Abstract
Background
Regenerative medicine holds promise for restoring voice in patients with vocal fold scarring. As experimental treatments approach clinical translation, several considerations remain. Our objective was to evaluate efficacy and biocompatibility of four bone marrow mesenchymal stromal cell (BM-MSC) and tunable hyaluronic acid based hydrogel (HyStem-VF) treatments for vocal fold scar using clinically acceptable materials, a preclinical sample size and a dosing comparison.
Methods
Vocal folds of 84 rabbits were injured and injected with four treatment variations (BM-MSC, HyStem-VF, and BM-MSC in HyStem-VF at two concentrations) 6 weeks later. Efficacy was assessed with rheometry, real-time polymerase chain reaction (PCR) and histology at 2, 4 and 10 weeks following treatment. Lung, liver, kidney, spleen and vocal folds were screened for biocompatibility by a pathologist.
Results and discussion
Persistent inflammation was identified in all hydrogel-injected groups. The BM-MSC alone treatment appeared to be the most efficacious and safe, providing an early resolution of viscoelasticity, gene expression consistent with desirable extracellular matrix remodeling (less fibronectin, collagen 1α2, collagen 3, procollagen, transforming growth factor [TGF]β1, alpha smooth muscle actin, interleukin-1β, interleukin-17β and tumor necrosis factor [TNF] than injured controls) and minimal inflammation. Human beta actin expression in BM-MSC–treated vocal folds was minimal after 2 weeks, suggesting that paracrine signaling from the BM-MSCs may have facilitated tissue repair.
Keywords: elasticity, fibrosis, hyaluronic acid, hydrogel, mesenchymal stromal cells, rheology, viscosity, vocal cord
Introduction
Reliable treatments for vocal fold scarring are needed [1,2]. In pre-clinical investigations, bone marrow mesenchymal stromal cell (BM-MSC) and hyaluronic acid hydrogel therapies have shown promise for vocal fold regeneration through enhanced tissue viscoelasticity, extracellular matrix (ECM) profiles, and immunomodulation [3–10]. Prior to the development of a cell and/or gel-based therapy for clinical use, a number of practical considerations need to be addressed. First, because the BM-MSC dosing literature is based on intravenous injection or direct delivery to other organs, dosing for local delivery to the vocal fold needs to be investigated [11–13]. Second, bio-compatibility of BM-MSCs and hyaluronic acid hydrogels has not been evaluated in an in vivo model of vocal fold scar, yet it is critical given the location of the larynx [14]. The vocal folds are located at the entrance to the airway, so a severe inflammatory response in these tissues could be fatal [15]. Third, cell and gel products intended for clinical applications will need to be produced from materials suitable for human use. Leaders in tissue engineering have identified the importance of manufacturing and scale-up during product development, encouraging therapeutic materials to be reproducible and generated under Current Good Manufacturing Practices (cGMP) [16]. These three fundamental elements have been incorporated into our current investigation.
In the present work, we evaluated pre-clinical efficacy and biocompatibility of four cell and hydrogel treatments (BM-MSC, HyStem-VF, and two doses of BM-MSCs seeded in HyStem-VF) in an in vivo vocal fold scar model using materials amenable to clinical use. Efficacy was assessed in the vocal folds with oscillatory shear stress rheometry, gene expression, and histology. Biocompatibility was screened in the lung, liver, kidney, spleen, and vocal folds by a veterinary histopathologist to rule out significant cell migration and tumor formation. Cells were expanded and tested using a manufacturing process and quality control test methods that meet cGMP protocols for human clinical trials. Hydrogel components were produced in a cGMP-compliant facility using materials that were United States Pharmacopeia and National Formulary (USP/NF) grade or better. To our knowledge, this is the first pre-clinical investigation of a cell-based vocal fold scarring treatment that uses clinical-grade materials, biocompatibility testing and dose response to support a future clinical trial.
Materials and methods
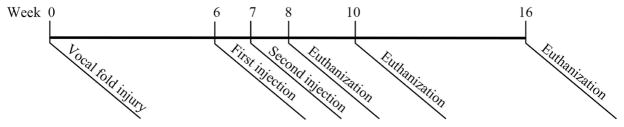
Eighty-four rabbits were included in the study, with 18 rabbits in each of the four experimental groups, nine rabbits in the control group, and three rabbits that were added to replace rabbits that perished once the study had begun. At the beginning of the study, bilateral vocal fold mucosal injury was created in the treatment groups, and unilateral injury was created in the controls (Figure 1). Treatments included Gel Only (GEL), Cells Only (CELL), Cell and Gel: Low Dose (LD) and Cell and Gel: High Dose (HD) (Table I). For each control rabbit, the uninjured vocal fold received no treatment and was included in the Uninjured group, and the injured vocal fold was injected with the diluent and included in the Injured group. Each rabbit (Injured, GEL, CELL, LD and HD) received two injections 1 week apart at weeks 6 and 7 (Table I, Figure 1). One third of the rabbits in each group were euthanized at weeks 8, 10 and 16 (which was 2 weeks, 4 weeks and 10 weeks following the initial treatment).
Figure 1.
Experimental Paradigm True vocal fold injury was created at week 0 (controls = unilateral injury; treatment groups = bilateral injury). At weeks 6 and 7, cell/gel treatments were injected (Table I). One third of the animals in each treatment group were euthanized 2 weeks, 4 weeks and 10 weeks following the initial treatment.
Table I.
Treatment formulations.
| Group | Injection volume | Injection |
|---|---|---|
| Uninjured | 0 μL | None |
| Injured | 150 μL | Diluenta |
| GEL | 150 μL | HyStem-VFb |
| CELL | 100 μL | 3 × 105 cells (14.98 μL) in diluentc (85.02 μL) |
| LD | 100 μL | 1.5 × 105 cells (14.98 μL), HyStem-VFd (85.02 μL) |
| HD | 100 μL | 3 × 105 cells (14.98 μL), HyStem-VFd (85.02 μL) |
Treatment formulations and injection volumes are provided by group.
Diluent: Plasma-Lyte + 10% rabbit serum albumin.
Hystem-VF: Glycosil (CMHA-S; 1.2% wt/vol), Gelin-S (thiol-modified gelatin; 0.06% wt/vol) and Extralink (PEGDA; 0.8% wt/vol).
BM-MSC isolation and expansion
Human BM-MSCs were obtained from Waisman Biomanufacturing and expanded and tested using a manufacturing process and quality control test methods that are similar to those used for cGMP protocols for human clinical trials. Cells were originally derived from the iliac crest of a healthy 22-year-old female. BM-MSCs were cultured in Alpha Minimum Essential Medium that was supplemented with 10% fetal bovine serum (FBS) and 1× Glutamax. Cells were cryo-preserved in 2 mL Corning cryogenic vials at two concentrations (1 × 107 cells/mL for LD; 2 × 107 cells/mL for HD). The final formulation for both concentrations included Plasma-Lyte, 10% rabbit serum albumin, and 2.5% dimethyl sulfoxide (DMSO).
