Abstract
Background
Mild cognitive impairment and dementia are common, clinically important features of Parkinson's disease (PD). The underlying disease pathology is heterogeneous and not yet well characterized. Biomarkers for cognitive impairment in PD could aid in diagnostic and prognostic evaluation and in the development of new cognitive enhancing treatments.
Objective
To examine the relationship between CSF markers and cognition in a large, multicentre, cohort study of early, untreated PD, and compare marker concentrations between PD patients with and without MCI and healthy, age-matched controls.
Methods
414 early, untreated PD (34% with mild cognitive impairment) and 189 healthy, cognitively intact controls with baseline neuropsychological testing and CSF abeta42, t-tau, p-tau181 and α-synuclein results were included. Multiple linear regression models were constructed with a composite cognition factor, or memory-, or visuospatial- or executive-attention domains as dependent variables, and CSF markers, demographic characteristics and MDS-UPDRS III score as predictors.
Results
Lower α-synuclein was associated with reduced performance on the executive-attention domain and the composite cognition factor in the whole PD-group. Abeta42 was significantly decreased in PD with mild cognitive impairment compared with controls after adjusting for covariates, while values in PD without MCI were identical to healthy controls.
Conclusions
The association between reduced CSF α-synuclein concentrations and cognition suggests that α-synuclein pathology contributes to early cognitive impairment in PD, in particular to executive-attentional dysfunction. Longitudinal analyses are needed to determine if this and other CSF biomarkers in early Parkinson's disease are associated with the risk of future cognitive decline and dementia.
Keywords: Parkinson disease, cerebrospinal fluid, Mild cognitive impairment, alpha-synuclein, amyloid beta-peptides, tau Proteins
INTRODUCTION
Mild cognitive impairment and dementia are common in Parkinson's disease (PD) [1], with heterogeneous cognitive profiles and underlying disease pathology [2]. In addition to the defining Lewy bodies and Lewy neurites, coexisting Alzheimer's disease (AD) pathology and vascular lesions are common in PD [3, 4] and there is evidence that all these pathologies contribute to cognitive impairment, and may have additive effects [4, 5]. Preliminary in-vivo evidence supports this, with PD patients with higher amyloid burden (e.g. higher amyloid radioligand Pittsburgh compound B-retention on PET imaging) experiencing more rapid cognitive decline [6].
Cerebrospinal fluid (CSF) markers beta-amyloid42 (abeta42), total tau protein (t-tau), phosphorylated tau protein (p-tau), and α-synuclein reflect pathophysiological changes relevant to cognition in PD. Several studies have reported a correlation between decreased abeta42 and cognitive impairment in PD [7–11], including phonemic [7, 11] and semantic fluency [7], and memory [8, 10]. Decreased CSF abeta42 also predicts more rapid cognitive decline and development of dementia in PD [11–15]. Associations between cognitive impairment and increased t-tau, p-tau181 [7] and α-synuclein have also been found [15, 16]. These CSF biomarkers can therefore potentially predict cognitive decline, and could also aid in the development of new cognitive enhancing treatments, and inform selection of patients to receive such treatments when available. However, most previous studies included small samples of patients from a single center.
Using data from a large multicenter-study, we aimed to examine the relationship between CSF markers and cognition in newly diagnosed PD patients, and compare marker concentrations between PD patients with and without MCI and healthy, age-matched controls. Based on previous evidence we hypothesized that cognitive impairment in PD would correlate with decreased abeta42 and α-synuclein, and increased tau and p-tau181 concentrations in CSF.
METHODS
PPMI and clinical procedures
Parkinson's Progression Markers Initiative (PPMI) is an international project focusing on development of biomarkers of progression in PD [17, 18] (see also ppmi-info.org). At baseline, there were 423 patients with untreated PD without dementia and 196 cognitively intact control subjects from 24 clinical sites (data was downloaded June 2014). Data from the screening (<45 days prior to baseline) and baseline visits were used for this study. 417 PD patients and 189 controls had results on CSF analysis from either baseline or V01 (the next study visit - three months after baseline, 3 PD patients and one control had only V01 results) and were included in the current study. In addition three PD patients were excluded due to incomplete test results, resulting in 414 PD and 189 controls. All participants in the study completed an extensive evaluation battery, including neurological examination, MRI imaging, DaTscan imaging, and neuropsychological examination [18, 19]. The study was approved by the medical ethical committees at the respective centers, and written informed consent was obtained from all participants.
Neuropsychological assessment
The neuropsychological assessment, described in detail in a previous study [19], included tests of visuospatial functions (15 item-version of the Benton's judgment of Line Orientation test), verbal memory (Hopkins Verbal Learning Test Revised), executive function (three semantic fluency tests; number of animals, vegetables and fruits mentioned in 60 seconds), and attention (Letter Number Sequencing Test and Symbol Digit Modalities Test). In addition subtests from the Montreal Cognitive Assessment (MoCA) were used as previously described [19].
