbc.M111.257568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond) 2012;36:387–96. doi: 10.1038/ijo.2011.119. [DOI] [PubMed] [Google Scholar]
- 168.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012;20:1444–8. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 169.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. The American journal of clinical nutrition. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 170.Soares MJ, Chan She Ping-Delfos W, Ghanbari MH. Calcium and vitamin D for obesity: a review of randomized controlled trials. European journal of clinical nutrition. 2011;65:994–1004. doi: 10.1038/ejcn.2011.106. [DOI] [PubMed] [Google Scholar]
- 171.Boon N, Hul GB, Sicard A, et al. The effects of increasing serum calcitriol on energy and fat metabolism and gene expression. Obesity (Silver Spring) 2006;14:1739–46. doi: 10.1038/oby.2006.200. [DOI] [PubMed] [Google Scholar]
- 172.Peterson LA, Zeng X, Caufield-Noll CP, Schweitzer MA, Magnuson TH, Steele KE. Vitamin D status and supplementation before and after bariatric surgery: a comprehensive literature review. Surg Obes Relat Dis. 2016 doi: 10.1016/j.soard.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 173.Rueda S, Fernandez-Fernandez C, Romero F, Martinez de Osaba J, Vidal J. Vitamin D, PTH, and the metabolic syndrome in severely obese subjects. Obes Surg. 2008;18:151–4. doi: 10.1007/s11695-007-9352-3. [DOI] [PubMed] [Google Scholar]
- 174.Olsson K, Saini A, Stromberg A, et al. Evidence for Vitamin D Receptor Expression and Direct Effects of 1alpha,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology. 2016;157:98–111. doi: 10.1210/en.2015-1685. [DOI] [PubMed] [Google Scholar]
- 175.Wang Y, DeLuca HF. Is the vitamin d receptor found in muscle? Endocrinology. 2011;152:354–63. doi: 10.1210/en.2010-1109. [DOI] [PubMed] [Google Scholar]
- 176.Girgis CM, Mokbel N, Cha KM, et al. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology. 2014;155:3227–37. doi: 10.1210/en.2014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Girgis CM, Clifton-Bligh RJ, Mokbel N, Cheng K, Gunton JE. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology. 2014;155:347–57. doi: 10.1210/en.2013-1205. [DOI] [PubMed] [Google Scholar]
- 178.Boland RL. VDR activation of intracellular signaling pathways in skeletal muscle. Molecular and cellular endocrinology. 2011;347:11–6. doi: 10.1016/j.mce.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 179.Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocrine reviews. 2013;34:33–83. doi: 10.1210/er.2012-1012. [DOI] [PubMed] [Google Scholar]
- 180.Bauman VK, Valinietse MY, Babarykin DA. Vitamin D3 and 1,25-dihydroxyvitamin D3 stimulate the skeletal muscle-calcium mobilization in rachitic chicks. Archives of biochemistry and biophysics. 1984;231:211–6. doi: 10.1016/0003-9861(84)90380-1. [DOI] [PubMed] [Google Scholar]
- 181.Santillan G, Katz S, Vazquez G, Boland RL. TRPC3-like protein and vitamin D receptor mediate 1alpha,25(OH)2D3-induced SOC influx in muscle cells. The international journal of biochemistry & cell biology. 2004;36:1910–8. doi: 10.1016/j.biocel.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 182.Capiati D, Benassati S, Boland RL. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. Journal of cellular biochemistry. 2002;86:128–35. doi: 10.1002/jcb.10191. [DOI] [PubMed] [Google Scholar]
- 183.Annweiler C, Schott AM, Montero-Odasso M, et al. Cross-sectional association between serum vitamin D concentration and walking speed measured at usual and fast pace among older women: the EPIDOS study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:1858–66. doi: 10.1002/jbmr.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bhat M, Kalam R, Qadri SS, Madabushi S, Ismail A. Vitamin D deficiency-induced muscle wasting occurs through the ubiquitin proteasome pathway and is partially corrected by calcium in male rats. Endocrinology. 2013;154:4018–29. doi: 10.1210/en.2013-1369. [DOI] [PubMed] [Google Scholar]
- 185.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. The New England journal of medicine. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 186.Latham NK, Anderson CS, Reid IR. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. Journal of the American Geriatrics Society. 2003;51:1219–26. doi: 10.1046/j.1532-5415.2003.51405.x. [DOI] [PubMed] [Google Scholar]
- 187.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Murad MH, Elamin KB, Abu Elnour NO, et al. Clinical review: The effect of vitamin D on falls: a systematic review and meta-analysis. The Journal of clinical endocrinology and metabolism. 2011;96:2997–3006. doi: 10.1210/jc.2011-1193. [DOI] [PubMed] [Google Scholar]
- 189.Knutsen KV, Madar AA, Lagerlov P, et al. Does vitamin D improve muscle strength in adults? A randomized, double-blind, placebo-controlled trial among ethnic minorities in Norway. The Journal of clinical endocrinology and metabolism. 2014;99:194–202. doi: 10.1210/jc.2013-2647. [DOI] [PubMed] [Google Scholar]