Vocal fold injury
The University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee approved all protocol procedures. Adult female New Zealand White rabbits (2.5–3.5 kg, Harlan Laboratories) were sedated with ketamine (10 mg/kg) and dexmedetomidine (0.20 mg/kg). Preprocedurally, buprenorphine (0.05 mg/kg) and supplemental O2 were administered. The larynx was visualized under microscopic direct laryngoscopy with a Pilling pediatric endoscope (Pilling). A 2-mm forceps (MicroFrance) was used to remove a portion of the mid-membranous true vocal fold. Injury was unilateral in control rabbits and bilateral in treatment rabbits. Following the procedure, dexmedetomodine was reversed with atipimazole (1× the volume of dexmedetomodine).
Treatment
Six weeks after biopsy, the rabbits were anesthetized and their larynges were visualized as above. A 24-gauge needle (Merz Aesthetics, 9010M1) was used to inject cell/gel combinations into the injured region (Table I). Injured rabbits were injected unilaterally with 150 μL of the diluent at weeks 6 and 7. Uninjured vocal folds did not receive injections throughout the study. GEL rabbits received bilateral 150 μL injections of HyStem-VF (Glycosil: CMHA-S 1.2% weight per volume [wt/vol]; Gelin-S: thiol-modified gelatin 0.06% wt/vol; Extralink: PEGDA 0.8% wt/vol) [5,14,17] at weeks 6 and 7. The hydrogel was allowed to cross-link for approximately 13 min prior to injection, to prevent egress from the injection site. CELL rabbits received bilateral 100 μL injections of 3 × 105 human BM-MSCs suspended in the diluent at weeks 6 and 7. Cells were thawed to passage 6 just prior to injection. Rabbits in the LD group received bilateral 100 μL injections of 1.5 × 105 human BM-MSCs suspended in HyStem-VF at weeks 6 and 7. Cells were thawed just prior to injection, and mixed with the gel using a pipette. Rabbits in the HD group received bilateral 100 μL injections of 3 × 105 human BM-MSCs suspended in HyStem-VF at weeks 6 and 7.
Euthanization
One third of the animals in each treatment group were euthanized at 2 weeks, 4 weeks and 10 weeks following the initial treatment with an intravenous administration of Beuthanasia-D (0.05 mg/kg). Vocal folds (including the thyroarytenoid muscle and arytenoid cartilage), lungs, liver, kidneys and spleen were removed. In each treatment group (GEL, CELL, LD and HD) at each time point, at least four of the vocal folds were reserved for rheology, three of the vocal folds were reserved for immunohistochemistry, and three of the vocal folds were reserved for RT-polymerase chain reaction (PCR). At each time point, one Injured vocal fold was reserved for each of the assays (rheology, immunohistochemistry and RT-PCR) and one Uninjured vocal fold was reserved for each of the assays.
Rheology
Vocal folds from each treatment group were stored at −80°C prior to testing, then thawed and hydrated in a 1% phosphate-buffered saline (PBS) solution at room temperature. Additionally, seven uninjured, untreated vocal folds from New Zealand white rabbits were included for comparison; rheological testing was performed on these vocal folds at the same time as the treated vocal folds. Rheological testing was performed with a Bohlin Gemini-150 controlled stress rotational rheometer (Malvern Instruments Ltd) with an 8-mm parallel plate system. Temperature at the bottom plate was maintained at 37°C using a water jacket attached to a F25-ME external heating and circulation unit (Julabo USA Inc). To encourage tissue adhesion, 220 grit sandpaper (Norton Abrasives) was attached to both plates with Permabond 105 (Permabond LLC). Sandpaper was replaced every three samples. Excess tissue was trimmed. Samples were placed between the plates, and the upper plate was lowered in increments of approximately 100 μm until the normal force applied to the tissue remained >25 g for at least 5 seconds. Gap size was then decreased by an additional 20%. Tissue hydration was maintained by applying 1% PBS as needed. Frequency sweeps were performed using a controlled-stress paradigm from 0.01–10 Hz, taking 7 sample points every decade. Elastic shear moduli (G′) and viscous moduli (G″) were computed from the results. All rheological measurements were taken without knowledge of the treatment condition.
Real-time PCR
Vocal folds were stored overnight in RNA later RNA Stabilization Reagent (Qiagen) at 4°C, then transferred to a −80°C freezer. For each treatment group, three rabbits per time point (2, 4 and 10 weeks following injury) were included. For each control group, one rabbit per time point was included.
Vocal fold tissue was homogenized using a motorized mortar and pestle (Pellet Pestle; Kontes). Total RNA was isolated using the RNeasy Mini plus Kit (Qiagen) with on-column DNase digestion. Two micrograms of total RNA were reverse-transcribed using QuantiTect Reverse Transcription kit (Qiagen) to generate complementary DNA (cDNA). Real-time amplification for all genes except osteocalcin was performed in a LightCycler 1.5 System (Roche) for 45 cycles (95°C for 10 seconds, 50°C–61°C dependent on the primer melting temperature for 5 seconds, 72°C for 10 seconds). A LightCycler FastStart DNA MasterPlus SYBR Green I (Roche) kit was used in conjunction with primer pairs (Supplementary Table A). Primer specificity was confirmed by melting curves. Exact amplification efficiencies of target and reference genes were assessed by LightCycler software before normalized gene expression levels were calculated. Real-time amplification of osteocalcin was performed in an Applied Biosystems 7500 Fast Real-Time PCR instrument for 40 cycles (95°C for 15 seconds, 95°C for 2 minutes, 55°C for 15 seconds, 72°C for 1 minute) using the SYBR Select Master Mix (Life Technologies). Rabbit ulna cDNA was included as a positive control to assist interpretation of vocal fold results. The standard curve method was used to quantify expression of all genes. Results are shown by the messenger RNA (mRNA) concentration of the target gene (ng/μL) normalized by rabbit β-actin mRNA (ng/μL). All reactions were performed in triplicate.
Histology
Three rabbits in each treatment group and one control rabbit from each time point (2, 4 and 10 weeks) were included for histological analysis. Larynges were hemisected, fixed in 10% neutral buffered formalin for 24 h at room temperature, processed and embedded in paraffin, and coronally sectioned at 5 μm with a microtome (Leitz). Three true vocal fold sections from each rabbit were stained with Masson’s Trichrome to identify collagen content (Newcomer Supply), Verhoeff-Van Giesen to identify elastic fibers (EVG, Diagnostic Biosystem) and Alcian blue stain, pH 2.5, with a hyaluronidase digestion on a serial slide to identify hyaluronic acid (Newcomer Supply). Slides were imaged with a light microscope (E-600, Nikon) and digital camera (Olympus DP-71) at 40× magnification. The amount and intensity of staining in the thickest part of the lamina propria were quantified with MetaMorph 7.5 (Molecular Devices). Areas of clearly stain-expressing cells in the uninjured tissue relative to any background staining were used to establish a threshold. Percentage of staining was calculated by normalizing the area of stained pixels, as determined by the threshold, to the total area of the lamina propria in each image. The average intensity of the staining was determined through Metamorph functions, quantifying the relative background staining between sections of the same stain. Staining and intensity quantifications were performed in triplicate. Ten percent of the slides were re-measured to examine intra-rater reliability.
