Abstract
Introduction
Few hospitals treat patients’ tobacco dependence. To be effective, hospital-initiated cessation interventions must provide at least 1 month of supportive contact post-discharge.
Study design
Individually randomized clinical trial. Recruitment commenced July 2011; analyses were conducted October 2014–June 2015.
Setting/participants
The study was conducted in two large Midwestern hospitals. Participants included smokers who were aged ≥18 years, planned to stay quit after discharge, and spoke English or Spanish.
Intervention
Hospital-based cessation counselors delivered the intervention. For patients randomized to warm handoff, staff immediately called the quitline from the bedside and handed the phone to participants for enrollment and counseling. Participants randomized to fax were referred on the day of hospital discharge.
Main outcome measures
Outcomes at 6 months included quitline enrollment/adherence, medication use, biochemically verified cessation, and cost effectiveness.
Results
Significantly more warm handoff than fax participants enrolled in quitline (99.6% vs 59.6%; relative risk, 1.67; 95% CI=1.65, 1.68). One in four (25.4% warm handoff, 25.3% fax) were verified to be abstinent at 6-month follow-up; this did not differ significantly between groups (relative risk, 1.02; 95% CI=0.82, 1.24). Cessation medication use in the hospital and receipt of a prescription for medication at discharge did not differ between groups; however, significantly more fax participants reported using cessation medication post-discharge (32% vs 25%, p=0.01). The average incremental cost-effectiveness ratio of enrolling participants into warm handoff was $0.14. Hospital-borne costs were significantly lower in warm handoff than in fax ($5.77 vs $9.41, p<0.001).
Conclusions
One in four inpatient smokers referred to quitline by either method were abstinent at 6 months post-discharge. Among motivated smokers, fax referral and warm handoff are efficient and comparatively effective ways to link smokers with evidence-based care. For hospitals, warm handoff is a less expensive and more effective method for enrolling smokers in quitline services.
Introduction
Hospitals are important but untapped venues for reaching and treating smokers. An estimated 1.1 million smokers in the United Kingdom and 6.5 million smokers in the U.S. are hospitalized each year.1,2 Few hospitals provide assistance in quitting,3 even though effective interventions exist.4 Guidelines in many countries, however, recommend that hospitals integrate smoking-cessation interventions into routine care5 and there are increasing regulatory pressures on hospitals to do so.6–8 In the U.S., healthcare reform recently placed new resources in the hands of patients and providers by mandating coverage of evidence-based cessation services and expanding public and private insurance coverage.9
To help smokers quit, hospitals must provide at least 1 month of supportive contact post-discharge.4 Referral to tobacco quitlines is in many ways an ideal method for hospitals to provide that follow-up. Quitlines are available—free of charge—in a number of countries throughout the world.10,11 Quitlines are effective and cost effective for smoking cessation,12–14 accessible for smokers with telephones, and are undersubscribed and eager to increase their reach.15,16
A number of U.S. hospitals have begun referring smokers to quitlines via fax referral.17–19 This process typically involves identifying smokers, assessing for willingness to quit, completing a fax referral form, and faxing the form to the quitline. The quitline then proactively calls to register the patient and provide counseling. Observational studies, however, have found that only 16%–53% of smokers who are fax referred actually register for services.20–23 No studies have yet reported cessation rates among fax-referred smokers.
“Warm handoff” is another promising strategy for effecting transitions in health care. In a warm handoff, patients who screen positive for health issues are immediately introduced to a specialty care provider for on-the-spot enrollment and treatment. At present, no clinical trials of the effectiveness and cost effectiveness of warm handoffs for treating tobacco dependence have been published. One study that focused on the treatment of substance use disorders reported that warm handoff achieved 80%–90% enrollment rates.24,25 Preliminary results from one hospital-based smoking-cessation trial examining warm handoff versus provision of a quitline phone number reported biochemically verified abstinence rates of 7.5%, with no differences across groups.26 These findings were disappointing, but the results were very preliminary—reported in 2015 conference proceedings—and the effects on enrollment, treatment adherence, and costs of care remain unknown.
The objective of this study was to determine the relative effectiveness, and cost effectiveness, of warm handoff versus fax referral for transitioning inpatient smokers to post-discharge care. This study is one of six studies in the U.S. Consortium of Hospitals Advancing Research on Tobacco,27 which were designed to test methods for implementing cessation guidelines in real-world hospital settings.
Methods
The trial employed a two-arm, individually randomized design to examine the impact of warm handoff on enrollment in quitline services and biochemically verified cessation at 6 months post-enrollment. Participants were smokers admitted to two large hospitals in Kansas with dedicated tobacco treatment interventionists on staff. The study protocol28 provides an in-depth description of the study design and methods. The IRBs at both hospitals approved study protocols and all participants provided consent.
Eligibility criteria included planning to stay quit post-discharge, smoking any cigarettes within the past 30 days, being aged ≥18 years, speaking English or Spanish, having access to a telephone post-discharge, having no other household member participating in the trial, not currently being pregnant, and having no comorbidity or health issue preventing full participation.
Identification and Recruitment
Hospital treatment staff identified potential study participants via three sources in the electronic health record (EHR): a complete list of tobacco users in the hospital, a list of tobacco users who requested tobacco treatment, and a list of tobacco users whose providers ordered tobacco treatment. At the bedside, staff described the study and screened for eligibility/interest in participating. Among consenting participants, staff conducted a brief baseline assessment, conducted random assignment via a tablet computer, and provided intervention/referral according to the study arm to which the patient was assigned. Randomization was individual and was blocked by hospital site and recruitment method to ensure equal proportions of patients were assigned to each study arm within each hospital and across each EHR source.
Intervention and Control Arm Procedures
Patients in both study arms received the hospitals’ standard cessation brochure with information and resources for quitting smoking. Hospital staff providing treatments were part of UKanQuit, a tobacco treatment service funded by the hospital.29
For the fax referral group, UKanQuit staff standard hospital screening and intervention procedures included: (1) assessing withdrawal; (2) adjusting inpatient nicotine replacement to enhance patient comfort; and (3) providing assistance in quitting, which included developing a quit plan and arranging medication prescriptions on discharge. Staff fax referred patients to the quitline on the day they were discharged from the hospital.
For the warm handoff group, during the initial brief intervention, UKanQuit staff assessed withdrawal, adjusted nicotine replacement to ensure patient comfort, and described warm handoff procedures. UKanQuit staff then performed the handoff by calling the quitline, notifying the quitline that an inpatient was on the line, transferring the call to the patients’ mobile or bedside hospital phone for enrollment and an initial counseling session, and then leaving the room. After the quitline session, the counselor checked back with the patient to follow up on decisions made during the counseling session, such as arranging for medication scripts on discharge.
