Abstract
Intracerebral hemorrhage (ICH) is a severe type of stroke causing neurological dysfunction with high mortality rate. Depression is one of the most common complications of ICH. In the present study, the effects of treadmill exercise on ICH-induced depressive symptoms in relation with apoptosis were investigated using rats. ICH rat model was induced by injection of collagenase into the hippocampus using stereotaxic instrument. Open field test for activity and forced swimming test for depressive symptoms were conducted. Apoptosis in the hippocampus was detected using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay, immunohistochemistry for caspase-3, and western blot for Bcl-2 and Bax. Western blot analysis for 5-hydroxy-tryptamine (5-HT, serotonin) and tryptophan hydroxylase (TPH) in the dorsal raphe was also conducted for biomarkers of depression. In the present results, immobility time was increased and climbing time was decreased by induction of ICH and treadmill exercise inhibited immobility time and increased climbing time in ICH rats. DNA fragmentation and caspase-3 expression in the hippocampal dentate gyrus were enhanced by induction of ICH and treadmill exercise suppressed ICH-induced DNA fragmentation and caspase-3 expression. Bax expression in the hippocampus was increased by induction of ICH and treadmill exercise inhibited Bax expression in the ICH rats. Expressions of 5-HT and TPH in the dorsal raphe were decreased by induction of ICH and treadmill exercise increased expressions of 5-HT and TPH in the ICH rats. In the present study, treadmill exercise ameliorated depressive symptoms through inhibiting apoptosis.
Keywords: Intracerebral hemorrhage, Treadmill exercise, Apoptosis, Depression, Hippocampus, Dorsal raphe
INTRODUCTION
Intracerebral hemorrhage (ICH) is a severe type of stroke causing neurological dysfunction with high a mortality rate. The mortality of ICH is higher than that of ischemic stroke (Ferro, 2006). Surgical therapy for ICH is not effective and effective drugs for ICH have not been developed. Alternative and complementary therapeutic strategies for ICH have been suggested. Neuronal cell death in the ICH is known to be induced by apoptotic mechanism (Hwang et al., 2013; Lee et al., 2003).
Apoptosis is a form of cell death that constitutes cell replacement, tissue remodeling, and the removal of damaged cells. Apoptosis is triggered by a variety of stimuli (Thompson, 1995), however, inappropriate or excessive apoptosis causes many neurodegenerative disorders, including stroke (Hwang et al., 2013; Lee et al., 2003). Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining detects DNA fragmentation that is a hallmark of apoptosis (Ko et al., 2009; Sung et al., 2010). The caspases, a family of 14 cysteine proteases, are essential players in apoptotic cell death both as initiators (caspase- 2, -8, -9, and -10) and executioners (caspase-3, -6, and -7) (Reed, 2000). Cell death in the parenchyma occurs via apoptotic mechanisms during ICH, and apoptotic cell death is induced by caspase-3 activation in cells adjacent to the hematoma (Hwang et al., 2013; Lee et al., 2003). In addition to caspases, the Bcl-2 family proteins also regulate apoptosis. The Bcl-2 family proteins are classified into antiapoptotic proteins, such as Bcl-2 and Bcl-2XL, and pro-apoptotic proteins, such as Bax and Bid. The balance between pro-apoptotic and anti-apoptotic Bcl-2 family members determines the mitochondrial response to apoptotic stimuli (Hwang et al., 2013; Upadhyay et al., 2003).
Serotonergic system is implicated in many psychiatric disorders, such as anxiety, mood and eating disorders, migraine, and depression (Bannai et al., 2007; Cooper et al., 2008). Serotonergic system inhibits proactive coping responses including aggression and escape behaviors, and it also facilitates passive-submissive behaviors including fear- and anxiety-like behaviors (Bannai et al., 2007; Cooper et al., 2008; Graeff and Zangrossi, 2010).
Depression is one of the most common complications of ICH. Depression is a decreased mood state and it also disturbs cognitive and physical functions. Depression is closely associated with down-regulation of serotonergic neurotransmitters, such as 5-hydroxytryptamine (5-HT, serotonin), in the dorsal raphe of the brain. Antidepressant drugs reduce depressive symptoms through enhancing 5-HT level in the dorsal raphe (Kim et al., 2002). Tryptophan hydroxylase (TPH) catalyzes the rate-limiting step of 5-HT biosynthesis in the serotonergic neurons, therefore 5-HT and TPH expressions are related with the severity of depression. Depressed suicide victims showed higher TPH immunoreactivity in the dorsal raphe compared to the control subjects (Underwood et al., 1999). Chamas et al. (2004) showed that repeated immobilization stress increased TPH mRNA and protein concentration.