Histopathology
Vocal folds, lung, liver, kidney and spleen were fixed in 10% neutral buffered formalin, processed and embedded in paraffin, and sectioned at 5 μm with a microtome (Leitz). Three rabbits from each treatment group and two controls at week 16 were included. All tissue sections were stained with hematoxylin and eosin (H-E) and evaluated by a veterinary pathologist with light microscopy. Vocal fold sections were assessed for presence of gel, and degree of edema, fibrosis and inflammation (none, minimal, mild, moderate, severe). Lung, liver, kidney and spleen were examined for abnormalities (e.g., pulmonary edema/hemorrhage, hepatic extramedullary hematopoiesis) and for the appearance of BM-MSCs in H-E–stained sections.
Statistical analysis
To evaluate the rheology data, Mixed Models were fitted to compare the slope and intercept of the lines representing the modulus by frequency relationship for each group. Groups were defined by the treatment by time combination. Differences of least squares means were used to evaluate pair-wise differences for significant effects. For histology and RT-PCR, repeated measures analysis of variance (ANOVA) was used to examine treatment and time post-treatment as main effects and to evaluate the interaction of treatment * time. Differences of least squares means were used to evaluate pairwise differences for significant effects for all gene and histology data. Intra-rater agreement estimates were produced using a mixed-effects model with SAS software procedure PROC MIXED. The overall α level for testing was set at 0.05. All results were obtained using SAS statistical software (Version 9.2, SAS Institute Inc.).
Results
Rheology
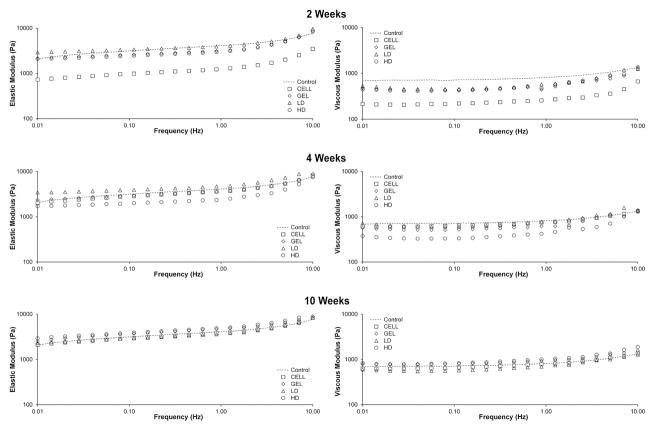
Slope of the lines representing the modulus by frequency relationship were not significantly different between the groups for elastic (G′) or viscous (G″) moduli. Intercepts were significantly different between groups for elastic moduli (F(12,50) = 2.97; P = 0.0034) and viscous moduli (F(12,50) = 2.43; P = 0.0143). Two, 4 and 10 weeks following treatment, the elastic moduli of all treatment groups did not differ from uninjured controls (n = 7) with two exceptions (Figure 2). Two weeks following the treatment, CELL vocal folds were significantly less stiff than uninjured controls (P = 0.001), and 4 weeks following treatment, LD vocal folds were more stiff than uninjured controls (P = 0.026). Two, 4 and 10 weeks following treatment, the viscous moduli of all treatment groups did not differ from controls with one exception. Two weeks following the treatment, CELL vocal folds were less viscous than controls (P = 0.001).
Figure 2.
Elastic (G′) and viscous moduli (G″) of all treatment groups did not differ from uninjured controls 10 weeks following treatment. G′ and G″ for all treatment groups (CELL, GEL, LD and HD) and uninjured control vocal folds at 2, 4 and 10 weeks following treatment are included. The uninjured control vocal folds were obtained from seven New Zealand white rabbits that had not undergone vocal fold injury or treatment (see Materials and Methods). Data are shown in log-log plots, the standard method for representing rheology data in voice literature. Due to the logarithmic scale, group variances were not included.
Histology
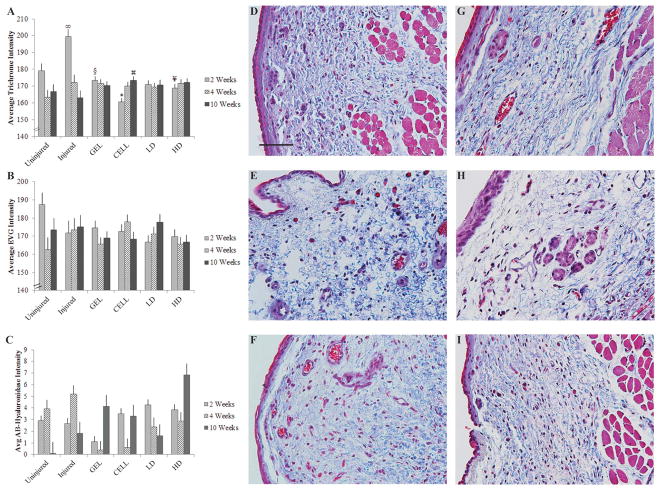
For average trichrome intensity, there was a significant interaction effect for treatment and time post-injury (F(10,23) = 6.20; P = 0.0001), but no significant main effects (Figure 3). Two weeks following treatment, CELL vocal folds had less trichrome staining than all other groups (ps < 0.05), and HD had less trichrome staining than Uninjured and Injured groups (ps < 0.05), GEL had less trichrome staining than Injured vocal folds (P < 0.0001) and Injured had more trichrome staining than LD and Uninjured vocal folds (ps < 0.001). Ten weeks following treatment, CELL vocal folds had more trichrome staining than Injured vocal folds (P < 0.05). EVG and hyaluronic acid did not differ with treatment or time post-treatment, and there were no significant interaction effects (Figure 3). For staining intensity calculations, interclass coefficient for intra-rater reliability was 0.97 for trichrome, 0.99 for EVG, 0.99 for Alcian blue and 0.99 for hyaluronidase digestion.
Figure 3.
Trichrome staining differed by treatment group and weeks post-injury, but there were no significant effects for EVG or Alcian Blue. Average trichrome (A), EVG (B) and Alcian Blue with hyaluronidase digestion (AB-Hyaluronidase; C) staining intensities are included for two control groups (Uninjured and Injured) and four treatment groups (GEL, CELL, LD and HD). Data are shown as mean ± standard error. *P < 0.05 compared with all other groups at 2 weeks following treatment; ∞P < 0.05 compared with Uninjured, LD at 2 weeks following treatment; ¥P < 0.05 compared with Uninjured, Injured at 2 weeks following treatment; §P < 0.05 compared with Injured at 2 weeks following treatment;
 P < 0.05 compared with Injured at 10 weeks following treatment. Representative histological images of trichrome-stained Uninjured (D), Injured (E), CELL (F), GEL (G), LD (H) and HD (I) vocal fold are shown 10 weeks following treatment (40× magnification; scale bar = 100 μm).
