Abstract
Purpose
We conducted a nationwide cohort study to investigate the relationship between systemic lupus erythematosus (SLE) and the risk of incident respiratory failure.
Methods
From the National Health Insurance Research Database, we identified 11 533 patients newly diagnosed with SLE and 46 132 controls without SLE who were randomly selected through frequency-matching according to age, sex, and index year. Both cohorts were followed until the end of 2011 to measure the incidence of incident respiratory failure, which was compared between the 2 cohorts through a Cox proportional hazards regression analysis.
Results
The adjusted hazard ratio (aHR) of incident respiratory failure was 5.80 (95% confidence interval [CI] = 5.15–6.52) for the SLE cohort after we adjusted for sex, age, and comorbidities. Both men (aHR = 3.44, 95% CI = 2.67–4.43) and women (aHR = 6.79, 95% CI = 5.93–7.77) had a significantly higher rate of incident respiratory failure in the SLE cohort than in the non-SLE cohort. Both men and women aged <35 years (aHR = 31.2, 95% CI = 21.6–45.2), 35–65 years; (aHR = 6.19, 95% CI = 5.09–7.54) and ≥65 years (aHR = 2.35, 95% CI = 1.92–2.87) had a higher risk of incident respiratory failure in the SLE cohort. Moreover, the risk of incident respiratory failure was higher in the SLE cohort than the non-SLE cohort, for subjects with (aHR = 2.65, 95% CI = 2.22–3.15) or without (aHR = 9.08, 95% CI = 7.72–10.7) pre-existing comorbidities. In the SLE cohort, subjects with >24 outpatient visits and hospitalizations per year had a higher incident respiratory failure risk (aHR = 21.7, 95% CI = 18.0–26.1) compared with the non-SLE cohort.
Conclusion
Patients with SLE are associated with an increased risk of incident respiratory failure, regardless of their age, sex, and pre-existing comorbidities; especially medical services with higher frequency.
Introduction
Respiratory failure is a common cause of admission to intensive care units [1]. Respiratory failure can be divided into 1) acute respiratory failure (ICD-9-CM code 518.81) which is hypoxaemic (arterial oxygen tension, PaO2 < 60 mmHg) with or without hypercapnia (arterial carbon dioxide tension, PaCO2 > 50 mmHg), develops within minutes or hours (pH <7.3 and the value of the bicarbonate ion is normal) in patients with loss of the ability to ventilate adequately or to provide sufficient oxygen to the blood and systemic organs; 2) chronic respiratory failure (ICD-9-CM code 518.83) which is hypercapnic and hypoxaemic, develops over several days or longer (normal or slightly decreased pH and the value of the bicarbonate ion increased) in patients with existing respiratory disease; and 3) acute on chronic respiratory failure (ICD-9-CM code 518.84) which is an acute deterioration in an individual with chronic respiratory failure (PaCO2 > 50 mmHg, pH <7.3 and the value of the bicarbonate ion increased; hypoxaemic) [2–4].
Systemic lupus erythematosus (SLE) is more prevalent in women, particularly of child-bearing age [5]. A recent population-based study in Taiwan reported that the average incidence of SLE cases between 2003 and 2008 was 4.87 per 100 000 person-years [5]. SLE is an autoimmune disease that affects multiple organ systems [6] such as the heart, joints, skin, lungs [7,8], chest wall, pleura, vessels [9], liver, kidneys [10], and nervous system [11]. SLE is associated with an increased risk of incident lung diseases (e.g., obstructive airway disease [7,12], pneumonia [8], and pulmonary embolism [13]), pleurisy and pleural effusion [14], and incident atherosclerosis-related diseases (e.g., hypertension [15], diabetes [16], stroke [15,17], and end-stage renal diseases; ESRD [18]). These SLE complications may lead to acute hypoxaemic failure (e.g., pneumonia [19]) with or without hypercapnia (e.g., asthma [7]), chronic respiratory failure (e.g., chronic obstructive pulmonary disease; COPD [12]), or acute on chronic respiratory failure (e.g., interstitial lung disease [14], stroke with hyperlipidemia [20], and ESRD [21] with alveolar edema) [3].
Young patients with the onset of SLE, stroke, hypertension, or ESRD have a poor prognosis [22]. Patients with SLE may exhibit severe deterioration of respiratory function such as incident respiratory failure [23]. Deterioration of major organ function may be directly caused by the disease [24], even in patients without pre-existing comorbidities [25]. We hypothesized that SLE is associated with an increased risk of lung [26] or cardiovascular diseases [20] or exacerbates pre-existing airway or vascular diseases [7,12,13], thus contributing to incident respiratory failure (Fig 1). To the best of our knowledge, this is the first English-language study evaluating the relationship between SLE and the risk of incident respiratory failure in the general population.
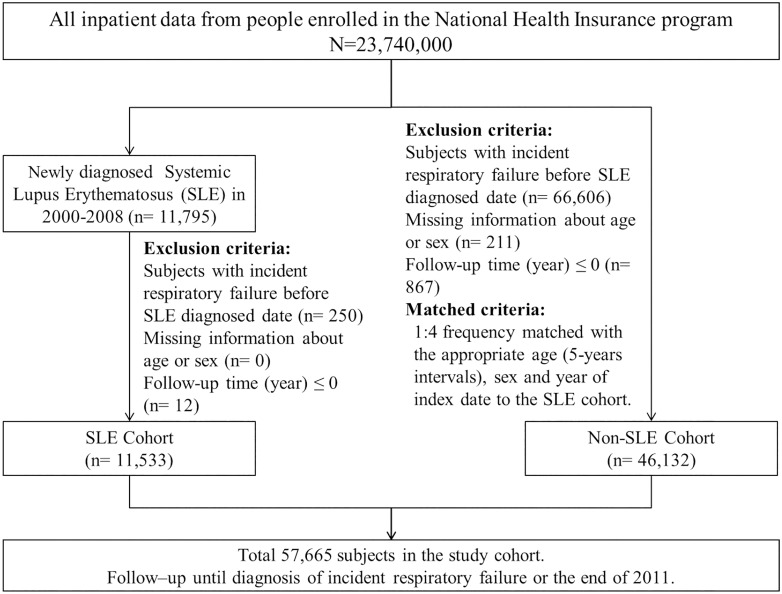
Fig 1. Flowchart presenting the process of selecting the study subjects.
Material and Methods
Data source
Taiwan’s National Health Insurance (NHI) program is a universal insurance program that was launched in 1995. Enrollment is mandatory to ensure adequate risk pooling and broad-based fund collection. Since 2007, the NHI has covered approximately 99% of Taiwan’s population [27]. The National Health Research Institutes (NHRI) obtained the enrollment files and original reimbursement claims data of NHI enrollees to build the National Health Insurance Research Database (NHIRD). The NHRI releases information for research in medicine and health care. We used 2 types of data from the NHIRD in the current study: the Registry for Catastrophic Illness Patient Database (RCIPD) and the inpatient claims data of NHI enrollees from 1997 to 2011. The NHRI protects personal information in the NHIRD by encrypting the identification codes of patients and medical facilities before releasing the data. The diagnoses of diseases in the NHIRD are based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes.