Exercise is known to ameliorate apoptosis in various neurodegenerative disorders (Heo et al., 2014; Kim et al., 2014; Lee et al., 2003). Ameliorating effect of exercise on depression has been reported (Newman and Motta, 2007; Perraton et al., 2010), however, the effects of exercise on ICH-induced depressive symptoms are unclear.
In the present study, the effects of treadmill exercise on ICH-induced depressive symptoms in relation with apoptosis were investigated using rats. For this study, open field test for activity and forced swimming test for depressive symptoms were conducted. Apoptosis in the hippocampus was detected using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay, immunohistochemistry for caspase-3, and western blot for Bcl-2 and Bax. Biomarkers for the depression were assessed by Western blot analysis for 5-HT and TPH in the dorsal raphe.
MATERIALS AND METHODS
Experimental animals and treatments
All experiments were performed by the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. Seven weeks of aged Sprague-Dawley rats (210±10 g) were randomly divided into five groups (n=10 in each group): the sham-operation group, the sham-operation and treadmill exercise group, the hemorrhage-induction group, and the hemorrhage-induction and treadmill exercise group.
Induction of collagenase-induced ICH
ICH was induced according to the previously described method (Hwang et al., 2013). To induce ICH, the rats were anesthetized with Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories, Carros, France) and placed in a stereotaxic frame. The needle of a 10-μL Hamilton syringe (Micro 701, Hamilton Co., Reno, NV, USA) was inserted through a burr hole into the right hippocampus to the following coordinates: 2.2 mm anterior, 2.2 mm lateral, 4.2-mm depth to bregma. One-microliter distilled water containing 0.2 U collagenase (Type 4, Sigma Chemical Co., St Louis, MO, USA) was infused over 1 min. The needle remained in place for an additional 3 min following the infusion, and then was withdrawn slowly.
Exercise protocol
The rats in the exercise groups were forced to run on a motorized treadmill for 30 min once a day for 2 weeks. The exercise load consisted of running at a speed of 2 m/min for the first 5 min, 5 m/min for the next 5 min, and 8 m/min for the last 20 min, with a 0° inclination. The rats in the control groups were left on the treadmill without running for the same period as the exercise groups.
Open field test
Activity was determined using the open field test according to the previously described method (Kim et al., 2011). The animals were randomly assigned to an order of testing and placed in a white square open field arena (100 cm × 100 cm) made of wood. It was enclosed with 40-cm-high walls and placed under strong illumination (200 lux). The arena was divided into 25 squares (each square is 20 cm × 20 cm), defined as 9 central and 16 peripheral squares. The animal was placed in the center of the arena and left free to explore the environment for 1 min. After that time, the numbers of squares that the rat crossed were recorded for 5 min.
Forced swimming test
Forced swimming test was conducted according to the previously described method (Sung et al., 2010). Experimental animals were dropped individually into glass cylinders. The glass cylinder (height, 50 cm; diameter, 15 cm) contained water at a temperature of 27°C. The water depth was 30 cm. All of the experimental gerbils underwent a pretest for 15 min to eliminate the acute stress of the water and to provide the animals with the ability to adapt to the water. Twenty-four hr after pretest, the animals were tested for 5 min. During the test session, the climbing time and the immobility time were analyzed using a Smart version 2.5 video tracking system (Panlab, Barcelona, Spain). Immobility behavior was defined to occur when no additional activity was observed other than the actions needed to keep the animals’ head above the water. Climbing behavior consisted of upward-directed movements of the forepaws along the side of the swim chamber.
Preparation of tissues
The animals were sacrificed immediately after determining the forced swimming test. The rats were anesthetized using Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories), transcardially perfused with 50-mM phosphate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100-mM phosphate buffer (pH, 7.4). Brains were dissected, and storage overnight same fixative, it was transferred to 30% sucrose for cryoprotection. For the immunohistochemistry, the slices were coronal sectioned at 40 μm thick using a cryostat (Leica, Nussloch, Germany). Ten slice sections on average in the hippocampus were collected from each rat. The sections of 2.5 mm to 2.7 mm posterior from the bregma were used for immunohistochemistry.