The test scores obtained by PD patients were, for tests resulting in continuous scores, transformed to z-scores using the means and standard deviations from the control group. Three cognitive domains were constructed by averaging z-scores from different tests of the same domain, memory (z- scores of immediate and delayed recall, Hopkins Verbal Learning test), attention/executive function (z- scores of Semantic fluency, Phonemic fluency, Symbol Digit Modalities Test and Letter Number Sequencing) and visuospatial function (only Benton Judgement of line orientation). In order to estimate a global composite cognition score representing only common variance in all cognitive tests, a single regression-based factor score derived from a principal component analysis of raw scores from all tests was computed based on the full sample of patients and controls in this study. This factor score represented 34% of the variance in the full set of cognitive tests.
Diagnosis of MCI
PD patients were classified as having MCI (PD-MCI) according to an approximation to the level II criteria of the Movement Disorder Society Task Force criteria for MCI in PD [20] as described in detail previously [19]. As PPMI was designed prior to the publication of these criteria, it does not include two comprehensive neuropsychological tests for all domains. Thus some MoCA items were included in the MCI classification. The language domain was assessed with sentence repetition, abstraction and naming from MoCA, executive function with the semantic fluency tests in addition to phonemic fluency and Trail Making from MoCA, visuospatial function with Benton's judgment of Line Orientation and the clock and cube from MoCA, memory with total immediate recall and delayed recall from Hopkins Verbal Learning test and attention with Symbol Digit Modalities Test and Letter Number Sequencing in addition backward digit span, vigilance and serial 7 s from MoCA. Briefly, impairment was defined as a score more than 2.0 standard deviations below the mean for controls for the tests with a continuous score or a result below the maximum possible score on selected MoCA subtests. Patients were defined as having MCI if they showed impairment on two or more tests, regardless of domain, and classified as single domain or multiple domain MCI as recommended [20]. Patients not fulfilling criteria for MCI were classified as having normal cognition (PD-NC).
Analysis of CSF samples
Details about lumbar puncture and sample processing can be found in the PPMI biologics manual on the PPMI website (http://ppmi-info.org). Never before thawed CSF samples were analyzed as described in detail previously [18] for biomarkers abeta42, t-tau and tau phosphorylated at threonine number 181 using a Multiplex xMap Luminex platform, and Research-Use-Only Innogenetics immunoassay kits from Fujirebio/Innogenetics. A-synuclein was analyzed using an ELISA assay commercially available from Covance.
The levels of α-synuclein in blood cells are much higher than in CSF, thus blood contamination might have an important impact on CSF α-synuclein concentrations [21]. Hemoglobin (Hb) concentrations in CSF were therefore assessed using an ELISA assay with reagents from Bethyl Laboratories [18], with standards in a range from 7.5–125 ng/ml, each sample was analyzed at 3 dilutions to secure that at least one of the dilutions fell within the range of the assay standards. 306 patients and controls had CSF Hb concentrations below detection limit, and for 131 no CSF Hb concentrations were available. For some individuals, more than one CSF hemoglobin concentration was reported in the PPMI database from the baseline lumbar puncture. The one closest in date to the α-synuclein analysis was included in this analysis.
In line with this, CSF α-synuclein and CSF Hb concentrations were significantly correlated (all PPMI samples, including the subjects without evidence of dopaminergic dysfunction, Spearman Correlation coefficient 0.092, p = 0.036). The scatterplot suggested correlation was driven by a few outliers with high α-synuclein. After exclusion of 5 subjects with α-synuclein concentrations higher than 3 standard deviations above the mean, there was no longer a significant correlation between α-synuclein and hemoglobin. The dataset was divided into four groups, (Hb below detection limit, Hb > detection limit <200 ng/ml, Hb >200 ng/ml and Hb missing) and concentration of α-synuclein was found to be similar in these groups by the Kruskal-Wallis test for independent samples (p = 0.150). Thus the 5 PD subjects with concentrations above mean+3 SD were excluded from the multiple regression models with cognitive functions as dependent variables. APOE genotyping was performed using allele-specific oligonucleotide probes, labeled with fluorogenic reporter dyes (TaqMan method).
Statistical analysis
Data were analyzed using SPSS Statistic, mainly version 22. The CSF results were tested for normality using the Kolmogorov-Smirnov statistic, and were not normally distributed (results not shown). To compare demographic variables and CSF markers between groups, Students t-test, Mann-Whitney test or Chi-square test were used as applicable (Table 1.). Thereafter, multiple linear regression models were constructed to assess the effect of CSF markers on cognition in the PD patients. First, unadjusted analyses were preformed with only the CSF markers vs the cognitive variables. As a next step the analysis were adjusted for potential confounders; age, education, gender and APOE status (presence of APOE ε4 or APOE ε2 allele) and total MDS-UPDRS III score. Four separate models were created, with the composite cognition score, memory domain, visuospatial domain and executive-attention domain as dependent variables. APOE-status did not have any significant effects on any of the dependent variables, and was removed from the final analysis. Only patients without missing values were included in the analyses, n = 403 (5 excluded to due to extreme α-synuclein values, two due to missing p-tau, 4 due to missing t-tau results.).