- 190.Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes—authors’ reply. Lancet Diabetes Endocrinol. 2014;2:364–5. doi: 10.1016/S2213-8587(14)70100-7. [DOI] [PubMed] [Google Scholar]
- 191.Sinha A, Hollingsworth KG, Ball S, Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. The Journal of clinical endocrinology and metabolism. 2013;98:E509–13. doi: 10.1210/jc.2012-3592. [DOI] [PubMed] [Google Scholar]
- 192.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. Jama. 2010;303:1815–22. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 193.Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly High-Dose Vitamin D Treatment for the Prevention of Functional Decline: A Randomized Clinical Trial. JAMA Intern Med. 2016;176:175–83. doi: 10.1001/jamainternmed.2015.7148. [DOI] [PubMed] [Google Scholar]
- 194.Bikle DD. Vitamin D and the skin: Physiology and pathophysiology. Reviews in endocrine & metabolic disorders. 2012;13:3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lehmann B, Meurer M. Extrarenal sites of calcitriol synthesis: the particular role of the skin. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2003;164:135–45. doi: 10.1007/978-3-642-55580-0_9. [DOI] [PubMed] [Google Scholar]
- 196.Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. The Journal of biological chemistry. 2003;278:38084–93. doi: 10.1074/jbc.M307028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bikle DD, Nemanic MK, Whitney JO, Elias PW. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986;25:1545–8. doi: 10.1021/bi00355a013. [DOI] [PubMed] [Google Scholar]
- 198.Pillai S, Bikle DD, Elias PM. 1,25-Dihydroxyvitamin D production and receptor binding in human keratinocytes varies with differentiation. The Journal of biological chemistry. 1988;263:5390–5. [PubMed] [Google Scholar]
- 199.Li YC, Pirro AE, Amling M, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9831–5. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yoshizawa T, Handa Y, Uematsu Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nature genetics. 1997;16:391–6. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 201.Malloy PJ, Pike JW, Feldman D. The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocrine reviews. 1999;20:156–88. doi: 10.1210/edrv.20.2.0359. [DOI] [PubMed] [Google Scholar]
- 202.Bikle DD, Chang S, Crumrine D, et al. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. The Journal of investigative dermatology. 2004;122:984–92. doi: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- 203.Malloy PJ, Feldman D. Genetic disorders and defects in vitamin d action. Endocrinol Metab Clin North Am. 2010;39:333–46. doi: 10.1016/j.ecl.2010.02.004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lien WH, Polak L, Lin M, Lay K, Zheng D, Fuchs E. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nature cell biology. 2014;16:179–90. doi: 10.1038/ncb2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Choi YS, Zhang Y, Xu M, et al. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell stem cell. 2013;13:720–33. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lim X, Tan SH, Koh WL, et al. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 2013;342:1226–30. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shah S, Islam MN, Dakshanamurthy S, et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Molecular cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 208.Palmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM. The Vitamin D Receptor Is a Wnt Effector that Controls Hair Follicle Differentiation and Specifies Tumor Type in Adult Epidermis. PloS one. 2008;3:e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cianferotti L, Cox M, Skorija K, Demay MB. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9428–33. doi: 10.1073/pnas.0702884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hawker NP, Pennypacker SD, Chang SM, Bikle DD. Regulation of human epidermal keratinocyte differentiation by the vitamin D receptor and its coactivators DRIP205, SRC2, and SRC3. The Journal of investigative dermatology. 2007;127:874–80. doi: 10.1038/sj.jid.5700624. [DOI] [PubMed] [Google Scholar]
- 211.Oda Y, Uchida Y, Moradian S, Crumrine D, Elias PM, Bikle DD. Vitamin D receptor and coactivators SRC2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. The Journal of investigative dermatology. 2009;129:1367–78. doi: 10.1038/jid.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Oda Y, Chalkley RJ, Burlingame AL, Bikle DD. The transcriptional coactivator DRIP/mediator complex is involved in vitamin D receptor function and regulates keratinocyte proliferation and differentiation. The Journal of investigative dermatology. 2010;130:2377–88. doi: 10.1038/jid.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Oda Y, Hu L, Bul V, Elalieh H, Reddy JK, Bikle DD. Coactivator MED1 ablation in keratinocytes results in hair-cycling defects and epidermal alterations. The Journal of investigative dermatology. 2012;132:1075–83. doi: 10.1038/jid.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bikle DD, Xie Z, Tu CL. Calcium regulation of keratinocyte differentiation. Expert review of endocrinology & metabolism. 2012;7:461–72. doi: 10.1586/eem.12.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Oda Y, Tu CL, Pillai S, Bikle DD. The calcium sensing receptor and its alternatively spliced form in keratinocyte differentiation. The Journal of biological chemistry. 1998;273:23344–52. doi: 10.1074/jbc.273.36.23344. [DOI] [PubMed] [Google Scholar]