TUNEL staining
To visualize DNA fragmentation, a marker of apoptosis, TUNEL staining was performed using an In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to the manufacturer’s protocol (Ko et al., 2009; Sung et al., 2010). The sections were postfixed in ethanol-acetic acid (2:1) and rinsed. The sections were then incubated with proteinase K (100 μg/mL), rinsed, and incubated in 3% H2O2, permeabilized with 0.5% Triton X-100, rinsed again, and incubated in the TUNEL reaction mixture. The sections were rinsed and visualized using Converter-POD with 0.03% 3, 3′-diaminobenzidine (DAB). Mayer’s hematoxylin (DAKO, Glostrup, Denmark) was used as a counter-stain, and the sections were mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature and dehydrated through a gradient of ethanol and covered with coverslips using Permount (Fisher Scientific, New Jersey, NJ, USA).
Immunohistochemistry for caspase-3
To visualize caspase-3 expression, caspase-3 immunohistochemistry was performed according to the previously described method (Kim et al., 2010; Ko et al., 2009). The sections were selected from each brain and incubated overnight with mouse anti-caspase-3 antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and then with biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) and the Vector Elite ABC kit (1:100; Vector Laboratories) were amplified for another 1 hr. Antibody-biotin-avidin-peroxidase complexes were visualized using 0.03% DAB, and the sections were mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature and dehydrated through a gradient of ethanol and covered with coverslips using Permount (Fisher Scientific).
Immunohistochemistry for 5-HT and TPH
Immunohistochemistry for 5-HT and TPH was performed according to the previously described method (Seo et al., 2011; Sung et al., 2010). Briefly, the sections were incubated in PBS for 10 min and washed 3 times, again with PBS and then incubated in 1% H2O2 for 30 min. Next, the sections were incubated overnight with rabbit anti-5-HT antibody (Immunostar, Hudson, NY, USA) at a dilution of 1:500 for visualization of 5-HT expression or with mouse anti-TPH antibody (Oncogene Research Product, Cambridge, UK) at a dilution of 1:1,000 for visualization of TPH expression. The sections were then incubated for 1 hr with biotinylated antirabbit secondary antibody or with antimouse secondary antibody (Vector Laboratories). The sections were subsequently incubated with avidin-biotin-peroxidase complex (Vector Laboratories) for 1 hr at room temperature. Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.05% DAB (Sigma Chemical Co.) and 0.01% H2O2 in 50-mM Tris-buffer (pH, 7.6) for approximately 3 min. The sections were then mounted on gelatin-coated glass slides. The slides were air-dried overnight at room temperature and dehydrated through a gradient of ethanol and covered with coverslips using Permount (Fisher Scientific).
Western blot analysis
Western blotting for Bcl-2 and Bax was performed according to the previously described method (Kim et al., 2010). The hippocampal tissues were dissected and collected, and then were immediately frozen at −70°C. The right hemisphere were homogenized on ice, and lysed in a lysis buffer containing 50-mM N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (pH, 7.5), 150-mM NaCl, 10% glycerol, 1% Triton X-100, 1-mM phenylmethylsulfonyl fluoride, 1-mM ethylene glycol tetraacetic acid, 1.5-mM MgCl2·6H2O, 1-mM sodium orthovanadate, and 100-mM sodium fluoride. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Hercules, CA, USA). Protein samples (30 μg) were separated on sodium dodecyl sulfate-polyacrylamide gel and transferred onto a nitrocellulose membrane. The membranes were incubated with 5% skim milk in Tris-buffered saline containing 0.1% Tween-20 and then incubated overnight at 4°C with the following primary antibodies: mouse anti-β-actin, anti-Bcl-2, and anti-Bax antibodies (1:1,000; Santa Cruz Biotechnology). Subsequently, membranes were incubated for 1 hr with attempt secondary antibodies (1:2,000; Vector Laboratories), and band detection was performed using the enhanced chemiluminescence detection kit (Santa Cruz Biotechnology).
Data analysis
For the Western blot analysis, the detected bands were calculated densitometrically using Molecular Analyst, version 1.4.1 (Bio-Rad). The area of the hippocampal dentate gryus was measured by Image-Pro Plus image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA). The numbers of TUNEL-positive and caspase-3-positive cells in the hippocampal dentate gryus were counted hemilaterally under a light microscope (Olympus, Tokyo, Japan), and they were expressed as the numbers of cells/mm2 of the hippocampal dentate gryus. The numbers of 5-HT-positive and TPH-positive cells in the dorsal raphe were counted hemilaterally under a light microscope (Olympus), and they were expressed as the numbers of cells/section of the dorsal raphe. Statistical analysis was performed using one-way analysis of variance followed by Duncan post hoc test, and the results are expressed as the mean±standard error of the mean. Significance was set as P<0.05.