Table 1.
Demographics, cognition and CSF marker concentrations
| PD (all patients) N= 414 | Healthy controls (HC) N= 189 | PD mild cognitive impairment (PD-MCI) N= 140 | PD normal cognition (PD-NC) N= 274 | |
|---|---|---|---|---|
| Sex (% male)¤ | 66 % (273/414) | 63% (120/189) | 67% (94/140) | 65% (179/274) |
| Age (mean, SD)# | 61.3 (9.7) | 60.2 (11.3) | 63.11 (9.33)d,f | 60.3 (9.70) |
| Education (mean, SD)# | 15.6 (3.0) | 16.0 (2.9) | 14.8 (3.31)e,h | 16.0 (2.72) |
| APOE-genotype, percent¥ | ε2/ε2 : 1%, ε2/ε3 : 14%, ε3/ε3 : 59%, ε3/ε4 : 23%, ε2/ε4 : 2%, ε4/ε4 : 2%. 38 patients without results. | ε2/ε2 : 2%, ε2/ε3 : 9%, ε3/ε3 : 62%, ε3/ε4 : 21%, ε2/ε4 : 4%, ε4/ε4 : 2%. 17 controls without results. | ε2/ε2 : 1%, ε2/ε3 : 11%, ε3/ε3 : 61%, ε3/ε4 : 23%, ε2/ε4 : 2%, ε4/ε4 : 2%. 16 patients without results. | ε2/ε2 : 0%, ε2/ε3 : 15%, ε3/ε3 : 58%, ε3/ε4 : 23%, ε2/ε4 : 2%, ε4/ε4 : 2%. 22 patients without results. |
| Months since PD diagnosis (median, quartiles)§ | 4.0 (2.0–8.0) | N.a. | 4.0 (2.0–9.0) | 4.0 (2.0–8.0) |
| UPDRS III (median, quartiles)§ | 20 (15–26)b | 0 (0–2) | 22 (16–27)c,h | 19 (14–26)j |
| Abeta42 (pg/ml) (median, quartiles)§ | 367.15 (305.13–426.55) | 378.50 (309.75–439.75) | 367.65 (297.55–438.50) | 366.80 (308.30–423.25) |
| T-tau(pg/ml) (median, quartiles)§ | 40.85 (32.30–52.65)a | 44.80 (35.20–64.60) | 42.50 (32.08–53.48)f | 40.30 (32.38–51.63)i |
| P-tau181(pg/ml) (median, quartiles)§ | 12.20 (9.30–18.50)a | 14.00 (11.10–21.50) | 13.10 (8.93–19.93)f | 12.05 (9.50–17.90)j |
| A-synuclein (pg/ml) (median, quartiles)§ | 1709.92 (1308.09–2171.00)b | 1939.78 (1363.80–2555.73) | 1581.12 (1219.75–2143.54)h | 1755.26 (1342.58–2185.54)i |
| Composite cognition factor (mean, (median), SD) | −0.14,(−0.06), (1.00) | N.a. | −0.76,(−0.72), (1.05) | 0.18, (0.16), (0.81) |
| Visuospatial function (mean, (median), SD) | −0.18, (−0.06), (1.08) | N.a. | −0.56, (−0.06), (1.27) | 0.02, (0.44), (0.90) |
| Memory function, (mean, (median), SD) | −0.38, (−0.29), (1.02) | N.a. | −0.93, (−0.89), (1.12) | −0.1, (−0.17), (0.84) |
| Executive-attention function (mean, (median), SD) | −0.29, (−0.29), (0.71) | N.a. | −0.69, (−0.73), (0.72) | −0.08, (−0.15), (0.61) |
| Benton judgment of line orientation (mean, SD)# | 12.8 (2.1) | 13.1 (2.0) | 12.0 (2.5)e,h | 13.2 (1.8) |
| Total immediate recall (HVLT) (mean, SD)# | 24.4 (5.0)b | 26.1 (4.5) | 21.8 (5.3)e,h | 25.8 (4.3) |
| Delayed recall (HVLT) (mean, SD)# | 8.4 (2.5)b | 9.3 (2.3) | 7.2 (2.9)e,h | 9.0 (2.1) |
| Letter-number sequencing (mean, SD)# | 10.6 (2.6) | 10.9 (2.6) | 9.4 (2.6)e,h | 11.2 (2.4) |
| Semantic fluency – sum of the three tests (mean, SD)# | 48.8 (11.6)a | 51.9 (11.3) | 44.0 (11.3)e,h | 51.3 (11.0) |
| Phonemic fluency (MoCA) (mean, SD)# | 13.1 (4.7)a | 14.2 (4.3) | 11.6 (4.8)e,h | 13.9 (4.4) |
| Symbol Digit Modalities Test (mean, SD)# | 41.3 (9.7)b | 46.8 (10.7) | 37.1 (11.1)e,h | 43.4 (8.1)j |
Sex, age and education are mean (SD). UPDRS III, months since PD diagnosis, abeta42, t-tau, p-tau181 and α-synuclein are median (25th–75th quartile). Months since PD diagnosis are months between PD diagnosis and the baseline examination. SD = standard deviation. N.a. = not applicable.