P < 0.05 compared with Injured at 10 weeks following treatment. Representative histological images of trichrome-stained Uninjured (D), Injured (E), CELL (F), GEL (G), LD (H) and HD (I) vocal fold are shown 10 weeks following treatment (40× magnification; scale bar = 100 μm).
RT-PCR
RT-PCR results for human β-actin (Figure 4), extra-cellular matrix genes (Figure 5, Supplementary Table B) and inflammatory and tissue remodeling genes (Figure 6, Supplementary Table C) are provided. There was a significant interaction effect (Treatment * Weeks) for collagen 1 alpha 2 (F(10,24) = 9.97; P < 0.0001), collagen 3 (F(10,24) = 2.98; P = 0.014), procollagen alpha 2 (F(10,24) = 5.15; P = 0.0005), fibronectin (F(10,24) = 2.90; P = 0.016), fibromodulin (F(10,24) = 13.38; P < 0.0001), HA synthase (HAS; F(10,24) = 6.70; P < 0.0001), hyaluronidase 2 (HYAL2; F(10,24) = 12.14; P < 0.0001), lipoprotein lipase (F(10,83) = 2.85; P = 0.004), interleukin 1 beta (IL-1β; F (10,24) = 9.05; P < 0.0001), interleukin 17 beta (IL-17β; F(10,24) = 10.10; P < 0.0001), interferon gamma (IFN-γ; F(10,24) = 2.41; P = 0.038), alpha smooth muscle actin (α-SMA; F(10,24) = 4.81; P = 0.0008) and tumor necrosis factor (TNF; F(10,24) = 15.28; P < 0.0001). There was a significant main effect of treatment on transforming growth factor beta 1 (TGF-β1; F(5,24) = 15.49; P < 0.0001), and osteocalcin when rabbit ulna was included and not included in the model (F(6,85) = 4039.40; P < 0.0001; F(5,83) = 3.78; P = 0.004, respectively).
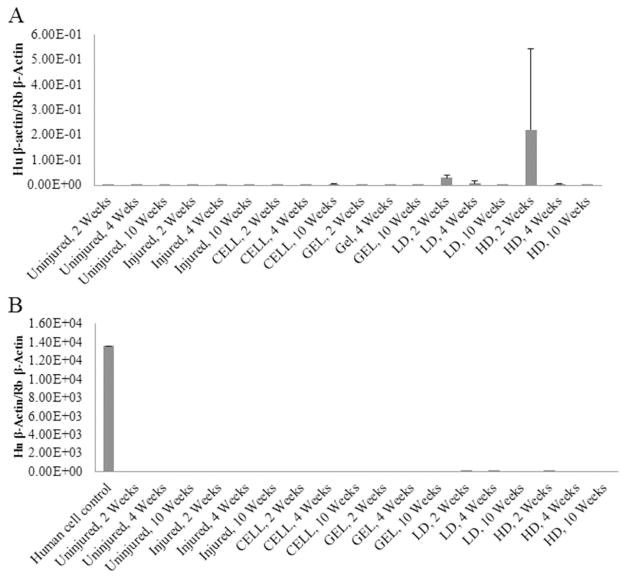
Figure 4.
Human β-Actin expression was greatest in HD at 2 weeks (A). Human β-actin fold changes are reported with respect to rabbit beta actin expression. β-Actin expression in human cells was included as a positive control and shown in (B) to demonstrate the scale of (A). Data are shown as mean ± standard deviation.
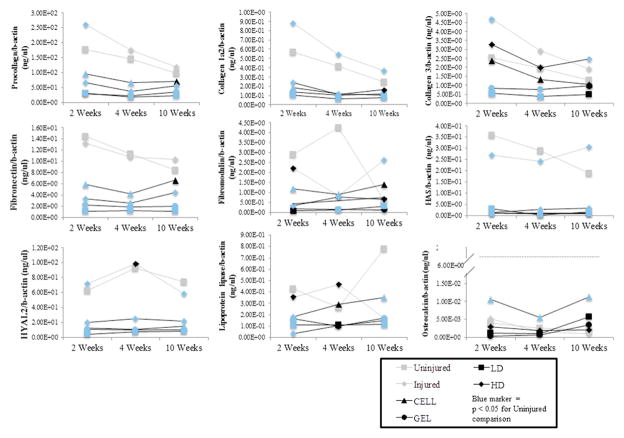
Figure 5.
ECM gene expression. RT-PCR results for pro-collagen alpha 2, collagen 1 alpha 2, collagen 3, fibronectin, fibromodulin, lipoprotein lipase, osteocalcin, hyaluronic acid synthase, hyaluronidase 2 and osteocalcin at 2, 4 and 10 weeks following treatment for all control and treatment groups. Fold changes are reported with respect to human beta actin expression. Data are shown as mean ± standard deviation. Significant pair-wise comparisons to Uninjured Controls are indicated with blue symbols (P < 0.05). All other significant pair-wise comparisons are provided in Supplementary Table B.
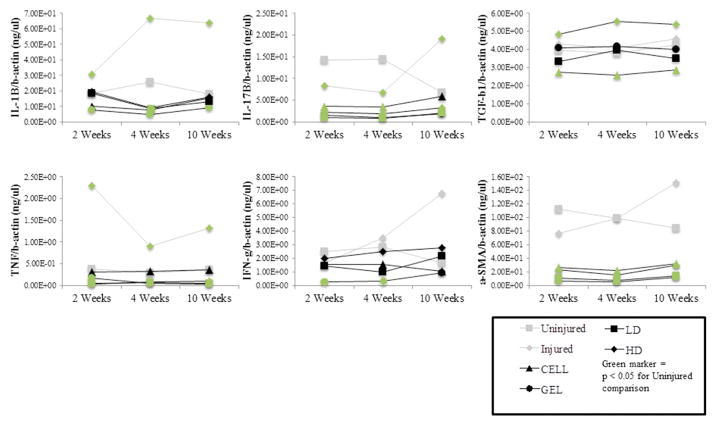
Figure 6.
Inflammatory and tissue remodeling gene expression. RT-PCR results for interleukin 1β, interleukin 17β, transforming growth factor β1, tumor necrosis factor, interferon γ and smooth muscle actin alpha at 2, 4 and 10 weeks following treatment for all control and treatment groups. Fold changes are reported with respect to human beta actin expression. Data are shown as mean ± standard deviation. Significant pair-wise comparisons to Uninjured Controls are indicated with green symbols (P < 0.05). All other significant pair-wise comparisons are provided in Supplementary Table C.
