Abstract
Introduction
This study determined the effectiveness of the Tobacco Tactics intervention.
Design/setting/participants
This was a pragmatic, quasi-experimental study conducted from 2010 to 2013 and analyzed from 2014 to 2015 in five Michigan community hospitals; three received the Tobacco Tactics intervention, and two received usual care. Smokers (N=1,528) were identified during hospitalization, and sent surveys and cotinine tests after 6 months. Changes in pre- to post-intervention quit rates in the intervention sites were compared with usual care control sites.
Intervention
The toolkit for nurses included: (1) 1 continuing education unit contact hour for training; (2) a PowerPoint presentation on behavioral and pharmaceutical interventions; (3) a pocket card entitled “Helping Smokers Quit: A Guide for Clinicians”; (4) behavioral and pharmaceutical protocols; and (5) a computerized template for documentation. The toolkit for patients included: (1) a brochure; (2) a cessation DVD; (3) the Tobacco Tactics manual; (4) a 1–800-QUIT-NOW card; (5) nurse behavioral counseling and pharmaceuticals; (6) physician reminders to offer brief advice to quit coupled with medication sign-off; and (7) follow-up phone calls by trained hospital volunteers.
Main outcome measures
The effectiveness of the intervention was measured by 6-month 30-day point prevalence, self-reported quit rates with NicAlert® urinary biochemical verification (48-hour detection period), and the use of electronic medical record data among non-responders.
Results
There were significant improvements in pre- to post-intervention self-reported quit rates (5.7% vs 16.5%, p<0.001) and cotinine-verified quit rates (4.3% vs 8.0%, p<0.05) in the intervention sites compared with no change in the control sites. Propensity-adjusted multivariable analyses showed a significant improvement in self-reported 6-month quit rates from the pre- to post-intervention time periods in the intervention sites compared to the control sites (p=0.04) and a non-statistically significant improvement in the cotinine-verified 6-month quit rate.
Conclusions
The Tobacco Tactics intervention, which meets the Joint Commission standards for inpatient smoking, has the potential to significantly decrease smoking among inpatient smokers.
Introduction
Numerous studies have shown that inpatient cessation programs, including those delivered by nurses, have the potential to reach a large number of smokers and are highly efficacious.1,2 Inpatient smoking programs capitalize on a teachable moment, take advantage of cessation induced by hospital smoking bans, and enroll a higher proportion of patients who smoke compared with quit lines.3–5 However, a wide gap exists between efficacious interventions and the implementation of such interventions in routine clinical practice.
To bridge the implementation gap, this pragmatic trial was designed to teach real-world inpatient nurses how to conduct tobacco-cessation interventions. Over the years, the authors have developed, tested, and refined the nurse-administered Tobacco Tactics intervention,6–10 which is based on the Agency for Healthcare Research and Quality Guidelines for Smoking Cessation.11 As one of six NIH-supported Consortium of Hospitals Advancing Research on Tobacco studies, this study determined the effectiveness of the nurse-administered Tobacco Tactics intervention among five Trinity Health community hospitals (three intervention and two usual care control) using 6-month self-reported and biochemically confirmed smoking cessation as the primary outcome.
Methods
Design
The protocol of the study has been previously described.12 In brief, this study, conducted from 2010 to 2013 and analyzed from 2014 to 2015, was a pragmatic, quasi-experimental trial initially conducted and analyzed in six Michigan Trinity Health community hospitals (matched on size and number of minority patients), of which three were to receive the nurse-administered Tobacco Tactics intervention and three were to receive usual care, although data from one of the control hospitals was not useable owing to a protocol deviation. In order to eliminate investigator bias, a random number generator was used to assign the hospitals to experimental or control conditions. Although medical surgical units were the primary targeted units, the leaders at the hospitals were allowed to include additional units. However, nurses and patients on non-targeted units were not followed.
Committed to those who are poor and underserved in its communities, Trinity Health is one of the largest multi-institutional Catholic healthcare delivery systems in the nation, serving people and communities in 21 states from coast to coast with 91 hospitals and 124 continuing care locations. Five Trinity Health hospitals in Michigan were included in the study. Inclusion criteria for the study were inpatients that:
smoked a cigarette within 1 month prior to hospitalization;
were aged ≥18 years; and
had a projected hospital stay of ≥24 hours.
Excluded were smokers that were:
involved in a concurrent smoking-cessation trial;
non-English speaking; or
not cognitively or physically able to participate.
This study was approved by the IRBs at the University of Michigan and the Trinity Health hospitals.
To obtain population quit rates throughout the study, all inpatient smokers were identified from the electronic medical record (EMR) and approached by a research assistant to provide written informed consent to surveys and a cotinine test. Using a modified Dillman et al. approach,13 patients were initially simultaneously mailed a survey and a NicAlert® cotinine test strip 6 months after discharge to determine current smoking status. During the pre-intervention period, the authors learned that patients were “turned off” by the cotinine strips and only one person returned a cotinine test without a survey, so beginning in the transition period cotinine strips were sent to only those that first returned a survey. Participants were given $10 for each survey and $20 for return of a cotinine test. For those that did not return surveys or cotinine tests, research assistants (not blinded) made follow-up calls to obtain at the very least quit status. For those who could not be contacted by telephone, a programmer (blinded) downloaded smoking status and quit date from the EMR and a research nurse (not blinded) reviewed text notes for patients that were readmitted to the hospital during the 5 to 8–month follow-up period to estimate participants’ 6-month cessation status.
Midway through the study in the intervention hospitals only, 76% (1,028/1,352) of targeted inpatient registered nurses and licensed practical nurses, along with 317 additional, non-targeted providers (for a grand total of 1,345 providers) were provided a 1-hour training in the Tobacco Tactics intervention. The nurses were aware of the intervention status of their hospitals. Although usual care was standard of care in the control hospitals, the intervention was standard of care in the intervention hospitals and all smokers, regardless of whether they enrolled in the study, were eligible to receive the intervention. Because there was no actual intervention in the control hospitals, the pre-intervention patients were the first half of those enrolled, and the post-intervention were the second half of those enrolled. In this way, receipt of services and quit rates for all patients were determined pre-intervention, during training, and post-intervention in both intervention and control groups. At the end of the study, nurses in the usual care control hospitals were also trained to conduct the intervention.
The Tobacco Tactics toolkit for nurses included:
1 continuing education unit contact hour for training;
a PowerPoint presentation on behavioral and pharmaceutical interventions;
a pocket card entitled “Helping Smokers Quit: A Guide for Clinicians” developed by U.S. DHHS, Public Health Service;
behavioral and pharmaceutical protocols; and
a computerized template for nurse documentation.
The Tobacco Tactics toolkit for patients included:
a brochure;
a cessation DVD;
the Tobacco Tactics manual14;
a 1–800-QUIT-NOW card;
nurse behavioral counseling and pharmaceuticals;
a physician reminder to offer brief advice to quit coupled with medication sign-off; and
follow-up phone calls.
When the nurse charted on the documentation template that the patient was given the Tobacco Tactics manual, the EMR was programmed to add the patient’s name and phone number to a list that was forwarded to Voluntary Services twice weekly. Trained volunteers provided peer telephone cessation counseling to patients at 2, 7, 14, 21, and 30 days after discharge. The telephone counseling was guided by a script and focused on behavioral support,15 which included the 3R’s (Remind, Rehearse, Reward) and the 4D’s (Delay, Deep Breathing, Drink Water, and Distract).16,17
In the Trinity Health system, all inpatients were screened for smoking on the nursing assessment. Nurses were instructed to give smokers brief advice to stop smoking. Qualitative comments from nurses indicated that smoking medications were rarely prescribed during hospitalization.
Measures
The effectiveness of the intervention was measured by two co-primary outcomes: 30-day point prevalence abstinence, self-reported quit rates (taken from either the 6-month follow-up mail or phone surveys or the EMR), and biochemical verification of smoking cessation using urinary NicAlert® tests (48-hour detection period), both collected 5–8 months post-discharge. The outcome measures were agreed upon by all investigators across the Consortium of Hospitals Advancing Research on Tobacco studies and were thought to be the most accurate and conservative.18 Covariates included demographic characteristics, discharge diagnosis and discharge comorbidities using standard ICD-9 code categories,19 the Alcohol Use Disorders Identification Test-C for alcohol use,20 self-rated functional health and well-being via the Short Form 36,21 and health status via the EuroQol-5D-5L.22
Statistical Analysis
Summary statistics based on means and SDs or frequencies and percentages were used to characterize the sample distribution for all variables. To compare differences in participant characteristics between the pre- and post-intervention time period, paired t-tests for continuous variables and McNemar’s tests for categorical variables were used. To compare the difference between sites at each time point, ANOVA for continuous variables and chi-square test for categorical variables were used.