Quitline services study participants were connected with the Kansas Department of Health and Environment public quitline services for counseling. The Department contracts with Alere Wellbeing to provide the quitline services. Alere provided enrollees with mailed materials and up to five proactive counseling calls. Alere made five attempts to reach enrollees to complete each of the five calls. Although quitline registration staff were aware of participants’ referral methods, once participants were registered and transferred, Alere counselors were blind to study arm and all quitline services were the same across study groups.
Research staff assessed fidelity on 10% of UKanQuit staff interventions using an intervention checklist. Staff calculated the percentage of steps conducted correctly within each study arm, and reported performance back to UKanQuit staff to encourage protocol adherence.
Data Collection and Data Management
Baseline assessment was conducted prior to randomization by UKanQuit staff. Follow-up assessments were conducted by research assistants, blinded to study allocation, at 1 month ($20) and 6 months ($50) post-randomization. Participants who provided salivary cotinine or carbon monoxide (CO) samples were reimbursed $100. The study commenced July 2011; analyses were conducted October 2014–June 2015.
Study Measures
The baseline survey included race and ethnicity, highest level of education, tobacco use characteristics, Heaviness of Smoking Index,30 Alcohol Use Disorder Identification Test,31 and the Patient Health Questionnaire-232 to screen for depression. Birth date, sex, and health insurance were collected from the EHR.
Primary and secondary diagnoses, procedures, and length of stay were collected from the EHR. ICD-9 codes for primary diagnoses were collapsed into the major ICD-9 categories. ICD-9 codes 290–319 were used to identify patients with primary or secondary psychiatric disorders. Diagnosis-Related Group codes were used to identify participants who had undergone cardiac or cerebrovascular surgery.33,34
Main outcome measures included enrollment in quitline (from Alere), self-reported abstinence at 1 and 6 months, and verified abstinence at 6 months. All participants with missing data, or who provided self-reported abstinence but failed to verify abstinence, were counted as smokers. Abstinence at 1 and 6 months was assessed by asking participants if they had smoked any cigarettes, even a puff, in the past 7 days (7-day point prevalence abstinence).35 Abstinence was verified via salivary cotinine, CO, or proxy. Participants with ≤15 ng/mL salivary cotinine were considered abstinent.36 Those still taking nicotine replacement were asked to verify abstinence with an expired CO sample. Those with ≤10 ppm were considered abstinent.36 Staff contacted proxies to verify abstinence among participants who did not provide cotinine or CO. At the time of study implementation, proxy verification was considered a valid method for confirming abstinence.37–39 A recent analysis of proxy verification in a clinical trial suggests it is no more valid than self-report.40 To examine the impact of proxy verification, the proportions of patients who verified abstinence via cotinine, CO, and proxy are reported.
Secondary outcomes included the number and timing of quitline calls completed (from Alere), in-hospital medication use and provision of a prescription at discharge (from the EHR), and post-discharge medication use (from participant self-report).
UKanQuit staff documented time spent in inpatient counseling. Alere provided time for quitline enrollment and quitline counseling per participant. Personnel time was valued at the national mean wage for health educator (occupational code 21-1091)41 plus 25% fringe benefit rate. Patient self-reports42,43 provided the type, dose, and the number of weeks each medication was used. Pharmacotherapy costs were based upon retail prices from an online pharmacy website. A standard per-minute charge was assigned to fax and telephone costs.
Statistical Analysis
The main outcome measure was verified abstinence at 6 months post-enrollment. Sample size was calculated using formulas from Fleiss et al.44 with estimated intent-to-treat quit rates of 9% in the control group and 15% in the treatment group, based on prior studies.20,45 A minimum of 994 participants were needed to test primary effects, assuming a two-tailed α =0.05 and power=0.80. Because patients were experiencing higher than expected mortality (6%) early in study implementation, an additional 6% of patients (n=60) were added to the sample, resulting in a total of 1,054 participants.
All analyses were conducted using two-sided tests for statistical significance. Chi-square tests were used to detect differences in proportions between groups. Independent sample t-tests were used to detect mean differences between groups. For the main outcome of verified cessation, logistic regression, including any characteristics that were statistically significant at baseline, was used to calculate AORs. To examine the impact of proxy verification on outcome, a sensitivity analysis was conducted in which all participants with proxy-confirmed abstinence were counted as smokers. For ease of interpretation, relative risk ratios (RRs) are also reported.
The primary cost-effectiveness analysis was set up as an incremental cost-effectiveness ratio to evaluate the added cost per additional: (1) enrollee in quitline; and (2) quitter for warm handoff versus fax referral. An incremental cost-effectiveness ratio demonstrates the additional cost needed to achieve a better outcome when an intervention is more expensive and more effective.46 If outcomes are equivalent between treatment arms, then the cost-effectiveness analysis defaults to a cost-minimization approach whereby the less expensive option is considered the more cost-effective intervention. The perspective of the analyses was a modified societal viewpoint that included costs regardless of who incurred them during the inpatient stay, post-discharge (outpatient counseling), and pharmacotherapy. Given that all costs were short term, they were not discounted. Independent sample t-tests were used to detect mean cost differences between groups.
