Abstract
The cellular and molecular basis of long-term memory in vertebrates remains poorly understood. Knowledge regarding long-term memory has been impeded by the enormous complexity of the vertebrate brain, particularly the mammalian brain, as well as by the relative complexity of the behavioral alterations examined in most studies of long-term memory in vertebrates. Here, we demonstrate a long-term form of nonassociative learning—specifically, long-term habituation (LTH)—of a simple reflexive escape response, the C-start, in zebrafish larvae. The C-start is triggered by the activation of one of a pair of giant neurons in the zebrafish’s hindbrain, the Mauthner cells. We show that LTH of the C-start requires the activity of NMDA receptors and involves macromolecular synthesis. We further show that the long-term habituated reflex can by rapidly dishabituated by a brief tactile stimulus. Our results set the stage for rigorous, mechanistic investigations of the long-term memory for habituation of a reflexive behavioral response, one that is mediated by a relatively simple, neurobiologically tractable, neural circuit. Moreover, the demonstration of NMDAR and transcriptionally dependent LTH in a translucent vertebrate organism should facilitate the use of optical recording, and optogenetic manipulation, of neuronal activity to elucidate the cellular basis of a long-term vertebrate memory.
Keywords: Zebrafish, Memory, Learning, NMDAR, Habituation
1. Introduction
The capability to form long-term memories confers a selective advantage by allowing organisms to more effectively operate in a dynamic environment. Consequently, this ability extends even to organisms with extremely simple nervous systems, which are able to store, maintain, and utilize information for relatively long periods of time (2002; Timbers & Rankin, 2011). Some of the cellular and molecular pathways that mediate long-term memory formation have been elucidated, and these display a striking evolutionary conservation (Bourtchuladze, Frenguelli, Blendy, Cioffi, Schutz, & Silva, 1994; Busto, Guven-Ozkan, Fulga, Van Vactor, & Davis, 2015; Cai, Pearce, Chen, & Glanzman, 2011; Dash, Hochner, & Kandel, 1990; Drier, Tello, Cowan, Wu, Blace, Sacktor, & Yin, 2002; Glanzman, 2013; Guan, Giustetto, Lomvardas, Kim, Miniaci, Schwartz, Thanos, & Kandel, 2002; Lakhina, Arey, Kaletsky, Kauffman, Stein, Keyes, Xu, & Murphy, 2015; Levenson & Sweatt, 2005; Sacktor, 2008; Silva, Kogan, Frankland, & Kida, 1998; Timbers & Rankin, 2011; Yin, Del Vecchio, Zhou, & Tully, 1995; Yin, Wallach, Del Vecchio, Wilder, Zhou, Quinn, & Tully, 1994). Commonly, long-term memories require the transcription of new gene products, as well as the translation of existing terminally localized mRNA into proteins (Goelet, Castellucci, Schacher, & Kandel, 1986; Kandel, Dudai, & Mayford, 2014), and result in morphological rearrangements associated with the modification of synapses (Bailey, Kandel, & Harris, 2015). But, to date, we only have a rudimentary understanding of the biological processes that mediate long-term memory.
Invertebrates have been used extensively by neurobiologists to study long-term memory (Bailey, Kandel, & Si, 2004; Kandel et al., 2014). A major advantage of studying long-term memory in invertebrate organisms is that the cellular and molecular changes that mediate the learned behavioral changes can typically be studied in well-defined neural circuits. A paradigmatic example of this situation is long-term modulation of the gill- and siphon-withdrawal reflex in the mollusk Aplysia. This reflex exhibits two forms of long-term nonassociative memory, long-term habituation (LTH) (Carew, Pinsker, & Kandel, 1972; Ezzeddine & Glanzman, 2003) and long-term sensitization (Pinsker, Hening, Carew, & Kandel, 1973). Both LTH (Bailey & Chen, 1988a; Castellucci, Carew, & Kandel, 1978) and long-term sensitization (Bailey & Chen, 1988a; b; Frost, Castellucci, Hawkins, & Kandel, 1985) are associated with, and due, in part, to electrophysiological and morphological changes at an identified synaptic connection, the monosynaptic connection between the sensory and motor neurons that mediate the reflex. This fact, coupled with the ability to recapitulate these learning-related cellular changes in dissociated cell culture (Glanzman, Kandel, & Schacher, 1990; Montarolo, Goelet, Castellucci, Morgan, Kandel, & Schacher, 1986; Montarolo, Kandel, & Schacher, 1988; Schacher, Kandel, & Montarolo, 1993), has enormously facilitated the acquisition of critical insights into the fundamental molecular mechanisms of long-term memory (Bailey et al., 2004; Kandel, 2001; Kandel et al., 2014). By contrast, progress toward a mechanistic understanding of long-term memory in vertebrates has been impeded by the difficulty of unambiguously ascribing specific learned behavioral changes to changes at specific cellular sites in the brain (as opposed to brain regions).
Recently, the zebrafish has begun to show considerable potential as a vertebrate system with significant —and, in some respects, unique—advantages for cell biological investigations of long-term memory. First, new technologies allow for the targeted deletion of genes in zebrafish (Bedell, Wang, Campbell, Poshusta, Starker, Krug, Tan, Penheiter, Ma, Leung, Fahrenkrug, Carlson, Voytas, Clark, Essner, & Ekker, 2012; Dahlem, Hoshijima, Jurynec, Gunther, Starker, Locke, Weis, Voytas, & Grunwald, 2012; Meng, Noyes, Zhu, Lawson, & Wolfe, 2008; Moore, Reyon, Sander, Martinez, Blackburn, Khayter, Ramirez, Joung, & Langenau, 2012). Second, larval zebrafish are translucent, which facilitates optical methods of recording neural activity (Aizenberg & Schuman, 2011; Bundschuh, Zhu, Schärer, & Friedrich, 2012; Friedrich & Korsching, 1997; Higashijima, Masino, Mandel, & Fetcho, 2003; Niell & Smith, 2005; O’Malley, Kao, & Fetcho, 1996; Warp, Agarwal, Wyart, Friedmann, Oldfield, Conner, Del Bene, Arrenberg, Baier, & Isacoff, 2012), as well as optogenetic manipulation of neuronal activity (Arrenberg, Del Bene, & Baier, 2009; Bundschuh et al., 2012; Douglass, Kraves, Deisseroth, Schier, & Engert, 2008; Fajardo, Zhu, & Friedrich, 2013; Portugues, Severi, Wyart, & Ahrens, 2013; Wyart, Del Bene, Warp, Scott, Trauner, Baier, & Isacoff, 2009; Zhu, Narita, Bundschuh, Fajardo, Scharer, Chattopadhyaya, Bouldoires, Stepien, Deisseroth, Arber, Sprengel, Rijli, & Friedrich, 2009), in the intact nervous system. Third, zebrafish can be treated with pharmacological agents directly through bath application, thereby enabling the rapid screening of behavior (Goldsmith, 2004; Langheinrich, 2003; Stewart & Kalueff, 2012). Finally, the zebrafish possesses an escape reflex, the so-called “C-start”, which is mediated by a relatively simple and well-understood neuronal circuit; importantly, the C-start is triggered by activation of one of a pair of large, identified neurons in the fish’s hindbrain, the Mauthner cells (Eaton, Lee, & Foreman, 2001; Issa, O’Brien, Kettunen, Sagasti, Glanzman, & Papazian, 2011; Korn & Faber, 2005; Medan & Preuss, 2014). The C-start therefore represents a vertebrate behavior that is invertebrate-like in the simplicity of its neural basis.