A time by site interaction term was the focus of the multivariable model, as this term represents the effect of the intervention at sites receiving the intervention relative to the control sites, controlling for covariates. A propensity score (commonly used in effectiveness studies)23,24 was calculated, which allowed for the aggregation of multiple covariates that could not be individually included in the model given the available sample size. Key demographic characteristics, including age, sex, and race, as well as all primary diagnoses and comorbid conditions were included a priori in the propensity score. Other variables included in the propensity score were those that were statistically significantly different between:
intervention versus control sites;
pre-intervention versus post-intervention within sites; or
pre-intervention and control sites combined versus post-intervention in intervention sites only (those that did not have the propensity to receive the intervention compared with those that did not have the propensity to receive the intervention).
Based upon these criteria, the following variables (in addition to those named above) were included in the propensity score calculation: number of cigarettes smoked per day, number of times quit smoking, insurance status, use of other tobacco products, problematic alcohol use, ability to conduct self-care, ability to maintain usual activities, and self-rated health.
Generalized linear mixed models (PROC GLIMMIX) were used to estimate the association between self-reported smoking cessation and cotinine-validated cessation and intervention status, while accounting for the clustering of patients within hospital units. The predictors in the model were the propensity score, intervention site (versus control site), time (post- versus pre-implementation), and interaction between time and site. Penalized imputation (assuming subjects with missing data had not quit) was used for any subject who was missing post-intervention data. Given the fairly small number (n=34) and uneven sizes of hospital units, the Morel correction for generalized estimating equations was applied25 and the two units that had only recruited one participant were excluded. To ensure that the propensity score was used appropriately, the authors explored overlap in the distribution of the propensity scores among patients in the pre- versus post-intervention groups and whether its inclusion in the multivariable modeling decreased the Akaike information criterion fit statistic. All analyses were conducted using SAS/STAT, version 9.3.
The sample size was based on power analysis conducted with PASS 2008 software for logistic regression analyses to detect a difference of 10% in quit rates between the intervention and control sites, considering the uneven mix of sample sizes between those two groups, and then adjusted for a design effect to reflect the effect of clustering of the data within 34 hospital units. With an intraclass correlation of 0.03, the study needed 1,128 subjects to obtain 80% power. Thus, with the actual analytic sample size of 1,336, the power would be sufficient for detecting the target effect.
Results
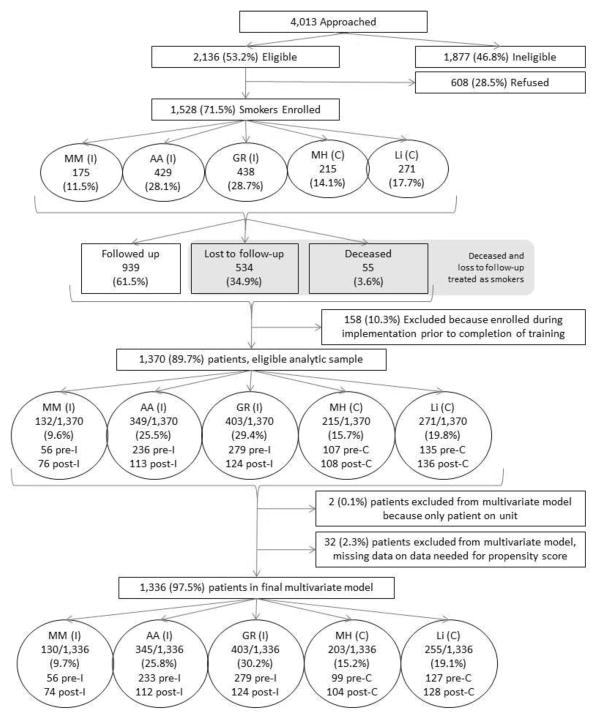
Figure 1 shows the recruitment and retention flowchart for the three intervention and two control hospitals. A total of 4,013 inpatients were approached, of which 2,136 were eligible. Of eligible patients, 1,528 were enrolled in the study between October 2011 and May 2013, resulting in a 71.5% participation rate. Of these, 158 were in the transition period and were not included in the final analyses. Of the remaining 1,370 (89.7%) enrolled participants, two were excluded from multivariable analysis because they were the only participants recruited on their unit, and 32 participants were excluded because of missing data necessary to calculate a propensity score. Thus, there were 1,336 participants in the final multivariable analyses.
Figure 1.
Recruitment and retention flowchart for intervention and control hospitals from 2011–2013 where “pre” represents pre-intervention and “post” represents post-intervention.
MM, Muskegon Mercy; AA, Ann Arbor; GR, Grand Rapids; MH, Muskegon Hackley; Li, Livonia
The patients in the intervention and control sites were followed fairly concurrently, although differences in time to IRB approval, hiring staff, recruitment rates, training nurses, among other factors, resulted in some variations among the sites. The pre-intervention periods all started within about 3 months of one another (range, October 24, 2011 to February 8, 2012) and ended within 7 months of one another (range, February 20, 2012 to September 20, 2012). The post-intervention periods all started with 5 months of one another (range, May 31, 2012 to October 26, 2012) and all ended the same month (May 7, 2013, which was the study close), except one site that ended on January 30, 2013 because they met their pre-specified quota early.
Among the five sites, 175 (11.5%) patients were enrolled at Muskegon Mercy, 429 (28.1%) at Ann Arbor, 438 (28.7%) at Grand Rapids, 215 (14.1%) at Muskegon Hackley, and 271 (17.7%) at Livonia. The first three of these hospitals were intervention sites that had 571 in the pre-intervention period and 313 in the post-intervention period. The latter two hospitals were control sites that had 242 in the pre-intervention period and 244 in the post-intervention period.
Of the enrolled participants, 534 (34.9%) were lost to follow-up, 55 (3.6%) died, and 939 (61.5%) returned follow-up surveys or had their smoking status downloaded/abstracted from the EMR. Of the entire sample of 1,528, there were 170 participants that had missing outcome data, were readmitted to the hospital during the 6-month follow-up period, and had smoking data available. Of these, 155 (92.2%) were identified as current smokers. Ten were identified as having quit for 30 days using a variable that indicated date of quit or EMR abstraction by the research nurse. The remaining five identified as quit without a quit date were imputed as quit, as four of five were either in the pre-intervention period or post-intervention control; this was thought to be more accurate than classifying them as smokers, especially because it biased the results to the null.
Overall, non-responders were more likely to be male (p<0.001), employed (p<0.01), have a primary diagnosis of mental disorder (p=0.001), have a shorter length of stay (p<0.05), and have poorer self-rated health (p<0.05).
The average age of patients was 47.9 (SD=14.7) years, 49% were male, 77% were white, and 68% were unmarried. Less than half completed some college or higher (43%), and only 29% were currently employed. Participants smoked an average of 15.4 (SD=12.0) cigarettes per day, had quit an average of 3.4 (SD=18.5) times prior to the research study, and approximately 10% used other tobacco products. The most common discharge diagnoses were diseases of the digestive system (13.1%), diseases of the circulatory system (13.1%), injury and poisoning (9.9%), and diseases of the respiratory system (8.9%). The most common comorbidities were endocrine, nutritional, and metabolic diseases and immunity disorders (60.2%); diseases of the circulatory system (53.3%); mental disorders (50.4%); diseases of the digestive system (35.5%); symptoms, signs, and ill-defined conditions (35.4%); and diseases of the respiratory system (34.3%). There were various differences among the five hospitals and also pre- and post-intervention patients within the hospitals; these variables were among those added to the propensity score (Table 1).
Table 1.
