Abstract
Posterior fossa syndrome is a severe transient loss of language that frequently complicates resection of tumors of the cerebellum. The associated pathophysiology and relevant anatomy to this language deficit remains controversial. We performed a retrospective analysis of all cerebellar tumor resections at Seattle Children's Hospital from 2010 to 2015. Diffusion tensor imaging was performed on each of the patients as part of their clinical scan. Patients included in the study were divided into groups based on language functioning following resection: intact (N = 19), mild deficit (N = 19), and posterior fossa syndrome (N = 9). Patients with posterior fossa syndrome showed white matter changes evidenced by reductions in fractional anisotropy in the left and right superior cerebellar peduncle following resection, and these changes were still evident 1-year after surgery. These changes were greater in the superior cerebellar peduncle than elsewhere in the cerebellum. Prior to surgery, posterior fossa patients did not show changes in fractional anisotropy however differences were observed in mean and radial diffusivity measures in comparison to other groups which may provide a radiographic marker of those at greatest risk of developing post-operative language loss.
Keywords: Diffusion tensor imaging, Posterior fossa syndrome (PFS), Cerebellar mutism syndrome (CMS), Tumor
Abbreviations: AD, axial diffusivity; AP, anterior-posterior; CBW, cerebellar white matter; CTC, cerebellar-thalamic-cortical; FA, fractional anisotropy; KW, kruskal-wallis; MCP, middle cerebellar peduncle; MD, mean diffusivity; MPRAGE, Magnetization Prepared Rapid Acquisition Gradient Echo; PFS, posterior fossa syndrome; RD, radial diffusivity; RESTORE, Robust Estimation of Tensors by Outlier Rejection; SCP, superior cerebellar peduncle; SWI, Susceptibility weighted imaging; TORTOISE, Tolerably Obsessive Registration and Tensor Optimization Indolent Software Ensemble; TE, echo time; TR, relaxation time
Highlights
-
•
Language deficit associated with posterior fossa syndrome (PFS) remains controversial.
-
•
Diffusion tensor imaging of PFS was analyzed pre-op, post-op and 1-year post resection.
-
•
PFS patients show white matter change in the superior cerebellar peduncles.
-
•
No white matter change was observed prior to surgery.
-
•
Findings were evident post-op and remained at 1 year follow up.
1. Introduction
Aggressive treatment of pediatric midline posterior fossa tumors is a critical part of neuro-oncologic care but can be complicated by characteristic deficits, including severe loss of language ability and other neuropsychological dysfunction. Having first been described 20–30 years prior in sizable cases series (Pollack et al., 1995, Rekate et al., 1985), this is one of several cerebellar disease processes known to create language deficits (Baillieux et al., 2007, Ersahin et al., 1997, Fujisawa et al., 2005). In the largest series to date, 25% of patients undergoing cerebellar tumor resection showed symptoms of this language loss (Robertson et al., 2006b) and incidence has been reported as high at 40% (Gudrunardottir et al., 2010, Küper and Timmann, 2013). As tumors located in the posterior fossa represent 50–70% of solid tumors in children (Gajjar et al., 2004), major loss of language ability represents a major burden in cancer treatment side effects to patients and their families.
Beyond an understanding of its clinical significance, little consensus exists around this condition. Authors refer to this loss of language by multiple names. These include: cerebellar mutism, ataxic mutism, akinetic mutism, cerebellar mutism syndrome, syndrome of mutism and subsequent dysarthria, transient cerebellar mutism, and posterior fossa syndrome. Though efforts have been made to systematize more precise definition of each term, a significant overlap of the usage exists in the literature. Much of this codification effort focuses on the symptoms of language change and on frequently occurring comorbid findings. There is no formally agreed upon criteria regarding the degree of language loss with some investigators emphasizing a strict definition of mutism as complete language loss and others allowing for a broader definition of language deficit. Particular focus has been given to the timing of language changes, requiring that language loss occur 1–2 days following surgery (Dailey et al., 1995), while others allow for development immediately post-operatively (Robertson et al., 2006b). Additionally, the duration of language deficit is reported as limited to days to several months (Ildan et al., 2002). Still further disagreement exists regarding the presence of other neurological symptoms, with various authors including or excluding patients with dysarthria, dysphagia, incontinence, emotional lability, dysmetria, long tract signs and cranial nerve deficits (Gudrunardottir et al., 2011, Tamburrini et al., 2015). For the purposes of this paper we use the terminology posterior fossa syndrome (PFS), in which we refer to severe language loss following cerebellar tumor resection with the possible presence of the above mentioned comorbidities if not otherwise explainable by a lesion outside of the cerebellum.
The pathophysiological cause of PFS remains unclear. A number of non-mutually exclusive mechanisms have been proposed. Some of these mechanism attempt to account for the transient nature the mutism. SPECT and PET case studies have shown hypoperfusion of the cerebellum as well as cortical language and motor areas that resolved with mutism symptoms leading some to speculate a role for vasospasm in the transient language loss (Gedik et al., 2014). Other have reported increased areas of the edema which resolved with language improvement (Pollack et al., 1995). Still others have speculated that transient neurotransmitter or transient changes in autoregulation due to thermal injury may explain the syndrome (Siffert et al., 2000). Furthermore, it has been hypothesized that there may in fact be more permanent white mater changes resulting from direct damage from surgical resection, axonal injury, and/or damage to the white matter from an inflammatory response (Avula et al., 2015b, Gudrunardottir et al., 2010).
Additionally, a number of different anatomical regions have been implicated in the pathogenesis of this disorder. An early hypothesis of pathogenesis focused on the mechanisms of surgical disruption to the tracts in the vermis (Dailey et al., 1995, Zaheer and Wood, 2010). This led some surgeons to adopt a telovelar approach rather than sectioning the vermis when excising cerebellar tumors (Mussi and Rhoton, 2000). However, prospective studies in which the vermis was spared failed to show reproducible evidence of reduction in PFS incidence (Zaheer and Wood, 2010). Recently, increased focus has been placed upon the disruption of the ascending projections from the cerebellar nuclei as a cause of the clinical condition. The middle cerebellar peduncle was implicated in an early description of PFS (Pollack et al., 1995). More recent studies implicate ascending signal through the superior cerebellar peduncle (SCP) in the cerebellar (dental)-thalamic-cortical pathway (Avula et al., 2015a, Morris et al., 2009, Ojemann et al., 2013). An animal model has similarly implicated dentate outflow (Buzunov et al., 2010) in the pathogenesis of PFS. Projections from the cerebellum, especially the dentate nucleus, ascend through the superior cerebellar peduncle and decussate in the rostral pons and midbrain. These projections then travel to the ventral lateral nucleus of the thalamus and project broadly to cortical regions including the areas associated with language production in the frontal and temporal lobes.