Results
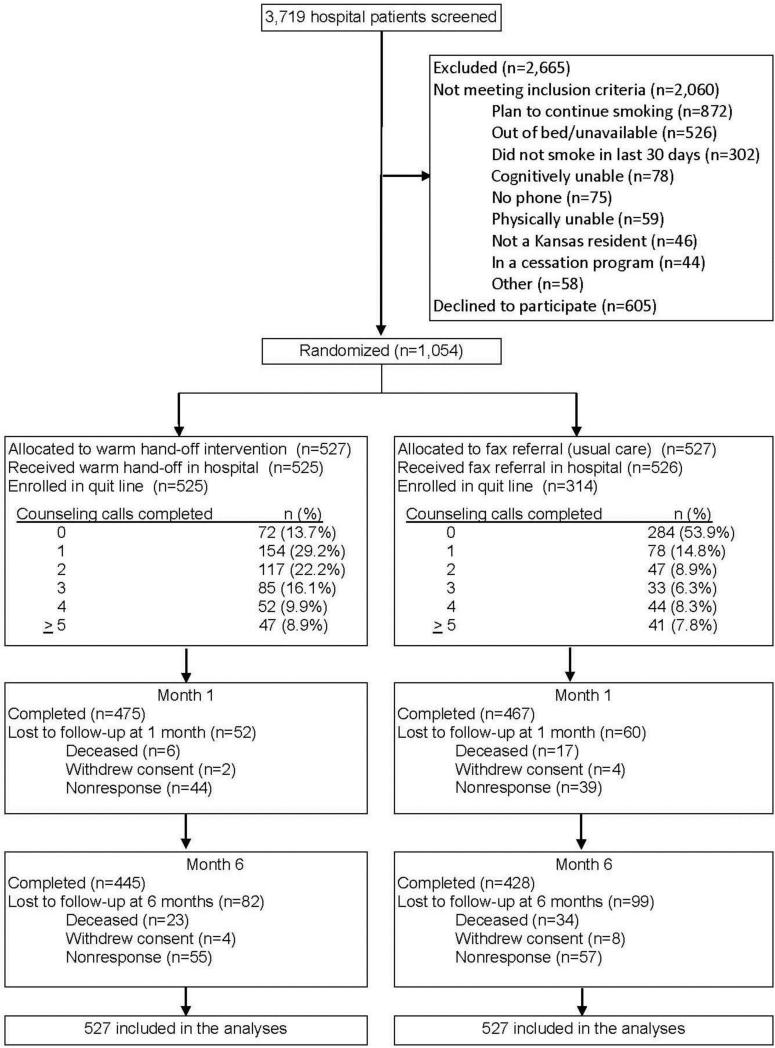
Of 3,719 individuals identified as smokers, 2,060 were ineligible, 605 declined to participate, and 1,054 provided consent and were enrolled in the trial (Figure 1). The top reason for ineligibility was planning to continue smoking after leaving the hospital (n=892; 42% of ineligibles). The proportion of participants reached for follow-up was 89% at Month 1 and 85% at Month 6. Randomization resulted in groups with similar baseline characteristics (Table 1) except for alcohol use (p=0.04), living with another smoker (p=0.03), and the Heaviness of Smoking Index (p=0.04). These, accordingly, were included in models evaluating outcomes.
Figure 1.
EQUIP CONSORT diagram.
Table 1.
Baseline Characteristics and Primary Reason for Hospitalization for Fax Referral and Warm Hand-Off Participants
| Variable | Total (n=1,054) | Fax (n=527) | Warm handoff (n=527) |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 49.89 (12.93) | 50.04 (12.36) | 49.74 (13.49) |
| Female, no. (%) | 581 (55.12%) | 291(55.22%) | 290 (55.03%) |
| Education <High school, no. (%) | 231 (21.92%) | 116 (22.01%) | 115 (21.82%) |
| Race | |||
| White, no. (%) | 719 (68.22%) | 356 (67.55%) | 363 (68.88%) |
| African American, no. (%) | 262 (24.86%) | 131(24.86%) | 131 (24.86%) |
| Other, no. (%) | 73 (6.93%) | 40(7.59%) | 33 (6.26%) |
| Latino, no. (%) | 62 (5.91%) | 29 (5.52%) | 33 (6.30%) |
| Live with other smoker, no. (%) | 523 (49.62%) | 244 (46.30%) | 279 (52.94%) |
| Hospital treatment | |||
| Reason for admission | |||
| Circulatory system, no. (%) | 261 (24.76%) | 131 (24.86%) | 130 (24.67%) |
| Respiratory system, no. (%) | 121 (11.48%) | 61 (11.57%) | 60 (11.39%) |
| Neoplasms, no. (%) | 50 (4.74%) | 25 (4.74%) | 25 (4.74%) |
| Mental disorders, no. (%) | 22 (2.09%) | 9 (1.71%) | 13 (2.47%) |
| Other, no. (%) | 600 (56.93%) | 301 (57.12%) | 299 (56.74%) |
| Psychiatric co-morbidity, no. (%) | 681 (64.6%) | 325 (61.7%) | 356 (67.6%) |
| Cardiac and cerebrovascular surgery, no. (%) | 121 (11.48%) | 58 (11.01%) | 63 (11.95%) |
| Primary insurance | |||
| Medicaid, no. (%) | 355 (33.68%) | 182 (34.54%) | 173 (32.83%) |
| Medicare, no. (%) | 314 (29.79%) | 148 (28.08%) | 166 (31.50%) |
| Private, no. (%) | 306 (29.03%) | 160 (30.36%) | 146 (27.70%) |
| Veterans Administration, no. (%) | 12 (1.14%) | 5 (0.95%) | 7 (1.33%) |
| Self-pay/none, no. (%) | 67 (6.36%) | 32 (6.07%) | 35 (6.64%) |
| Length of stay (hours), mean (SD) | 134.88 (133.10) | 137.3 (135.3) | 132.5 (131.0) |
| Emergency admissions, no. (%) | 630 (59.8%) | 321 (60.9%) | 309 (58.6%) |
| Health behavior and psychosocial measures | |||
| Alcohol use disorder (AUDIT-C), no. (%) | 323 (30.65%) | 146 (27.70%) | 177 (33.59%) |
| Possible depression (PHQ-2), no. (%) | 566 (53.80%) | 291 (55.32%) | 275 (52.28%) |
| Tobacco use | |||
| Cigarettes per day mean (SD) | 15.69 (11.05) | 16.37 (11.65) | 15.02 (10.37) |
| Daily smoking (>25/30 days), no. (%) | 759 (72.01%) | 368 (69.83%) | 391 (74.19%) |
| Use e-cigarettes in the past 30 days, no. (%) | 130 (12.33%) | 64 (12.14%) | 66 (12.52%) |
| Smoke within 30 mins of waking, no. (%) | 753 (71.71%) | 384 (72.87%) | 369 (70.55%) |
| Heavy smoking index (HSI) > 4, no. (%) | 347 (32.92%) | 189 (35.86%) | 158 (29.98%) |
| Confidence to quit/stay quit (possible range 1 – 5), mean (SD) | 3.79 (1.12) | 3.81 (1.15) | 3.78 (1.09) |
| Use other forms of tobacco in past 30 days, no. (%) | 82 (7.78%) | 46 (8.73%) | 36 (6.83%) |
| Used tobacco in the hospital, no. (%) | 53 (5.7%) | 27 (5.8%) | 26 (5.5%) |
Note: Boldface indicates statistical significance (p<0.05).