Bivariate Significance Tests of Covariates by Site and Intervention Status (N=1,370)
| Muskegon Mercy (N=132) |
Ann Arbor (N=349) |
Grand Rapids (N=403) |
Muskegon Hackley (N=215) |
Livonia (N=271) | Site main effect p- value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Pre- interve ntion |
Post- interve ntion |
Pre- interve ntion |
Post- interve ntion |
Pre- intervent ion |
Post- interve ntion |
Pre- intervent ion |
Post- intervent ion |
Pre- intervent ion |
Post- interventi on |
||
|
| |||||||||||
| n=56 | n=76 | n=236 | n=113 | n=279 | n=124 | n=107 | n=108 | n=135 | n=136 | ||
| Age | |||||||||||
| Mean (SD) | 49.5 (12.9) | 52.1 (12.8) | 53.3 (12.4) | 54.3 (12) | 49.4 (13.3) | 49.4 (13) | 39.4 (15.7) | 36.3 (13.8) | 43.9 (16.4) | 45.8 (14.9) | |
| Time p- valuea | 0.245 | 0.486 | 0.988 | 0.131 | 0.322 | <0.001 *** | |||||
| Mean # cigarettes/day | |||||||||||
| Mean (SD) | 15.1 (10.7) | 13.4 (7.5) | 16.7 (8.9) | 15.5 (9.3) | 14.5 (13.4) | 11.8 (8.2) | 16.9 (16.2) | 14.2 (13.2) | 16.7 (12.9) | 17.8 (14.6) | |
| Time p- value | 0.275 | 0.282 | 0.013* | 0.179 | 0.534 | 0.001*** | |||||
| Number of past quit attempts | |||||||||||
| Mean (SD) | 7.6 (40.0) | 3.3 (4.4) | 1.4 (3.8) | 0.6 (0.8) | 4.7 (21.3) | 1.6 (2.6) | 2.1 (3.3) | 2.3 (3.6) | 6.3 (32.6) | 5.7 (26.7) | |
| Time p- value | 0.350 | 0.001*** | 0.016* | 0.762 | 0.865 | 0.014* | |||||
| Other tobacco use | 9 (16.1) | 4 (5.3) | 20 (8.5) | 3 (2.7) | 34 (12.2) | 7 (5.6) | 15 (14.0) | 5 (4.6) | 21 (15.6) | 19 (14.2) | |
| Time p- value | 0.073 | 0.040* | 0.045* | 0.018* | 0.751 | 0.020* | |||||
| Sex | |||||||||||
| Male | 26 (46.4% ) | 41 (53.9% ) | 121 (51.3% ) | 46 (40.7% ) | 159 (57.0%) | 58 (46.8% ) | 36 (33.6%) | 29 (26.9%) | 82 (60.7%) | 69 (50.7%) | |
| Time p- value | 0.380 | 0.065 | 0.058 | 0.278 | 0.097 | <0.001 *** | |||||
| Race | |||||||||||
| White | 41 (73.2% ) | 57 (75%) | 197 (83.5% ) | 91 (80.5% ) | 207 (74.2%) | 76 (61.3% ) | 74 (69.2%) | 83 (76.9%) | 116 (85.9%) | 109 (80.1%) | |
| Time p- value | 0.817 | 0.498 | 0.009** | 0.204 | 0.205 | <0.001 *** | |||||
| Marital status | |||||||||||
| Married/Domestic Partner | 25 (44.6% ) | 22 (28.9% ) | 91 (38.6% ) | 49 (43.8% ) | 88 (31.5%) | 27 (21.8% ) | 30 (28.3%) | 34 (31.5%) | 26 (19.3%) | 43 (31.6%) | |
| Time p- value | 0.063 | 0.356 | 0.045* | 0.611 | 0.019* | 0.001*** | |||||
| Education status | |||||||||||
| High school or less | 32 (57.1% ) | 47 (61.8% ) | 131 (55.7% ) | 55 (48.7% ) | 163 (58.4%) | 79 (63.7% ) | 72 (68.6%) | 61 (57%) | 67 (51.5%) | 58 (43.6%) | |
| Time p- value | 0.586 | 0.216 | 0.317 | 0.082 | 0.198 | 0.003* | |||||
| Employment status | |||||||||||
| Employed | 17 (30.4% ) | 15 (19.7% ) | 67 (28.4% ) | 29 (25.7% ) | 87 (31.2%) | 35 (28.2% ) | 24 (22.4%) | 34 (31.8%) | 39 (29.3%) | 46 (34.3%) | |
| Time p- value | 0.159 | 0.593 | 0.551 | 0.124 | 0.380 | 0.471 | |||||
| Problematic alcohol use | |||||||||||
| 25 (44.6) | 25 (33.3) | 64 (27.2) | 21 (18.6) | 113 (40.5) | 38 (30.6) | 16 (15.2) | 38 (35.8) | 63 (47.4) | 64 (48.1) | ||
| Time p- value | 0.187 | 0.079 | 0.059 | 0.001*** | 0.902 | <0.001 *** | |||||
| Primary insurance | <0.001 *** | ||||||||||
| Self- pay/None | 3 (5.4%) | 15 (19.7% ) | 38 (16.1% ) | 18 (15.9% ) | 56 (20.1%) | 27 (21.8% ) | 18 (16.8%) | 16 (14.8%) | 28 (20.7%) | 15 (11.0%) | |
| Medicare | 19 (33.9% ) | 36 (47.4% ) | 97 (41.1% ) | 42 (37.2% ) | 87 (31.2%) | 34 (27.4% ) | 28 (26.2%) | 11 (10.2%) | 48 (35.6%) | 54 (39.7%) | |
| Medicaid | 13 (23.2% ) | 6 (7.9%) | 23 (9.8%) | 7 (6.2%) | 45 (16.1%) | 35 (28.2% ) | 42 (39.3%) | 42 (38.9%) | 9 (6.7%) | 10 (7.4%) | |
| VA | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | |
| Other public | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 6 (5.31% ) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 10 (9.3%) | 4 (3.0%) | 5 (3.7%) | |
| Private | 21 (37.5% ) | 15 (19.7% ) | 78 (33.1% ) | 40 (35.4% ) | 91 (32.6%) | 28 (22.6% ) | 19 (17.8%) | 29 (26.9%) | 46 (34.1%) | 51 (37.5%) | |
| Time p- value | 0.001*** | 0.007** | 0.021* | 0.001*** | 0.34 | ||||||
| Primary discharge diagnosis | |||||||||||
| Infectious diseasesc | 1 (1.8%) | 8 (10.5% ) | 7 (3%) | 5 (4.4%) | 31 (11.1%) | 25 (20.2% ) | 7 (6.5%) | 6 (5.6%) | 0 (0.0%) | 6 (4.4%) | |
| Time p- value | 0.078 | 0.535 | 0.015* | 0.762 | 0.030* | <0.001 *** | |||||
| Neoplasmsd | 0 (0.0%) | 0 (0.0%) | 12 (5.1%) | 10 (8.8%) | 11 (3.9%) | 3 (2.4%) | 5 (4.7%) | 2 (1.9%) | 0 (0.0%) | 2 (1.5%) | |
| Time p- value | 1.000 | 0.176 | 0.564 | 0.280 | 0.498 | <0.001 *** | |||||
| Endocrine disorderse | 3 (5.4%) | 2 (2.6%) | 14 (5.9%) | 5 (4.4%) | 15 (5.4%) | 12 (9.7%) | 1 (0.9%) | 8 (7.4%) | 3 (2.2%) | 3 (2.2%) | |
| Time p- value | 0.650 | 0.561 | 0.111 | 0.035* | 1.000 | 0.095 | |||||
| Diseases of the bloodf | 1 (1.8%) | 0 (0.0%) | 1 (0.4%) | 2 (1.8%) | 3 (1.1%) | 1 (0.8%) | 0 (0.0%) | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) | |