All significant pair-wise comparisons are provided in Supplementary Tables B and C, and those involving Uninjured vocal folds at 10 weeks following treatment are included here. At 10 weeks, Uninjured vocal folds expressed more HYAL2 and lipoprotein lipase than all treatment groups and Injured vocal folds. Also at 10 weeks, Uninjured vocal folds expressed more α-SMA and HAS than all treatment groups, and more procollagen, fibronectin, TNF and IL-17β than GEL, LD and HD. At 10 weeks, Uninjured expressed more collagen 1 alpha 2 than GEL, CELL and LD, and less collagen 3 than HD. At 10 weeks, Uninjured vocal folds expressed more IL-1β than GEL and more fibromodulin than LD. Also at 10 weeks, Uninjured expressed less IFN-γ, IL-17β, IL-1β and fibromodulin than Injured vocal folds and less fibromodulin than CELL.
Uninjured vocal folds expressed significantly less TGF-β1 than HD and more TGF-β1 than CELL. Osteocalcin expression was significantly higher in rabbit ulna than all groups. When rabbit ulna was excluded from the model, CELL expressed more osteocalcin than all groups.
There were no significant effects associated with human β-actin expression for the treatment and control groups. A human cell sample was included as a positive control, but was not included in statistical analysis.
Histopathology
In the vocal fold sections, the veterinary pathologist identified gel in 0% of controls and CELL, and 100% of GEL, LD and HD (Table II). Inflammation was reported in 33% of CELL and 100% of GEL, LD and HD. Edema was found in 50% of controls, 67% of CELL and 33% of HD. Fibrosis was identified in 50% of controls, 100% of CELL and 67% of HD. Degree of inflammation, edema and fibrosis is reported in Table II. The veterinary pathologist reported a close association between the presence of gel and granulomatous/pyogranulomatous and lymphocytic inflammation in the GEL, LD and HD groups.
Table II.
Vocal fold histopathology at 10 wk post-treatment.
| Control (n = 2)a | GEL (n = 3) | CELL (n = 3) | LD (n = 3) | HD (n = 3) | |
|---|---|---|---|---|---|
| Presence of gel | 0% | 100% | 0% | 100% | 100% |
| Inflammation | 0% | 100% (moderate, marked, marked) | 33% (mild) | 100% (marked, marked, marked) | 100% (mild, moderate, marked) |
| Edema | 50% (mild) | 0% | 67% (minimal, mild) | 0% | 33% (mild) |
| Fibrosis | 50% (mild) | 0% | 100% (minimal, minimal, moderate | 0% | 67% (mild, mild) |
A histopathologist’s ratings for the presence of gel, inflammation, edema and fibrosis in H-E–stained slides of vocal folds from control, GEL, CELL, LD and HD groups.
Controls included one Injured, and one Uninjured vocal fold.
In the lung, liver, kidney and spleen tissues, the pathologist reported no apparent foci of BM-MSCs or tumor growth secondary to BM-MSCs 10 weeks following treatment. The pathologist detected other abnormalities in all groups (e.g., pulmonary edema) that she attributed to conditions (e.g., euthanasia) that were unrelated to the experimental protocol.
Rabbit attrition
Nine rabbits died during the experiment. Five deaths were due to laryngeal stridor secondary to inflammation and edema that was associated with the treatments (GEL = 2, LD = 2, HD = 1). Two deaths were associated with anesthesia (control = 2). Two deaths were related to aspiration of food/fecal material from mouth that was found incidentally prior to surgical procedures (GEL = 1, HD = 1). This is a common occurrence in rabbit research. Removal of the material was attempted every time it was found, with two rabbits not recovering from removal attempts. Three rabbits were added so that all treatment groups had at least 15 surviving participants (GEL = 15 surviving participants, CELL = 18 surviving participants, LD = 16 surviving participants, HD = 17 surviving participants, Uninjured/Injured controls = 9 surviving participants).
Discussion
To date, no clinical trial of MSC-based therapy for vocal fold scar has been published. Our pre-clinical project was designed with US Food and Drug Administration advisement to facilitate the development of clinically translatable therapy. We addressed several previously unexplored considerations, including the biocompatibility of BM-MSCs and hyaluronic acid hydrogels in an established in vivo model, the production of materials using methods similar to cGMP, a large sample size, a dosing comparison and inclusion of both cell-only and gel-only controls.
Our primary functional assessment of the four treatments was to measure tissue viscoelasticity with controlled stress rheometry. As the mechanical properties of the vocal fold mucosa largely determine vocal quality and clinical pathology, rheology is becoming a standard functional test in pre-clinical studies [18–20]. Ten weeks following treatment, the elastic and viscous moduli of vocal folds in each treatment group (GEL, CELL, LD and HD) did not statistically differ from uninjured controls (n = 7), suggesting that all treatments yielded desirable mechanical properties in the long-term. One of the two deviations from this trajectory was that CELL-treated vocal folds were significantly less stiff (G′) and less viscous (G″) than uninjured controls at 2 weeks. As the second injection (week 7) was performed just 1 week prior to rheology testing (week 8), potentially some of the thin liquid diluent from the cell injection remained in the site, distorting the biomechanical measurements for the CELL group. By the later time points, the biomechanical data for the CELL group did not differ from uninjured controls, potentially related to the diluent being more fully absorbed into the tissue.
Due to the small sample size of injured vocal folds in this study, we compared our rheology treatment data to injured, untreated controls from a previous investigation [7]. In the previous investigation, injured rabbit vocal folds were injected with saline, and rheological testing was performed 2 months later. The injured, saline-treated vocal folds (n = 5 vocal folds) were more than a magnitude stiffer (increased G′) and more viscous (increased G″) than all treatment groups in the present study. Specifically, G′ of the injured, untreated vocal folds in the previous investigation was between 10,000 and 100,000 Pa at 0.01–10 Hz, and G′ of the treatment groups in the present investigation was <10,000 Pa (0.01–10.0 Hz). G″ of the injured, untreated vocal folds from the previous investigation was between 1000 and 10,000 Pa (0.01–10.0 Hz) and G″ from the present investigation was <1000 Pa (0.01–10.0 Hz) for all treatment groups [7]. These data suggest that the elastic and viscous moduli of all of the treatment groups in the present study are substantially improved as compared with historical injured controls.
The favorable CELL data agree with a previous comparison of three treatments (vocal fold fibro-blasts, HyStem-VF and fibroblasts seeded in HyStem-VF) for chronic rabbit vocal fold scar in which the cells alone therapy resulted in significantly less stiff and less viscous tissue than injured controls at 8 weeks [7]. This agreement may suggest that vocal fold fibroblasts and BM-MSCs have a similar potential for regenerating vocal fold tissue. It has been established that fibro-blasts and BM-MSC have similar morphology, cell surface markers, differentiation potential and immunologic properties [21–24].