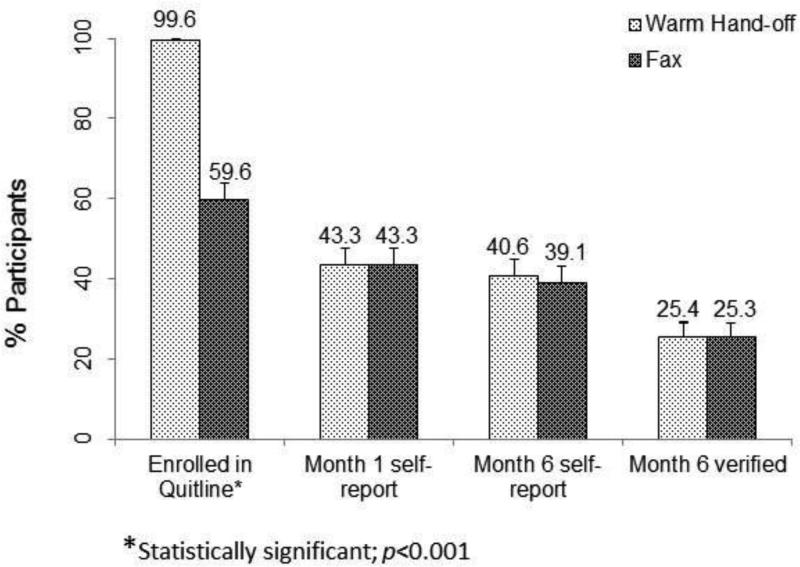
Significantly more warm handoff participants than fax participants enrolled in quitline services (99.6% vs 59.6%; AOR=177.18, 95% CI=43.70 718.41; p<0.001; RR=1.67, 95% CI=1.65, 1.68). One in four study participants (25.4% warm handoff, 25.3% fax) were verified to be abstinent at 6-month follow-up; this did not, however, differ significantly between groups (AOR=1.02, 95% CI=0.77, 1.35; p=0.88; RR=1.02, 95% CI=0.82, 1.24).
The proportions of participants who self-reported abstinence and verified by cotinine, CO, and proxy were 94.1%, 3.0%, and 2.9%, respectively, in the warm handoff group and 93.5%, 2.7%, and 3.8%, respectively, in the fax group. Counting proxy-verified participants as smokers, the proportion of abstinent smokers in each study arm would drop to 23.7% in warm handoff and 21.6% fax, which still does not differ significantly between groups (AOR=1.12, 95% CI=0.84, 1.40; p=0.42; RR=1.10, 95% CI=0.88, 1.37) (Figure 2).
Figure 2.
Enrollment and abstinence outcomes.
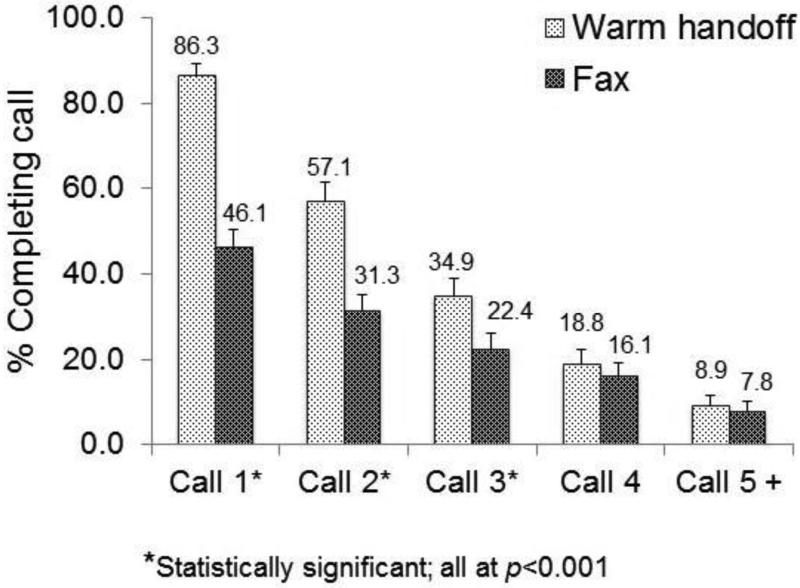
Note: Participants referred via warm hand-off completed more quitline calls (mean=2.05, SD=1.40) than fax-referred patients (mean=1.23, SD=1.63) (p<0.001) Participants referred via warm hand-off were more likely to complete calls 1-3 than fax referred participants (p<0.001); there were no differences for calls 4 and 5.
Overall, more quitline counseling sessions were completed by warm handoff patients (mean=2.08, SD=1.53) than fax-referred patients (mean=1.25, SD=1.71) (p<0.001) (Figure 3). Both groups experienced similar rates of attrition between the first and second counseling calls: Fax referral participation dropped from 46.1% to 31.3% for an attrition rate of 32.0% and warm handoff dropped from 86.3% to 57.1% for an attrition rate of 34.0% (p=0.93).
Figure 3.
Quitline counseling participation, by study arm.
Warm handoff participants received their first quitline sessions in the hospital. Comparing only received post-discharge counseling calls, there were no significant differences between study arms: Warm handoff patients received an average of 1.30 (SD=1.39) calls and fax-referred patients received 1.25 (SD=1.71) calls (p=0.61). Of note, the number of days until completion of the first post-discharge counseling call, among participants who took that call, were 16.89 (median=6) for fax participants, and 28.49 (median=15) for warm handoff; this difference was significant (SD=28.78 and SD=30.15, respectively, p<0.0001).
One in four (25%) participants used some form of cessation medication in the hospital; this did not differ significantly between warm handoff and fax (23% vs 26%, p=0.23). A somewhat higher percentage (31%) of patients received prescriptions for cessation medication at discharge; likewise, this did not differ significantly between warm handoff and fax (30% vs 32%, p=0.66). Significantly more participants in the fax referral group reported they had used some form of cessation medication post-discharge (32% vs 25%, p=0.01).
An online Appendix provides details on study costs. The cost borne by the hospital was significantly less in the warm handoff arm than in the fax arm ($5.77 vs $9.41, respectively, p<0.001). Total counselor time costs (inpatient and outpatient) were higher in warm handoff compared with fax ($26.50 vs $22.39, p<0.001), which in turn generated slightly higher telephone costs ($1.36 vs $0.86, p<0.001). There was no significant difference in pharmaceutical costs between treatment arms ($61.86 for warm handoff and $65.95 for fax). An incremental cost-effectiveness ratio was not calculated as abstinence did not differ significantly across groups. Therefore, the cost-minimization approach ensued. The societal per-participant cost of the intervention did not differ by arm (warm handoff, $89.11; fax, $89.57; p=0.96), for a cost per abstinent participant of $353.23 for warm handoff and $352.53 for fax.