RESULTS
Effect of treadmill exercise on activity in the open field test
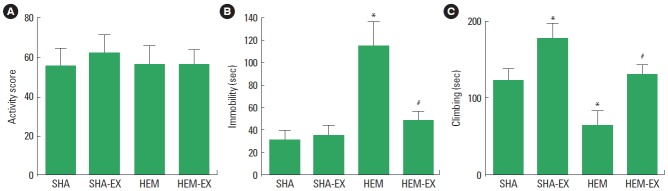
The activity score in the open field test is presented in Fig. 1A. In the activity, there was no statistical significance among the groups.
Fig. 1.
Effects of treadmill exercise on activity and depression-like behaviors. (A) Activity in the open field test. (B) Immobility time in the forced swimming test. (C) Climbing time in the forced swimming test. SHA, sham-operation group; SHA-EX, sham-operation and treadmill exercise group; HEM, hemorrhage-induction group; HEM-EX, hemorrhage-induction and treadmill exercise group. Values are presented as mean±standard error of the mean. *P<0.05 compared to the SHA group. #P<0.05 compared to the HEM group.
Effect of treadmill exercise on depression-like behaviors in the forced swimming test
The immobility time in the forced swimming test is presented in Fig. 1B. The immobility time was increased by induction of ICH (P<0.05) and treadmill exercise inhibited ICH-induced increment of immobility time (P<0.05).
The climbing time in the forced swimming test is presented in Fig. 1C. The climbing time was decreased by induction of ICH (P<0.05) and treadmill exercise increased climbing time in the normal and ICH rats (P<0.05).
Effect of treadmill exercise on the number of TUNEL-positive cells in the hippocampal dentate gyrus
The number of TUNEL-positive cells is presented in Fig. 2B. Induction of ICH enhanced DNA fragmentation in the hippocampal dentate gyrus (P<0.05) and treadmill exercise suppressed ICH-induced DNA fragmentation (P<0.05). In the normal rats, treadmill exercise exerted no significant effect on the DNA fragmentation.
Fig. 2.
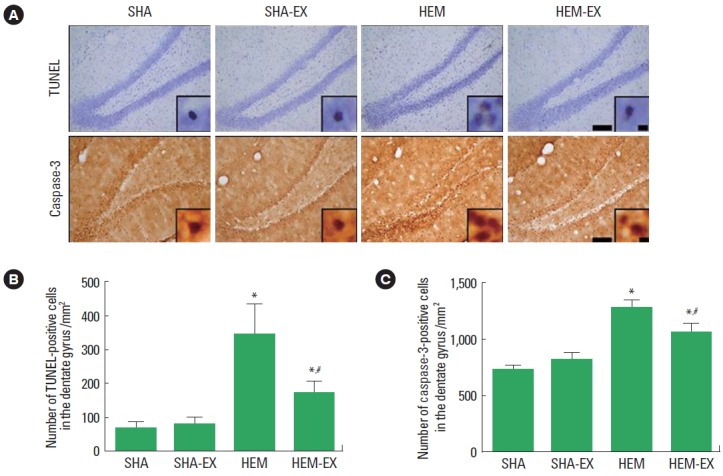
Effects of treadmill exercise on the numbers of terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL)-positive cells and active caspase-3-positive cells in the hippocampal dentate gyrus. (A) Photomicrographs showing TUNEL-positive and caspase-3-positive cells in the hippocampal dentate gyrus (immunohistochemistry). Scale bar=200 μm, scale bar in enclosed images=10 μm. (B, C) The numbers of TUNEL-positive and caspase-3-positive cells in the hippocampal dentate gyrus. SHA, sham-operation group; SHA-EX, sham-operation and treadmill exercise group; HEM, hemorrhage-induction group; HEM-EX, hemorrhage-induction and treadmill exercise group. Values are presented as mean±standard error of the mean. *P<0.05 compared to the SHA group. #P<0.05 compared to the HEM group.
Effect of treadmill exercise on the number of caspase-3-positive cells in the hippocampal dentate gyrus
The number of caspase-3-positive cells is presented in Fig. 2C. Induction of ICH enhanced caspase-3 expression in the hippocampal dentate gyrus (P<0.05) and treadmill exercise suppressed ICH-induced caspase-3 expression (P<0.05). In the normal rats, treadmill exercise exerted no significant effect on the caspase-3 expression.