Of course, these advantages would be moot for functional analyses of learning and memory if zebrafish could not learn. However, zebrafish, like all teleost fish, exhibit significant and well-documented learning capabilities (Al-Imari & Gerlai, 2008; Colwill, Raymond, Ferreira, & Escudero, 2005; Karnik & Gerlai, 2012; Sison & Gerlai, 2010; Williams, White, & Messer, 2002). Furthermore, the capacity for both nonassociative and associative learning is present even in zebrafish larvae (Aizenberg & Schuman, 2011; Best, Berghmans, Hunt, Clarke, Fleming, Goldsmith, & Roach, 2008; Hinz, Aizenberg, Tushev, & Schuman, 2013; Roberts, Bill, & Glanzman, 2013; Roberts, Reichl, Song, Dearinger, Moridzadeh, Lu, Pearce, Esdin, & Glanzman, 2011; Wolman & Granato, 2011). Recently, long-term memory has been documented in larval zebrafish (Hinz et al., 2013; Wolman, Jain, Liss, & Granato, 2011). Here, we show that a simple reflex, the C-start, can undergo LTH in zebrafish larvae. We further demonstrate that this form of persistent nonassociative behavioral modification depends critically upon NMDA receptor-mediated neurotransmission and requires the synthesis of new macromolecules.
2. Materials and methods
2.1 Animals
The zebrafish (Danio rerio, wild type TL strain) was used in all experiments. The adult fish were cared for and maintained at the UCLA Zebrafish Core Facility using standard methods. The adults were bulk mated; the embryos were collected the following morning, placed a Petri dish (density, 80 embryos per dish) containing E3 medium for zebrafish embryos (5 mM NaCl, 0.33 mM MgCl2, 0.33 mM CaCl2, 0.17 mM KCl, 10–5% methylene blue, pH 7.2), and kept in a darkened incubator at 28.5°C for 5 d. The E3 (control) medium was changed daily, and sick or dead embryos were removed from the Petri dish.
2.2 Behavioral recording and training
Immediately prior to training the larvae were put into individual wells (~3 ml of control medium) and the wells were placed on a light box (Gagne Inc., Johnson City NY) in a lighted room. The larvae were left on the light box, undisturbed, for 1 h prior to the onset of the pretests. A speaker, which was positioned next to the wells, was used to deliver auditory/vibrational pulses to the larvae; each pulse consisted of a 1 ms, 200 Hz ramp wave (109 dB). The responses of the larvae to the auditory pulses were recorded using a TroubleShooter TS100MS high-speed camera (Fastec Imaging, San Diego, CA) at 1000 frames/s. A response to an auditory pulse was deemed a C-start response if the event occurred ≤ 25 ms after the stimulus and began with the characteristic C-bend (Eaton et al., 2001). A trained observer coded the C-start as a “1” for a response or a “0” for no response. A blinded observer coded any ambiguous responses. (Note that ambiguous responses represented a small percentage of the total.) Prior to habituation training 5 pulses (5 min interpulse interval) were delivered to the larvae (Pretest, Figure 1). Habituation training began 5 min after the last pretest pulse, and consisted of 6 blocks of stimulation followed by 20 min with no stimulation (interblock interval). Each block of training comprised 8 bouts of auditory stimulation followed by 3 min with no stimulation (interbout interval); each bout of stimulation, in turn, comprised 120 pulses delivered at 1 Hz. Thus, during habituation training a fish received 5,760 auditory pulses over about a period of about five-and-a-half hours. In the initial set of experiments the larvae were left on the light box with the room lights on and given posttests after 1 h. The stimulation protocol for the posttest was identical to that of the pretest. Untrained (Test Alone) fish were treated exactly like the trained fish, except that they were simply left on the light box, unstimulated, for the equivalent time amount of time as the habituation training period.
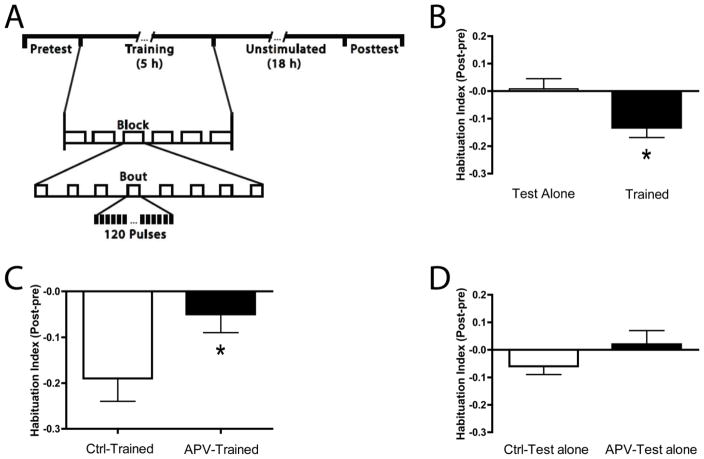
Figure 1.