Study population
Fig 1 presents a flow chart of the selection process. This study used a population-based cohort study design [28] and ICD-9-CM codes to define the status of SLE (ICD-9-CM code 710.0) [29], and incident respiratory failure (ICD-9-CM codes 518.81, 518.83, and 518.84). In Taiwan, patients with chronic inflammatory diseases, including those with SLE, can apply for a catastrophic illness certificate; in addition, patients with SLE who fulfill 4 or more diagnostic criteria based on the American College of Rheumatology criteria are eligible for this certificate [13]. The 11 criteria are malar rash; discoid rash; photosensitivity; oral ulcers; nonerosive arthritis; pleuritis or pericarditis; renal disorder, persistent proteinuria, or cellular casts in the urine; neurologic disorder, seizures, or psychosis; hematologic disorder, including hemolytic anemia, leukopenia, lymphopenia, or thrombocytopenia; the production of antinuclear antibody (Ab); and other immunologic disorders, including the production of anti-DNA, anti-Smith or antiphospholipid Abs. Patients with an application showing a catastrophic illness certificate are exempted from copayments. All certificate applications are scrutinized through peer review. We used the RCIPD to identify 11 533 patients with incident SLE in the claims data between 2000 and 2008; the date of SLE diagnosis was used as the index date [30]. For each patient with SLE, 4 controls frequency-matched by age (5-y intervals), sex, and index year were selected from patients without SLE (n = 46 132) to comprise the non-SLE cohort. The dates of randomly selected outpatient or inpatient visits during the index years were selected as the index dates for the non-SLE cohort. Patients were excluded if they had experienced incident respiratory failure before the index date (SLE diagnosis date) or had missing data. We analyzed the development of incident respiratory failure in the SLE cohort compared with that in the non-SLE cohort. The follow-up person-years were calculated at the end of 2011 for each patient or until the diagnosis of incident respiratory failure (n = 1182), withdrawal from the insurance system (n = 3256; 5.65%), or death (n = 18 683; 3.24%). Adjustment was made for pre-existing comorbidities, namely hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM code 272), diabetes (ICD-9-CM code 250) [31], COPD (ICD-9-CM codes 491, 492, and 496), asthma (ICD-9-CM codes 493 and 494), stroke (ICD-9-CM codes 430–438), ischemic heart disease (IHD; ICD-9-CM codes 410–414) [32], pneumonia (ICD-9-CM codes 480–487) [33,34], ESRD (ICD-9-CM code 585), and pulmonary embolism (ICD-9-CM code 415.1), present before the index date.
We used propensity score matching [35] to confirm the results. In addition, we corrected for various risk factors, including sex, age, hypertension, hyperlipidemia, diabetes, COPD, asthma, pneumonia, stroke, ESRD, IHD, pulmonary embolism, and index year, in both cohorts at a 1:3 ratio.
Ethics Statement
The NHIRD encrypts patients’ personal information to protect their privacy and thus provides researchers with anonymous identification numbers associated with relevant claims information, including sex, birth date, medical services received, and prescriptions. Therefore, patient consent is not required in order to access the NHIRD. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH104-REC2-115). The IRB also specifically waived the consent requirement.
Data Availability Statement
All data and related metadata were deposited in an appropriate public repository (http://nhird.nhri.org.tw/en/index.html). The study population data obtained from the NHIRD (http://nhird.nhri.org.tw/en/index.html) are maintained by the NHRI (http://nhird.nhri.org.tw/), a nonprofit foundation established by the Taiwan government. Only citizens of Taiwan who fulfill the requirements of conducting research projects are eligible to apply for data from the NHIRD. The use of the NHIRD is limited to research purposes only. Applicants must follow Taiwan’s Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of the National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and his or her supervisor upon application submission. All applications are reviewed for approval prior to data release.
Statistical Analysis
We used the chi-squared test to compare demographic characteristics such as sex, age (< 35, 35–65, and ≥ 65 y), and history of comorbidities between the SLE and non-SLE cohorts. The mean age of the patients in both cohorts was analyzed using the Student t test. A Poisson regression model was used to estimate the incidence rate ratio (IRR) and 95% confidence interval (CI) of incident respiratory failure in both cohorts. The crude hazard ratios (HRs) of patients with SLE and those without SLE were measured using Cox proportional hazards regression models. After adjustment for potential risk factors (i.e., age, sex, hypertension, hyperlipidemia, diabetes, COPD, asthma, pneumonia, stroke, IHD, pulmonary embolism, and ESRD), the risk was presented as an adjusted HR (aHR) of the SLE cohort by using multivariable Cox proportional hazards regression and compared with that of the non-SLE cohort. The continuous variable of age and dichotomous variables of sex, hypertension, hyperlipidemia, diabetes, COPD, asthma, pneumonia, stroke, IHD, pulmonary embolism, and ESRD were included in the multivariable Cox proportional hazards regression. In addition, the association between the number of medical visits (outpatient visits and hospitalizations) per year resulting from SLE exacerbation and the risk of respiratory failure were calculated. The cumulative respiratory failure incidence rate was calculated for each cohort. Kaplan–Meier analysis was used to estimate the cumulative incidence rate of incident respiratory failure for each subgroup, and a log-rank test was performed to evaluate any between-groups differences.
Propensity scores [35] were calculated using logistic regression to estimate the probability of SLE assignment according to the baseline variables including year of SLE diagnosis, sex, age, and history of comorbidities. After propensity score matching, we performed Cox proportional hazards model stratification of matched pairs to estimate differences in the risk of incident respiratory failure between the SLE and non-SLE cohorts. All statistical analyses were performed using the Statistical Analysis Software Version 9.4 (SAS Institute Inc., NC, USA); a 2-tailed P value of < .05 was considered statistically significant.
Results
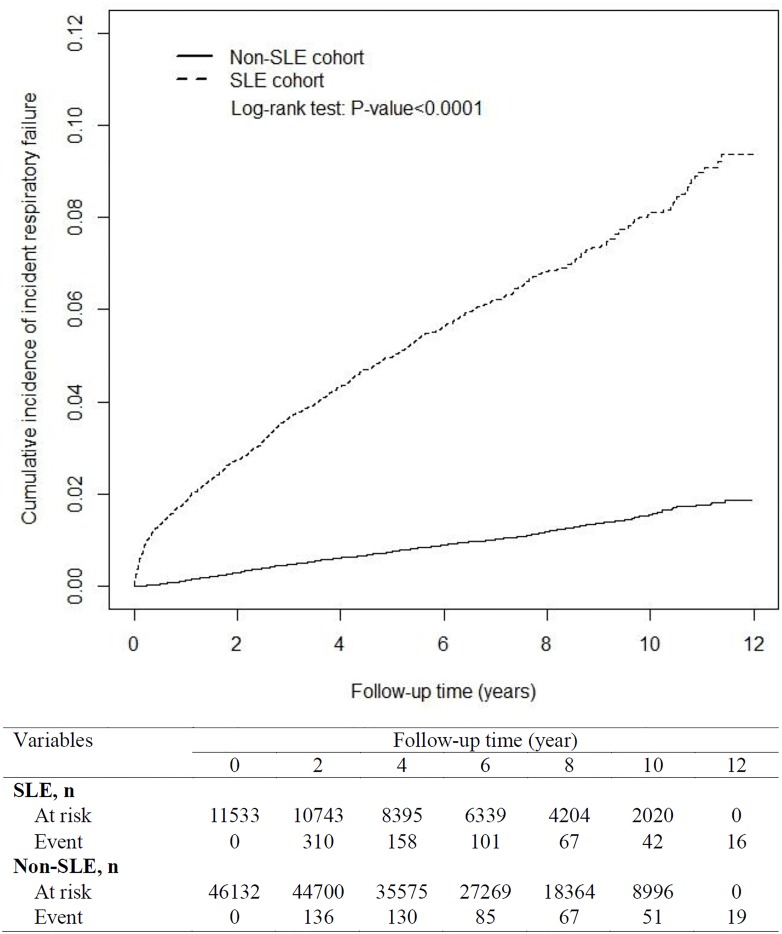
Table 1 shows the demographic characteristic variables in the 2 cohorts. Both cohorts had similar sex and age distributions (chi-squared test: P = .99) and higher proportions of women (88.0%) and patients older than 35 years (53.2%). The mean age of the patients was 35.9 years in both cohorts (Student t test: P = .93)[36]. The SLE cohort also included patients with childhood onset of SLE, and the number of SLE patients younger than 10 years was 139. Compared with the non-SLE cohort, the SLE cohort had a higher percentage of patients with hypertension (2.61% vs. 7.98%), hyperlipidemia (0.74% vs. 2.28%), diabetes (1.64% vs. 2.19%), COPD (0.41% vs. 0.79%), asthma (0.72% vs. 1.23%), stroke (1.09% vs. 2.46%), IHD (2.35% vs. 4.13%), pneumonia (1.77% vs. 7.46%), ESRD (0.17% vs. 0.64%), and pulmonary embolism (0.09% vs. 1.07%; chi-squared test: P < .0001). The mean follow-up period was 6.84 years for the non-SLE cohort and 6.47 years for the SLE cohort. By the end of the follow-up period, the cumulative incidence of incident respiratory failure was higher in the SLE cohort than in the non-SLE cohort (Fig 2).