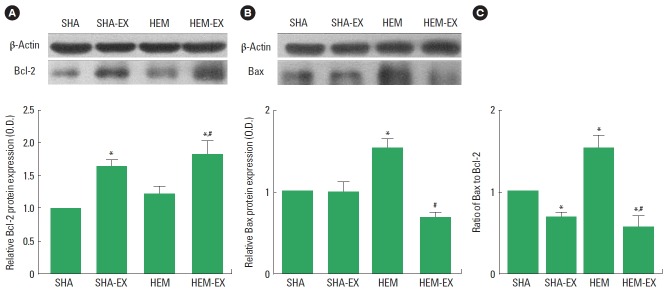
Effect of treadmill exercise on Bcl-2 and Bax expressions in the hippocampus
The level of Bcl-2 is presented in Fig. 3A. These results showed that treadmill exercise increased Bcl-2 expression in the hippocampus both in the normal and ICH-induced rats (P<0.05). Induction of ICH exerted no significant effect on Bcl-2 expression.
Fig. 3.
Effects of treadmill exercise on the Bcl-2 and Bax expressions in the hippocampus. (A) Bcl-2 expression in the hippocampus. (B) Bax expression in the hippocampus. (C) Ratio of Bax to Bcl-2 in the hippocampus. SHA, sham-operation group; SHA-EX, sham-operation and treadmill exercise group; HEM, hemorrhage-induction group; HEM-EX, hemorrhage-induction and treadmill exercise group. Values are presented as mean±standard error of the mean. *P<0.05 compared to the SHA group. #P<0.05 compared to the HEM group.
The level of Bax is presented in Fig. 3B. Induction of ICH increased Bax expression in the hippocampus (P<0.05) and treadmill exercise inhibited Bax expression in the ICH rats (P<0.05).
The ratio of Bax to Bcl-2 is presented in Fig. 3C. Induction of ICH enhanced the ratio of Bax to Bcl-2in the hippocampus (P<0.05) and treadmill exercise inhibited the ratio of Bax to Bcl-2 in the normal and ICH rats (P<0.05).
Effect of treadmill exercise on 5-HT synthesis and TPH expression in the dorsal raphe
The number of 5-HT-positive cells is presented in Fig. 4B. 5-HT expression in the dorsal raphe was decreased by induction of ICH (P<0.05) and treadmill exercise increased 5-HT expression in the ICH rats.
Fig. 4.
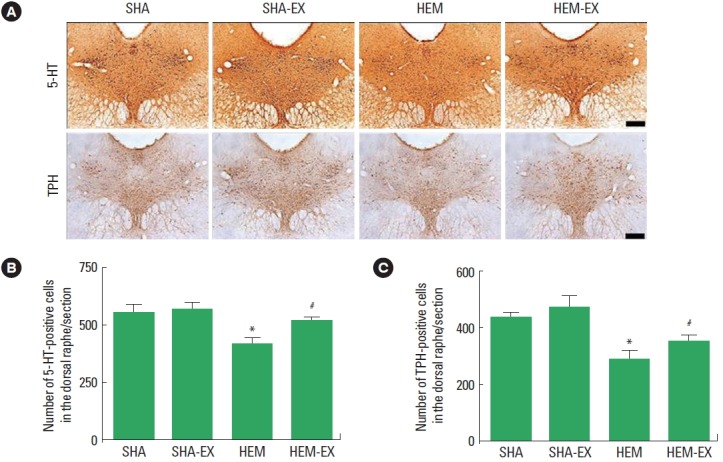
Effects of treadmill exercise on the expressions of 5-hydroxytryptamine (5-HT) and tryptophan hydroxylase (TPH) in the dorsal raphe. (A) Photomicrographs showing 5-HT and TPH expressions in the dorsal raphe (immunohistochemistry). Scale bar=200 μm. (B, C) The numbers of 5-HT-positive and TPH-positive cells in the dorsal raphe. SHA, sham-operation group; SHA-EX, sham-operation and treadmill exercise group; HEM, hemorrhage-induction group; HEM-EX, hemorrhage-induction and treadmill exercise group. Values are presented as mean±standard error of the mean. *P<0.05 compared to the SHA group. #P<0.05 compared to the HEM group.
The number of TPH-positive cells is presented in Fig. 4C. TPH expression in the dorsal raphe was decreased by induction of ICH (P<0.05) and treadmill exercise increased TPH expression in the ICH rats.