Habituation of the C-start in larval zebrafish expressed at 1 h posttraining requires NMDA receptor activity. (A) Experimental protocol used to demonstrate 1 h habituation. The Habituation Index = the mean posttest response rate minus mean pretest response rate. See the Materials and methods for additional information. All fish were 5 d post-fertilization (dpf) at the start of the experiments. After the Pretest period, during which the larvae were given 5 auditory pulses separated by 5 min, trained larvae received 6 blocks of habituation training, separated by 20 min. Each block comprised 8 bouts of auditory pulses (120 pulses per bout at 1 Hz, 3 min interbout interval). Following habituation training there was a 1 h period, during which the larvae were unstimulated, followed by the Posttest. The Posttest was identical to the Pretest. Untrained (Test Alone) larvae were treated identically to the Trained larvae, except that they received no stimulation prior to the rest period. See the Materials and methods for additional details. (B) ITH of the C-start. The Trained group exhibited significantly fewer escape responses than did the Test Alone group (asterisk). (C) Effect of NMDA receptor blockade on ITH. The APV-Trained group exhibited significantly less habituation than the Ctrl-Trained group (asterisk). (D) APV exposure did not affect the baseline responsiveness of zebrafish larvae. There was no significant difference between the two groups of untrained larvae (APV-Test Alone and Ctrl-Test Alone) with respect to the number of C-starts elicited.
In a second set of experiments the fish were given posttests at both 1 h and 18 h after the training/control (test alone) period. Here, after the 1 h posttest the light bulb in the light box and the room lights were switched off, and the larvae were left in their wells in the dark, undisturbed, for 16 h. Then the lights in the room were turned on, as was the light in the light box; the larvae were allowed to acclimate to the light for 1 h, after which they were given the posttest stimulation.
A third experimental protocol was identical to that in the second set of experiments, except that there was only a single posttest at 18 h following the training/test alone period. The 1 h posttest was omitted, and the larvae were left unstimulated in the dark for 17 h rather than 16 h.
2.3 Drug treatment
Drugs were obtained from Sigma (St. Louis, MO). DL-2-amino-5-phosphonopentanoic acid (DL-APV), D-2-amino-5-phosphonopentanoic acid (D-APV) and cyclohexamide were dissolved in control medium. 5,6-Dichlorobenzimidazole 1-β-D-ribofuranoside (DRB) was dissolved in DMSO and then diluted in control medium to a final concentration of 0.2% DMSO. In experiments using APV, larvae were placed into individual wells containing either the drug or control medium for 24 h. Then, immediately before the beginning of the pretest, the solutions were replaced with fresh APV/control medium, and the larvae remained in these solutions throughout the experiment. In experiments using cyclohexamide and DRB, the drug was added in the appropriate concentration to the individual wells immediately following the last pretest, and habituation training/test alone treatment was begun 15 min later. After the end of the habituation/test alone regimen, the drug/control medium was washed out of the wells, and larvae were given the posttest 18 h later.
2.4 Cold shock
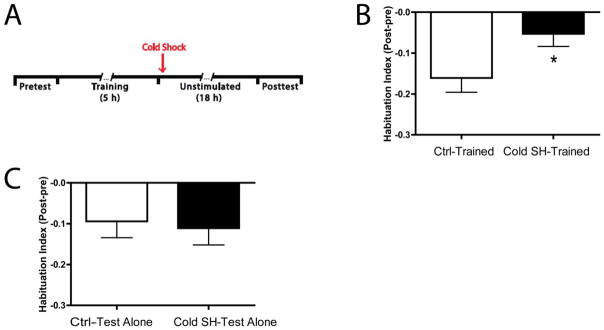
Immediately after habituation training/test alone treatment the larvae were separated into four groups. Larvae in two groups (Ctrl-Trained and Ctrl-Test Alone) were removed from their training wells and placed into new individual wells that contained control medium at room temperature for 2 min. Larvae in the other two group (Cold SH-Trained and Cold SH-Test Alone) were removed from their training wells and placed individually into wells that contained control medium chilled to 6–8° C degrees for 2 min. Afterwards, all fish were returned to their original training wells and left undisturbed until the posttest.
2.5 Dishabituation
To dishabituate the C-start, fish were lightly touched with a broom bristle, applied by hand, 15 min prior to the Posttest.
2.6 Statistics
A habituation index (HI), the mean pretest response rate subtracted from the mean posttest response rate, was calculated for each fish. Two-tailed, unpaired t-tests, one-way ANOVAs, and repeated measures ANOVAs were performed using the HI data for statistical comparisons. Student Newman-Keuls tests were used for post-hoc analysis of significant ANOVAs.
3. Results
3.1 LTH of the C-Start reflex requires NMDA receptor activity
We previously showed that a spaced training protocol elicited a short-term (≤ 25 min) form of habituation of the C-start reflex (Roberts et al., 2011). Here, we find that by training with an “on-off” pattern of auditory stimulation (Figure 1A) we can induce habituation that persisted for ≥ 1 h (Figure 1B), indicated by the Habituation Index (HI: mean posttest response rate minus the mean pretest response rate). Larvae that received habituation training (Trained, n = 48) responded significantly less (HI = −0.13 ± 0.04) to auditory pulses at 1 h after training than did larvae (Test Alone, n = 24), tested at the equivalent experimental time, that received no habituation training (HI = 0.08 ± 0.04; t = 2.42, p < 0.05).
This more persistent habituation learning may represent a form of intermediate-term memory (ITM), and we therefore term it intermediate-term habituation (ITH) here. Additional work will be necessary to ascertain whether the habituation memory at the 1 h test point meets the hallmark criteria of ITM as described in other systems (Ghirardi, Montarolo, & Kandel, 1995; Sutton, Masters, Bagnall, & Carew, 2001). To determine whether ITH of the C-start requires activation of NMDA receptors for its induction, as does short-term habituation (Roberts et al., 2011), we incubated larvae in 200 μM DL-APV (APV-Trained, n = 24) or E3 (control [Ctrl]) medium (Ctrl-Trained, n = 24) for 24 h prior to the onset of the training/testing; the larvae remained in the APV/control solution throughout the training/test alone regimens. The APV-Trained larvae were significantly more responsive (HI = −0.05 ± 0.04) than the Ctrl-Trained larvae (HI = −0.19 ± 0.05; t = 2.09, p < 0.05), indicating that NMDA receptor signaling was required for ITH (Figure 1C). We tested whether APV nonspecifically enhanced the responsiveness of untrained fish by incubating larvae in 200 μM DL-APV (APV-Test Alone, n = 24) or control solution (Ctrl-Test Alone, n = 24) for 24 h prior to the onset of the test alone experimental protocol. No significant difference was observed between the APV-Test Alone group (HI = 0.02 ± 0.05) and the Ctrl-Test Alone group (HI = −0.06 ± 0.03; t = 1.19, p > 0.2) (Figure 1D); therefore, APV did not produce deleterious effects (e.g., damage to the auditory pathway or induction of fatigue) that might account for its ability to block ITH, or otherwise alter the baseline responsiveness of the larvae.