The favorable rheological results for the GEL group were consistent with previous investigations of HyStem-VF. Rabbit vocal folds treated with HyStem-VF were less stiff and less viscous than injured controls in the short-term (3 weeks) [5,8] and long-term (6 months) [9] when injected at the time of injury. The robustness of our GEL data is underscored by the comparison with Uninjured as well as Injured controls. Collagen makes up approximately 40% of the protein content of the healthy vocal fold lamina propria, and is considered to be a major contributor to vocal fold scar and viscoelasticity [25–27]. Injured controls at 2 weeks had the most collagen staining and gene expression of procollagen alpha 2, collagen 1 alpha 2 and collagen 3 of all the groups, consistent with the elevated collagen levels typically reported for vocal fold injury [27]. When evaluating which treatment might yield the most favorable collagen levels, the CELL data should be considered. Two weeks following treatment, CELL had significantly lower histological collagen levels than all treatment and control groups, as well as the most favorable viscoelastic measurements (G′ and G″) of all the groups. These findings are consistent with the Thibeault et al. 2008 comparison of three cell and HyStem-VF treatments for vocal fold scar, wherein the cells alone treatment resulted in the lowest collagen levels and the best biomechanical outcomes [7]. At 10 weeks, the viscoelastic properties of the CELL group had been restored, but they had more collagen staining than Injured controls, which appears to be a contradiction. The relationship between vocal fold viscoelasticity and collagen has historically been unclear. Various in vitro, animal and human studies have suggested a positive, negative or no relationship between collagen density and vocal fold viscoelasticity [27–32]. This may suggest that viscoelasticity is influenced by other collagen parameters, such as the degree of cross-linking or fiber alignment [33]. The trichrome-stained images (Figure 3) reveal that the collagens in the Injured group are more disorganized and in thicker bundles than the other groups, consistent with previous investigations [27].
Fibronectin is a glycoprotein that binds cells to ECM molecules such as collagen, and its presence is required during collagen 1 fibrillogenesis [34,35]. Elevated fibronectin is typically associated with vocal fold scarring [27]. In our study, Injured controls expressed more fibronectin than all treatment groups (CELL, GEL, LD and HD) at all time points. Furthermore, there was a direct relationship between fibronectin and collagen 1 alpha 2 transcription for each group, with the Injured vocal folds expressing more of these two genes than all treatment groups at 2, 4 and 10 weeks. This may suggest that an abundance of fibronectin promotes collagen 1 deposition in injured vocal fold tissue, and that one therapeutic mechanism underlying BM-MSC and HyStem-VF treatments relates to a reduced level of fibronectin.
Hyaluronic acid is a water-binding, space-filling lubricant thought to be a critical contributor to vocal fold viscoelasticity [20,36–38]. In our study, histological levels of HA did not differ for any of the groups. Gene expression levels associated with HAS and breakdown (HYAL2) were significantly lower in all treatment groups at all time points as compared with Injured and Uninjured controls. These findings were consistent with previous studies in which levels of HA protein, HAS2 and HYAL2 were the same or lower in HyStem-VF–treated vocal folds than controls from 5 days to 6 months following treatment [6,8,9]. Despite the HA gene and protein levels in the present study, all of the treatments restored vocal fold viscoelasticity to baseline levels by the end of the study. The role of HA in repairing the vocal fold following injury is complex and incompletely understood, and likely involves interaction between multiple molecules (ECM proteins, cytokines, and so on) [27].
TGFβ-1 is a powerful mediator of ECM synthesis and degradation during wound healing. Following tissue injury, many different cells (macrophages, platelets, leukocytes, fibroblasts and MSCs) release TGFβ. TGFβ-1 stimulates fibroblasts to deposit collagen, inhibit collagenase and induce α-SMA expression [39–41]. Elevated TGFβ1 levels are found in fibrotic vocal fold, liver, lung, kidney, retina and skin [40,42,43]. Dermal scarring can be reduced when exogenous antibodies are delivered to a wound to neutralize TGFβ-1 [44]. In the present study, the CELL group expressed significantly lower levels of TGFβ-1 than all control and treatment groups, and HD expressed higher levels than all groups. In the HD condition, we suspect that the significant inflammation caused by the hydrogel stimulated TGFβ-1 release from the high dose of MSCs or through paracrine signaling with inflammatory cells [45,46]. The abundance of TGFβ-1 in HD tissue may have been responsible for the elevated transcription of pro-collagen and collagen 3 relative to the other treatments. The dearth of TGFβ-1 transcription in the CELL group is consistent with the lower α-SMA transcription relative to Injured controls at all time points.
RT-PCR was used to detect MSC-derivatives for bone and fat lineages in the vocal folds. We assume that osteogenic products would have an undesirable effect on vocal fold viscoelasticity [20], and that adipogenic products would have a desirable effect [47–49]. Osteocalcin was expressed 452-fold greater in rabbit ulna than the next highest-expressing group (CELL, 10 weeks). When rabbit ulna was omitted from our statistical model, CELL-treated vocal folds expressed significantly more osteocalcin (0.0113 ng/μL mRNA) than all other groups. It is unknown if this expression level would be clinically meaningful; however, the favorable rheological measurements of the CELL group suggest that it would have a negligible functional impact. The CELL group may have expressed higher levels of osteocalcin due to a low-level, constitutive gene expression in the BM-MSCs [50]. The CELL group also expressed more lipoprotein lipase than all other treatment groups at 10 weeks. Potentially the similarity in matrix stiffness of native vocal fold and fat directed the injected BM-MSCs toward a fat lineage due to a latent mechanosensitivity [47,51,52].
As severe swelling in the vocal folds could fatally restrict the airway, RT-PCR and histopathology were used to evaluate the inflammatory response [17,43,53]. At 10 weeks following initial treatment, Injured controls expressed higher levels of pro-inflammatory cytokines (IL-1β, IL-17β, IFN-γ and TNF) than all groups. In the treated groups, expression of IL-1β, IL-17β, IFN-γ and TNF had returned to the baseline levels found in Uninjured controls by 10 weeks, suggesting that an inflammatory response was not detected at the transcriptional level.
The histopathology results revealed moderate to marked inflammation in all of the vocal folds treated with HyStem-VF (GEL, LD and HD; n = 3) at 10 weeks following the initial treatment. The histopathologist identified inflammation in the tissue sections by specific immune cells (e.g., leukocytes, macrophages) that suggest the presence of an antigen. As moderate to marked inflammation is an unacceptable potential consequence of a clinical treatment, further investigation into the causes of this HyStem-VF result is warranted. One of the reasons that persistent inflammation is an important clinical consideration is that MSCs can change their phenotype in response to their microenvironment. BM-MSCs are sensitive to biochemical signals at sites of injury and inflammation, and can be polarized toward anti- or pro-inflammatory phenotypes that can have a myriad of downstream effects [54,55]. In our study, differences between the combination treatment groups (LD, HD) and the CELL group could be related to inflammation-induced MSC phenotype changes. Further investigations are needed to define these cues and signaling pathways to better direct vocal fold tissue engineering efforts.