DISCUSSION
Intracerebral injection of collagenase into the hippocampus induces a lesion with triggered apoptotic neuronal cell death in the hippocampus (Hwang et al., 2013; Lee et al., 2003; Suh et al., 2011). Apoptosis plays a key role in the neuronal cell death by stroke (Matsushita et al., 2000; Qureshi et al., 2003). Induction of ICH in rats induced neuronal cell death, and apoptosis is closely implicated in ICH-induced neuronal cell death (Gong et al., 2001; Matsushita et al., 2000). The morphological characteristics of apoptosis are cell shrinkage, chromatin condensation, membrane blebbing, internucleosomal DNA fragmentation, and formation of apoptotic bodies (Li et al., 1995). Up-regulation and activation of caspase-3 are the important hallmark of apoptosis following ischemic and hemorrhagic brain insults (Benchoua et al., 2004; Suh et al., 2011). Increment in the numbers of TUNEL-positive and caspase-3-positive cells in the hippocampus represent enhancement of apoptotic neuronal cell death in the hippocampus (Lee et al., 2003; Suh et al., 2011).
In the present study, induction of ICH enhanced DNA fragmentation and caspase-3 expression in the hippocampal dentate gyrus. Treadmill exercise suppressed ICH-induced DNA fragmentation and caspase-3 expression. In the normal rats, treadmill exercise exerted no significant effect on the DNA fragmentation caspase-3 expression. These results demonstrated that ICH induced apoptotic neuronal cell death in the hippocampus and treadmill exercise inhibited ICH-induced apoptosis.
Bcl-2 family proteins have one or more Bcl-2 homology domains and regulate intracellular apoptotic signal transduction by modulating mitochondrial membrane permeability. Bax is pro-apoptotic protein, and Bax eliminates the mitochondrial membrane potential by increasing the permeability transition pore and facilitating the release of cytochrome c. Conversely, Bcl-2 is antiapoptotic protein, and Bcl-2 conserves the membrane potential and inhibit the release of cytochrome c (Sugawara et al., 2004). Bcl-2 member inhibits apoptosis by preventing the release of cytochrome c from mitochondria, however, Bcl-2 forms heterodimers with the pro-apoptotic member Bax, and they are incapacitated from their protective function (Kuwana and Newmeyer, 2003). Thus, the balance of Bax to Bcl-2 is one of the crucial factors determining whether the cells undergo apoptosis (Upadhyay et al., 2003). Up-regulation of Bcl-2 expression suggests neuroprotection (Engelhard et al., 2003) and down-regulation of Bax expression represents antiapoptotic effect (Il’inykh et al., 2008).
In the present study, induction of ICH increased Bax expression in the hippocampus and treadmill exercise inhibited Bax expression in the ICH rats. Induction of ICH did not exert any effects on the Bcl-2 expression. As the results, induction of ICH enhanced the ratio of Bax to Bcl-2 in the hippocampus and treadmill exercise inhibited the ratio of Bax to Bcl-2 ratio in the ICH rats. These results showed that ICH initiated apoptosis through enhancing Bax expression in the hippocampus and treadmill exercise inhibited Bax-mediated apoptosis.
Increased activity was observed in the attention deficit/hyperactivity disorder rats (Kim et al., 2011). In the present study, activity was not changed by induction of ICH and treadmill exercise exerted no effect on activity. These results reveal that ICH is not associated with activity.
Immobility time and climbing time in the forced swimming test are interpreted as an indicator determining depression. Increment in immobility time represents the failure of persistence in escape-directed behavior (Cryan et al., 2002). Alcohol dependence and/or abstinence increased in immobility time and decreased in climbing time in the forced swimming test, showing depression-like behaviors (Stevenson et al., 2009). In the maternal rats separated from their pups, a decrement of climbing time and an increment of immobility time were observed, representing depression-like behaviors (Sung et al., 2010).
In the present study, immobility time was increased and climbing time was decreased by induction of ICH and treadmill exercise inhibited immobility time and increased climbing time in ICH rats. These results demonstrated that ICH evoked depression-like behaviors and treadmill exercise ameliorated these symptoms.
Reduced brain 5-HT concentration is highly correlated with the one of the underlying mechanisms of depression (Kim et al., 2002; Yang et al., 2008; Vasudeva et al., 2011). TPH is an enzyme that regulates the synthesis of the 5-HT, and TPH level was higher in the depressed suicides, suggesting that serotonin impairment in the suicides might be due to the hypofunction of serotonin synthesizing enzyme (Boldrini et al., 2005).