- 216.Tu CL, Chang W, Bikle DD. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. The Journal of biological chemistry. 2001;276:41079–85. doi: 10.1074/jbc.M107122200. [DOI] [PubMed] [Google Scholar]
- 217.Tu CL, Crumrine DA, Man MQ, et al. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. The Journal of investigative dermatology. 2012;132:2350–9. doi: 10.1038/jid.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Oda Y, Tu CL, Menendez A, Nguyen T, Bikle DD. Vitamin D and calcium regulation of epidermal wound healing. The Journal of steroid biochemistry and molecular biology. 2015 doi: 10.1016/j.jsbmb.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bikle DD. Vitamin D receptor, a tumor suppressor in skin. Can J Physiol Pharmacol. 2015;93:349–54. doi: 10.1139/cjpp-2014-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schon MP, Boehncke WH. Psoriasis. The New England journal of medicine. 2005;352:1899–912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 221.Perez A, Chen TC, Turner A, et al. Efficacy and safety of topical calcitriol (1,25-dihydroxyvitamin d3) for the treatment of psoriasis. The British journal of dermatology. 1996;134:238–46. [PubMed] [Google Scholar]
- 222.Kragballe K, Beck HI, Sogaard H. Improvement of psoriasis by a topical vitamin D3 analogue (MC 903) in a double-blind study. The British journal of dermatology. 1988;119:223–30. doi: 10.1111/j.1365-2133.1988.tb03204.x. [DOI] [PubMed] [Google Scholar]
- 223.van de Kerkhof PC, Berth-Jones J, Griffiths CE, et al. Long-term efficacy and safety of tacalcitol ointment in patients with chronic plaque psoriasis. The British journal of dermatology. 2002;146:414–22. doi: 10.1046/j.1365-2133.2002.04567.x. [DOI] [PubMed] [Google Scholar]
- 224.Barker JN, Ashton RE, Marks R, Harris RI, Berth-Jones J. Topical maxacalcitol for the treatment of psoriasis vulgaris: a placebo-controlled, double-blind, dose-finding study with active comparator. The British journal of dermatology. 1999;141:274–8. doi: 10.1046/j.1365-2133.1999.02975.x. [DOI] [PubMed] [Google Scholar]
- 225.Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. The Journal of clinical investigation. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Debinska A, Sikorska-Szaflik H, Urbanik M, Boznanski A. The role of vitamin D in atopic dermatitis. Dermatitis. 2015;26:155–61. doi: 10.1097/DER.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 227.Ingen-Housz-Oro S, Boudou P, Bergot C, et al. Evidence of a marked 25-hydroxyvitamin D deficiency in patients with congenital ichthyosis. J Eur Acad Dermatol Venereol. 2006;20:947–52. doi: 10.1111/j.1468-3083.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 228.Thacher TD, Fischer PR, Pettifor JM, Darmstadt GL. Nutritional rickets in ichthyosis and response to calcipotriene. Pediatrics. 2004;114:e119–23. doi: 10.1542/peds.114.1.e119. [DOI] [PubMed] [Google Scholar]
- 229.Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med. 2007;13:117–24. doi: 10.1016/j.molmed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 230.Bikle DD. Vitamin D and immune function: understanding common pathways. Current osteoporosis reports. 2009;7:58–63. doi: 10.1007/s11914-009-0011-6. [DOI] [PubMed] [Google Scholar]
- 231.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. Journal of immunology. 2007;179:1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 232.Sigmundsdottir H, Pan J, Debes GF, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–93. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 233.Bikle DD, Pillai S, Gee E, Hincenbergs M. Regulation of 1,25-dihydroxyvitamin D production in human keratinocytes by interferon-gamma. Endocrinology. 1989;124:655–60. doi: 10.1210/endo-124-2-655. [DOI] [PubMed] [Google Scholar]
- 234.Bikle DD, Pillai S, Gee E, Hincenbergs M. Tumor necrosis factor-alpha regulation of 1,25-dihydroxyvitamin D production by human keratinocytes. Endocrinology. 1991;129:33–8. doi: 10.1210/endo-129-1-33. [DOI] [PubMed] [Google Scholar]
- 235.Pryke AM, Duggan C, White CP, Posen S, Mason RS. Tumor necrosis factor-alpha induces vitamin D-1-hydroxylase activity in normal human alveolar macrophages. Journal of cellular physiology. 1990;142:652–6. doi: 10.1002/jcp.1041420327. [DOI] [PubMed] [Google Scholar]