| Time p- value | 0.424 | 0.246 | 1.000 | 1.000 | 1.000 | 0.583 | |||||
| Mental disordersg | 0 (0.0%) | 1 (1.3%) | 8 (3.4%) | 1 (0.9%) | 8 (2.9%) | 2 (1.6%) | 0 (0.0%) | 0 (0.0%) | 36 (26.7%) | 40 (29.4%) | |
| Time p- value | 1.000 | 0.281 | 0.421 | 1.000 | 0.615 | <0.001 *** | |||||
| Diseases of the nervous systemh | 2 (3.6%) | 0 (0.0%) | 13 (5.5%) | 6 (5.3%) | 7 (2.5%) | 4 (3.2%) | 5 (4.7%) | 0 (0.0%) | 2 (1.5%) | 2 (1.5%) | |
| Time p- value | 0.178 | 0.939 | 0.743 | 0.029* | 1.000 | 0.029* | |||||
| Diseases of the circulatory systemi | 9 (16.1% ) | 12 (15.8% ) | 39 (16.5% ) | 22 (19.5% ) | 43 (15.4%) | 20 (16.1% ) | 11 (10.3%) | 7 (6.5%) | 5 (3.7%) | 11 (8.1%) | |
| Time p- value | 0.965 | 0.498 | 0.855 | 0.315 | 0.126 | <0.001 *** | |||||
| Diseases of the respiratory systemj | 4 (7.1%) | 7 (9.2%) | 26 (11%) | 14 (12.4% ) | 29 (10.4%) | 11 (8.9%) | 6 (5.6%) | 4 (3.7%) | 10 (7.4%) | 11 (8.1%) | |
| Time p- value | 0.759 | 0.706 | 0.637 | 0.538 | 0.834 | 0.071 | |||||
| Diseases of the digestive systemk | 12 (21.4% ) | 15 (19.7% ) | 32 (13.6% ) | 10 (8.8%) | 47 (16.8%) | 17 (13.7% ) | 11 (10.3%) | 14 (13%) | 5 (3.7%) | 17 (12.5%) | |
| Time p- value | 0.812 | 0.206 | 0.427 | 0.540 | 0.008** | 0.004** | |||||
| Diseases of the genitourinary systeml | 0 (0.0%) | 2 (2.6%) | 12 (5.1%) | 4 (3.5%) | 12 (4.3%) | 6 (4.8%) | 6 (5.6%) | 3 (2.8%) | 4 (3.0%) | 1 (0.7%) | 0.197 |
| Time p- value | 0.508 | 0.518 | 0.809 | 0.332 | 0.214 | ||||||
| Perinatal diseasem | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.9%) | 1 (0.4%) | 0 (0.0%) | 29 (27.1%) | 43 (39.8%) | 1 (0.7%) | 2 (1.5%) | |
| Time p- value | 1.000 | 0.324 | 1.000 | 0.048* | 1.000 | <0.001 *** | |||||
| Diseases of the skinn | 1 (1.8%) | 2 (2.6%) | 9 (3.8%) | 5 (4.4%) | 8 (2.9%) | 7 (5.6%) | 2 (1.9%) | 1 (0.9%) | 10 (7.4%) | 11 (8.1%) | |
| Time p- value | 1.000 | 1.000 | 0.252 | 0.621 | 0.834 | 0.006** | |||||
| Diseases of the musculos keletal systemo | 13 (23.2% ) | 15 (19.7% ) | 20 (8.5%) | 11 (9.7%) | 10 (3.6%) | 1 (0.8%) | 3 (2.8%) | 2 (1.9%) | 13 (9.6%) | 7 (5.1%) | |
| Time p- value | 0.629 | 0.699 | 0.184 | 0.683 | 0.158 | <0.001 *** | |||||
| Congenital anomalies p | 3 (5.4%) | 6 (7.9%) | 11 (4.7%) | 4 (3.5%) | 21 (7.5%) | 3 (2.4%) | 6 (5.6%) | 3 (2.8%) | 38 (28.1%) | 12 (8.8%) | |
| Time p- value | 0.733 | 0.782 | 0.046* | 0.332 | <0.001*** | <0.001 *** | |||||
| Injury and poisonings | 7 (12.5% ) | 5 (6.6%) | 30 (12.7% ) | 12 (10.6% ) | 31 (11.1%) | 12 (9.7%) | 13 (12.1%) | 11 (10.2%) | 6 (4.4%) | 8 (5.9%) | |
| Time p- value | 0.242 | 0.574 | 0.667 | 0.647 | 0.593 | 0.052 | |||||
| Supplementary classification factors influencin health status and contact with health services | 0 (0.0%) | 1 (1.3%) | 2 (0.8%) | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) | 2 (1.9%) | 1 (0.9%) | 2 (1.5%) | 2 (1.5%) | |
| Time p- value | 1.000 | 1.000 | 1.000 | 0.621 | 1.000 | 0.217 | |||||
| Comorbidities (Secondary discharge diagnoses) | |||||||||||
| Infectious diseasesc | 9 (16.1% ) | 10 (13.2% ) | 26 (11.0% ) | 14 (12.4% ) | 39 (14.0%) | 29 (23.4% ) | 12 (11.2%) | 11 (10.2%) | 22 (16.3%) | 19 (14.0%) | |
| Time p- value | 0.637 | 0.706 | 0.020 | 0.807 | 0.593 | 0.141 | |||||
| Neoplasmsd | 5 (8.9%) | 5 (6.6%) | 9 (3.8%) | 6 (5.3%) | 21 (7.5%) | 6 (4.8%) | 3 (2.8%) | 5 (4.6%) | 3 (2.2%) | 3 (2.2%) | |
| Time p- value | 0.743 | 0.576 | 0.319 | 0.721 | 1.000 | 0.039* | |||||
| Endocrine disorderse | 37 (66.1% ) | 53 (69.8% ) | 136 (57.6% ) | 76 (67.3% ) | 188 (67.4%) | 90 (72.6% ) | 54 (50.5%) | 48 (44.4%) | 74 (54.8%) | 69 (50.7%) | |
| Time p- value | 0.655 | 0.085 | 0.298 | 0.377 | 0.501 | <0.001 *** | |||||
| Diseases of the bloodf | 11 (19.6% ) | 17 (22.4% ) | 50 (21.2% ) | 26 (23.0% ) | 88 (31.5%) | 34 (27.4% ) | 26 (24.3%) | 17 (15.7%) | 26 (19.3%) | 20 (14.7%) | |
| Time p- value | 0.705 | 0.700 | 0.406 | 0.117 | 0.318 | 0.001*** | |||||
| Mental disordersg | 21 (37.5% ) | 27 (35.5% ) | 110 (46.6% ) | 47 (41.6% ) | 156 (55.9%) | 71 (57.3% ) | 47 (43.9%) | 45 (41.7%) | 51 (37.8%) | 53 (39.0%) | |
| Time p- value | 0.816 | 0.378 | 0.802 | 0.738 | 0.840 | <0.001 *** | |||||
| Disease of the nervous systemh | 14 (25.0% ) | 23 (30.3% ) | 70 (29.7% ) | 32 (28.3% ) | 81 (29.0%) | 48 (38.7% ) | 26 (24.3%) | 25 (23.1%) | 43 (31.9%) | 32 (23.5%) | |
| Time p- value | 0.506 | 0.796 | 0.055 | 0.843 | 0.126 | 0.290 | |||||
| Diseases of the circulatory systemi | 35 (62.5% ) | 47 (61.8% ) | 153 (64.8% ) | 63 (55.8% ) | 172 (61.6%) | 77 (62.1% ) | 35 (32.7%) | 21 (19.4%) | 63 (46.7%) | 64 (47.1%) | |
| Time p- value | 0.939 | 0.102 | 0.932 | 0.027* | 0.948 | <0.001 *** | |||||
| Diseases of the respiratory systemj | 19 (33.9% ) | 30 (39.5% ) | 74 (31.4% ) | 42 (37.2% ) | 115 (41.2%) | 63 (50.8% ) | 31 (29.0%) | 23 (21.3%) | 40 (29.6%) | 33 (24.3%) | |
| Time p- value | 0.515 | 0.281 | 0.074 | 0.194 | 0.320 | <0.001 *** | |||||