Groups compared by the Pearson Chi-Square test,
Groups compared by the t-test for independent samples.
Groups compared by the Fisher's exact test.
Groups compared by the Mann Whitney test.
P-values: a = PD vs HC <0.01, b = PD vs HC <0.001, c = PD-MCI vs PD-NC <0.05, d = PD-MCI vs PD-NC <0.01, e = PD-MCI vs PD-NC <0.001, f = PD-MCI vs HC < 0.05, g = PD-MCI vs HC <0.01, h = PD-MCI vs HC <0.001, i = PD-NC vs HC <0.01, j = PD-NC vs HC <0.001.
Multinomial logistic regression was conducted to compare marker concentrations between PD-MCI, PD-NC and controls while adjusting for the influence of other markers, gender, age, education and MDS-UPDRS III scores, with a combination of forced entry (demographics and UPDRSIII) and stepwise (CSF markers) inclusion of predictors. The regression analysis were checked for multicollinearity using the approach recommended by Belsey and Kuh, and for leverage, heteroscedacity, and normality of residuals, and did not violate assumptions, although there was a tendency for non-normality of residuals for the analysis of the visuospatial variable.
RESULTS
Demographics, CSF markers and MCI classification
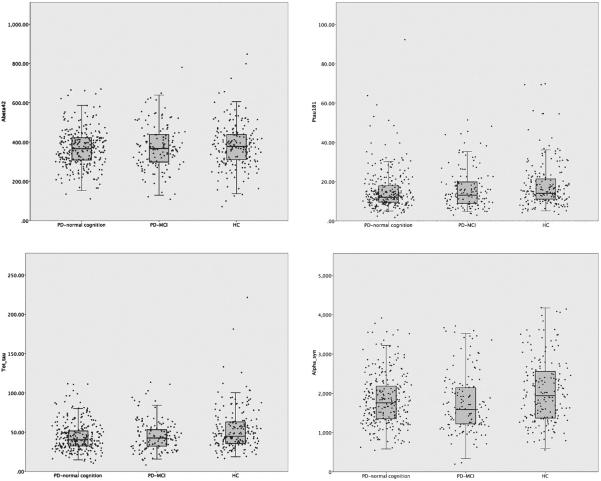
For demographics and results of CSF analysis, see Table 1 and Fig. 1. Briefly, there were no significant differences in gender, age or education between PD and healthy controls (HC). T-tau, p-tau181 and α-synuclein concentrations were lower in PD than HC (p = 0.001, p = 0.001 and p < 0.001), whereas abeta42 concentrations did not differ between groups in unadjusted analyses. Both t-tau (Spearman's rho correlation coefficient = 0.209, p < 0.001) and α-synuclein (rho = 0.123, p = 0.002) concentrations were significantly associated with age, and the CSF markers were all significantly correlated with each other (results not shown).
Fig. 1.
CSF marker concentrations in PD with normal cognition, PD-MCI and healthy controls, including scatter plots. PD-MCI = PD with mild cognitive impairment, HC= healthy controls. Each dot in the scatter plots represents an individual patient.
There were 140 PD-MCI patients (34%) and 274 PD-NC. Amongst PD-MCI patients, 10 (7%) had single domain MCI and 130 (93%) had multiple domain MCI. The PD-MCI patients were significantly older (p = 0.005), had less formal education (p < 0.001) and higher UPDRS scores (p = 0.013) than PD-NC. Both t-tau, p-tau181 and α-synuclein were reduced in the both the whole PD-group, PD-MCI and PD-NC compared to healthy controls. In the fully adjusted model, abeta42 concentrations were significantly lower in PD-MCI (p = 0.03) compared with HC, whereas the difference was not significant between PD-NC and HC (p = 0.06) or between the whole PD-group and HC. No significant differences were found in CSF marker concentrations between PD-MCI and PD-NC, between multiple domain and single domain MCI, or between multiple domain MCI and PD-NC in un-adjusted or fully adjusted analyses (results not shown).
Relationship between CSF marker candidates and the cognitive domains
We then analyzed associations between CSF markers and the four cognitive measures in the PD group using multiple regressions (for details see Table 2). All full models were significant. In the final model with all predictors, low α-synuclein was significantly associated with both decreased composite cognitive score (standardized beta = 0.132, p = 0.02) and reduced score on the executive-attention domain (standardized beta = 0.137, p = 0.02), and associated at trend level with decreased score in the memory-(standardized beta 0.109, p = 0.07) and visuospatial domains (standardized beta = 0.118, p = 0.06). Higher t-tau was significantly associated with impaired executive-attention- and visuospatial function in the first models with only CSF markers as predictors (standardized beta = −0.216, p = <0.01 and standardized beta = −0.181, p < 0.01)). However, the association was no longer significant after adjustment for confounders (standardized beta = −0.084, p = 0.17 and standardized beta = −0.094, p = 0.16, respectively). There were no significant associations between abeta42 or p-tau 181 and cognitive domains.