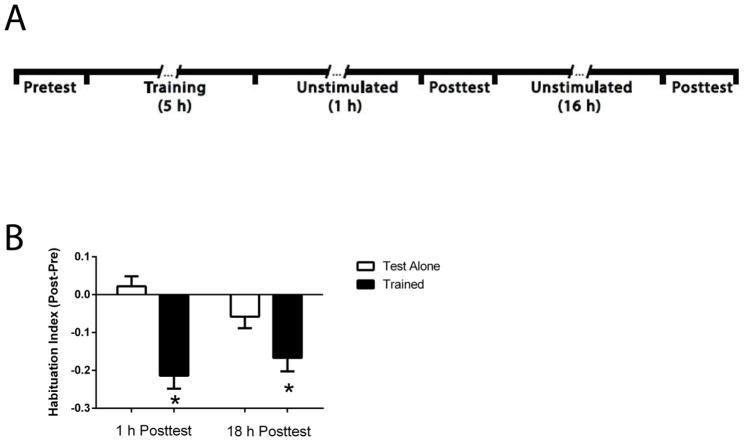
To determine whether the habituation induced by our training persisted for > 1 h, we performed another experiment, using the same training regimen, in which larvae were tested at 18 h, as well as at 1 h, posttraining (Figure 2A). Larvae that received the habituation training (Trained group) exhibited fewer C-starts at both 1 h and 18 h after training (HI = −0.21 ± 0.03 and −0.17 ± 0.04, respectively) than did larvae that only received test stimulation (Test Alone group; HI = 0.02 ± 0.03 and −0.06 ± 0.03 for the 1 h and 18 h Posttests, respectively) (Figure 2B). A repeated-measures ANOVA performed on the two posttest trials yielded a significant main effect for the group (F[1,142] = 26.3; p < 0.05). One-Way ANOVAs at each posttest confirmed that there was more habituation in the Trained group compared to the Test Alone group (p < 0.05 for both test times).
Figure 2.
Habituation of the C-start in zebrafish larvae can persist for ≥ 18 h. (A) Experimental protocol for the fish that received habituation training. Here, posttests occurred at 1 h and 18 h after training. The Test Alone group did not receive habituation training but was otherwise treated identically to the Trained group. After the 1 h Posttest, larvae were left in the dark, unstimulated, for 15 h. Then the room lights were turned on for 1 h after which there was a second posttest. (B) LTH of the C-start. The trained larvae exhibited significantly greater habituation at both the 1 h and 18 h Posttests than did the larvae given the test alone treatment (asterisks).
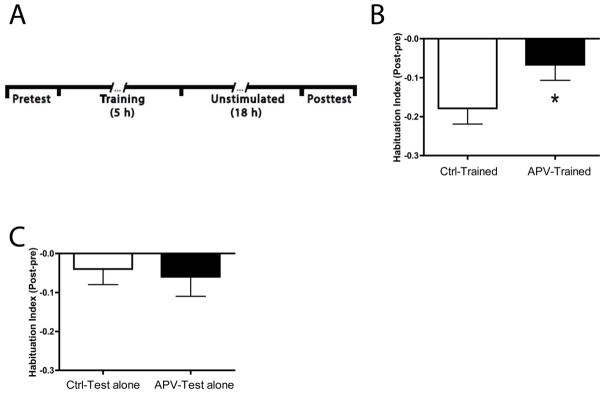
We then tested whether NMDA receptor signaling was necessary for the habituation expressed at 18 h posttraining (hereafter, LTH). Larvae were given habituation training after 24 h of incubation in either 100 μM D-APV (APV-Trained, n = 48) or control solution (Ctrl-Trained, n = 47); as before, the fish remained in the drug/control solutions throughout the training and testing. The Ctrl-Trained group exhibited significantly more habituation (HI = −0.18 ± 0.04; Figure 3B) than the APV-Trained group (HI = −0.07 ± 0.04; t = 2.00, p < 0.05) at 18 h. No statistical differences were observed between untrained APV (APV-Test Alone, n = 35) and Control (Ctrl-Test Alone, n = 36) groups (HI = −0.06 ± 0.05 and −0.04 ± 0.04, respectively; t = 0.30, p > 0.7) (Figure 3C). Thus, the drug appeared not to have nonspecific effects that could account for its disruption of LTH.
Figure 3.
LTH of the C-start in zebrafish expressed depends on NMDA receptor activity. (A) Experimental protocol for LTH that included only a single posttest at 18 h after training/test alone treatment. (B) Effect of NMDA receptor blockade on LTH. The larvae treated with APV (APV-Trained) exhibited significantly less habituation than larvae treated with the control solution (Ctrl-Trained) 18 h after the end of training (asterisk). (C) APV treatment alone did not alter the responsiveness of larvae. There was no difference in responsiveness between two untrained groups, one given the test alone protocol in control solution and another given the test alone protocol in APV.