In the present study, expressions of 5-HT and TPH in the dorsal raphe were decreased by induction of ICH and treadmill exercise increased expressions of 5-HT and TPH in the ICH rats. These results confirmed that ICH induced depressive state and treadmill exercise alleviated depressive state.
Here in this study, ICH initiated apoptosis in the hippocampus and ICH also induced depressive symptoms. In contrast, treadmill exercise ameliorated depressive symptoms through inhibiting apoptosis. Based on the present results, it possibility that treadmill exercise can be used as the therapeutic strategy for the alleviation of depression with antiapoptotic effect in ICH patients.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology (Berl) 2007;193:295–304. doi: 10.1007/s00213-007-0780-5. [DOI] [PubMed] [Google Scholar]
- Benchoua A, Braudeau J, Reis A, Couriaud C, Onténiente B. Activation of proinflammatory caspases by cathepsin B in focal cerebral ischemia. J Cereb Blood Flow Metab. 2004;24:1272–1279. doi: 10.1097/01.WCB.0000140272.54583.FB. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- Chamas FM, Underwood MD, Arango V, Serova L, Kassir SA, Mann JJ, Sabban EL. Immobilization stress elevates tryptophan hydroxylase mRNA and protein in the rat raphe nuclei. Biol Psychiatry. 2004;55:278–283. doi: 10.1016/s0006-3223(03)00788-1. [DOI] [PubMed] [Google Scholar]
- Cooper MA, McIntyre KE, Huhman KL. Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology. 2008;33:1236–1247. doi: 10.1016/j.psyneuen.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Engelhard K, Werner C, Eberspächer E, Bachl M, Blobner M, Hildt E, Hutzler P, Kochs E. The effect of the alpha 2-agonist dexmedetomidine and the N-methyl-D-aspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003;96:524–531. doi: 10.1097/00000539-200302000-00041. [DOI] [PubMed] [Google Scholar]
- Ferro JM. Update on intracerebral haemorrhage. J Neurol. 2006;253:985–999. doi: 10.1007/s00415-006-0201-4. [DOI] [PubMed] [Google Scholar]
- Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48:875–882. doi: 10.1097/00006123-200104000-00037. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Zangrossi H., Jr The dual role of serotonin in defense and the mode of action of antidepressants on generalized anxiety and panic disorders. Cent Nerv Syst Agents Med Chem. 2010;10:207–217. doi: 10.2174/1871524911006030207. [DOI] [PubMed] [Google Scholar]
- Heo YM, Shin MS, Lee JM, Kim CJ, Baek SB, Kim KH, Baek SS. Treadmill exercise ameliorates short-term memory disturbance in scopolamine-induced amnesia rats. Int Neurourol J. 2014;18:16–22. doi: 10.5213/inj.2014.18.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang L, Choi IY, Kim SE, Ko IG, Shin MS, Kim CJ, Kim SH, Jin JJ, Chung JY, Yi JW. Dexmedetomidine ameliorates intracerebral hemorrhage-induced memory impairment by inhibiting apoptosis and enhancing brain-derived neurotrophic factor expression in the rat hippocampus. Int J Mol Med. 2013;31:1047–1056. doi: 10.3892/ijmm.2013.1301. [DOI] [PubMed] [Google Scholar]
- Il’inykh FA, Bannova AV, Kalinina TS, Dygalo NN. Effects of ligands of alpha2-adrenoceptors on mRNA level of apoptotic proteins in the developing rat brain. Izv Akad Nauk Ser Biol. 2008;(1):104–109. [PubMed] [Google Scholar]
- Kim BK, Shin MS, Kim CJ, Baek SB, Ko YC, Kim YP. Treadmill exercise improves short-term memory by enhancing neurogenesis in amyloid beta-induced Alzheimer disease rats. J Exerc Rehabil. 2014;10:2–8. doi: 10.12965/jer.140086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Heo HI, Kim DH, Ko IG, Lee SS, Kim SE, Kim BK, Kim TW, Ji ES, Kim JD, Shin MS, Choi YW, Kim CJ. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neurosci Lett. 2011;504:35–39. doi: 10.1016/j.neulet.2011.08.052. [DOI] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45:357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Kim SW, Park SY, Hwang O. Up-regulation of tryptophan hydroxylase expression and serotonin synthesis by sertraline. Mol Pharmacol. 2002;61:778–785. doi: 10.1124/mol.61.4.778. [DOI] [PubMed] [Google Scholar]