Discussion
Warm handoff was more effective than fax referral at enrolling hospitalized smokers in quitline services. This, however, did not translate into any advantage in quitting. Both study arms, but especially fax referral, yielded much higher quit rates than projected, resulting in similar outcomes across the study arms. The costs for both interventions were the same; hospitals, however, bore less of the costs for the warm handoff. Both interventions meet Joint Commission and Centers for Medicare and Medicaid Services measures for tobacco treatment, and both appear to be good options for providing post-discharge care.
This trial contributes to the science of tobacco treatment by describing quit rates among hospitalized smokers referred to a tobacco quitline. The absolute intent-to-treat quit rates (41% self-reported, 25% biochemically verified) were much higher than projected outcomes for each study arm, which were based on a 2007 Cochrane review of hospital trials.47 This may be due to greater availability of cessation pharmacotherapies. Varenicline came to the market in May of 2006, and the Affordable Care Act of 2010 greatly expanded private insurance and Medicaid coverage of cessation medications.48
Abstinence rates were also much higher than outcomes reported in quitline studies in non-hospital samples, which average 12% self-reported intent to treat.13 Hospitalized smokers may be better connected with the healthcare system and suffering the consequences of tobacco use, which might give them more resources and motivation to quit. The present study's abstinence rates compare favorably to intervention arm abstinence rates of other hospital cessation trials— including recently conducted trials—which average 29%.4
It was hypothesized that warm handoff would achieve higher enrollment, higher post-discharge counseling exposure, and higher quit rates compared with fax referral. The first hypothesis was supported, but the second and third were not. This can be attributed to several reasons. First, robust inpatient counseling, provided by UKanQuit, may have boosted quit rates among participants in the fax arm.
Second, in the present study, 60% of fax-referred patients enrolled in quitline, far exceeding the enrollment rate of 41% in a previous hospital-based study.23 The high rates of enrollment via fax-referral were possibly due to UKanQuit staff's strong fax referral procedures, which included clearly describing the fax referral and quitline counseling processes to patients. Moreover, prior studies were conducted over multiple sites and did not assess or provide feedback on referral fidelity. Fidelity procedures in the present study ensured that staff provided consistent, high-quality referrals.
Third, participant attrition rendered post-discharge exposure to counseling, which is key to maintaining abstinence, equivalent across both groups. The counseling attrition rates between the first and second counseling calls in the present study (32.6% for fax-referral and 37.3% for warm handoff) were similar to outpatient quitline studies, which ranged from 21% to 48%,49,50 and were somewhat lower than the 48% attrition between Calls 1 and 2 found in one hospital-based study.23 However, warm handoff participants received their first quitline call as inpatients, whereas fax-referred participants received their first counseling call post-discharge. Hence, for warm handoff participants, the typical attrition that usually occurs after Call 1 occurred in the transition between inpatient and post-discharge care.
Fourth, groups also differed on how much time elapsed between discharge and the first post-discharge call. Warm handoff participants may have scheduled their second call further out to permit recovery time. Post-hoc analyses of predictors of time to first discharge call might shed light on factors that influence that delay.
In the future, quitlines should consider how they can best reach out to hospital smokers post-discharge. A call within the first week of a quit attempt post-discharge would be ideal, as many smokers relapse within the first week of discharge.51 Until smokers feel able to participate in real-time counseling, asynchronous methods for interacting with smokers, such as text messaging or other mobile or Internet methods,52 might help them stay committed to quitting during their post-hospital recuperation. Text messaging has the added advantage of being a low-cost, effective method for smoking cessation that is globally affordable.53,54
Several factors could account for the somewhat higher self-reported use of post-discharge medication among fax participants. Fax participants received their inpatient counseling face to face from UKanQuit staff, whereas warm handoff participants received most of their inpatient counseling from telephone quitline counselors. UKanQuit counselors may have given greater emphasis to the importance of using medication post-discharge.
Although there was a slightly higher counseling cost in the warm handoff arm, it was offset by a slightly higher cost for pharmacotherapy in the fax arm. Ultimately, there was no difference in costs, from a societal perspective, between treatment arms. Different healthcare providers or perspectives, however, might prefer one approach over another based upon which direct costs they would incur. For instance, hospitals might prefer the warm handoff approach as it required less staff time (costs), which would shift the inpatient counseling costs to the quitline. The quitline, however, might prefer to have those costs incurred by the hospital unless they are fully reimbursed for those additional minutes. All told, however, either referral method should extend the reach and efficiency of quitlines, because the steady stream of enrollees could reduce the costs associated with campaigns to promote quitline utilization.55
Limitations
The main limitation rests with the decision to have warm handoff participants complete their first counseling call in the hospital. The first counseling call involves assessment, treatment planning, and counseling and is usually the lengthiest session. On the one hand, this decision ensured that most warm handoff participants received at least one counseling session. On the other hand, undergoing enrollment and counseling during a busy hospital stay may have been overwhelming for inpatients—especially those with more-severe conditions. Post hoc analyses of the impact of condition severity of treatment adherence could evaluate the degree to which recovery demands affected participation. Future studies could evaluate customizing the quitline experience to better meet the hospital context. This could involve a warm handoff for enrollment to quitline, followed by brief motivational counseling focused on the importance of participating in counseling post-discharge, as most (67%) smokers planning to stay quit relapse within 1 month of leaving the hospital.51 Subsequent calls post-discharge could fully address the specific needs, challenges, and issues of smokers discharged from hospitals.
A second limitation involved permitting only smokers who were ready to quit to participate. This precluded testing the effects of warm handoff among unmotivated smokers. Warm handoff might be relatively more effective, compared with fax referral, for helping unmotivated smokers quit owing to the opportunity to sample what quitlines have to offer.
Last, the study was conducted in only two hospitals, and was implemented by a free-standing tobacco treatment service, which may limit generalizability. Warm handoff, however, may be more generalizable than fax referral as warm handoff does not require specialized training in tobacco intervention to implement.