BM-MSCs were not identified in the vocal fold sections by the histopathologist, and human β-actin expression did not statistically differ among any of the treatment or control groups. These data suggest that there was not significant cell engraftment in the groups that were injected with BM-MSC (CELL, LD and HD). Tissue repair may have been related to BM-MSCs releasing soluble factors that stimulated paracrine pathways of remodeling, rather than cell engraftment and differentiation [56,57]. The lack of BM-MSCs found elsewhere (lung, liver, kidney and spleen) adds further clinical interest for the BM-MSC treatments.
Several limitations in this investigation warrant discussion. The sample size of the Uninjured and Injured groups was small for IHC and RT-PCR. Three rabbits were included for each treatment group for each time point (2, 4 and 10 weeks), but only one rabbit was included per time point for Uninjured and Injured. This likely contributed to variability in the control groups across time. However, sample size was not a limitation for the rheological data, as seven uninjured vocal folds were included for analysis.
Another limitation was that injection volumes of the treatments varied by group. Injured and GEL received two 150 μL injections, whereas CELL, LD and HD received two 100 μL injections (Table I). This protocol change was initiated due to a greater than expected rabbit attrition rate that was associated with airway edema. Reducing the injection volume for the remaining groups (CELL, LD and HD) resolved the attrition issue. It is not known if the two injection volumes caused inter-group differences.
Conclusion
All four treatments (CELL, GEL, LD and HD) resulted in tissue viscoelasticity that had been restored to uninjured levels by the end of the study. The persistent and moderate to marked inflammation observed in the HyStem-VF groups (GEL, LD and HD) indicates poor biocompatibility, despite the clinical-grade hydrogel. It is unclear if the poorer outcomes in the gel-injected groups were due to the severity of inflammation exceeding the reparative capacity of the BM-MSCs, or if the BM-MSCs had a reduced capacity to stimulate paracrine signaling with the native tissue because they were trapped in the hydrogel. Overall, the data suggest that the injection of BM-MSCs without a carrier gel yielded the best outcomes, with an early recovery of vocal fold elasticity and viscosity, as well as a gene expression profile consistent with favorable wound healing. Gene expression and histology indicated that the BM-MSC engraftment in the CELL vocal folds was low after 2 weeks, suggesting that the injected cells promoted tissue repair by stimulating paracrine mechanisms rather than through long-term engraftment.
Acknowledgments
The authors thank Denise Schwahn, PhD, DVM, for histopathology examination, Glen Leverson, PhD, for statistical analysis, Drew Roennenberg, MS, for histology assistance and John Centanni, PhD, for study design assistance. This project has been funded in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201000010C, and from the National Institutes of Health NIDCD (R01 DC4336, F31 DC012973).
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.jcyt.2016.07.005.
Footnotes
Disclosure of interests: The authors do not have any conflicts of interest to disclose.
References
- 1.Benninger MS, Alessi D, Archer S, Bastian R, Ford C, Koufman J, et al. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg. 1996;115:474–82. doi: 10.1177/019459989611500521. [DOI] [PubMed] [Google Scholar]
- 2.Welham N, Choi SH, Dailey SH, Ford CN, Jiang JJ, Bless DM. Prospective multi-arm evaluation of surgical treatments for vocal fold scar and pathologic sulcus vocalis. Laryngoscope. 2011;121:1252–60. doi: 10.1002/lary.21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hertegård S, Cedervall J, Svensson B, Forsberg K, Maurer FHJ, Vidovska D, et al. Viscoelastic and histologic properties in scarred rabbit vocal folds after mesenchymal stem cell injection. Laryngoscope. 2006;116:1248–54. doi: 10.1097/01.mlg.0000224548.68499.35. [DOI] [PubMed] [Google Scholar]
- 4.Svensson B, Nagubothu RS, Cedervall J, Le Blanc K, Ährlund-Richter L, Tolf A, et al. Injection of human mesenchymal stem cells improves healing of scarred vocal folds: analysis using a xenograft model. Laryngoscope. 2010;120:1370–5. doi: 10.1002/lary.20926. [DOI] [PubMed] [Google Scholar]
- 5.Duflo S, Thibeault S, Li W, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12:2171–80. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 6.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich G. Effect of a synthetic extracellular matrix on vocal fold lamina propria gene expression in early wound healing. Tissue Eng. 2006;12:3201–7. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- 7.Thibeault S, Klemuk SA, Smith ME, Leugers C, Prestwich G. In vivo comparison of biomimetic approaches for tissue regeneration of the scarred vocal fold. Tissue Eng Part A. 2008;15:1481–7. doi: 10.1089/ten.tea.2008.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen JK, Thibeault S, Walsh JF, Xiao ZSHU, Prestwich GD. In vivo engineering of the vocal fold extracellular matrix with injectable hyaluronic acid hydrogels: early effects on tissue repair and biomechanics in a rabbit model. Ann Otol Rhinol Laryngol. 2005;114:662–70. doi: 10.1177/000348940511400902. [DOI] [PubMed] [Google Scholar]
- 9.Thibeault S, Klemuk SA, Chen X, Quinchia Johnson BH. In vivo engineering of the vocal fold ECM with injectable HA hydrogels—late effects on tissue repair and biomechanics in a rabbit model. J Voice. 2010;25:249–53. doi: 10.1016/j.jvoice.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson SE, King SN, Kim J, Chen X, Thibeault S, Hematti P. The effect of mesenchymal stromal cell–hyaluronic acid hydrogel constructs on immunophenotype of macrophages. Tissue Eng Part A. 2011;17:2463–71. doi: 10.1089/ten.tea.2010.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mastri M, Lin H, Lee T. Enhancing the efficacy of mesenchymal stem cell therapy. World J Stem Cells. 2014;6:82–93. doi: 10.4252/wjsc.v6.i2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson JD, Bertaso AG, Psaltis PJ, Frost L, Carbone A, Paton S, et al. Impact of timing and dose of mesenchymal stromal cell therapy in a preclinical model of acute myocardial infarction. J Card Fail. 2013;19:342–53. doi: 10.1016/j.cardfail.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014;54:1418–37. doi: 10.1111/trf.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Thibeault S. Biocompatibility of a synthetic extracellular matrix on immortalized vocal fold fibroblasts in 3-D culture. Acta Biomater. 2010;6:2940–8. doi: 10.1016/j.actbio.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson PC, Mikos AG, Fisher JP, Jansen JA. Strategic directions in tissue engineering. Tissue Eng. 2007;13:2827–37. doi: 10.1089/ten.2007.0335. [DOI] [PubMed] [Google Scholar]
- 17.Thibeault S, Duflo S. Inflammatory cytokine responses to synthetic extracellular matrix injection to the vocal fold lamina propria. Ann Otol Rhinol Laryngol. 2008;117:221–6. doi: 10.1177/000348940811700310. [DOI] [PubMed] [Google Scholar]