| Diseases of the digestive systemk | 21 (37.5% ) | 22 (28.9% ) | 92 (39.0% ) | 45 (39.8% ) | 120 (43.0%) | 50 (40.3% ) | 31 (29.0%) | 22 (20.4%) | 38 (28.1%) | 45 (33.1%) | |
| Time p- value | 0.300 | 0.880 | 0.614 | 0.143 | 0.378 | <0.001 *** | |||||
| Diseases of the genitourinary systeml | 12 (21.4% ) | 24 (31.6% ) | 41 (17.4% ) | 25 (22.1% ) | 77 (27.6%) | 35 (28.2% ) | 21 (19.6%) | 9 (8.3%) | 29 (21.5%) | 23 (16.9%) | |
| Time p- value | 0.196 | 0.289 | 0.897 | 0.017* | 0.339 | <0.001 *** | |||||
| Perinatal diseasesm | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.4%) | 1 (0.8%) | 27 (25.2%) | 43 (39.8%) | 1 (0.7%) | 2 (1.5%) | |
| Time p- value | n/a | n/a | 0.521 | 0.023* | 1.000 | <0.001 *** | |||||
| Diseases of the skinn | 6 (10.7% ) | 7 (9.2%) | 24 (10.2% ) | 8 (7.1%) | 21 (7.5%) | 6 (4.8%) | 5 (4.7%) | 9 (8.3%) | 12 (8.9%) | 15 (11.0%) | |
| Time p- value | 0.774 | 0.349 | 0.319 | 0.277 | 0.556 | 0.403 | |||||
| Diseases of the musculos keletal systemo | 17 (30.4% ) | 32 (42.1% ) | 66 (28.0% ) | 27 (23.9% ) | 56 (20.1%) | 34 (27.4% ) | 22 (20.6%) | 10 (9.3%) | 38 (28.1%) | 31 (22.8%) | |
| Time p- value | 0.167 | 0.421 | 0.102 | 0.020* | 0.312 | <0.001 *** | |||||
| Congenital anomalies p | 1 (1.8%) | 0 (0.0%) | 2 (0.8%) | 1 (0.9%) | 3 (1.1%) | 1 (0.8%) | 1 (0.9%) | 2 (1.9%) | 2 (1.5%) | 0 (0.0%) | |
| Time p- value | 0.424 | 1.000 | 1.000 | 1.000 | 0.247 | 0.955 | |||||
| Ill-defined conditions r | 19 (33.9% ) | 26 (34.2% ) | 84 (35.6% ) | 29 (25.7% ) | 127 (45.5%) | 52 (41.9% ) | 33 (30.8%) | 27 (25.0%) | 44 (32.6%) | 44 (32.4%) | |
| Time p- value | 0.973 | 0.064 | 0.504 | 0.340 | 0.966 | <0.001 *** | |||||
| Injury and poisonings | 9 (16.1% ) | 13 (17.1% ) | 30 (12.7% ) | 20 (17.7% ) | 68 (24.4%) | 39 (31.5% ) | 22 (20.6%) | 10 (9.3%) | 24 (17.8%) | 19 (14.0%) | |
| Time p- value | 0.875 | 0.213 | 0.137 | 0.020* | 0.391 | <0.001 *** | |||||
| Comorbid supplementary classification factors influencing health status and contact with health services | 32 (57.1% ) | 40 (52.6% ) | 179 (75.8% ) | 92 (81.4% ) | 193 (69.2%) | 81 (65.3% ) | 65 (60.7%) | 81 (75.0%) | 9 (6.7%) | 73 (53.7%) | <0.001 *** |
| Time p- value | 0.607 | 0.243 | 0.444 | 0.025* | <0.001*** | ||||||
| Self-rated health status | |||||||||||
| Excellent | 2 (3.6%) | 1 (1.3%) | 6 (2.5%) | 1 (0.9%) | 3 (1.1%) | 1 (0.8%) | 8 (7.5%) | 7 (6.5%) | 5 (3.7%) | 4 (2.9%) | |
| Very good | 3 (5.4%) | 4 (5.3%) | 16 (6.8%) | 6 (5.3%) | 24 (8.6%) | 6 (4.8%) | 25 (23.4%) | 22 (20.4%) | 14 (10.4%) | 14 (10.3%) | |
| Good | 9 (16.1% ) | 32 (42.7% ) | 79 (33.5% ) | 33 (29.2% ) | 88 (31.5%) | 35 (28.2% ) | 32 (29.9%) | 46 (42.6%) | 47 (35.1%) | 53 (39%) | |
| Fair | 22 (39.3% ) | 21 (28.0% ) | 76 (32.2% ) | 44 (38.9% ) | 96 (34.4%) | 51 (41.1% ) | 22 (20.6%) | 27 (25%) | 43 (32.1%) | 41 (30.1%) | |
| Poor | 20 (35.7% ) | 17 (22.7% ) | 59 (25%) | 29 (25.7% ) | 68 (24.4%) | 31 (25.0% ) | 20 (18.7%) | 6 (5.6%) | 25 (18.7%) | 24 (17.6%) | |
| Time p- value | 0.060 | 0.598 | 0.544 | 0.029* | 0.971 | <0.001 *** | |||||
| Self-care activities | |||||||||||
| No problem | 29 (51.8% ) | 46 (60.5% ) | 144 (61.0% ) | 84 (74.3%) | 191 (68.5% ) | 88 (71.0%) | 71 (664%) | 82 (75.9%) | 90 (69.2%) | 95 (71.4% ) | |
| Slight Problem | 12 (21.4% ) | 15 (19.7% ) | 33 (14.0% ) | 13 (11.5%) | 47 (16.9% ) | 16 (12.9%) | 19 (17.8%) | 16 (14.8%) | 24 (18.5%) | 17 (12.8% ) | |
| Moderate Problem | 7 (12.5% ) | 6 (7.9%) | 34 (14.4% ) | 5 (4.4%) | 24 (8.6%) | 6 (4.8%) | 6 (5.6%) | 7 (6.5%) | 12 (9.2%) | 12 (9.0%) | |
| Severe Problem | 8 (14.3% ) | 6 (7.9%) | 21 (8.9%) | 11 (9.7%) | 5 (1.8%) | 6 (4.8%) | 6 (5.6%) | 3 (2.8%) | 3 (2.3%) | 5 (3.8%) | |
| Unable | 0 (0.0%) | 3 (4.0%) | 4 (1.7%) | 0 (0.0%) | 12 (4.3%) | 8 (6.5%) | 5 (4.7%) | 0 (0.0%) | 1 (0.8%) | 4 (3.0%) | <0.001*** |
| Time p- value | 0.328 | 0.024* | 0.179 | 0.130 | 0.463 | ||||||
| Usual activities | |||||||||||
| No problems | 14 (25.0% ) | 25 (32.9% ) | 114 (48.3% ) | 52 (46.0%) | 121 (43.4% ) | 55 (44.4%) | 45 (42.1%) | 54 (50.0%) | 54 (40.6%) | 66 (49.3% ) | |
| Slight Problem | 17 (30.4% ) | 18 (23.7% ) | 49 (20.8% ) | 36 (31.9%) | 64 (22.9% ) | 14 (11.3%) | 27 (25.2%) | 27 (25.0%) | 30 (22.6%) | 18 (13.4% ) | |
| Moderate problem | 9 (16.1% ) | 15 (19.7% ) | 47 (19.9% ) | 17 (15.0%) | 32 (11.5% ) | 6 (4.8%) | 16 (15.0%) | 12 (11.1%) | 28 (21.1%) | 21 (15.7% ) | |
| Severe Problem | 9 (16.1% ) | 12 (15.8% ) | 24 (10.2% ) | 8 (7.1%) | 11 (3.9%) | 6 (4.8%) | 7 (6.5%) | 12 (11.1%) | 12 (9.0%) | 12 (9.0%) | |
| Unable | 7 (12.5% ) | 6 (7.9%) | 2 (0.9%) | 0 (0.0%) | 51 (18.3% ) | 43 (34.7%) | 12 (11.2%) | 3 (2.8%) | 9 (6.8%) | 17 (12.7% ) | <0.001*** |
| Time p- value | 0.707 | 0.153 | <0.001*** | 0.088 | 0.105 | ||||||
Note: Boldface indicates statistical significance (*p<0.05, **p<0.01, ***p<0.001)
Time p-value represents pre- post-intervention time period differences within site.
Two-item Fagerström Test for Nicotine Dependence (FTND) scores can range from 0 to 6, with a score >4 considered nicotine dependent.