- 236.Gyetko MR, Hsu CH, Wilkinson CC, Patel S, Young E. Monocyte 1 alpha-hydroxylase regulation: induction by inflammatory cytokines and suppression by dexamethasone and uremia toxin. J Leukoc Biol. 1993;54:17–22. doi: 10.1002/jlb.54.1.17. [DOI] [PubMed] [Google Scholar]
- 237.Ren S, Nguyen L, Wu S, Encinas C, Adams JS, Hewison M. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. The Journal of biological chemistry. 2005;280:20604–11. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- 238.Vidal M, Ramana CV, Dusso AS. Stat1-vitamin D receptor interactions antagonize 1,25-dihydroxyvitamin D transcriptional activity and enhance stat1-mediated transcription. Molecular and cellular biology. 2002;22:2777–87. doi: 10.1128/MCB.22.8.2777-2787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. The Journal of steroid biochemistry and molecular biology. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 240.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. The Journal of pharmacology and experimental therapeutics. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 241.Joshi S, Pantalena LC, Liu XK, et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Molecular and cellular biology. 2011;31:3653–69. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Palmer MT, Lee YK, Maynard CL, et al. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. The Journal of biological chemistry. 2011;286:997–1004. doi: 10.1074/jbc.M110.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Keating P, Munim A, Hartmann JX. Effect of vitamin D on T-helper type 9 polarized human memory cells in chronic persistent asthma. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2014;112:154–62. doi: 10.1016/j.anai.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 244.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. Journal of immunology. 2001;167:1945–53. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 245.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 246.Alroy I, Towers TL, Freedman LP. Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Molecular and cellular biology. 1995;15:5789–99. doi: 10.1128/mcb.15.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cippitelli M, Santoni A. Vitamin D3: a transcriptional modulator of the interferon-gamma gene. European journal of immunology. 1998;28:3017–30. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 248.Riis JL, Johansen C, Gesser B, et al. 1alpha,25(OH)(2)D(3) regulates NF-kappaB DNA binding activity in cultured normal human keratinocytes through an increase in IkappaBalpha expression. Archives of dermatological research. 2004;296:195–202. doi: 10.1007/s00403-004-0509-9. [DOI] [PubMed] [Google Scholar]
- 249.Dong X, Lutz W, Schroeder TM, et al. Regulation of relB in dendritic cells by means of modulated association of vitamin D receptor and histone deacetylase 3 with the promoter. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16007–12. doi: 10.1073/pnas.0506516102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mathieu C, Van Etten E, Gysemans C, et al. In vitro and in vivo analysis of the immune system of vitamin D receptor knockout mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16:2057–65. doi: 10.1359/jbmr.2001.16.11.2057. [DOI] [PubMed] [Google Scholar]
- 251.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404–12. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- 252.Amuchastegui S, Daniel KC, Adorini L. Inhibition of acute and chronic allograft rejection in mouse models by BXL-628, a nonhypercalcemic vitamin D receptor agonist. Transplantation. 2005;80:81–7. doi: 10.1097/01.tp.0000164619.49828.7a. [DOI] [PubMed] [Google Scholar]
- 253.Zhang Z, Hener P, Frossard N, et al. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1536–41. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Topilski I, Flaishon L, Naveh Y, Harmelin A, Levo Y, Shachar I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. European journal of immunology. 2004;34:1068–76. doi: 10.1002/eji.200324532. [DOI] [PubMed] [Google Scholar]
- 255.Wittke A, Weaver V, Mahon BD, August A, Cantorna MT. Vitamin D receptor-deficient mice fail to develop experimental allergic asthma. Journal of immunology. 2004;173:3432–6. doi: 10.4049/jimmunol.173.5.3432. [DOI] [PubMed] [Google Scholar]
- 256.Bieber T. Atopic dermatitis. The New England journal of medicine. 2008;358:1483–94. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 257.Hartmann B, Riedel R, Jorss K, et al. Vitamin D receptor activation improves allergen-triggered eczema in mice. The Journal of investigative dermatology. 2012;132:330–6. doi: 10.1038/jid.2011.296. [DOI] [PubMed] [Google Scholar]
- 258.Ehrchen J, Helming L, Varga G, et al. Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:3208–18. doi: 10.1096/fj.06-7261com. [DOI] [PubMed] [Google Scholar]