Given the lack of clarity regarding diagnostic criteria, pathophysiology and associated anatomy, it is logical that disagreement in the literature exists regarding risk factors for posterior fossa syndrome. A number of pre-operative radiographic findings have been associated with development of PFS, but not in a consistent fashion. Location of tumor in the midline has been associated with increased risk of mutism; however, it has also been occasionally reported in resections of tumors of the lateral cerebellum (Gelabert-Gonzalez and Fernandez-Villa, 2001). Some studies have reported tumor size is associated with PFS (Catsman-Berrevoets et al., 1999), while other investigators did not find an association with size (Robertson et al., 2006a, Wells et al., 2010). A more superior tumor location and compression of the brainstem have also been shown to be associated with increased risk of PFS (McMillan et al., 2008, Morris et al., 2009).
Diffusion imaging techniques have played a role in investigating potential pathophysiological basis of PFS. These efforts have included diffusion-weighted studies (Avula et al., 2015a) and diffusion-tensor/tractography techniques (Law et al., 2012, Morris et al., 2009, Ojemann et al., 2013, Soelva et al., 2012, van Baarsen et al., 2013). They have predominately compared post-operative scans to clinical variables of the condition, and have generally supported the hypothesis that PFS results from perturbations in the ascending cerebellar-thalamic-cortical (CTC) projections. However, each study presents only a specific time period in relation to symptoms and resection with some focusing on the immediate post-operative scan (Law et al., 2012, Ojemann et al., 2013) or a scan performed over a year after PFS onset (Soelva et al., 2012). One study presents pre- and post-op conventional imaging findings but only post-op DTI finings (Morris et al., 2009), while another presents both pre- and post-op DTI findings (Ojemann et al., 2013) but with no long term follow-up.
We employed a retrospective longitudinal, serial imaging study of patients with PFS to further investigate underlying pathophysiological changes involved with this syndrome. We compared these patients with both functionally intact tumor controls and those with only mild language deficit following resection. This study employs diffusion tensor imaging (DTI) at three time points across all three groups. Imaging was performed prior to surgical resection, immediately following resection, and at the 1-year interval to examine white matter changes in the (CTC) projections. This is a novel study in that we assessed patients throughout their clinical course from tumor diagnosis, through development of PFS, and to language resolution. By analyzing pre-operative DTI scans, we also investigated whether radiographic and clinical markers exist that can aide in the early identification of patients most at risk of developing PFS. Immediate post-surgical resection scans allow us to assess white matter changes contemporaneously with mutism development to determine potentially disrupted fiber tracts.
Finally, we evaluated DTI parameters at 1 year post-resection to characterize the evolution of white matter changes. The aim of this longitudinal study is to analyze the previously proposed theories on the pathophysiology of cerebellar mutism by following the progression of the condition.
2. Materials and methods
2.1. Patient characteristics
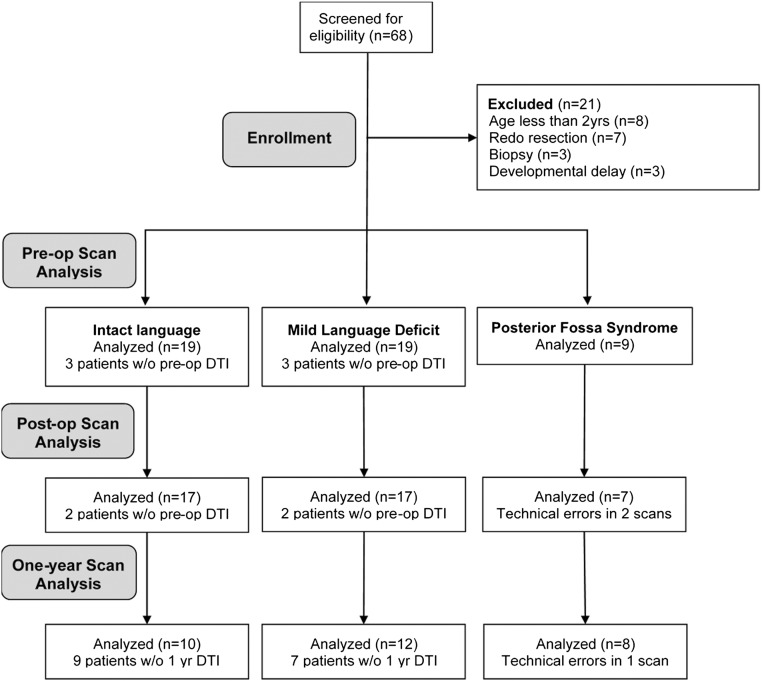
All patients undergoing resection of intraparenchymal cerebellar tumors at Seattle Children's Hospital (SCH) from June 2010 through June 2015 were retrospectively reviewed (Fig. 1). The study protocol was approved by the SCH institutional review board prior to accessing patient records. Inclusion criteria were presence of an intrinsic tumor of the cerebellum that involved the midline and was radically resected at our institution. Exclusion criteria included redo resection, surgery involving only biopsy, or age < 2 years. Patients with pre-existing co-morbid neurological disease or severe development delays were also excluded. Clinical information was gleaned retrospectively from review of the patient's electronic medical record. Patients were divided into three groups based on post-operative verbal performance. The verbally intact group (n = 19) demonstrated intact verbally fluency after surgery and unchanged cognitive ability. The mild language impairment group (n = 19) included patients who continued to speak in multi-word sentences but showed some decrement in cognitive performance (e.g., shorter sentence length, trouble with orientation questions). The posterior fossa syndrome (PFS) group (n = 9) included the patients with complete loss of language production or production of only single words. Concern for PFS must have been raised contemporaneously by qualified medical personnel including their neurosurgeon, rehab medicine physician, oncologist, or speech pathologist documented in the patient's record.
Fig. 1.
Consort diagram of enrolled patients, pre-operative, post-operative, and 1-year follow-up.