Infectious diseases (ICD9 codes 001–139)
Neoplasms (ICD9 codes 140–239)
Endocrine disorders (ICD9 codes 240–279)
Diseases of the blood (ICD9 codes 280–289)
Mental disorders (ICD9 codes 290–319)
Diseases of the nervous system (ICD9 codes 320–389)
Diseases of the circulatory system (ICD9 codes 390–459)
Diseases of the respiratory system (ICD9 codes 460–519)
Diseases of the digestive system (ICD9 codes 520–579)
Diseases of the genitourinary system (ICD9 codes 580–629)
Perinatal diseases (ICD9 codes 630–679)
Diseases of the skin (ICD9 codes 680–709)
Diseases of the musculoskeletal system (ICD9 codes 710–739)
Congenital anomalies (ICD9 codes 740–759)
Ill-defined conditions (ICD9 codes 780–799)
Injury and poisoning (ICD9 codes 800–999)
The propensity-adjusted quit rates by site and time period are shown in Table 2 and were very similar to the unadjusted quit rates (data not shown). When combining together all of the intervention sites, there was a significant increase in pre- to post-intervention 6-month self-reported quit rates (5.7% vs 16.5%, p<0.001), whereas there was no significant change in the control group (4.3% vs 5.3%, p=0.688). Cotinine tests were sent to 1,372 participants, but only 448 (33%) were returned, as patients commented on follow-up phone calls that they were “turned off” by the urinary cotinine strip. Nonetheless, the tests also showed significantly higher pre- to post-intervention quit rates in the intervention sites (4.3% vs 8.0%, p=0.037) compared with the control sites (1.8% vs 2.5%, p=0.683).
Table 2.
Propensity Score Adjusted Quit Rates by Site and Pre- Versus Post-Intervention Time Point
| Overall self-reported quit rates (N=1,336) | Intervention sites N=878 | Control sites N=458 | |||
|---|---|---|---|---|---|
| Pre-intervention (N=794) | 5.7% | 4.3% | |||
| Post-intervention (N=542) | 16.5% | 5.3% | |||
| p-value | <0.001*** | 0.688 | |||
| Cotinine-verified quit rate (N=1,181)* | N=776 | N=405 | |||
| Pre-intervention (N=794) | 4.3% | 1.8% | |||
| Post-intervention (N=387) | 8.0% | 2.5% | |||
| p-value | 0.037** | 0.683 | |||
| Muskegon Mercy N=130 | Ann Arbor N=345 | Grand Rapids N=403 | Muskegon Hackley N=203 | Livonia N=255 | |
| Self-reported quit rate (N=1,336) | |||||
| Pre-intervention (N=794) | 6.5% | 6.8% | 5.4% | 5.0% | 3.5% |
| Post-intervention (N=542) | 14.8% | 14.2% | 21.6% | 6.9% | 3.5% |
| p-value | 0.126 | 0.032** | <0.001* | 0.619 | 0.997 |
| Muskegon Mercy N=111 | Ann Arbor N=306 | Grand Rapids N=359 | Muskegon Hackley N=180 | Livonia N=225 | |
| Cotinine-verified quit rate (N=1,181) | |||||
| Pre-intervention (N=794) | 6.6% | 5.5% | 3.4% | 3.5% | 0.38% |
| Post-intervention (N=387) | 8.7% | 7.1% | 10.2% | 5.7% | 0.0% |
| p-value | 0.59 | 0.52 | 0.029** | 0.503 | 0.64 |
In terms of hospital-specific quit rates, there were significant improvements in pre- to post-intervention self-reported quit rates in the Ann Arbor (6.8% vs 14.2%, p=0.032) and Grand Rapids (5.4% vs 21.6%, p<0.001) intervention sites, while results for the Muskegon Mercy smallest intervention site were in the expected direction (6.5% vs 14.8%, p=0.126). There were no significant changes in the self-reported quit rates in the control sites. The only increase in cotinine-verified quit rates were in the Grand Rapids largest intervention site (3.4% vs 10.2%, p=0.029).
Overall, the 448 cotinine tests had a sensitivity of 96% and a specificity of 86%, compared with self-reported 7-day point prevalence abstinence. Excluding the 147 cotinine tests returned in the transition period, there were no significant differences in cotinine strip return rates between quitters and non-quitters and no differential misreporting pre- versus post-intervention or intervention versus control arm. Cotinine tests showed that 3.5% (7/200) and 4.0% (4/101) misreported quitting in the pre- versus post-intervention periods, respectively, and 3.6% (7/193) and 3.7% (4/108) misreported quitting in the intervention versus control arms, respectively.
Analyses demonstrated considerable overlap in propensity score among those who did and did not receive the intervention. The propensity score improved the model fit (Akaike information criterion, 7418.11) in models without the propensity score as compared with (Akaike information criterion, 7218.40) models with the propensity score. Thus, the propensity score was retained in the final models.
As shown in Table 3, the propensity-adjusted multivariable analyses showed that there was significant improvement in self-reported 6-month quit rates from the pre- to post-intervention time periods in the intervention sites as compared with the control sites (p=0.04). There was a non-significant improvement in cotinine-verified 6-month quit rate; the magnitude of the effect of the intervention time by site term was only about half of that for the self-reported model.
Table 3.
Propensity-Adjusted GEE Models of Smoking Cessation Including Interaction of Intervention Status by Time Perioda
| Self-reported 6-month cessation (N=1,336) | Cotinine-verified 6-month cessation (N=1,181) | |||
|---|---|---|---|---|
|
| ||||
| Beta (SE) | p-value | Beta (SE) | p-value | |
| Intercept | −2.82 (0.34) | <0.0001 | −3.63 (0.69) | <0.0001 |
| Intervention sites (versus Control sites) | −0.10 (0.45) | 0.82 | 0.32 (0.73) | 0.66 |
| Post-intervention time period (versus pre-intervention) | 0.16 (0.34) | 0.65 | 0.24 (0.91) | 0.79 |
| Intervention sites X Post-intervention time period | 0.90 (0.43) | 0.04* | 0.46 (1.00) | 0.65 |
Note: Boldface indicates statistical significance (*p<0.05)
Propensity score includes: age, sex, race, number of cigarettes per day, number of times quit smoking, insurance status, use of other tobacco, problematic alcohol use, ability to conduct self-care, ability to maintain usual activities, self-rated health, primary diagnosis, and comorbid health conditions.
Discussion
This study is one of the few pragmatic trials that have evaluated the effectiveness of a smoking-cessation intervention for inpatient smokers in real-world hospital settings using real-world providers, primarily nurses. Both the intervention and usual care quit rates were slightly lower than other studies,26–30 where the ranges were 26%–41% for the intervention groups compared with 9%–20% in the usual care group across all studies. The reason for the lower rates in both groups in this study versus other published studies may be because the other studies were mostly (but not all) RCTs conducted in large academic centers and one was with cardiac patients known to have higher quit rates,31 whereas this study was a pragmatic trial conducted in community hospitals that included all smokers regardless of motivation to quit. In all the studies, including this one, quit rates nearly doubled in the intervention site and in some cases tripled. The Grand Rapids intervention site did particularly well, and although there were no noted differences in fidelity across sites, the Grand Rapids site did open the training up to outpatient nurses, which may have provided additional follow-up for some patients.
The Tobacco Tactics intervention was found to be effective and meets the fairly new Joint Commission standards, which apply to all inpatient smokers and include tobacco use screening, treatment in the hospital, treatment at discharge, and follow-up telephone contact.32 Though this study trained staff nurses to integrate smoking-cessation services into their routine care, other inpatient studies have used dedicated cessation counselors to provide services.29,33 In the authors’ experience, it is unlikely that hospitals will hire dedicated smoking-cessation counselors.
Another way to employ dedicated smoking-cessation counselors is referral to telephone quit lines (albeit referral alone may not meet Joint Commission standards). Telephone quit lines have been shown to be highly effective, but reach only 6%–10% of smokers, primarily those most motivated to quit.5 The Tobacco Tactics intervention was offered to all inpatient smokers regardless of motivation to quit. Proactive outreach to smokers has been shown to improve quit rates.34
Nurses, the largest group of frontline providers, have the potential to reach a large number of captive inpatient smokers. Nurses have rapport with patients and can relate the patient’s smoking to their medical condition. In addition, nurses are educated in health education and can relate the patient’s smoking behavior to their medical comorbidities, thereby enhancing motivation to quit. Moreover, nurses can work with physicians to initiate medications and write nursing notes.