Table 2.
Results from the multiple regression analysis, all PD patients included
| Composite cognition | Executive/attention | Visuospatial function | Memory | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Step | Predictor | Beta | P | Beta | p | Beta | p | Beta | p |
| 1. | abeta42 | 0.071 | 0.189 | 0.068 | 0.211 | 0.067 | 0.220 | 0.020 | 0.719 |
| t-tau | −0.187 | 0.006 | −0.216 | 0.001 | −0.181 | 0.008 | −0.128 | 0.062 | |
| p-tau181 | 0.038 | 0.487 | 0.050 | 0.364 | −0.008 | 0.891 | 0.028 | 0.618 | |
| α-synuclein | 0.165 | 0.014 | 0.174 | 0.009 | 0.161 | 0.016 | 0.136 | 0.044 | |
| 2. | Abeta42 | −0.015 | 0.752 | −0.005 | 0.922 | 0.064 | 0.214 | −0.055 | 0.259 |
| T-tau | −0.058 | 0.327 | −0.084 | 0.172 | −0.094 | 0.155 | −0.023 | 0.715 | |
| P-tau181 | 0.029 | 0.525 | 0.037 | 0.443 | −0.026 | 0.612 | 0.022 | 0.657 | |
| α-synuclein | 0.132 | 0.019 | 0.137 | 0.020 | 0.118 | 0.058 | 0.109 | 0.068 | |
| Gender (1 = F, 2 =M) | −0.251 | <0.001 | −0.153 | <0.001 | 0.230 | <0.001 | −0.246 | <0.001 | |
| Age (in years) | −0.382 | <0.001 | −0.372 | <0.001 | −0.132 | 0.009 | −0.323 | <0.001 | |
| Education (in years) | 0.294 | <0.001 | 0.294 | <0.001 | 0.238 | <0.001 | 0.256 | <0.001 | |
| UPDRS III total | −0.104 | 0.014 | −0.062 | 0.158 | −0.116 | 0.014 | −0.085 | 0.057 | |
Results from the multiple linear regression models of the effects of CSF markers on cognition in the PD patients. In step 1 only the CSF markers were included as predictors vs composite cognition and the cognitive domains as dependent variables. In step 2 potential confounding variables were added. Only patients without missing values were included in the analyses, n = 403 (5 excluded to due to extreme α-synuclein values, two due to missing p-tau, 4 due to missing t-tau results.). The reported beta-values are standardized.
DISCUSSION
This is the largest study exploring the association between CSF biomarkers and cognition in PD, and one of few to explore if α-synuclein is associated with cognitive impairment. The main finding was a significant association between reduced α-synuclein and reduced composite cognition and executive-attention domain tests scores. The association between higher t-tau concentration and impairment in the executive-attention and visuospatial domains were no longer significant after the demographic predictors were added to the model, most likely because t-tau concentrations increase with increasing age [22]. CSF abeta42 was reduced in PD-MCI, but not in PD-NC compared with HC in the fully adjusted multinomial logistic regression model, suggesting a possible association between abeta42 and cognition. T-tau, p-tau181 and α-synuclein were lower in PD than HC, but were not associated with MCI.
We report a novel finding of an association between reduced CSF α-synuclein and cognitive impairment in untreated, newly diagnosed PD-patients, but the impact of our finding is limited by the relatively small standardized beta values and the fact that we did not correct for multiple testing. Even so, this suggests that Lewy-body pathology is associated with cognitive impairment in early PD. We speculate that involvement of striato-prefrontal circuits contributes to executive-attentional impairment. This hypothesis is consistent with recent findings in the same cohort of an association between reduced dopamine transporter SPECT binding and executive dysfunction [23], and also with findings that frontal-executive dysfunction evolves independently of impairment of other cognitive domains on a dopaminergic basis [24].
Strengths to our study is the large number of patients, the extensive examination battery including DaTscans, the standardized procedures, and the longitudinal design which increases diagnostic accuracy. Future longitudinal studies will show whether the CSF-values can predict rate of cognitive decline and time to dementia.