2.2. Imaging methods
Imaging was acquired as part of routine diagnostic evaluation on either a 1.5 T or 3 T Siemens MRI scanner (Erlangen, Germany) at the Seattle Children's Hospital. Given the narrow selection of scanner hardware, all results were verified as to have not arisen due to difference in field strengths. Scan protocol included the following sequences and scan parameters: MPRAGE (3D Structural T1 weighted image), T2, FLAIR, SWI and diffusion weighted images. The MPRAGE and diffusion pulse sequences were used for study analysis and employed the following parameters. MPRAGE was collected with a 1 mm slice thickness, field of view 256 × 256 mm, TR 1879 ms, TE 3.3 ms. Diffusion weighted imaging was collected with a single-shot, echo planar imaging sequence consisting of four b0 unweighted images and 10 non-collinear directions acquired four times with a b-value of 1000 s/mm2, field of view 256 × 256 mm, 3 mm slice thickness, TR 5800 ms, TE 96 ms. Total scan time was approximately 30 min, with approximately 5 min of time dedicated to DTI acquisition. Scans were analyzed from three time points: prior to surgery (within 7 days pre-op); immediately following the surgical resection (within 48 h post-op); and at 1 year (9–15 months) from date of resection (and after the resolution of language changes).
Each patient's pre-operative scan was reviewed for evidence of hydrocephalus by a neuroradiologist as part of their clinical care. These scans were reviewed prior to tumor resection and therefore the radiologist was blind to the development of PFS. In addition to reviewing the radiologist report, evidence of hydrocephalus findings were verified through visual inspection by a senior neurosurgical resident physician.
2.3. Image post processing
DTI data was processed with the analytical methods constructed by Dr. Carlo Pierpaoli and colleagues at the NIH using TORTOISE - Tolerably Obsessive Registration and Tensor Optimization Indolent Software Ensemble (Pierpaoli et al., 2010). Briefly, TORTOISE is a freely available, public access software that provides a comprehensive post-processing pipeline which applies rigorous correction for echo-planar imaging distortion, susceptibility, motion, and eddy current distortion (Rohde et al., 2004), and robust estimation of tensors by outlier rejection (RESTORE) (Chang et al., 2012). For EPI correction distortion, the diffusion images were registered to the 1 mm isotropic T1 using non-linear b-splines. Eddy current and motion distortion were corrected using standard affine transformations followed by re-orientation of the b-matrix for the rotational aspect of the rigid body motion. RESTORE was then used to estimate the tensor and subsequent DTI metrics.
Following this post-processing, 3D image stacks were introduced into DTIstudio (Oishi et al., 2009, Zhang et al., 2010) for segmentation of the DTI atlas (Oishi et al., 2011) on to each patients DTI data set. DTI studio allowed for the extraction of DTI metrics within each 3D-atlas-based region of interest providing a comprehensive sampling throughout the entire brain into 189 regions including ventricular space. In this process, the DTI atlas is transformed to fit each patient's scan in order to compensate for anatomical changes due to disease processes (e.g., hydrocephalous, edema, tumor mass effect). The ROI's derived from the DTI atlas were then visually inspected to assure quality of fit of the scan. The DTI metric used for primary analysis was fractional anisotropy (FA) while mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were examined in an exploratory fashion. Selection of regions was limited to regions of white matter as the main hypothesis regarding DTI was that changes in white matter integrity observed with FA would relate to PFS presentation. For white matter segmentation, FA images were threshold at 0.2 or greater.
The investigation was limited to the left and right superior cerebellar peduncle (SCP), middle cerebellar peduncle (MCP), and cerebellar white mater (CBW), areas known to encompass the main anatomical regions implicated in PFS. The SCP comprises the primary ascending efferent fibers from the cerebellum to the thalamus and cortex. The MCP carries the primary cerebellar afferent fibers descending from the cortex and language areas. The CBW was chosen because it contains the vermis and paravermian areas. The inferior cerebellar peduncle was excluded due to its primary descending projection.
A number of risk factors on pre-operative imaging have been reported in the literature. These include hydrocephalous, tumor location, tumor volume, and brainstem compression. The longitudinal nature of this study makes it uniquely positioned to investigate these factors. The method of evaluating each is discussed below.
2.4. Tumor location and volume calculation methods
Tumor location was analyzed by comparing the ratio of tumor in the superior and inferior to the half of the cerebellum relative to the apex of the four ventricle as described previously (Soelva et al., 2012). Tumor volume was calculated using the ABC/2 method (Kothari et al., 1996). Briefly, the widest diameter of the tumor was measured on the axial slice with the maximal tumor area (A). A second diameter was then measured perpendicular to this line (B). Finally axial slices containing the tumor were counted. Axial slices with tumor area > 75% of the maximal area were counted as one slice. Slices with 25–75% of the maximal area were counted as 0.5 slices. Slices with < 25% of the maximal area were not counted. This count was then multiplied by slice thickness (C) and divided by two.
2.5. Brainstem compression calculation methods
Brainstem compression was calculated by measuring the largest anterior-posterior diameter distance across the pons (McMillan et al., 2008). All measurements were made in the midline from the basilar artery to the floor of the fourth ventricle. When the fourth ventricle was effaced by tumor mass, measurements were made in the midline from the basilar artery to the anterior most extent of the tumor or cyst. Measurements were made on the pre-op and post-op scans. The difference between these two measurements were calculated for each patient and analyzed as a separate variable.
2.6. Statistical methods
All data were analyzed using GraphPad Prism version 6 (GraphPad Software). Differences between DTI metrics across the three groups were assessed using Kruskal-Wallis ANOVA given the nonparametric nature of the group distributions. Significant differences observed between groups were followed with pair-wise comparison by Dunn's correction for multiple comparisons. Spearman correlations were employed to examine relationships between imaging results and other clinically relevant measures to explore specificity of the overall study findings.
3. Results
In total, scans from 47 patients across three time points (pre-op, post-op, 1 year) were analyzed (Fig. 1). No differences were seen in gender, handedness, or tumor pathology across outcome groups (Table 1). PFS patients were significantly younger in comparison to the intact language and mild deficit groups. Therefore, subgroup analysis of all primary study findings was completed to investigate any potential contribution of age on the overall results.
Table 1.