It is important to note that the study was not designed to test the efficacy of post-discharge telephone quitline counseling. It may be that smokers who had not received either fax or warm handoff to quitline would have quit at the same rates as those who had been referred. One trial examined the impact of quitline versus usual care on cessation among hospitalized patients and found no significant differences in 6-month abstinence (30.2% and 30.8%, respectively).56 The outcome was self-report only, utilized one quitline, and no details are yet available on quitline enrollment, engagement, or study costs. Hence, although quitline has been shown to be effective among community-dwelling smokers, its effectiveness for hospitalized smokers has yet to be definitively established. It is important to note, however, that most hospital trials that were effective employed telephone counseling to provide post-discharge follow-up.4 Should quitlines, as currently designed, prove to be ineffective for inpatients, quitlines could develop and test new protocols that better approximate the telephone procedures used in successful hospital trials.
Conclusions
Warm handoff is an excellent tool for linking patients to phone-based treatment, but the conditions under which this tool might be most effective remain unclear. Because warm handoff provided a “try it, you'll like it” opportunity to sample quitline services, it could be especially effective among treatment-naïve or -resistant smokers.
Low- and middle-income countries could readily adopt these interventions. The use of mobile communications is prevalent globally.57 Integrating quitlines into health services such as hospital care can help care providers to provide high-quality tobacco treatment by providing a reliable and accessible referral option.55 Hospital referral to quitline might be less effective in low- and middle-income countries, as they typically employ reactive quitlines that are not as effective as proactive quitlines.13
It is likely that hospitals will turn to quitlines—where they exist—to deliver post-discharge care to tobacco users. Should this occur, hospitalized smokers may become major consumers of quitline counseling. Among motivated smokers, fax referral and warm handoff are efficient and comparatively effective ways to link smokers with quitlines. Moreover, hospitals without dedicated tobacco treatment staff could use warm handoff to quitlines to deliver expert care to patients both during and after their hospital stay.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the efforts of Brian Hernandez, Genevieve Casey, Lisa Silverman, Terri Tapp, Andrea Elyachar, Sharon Fitzgerald, and Hope Krebill for conducting the trial. The authors thank the State of Kansas Tobacco Use Prevention Program of the Kansas Department of Health and Environment for their advice in designing the study and administration of the Kansas tobacco quitline, KanQuit. The authors also thank the care providers and electronic health record data managers of the University of Kansas Hospital and Stormont Vail Regional Health Care for their support and collaboration on study implementation. The study clinical trials registration number is NCT01305928.
This work was supported solely by funding from the National Heart, Lung, and Blood Institute (U01 HL105232-01). Studies funded under the NIH U01 mechanism represent a consortium across studies and across one or more NIH Institutes. As such, NIH Institutes necessarily take part in the discussion on the design and conduct of all studies, although final decision on all aspects of the study rests with the principal investigator. NIH staff have had a chance to comment on the manuscript while in preparation and prior to submission, but all decisions on whether, where and when to submit the manuscript were made by the principal investigator.
None of the authors have institutional or corporate affiliations that conflict with this study, and no financial disclosures were reported by the authors of this paper. The study commenced after approval of IRBs at both hospitals (KUMC HSC 123456, SV HSC IRB00008059) and all participants provided consent. Selected findings were presented at the Society for Research on Nicotine and Tobacco Annual Conference, February 2015, Philadelphia, PA.
This work was supported solely by funding from the National Heart, Lung, and Blood Institute (U01 HL105232-01). Studies funded under the NIH U01 mechanism represent a consortium across studies and across one or more NIH Institutes. As such, NIH Institutes necessarily take part in the discussion on the design and conduct of all studies, although final decision on all aspects of the study rests with the principal investigator. NIH staff have had a chance to comment on the manuscript while in preparation and prior to submission, but all decisions on whether, where and when to submit the manuscript were made by the principal investigator.
KPR oversaw all aspects of the study, completed the first draft of the manuscript, collected feedback and approval from all authors, and submitted the final version of the manuscript. BF assisted with study design and coordinated collection of medical record data. TIS designed and conducted the cost-effectiveness analyses. LM was the project director and, as such, directed all aspects of day-to-day project operations. NN managed the study database and generated all descriptive data. TB was scientific director for Alere Wellbeing's participation in the trial. TSS worked with NN and KJP to conduct study analyses and interpret results. KJP selected methods for testing the study hypotheses and conducted outcome analyses. BHC helped with study design and in outlining the quitline contribution to the trial. BM coordinated Alere Wellbeing's implementation of quitline counseling in the trial. EFE contributed to the study design and served as the medical director for the trial. CC coordinated the state quitline and advised the team regarding the state quitline protocols and configuration. DJC recruited Stormont Vail and assisted with replication of UKanQuit and data collection at the second site. MM facilitated all aspects of the trial at Stormont Vail. All authors reviewed drafts of the manuscript and approved the final manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Szatkowski L, Murray R, Hubbard R, Agrawal S, Huang Y, Britton J. Prevalence of smoking among patients treated in NHS hospitals in England in 2010/2011: a national audit. Thorax. 2015;70(5):498–500. doi: 10.1136/thoraxjnl-2014-206285. http://dx.doi.org/10.1136/thoraxjnl-2014-206285. [DOI] [PubMed] [Google Scholar]
- 2.Orleans CT, Kristeller JL, Gritz ER. Helping hospitalized smokers quit: new directions for treatment and research. J Consult Clin Psychol. 1993;61(5):778–789. doi: 10.1037//0022-006x.61.5.778. http://dx.doi.org/10.1037/0022-006X.61.5.778. [DOI] [PubMed] [Google Scholar]
- 3.Freund M, Campbell E, Paul C, et al. Smoking care provision in hospitals: a review of prevalence. Nicotine Tob Res. 2008;10(5):757–774. doi: 10.1080/14622200802027131. http://dx.doi.org/10.1080/14622200802027131. [DOI] [PubMed] [Google Scholar]
- 4.Rigotti NA, Clair C, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012;5:CD001837. doi: 10.1002/14651858.CD001837.pub3. http://dx.doi.org/10.1002/14651858.cd001837.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Society of Research on Nicotine and Tobacco. treatobacco.net [April 13, 2014]; Treatobacco.net is a unique source of evidence-based data and practical support for the treatment of tobacco dependence. 2014 www.treatobacco.net/.
- 6.The Joint Commission [June 29, 2015];Tobacco Treatment. 2014 www.jointcommission.org/tobacco_treatment/.
- 7.Fiore MC, Goplerud E, Schroeder SA. The Joint Commission's new tobacco-cessation measures--will hospitals do the right thing? N Engl J Med. 2012;366(13):1172–1174. doi: 10.1056/NEJMp1115176. http://dx.doi.org/10.1056/NEJMp1115176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Medicare and Medicaid Services [June 29, 2015];The Medicare and Medicaid Electronic Health Record (EHR) Incentive Programs: STAGE 2 TOOLKIT. www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/Stage2_Toolkit_EHR_0313.pdf.