Table 1. Comparison of the demographics and health status between the SLE and non-SLE cohorts at baseline.
| SLE | |||||
|---|---|---|---|---|---|
| No (N = 46132) | Yes (N = 11533) | p value | |||
| Variables | n | % | n | % | |
| Sex | 0.99 | ||||
| Women | 40592 | 88.0 | 10148 | 88.0 | |
| Men | 5540 | 12.0 | 1385 | 12.0 | |
| Age, year | 0.99 | ||||
| <35 | 24532 | 53.2 | 6133 | 53.2 | |
| 35–65 | 18608 | 40.3 | 4652 | 40.3 | |
| ≥65 | 2992 | 6.49 | 748 | 6.49 | |
| Mean (SD) # | 35.9 (16.3) | 35.9 (16.2) | 0.93 | ||
| Comorbidity | |||||
| Hypertension | 1202 | 2.61 | 920 | 7.98 | <.0001 |
| Hyperlipidemia | 342 | 0.74 | 263 | 2.28 | <.0001 |
| Diabetes | 758 | 1.64 | 252 | 2.19 | <.0001 |
| COPD | 187 | 0.41 | 91 | 0.79 | <.0001 |
| Asthma | 334 | 0.72 | 142 | 1.23 | <.0001 |
| Stroke | 502 | 1.09 | 284 | 2.46 | <.0001 |
| IHD | 1083 | 2.35 | 476 | 4.13 | <.0001 |
| Pneumonia | 817 | 1.77 | 860 | 7.46 | <.0001 |
| ESRD | 79 | 0.17 | 74 | 0.64 | <.0001 |
| Pulmonary Embolism | 42 | 0.09 | 123 | 1.07 | <.0001 |
| Mean of follow-up years (SD) # | 6.84 (3.04) | 6.47 (3.21) | <.0001 | ||
SLE, systemic lupus erythematosus; COPD, chronic obstructive pulmonary disease; IHD, ischemic heart disease; ESRD, end-stage renal disease; chi-squared test;
#Student t tests.
Fig 2. Cumulate incidence of incident respiratory failure between the SLE and non-SLE cohorts, obtained using the Kaplan–Meier model.
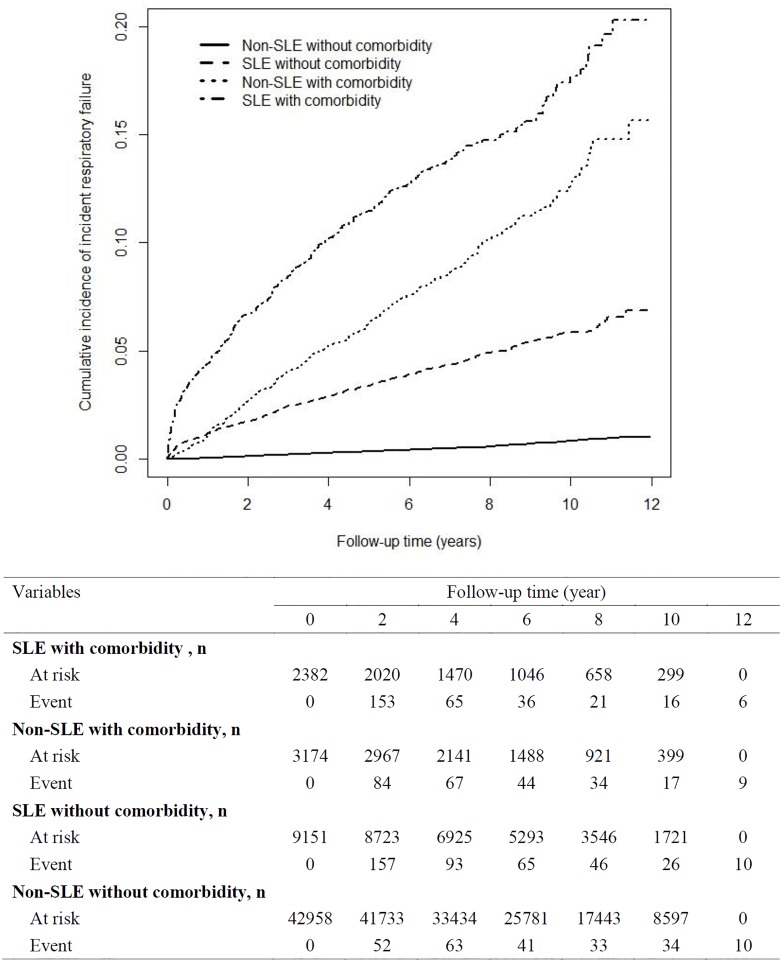
In the SLE cohort, the overall IRR for incident respiratory failure was 6.02 (95% CI = 5.72–6.33) and the aHR was 5.80 (95% CI = 5.15–6.52; Table 2). In the sex-stratified analysis, the women with SLE exhibited a 6.79-fold (95% CI = 5.93–7.77) increased risk of incident respiratory failure compared with the women without SLE. Moreover, men with SLE exhibited a 3.44-fold (95% CI = 2.67–4.43) increased risk of incident respiratory failure compared with the men without SLE. The incidence rate of incident respiratory failure increased with age. Regardless of age group (<35, 35–65, or ≥65 y), patients with SLE had an increased risk of incident respiratory failure than did those without SLE (<35 y: aHR = 31.2, 95% CI = 21.6–45.2; 35–65 y: aHR = 6.19, 95% CI = 5.09–7.54; ≥65 y: aHR = 2.35, 95% CI = 1.92–2.87). In both cohorts, the incidence rate of incident respiratory failure was higher among the patients with pre-existing comorbidities. Among those without pre-existing comorbidities, the risk of incident respiratory failure was 9.08-fold (95% CI = 7.72–10.7) higher in the SLE cohort than the non-SLE cohort. Among those with pre-existing comorbidities, the SLE cohort still exhibited an increased risk of incident respiratory failure compared with the non-SLE cohort (aHR = 2.65, 95% CI = 2.22–3.15) after adjustment for potential risk factors. Fig 3 shows the cumulative incidence of incident respiratory failure in the 4 subgroups: 1) the SLE with pre-existing comorbidities, 2) the non-SLE with pre-existing comorbidities, 3) the SLE without pre-existing comorbidities and 4) the non-SLE without pre-existing comorbidities. Fig 3 display the highest cumulative incidence of incident respiratory failure was in the SLE with pre-existing comorbidities.