Patient characteristics (*P < 0.05 post-hoc, Dunn's correction).
| Patient characteristics | All | Intact | Mild deficit | PFS |
|---|---|---|---|---|
| N | 47 | 19 (40%) | 19 (40%) | 9 (19%) |
| AGE | 9.7 ± 4.8 | 10.5 ± 5.4 | 10.8 ± 4.1 | 5.7* ± 2.8 |
| Gender (% male) | 24 (51) | 10 (53%) | 9 (47%) | 9 (47%) |
| Handedness (R) | 39 (83%) | 16 (84%) | 16 (84%) | 6 (66%) |
| Path: pilocytic | 28 (60%) | 12 (63%) | 11 (58%) | 5 (56%) |
| Path: medullo | 14 (30%) | 5 (26%) | 5 (26%) | 4 (44%) |
| Hydrocephalous | 40 (85%) | 16 (85%) | 16 (85%) | 8 (88%) |
3.1. Immediate post-operative changes in DTI metrics
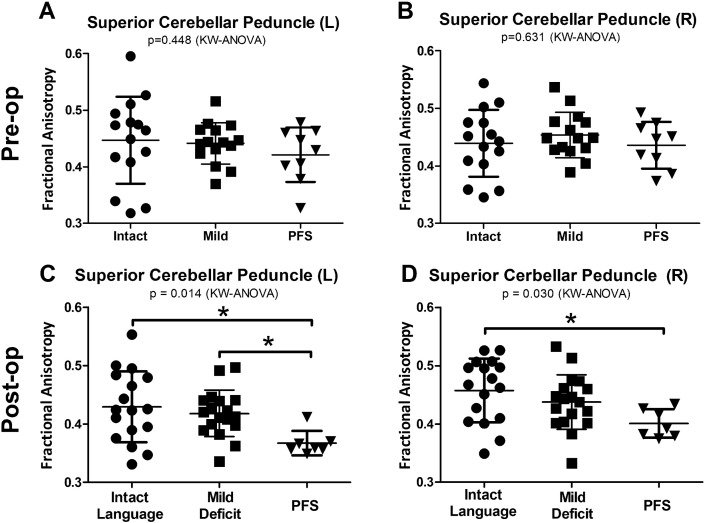
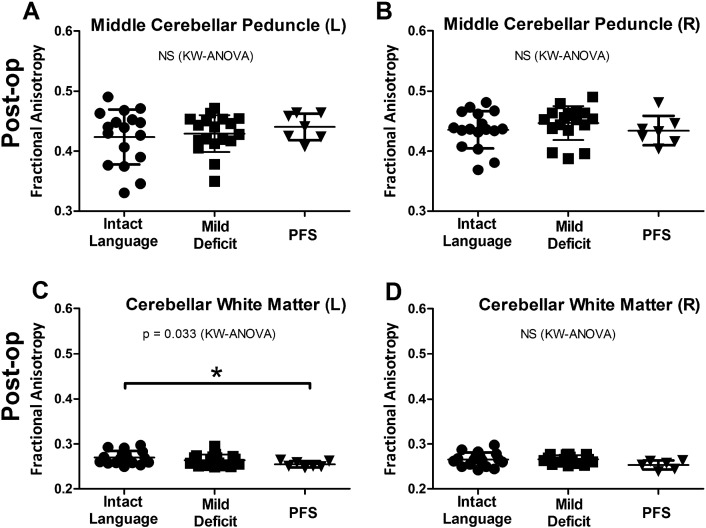
Post-operative scans were performed in the first 48 h following tumor resection, typically within 24 h. While no difference was seen in FA prior to resection (Fig. 2A and B), PFS patients showed reduced FA in both the left (P = 0.014) and right SCP (P = 0.030) by Kruskal-Wallis ANOVA and remained significant after Dunn's correction for multiple comparisons (Fig. 2C and D). These changes appeared to be fairly specific to the SCP, as similar findings were not observed when comparing the left (P = 0.688) or right (P = 0.274) MCP across groups (Fig. 3A and B). Additionally, no difference was observed in the right CBW (P = 0.084) and while a significant difference was seen in the left CBW (P = 0.033) it was quite weak (effect size PFS to verbally intact group = 0.015) compared to the left SCP (PFS to verbally intact = 0.063).
Fig. 2.
PFS patients show reduced fractional anisotropy (FA) in the superior cerebellar peduncle (SCP) on post-operative but not pre-operative scans. Prior to resection, the left SCP (A) and right SCP (B) showed no difference between language groups. In the immediate post-operative period, the left SCP (C) showed reduced FA in PFS patients compared to both verbally intact and mildly impaired patients. The right SCP (D) showed reduced FA in PFS patients compared to the verbally intact group. KW = Kruskal-Wallis. *Significance after post-hoc Dunn's correction for multiple comparisons (P < 0.05).
Fig. 3.
DTI changes show specificity for the SCP. Patients who suffered PFS showed no FA changes in the MCP (A and B) compared to other patients. In the cerebellar white matter, PFS patients show reduced FA on the left (C) and no change on the right (D). KW = Kruskal-Wallis. *Significance after post-hoc Dunn's correction for multiple comparisons (P < 0.05).
3.2. One year changes in DTI metrics
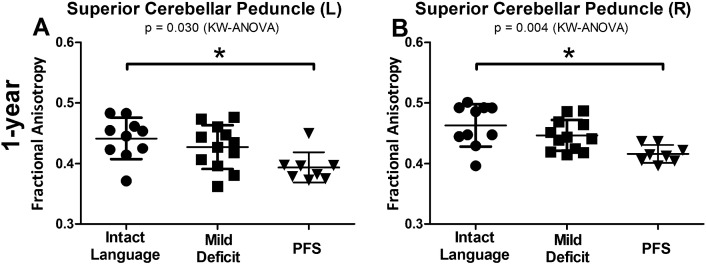
After 1-year from the resection and resolution of language symptoms, patients who suffered PFS continued to show reductions in FA in the bilateral SCP (left P = 0.030, right P = 0.004) (Fig. 4A–B). There were no differences in the left or right MCP (P = 0.672 and P = 0.673, respectively) or right CBW (P = 0.06). There was a difference in the left CBW (P = 0.025) but again with a small effect size (effect size PFS to verbally intact group = 0.013) compared to the left SCP (PFS to verbally intact = 0.045).
Fig. 4.
DTI changes persist 1-year post surgery. One year following surgery, patients who suffered PFS showed reduced FA in both the left (left) and right (right) SCP compared to patients who were immediately verbally intact. KW = Kruskal-Wallis. *Significance after post-hoc Dunn's correction for multiple comparisons (P < 0.05).