Smokers have an increased risk of morbidity and mortality, resulting in more hospital stays, longer hospital stays, and greater expenses per admission than non-smokers.35 A large number of participants had diseases of the circulatory and respiratory system, which are often smoking-related. Patients who have experienced health-related consequences from smoking have an increased interest in smoking cessation, especially within the first year of diagnosis.36 When a patient experiences a life-altering event related to their comorbidities, they may be frightened and willing to make changes in their life to prevent future reoccurrences.37
About half of the participants had mental health comorbidities and those with mental health comorbidities commonly smoke more heavily, have higher nicotine dependence, experience more withdrawal symptoms, and have lower quit rates than those without mental health disorders.38 Smoking rates have been shown to vary by demographic groups and the sample was fairly well represented with equal numbers of men and women, one-quarter being non-white, and more than half having a high school education or less. Future papers from this study will evaluate the effects of the intervention on selected diagnostic and demographic subgroups enrolled in the study.
Limitations
Though real-world, non-randomized, effectiveness trials are more feasible and allow for implementation in real-world environments, the lack of randomization at the patient level may make interpretation of the results more difficult. Although data from one of the control hospitals were not useable, the patient sample size was large enough to allow clinically and statistically meaningful comparisons with the intervention hospitals. The research assistants and research nurse were not blinded, which could potentially introduce bias. The participation rate was high (71.5%), and although the follow-up rate was somewhat lower at 61.5%, the follow-up rate was similar to other inpatient smoking-cessation trials.39,40 Classifying surviving non-responders as smokers is common in smoking studies, but may bias the results.41 The lack of significant changes in the cotinine-verified quit rates in the multivariable model was likely due to the low return rate of cotinine strips, and subsequent potential overestimation of non-quitting in the case of unreturned strips.
Conclusions
This study showed that there were significant improvements in 6-month quit rates from the pre- to post-intervention time periods in the intervention sites compared with the control sites. Although hospitalization itself can be a catalyst for quitting, once trained, nurses are capable of improving quit rates beyond usual care. The Tobacco Tactics intervention meets the Joint Commission standards for treatment of inpatient smokers.
Acknowledgments
A special acknowledgement goes to co-author, David Ronis, who was the methodological mentor to Sonia Duffy as she developed the Tobacco Tactics intervention over the years, provided statistical analyses for the vast majority of papers related to the Tobacco tactics intervention, and directed the complicated propensity adjusted analyses for this paper; unfortunately he passed away unexpectedly and did not to see this publication to print.
This work has been greatly enhanced by the invaluable expertise of the personnel from all of the Consortium of Hospitals Advancing Research on Tobacco research sites as well as William Riley, PhD, Catherine (Kate) Stoney, PhD, and other NIH personnel. The authors would like to express our heartfelt appreciation to the nurses and other staff who included the intervention in their already busy work schedules. The study could not have been accomplished without the hard work of our research staff and staff from the Trinity Health System, including Elizabeth Murphy, Sue Klotz, Lucinda Wilkinson, Rebecca Masters, Rita Ferguson, Sonya Hilbrand, Danielle Poliski, John Ryan Scott, Julia Ferry, Elizabeth Tyrpak, Jennessa Rooker, Cody Carey, Tina Maxbauer, Seung Hee Choi, Samantha Louzon, and Andrea Waltje. Lastly, we would like to thank the patients who participated in this study. Permission has been obtained by the corresponding author from all people named in the acknowledgments.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or NIH.
The sponsor National Heart, Lung, and Blood Institute (NIH) had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
The project described was supported by Award Number UO1HL105218 from the National Heart, Lung, and Blood Institute and approved by the following IRBs: The University of Michigan, January 26, 2011; Saint Mary’s Health Care, September 27, 2011; Saint Joseph Mercy Health Care System, November 16, 2011; and Mercy Health, September 14, 2011. SD conceptualized the study, implemented the study, participated in analysis, and drafted the manuscript. DR provided oversight of data collection and integrity and directed data analysis. CK conducted the propensity-adjusted analyses. LE served as the study project manager. SH conducted univariate and bivariate data analysis. JY provided specialized consultation for the propensity-adjusted analyses. PT provided oversight in the implementation of the study in the Trinity Health system. CW provided oversight in the implementation of the study in the St. Mary’s Grand Rapids site. KM provided oversight in the implementation of the study at the Mercy Health Muskegon site. LF provided oversight in the implementation of the study at the St. Joseph Mercy Ann Arbor site. DG provided oversight in the implementation of the study at the St. Mary’s Livonia site. NJ assisted with the writing of the paper. All authors read and approved the manuscript.
Trial registration: Dissemination of Tobacco Tactics for Hospitalized Smokers (NCT01309217).
Footnotes
The article contents were previously presented at the Society for Nicotine and Tobacco Research 21st Annual Meeting in Philadelphia, PA that was held on February 25–28, 2015.
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rigotti NA, Clair C, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012;5:CD001837. doi: 10.1002/14651858.CD001837.pub3. http://dx.doi.org/10.1002/14651858.cd001837.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice VH, Hartmann-Boyce J, Stead LF. Nursing interventions for smoking cessation. Cochrane Database Syst Rev. 2013;(8) doi: 10.1002/14651858.CD001188.pub4. [DOI] [PubMed] [Google Scholar]
- 3.Simon JA, Carmody TP, Hudes ES, Snyder E, Murray J. Intensive smoking cessation counseling versus minimal counseling among hospitalized smokers treated with transdermal nicotine replacement: a randomized trial. Am J Med. 2003;114(7):555–562. doi: 10.1016/s0002-9343(03)00081-0. http://dx.doi.org/10.1016/S0002-9343(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 4.Taylor CB, Curry SJ. Implementation of evidence-based tobacco use cessation guidelines in managed care organizations. Ann Behav Med. 2004;27(1):13–21. doi: 10.1207/s15324796abm2701_3. http://dx.doi.org/10.1207/s15324796abm2701_3. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman A, Augustson E, Davis KIA, Finney Rutten LJ. Awareness and Use of Tobacco Quitlines: Evidence from the Health Information National Trends Survey. J Health Commun. 2010;15(Suppl 3):264–278. doi: 10.1080/10810730.2010.526172. http://dx.doi.org/10.1080/10810730.2010.526172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vick L, Duffy SA, Ewing LA, Rugen K, Zak C. Implementation of an inpatient smoking cessation programme in a Veterans Affairs facility. J Clin Nurs. 2013;22(5–6):866–880. doi: 10.1111/j.1365-2702.2012.04188.x. [DOI] [PubMed] [Google Scholar]
- 7.Duffy SA, Ronis DL, Valenstein M, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2203–2208. doi: 10.1158/1055-9965.EPI-05-0880. http://dx.doi.org/10.1158/1055-9965.EPI-05-0880. [DOI] [PubMed] [Google Scholar]
- 8.Duffy SA, Ronis DL, Richardson C, et al. Protocol of a randomized controlled trial of the Tobacco Tactics website for operating engineers. BMC Public Health. 2012;12:335. doi: 10.1186/1471-2458-12-335. http://dx.doi.org/10.1186/1471-2458-12-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy SA, Ronis DL, Karvonen-Gutierrez CA, et al. Effectiveness of the tobacco tactics program in the Department of Veterans Affairs. Ann Behav Med. 2014;48(2):265–274. doi: 10.1007/s12160-014-9605-z. http://dx.doi.org/10.1007/s12160-014-9605-z. [DOI] [PubMed] [Google Scholar]
- 10.Duffy SA, Karvonen-Gutierrez CA, Ewing LA, Smith PM Veterans Integrated Services Network (VISN) 11 Tobacco Tactics Team. Implementation of the Tobacco Tactics Program in the Department of Veterans Affairs. J Gen Intern Med. 2010;25(Suppl 1):3–10. doi: 10.1007/s11606-009-1075-9. http://dx.doi.org/10.1007/s11606-009-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobacco Use and Dependence Guideline Panel. [Accessed March 7, 2016];Treating Tobacco Use and Dependence: 2008 Update. 2008 http://www.ncbi.nlm.nih.gov/books/NBK63952/
- 12.Duffy SA, Ronis DL, Titler MG, et al. Dissemination of the nurse-administered Tobacco Tactics intervention versus usual care in six Trinity community hospitals: study protocol for a comparative effectiveness trial. Trials. 2012;13:125. doi: 10.1186/1745-6215-13-125. http://dx.doi.org/10.1186/1745-6215-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillman DA, Smyth JD, Christian LM. Internet, Mail and Mixed-Mode Surveys: The Tailored Design Method. 3. 2009. [Google Scholar]
- 14.Ewing LA, Karvonen-Gutierrez CA, Noonan D, Duffy SA. Development of the Tobacco Tactics logo: From thumb prints to press. Tob Induc Dis. 2012;10(1):6. doi: 10.1186/1617-9625-10-6. http://dx.doi.org/10.1186/1617-9625-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffy SA, Ewing LA, Louzon SA, Ronis DL, Jordan N, Harrod M. Evaluation and costs of volunteer telephone cessation follow-up counseling for Veteran smokers discharged from inpatient units: a quasi-experimental, mixed methods study. Tob Induc Dis. 2015;13(1):4. doi: 10.1186/s12971-015-0028-9. http://dx.doi.org/10.1186/s12971-015-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louisville Metro Department of Public Health and Wellness. [Accessed January 13, 2016];Remember the 3 R’s. https://louisvilleky.gov/sites/default/files/health_and_wellness/educationalmaterials/wk11-_three_rs_handout.pdf.