In contrast to our findings of a link between lower α-synuclein and cognitive impairment, lower α-synuclein concentrations were recently reported to be associated with preservation of cognitive function in two longitudinal studies [15, 16]. The first report came from the DATATOP trial, which initially was a randomized controlled trial of deprenyl and α-tocopherol, including newly diagnosed PD patients without dementia. The main finding from the study was that higher α-synuclein values predicted faster decline in several cognitive tests. Surprisingly, the associations go in different directions in the two study phases : in one phase decreased α-synuclein was associated with reduced score on delayed verbal recall, while in the second phase decreased α-synuclein was associated with increased test recall score. In that study patients were recruited in 1987 and 1988, and CSF had been stored for >20 years before analyses. Compared to our study, a different α-synuclein assay was used in the DATATOP trial and the mean concentrations were different; DATATOP: Mean 630 pg/ml; PPMI: 1845 pg/ml in PD patients. The second report, a part of the Swedish BioFinder Study [15], found significant correlations between higher α-synuclein concentrations at baseline and worsening of cognitive processing speed (A Quick Test of Cognitive Speed). However, these patients were not newly diagnosed, having a mean disease duration of 7 years at baseline – thus being both older (median 67.5 years) and already on dopaminergic medication, all in all comparable to DATATOP phase 2. Again a different α-synuclein assay was used, and concentrations were surprisingly much lower than in the PPMI and DATATOP, with PD patients having median 50.5 pg/ml. All this might have affected the results. Future studies will clarify whether the discrepancy between findings is caused by the assays or different disease stages of the patients.
α-synuclein is a protein found in abundance in the human brain, and a main constituent of the Lewy bodies hallmark to PD, but the exact function is yet poorly understood. Similarly, the mechanism that leads to the decrease of α-synuclein in CSF in PD remains to be clarified, and thus it is not known whether this is due to a-synuclein pathology, influx of a-synuclein in the Lewy bodies, altered release of a-synuclein from synapses or or other mechanisms. Mixed pathologies are common in older individuals and thus might cloud the reduced concentration of α-synuclein in PD and other synucleinopathies. More research into α-synuclein metabolism and PD pathogenesis is necessary to establish whether CSF measurements of total α-synuclein or maybe oligomeric or post translationally modified forms provide more information. Using AD as an analogy, the usefulness of measuring total amounts of beta amyloid-peptides in CSF is limited, the marker became useful when it was discovered that the 42 amino acid form was key in disease pathogenesis.
Several studies have found correlations between decreased CSF abeta42 levels and memory impairment [8, 10], but in the current study, such an association was not found. These discrepant findings may be due to differences in the cohorts, since our cohort tended to be younger and less cognitively impaired. For example, mean MoCA score was 27.1 in our cohort, compared to MoCA= 25.3 in one study [10], and a MMSE score of 27.8 in another [8]; this MMSE score corresponds approximately to a MoCA score of 23–24 [25]. Although we did not observe significant differences between abeta42 concentrations in PD with or without MCI, PD-MCI but not PD-NC differed significantly from HC in the adjusted model. Abeta42 thus seems to be decreased in at least a subset of early PD patients with cognitive impairment. Future studies of the cohort will determine whether the previously reported finding that a more Alzheimer-like CSF marker profile with low abeta42 levels can predict future cognitive decline in PD can be confirmed [11–15]. Taken together, the available literature suggest that abeta-pathology is important for memory impairment in older and more advanced PD-patients but less so in early PD.
In addition to cohort differences, discrepancies between different CSF studies could be explained by pre-analytical and analytical variation. The CSF markers, especially abeta42 have a great tendency to adhere to surfaces including polypropylene [26]. Findings may also be influenced by the different analysis methods that were used. For example, in some studies Innotest ELISAs were used [8, 10] in contrast to the Luminex xMAP platform used in PPMI.
Another methodological factor potentially influencing our findings is the multi-center design of PPMI with a large number of clinical sites. Slight differences in pre-analytical sample handling between centers could decrease the signal-to-noise ratio, influencing especially abeta42 [26]. One limitation of the PPMI is the restricted neuropsychological test battery, since the study was designed prior to the publication of the MCI criteria [20]. Thus adjustments had to be made for the classification of MCI such as including subtests from MoCA without age- and education adjusted norms. Our MCI classification was thus not adjusted for age and education, so there is a risk that some of the elderly, less educated patients were incorrectly classified as PD-MCI and some of the younger patients with high education wrongfully as PD-NC. However, when comparing markers concentrations, we adjusted for age, education and UPDRS III scores to avoid this problem. Finally, HC were required to have a MoCA score of 27 or higher, which increases the risk of a super-normal HC group and this in turn might have influenced marker concentrations. However, this would not influence the association between CSF markers and cognition in the PD group.
More research is needed to clarify the link between CSF markers and cognitive domain performance, motor function and structural brain changes. Standardization of pre-analytical and analytical factors is crucial for further advances in this field. CSF analyses can potentially increase our understanding of the mechanisms underlying early cognitive impairment in PD, by disentangling the relative contribution of α-synuclein pathology and concomitant AD pathology as well as other factors.
ACKNOWLEDGMENTS
Data used in the preparation of this article were obtained from the Parkinson's Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation for Parkinson's Research and funding partners, including Abbvie, Avid Radiopharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal, Roche, Servier and UCB.