Table 2. Incidence and adjusted HR of incident respiratory failure between the non-SLE and SLE cohorts, stratified by sex, age, and comorbidity.
| SLE | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | Compared to non-SLE cohort | ||||||
| Variables | Event | PY | Rate | Event | PY | Rate | IRR (95% CI) | Adjusted HR (95% CI) |
| Overall | 488 | 315622 | 1.55 | 694 | 74616 | 9.30 | 6.02(5.72–6.33)** | 5.80(5.15–6.52)*** |
| Sex | ||||||||
| Women | 346 | 279566 | 1.24 | 579 | 66279 | 8.74 | 7.06(6.68–7.46)*** | 6.79(5.93–7.77)*** |
| Men | 142 | 36056 | 3.94 | 115 | 8337 | 13.8 | 3.50(3.04–4.04)*** | 3.44(2.67–4.43)*** |
| Age, year | ||||||||
| <35 | 32 | 168804 | 0.19 | 264 | 41205 | 6.41 | 33.8(30.2–37.8)*** | 31.2(21.6–45.2)*** |
| 35–65 | 164 | 129503 | 1.27 | 280 | 30076 | 9.31 | 7.35(6.78–7.97)*** | 6.19(5.09–7.54)*** |
| ≥65 | 292 | 17314 | 16.9 | 150 | 3335 | 45.0 | 2.67(2.26–3.15)*** | 2.35(1.92–2.87)*** |
| Comorbidity | ||||||||
| No | 233 | 296729 | 0.79 | 397 | 61409 | 6.46 | 8.23(7.78–8.71)*** | 9.08(7.72–10.7)*** |
| Yes | 255 | 18893 | 13.5 | 297 | 13206 | 22.5 | 1.67(1.45–1.91)*** | 2.65(2.22–3.15)*** |
SLE, Systemic Lupus Erythematosus; PY: person-year; Rate: incidence rate (per 1,000 person-y); IRR, incidence rate ratio; HR, hazard ratio; multiple analysis including age, sex, and comorbidities;
**P < 0.01,
***P < 0.001.
Fig 3. Cumulative incidence of incident respiratory failure in the different subgroups.
Table 3 presents the comorbidity-stratified analysis. Compared to the non-SLE cohort, the incidence of incident respiratory failure was higher in those without pre-existing comorbidities (All P values < .001) among the SLE cohort. Meanwhile, the incidence of incident respiratory failure was higher in those with pre-existing comorbidities except ESRD and pulmonary embolism among the SLE cohort also (All P values < .001; except ESRD and pulmonary embolism).
Table 3. Incidence and adjusted HR of incident respiratory failure between the non-SLE and SLE cohorts, stratified by comorbidity type.
| SLE | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | Compared to non-SLE cohort | ||||||
| Variables | Event | PY | Rate | Event | PY | Rate | IRR (95% CI) | Adjusted HR (95% CI) |
| Hypertension | ||||||||
| No | 351 | 309067 | 1.14 | 548 | 70062 | 7.82 | 6.89(6.53–7.26)*** | 6.78(5.91–7.78)*** |
| Yes | 137 | 6555 | 20.9 | 146 | 4553 | 32.1 | 1.53(1.24–1.90)*** | 2.90(2.25–3.74)*** |
| Hyperlipidemia | ||||||||
| No | 463 | 313686 | 1.48 | 660 | 73119 | 9.03 | 6.12(5.81–6.44)*** | 5.76(5.10–6.50)*** |
| Yes | 25 | 1936 | 12.9 | 34 | 1496 | 22.7 | 1.76(1.14–2.72)** | 5.53(3.04–10.1)*** |
| Diabetes | ||||||||
| No | 388 | 311580 | 1.25 | 642 | 73489 | 8.74 | 7.02(6.66–7.40)*** | 6.55(5.76–7.45)*** |
| Yes | 100 | 4041 | 24.8 | 52 | 1127 | 46.2 | 1.87(1.38–2.52)*** | 2.34(1.64–3.33)*** |
| COPD | ||||||||
| No | 452 | 314718 | 1.44 | 670 | 74231 | 9.03 | 6.28(5.97–6.62)*** | 6.06(5.37–6.85)*** |
| Yes | 36 | 904 | 39.8 | 24 | 385 | 62.4 | 1.57(0.88–2.78) | 2.47(1.39–4.41)*** |
| Asthma | ||||||||
| No | 456 | 313781 | 1.45 | 661 | 73909 | 8.94 | 6.15(5.85–6.48)*** | 5.90(5.23–6.67)*** |
| Yes | 32 | 1841 | 17.4 | 33 | 707 | 46.7 | 2.69(1.71–4.22)*** | 3.46(2.06–5.81)*** |
| Stroke | ||||||||
| No | 408 | 313045 | 1.30 | 642 | 73301 | 8.76 | 6.72(6.38–7.08)*** | 6.38(5.62–7.25)*** |
| Yes | 80 | 2576 | 31.1 | 52 | 1314 | 39.6 | 1.27(0.90–1.81) | 2.32(1.58–3.40)*** |
| IHD | ||||||||
| No | 361 | 308495 | 1.17 | 622 | 71742 | 8.67 | 7.41(7.02–7.82)*** | 7.08(6.19–8.08)*** |
| Yes | 127 | 7126 | 17.8 | 72 | 2874 | 25.1 | 1.41(1.09–1.81)** | 2.11(1.57–2.85)*** |
| Pneumonia | ||||||||
| No | 452 | 311281 | 1.45 | 588 | 70098 | 8.39 | 5.78(5.49–6.08)*** | 5.85(5.17–6.63)*** |
| Yes | 36 | 4340 | 8.29 | 106 | 4518 | 23.5 | 2.83(2.08–3.85)*** | 4.21(2.83–6.27)*** |
| ESRD | ||||||||
| No | 478 | 315240 | 1.52 | 683 | 74193 | 9.21 | 6.07(5.77–6.39)*** | 5.86(5.20–6.61)*** |
| Yes | 10 | 381 | 26.2 | 11 | 423 | 26.0 | 0.99(0.46–2.14) | 1.96(0.69–5.56) |
| Pulmonary Embolism | ||||||||
| No | 480 | 315360 | 1.52 | 678 | 73949 | 9.17 | 6.02(5.72–6.34)*** | 5.90(5.24–6.65)*** |
| Yes | 8 | 262 | 30.5 | 16 | 667 | 24.0 | 0.79(0.37–1.66) | 1.26(0.45–3.52) |
SLE, systemic lupus erythematosus; COPD, chronic obstructive pulmonary disease; IHD, ischemic heart disease; ESRD, end-stage renal disease; PY: person-year; Rate: incidence rate (per 1,000 person-y); IRR, incidence rate ratio; HR, hazard ratio; multivariable analysis including age, sex, and comorbidities;
**P < .01,
***P < .001.
Table 4 presents the association between the risk of incident respiratory hortfailure and the number of medical visits per year by patients with SLE. The risk of incident respiratory failure was highest in the SLE patients with more than 24 outpatient visits and hospitalizations (aHR = 21.7, 95% CI = 18.0–26.1). The risk of incident respiratory failure was higher in patients who had a higher annual medical visit and hospitalization frequency.