- 259.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal immunology. 2009;2:403–11. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rajapakse R, Mousli M, Pfaff AW, et al. 1,25-Dihydroxyvitamin D3 induces splenocyte apoptosis and enhances BALB/c mice sensitivity to toxoplasmosis. The Journal of steroid biochemistry and molecular biology. 2005;96:179–85. doi: 10.1016/j.jsbmb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 261.Ryz NR, Patterson SJ, Zhang Y, et al. Active vitamin D (1,25-dihydroxyvitamin D3) increases host susceptibility to Citrobacter rodentium by suppressing mucosal Th17 responses. American journal of physiology Gastrointestinal and liver physiology. 2012;303:G1299–311. doi: 10.1152/ajpgi.00320.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol. 2010;9:599–612. doi: 10.1016/S1474-4422(10)70086-7. [DOI] [PubMed] [Google Scholar]
- 263.Knip M, Virtanen SM, Akerblom HK. Infant feeding and the risk of type 1 diabetes. The American journal of clinical nutrition. 2010;91:1506S–13S. doi: 10.3945/ajcn.2010.28701C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Littorin B, Blom P, Scholin A, et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS) Diabetologia. 2006;49:2847–52. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 265.Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Progress in biophysics and molecular biology. 2006;92:60–4. doi: 10.1016/j.pbiomolbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 266.Lee YH, Bae SC. Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: a meta-analysis. Clinical and experimental rheumatology. 2016 [PubMed] [Google Scholar]
- 267.Kamen D, Aranow C. Vitamin D in systemic lupus erythematosus. Current opinion in rheumatology. 2008;20:532–7. doi: 10.1097/BOR.0b013e32830a991b. [DOI] [PubMed] [Google Scholar]
- 268.Goswami R, Marwaha RK, Gupta N, et al. Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: a community-based survey. The British journal of nutrition. 2009;102:382–6. doi: 10.1017/S0007114509220824. [DOI] [PubMed] [Google Scholar]
- 269.Chiu CY, Huang SY, Peng YC, et al. Maternal vitamin D levels are inversely related to allergic sensitization and atopic diseases in early childhood. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2015;26:337–43. doi: 10.1111/pai.12384. [DOI] [PubMed] [Google Scholar]
- 270.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 271.Quirk SK, Rainwater E, Shure AK, Agrawal DK. Vitamin D in atopic dermatitis, chronic urticaria and allergic contact dermatitis. Expert review of clinical immunology. 2016:1–9. doi: 10.1586/1744666X.2016.1171143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Castro M, King TS, Kunselman SJ, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. Jama. 2014;311:2083–91. doi: 10.1001/jama.2014.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yang J, Liu L, Zhang Q, Li M, Wang J. Effect of vitamin D on the recurrence rate of rheumatoid arthritis. Experimental and therapeutic medicine. 2015;10:1812–6. doi: 10.3892/etm.2015.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bizzarri C, Pitocco D, Napoli N, et al. No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes care. 2010;33:1962–3. doi: 10.2337/dc10-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kim G, Bae JH. Vitamin D and atopic dermatitis: A systematic review and meta-analysis. Nutrition. 2016 doi: 10.1016/j.nut.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 276.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 277.Schauber J, Gallo RL. The vitamin D pathway: a new target for control of the skin’s immune response? Experimental dermatology. 2008;17:633–9. doi: 10.1111/j.1600-0625.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Rook GA, Steele J, Fraher L, et al. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–63. [PMC free article] [PubMed] [Google Scholar]
- 279.Adams JS, Ren S, Liu PT, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. Journal of immunology. 2009;182:4289–95. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Davies PD, Brown RC, Woodhead JS. Serum concentrations of vitamin D metabolites in untreated tuberculosis. Thorax. 1985;40:187–90. doi: 10.1136/thx.40.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 282.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 283.Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. The New England journal of medicine. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 284.Martineau AR, Timms PM, Bothamley GH, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377:242–50. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis] A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC infectious diseases. 2013;13:22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Daley P, Jagannathan V, John KR, et al. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. The Lancet Infectious diseases. 2015;15:528–34. doi: 10.1016/S1473-3099(15)70053-8. [DOI] [PubMed] [Google Scholar]
- 287.Tukvadze N, Sanikidze E, Kipiani M, et al. High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. The American journal of clinical nutrition. 2015;102:1059–69. doi: 10.3945/ajcn.115.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]