Financial Disclosures: Dr. Skogseth reports no disclosures. Dr. Bronnick reports no disclosures. Dr. Pereira has received a Marie Curie Intra-European Fellowship and project grants from Swedish non-governmental and industry independent sources; Alzheimerfonden, Stiftelsen för ålderssjukdomar, Loo och Hans Ostermans stiftelse and Lindhes Advokatbyra AB. Dr. Mollenhauer is member of the executive steering committee of the Parkinson Progression Marker Initiative of the Michael J. Fox Foundation for Parkinson's Research. She has received independent research grants from TEVA-Pharma, and honoraria for consultancy from GE Healthcare, Roche, AbbVie, TEVA-Pharma, for presentations from GlaxoSmithKline, Orion Pharma, TEVA-Pharma and travel costs from TEVA-Pharma. She has received grants from the BMBF, EU, Deutsche Parkinson Vereinigung, Michael J. Fox Foundation for Parkinson's Research, Stifterverband für die deutsche Wissenschaft, and has scientific collaborations with Roche, Ely Lilly, Covance/BioLegend and Biogen Idec. Dr. Weintraub is part of the scientific advisory boards: Commercial - Pfizer, CHDI, Teva Pharmaceuticals, Avanir Pharmaceuticals, Merck, Eli Lilly and Company, Biogen, Lundbeck Inc. He is part of the Editorial Board of Movement Disorders Journal and receives: Funding for Travel or Speaker Honoraria: Commercial - Teva Pharmaceuticals; Research Support, Commercial Entities: Novartis Pharmaceuticals, PI, 2011-; Research Support from Government Entities: NINDS, P50 NS053488, Investigator, 2007- NINDS, R01NS065087, Investigator, 2009- NIA, RO1AG031348, Investigator, 2008-; Research Support, Foundations and Societies: Michael J. Fox Foundation for Parkinson's Research, PI, 2009–2011, Michael J. Fox Foundation for Parkinson's Research, PI, 2011-Michael J. Fox Foundation for Parkinson's Research, Investigator, 2011-; License Fee Payments, Technology or Inventions: Licensing fees from the University of Pennsylvania for licensing of Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease.
Dr. Fladby reports no disclosures. Dr. Aarsland serves on scientific advisory boards for Lundbeck Inc. and Merck Serono; has received funding for travel and speaker honoraria from Lundbeck Inc., Novartis, GE Healthcare, and GlaxoSmithKline; serves on the editorial boards of International Psychogeriatrics, Movement Disorders, and the Journal of Neurology, Neurosurgery, and Psychiatry; and receives research support from Lundbeck Inc. and Merck Serono.
Author's Roles: RES, KB, JBP and DA were involved in the conception and design of the study, processing, analysis and interpretation of the data, and drafting/revising the manuscript for content. Statistical analyses were conducted by RES, KB and JBP. BM, TF and DW were involved in the interpretation of the data, and drafting/revising the manuscript for content. All authors have given final approval of the submitted version.
REFERENCES
- [1].Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson's disease: Diagnosis, biomarkers, and treatment. Lancet Neurol. 2012;11:697–707. doi: 10.1016/S1474-4422(12)70152-7. [DOI] [PubMed] [Google Scholar]
- [2].Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9:1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- [3].Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van Deerlin V, Lee VM, Leverenz JB, Montine TJ, Duda JE, Hurtig HI, Trojanowski JQ. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72:587–598. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kandiah N, Zainal NH, Narasimhalu K, Chander RJ, Ng A, Mak E, Au WL, Sitoh YY, Nadkarni N, Tan LC. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson's disease. Parkinsonism Relat Disord. 2014;20:1203–1208. doi: 10.1016/j.parkreldis.2014.08.024. [DOI] [PubMed] [Google Scholar]
- [5].Compta Y, Parkkinen L, Kempster P, Selikhova M, Lashley T, Holton JL, Lees AJ, Revesz T. The significance of alpha-synuclein, amyloid-beta and tau pathologies in Parkinson's disease progression and related dementia. Neurodegener Dis. 2014;13:154–156. doi: 10.1159/000354670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gomperts SN, Locascio JJ, Rentz D, Santarlasci A, Marquie M, Johnson KA, Growdon JH. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013;80:85–91. doi: 10.1212/WNL.0b013e31827b1a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Compta Y, Marti MJ, Ibarretxe-Bilbao N, Junque C, Valldeoriola F, Munoz E, Ezquerra M, Rios J, Tolosa E. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson's disease. Mov Disord. 2009;24:2203–2210. doi: 10.1002/mds.22594. [DOI] [PubMed] [Google Scholar]