Table 4. Adjusted HR of incident respiratory failure associated with the annual frequency of outpatient visits and hospitalizations because of SLE exacerbation.
| Variables | N | Event | Rate | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Non-SLE cohort | 46132 | 488 | 1.55 | 1.00 | 1.00 |
| Number of outpatient visits and hospitalizations per year | |||||
| ≤ 11 | 5302 | 178 | 4.85 | 3.14(2.65–3.73)*** | 3.09(2.60–3.67)*** |
| 12–17 | 3965 | 173 | 6.63 | 4.28(3.60–5.09)*** | 4.77(4.00–5.69)*** |
| 18–24 | 1548 | 173 | 19.5 | 12.4(10.4–14.7)*** | 11.4(9.57–13.7)*** |
| > 24 | 718 | 170 | 58.0 | 35.8(30.1–42.7)*** | 21.7(18.0–26.1)*** |
| p-value for trend | <.0001 | <.0001 |
SLE, Systemic Lupus Erythematosus; HR, hazard ratio; multivariable analysis including age, sex, and comorbidities;
***P < .001.
The propensity score-matching analysis [35] was used to reduce bias when investigating the association between incident respiratory failure outcomes and SLE. In total, 9841 and 29 523 patients were included in the SLE and non-SLE cohorts, respectively (Table 5). The standardized difference was low in the baseline potential risk in both cohorts (standardized difference < 0.1) [35]. After propensity score matching, the risk of incident respiratory failure remained higher in the SLE cohort than in the non-SLE cohort (aHR = 7.84, 95% CI = 5.82–10.6; Table 6).
Table 5. Comparison in demographic status and health status between SLE and non-SLE cohorts after propensity score matching.
| SLE | |||||
|---|---|---|---|---|---|
| No (N = 29523) | Yes (N = 9841) | Standardized difference | |||
| Variables | n | % | n | % | |
| Sex | 0.002 | ||||
| Women | 26257 | 88.9 | 8759 | 89.0 | |
| Men | 3266 | 11.1 | 1082 | 11.0 | |
| Age, year | 0.002 | ||||
| <35 | 16500 | 55.9 | 5501 | 55.9 | |
| 35–65 | 11435 | 38.7 | 3897 | 39.6 | |
| ≥65 | 1588 | 5.38 | 443 | 4.50 | |
| Mean (SD) # | 34.8 (15.7) | 34.6 (15.2) | 0.002 | ||
| Comorbidity | |||||
| Hypertension | 368 | 1.25 | 95 | 0.97 | 0.027 |
| Hyperlipidemia | 163 | 0.55 | 55 | 0.56 | 0.001 |
| Diabetes | 573 | 1.94 | 188 | 1.91 | 0.002 |
| COPD | 102 | 0.35 | 46 | 0.47 | 0.019 |
| Asthma | 188 | 0.64 | 71 | 0.72 | 0.010 |
| Stroke | 208 | 0.70 | 126 | 1.28 | 0.058 |
| IHD | 546 | 1.85 | 273 | 2.77 | 0.062 |
| Pneumonia | 134 | 0.45 | 49 | 0.50 | 0.006 |
| ESRD | 41 | 0.14 | 20 | 0.20 | 0.016 |
| Pulmonary Embolism | 2 | 0.01 | 2 | 0.02 | 0.012 |
SLE, systemic lupus erythematosus; COPD, chronic obstructive pulmonary disease; IHD, ischemic heart disease; ESRD, end-stage renal disease.
Table 6. Incidence and adjusted hazard ratio of incident respiratory failure between non-SLE and SLE cohorts by propensity score matching.
| SLE | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | Compared to non-SLE cohort | ||||||
| Variables | Event | PY | Rate | Event | PY | Rate | IRR (95% CI) | Hazard Ratio (95% CI) |
| Overall | 271 | 204242 | 1.33 | 488 | 65457 | 7.46 | 5.62(5.27–5.99)*** | 7.84(5.82–10.6)*** |
SLE, systemic lupus erythematosus; PY: person-year; Rate: incidence rate (per 1,000 person-years); IRR, incidence rate ratio;
***p<0.001.
Discussion
This study revealed that regardless of age, sex, or pre-existing comorbidities, the incidence of incident respiratory failure was higher in the SLE cohort than in the non-SLE cohort (aHR = 5.80, 95% CI = 5.15–6.52). SLE patients older than 65 years of age exhibited a higher incidence of incident respiratory failure, which is in contrast to the poor prognosis of young women [37] reported in a previous study [18]. A possible explanation may be that older patients in the SLE and non-SLE cohorts tended to have more primary complications, such as cardiovascular [11] and pulmonary lesions [38], resulting from the primary deterioration [38,39] of the patients in the SLE cohort; these primary complications may be risk factors for the onset of incident respiratory failure among older adults.
In this study, males [aHR = 3.44, Table 2] with SLE exhibited a higher risk of incident respiratory failure as did their female [aHR = 6.79,Table 2] counterparts [40]. Furthermore, the incidence of incident respiratory failure was higher in patients with SLE without pre-existing comorbidities than it was in those without SLE and pre-existing comorbidities [Fig 3]. The risk of incident respiratory failure in the SLE cohort without pre-existing the atherosclerosis-related diseases (e.g.; hypertension [aHR = 6.78], stroke [aHR = 6.38], IHD [aHR = 7.08]; Table 3) were still higher. These findings aid clinicians by revealing that men with SLE but without pre-existing comorbidities [25,41] could develop incident respiratory failure.
Systemic lupus erythematosus is protean in its manifestations and follows a relapsing and remitting course. No study has investigated the relationship of the numbers of SLE exacerbations and related hospital admissions with the risk of incident respiratory failure. We observed that an increased frequency of outpatient department visits and hospitalizations was associated with SLE exacerbation. This finding suggests that poor control of the deterioration of an obstructed airway, occlusion of the pulmonary vessel, and atherosclerosis of the arterial system or coronary artery were crucial factors for incident respiratory failure in the SLE cohort. The recurrence of SLE exacerbation causes primary cumulative damage [39] to the lung parenchyma (e.g., pneumonia) [19,42], airway (e.g., airway obstruction [12,26,43,44]), and pulmonary vessel (e.g., pulmonary embolism) [13,45], thus resulting in hypoxemia and incident respiratory failure [29] in SLE patients without pre-existing comorbidities.
Several factors may predispose patients with SLE to respiratory failure, including pulmonary lesions, such as those in the airway [12], vessels [19], interstitium [11], and pleura [41]; and extrapulmonary lesions such as those in the brain [11], kidneys [10], and heart [11]. In patients with SLE, the functions of these organs progressively deteriorate [46], which is associated with incident comorbidities [18,45,47]. In our study, the patients with SLE without pre-existing comorbidities (e.g., pulmonary embolism [aHR = 5.90], ESRD, [aHR = 5.86]; Table 3) had a higher risk of incident comorbidities with respiratory failure compared with the non-SLE cohort; this finding concurs with that of a previous study [48].