3.3. Longitudinal summary of SCP DTI metrics
While no significant difference was seen in FA values in the SCP pre-operatively, we did observe a significant increase in mean diffusivity (P = 0.008) and radial diffusivity (P = 0.0016) in the left SCP (Table 2) of PFS patients suggesting that patients at risk for PFS may exhibit white matter vulnerability in this region prior to resection (Kruskal-Wallis ANOVA). No trend was seen in the right SCP mean diffusivity (P = 0.850) or radial diffusivity (P = 0.372). No changes in other DTI metrics were observed with the exception of post-operative right axial diffusivity (P = 0.033), which was also reduced.
Table 2.
Summary of DTI findings in the superior cerebellar peduncle.
| DTI Metric | FA | MD | AD | RD | ||||
|---|---|---|---|---|---|---|---|---|
| Hemisphere | L | R | L | R | L | R | L | R |
| Pre-op | NS | NS | ↑ | NS | NS | NS | ↑ | NS |
| Post-op | ↓ | ↓ | NS | NS | NS | ↓ | NS | NS |
| 1-year | ↓ | ↓ | NS | NS | NS | NS | NS | NS |
Up or down arrow indicates direction of DTI signal change in PFS patients compared to intact tumor controls and is significant at P < 0.05 after post-hoc Dunn's correction.
3.4. Adjustment for age
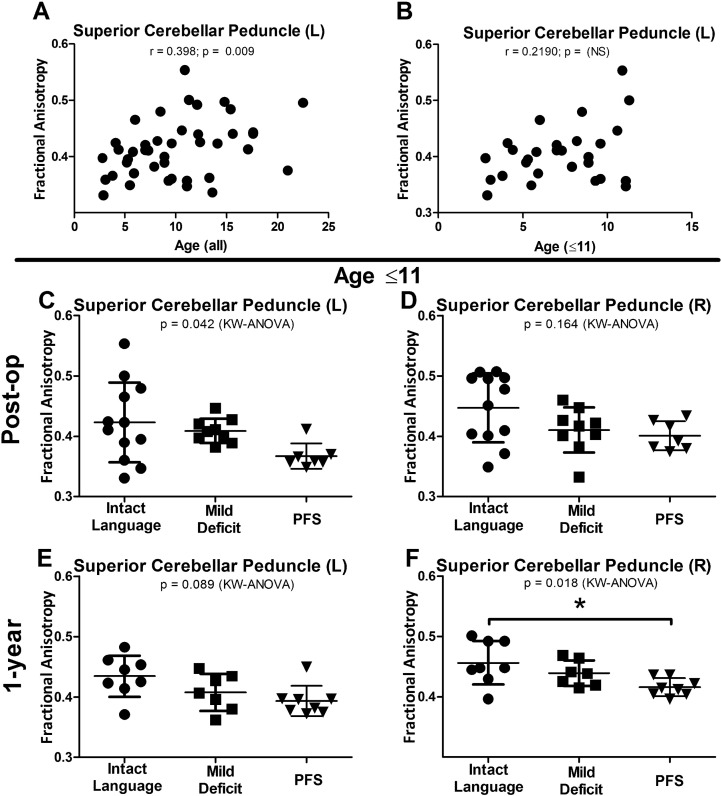
As noted in Table 1, the PFS group was significantly younger than the intact language and mild deficit language groups. In order to understand the contribution of age to the DTI findings in the SCP, spearman rank correlations were completed on the FA values in the SCP against the patient's age. A significant correlation was identified with this analysis (r = 0.398, P = 0.009, Fig. 5A) and as such, further subgroup analysis was undertaken to determine if the primary findings held after proper age matching. In this subset, FA and age no longer showed a significant correlation (r = 0.219, P = 0.263) (Fig. 5B). In the post-operative scan, FA reductions in the left SCP remained significant (P = 0.042) (Fig. 5C) while the change in the right SCP remained only slightly trending (P = 0.164) (Fig. 5D). Additionally, at 1-year post-op, the right SCP showed significant reductions in FA and following Dunn's correction between the PFS and intact language group (P = 0.018) (Fig. 5F) while the left SCP remained trending (P = 0.089) (Fig. 5E). Taken together, we interpret this subgroup analysis finding to mean that while younger age appears to be a risk factor for the development of PFS, DTI imaging findings suggest that there is a subset of patients, even after adjustment for age, that are vulnerable to this syndrome and that the evaluation of this advanced neuroimaging tool could provide greater insight into the identification of those at risk.
Fig. 5.
DTI changes remain after controlling for age differences.
FA values were found to correlate with age (A). SCP FA values are no longer significantly correlated following subgroup analysis matching for age across groups (B). Following age restriction, reductions in FA in the SCP remain significant (C and F) or trend towards significant (D and E). KW = Kruskal-Wallis. *Significance after post-hoc Dunn's correction for multiple comparisons (P < 0.05).
3.5. Consideration of other radiological risk factors
Presence of hydrocephalous did not relate to the development of PFS (Table 1). There was no significant difference or trend seen in its prevalence between the language groups (P = 0.741, Chi-square).
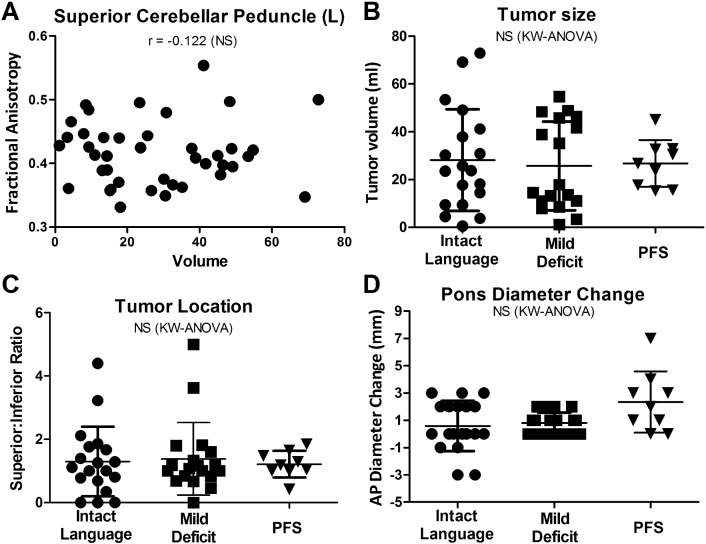
The relationship of tumor size to language group and FA values was assessed in order to determine if these imaging findings were merely a result of greater tumor volume. In contrast to previously reported findings (Catsman-Berrevoets et al., 1999) no size differences were observed between groups (P = 0.857, Fig. 6A), and no correlation existed between tumor volume and FA in the SCP (P = 0.448, Fig. 6B) suggesting that the reductions in FA in the SCP in PFS patients occurred independent of tumor size.