- [8].Alves G, Bronnick K, Aarsland D, Blennow K, Zetterberg H, Ballard C, Kurz MW, Andreasson U, Tysnes OB, Larsen JP, Mulugeta E. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson's disease: The Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2010;81:1080–1086. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- [9].Leverenz JB, Watson GS, Shofer J, Zabetian CP, Zhang J, Montine TJ. Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson's disease. Parkinsonism Relat Disord. 2011;17:61–64. doi: 10.1016/j.parkreldis.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yarnall AJ, Breen DP, Duncan GW, Khoo TK, Coleman SY, Firbank MJ, Nombela C, Winder-Rhodes S, Evans JR, Rowe JB, Mollenhauer B, Kruse N, Hudson G, Chinnery PF, O'Brien JT, Robbins TW, Wesnes K, Brooks DJ, Barker RA, Burn DJ, Group I-PS Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE-PD study. Neurology. 2014;82:308–316. doi: 10.1212/WNL.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Compta Y, Pereira JB, Rios J, Ibarretxe-Bilbao N, Junque C, Bargallo N, Camara A, Buongiorno M, Fernandez M, Pont-Sunyer C, Marti MJ. Combined dementia-risk biomarkers in Parkinson's disease: A prospective longitudinal study. Parkinsonism Relat Disord. 2013;19:717–724. doi: 10.1016/j.parkreldis.2013.03.009. [DOI] [PubMed] [Google Scholar]
- [12].Siderowf A, Xie SX, Hurtig H, Weintraub D, Duda J, Chen-Plotkin A, Shaw LM, Van Deerlin V, Trojanowski JQ, Clark C. CSF amyloid beta 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75:1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Alves G, Lange J, Blennow K, Zetterberg H, Andreasson U, Forland MG, Tysnes OB, Larsen JP, Pedersen KF. CSF Abeta42 predicts early-onset dementia in Parkinson disease. Neurology. 2014;82:1784–1790. doi: 10.1212/WNL.0000000000000425. [DOI] [PubMed] [Google Scholar]
- [14].Parnetti L, Farotti L, Eusebi P, Chiasserini D, De Carlo C, Giannandrea D, Salvadori N, Lisetti V, Tambasco N, Rossi A, Majbour NK, El-Agnaf O, Calabresi P. Differential role of CSF alpha-synuclein species, tau, and Abeta42 in Parkinson's Disease. Front Aging Neurosci. 2014;6:53. doi: 10.3389/fnagi.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hall S, Surova Y, Ohrfelt A, Zetterberg H, Lindqvist D, Hansson O. CSF biomarkers and clinical progression of Parkinson disease. Neurology. 2015;84:57–63. doi: 10.1212/WNL.0000000000001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stewart T, Liu C, Ginghina C, Cain KC, Auinger P, Cholerton B, Shi M, Zhang J, Parkinson Study Group DI Cerebrospinal fluid alpha-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am J Pathol. 2014;184:966–975. doi: 10.1016/j.ajpath.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Parkinson Progression Marker I The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS, Waligorska T, Taylor P, Pan S, Frasier M, Marek K, Kieburtz K, Jennings D, Simuni T, Tanner CM, Singleton A, Toga AW, Chowdhury S, Mollenhauer B, Trojanowski JQ, Shaw LM, Parkinson's Progression Markers I Association of cerebrospinal fluid beta-amyloid 1-42, T-tau, P-tau181, and alpha-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol. 2013;70:1277–1287. doi: 10.1001/jamaneurol.2013.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pereira JB, Svenningsson P, Weintraub D, Bronnick K, Lebedev A, Westman E, Aarsland D. Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology. 2014;82:2017–2025. doi: 10.1212/WNL.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, Aarsland D, Kulisevsky J, Rodriguez-Oroz MC, Burn DJ, Barker RA, Emre M. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Leverenz JB, Baird G, Montine TJ, Hancock AM, Hwang H, Pan C, Bradner J, Kang UJ, Jensen PH, Zhang J. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010;133:713–726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Randall C, Mosconi L, de Leon M, Glodzik L. Cerebrospinal fluid biomarkers of Alzheimer's disease in healthy elderly. Front Biosci (Landmark Ed) 2013;18:1150–1173. doi: 10.2741/4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Siepel FJ, Bronnick KS, Booij J, Ravina BM, Lebedev AV, Pereira JB, Gruner R, Aarsland D. Cognitive executive impairment and dopaminergic deficits in de novo Parkinson's disease. Mov Disord. 2014;29:1802–1808. doi: 10.1002/mds.26051. [DOI] [PubMed] [Google Scholar]
- [24].Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132:2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- [25].Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE. Comparative accuracies of two common screening instruments for classification of Alzheimer's disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 2013;9:529–537. doi: 10.1016/j.jalz.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Perret-Liaudet A, Pelpel M, Tholance Y, Dumont B, Vanderstichele H, Zorzi W, Elmoualij B, Schraen S, Moreaud O, Gabelle A, Thouvenot E, Thomas-Anterion C, Touchon J, Krolak-Salmon P, Kovacs GG, Coudreuse A, Quadrio I, Lehmann S. Risk of Alzheimer's disease biological misdiagnosis linked to cerebrospinal collection tubes. J Alzheimers Dis. 2012;31:13–20. doi: 10.3233/JAD-2012-120361. [DOI] [PubMed] [Google Scholar]