If patients with SLE develop pulmonary or cardiovascular lesions along with the pre-existing pulmonary disease (e.g., airway, parenchyma [12]), microvascular disease (e.g., diabetes [16,18]), or macrovascular disease (e.g., hypertension, stroke) [12,15,17], the dual effects may contribute to cumulative damage to the airway or system vessels [39,49], thereby increasing the risk of incident respiratory failure [3,29]. In our study, the SLE patients with pre-existing comorbidities (e.g., hypertension, [aHR = 2.90], diabetes [aHR = 2.34], COPD [aHR = 2.47], stroke [aHR = 2.32], and pneumonia [aHR = 4.21]; Table 3) exhibited a higher risk of the incident respiratory failure compared with the non-SLE cohort; this finding is in line with the findings of previous research.
Our findings alert clinicians to be aware of the effect of SLE on the risk of incident respiratory failure [29,42], particularly in patients who frequently receive medical services, regardless of age, sex, and pre-existing comorbidities.
Limitations
This study has several limitations that should be addressed when interpreting its findings. The NHIRD does not provide detailed lifestyle information, such as smoking history, body mass index, or physical activity level, all of which are potential confounding factors for this study. However, the SLE treatment and lifestyle modification of patients with SLE may implicate these factors in accelerated respiratory lesions in SLE. In addition, information on the severity of SLE, such as disease activity, functional impairment, and physical damage, may result in the underdiagnosis of hypertension, hyperlipidemia, or diabetes. Patients listed in the RCIPD receive resgular immunosuppressant therapy. The lack of detailed drug data, such as hydroxychloroquine and glucocorticosteroids, for adjusting the outcomes of interest could be another limitation. In addition, the clinical presentation of SLE may vary. Undiagnosed SLE [50] may affect the diagnosis of incident respiratory failure. Pleural lesions and interstitial lung disease are potential factors for respiratory failure [3]. In addition, SLE patients complicated with antiphospholipid Ab syndrome may be at a higher risk of developing respiratory failure [51]. Hypercoagulation state due to formation of antiphospholipid Ab can result in catastrophic diseases resulting from vascular occlusion in such diseases as stroke (n = 284), IHD (n = 476), ESRD (n = 74), and pulmonary embolism (n = 123) (Table 1). We could identify only approximately 8.3% of possible cases of aniphospholippid Ab syndrome from the current SLE cohort (n = 11 533 in Table 1). There is no specific ICD-9 code for antihopholipid Ab syndrome that could have assisted us in exploring this issue in the current study. Moreover, hemorrhagic alveolitis [52] is a rare disease [47] complicated with the antihopholipid Ab syndrome, which was not analyzed in the present study. The diagnosis of pneumonia [53] requires thoracic imaging, titer of the serum, sputum smear/culture, respiratory specimens, or tissues with pathological proof [42], which were not available in this study [54]. Finally, the prevalence of COPD [55] in young adults [56] is low [57], and, from an epidemiological perspective, the incidence of asthma is relatively lower in control groups. Asthma and COPD may indicate past respiratory failure [58] before the SLE diagnosis date. Therefore, patients with asthma or COPD were excluded from our study if they had past respiratory failure before the index date (SLE diagnosis date), which may explain the relatively lower frequency of asthma in the non-SLE group; thus, this is another confounding factor. These limitations warrant further investigation.
Strengths
In Taiwan, SLE is categorized as a catastrophic disease [12]; thus, the validity period of this disease is valid for life. Each patient in this study was individually followed up until the diagnosis of incident respiratory failure. In addition, the professional discipline care of SLE patients since 2007 is in line with the 1995–2007 period. Therefore, the coverage of the insurance program after 2007 is similar to that from 1995 to 2007, with no significant differences [59]. Furthermore, respiratory failure is also categorized as a catastrophic disease in Taiwan [60]. These 2 diseases should be scrutinized through peer review by a specialist [12]. Multidisciplinary teams (e.g., rheumatologist, cardiologist, pulmonologist, respiratory therapist and certified educator) guide the assessment, treatment, and holistic care of patients with incident SLE and subsequent incident respiratory failure [12,61]. This study adopted a nationwide population-based cohort longitudinal design to evaluate the risk of incident respiratory failure in a mostly Asian population with SLE. The findings can thus be generalized to the general population [62].
Conclusion
This study determined that patients with SLE are associated with an increased risk of incident respiratory failure, regardless of age, sex, and pre-existing comorbidities; this increased risk is particularly pronounced among patients who frequently receive medical services.
Supporting Information
(DOC)
Abbreviations
- aHR
adjusted hazard ratio
- CI
confidence interval
- NHIRD
National Health Insurance Research Database
- NHI
National Health Insurance
- NHRI
National Health Research Institutes
- LHID 2000
Longitudinal Health Insurance Database 2000
- SLE
systemic lupus erythematosus
- COPD
chronic obstructive pulmonary disease
- ESRD
end-stage renal disease
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
Data Availability
The data on the study population that were obtained from the NHIRD (http://nhird.nhri.org.tw/en/index.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/). The NHRI is a nonprofit foundation established by the government. Only citizens of the Republic of China who fulfill the requirements of conducting research projects are eligible to apply for the NHIRD. The use of NHIRD is limited to research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and his/her supervisor upon application submission. All applications are reviewed for approval of data release.