- 17.Green CJ. [Accessed January 13, 2016];Quit Smoking Aids - The 4D’s - Delay, Deep Breath, Drink Water and Do Something Else. 2010 http://ezinearticles.com/?Quit-Smoking-Aids---The-4Ds---Delay,-Deep-Breath,-Drink-Water-and-Do-Something-Else&id=3864747.
- 18.Riley WT, Stevens VJ, Zhu SH, Morgan G, Grossman D. Overview of the Consortium of Hospitals Advancing Research on Tobacco (CHART) Trials. 2012;13(122):122. doi: 10.1186/1745-6215-13-122. http://dx.doi.org/10.1186/1745-6215-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The International Classification of Diseases tR. Diseases and Injuries Tabular Index.
- 20.Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29(5):844–854. doi: 10.1097/01.alc.0000164374.32229.a2. http://dx.doi.org/10.1097/01.ALC.0000164374.32229.A2. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, editor. SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 22.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. http://dx.doi.org/10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. http://dx.doi.org/10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. http://dx.doi.org/10.1002/(SICI)1097-0258(19981015)17:19<2265::AID-SIM918>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Morel JG, Bokossa MC, Neerchal NK. Small sample correction for the variance of GEE estimators. Biometrical Journal. 2003;45(4):395–409. http://dx.doi.org/10.1002/bimj.200390021. [Google Scholar]
- 26.Rigotti N, Regan S, Levy D, et al. Sustained Care Intervention and Postdischarge Smoking Cessation Among Hospitalized Adults A Randomized Clinical Trial. JAMA. 2014;312(7):719–728. doi: 10.1001/jama.2014.9237. http://dx.doi.org/10.1001/jama.2014.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohiuddin SM, Mooss AN, Hunter CB, Grollmes TL, Cloutier DA, Hilleman DE. INtensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease. Chest. 2007;131(2):446–452. doi: 10.1378/chest.06-1587. http://dx.doi.org/10.1378/chest.06-1587. [DOI] [PubMed] [Google Scholar]
- 28.Chouinard MC, Robichaud-Ekstrand S. The effectiveness of a nursing inpatient smoking cessation program in individuals with cardiovascular disease. Nurs Res. 2005;54(4):243–254. doi: 10.1097/00006199-200507000-00006. http://dx.doi.org/10.1097/00006199-200507000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Gadomski AM, Gavett J, Krupa N, Tallman N, Jenkins P. Effectiveness of an inpatient smoking cessation program. J Hosp Med. 2011;6(1):E1–8. doi: 10.1002/jhm.641. http://dx.doi.org/10.1002/jhm.641. [DOI] [PubMed] [Google Scholar]
- 30.Reid RD, Mullen KA, Slovinec D’Angelo ME, et al. Smoking cessation for hospitalized smokers: an evaluation of the “Ottawa Model”. Nicotine Tob Res. 2010;12(1):11–18. doi: 10.1093/ntr/ntp165. http://dx.doi.org/10.1093/ntr/ntp165. [DOI] [PubMed] [Google Scholar]
- 31.Lando H, Hennrikus D, McCarty M, Vessey J. Predictors of quitting in hospitalized smokers. Nicotine Tob Res. 2003;5(2):215–222. doi: 10.1080/0955300031000083436. http://dx.doi.org/10.1080/0955300031000083436. [DOI] [PubMed] [Google Scholar]
- 32.Joint Commission on Accreditation of Healthcare Organizations. [Accessed February 2, 2012];Tobacco Treatment Measures (TTM) 2011 www.jointcommission.org/assets/1/6/Tobacco%20Treatment%20Measures%20List1.PDF.
- 33.Reid JL, Hammond D, Boudreau C, Fong GT, Siahpush M. Socioeconomic disparities in quit intentions, quit attempts, and smoking abstinence among smokers in four western countries: findings from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2010;12(Suppl 1):S20–33. doi: 10.1093/ntr/ntq051. http://dx.doi.org/10.1093/ntr/ntq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzelepis F, Paul CL, Walsh RA, McElduff P, Knight J. Proactive telephone counseling for smoking cessation: meta-analyses by recruitment channel and methodological quality. J Natl Cancer Inst. 2011;103(12):922–941. doi: 10.1093/jnci/djr169. http://dx.doi.org/10.1093/jnci/djr169. [DOI] [PubMed] [Google Scholar]
- 35.Haapanen-Niemi N, Miilunpalo S, Vuori I, Pasanen M, Oja P. The impact of smoking, alcohol consumption, and physical activity on use of hospital services. Am J Public Health. 1999;89(5):691–698. doi: 10.2105/ajph.89.5.691. http://dx.doi.org/10.2105/AJPH.89.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Twardella D, Loew M, Rothenbacher D, Stegmaier C, Ziegler H, Brenner H. The diagnosis of a smoking-related disease is a prominent trigger for smoking cessation in a retrospective cohort study. J Clin Epidemiol. 2006;59(1):82–89. doi: 10.1016/j.jclinepi.2005.05.003. http://dx.doi.org/10.1016/j.jclinepi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Boudreaux ED, Baumann BM, Camargo CA, Jr, O’Hea E, Ziedonis DM. Changes in smoking associated with an acute health event: theoretical and practical implications. Ann Behav Med. 2007;33(2):189–199. doi: 10.1007/BF02879900. http://dx.doi.org/10.1007/BF02879900. [DOI] [PubMed] [Google Scholar]
- 38.Solty H, Crockford D, White WD, Currie S. Cigarette smoking, nicotine dependence, and motivation for smoking cessation in psychiatric inpatients. Can J Psychiatry. 2009;54(1):36–45. doi: 10.1177/070674370905400107. [DOI] [PubMed] [Google Scholar]
- 39.Katz DA, Holman JE, Johnson SR, et al. Implementing Best Evidence in Smoking Cessation Treatment for Hospitalized Veterans: Results from the VA-BEST Trial. Jt Comm J Qual Patient Saf. 2014;40(11):493–503. doi: 10.1016/s1553-7250(14)40064-3. [DOI] [PubMed] [Google Scholar]
- 40.Gadomski AM, Stayton M, Krupa N, Jenkins P. Implementing a smoke-free medical campus: impact on inpatient and employee outcomes. J Hosp Med. 2010;5(1):51–54. doi: 10.1002/jhm.473. http://dx.doi.org/10.1002/jhm.473. [DOI] [PubMed] [Google Scholar]
- 41.Nelson DB, Partin MR, Fu SS, Joseph AM, An LC. Why assigning ongoing tobacco use is not necessarily a conservative approach to handling missing tobacco cessation outcomes. Nicotine Tob Res. 2009;11(1):77–83. doi: 10.1093/ntr/ntn013. http://dx.doi.org/10.1093/ntr/ntn013. [DOI] [PubMed] [Google Scholar]