- 9.McAfee T, Babb S, McNabb S, Fiore MC. Helping smokers quit--opportunities created by the Affordable Care Act. N Engl J Med. 2015;372(1):5–7. doi: 10.1056/NEJMp1411437. http://dx.doi.org/10.1056/NEJMp1411437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson CM, Zhu SH. Tobacco quitlines: looking back and looking ahead. Tob Control. 2007;16(Suppl 1):i81–86. doi: 10.1136/tc.2007.020701. http://dx.doi.org/10.1136/tc.2007.020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey L. An Overview of NAQC. National Network of Tobacco Cessation Quitlines -- 2006 Regional Meeting -- Western Region; San Diego, CA.. May 1-2, 2006.2006. [Google Scholar]
- 12.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Department of Health and Human Services; U.S. DHHS; Rockville, MD: 2008. [Google Scholar]
- 13.Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2013;8:CD002850. doi: 10.1002/14651858.CD002850.pub3. http://dx.doi.org/10.1002/14651858.cd002850.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25(1):73–78. doi: 10.1080/09595230500459537. http://dx.doi.org/10.1080/09595230500459537. [DOI] [PubMed] [Google Scholar]
- 15.Ossip-Klein DJ, McIntosh S. Quitlines in North America: evidence base and applications. Am J Med Sci. 2003;326(4):201–205. doi: 10.1097/00000441-200310000-00010. http://dx.doi.org/10.1097/00000441-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Swartz SH, Cowan TM, Klayman JE, Welton MT, Leonard BA. Use and effectiveness of tobacco telephone counseling and nicotine therapy in Maine. Am J Prev Med. 2005;29(4):288–294. doi: 10.1016/j.amepre.2005.06.015. http://dx.doi.org/10.1016/j.amepre.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 17.North American Quitline Consortium. Quitline Operations: A Practical Guide to Promising Approaches. North American Quitline Consortium; Phoenix, AZ: 2005. [Google Scholar]
- 18.Perry RJ, Keller PA, Fraser D, Fiore MC. Fax to quit: a model for delivery of tobacco cessation services to Wisconsin residents. WMJ. 2005;104(4):37–40, 44. [PubMed] [Google Scholar]
- 19.Bernstein SL, Jearld S, Prasad D, Bax P, Bauer U. Rapid implementation of a smokers' quitline fax referral service in an urban area. J Health Care Poor Underserved. 2009;20(1):55–63. doi: 10.1353/hpu.0.0112. http://dx.doi.org/10.1353/hpu.0.0112. [DOI] [PubMed] [Google Scholar]
- 20.Wadland WC, Holtrop JS, Weismantel D, Pathak PK, Fadel H, Powell J. Practice-based referrals to a tobacco cessation quit line: assessing the impact of comparative feedback vs general reminders. Ann Fam Med. 2007;5(2):135–142. doi: 10.1370/afm.650. http://dx.doi.org/10.1370/afm.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentz CJ, Bayley KB, Bonin KE, Fleming L, Hollis JF, McAfee T. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30(1):31–37. doi: 10.1016/j.amepre.2005.08.043. http://dx.doi.org/10.1016/j.amepre.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 22.Willett JG, Hood NE, Burns EK, et al. Clinical faxed referrals to a tobacco quitline: reach, enrollment, and participant characteristics. Am J Prev Med. 2009;36(4):337–340. doi: 10.1016/j.amepre.2008.12.004. http://dx.doi.org/10.1016/j.amepre.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Leuthard JL, Beebe LA, Halstead L, Olson KD, Roysdon JW. Increased evidence-based tobacco treatment through Oklahoma hospital system changes. Am J Prev Med. 2015;48(1 Suppl 1):S65–70. doi: 10.1016/j.amepre.2014.09.017. http://dx.doi.org/10.1016/j.amepre.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Tindall EJ. Ravenswood: Bringing Behavioralists into an FQHC. Vol. 2009. National Council Magazine; 2009. pp. 37–38. Winter. [Google Scholar]
- 25.Cummings NA, O'Donohue WT, Cummings JL. The financial dimension of integrated behavioral/primary care. J Clin Psychol Med Settings. 2009;16(1):31–39. doi: 10.1007/s10880-008-9139-2. http://dx.doi.org/10.1007/s10880-008-9139-2. [DOI] [PubMed] [Google Scholar]
- 26.Kadimpati S, Nolan M, Burke M, Schroeder DR, Warner DO. Use of Quitlines to Provide Primary Tobacco Interventions in Hospitalized Patients: A Population-Based Randomized Trial.. Paper presented at: Annual Conference of the Society for Research on Nicotine & Tobacco; Philadelphia, PA.. March, 2015. [Google Scholar]
- 27.Riley WT, Stevens VJ, Zhu SH, Morgan G, Grossman D. Overview of the Consortium of Hospitals Advancing Research on Tobacco (CHART). Trials. 2012;13:122. doi: 10.1186/1745-6215-13-122. http://dx.doi.org/10.1186/1745-6215-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richter KP, Faseru B, Mussulman LM, et al. Using “warm handoffs” to link hospitalized smokers with tobacco treatment after discharge: study protocol of a randomized controlled trial. Trials. 2012;13:127. doi: 10.1186/1745-6215-13-127. http://dx.doi.org/10.1186/1745-6215-13-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faseru B, Turner M, Casey G, et al. Evaluation of a hospital-based tobacco treatment service: outcomes and lessons learned. J Hosp Med. 2011;6(4):211–218. doi: 10.1002/jhm.835. http://dx.doi.org/10.1002/jhm.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Leon J, Diaz FJ, Becona E, Gurpegui M, Jurado D, Gonzalez-Pinto A. Exploring brief measures of nicotine dependence for epidemiological surveys. Addict Behav. 2003;28(8):1481–1486. doi: 10.1016/s0306-4603(02)00264-2. http://dx.doi.org/10.1016/S0306-4603(02)00264-2. [DOI] [PubMed] [Google Scholar]
- 31.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. http://dx.doi.org/10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 32.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. http://dx.doi.org/10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y, Yu C, Luo A, Huang Y, Warner DO. Perioperative tobacco interventions by Chinese anesthesiologists: practices and attitudes. Anesthesiology. 2010;112(2):338–346. doi: 10.1097/ALN.0b013e3181c91ee7. http://dx.doi.org/10.1097/ALN.0b013e3181c91ee7. [DOI] [PubMed] [Google Scholar]
- 34.United Kingdom Small Aneurysm Trial Participants Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1445–1452. doi: 10.1056/NEJMoa013527. http://dx.doi.org/10.1056/NEJMoa013527. [DOI] [PubMed] [Google Scholar]
- 35.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. http://dx.doi.org/10.1080/1462220031000070552. [PubMed] [Google Scholar]
- 36.Benowitz NL. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. http://dx.doi.org/10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Rennie DC, Dosman JA. The reliability of cigarette consumption reports by spousal proxies. Am J Public Health. 1995;85(12):1711–1712. doi: 10.2105/ajph.85.12.1711. http://dx.doi.org/10.2105/AJPH.85.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilpin EA, Pierce JP, Cavin SW, et al. Estimates of population smoking prevalence: self-vs proxy reports of smoking status. Am J Public Health. 1994;84(10):1576–1579. doi: 10.2105/ajph.84.10.1576. http://dx.doi.org/10.2105/AJPH.84.10.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyland A, Cummings KM, Lynn WR, Corle D, Giffen CA. Effect of proxy-reported smoking status on population estimates of smoking prevalence. Am J Epidemiol. 1997;145(8):746–751. doi: 10.1093/aje/145.8.746. http://dx.doi.org/10.1093/aje/145.8.746. [DOI] [PubMed] [Google Scholar]
- 40.Regan S, Reid ZZ, Kelley JH, et al. Smoking Status Confirmation by Proxy: Validation in a Smoking Cessation Trial. Nicotine Tob Res. 2016;18(1):34–40. doi: 10.1093/ntr/ntv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.U.S. Board of Labor Statistics [June 2014];May 2012 National Occupational Employment and Wage Estimates United States. 2012 www.bls.gov/oes/2012/may/oes_nat.htm.