- 18.Rousseau B, Sohn J, Montequin DW, Tateya I, Bless DM. Functional outcomes of reduced hyaluronan in acute vocal fold scar. Ann Otol Rhinol Laryngol. 2004;113:767. doi: 10.1177/000348940411301001. [DOI] [PubMed] [Google Scholar]
- 19.Rousseau B, Hirano S, Chan R, Welham N, Thibeault S, Ford CN, et al. Characterization of chronic vocal fold scarring in a rabbit model. J Voice. 2004;18:116–24. doi: 10.1016/j.jvoice.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Caton T, Thibeault S, Klemuk S, Smith ME. Viscoelasticity of hyaluronan and nonhyaluronan based vocal fold injectables: implications for mucosal versus muscle use. Laryngoscope. 2007;117:516–21. doi: 10.1097/MLG.0b013e31802e9291. [DOI] [PubMed] [Google Scholar]
- 21.Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy. 2012;14:516–21. doi: 10.3109/14653249.2012.677822. [DOI] [PubMed] [Google Scholar]
- 22.Hanson SE, Kim J, Johnson BHQ, Bradley B, Breunig MJ, Hematti P, et al. Characterization of mesenchymal stem cells from human vocal fold fibroblasts. Laryngoscope. 2010;120:546–51. doi: 10.1002/lary.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haniffa M, Wang X, Holtick U, Rae M, Isaacs JD, Dickinson AM, et al. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 24.Haniffa M, Collin MP, Buckley CD, Dazzi F. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica. 2009;94:258–63. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn M, Kobler JB, Zeitels SM, Langer R. Quantitative and comparative studies of the vocal fold extracellular matrix II: collagen. Ann Otol Rhinol Laryngol. 2006;115:225–32. doi: 10.1177/000348940611500311. [DOI] [PubMed] [Google Scholar]
- 26.Hirano S, Minamiguchi S, Yamashita M, Ohno T, Kanemaru S, Kitamura M. Histologic characterization of human scarred vocal folds. J Voice. 2009;23:399–407. doi: 10.1016/j.jvoice.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Hansen JK, Thibeault S. Current understanding and review of the literature: vocal fold scarring. J Voice. 2006;20:110–20. doi: 10.1016/j.jvoice.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Chhetri DK, Head C, Revazova E, Hart S, Bhuta S, Berke GS. Lamina propria replacement therapy with cultured autologous fibroblasts for vocal fold scars. Otolaryngol Head Neck Surg. 2004;131:864–70. doi: 10.1016/j.otohns.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Cedervall J, Ährlund-Richter L, Svensson B, Forsgren K, Maurer FHJ, Vidovska D, et al. Injection of embryonic stem cells into scarred rabbit vocal folds enhances healing and improves viscoelasticity: short-term results. Laryngoscope. 2007;117:2075–81. doi: 10.1097/MLG.0b013e3181379c7c. [DOI] [PubMed] [Google Scholar]
- 30.Thibeault S, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96–104. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 31.Thibeault S, Bless DM, Gray SD. Interstitial protein alterations in rabbit vocal fold with scar. J Voice. 2003;17:377–83. doi: 10.1067/s0892-1997(03)00064-x. [DOI] [PubMed] [Google Scholar]
- 32.Chan RW, Fu M, Young L, Tirunagari N. Relative contributions of collagen and elastin to elasticity of the vocal fold under tension. Ann Biomed Eng. 2007;35:1471–83. doi: 10.1007/s10439-007-9314-x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Xu B, Chow MJ. Experimental and modeling study of collagen scaffolds with the effects of crosslinking and fiber alignment. Int J Biomater. 2011;2011:172389. doi: 10.1155/2011/172389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engvall E, Ruoslahti E, Miller EJ. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978;147:1584–95. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan R, Gray SD, Titze I. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngol Head Neck Surg. 2001;124:607–14. doi: 10.1177/019459980112400602. [DOI] [PubMed] [Google Scholar]
- 37.Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791–809. doi: 10.1016/j.carres.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 38.Necas J, Bartosikova L, Brauner P, Kolar J. Hyaluronic acid (hyaluronan): a review. Vet Med (Praha) 2008;53:397–411. [Google Scholar]
- 39.Ignotz RA, Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–45. [PubMed] [Google Scholar]
- 40.Adzick NS, Lorenz HP. Cells, matrix, growth factors, and the surgeon. The biology of scarless fetal wound repair. Ann Surg. 1994;220:10–18. doi: 10.1097/00000658-199407000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 43.Lim X, Tateya I, Tateya T, Muñoz-Del-Río A, Bless DM. Immediate inflammatory response and scar formation in wounded vocal folds. Ann Otol Rhinol Laryngol. 2006;115:921–9. doi: 10.1177/000348940611501212. [DOI] [PubMed] [Google Scholar]
- 44.Shah M, Foreman DM, Ferguson MWJ. Neutralization of TGF-beta(1) and TGF-beta(2) or exogenous addition of TGF-beta(3) to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 45.Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142–9. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136–43. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan R, Titze I. Viscosities of implantable biomaterials in vocal fold augmentation surgery. Laryngoscope. 1998;108:725–31. doi: 10.1097/00005537-199805000-00019. [DOI] [PubMed] [Google Scholar]
- 48.Sataloff RT, Spiegel JR, Hawkshaw M, Rosen DC, Heuer RJ. Autologous fat implantation for vocal fold scar: a preliminary report. J Voice. 1997;11:238–46. doi: 10.1016/S0892-1997(97)80083-5. [DOI] [PubMed] [Google Scholar]
- 49.Shaw GY, Szewczyk MA, Searle J, Woodroof J. Autologous fat injection into the vocal folds: technical considerations and long-term follow-up. Laryngoscope. 1997;107:177–86. doi: 10.1097/00005537-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura A, Dohi Y, Akahane M, Ohgushi H, Nakajima H, Funaoka H, et al. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng Part C Methods. 2009;15:169–80. doi: 10.1089/ten.tec.2007.0334. [DOI] [PubMed] [Google Scholar]
- 51.Chan R, Titze I. Viscoelastic shear properties of human vocal fold mucosa: measurement methodology and empirical results. J Acoust Soc Am. 1999;106:2008. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 52.Engler A, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 53.Branski RC, Rosen CA, Verdolini K, Hebda PA. Biochemical markers associated with acute vocal fold wound healing: a rabbit model. J Voice. 2005;19:283–9. doi: 10.1016/j.jvoice.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Anton K, Banerjee D, Glod J. Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased IL-6 and CXCL10 secretion. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kota DJ, DiCarlo B, Hetz RA, Smith P, Cox CS, Olson SD. Differential MSC activation leads to distinct mononuclear leukocyte binding mechanisms. Sci Rep. 2014;4:4565. doi: 10.1038/srep04565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burdon TJ, Paul A, Noiseux N, Prakash S, Shum-Tim D. Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res. 2011;2011:207326. doi: 10.1155/2011/207326. [DOI] [PMC free article] [PubMed] [Google Scholar]