3.2 C-start response can be rapidly dishabituated following LTH
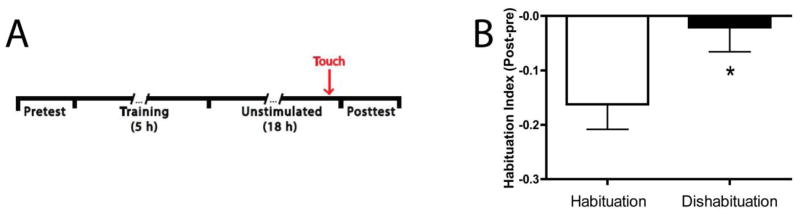
Long-term habituation in Aplysia can be rapidly reversed (dishabituated) by brief training with an arousing stimulus (Carew, Castellucci, & Kandel, 1979). We tested whether the depressed C-start in zebrafish larvae following training that induced LTH could be dishabituated. For this test we habituated two groups of larvae, after which each of the larvae in one group (Dishabituation, n = 48) was given a brief tactile stimulus (a light touch) 15 min prior to the posttest at 18 h, whereas the other group (Habituation, n = 48) was not disturbed prior to the posttest (Figure 4A). The Dishabituation group exhibited significantly less habituation (HI = −0.02 ± 0.05) compared to the habituation group (HI = −0.16 ± 0.05; t = 2.21, p < 0.05) (Figure 4B), indicating that LTH induced by auditory stimulation can be reversed by a single, brief tactile stimulus.
Figure 4.
LTH of the C-start can be rapidly reversed by a tactile stimulus. (A) Experimental protocol for demonstrating dishabituation of the C-start. Two groups of larvae were trained using the protocol shown in Figure 3A. Animals in the Dishabituation group received a brief touch (arrow) with a hand-held broom bristle 15 min prior to the Posttest. (B) Dishabituation following LTH. The touch dishabituated the C-start, as indicated by the comparison between the Dishabituation group (asterisk) and the group of larvae not given tactile stimulation prior to the Posttest (Habituation group).
3.3 Cold shock blocks LTH of the C-start
Cold shock can block the consolidation of long-term memory in flies (Tully, Preat, Boynton, & Del Vecchio, 1994), worms (Amano & Maruyama, 2011) and snails (Yamada, Sekiguchi, Suzuki, & Mizukami, 1992). It is believed that the disruptive effect of cold shock on long-term memory is due to its interference with protein synthesis. To determine whether LTH is sensitive to cold shock we compared larvae that received habituation training followed by cold shock (Cold SH-Trained, n = 68) to a group that receive habituation training alone (Ctrl-Trained, n = 71) (Figure 5A). The Cold SH-Trained larvae exhibited significantly less habituation (HI = −0.05 ± 0.03) compared to the Ctrl-Trained (HI = −0.16 ± 0.04; t = 2.32, p < 0.05) (Figure 5B). Cold shock had no apparent deleterious effects on the health of the fish as indicated by a comparison of reflex responsiveness in two groups of untrained fish, one exposed to cold shock (Cold SH-Test Alone, n = 36) and the other to control solution (Ctrl-Test Alone, n = 36) at room temperature (HI = −0.11 ± 0.04 and HI = −0.09 ± 0.04, respectively; t > 0.2, p > 0.7) (Figure 5C). These data indicate that cold shock disrupts LTH of the C-start and provide mechanistic evidence that the memory for LTH requires protein synthesis.
Figure 5.
LTH of the C-start is disrupted by cold shock. (A) Experimental protocol for testing the effect of cold shock on LTH. LTH training (Fig. 3A) was administered to two groups of larvae. One of the groups (Cold SH-Trained) received cold shock (exposure to control solution at 6–8°C for 2 min) immediately after training whereas the other group (Ctrl-Trained) did not. (B) Effect of posttraining cold shock on LTH. The expression of habituation at 18 h posttraining was blocked in the Cold SH-Trained larvae (asterisk). (C) Cold shock did not affect the baseline responsiveness of the larvae. There was no significant difference in the number of posttest C-starts evoked in untrained larvae that received the cold shock (Cold SH-Test Alone) and in untrained larvae that did not (Ctrl-Test Alone).
We attempted to explicitly test the requirement for protein synthesis by examining whether a protein synthesis inhibitor, cyclohexamide, would block LTH. We used a 10-μM concentration of the drug because this concentration had been previously shown by others to be effective in disrupting persistent memory in zebrafish larvae (Hinz et al., 2013; Wolman et al., 2011). Prior to attempting to block LTH with cyclohexamide, however, we tested the effect of prolonged exposure to the drug on the responsiveness of larvae. Fish at 5 days post-fertilization (dpf) were exposed to cyclohexamide for 6 h—the duration of the training period—and the threshold for eliciting the C-start was tested the following day. We observed a significant reduction of responsiveness in the larvae (p < 0.05, data not shown). Thus, prolonged exposure to the protein synthesis inhibitor appeared to adversely affect the health of the larvae. We did not examine the effect of lower concentrations of the drug, or of other protein synthesis inhibitors, on the larvae.
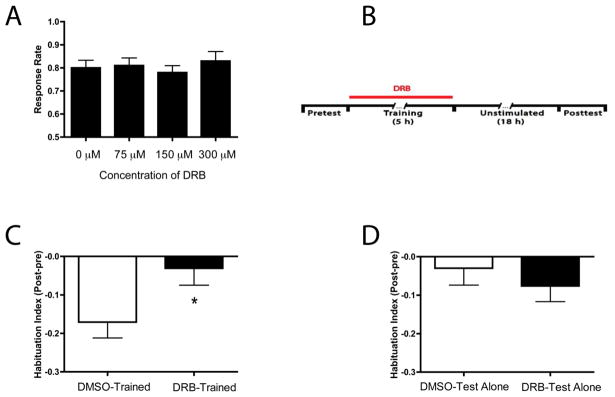
3.5 LTH of the C-start requires transcription
A hallmark of long-term memory is its dependence on gene transcription (Davis & Squire, 1984; Goelet et al., 1986). As a prelude to testing whether LTH of the C-start requires transcription, we assessed the effects of various concentrations of 5,6-Dichloro-1-β-D-ribofuranosylbenzimidazole (DRB), a reversible inhibitor of transcription, on the baseline responsiveness of zebrafish larvae. Fish (5 dpf) were exposed to various concentrations of DRB for 6 h, and the threshold for eliciting the C-start was determined the next day. We included groups of larvae exposed to 75 μM DRB (n = 29), 150 μM DRB (n = 30) or 300 μM DRB (n = 30), or to the vehicle (0.2% DMSO) alone (n = 31). Larval zebrafish appeared to tolerate DRB well. There were no significant differences in the response threshold among the four groups (F[3,155] = 0.49, p > 0.6; Fig. 6A).
Figure 6.