- Ko IG, Shin MS, Kim BK, Kim SE, Sung YH, Kim TS, Shin MC, Cho HJ, Kim SC, Kim SH, Kim KH, Shin DH, Kim CJ. Tadalafil improves short-term memory by suppressing ischemia-induced apoptosis of hippocampal neuronal cells in gerbils. Pharmacol Biochem Behav. 2009;91:629–635. doi: 10.1016/j.pbb.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–699. doi: 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Lee HH, Kim H, Lee MH, Chang HK, Lee TH, Jang MH, Shin MC, Lim BV, Shin MS, Kim YP, Yoon JH, Jeong IG, Kim CJ. Treadmill exercise decreases intrastriatal hemorrhage-induced neuronal cell death via suppression on caspase-3 expression in rats. Neurosci Lett. 2003;352:33–36. doi: 10.1016/j.neulet.2003.08.039. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M, Jiang N, Zhang ZG, Zaloga C. Induction of DNA fragmentation after 10 to 120 minutes of focal cerebral ischemia in rats. Stroke. 1995;26:1252–1257. doi: 10.1161/01.str.26.7.1252. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Meng W, Wang X, Asahi M, Asahi K, Moskowitz MA, Lo EH. Evidence for apoptosis after intercerebral hemorrhage in rat striatum. J Cereb Blood Flow Metab. 2000;20:396–404. doi: 10.1097/00004647-200002000-00022. [DOI] [PubMed] [Google Scholar]
- Newman CL, Motta RW. The effects of aerobic exercise on childhood PTSD, anxiety, and depression. Int J Emerg Ment Health. 2007;9:133–158. [PubMed] [Google Scholar]
- Perraton LG, Kumar S, Machotka Z. Exercise parameters in the treatment of clinical depression: a systematic review of randomized controlled trials. J Eval Clin Pract. 2010;16:597–604. doi: 10.1111/j.1365-2753.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suri MF, Ostrow PT, Kim SH, Ali Z, Shatla AA, Guterman LR, Hopkins LN. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery. 2003;52:1041–1047. [PubMed] [Google Scholar]
- Reed CJ. Apoptosis and cancer: strategies for integrating programmed cell death. Semin Hematol. 2000;37(4 Suppl 7):9–16. doi: 10.1016/s0037-1963(00)90055-6. [DOI] [PubMed] [Google Scholar]
- Seo JH, Sung YH, Kim KJ, Shin MS, Lee EK, Kim CJ. Effects of Phellinus linteus administration on serotonin synthesis in the brain and expression of monocarboxylate transporters in the muscle during exhaustive exercise in rats. J Nutr Sci Vitaminol (Tokyo) 2011;57:95–103. doi: 10.3177/jnsv.57.95. [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, Hodge CW. Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology. 2009;34:1209–1222. doi: 10.1038/npp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Fujimura M, Noshita N, Kim GW, Saito A, Hayashi T, Narasimhan P, Maier CM, Chan PH. Neuronal death/survival signaling pathways in cerebral ischemia. NeuroRx. 2004;1:17–25. doi: 10.1602/neurorx.1.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HJ, So SM, Na YG, Ko IG, Kim SE, Sung YH, Shin MS, Kim CJ, Cho YS, Kim KH. Neuroprotective effects of tamsulosin on intracerebral hemorrhage. Neural Regen Res. 2011;6:2505–2510. [Google Scholar]
- Sung YH, Shin MS, Cho S, Baik HH, Jin BK, Chang HK, Lee EK, Kim CJ. Depression-like state in maternal rats induced by repeated separation of pups is accompanied by a decrease of cell proliferation and an increase of apoptosis in the hippocampus. Neurosci Lett. 2010;470:86–90. doi: 10.1016/j.neulet.2009.12.063. [DOI] [PubMed] [Google Scholar]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Upadhyay D, Panduri V, Ghio A, Kamp DW. Particulate matter induces alveolar epithelial cell DNA damage and apoptosis: role of free radicals and the mitochondria. Am J Respir Cell Mol Biol. 2003;29:180–187. doi: 10.1165/rcmb.2002-0269OC. [DOI] [PubMed] [Google Scholar]
- Vasudeva RK, Lin RC, Simpson KL, Waterhouse BD. Functional organization of the dorsal raphe efferent system with special consideration of nitrergic cell groups. J Chem Neuroanat. 2011;41:281–293. doi: 10.1016/j.jchemneu.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]