Fig. 6.
Tumor size does not account for PFS Findings Observed on MRI.
Tumor size also did not correlate with FA (A) in the regions of interest and showed no difference between groups (B). No relationship was seen between the tumors superior-inferior position and PFS (C). Changes in pontine AP diameter as a marker of brainstem compression also showed no difference between groups (D). r = spearman correlation. KW = Kruskal-Wallis. *Significance after post-hoc Dunn's correction for multiple comparisons (P < 0.05).
Given the white matter changes seen in superior cerebellar structures, the location of the tumor was assessed by comparing the ratio of the tumor portion in the superior versus inferior cerebellum. Contrary to previously published studies (Soelva et al., 2012), no difference was noted between groups in the ratio of superior to inferior tumor location (P = 0.894, Fig. 6C).
Decreased pre-operative anterior-posterior (AP) diameter of the pons and change in AP diameter of the pons following tumor resection has previously been reported to predict PFS (McMillan et al., 2008). In our cohort of patients, no difference was noted between pre-operative AP diameter mean (Intact group = 17.6 ± 3.9, Mild = 17.8 ± 2.4, PFS = 16.0 ± 3.9; P = 0.495). There was also no difference in post-operative AP diameter (mean Intact group = 18.7 ± 3.91, Mild = 18.5 ± 2.22, PFS = 18.3 ± 4.80; P = 0.976). A trend was noted in the change of AP diameter between pre-operative and post-operative AP diameter (Fig. 6D) but did not reach significance (P = 0.098).
4. Discussion
The data presented here show marked changes in the white matter by DTI of the superior cerebellar peduncle in patients who suffer posterior fossa syndrome following resection of cerebellar tumors that was not present on pre-operative evaluation. These findings showed specificity to superior cerebellar peduncle. No changes were seen in the middle cerebellar peduncle after resection. A small change was seen in the left cerebellar white matter but the effect size was much smaller than that seen in the superior cerebellar peduncle. No change was seen in the right cerebellar white matter.
Our study found no relationship between PFS and other previously reported clinical and radiographic factors including patient handedness even though PFS has been previously reported to occur more frequently in left handed patients (Law et al., 2012).
Furthermore, no relationship was observed between size of the tumor and incidence of PFS; no correlation was discovered between size and FA changes. No relationship was seen between superior location of the tumor and language changes. Pre-operative compression of the brainstem did not relate to development of PFS, and changes observed in AP diameter of the pons did not reach significance While others have reported a predilection to PFS among patients with more high grade pathology (Soelva et al., 2012), in our study the pathological grade of the tumor also showed no-difference between language groups as did the diagnosis of hydrocephalus.
Conversely, age did appear to correlate with the presence of PFS and FA changes. While PFS patients were younger than both the verbally intact and mild deficit groups, this did not entirely explain the DTI findings as evidenced by the subgroup analysis in which reductions in FA were still observed in the age-matched group sample. While it is generally thought that PFS primarily occurs in pediatric patients, various reports have shown no correlation with age in this population (Mariën et al., 2013). In addition, pre-operative baseline FA was not different across groups suggesting the FA reductions observed on the post-operative scan in PFS patients were more likely associated with the resection than age alone. This implies that post-operative changes seen between the language groups cannot result entirely from differences in patient age. Thus, decreasing age likely represents a susceptibility to white matter changes associated with PFS, but is not the sole explanation for the changes observed. Maturation of white matter in the SCP may create a window of time when incomplete myelination leaves white matter more susceptible to traumatic or inflammatory injury.
The language deficit in PFS typically resolved after weeks to months. However, the white mater changes in the SCP observed with DTI immediately following resection were still present at one year from the time of resection. These findings would not be supportive of theories that attempt to exclusively explain PFS through transient phenomenon such as vasospasm, edema, neurotransmitter deficit, and temporary changes in autoregulation. While the causes of PFS may be multifactorial and include these mechanisms in the acute setting, longer lasting changes (such as trauma, axonal injury, or inflammatory change) may play a role as well. The presence of long lasting anatomical change is also consistent with the growing body of evidence that PFS patients show long-term emotional and intellectual changes despite resolution of mutism (Levisohn et al., 2000, Riva and Giorgi, 2000).
Pre-operative scans did not show FA changes, but did demonstrate alterations in other DTI metrics, specifically MD and RD. These findings do not yet accurately identify which patients will develop PFS, but do imply that patients who develop PFS may show a radiographic DTI marker prior to resection. If these finding were replicated more robustly in a larger data set with higher quality scans (e.g., greater number of directions), it may lead to the development of pre-operative radiographic markers of patients most at risk for PFS. These markers might allow surgeons greater accuracy in pre-operative discussions with patients' families regarding surgical risk and hospital course. Additionally, pre-operative markers could guide the approach and extent of surgical resection. In patients felt to represent a greater risk for PFS syndrome, surgeons might elect to alter their approach or technique to limit damage or irritation of the SCP, such as limiting the direct retraction or manipulation.
While this is the first longitudinal imaging analysis in PFS patients from pre-operative evaluation out to 1 year post-resection and functionally intact tumor controls to date, it is not without limitations. These include a limited sample size, clinical scan data not optimized for advanced imaging analysis such that more fine regions like the dentate nucleus could not be adequately sampled, and imbalanced age of patients per group. Additionally, data was recorded retrospectively which may induce inherit biases. Specific examples may include overlooking subtleties of clinical presentation and course observed. The diagnosis of PFS may be underreported in a retrospective analysis or alternate explanations of PFS-like behavior may be overlooked. Regardless of study design, lack of widespread consensus regarding definition of PFS limits the generalizability of any study on the subject. The diversity of presentations regarding onset of symptoms and co-morbid findings may represent at range of pathological mechanisms and anatomical changes. It may also contribute to the notable variance in reported incidence of PFS and its related syndromes.
Future directions of research on PFS would benefit from prospective studies allowing for careful documentation of co-morbid findings for more precise selection of mutism patients by symptomatology which may reveal more accurate anatomical and clinical relationships. Furthermore, multi-center studies of the clinical and radiographic (including DTI) findings of PFS with higher resolution DTI and isotropic voxel acquisition would provide data optimized for this quantitative post-processing and evaluation. Additionally, multi-center investigations would also involve pooled results of multiple surgical techniques which could lead to greater generalization of findings to the body of clinicians providing care for these patients. One could even propose altering surgical strategies to protect the SCPs, just as leaving small residual on the brainstem is an appropriate strategy in chemo- and radio-sensitive tumors.