The transcriptional inhibitor DRB blocks LTH. (A) Effect of prolonged exposure to DRB on the baseline responsiveness of zebrafish larvae. The number of C-starts evoked in larvae exposed to DRB (75 μM, 150 μM or 300 μM) did not differ significantly from that in larvae exposed simply to the DMSO vehicle solution (0 μM). (B) Experimental protocol for testing DRB’s effect on LTH. The drug was applied to the individual wells (300 μM concentration) immediately after the end of the Pretest, and the Training/Test Alone period commenced 15 min later. The drug remained in the wells throughout the Training/Test Alone period after which it was washed out of the wells with control (E3) medium. The larvae remained in control medium alone for the remainder of the experiment. (C) Effect of inhibiting transcription on habituation expressed at 18 h posttraining. LTH was blocked in the trained fish treated with DRB (asterisk). (D) Effect of exposure to DRB on the baseline responsiveness of larvae. The DRB treatment did not affect the number of C-starts evoked in the Posttest in untrained larvae, as indicated by the lack of a significant difference in the HI at 18 h between the DMSO-Test Alone and DRB-Test Alone groups.
We used 300 μM DRB to test the role of transcription in LTH. Larvae were given pretests in the control solution alone and then trained in the presence of either DRB in 0.2% DMSO (DRB-Trained, n = 39) or 0.2% DMSO alone (DMSO-Trained, n = 48) (Fig. 6B). We found that the DRB-Trained group exhibited significantly less habituation (HI = −0.03 ± 0.04) than did the DMSO Trained group (HI = −0.17 ± 0.04; t = 2.30, p < 0.05) (Fig. 6C). There were no significant differences in the responsiveness of an untrained group exposed to the drug (DRB-Test Alone, n = 42; HI = −0.08 ± 0.04) and an untrained group exposed to the vehicle alone (DMSO-Test Alone, n = 47; HI = −0.03 ± 0.04, p > 0.4) (Fig. 6D). These results indicate that LTH requires transcription of new gene products.
4. Discussion
Long-term habituation, albeit not of the C-start escape response, has been previously reported in both adult (Wong, Elegante, Bartels, Elkhayat, Tien, Roy, Goodspeed, Suciu, Tan, Grimes, Chung, Rosenberg, Gaikwad, Denmark, Jackson, Kadri, Chung, Stewart, Gilder, Beeson, Zapolsky, Wu, Cachat, & Kalueff, 2010) and larval zebrafish (Wolman et al., 2011). Wong et al. examined habituation of the responses, including exploration of the tank and freezing, of adult zebrafish to a novel tank. They observed that daily repetition of the exposure of an originally novel tank to the fish over the course of 7 d produced an increase in movement of the fish to the top of the tank and a steady decrease in freezing over exposures to the tank. Interestingly, anxiogenic drugs (caffeine and pentylenetetrazole) attenuated the habituation, but acute anxiolytic drugs (morphine and ethanol) had no effects on habituation. Wolman et al. (2011) studied habituation of a unique turning behavior in larval zebrafish, the O-bend, which is triggered by the sudden offset of the illumination of the fish’s chamber (“dark flash”). The larvae were trained using 120 min of dark flashes delivered at a 15 s ISI in either a massed or spaced presentation protocol; maximum habituation, persisting for 24 h, was observed using the spaced protocol. In support of the idea that this alteration of the O-bend represented long-term memory, bathing the larvae in cyclohexamide during the spaced habituation training blocked LTH. Notice that the O-bend is not mediated by the Mauthner cells, and the neural circuits that mediate this escape maneuver are not well-understood.
With specific respect to the C-start response, prior work has shown various forms of short-lived habituation of this neurobiologically tractable behavior in zebrafish larvae (Eaton, Farley, Kimmel, & Schabtach, 1977; Roberts et al., 2011; Wolman et al., 2011). The present data are the first to show, in either larvae or adult zebrafish, that the C-start can also undergo long-term (≥ 18 h) habituation. Support for the notion that the nonassociative learning exhibited by the fish in the present study is unambiguously long-term is provided by our finding that LTH was blocked by cold shock applied immediately after training and by training in a transcriptional inhibitor (DRB) (see Goelet et al., 1986). In studies of invertebrate memory, sensitivity to cold shock is commonly taken as evidence that a form of memory depends on protein synthesis (Amano & Maruyama, 2011; Tully et al., 1994; Yamada et al., 1992). Thus, our data, together with those from three previous studies (Andersson, Ek, & Olsson, 2015; Hinz et al., 2013; Wolman et al., 2011), indicate that even very young zebrafish possess the capacity for long-term memory.
We observed that LTH of the C-start was blocked by the NMDA receptor antagonist APV. Our results are therefore generally consistent with evidence from work on invertebrates (Das, Sadanandappa, Dervan, Larkin, Lee, Sudhakaran, Priya, Heidari, Holohan, Pimentel, Gandhi, Ito, Sanyal, Wang, Rodrigues, & Ramaswami, 2011; Ezzeddine & Glanzman, 2003) (but see Lau, Timbers, Mahmoud, & Rankin, 2013) and mammals (McNamara, Magidson, Linster, Wilson, & Cleland, 2008; Pellicano, Siciliano, & Sadile, 1993) that LTH requires activation of NMDA receptors.
Previous work has provided evidence for a role of NMDA receptors in short-lived habituation in zebrafish larvae. Best et al. (2007) examined habituation of startle-related movement by zebrafish larvae to 20–50 brief auditory stimuli delivered at interstimulus intervals of 1–20 s; they observed that the NMDA receptor antagonist memantine increased the distance moved per tone (attenuated habituation), whereas exposure of the larvae to NMDA together with memantine offset the enhancing effect of the antagonist. (Note that Best et al. gave multiple bouts—up to four—of some of the patterns of habituating stimuli, with the bouts separated by 15 min. The responses of the fish always recovered in between bouts, and thus there did not appear to be any buildup or prolongation of the duration of habituation due to iteration of the stimuli.) Wolman et al. (2011) and Roberts et al. (2011), using similar methods, demonstrated a brief form of habituation of the C-start to acoustic stimuli delivered at 0.2–1.0 Hz in larval zebrafish; this form, which endured for ≥ 1–3 min, but < 15 min, was termed short-term habituation by Wolman et al., and rapid habituation by Roberts et al. Using 10 spaced blocks of acoustic stimuli (1 Hz), the latter group discovered a second, more persistent, form of habituation of the C-start, which they called short-term, that endured for ≥ 25 min but < 1 h (Roberts et al., 2011).