- 42.Williams GC, McGregor H, Sharp D, et al. A Self-Determination Multiple Risk Intervention Trial to Improve Smokers' Health. J Gen Intern Med. 2006;21(12):1288–1294. doi: 10.1111/j.1525-1497.2006.00621.x. http://dx.doi.org/10.1111/j.1525-1497.2006.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams GC, McGregor HA, Sharp D, et al. Testing a self-determination theory intervention for motivating tobacco cessation: supporting autonomy and competence in a clinical trial. Health Psychol. 2006;25(1):91–101. doi: 10.1037/0278-6133.25.1.91. http://dx.doi.org/10.1037/0278-6133.25.1.91. [DOI] [PubMed] [Google Scholar]
- 44.Fleiss JL, Levin B, Cho Paik M. Statistical Methods for Rates and Proportions. Wiley-Interscience; Hoboken, NJ: 2003. http://dx.doi.org/10.1002/0471445428. [Google Scholar]
- 45.Munafo M, Rigotti N, Lancaster T, Stead L, Murphy M. Interventions for smoking cessation in hospitalised patients: a systematic review. Thorax. 2001;56(8):656–663. doi: 10.1136/thorax.56.8.656. http://dx.doi.org/10.1136/thorax.56.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haddix AC, Teutsch SM, Corso PS. Prevention effectiveness : a guide to decision analysis and economic evaluation. 2nd ed. Oxford University Press; Oxford ; New York: 2003. [Google Scholar]
- 47.Rigotti N, Munafo M, Stead L. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2007;(3):CD001837. doi: 10.1002/14651858.CD001837.pub2. http://dx.doi.org/10.1002/14651858.cd001837.pub2. [DOI] [PubMed]
- 48.Singleterry J, Jump Z, Lancet E, et al. State medicaid coverage for tobacco cessation treatments and barriers to coverage - United States, 2008-2014. MMWR Morb Mortal Wkly Rep. 2014;63(12):264–269. [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu SH, Tedeschi G, Anderson CM, et al. Telephone counseling as adjuvant treatment for nicotine replacement therapy in a “real-world” setting. Prev Med. 2000;31(4):357–363. doi: 10.1006/pmed.2000.0720. http://dx.doi.org/10.1006/pmed.2000.0720. [DOI] [PubMed] [Google Scholar]
- 50.Burns EK, Levinson AH, Deaton EA. Factors in nonadherence to quitline services: smoker characteristics explain little. Health Ed Behav. 2012;39(5):596–602. doi: 10.1177/1090198111425186. http://dx.doi.org/10.1177/1090198111425186. [DOI] [PubMed] [Google Scholar]
- 51.Mussulman L, Nazir N, Hall S, Richter KP. Time to relapse and predictors of relapse among hospitalized smokers.. 36th Annual Conference of the Association for Medical Education and Research on Substance Abuse; Bethesda, MD.. 2012. [Google Scholar]
- 52.Borrelli B, Bartlett YK, Tooley E, Armitage CJ, Wearden A. Prevalence and Frequency of mHealth and eHealth Use Among U.S. and UK Smokers and Differences by Motivation to Quit. J Med Internet Res. 2015;17(7):e164. doi: 10.2196/jmir.4420. http://dx.doi.org/10.2196/jmir.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abroms LC, Boal AL, Simmens SJ, Mendel JA, Windsor RA. A randomized trial of Text2Quit: a text messaging program for smoking cessation. Am J Prev Med. 2014;47(3):242–250. doi: 10.1016/j.amepre.2014.04.010. http://dx.doi.org/10.1016/j.amepre.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.West R, Raw M, McNeill A, et al. Health-care interventions to promote and assist tobacco cessation: a review of efficacy, effectiveness and affordability for use in national guideline development. Addiction. 2015;110(9):1388–1403. doi: 10.1111/add.12998. http://dx.doi.org/10.1111/add.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.WHO. Developing and improving national toll-free tobacco quit line services: a World Health Organization manual. France: 2011. ISBN: 978 92 4 150248 1. [Google Scholar]
- 56.Zhu S, Gamst A, Cummins S, et al. Helping Hospitalized Smokers Quit: A Factorial Design to Test the Effects of Telephone Counseling and Nicotine Patches.. Paper presented at: Annual Conference of the Society for Research on Nicotine & Tobacco; Philadelphia. March, 2015. [Google Scholar]
- 57.Lewis T, Synowiec C, Lagomarsino G, Schweitzer J. E-health in low- and middle-income countries: findings from the Center for Health Market Innovations. Bull World Health Organ. 2012;90(5):332–340. doi: 10.2471/BLT.11.099820. http://dx.doi.org/10.2471/BLT.11.099820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.