The presence of changes to superior structures of the cerebellum as opposed to the more lateral and inferior structures may provide surgical guidance to tumor resection. This finding may allow surgeons greater confidence in their dissection of the tele choroidea, inferior medullar velum and inferior cerebellum (e.g., vermis and inferior cerebellar peduncle). This greater inferior exposure may allow surgeons greater degrees of freedom when resecting the superior portion of the tumor where the patient's language may be most vulnerable. Thus contrary to previous trends, more aggressive manipulation of the inferior cerebellum may allow the surgeon to avoid PFS by allowing greater visualization and careful dissection of the SCP. This proposition requires further clinical investigation.
Mutism remains a common complication following resection of cerebellar tumors. Our work has used DTI findings to investigate the underlying pathophysiology and risk factors of this disease. By improving understanding of the anatomical changes associated with PFS, we hope to provide surgeons additional tools to safely remove cerebellar tumors and improve children's survival and functional outcome.
Funding
Support for this study was provided by the AANS/CNS Codman Neurotrauma and Critical Care Fellowship (08-0801).
Acknowledgements
We would like to thank Amy Anderson RN BSN for her contribution obtaining access to patient records, as well as Nicole Evans and Kody Zalewski for their contributions with initial image processing support.
References
- Avula S., Kumar R., Pizer B., Pettorini B., Abernethy L., Garlick D., Mallucci C. Diffusion abnormalities on intraoperative magnetic resonance imaging as an early predictor for the risk of posterior fossa syndrome. Neuro-Oncology. 2015:614–622. doi: 10.1093/neuonc/nou299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula S., Mallucci C., Kumar R., Pizer B. Posterior fossa syndrome following brain tumour resection: review of pathophysiology and a new hypothesis on its pathogenesis. Childs Nerv. Syst. 2015:1–9. doi: 10.1007/s00381-015-2797-0. [DOI] [PubMed] [Google Scholar]
- Baillieux H., Weyns F., Paquier P., De Deyn P.P., Marien P. Posterior fossa syndrome after a vermian stroke: a new case and review of the literature. Pediatr. Neurosurg. 2007;43:386–395. doi: 10.1159/000106388. [DOI] [PubMed] [Google Scholar]
- Buzunov E., Ojemann J.G., Robinson F.R. Rhesus macaque as an animal model for posterior fossa syndrome following tumor resection. Pediatr. Neurosurg. 2010;46:120–126. doi: 10.1159/000319008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsman-Berrevoets C.E., Van Dongen H.R., Mulder P.G., Paz y Geuze D., Paquier P.F., Lequin M.H. Tumour type and size are high risk factors for the syndrome of “cerebellar” mutism and subsequent dysarthria. J. Neurol. Neurosurg. Psychiatry. 1999;67:755–757. doi: 10.1136/jnnp.67.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L.C., Walker L., Pierpaoli C. Informed RESTORE: a method for robust estimation of diffusion tensor from low redundancy datasets in the presence of physiological noise artifacts. Magn. Reson. Med. 2012;68:1654–1663. doi: 10.1002/mrm.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey A.T., McKhann G.M., Berger M.S. The pathophysiology of oral pharyngeal apraxia and mutism following posterior fossa tumor resection in children. J. Neurosurg. 1995:467–475. doi: 10.3171/jns.1995.83.3.0467. [DOI] [PubMed] [Google Scholar]
- Ersahin Y., Mutluer S., Saydam S., Barcin E. Cerebellar mutism: report of two unusual cases and review of the literature. Clin. Neurol. Neurosurg. 1997;99:130–134. [PubMed] [Google Scholar]
- Fujisawa H., Yonaha H., Okumoto K., Uehara H., Ie T., Nagata Y., Suehiro E., Suzuki M. Mutism after evacuation of acute subdural hematoma of the posterior fossa. Childs Nerv. Syst. 2005;21:234–236. doi: 10.1007/s00381-004-0999-y. [DOI] [PubMed] [Google Scholar]
- Gajjar A., Hernan R., Kocak M., Fuller C., Lee Y., McKinnon P.J., Wallace D., Lau C., Chintagumpala M., Ashley D.M., Kellie S.J., Kun L., Gilbertson R.J. Clinical, histopathologic, and molecular markers of prognosis: toward a new disease risk stratification system for medulloblastoma. J. Clin. Oncol. 2004;22:984–993. doi: 10.1200/JCO.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Gedik G.K., Sari O., Koktekir E., Akdemir G. Fluorodeoxyglucose positron emission tomography/computed tomography findings in a patient with cerebellar mutism after operation in posterior fossa. Asian J. Surg. 2014 doi: 10.1016/j.asjsur.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Gelabert-Gonzalez M., Fernandez-Villa J. Mutism after posterior fossa surgery. Review of the literature. Clin. Neurol. Neurosurg. 2001;103:111–114. doi: 10.1016/s0303-8467(01)00125-1. [DOI] [PubMed] [Google Scholar]
- Gudrunardottir T., Sehested A., Juhler M., Schmiegelow K. Cerebellar mutism. Childs Nerv. Syst. 2010:355–363. doi: 10.1007/s00381-010-1328-2. [DOI] [PubMed] [Google Scholar]
- Gudrunardottir T., Sehested A., Juhler M., Grill J., Schmiegelow K. Cerebellar mutism: definitions, classification and grading of symptoms. Childs Nerv. Syst. 2011:1361–1363. doi: 10.1007/s00381-011-1509-7. [DOI] [PubMed] [Google Scholar]
- Ildan F., Tuna M., Erman T., Gocer A.I., Zeren M., Cetinalp E. The evaluation and comparison of cerebellar mutism in children and adults after posterior fossa surgery: report of two adult cases and review of the literature. Acta Neurochir. 2002;144:463–473. doi: 10.1007/s007010200067. [DOI] [PubMed] [Google Scholar]
- Kothari R.U., Brott T., Broderick J.P., Barsan W.G., Sauerbeck L.R., Zuccarello M., Khoury J. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- Küper M., Timmann D. Brain and Language. Elsevier Inc.; 2013. Cerebellar mutism; pp. 327–333. [DOI] [PubMed] [Google Scholar]