There is a puzzling inconsistency in the results from prior investigations of the role of NMDA receptors in short-lived forms of habituation of the C-start. Specifically, Wolman et al. (2011) reported that their short-term habituation was sensitive to a noncompetitive NMDA receptor antagonist (MK-801). In agreement with Wolman and colleagues, Roberts et al. (Roberts et al., 2011) observed that MK-801 produced a blockade of their briefer form of habituation (i.e., rapid habituation); but they found that, APV, a competitive NMDA receptor antagonist, did not disrupt rapid habituation, although it did block the more persistent form of habituation (i.e., short-term habituation) described in their study. The reason for the different effects of the noncompetitive and competitive NMDA receptor antagonists on brief habituation in the two studies is unclear. In a recent study of novel object recognition in zebrafish larvae Andersson and colleagues (2015) have reported that MK-801 blocks the induction of short-term (< 3 h) memory, as well as the retrieval of long-term (24 h), but not short-term, memory.
At present, we do not know the subcellular location(s) of the NMDA receptors whose activity is required for LTH of the C-start. It is likely, however, that the critical NMDA receptors reside at the excitatory club synapses between the 8th (auditory) nerve and the M-cell, or in the polysynaptic pathway that chemically inhibits the M-cell. Support for this idea comes from the demonstrations of long-term depression (LTD) of the excitatory synapses and long-term potentiation (LTP) of the inhibitory synapses in the M-cell circuit of the goldfish, a closely related species (Korn, Oda, & Faber, 1992; Yang & Faber, 1991); and prominent forms of both LTD and LTP are known to require NMDA receptor activity (Malenka & Bear, 2004).
A particularly intriguing finding of the present study is that the long-term habituated C-start in zebrafish larvae can undergo rapid dishabituation in response to a brief touch. Previously, Best et al. (2007) showed dishabituation of short-term, auditory-evoked startle-related movement of zebrafish to a light pulse. Wolman et al. also reported that cross-model stimulation, in this case with a tactile stimulus, can produce dishabituation of their short-term habituation of the C-start response. Our findings represent the first demonstration of dishabituation of a long-term habituated reflex in zebrafish. However, over 35 years ago, Carew et al. (1979) showed that a noxious stimulus, strong electrical shock of the neck, can produce rapid dishabituation of the long-term habituated defensive withdrawal reflex of Aplysia. In future work we plan to address whether dishabituation of LTH results from erasure of the habituation memory or, instead, the superimposition of a sensitizing process on the habituation (see Antonov, Kandel, & Hawkins, 2010; Groves & Thompson, 1970; Hawkins, Cohen, & Kandel, 2006; Thompson & Spencer, 1966).
In summary, we have demonstrated LTH of a neurobiologically simple reflexive response in zebrafish larvae. The extensive knowledge regarding the neural basis of this simple reflex (Eaton et al., 2001; Korn & Faber, 2005; Medan & Preuss, 2014) sets the stage for future rigorous investigations of the cellular and molecular mechanisms of long-term memory in a vertebrate system that is amenable to both optical monitoring of neuronal activity and optogenetic manipulation.
Highlights.
Demonstrated long-term habituation of the escape C-Start reflex in zebrafish larvae
Long-term habituation of the zebrafish C-Start reflex is NMDA receptor dependent
Long-term habituation of the C-Start reflex requires RNA transcription
Tactile stimuli can easily dishabituate long-term habituation of the C-Start reflex
Acknowledgments
This study was supported by the National Institute of Neurological Disorders and Stroke (NIH R01 NS029563).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenberg M, Schuman EM. Cerebellar-dependent learning in larval zebrafish. J Neurosci. 2011;31:8708–8712. doi: 10.1523/JNEUROSCI.6565-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Imari L, Gerlai R. Sight of conspecifics as reward in associative learning in zebrafish (Danio rerio) Behav Brain Res. 2008;189:216–219. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Amano H, Maruyama IN. Aversive olfactory learning and associative long-term memory in Caenorhabditis elegans. Learn Mem. 2011;18:654–665. doi: 10.1101/lm.2224411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson MÅ, Ek F, Olsson R. Using visual lateralization to model learning and memory in zebrafish larvae. Scientific Reports. 2015;5:8667. doi: 10.1038/srep08667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov I, Kandel ER, Hawkins RD. Presynaptic and postsynaptic mechanisms of synaptic plasticity and metaplasticity during intermediate-term memory formation inAplysia. J Neurosci. 2010;30:5781–5791. doi: 10.1523/JNEUROSCI.4947-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrenberg AB, Del Bene F, Baier H. Optical control of zebrafish behavior with halorhodopsin. Proc Natl Acad Sci USA. 2009;106:17968–17973. doi: 10.1073/pnas.0906252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Chen M. Long-term memory in Aplysia modulates the total number of varicosities of single identified sensory neurons. Proc Natl Acad Sci U S A. 1988a;85:2373–2377. doi: 10.1073/pnas.85.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Chen M. Long-term sensitization in Aplysia increases the number of presynaptic contacts onto the identified gill motor neuron L7. Proc Natl Acad Sci USA. 1988b;85:9356–9359. doi: 10.1073/pnas.85.23.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, Harris KM. Structural components of synaptic plasticity and memory consolidation. Cold Spring Harbor perspectives in biology. 2015;7 doi: 10.1101/cshperspect.a021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron. 2004;44:49–57. doi: 10.1016/j.neuron.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG, 2nd, Tan W, Penheiter SG, Ma AC, Leung AY, Fahrenkrug SC, Carlson DF, Voytas DF, Clark KJ, Essner JJ, Ekker SC. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491:114–118. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JD, Berghmans S, Hunt JJ, Clarke SC, Fleming A, Goldsmith P, Roach AG. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2008;33:1206–1215. doi: 10.1038/sj.npp.1301489. [DOI] [PubMed] [Google Scholar]