Funding Statement
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039 -005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1.Afessa B, Keegan MT, Mohammad Z, Finkielman JD, Peters SG. Identifying potentially ineffective care in the sickest critically ill patients on the third ICU day. Chest 2004;126: 1905–1909. [DOI] [PubMed] [Google Scholar]
- 2.Gunning KEJ. Pathophysiology of Respiratory Failure and Indications for Respiratory Support. Surgery (Medicine Publishing) 2003;21: 72–76. [Google Scholar]
- 3.Roussos C, Koutsoukou A. Respiratory failure. Eur Respir J Suppl 2003;47: 3s–14s. [DOI] [PubMed] [Google Scholar]
- 4.Han MK, McLaughlin VV, Criner GJ, Martinez FJ. Pulmonary diseases and the heart. Circulation 2007;116: 2992–3005. [DOI] [PubMed] [Google Scholar]
- 5.Yeh KW, Yu CH, Chan PC, Horng JT, Huang JL. Burden of systemic lupus erythematosus in Taiwan: a population-based survey. Rheumatol Int 2013;33: 1805–1811. 10.1007/s00296-012-2643-6 [DOI] [PubMed] [Google Scholar]
- 6.Lai NS, Tsai TY, Koo M, Huang KY, Tung CH, Lu MC. Patterns of Ambulatory Medical Care Utilization and Rheumatologist Consultation Predating the Diagnosis of Systemic Lupus Erythematosus: A National Population-Based Study. PLoS ONE 2014;9: e101485 10.1371/journal.pone.0101485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen TC, Tu CY, Lin CL, Wei CC, Li YF. Increased risk of asthma in patients with systemic lupus erythematosus. Am J Respir Crit Care Med 2014;189: 496–499. 10.1164/rccm.201310-1792LE [DOI] [PubMed] [Google Scholar]
- 8.Murray SG, Schmajuk G, Trupin L, Gensler L, Katz PP, Yelin EH, et al. National Lupus Hospitalization Trends Reveal Rising Rates of Herpes Zoster and Declines in Pneumocystis Pneumonia. PLoS ONE 2016;11: e0144918 10.1371/journal.pone.0144918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinicato NA, da Silva Cardoso PA, Appenzeller S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr Cardiol Rev 2013;9: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Han SS, Qin DD, Wu LH, Song Y, Yu F, et al. Renal Interstitial Arteriosclerotic Lesions in Lupus Nephritis Patients: A Cohort Study from China. PLoS ONE 2015;10: e0141547 10.1371/journal.pone.0141547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeleniewicz R, Suszek D, Majdan M. Clinical picture of late-onset systemic lupus erythematosus in a group of Polish patients. Pol Arch Med Wewn 2015;125: 538–544. [DOI] [PubMed] [Google Scholar]
- 12.Shen TC, Lin CL, Chen CH, Tu CY, Hsia TC, Shih CM, et al. Increased risk of chronic obstructive pulmonary disease in patients with systemic lupus erythematosus: a population-based cohort study. PLoS ONE 2014;9: e91821 10.1371/journal.pone.0091821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung WS, Lin CL, Chang SN, Lu CC, Kao CH. Systemic lupus erythematosus increases the risks of deep vein thrombosis and pulmonary embolism: a nationwide cohort study. J Thromb Haemost 2014;12: 452–458. 10.1111/jth.12518 [DOI] [PubMed] [Google Scholar]
- 14.Schneider F, Gruden J, Tazelaar HD, Leslie KO. Pleuropulmonary pathology in patients with rheumatic disease. Arch Pathol Lab Med 2012;136: 1242–1252. [DOI] [PubMed] [Google Scholar]
- 15.Ballocca F, D'Ascenzo F, Moretti C, Omede P, Cerrato E, Barbero U, et al. Predictors of cardiovascular events in patients with systemic lupus erythematosus (SLE): a systematic review and meta-analysis. Eur J Prev Cardiol. 2015;22:1435–41. 10.1177/2047487314546826 [DOI] [PubMed] [Google Scholar]
- 16.Santos MJ, Fonseca JE. Metabolic syndrome, inflammation and atherosclerosis—the role of adipokines in health and in systemic inflammatory rheumatic diseases. Acta Reumatol Port 2009;34: 590–598. [PubMed] [Google Scholar]
- 17.Kao AH, McBurney CA, Sattar A, Lertratanakul A, Wilson NL, Rutman S, et al. Relation of platelet C4d with all-cause mortality and ischemic stroke in patients with systemic lupus erythematosus. Transl Stroke Res 2014;5: 510–518. 10.1007/s12975-013-0295-9 [DOI] [PubMed] [Google Scholar]
- 18.Thomas G, Mancini J, Jourde-Chiche N, Sarlon G, Amoura Z, Harlé JR, et al. Mortality associated with systemic lupus erythematosus in France assessed by multiple-cause-of-death analysis. Arthritis Rheumatol 2014;66: 2503–2511. 10.1002/art.38731 [DOI] [PubMed] [Google Scholar]
- 19.Tektonidou MG, Wang Z, Dasgupta A, Ward MM. Burden of Serious Infections in Adults With Systemic Lupus Erythematosus: A National Population-Based Study, 1996–2011. Arthritis Care Res (Hoboken). 2015;67: 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenfeld SR, Kasturi S, Costenbader KH. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Semin Arthritis Rheum 2013;43: 77–95. 10.1016/j.semarthrit.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 21.Yu KH, Kuo CF, Chou IJ, Chiou MJ, See LC. Risk of end-stage renal disease in systemic lupus erythematosus patients: a nationwide population-based study. Int J Rheum Dis. 2016. February 10 10.1111/1756-185X.12828. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Mackie FE, Kainer G, Adib N, Boros C, Elliott EJ, Fahy R, et al. The national incidence and clinical picture of SLE in children in Australia—a report from the Australian Paediatric Surveillance Unit. Lupus 2015;24: 66–73. 10.1177/0961203314552118 [DOI] [PubMed] [Google Scholar]
- 23.Wiedermann FJ, Mayr A, Schobersberger W, Knotzer H, Sepp N, Rieger M, et al. Acute respiratory failure associated with catastrophic antiphospholipid syndrome. J Intern Med 2000;247: 723–730. [DOI] [PubMed] [Google Scholar]
- 24.Pardos-Gea J, Avegliano G, Evangelista A, Vilardell M, Ordi-Ros J. Cardiac manifestations other than valvulopathy in antiphospholipid syndrome: long-time echocardiography follow-up study. Int J Rheum Dis 2015;18: 76–83. 10.1111/1756-185X.12191 [DOI] [PubMed] [Google Scholar]
- 25.Fessler BJ, Boumpas DT. Severe major organ involvement in systemic lupus erythematosus. Diagnosis and management. Rheum Dis Clin North Am 1995;21: 81–98. [PubMed] [Google Scholar]
- 26.Al-Abbad AJ, Cabral DA, Sanatani S, Sandor GG, Seear M, Petty RE, et al. Echocardiography and pulmonary function testing in childhood onset systemic lupus erythematosus. Lupus 2001;10: 32–37. [DOI] [PubMed] [Google Scholar]
- 27.Cheng TM. Taiwan's new national health insurance program: genesis and experience so far. Health Aff (Millwood) 2003;22: 61–76. [DOI] [PubMed] [Google Scholar]
- 28.Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg 2010;126: 2234–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goss LB, Ortiz JR, Okamura DM, Hayward K, Goss CH. Significant Reductions in Mortality in Hospitalized Patients with Systemic Lupus Erythematosus in Washington State from 2003 to 2011. PLoS ONE 2015;10: e0128920 10.1371/journal.pone.0128920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin CY, Shih CC, Yeh CC, Chou WH, Chen TL, Liao CC. Increased risk of acute myocardial infarction and mortality in patients with systemic lupus erythematosus: two nationwide retrospective cohort studies. Int J Cardiol 2014;176: 847–851. 10.1016/j.ijcard.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 31.Yeh CC, Wang HH, Chou YC, Hu CJ, Chou WH, Chen TL, et al. High risk of gastrointestinal hemorrhage in patients with epilepsy: a nationwide cohort study. Mayo Clin Proc 2013;88: 1091–1098. 10.1016/j.mayocp.2013.06.024 [DOI] [PubMed] [Google Scholar]
- 32.Huang WS, Tseng CH, Lin CL, Tsai CH, Kao CH. Helicobacter pylori infection increases subsequent ischemic stroke risk: a nationwide population-based retrospective cohort study. QJM 2014;107: 969–975. 10.1093/qjmed/hcu117 [DOI] [PubMed] [Google Scholar]
- 33.Guevara RE, Butler JC, Marston BJ, Plouffe JF, File TM, Breiman RF. Accuracy of ICD-9-CM Codes in Detecting Community-acquired Pneumococcal Pneumonia for Incidence and Vaccine Efficacy Studies. Am J Epidemiol. 1999;149: 282–289. [DOI] [PubMed] [Google Scholar]