- Law N., Greenberg M., Bouffet E., Taylor M.D., Laughlin S., Strother D., Fryer C., McConnell D., Hukin J., Kaise C., Wang F., Mabbott D.J. Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro-Oncology. 2012:1294–1303. doi: 10.1093/neuonc/nos160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levisohn L., Cronin-Golomb A., Schmahmann J.D. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(Pt 5):1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- Mariën P., De Smet H.J., Wijgerde E., Verhoeven J., Crols R., De Deyn P.P. Posterior fossa syndrome in adults: a new case and comprehensive survey of the literature. Cortex. 2013:284–300. doi: 10.1016/j.cortex.2011.06.018. (Elsevier Ltd.) [DOI] [PubMed] [Google Scholar]
- McMillan H.J., Keene D.L., Matzinger M.A., Vassilyadi M., Nzau M., Ventureyra E.C.G. Brainstem compression: a predictor of postoperative cerebellar mutism. Childs Nerv. Syst. 2008:677–681. doi: 10.1007/s00381-008-0777-3. [DOI] [PubMed] [Google Scholar]
- Morris E.B., Phillips N.S., Laningham F.H., Patay Z., Gajjar A., Wallace D., Boop F., Sanford R., Ness K.K., Ogg R.J. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain. 2009:3087–3095. doi: 10.1093/brain/awp241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussi A.C., Rhoton A.L. Telovelar approach to the fourth ventricle: microsurgical anatomy. J. Neurosurg. 2000:812–823. doi: 10.3171/jns.2000.92.5.0812. [DOI] [PubMed] [Google Scholar]
- Oishi K., Faria A., Jiang H., Li X., Akhter K., Zhang J., Hsu J.T., Miller M.I., van Zijl P.C., Albert M., Lyketsos C.G., Woods R., Toga A.W., Pike G.B., Rosa-Neto P., Evans A., Mazziotta J., Mori S. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. NeuroImage. 2009;46:486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Faria A., Van Zijl P.C., Mori S. 2nd ed. Elsevier; Oxford, UK: 2011. MRI Atlas of Human White Matter. [Google Scholar]
- Ojemann J.G., Partridge S.C., Poliakov A.V., Niazi T.N., Shaw D.W., Ishak G.E., Lee A., Browd S.R., Geyer J.R., Ellenbogen R.G. Diffusion tensor imaging of the superior cerebellar peduncle identifies patients with posterior fossa syndrome. Childs Nerv. Syst. 2013:2071–2077. doi: 10.1007/s00381-013-2205-6. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C., Walker L., Irfanoglu M.O., Barnett A., Basser P., Chang L.-C., Koay C., Pajevic S., Rohde G., Sarlls J., Wu M. International Society for Magnetic Resonance in Medicince. 18th Annual Meeting. 2010. TORTOISE: an integrated software package for processing of diffusion MRI data. [Google Scholar]
- Pollack I.F., Polinko P., Albright A.L., Towbin R., Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery. 1995;37:885–893. doi: 10.1227/00006123-199511000-00006. [DOI] [PubMed] [Google Scholar]
- Rekate H.L., Grubb R.L., Aram D.M., Hahn J.F., Ratcheson R.A. Muteness of cerebellar origin. Arch. Neurol. 1985;42:697–698. doi: 10.1001/archneur.1985.04060070091023. [DOI] [PubMed] [Google Scholar]
- Riva D., Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(Pt 5):1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Robertson P.L., Muraszko K.M., Holmes E.J., Sposto R., Packer R.J., Gajjar A., Dias M.S., Allen J.C., Children's Oncology, G. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J. Neurosurg. 2006;105:444–451. doi: 10.3171/ped.2006.105.6.444. [DOI] [PubMed] [Google Scholar]
- Robertson P.L., Muraszko K.M., Holmes E.J., Sposto R., Packer R.J., Gajjar A., Dias M.S., Allen J.C., Group, C.a.s.O. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J. Neurosurg. 2006:444–451. doi: 10.3171/ped.2006.105.6.444. [DOI] [PubMed] [Google Scholar]
- Rohde G.K., Barnett A.S., Basser P.J., Marenco S., Pierpaoli C. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn. Reson. Med. 2004;51:103–114. doi: 10.1002/mrm.10677. [DOI] [PubMed] [Google Scholar]
- Siffert J., Poussaint T.Y., Goumnerova L.C., Scott R.M., LaValley B., Tarbell N.J., Pomeroy S.L. Neurological dysfunction associated with postoperative cerebellar mutism. J. Neuro-Oncol. 2000:75–81. doi: 10.1023/a:1006483531811. [DOI] [PubMed] [Google Scholar]
- Soelva V., Hernáiz Driever P., Abbushi A., Rueckriegel S., Bruhn H., Eisner W., Thomale U.-W. Fronto-cerebellar fiber tractography in pediatric patients following posterior fossa tumor surgery. Childs Nerv. Syst. 2012:597–607. doi: 10.1007/s00381-012-1973-8. [DOI] [PubMed] [Google Scholar]
- Tamburrini G., Frassanito P., Chieffo D., Massimi L., Caldarelli M., Di Rocco C. Cerebellar mutism. Childs Nerv. Syst. 2015:1841–1851. doi: 10.1007/s00381-015-2803-6. [DOI] [PubMed] [Google Scholar]
- van Baarsen K., Kleinnijenhuis M., Konert T., van Cappellen van Walsum A.-M., Grotenhuis A. Tractography demonstrates dentate-rubro-thalamic tract disruption in an adult with cerebellar mutism. Cerebellum. 2013:617–622. doi: 10.1007/s12311-013-0473-z. [DOI] [PubMed] [Google Scholar]
- Wells E.M., Khademian Z.P., Walsh K.S., Vezina G., Sposto R., Keating R.F., Packer R.J. Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: neuroradiographic features and origin. J. Neurosurg. Pediatr. 2010;5:329–334. doi: 10.3171/2009.11.PEDS09131. [DOI] [PubMed] [Google Scholar]
- Zaheer S.N., Wood M. Experiences with the telovelar approach to fourth ventricular tumors in children. Pediatr. Neurosurg. 2010:340–343. doi: 10.1159/000321539. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang J., Oishi K., Faria A.V., Jiang H., Li X., Akhter K., Rosa-Neto P., Pike G.B., Evans A., Toga A.W., Woods R., Mazziotta J.C., Miller M.I., van Zijl P.C., Mori S. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. NeuroImage. 2010;52:1289–1301. doi: 10.1016/j.neuroimage.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]