- 34.Drahos J, Vanwormer JJ, Greenlee RT, Landgren O, Koshiol J. Accuracy of ICD-9-CM codes in identifying infections of pneumonia and herpes simplex virus in administrative data. Ann Epidemiol. 2013;23:291–3. 10.1016/j.annepidem.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tien YC, Chiu YM, Liu MP. Frequency of Lost to Follow-Up and Associated Factors for Patients with Rheumatic Diseases. PLoS ONE 2016;11: e0150816 10.1371/journal.pone.0150816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol 2014;66: 369–378. 10.1002/art.38238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Celinska-Lowenhoff M, Musial J. Late-onset systemic lupus erythematosus: clinical manifestations, course, and prognosis. Pol Arch Med Wewn 2015;125: 497–499. [PubMed] [Google Scholar]
- 39.Catoggio LJ, Soriano ER, Imamura PM, Wojdyla D, Jacobelli S, Massardo L, et al. Late-onset systemic lupus erythematosus in Latin Americans: a distinct subgroup? Lupus 2015;24: 788–795. 10.1177/0961203314563134 [DOI] [PubMed] [Google Scholar]
- 40.Urowitz MB, Gladman D, Ibanez D, Bae SC, Sanchez-Guerrero J, Gordon C, et al. Atherosclerotic vascular events in a multinational inception cohort of systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010;62: 881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carmier D, Marchand-Adam S, Diot P, Diot E. Respiratory involvement in systemic lupus erythematosus. Rev Mal Respir 2010;27: e66–78. 10.1016/j.rmr.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 42.Chen M, Tian X, Qin F, Zhou J, Liu J, Wang M, et al. Pneumocystis Pneumonia in Patients with Autoimmune Diseases: A Retrospective Study Focused on Clinical Characteristics and Prognostic Factors Related to Death. PLoS ONE 2015;10: e0139144 10.1371/journal.pone.0139144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silberstein SL, Barland P, Grayzel AI, Koerner SK. Pulmonary dysfunction in systemic lupus erythematosus:prevalence classification and correlation with other organ involvement. J Rheumatol 1980;7: 187–195. [PubMed] [Google Scholar]
- 44.Keane MP, Lynch JP 3rd. Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax 2000;55: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuily S, Wahl D. Pulmonary hypertension in antiphospholipid syndrome. Curr Rheumatol Rep 2015;17: 478 10.1007/s11926-014-0478-8 [DOI] [PubMed] [Google Scholar]
- 46.Al Sawah S, Zhang X, Zhu B, Magder LS, Foster SA, Iikuni N, et al. Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus-the Hopkins Lupus Cohort. Lupus Sci Med 2015;2: e000066 10.1136/lupus-2014-000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kazzaz NM, Coit P, Lewis EE, McCune WJ, Sawalha AH, Knight JS. Systemic lupus erythematosus complicated by diffuse alveolar haemorrhage: risk factors, therapy and survival. Lupus Sci Med. 2015;2: e000117 10.1136/lupus-2015-000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Souza DC, Santo AH, Sato EI. Mortality profile related to systemic lupus erythematosus: a multiple cause-of-death analysis. J Rheumatol 2012;39: 496–503. 10.3899/jrheum.110241 [DOI] [PubMed] [Google Scholar]
- 49.Piga M, Casula L, Perra D, Sanna S, Floris A, Antonelli A, et al. Population-based analysis of hospitalizations in a West-European region revealed major changes in hospital utilization for patients with systemic lupus erythematosus over the period 2001–2012. Lupus. 2016;25:28–37. 10.1177/0961203315596597 [DOI] [PubMed] [Google Scholar]
- 50.Johnson AE, Gordon C, Hobbs FD, Bacon PA. Undiagnosed systemic lupus erythematosus in the community. Lancet 1996;347: 367–369. [DOI] [PubMed] [Google Scholar]
- 51.Asherson RA. Multiorgan failure and antiphospholipid antibodies: the catastrophic antiphospholipid (Asherson's) syndrome. Immunobiology 2005;210: 727–733. [DOI] [PubMed] [Google Scholar]
- 52.Cartin-Ceba R, Diaz-Caballero L, Al-Qadi MO, Tryfon S, Fervenza FC, Ytterberg SR, et al. Diffuse Alveolar Hemorrhage Secondary to Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: Predictors of Respiratory Failure and Clinical Outcomes. Arthritis Rheumatol 2016;68: 1467–1476. 10.1002/art.39562 [DOI] [PubMed] [Google Scholar]
- 53.Narata R, Wangkaew S, Kasitanon N, Louthrenoo W. Community-acquired pneumonia in Thai patients with systemic lupus erythematosus. Southeast Asian J Trop Med Public Health 2007;38: 528–536. [PubMed] [Google Scholar]
- 54.Yeh JJ, Neoh CA, Chen CR, Chou CY, Wu MT. A high resolution computer tomography scoring system to predict culture-positive pulmonary tuberculosis in the emergency department. PLoS ONE 2014;9: e93847 10.1371/journal.pone.0093847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung WS, Lin CL, Kao CH. Comparison of acute respiratory events between asthma-COPD overlap syndrome and COPD patients: a population-based cohort study. Medicine (Baltimore) 2015;94: e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baty F, Putora PM, Isenring B, Blum T, Brutsche M. Comorbidities and burden of COPD: a population based case-control study. PLoS ONE 2013;8: e63285 10.1371/journal.pone.0063285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inghammar M, Ekbom A, Engstrom G, Ljungberg B, Romanus V, Löfdahl CG, et al. COPD and the risk of tuberculosis—a population-based cohort study. PLoS ONE 2010;5: e10138 10.1371/journal.pone.0010138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dharmarajan K, Strait KM, Lagu T, Lindenauer PK, Tinetti ME, Lynn J, et al. Acute decompensated heart failure is routinely treated as a cardiopulmonary syndrome. PLoS ONE 2013;8: e78222 10.1371/journal.pone.0078222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsu YH, Chen HJ, Shen SC, Tsai WC, Hsu CC, Kao CH. Reduced Stroke Risk After Parathyroidectomy in End-Stage Renal Disease: A 13-Year Population-BasStudyed Cohort. Medicine (Baltimore) 2015;94: e936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nan-Ping Y, Yi-Hui L, Chi-Yu C, Jin-Chyr H, Y IL, Nien-Tzu C, et al. Comparisons of medical utilizations and categorical diagnoses of emergency visits between the elderly with catastrophic illness certificates and those without. BMC Health Serv Res 2013;13: 152 10.1186/1472-6963-13-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang WC, Tsai YH, Wei YF, Kuo PH, Tao CW, Cheng SL, et al. Wheezing, a significant clinical phenotype of COPD: experience from the Taiwan Obstructive Lung Disease Study. Int J Chron Obstruct Pulmon Dis 2015;10: 2121–2126. 10.2147/COPD.S92062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Booth CM, Rapoport B. Uptake of novel medical therapies in the general population. Curr Oncol 2011;18: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
The data on the study population that were obtained from the NHIRD (http://nhird.nhri.org.tw/en/index.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/). The NHRI is a nonprofit foundation established by the government. Only citizens of the Republic of China who fulfill the requirements of conducting research projects are eligible to apply for the NHIRD. The use of NHIRD is limited to research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and his/her supervisor upon application submission. All applications are reviewed for approval of data release.
All data and related metadata were deposited in an appropriate public repository (http://nhird.nhri.org.tw/en/index.html). The study population data obtained from the NHIRD (http://nhird.nhri.org.tw/en/index.html) are maintained by the NHRI (http://nhird.nhri.org.tw/), a nonprofit foundation established by the Taiwan government. Only citizens of Taiwan who fulfill the requirements of conducting research projects are eligible to apply for data from the NHIRD. The use of the NHIRD is limited to research purposes only. Applicants must follow Taiwan’s Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of the National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and his or her supervisor upon application submission. All applications are reviewed for approval prior to data release.