- Best JD, Berghmans S, Hunt JJFG, Clarke SC, Fleming A, Goldsmith P, Roach AG. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301489. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Bundschuh ST, Zhu P, Schärer YPZ, Friedrich RW. Dopaminergic modulation of mitral cells and odor responses in the zebrafish olfactory bulb. The Journal of Neuroscience. 2012;32:6830–6840. doi: 10.1523/JNEUROSCI.6026-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busto GU, Guven-Ozkan T, Fulga TA, Van Vactor D, Davis RL. microRNAs That Promote or Inhibit Memory Formation in Drosophila melanogaster. Genetics. 2015;200:569–580. doi: 10.1534/genetics.114.169623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Pearce K, Chen S, Glanzman DL. Protein kinase M maintains long-term sensitization and long-term facilitation in Aplysia. J Neurosci. 2011;31:6421–6431. doi: 10.1523/JNEUROSCI.4744-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew T, Castellucci VF, Kandel ER. Sensitization in Aplysia: restoration of transmission in synapses inactivated by long-term habituation. Science. 1979;205:417–419. doi: 10.1126/science.451611. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Pinsker HM, Kandel ER. Long-term habituation of a defensive withdrawal reflex in Aplysia. Science. 1972;175:451–454. doi: 10.1126/science.175.4020.451. [DOI] [PubMed] [Google Scholar]
- Castellucci VF, Carew TJ, Kandel ER. Cellular analysis of long-term habituation of the gill-withdrawal reflex of Aplysia californica. Science. 1978;202:1306–1308. doi: 10.1126/science.214854. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Raymond MP, Ferreira L, Escudero H. Visual discrimination learning in zebrafish (Danio rerio) Behav Processes. 2005;70:19–31. doi: 10.1016/j.beproc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Dahlem TJ, Hoshijima K, Jurynec MJ, Gunther D, Starker CG, Locke AS, Weis AM, Voytas DF, Grunwald DJ. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 2012;8:e1002861. doi: 10.1371/journal.pgen.1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Sadanandappa MK, Dervan A, Larkin A, Lee JA, Sudhakaran IP, Priya R, Heidari R, Holohan EE, Pimentel A, Gandhi A, Ito K, Sanyal S, Wang JW, Rodrigues V, Ramaswami M. Plasticity of local GABAergic interneurons drives olfactory habituation. Proc Natl Acad Sci USA. 2011;108:E646–E654. doi: 10.1073/pnas.1106411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Douglass AD, Kraves S, Deisseroth K, Schier AF, Engert F. Escape behavior elicited by single, channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons. Curr Biol. 2008;18:1133–1137. doi: 10.1016/j.cub.2008.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drier EA, Tello MK, Cowan M, Wu P, Blace N, Sacktor TC, Yin JC. Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster. Nat Neurosci. 2002;5:316–324. doi: 10.1038/nn820. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Farley RD, Kimmel CB, Schabtach E. Functional development in the Mauthner cell system of embryos and larvae of the zebra fish. J Neurobiol. 1977;8:151–172. doi: 10.1002/neu.480080207. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Lee RKK, Foreman MB. The Mauthner cell and other identified neurons of the brainstem escape network of fish. Progress in Neurobiology. 2001;63:467–485. doi: 10.1016/s0301-0082(00)00047-2. [DOI] [PubMed] [Google Scholar]
- Ezzeddine Y, Glanzman DL. Prolonged habituation of the gill-withdrawal reflex in Aplysia depends on protein synthesis, protein phosphatase activity, and postsynaptic glutamate receptors. J Neurosci. 2003;23:9585–9594. doi: 10.1523/JNEUROSCI.23-29-09585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo O, Zhu P, Friedrich RW. Control of a specific motor program by a small brain area in zebrafish. Front Neural Circuits. 2013;7 doi: 10.3389/fncir.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Frost WN, Castellucci VF, Hawkins RD, Kandel ER. Monosynaptic connections made by the sensory neurons of the gill- and siphon-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc Natl Acad Sci USA. 1985;82:8266–8269. doi: 10.1073/pnas.82.23.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi M, Montarolo PG, Kandel ER. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of Aplysia. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- Glanzman DL. PKM and the maintenance of memory. F1000 biology reports. 2013;5:4. doi: 10.3410/B5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman DL, Kandel ER, Schacher S. Target-dependent structural changes accompanying long-term synaptic facilitation in Aplysia neurons. Science. 1990;249:799–802. doi: 10.1126/science.2389145. [DOI] [PubMed] [Google Scholar]
- Goelet P, Castellucci VF, Schacher S, Kandel ER. The long and the short of long-term memory—a molecular framework. Nature. 1986;322:419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Goldsmith P. Zebrafish as a pharmacological tool: the how, why and when. Curr Opin Pharmacol. 2004;4:504–512. doi: 10.1016/j.coph.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, Thanos D, Kandel ER. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Cohen TE, Kandel ER. Dishabituation in Aplysia can involve either reversal of habituation or superimposed sensitization. Learn Mem. 2006;13:397–403. doi: 10.1101/lm.49706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, Masino MA, Mandel G, Fetcho JR. Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator. J Neurophysiol. 2003;90:3986–3997. doi: 10.1152/jn.00576.2003. [DOI] [PubMed] [Google Scholar]
- Hinz FI, Aizenberg M, Tushev G, Schuman EM. Protein synthesis-dependent associative long-term memory in larval zebrafish. J Neurosci. 2013;33:15382–15387. doi: 10.1523/JNEUROSCI.0560-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa FA, O’Brien G, Kettunen P, Sagasti A, Glanzman DL, Papazian DM. Neural circuit activity in freely behaving zebrafish (Danio rerio) J Exp Biol. 2011;214:1028–1038. doi: 10.1242/jeb.048876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Karnik I, Gerlai R. Can zebrafish learn spatial tasks? An empirical analysis of place and single CS US associative learning. Behav Brain Res. 2012;233:415–421. doi: 10.1016/j.bbr.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H, Faber DS. The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron. 2005;47:13–28. doi: 10.1016/j.neuron.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Korn H, Oda Y, Faber DS. Long-term potentiation of inhibitory circuits and synapses in the central nervous system. Proc Natl Acad Sci USA. 1992;89:440–443. doi: 10.1073/pnas.89.1.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhina V, Arey Rachel N, Kaletsky R, Kauffman A, Stein G, Keyes W, Xu D, Murphy Coleen T. Genome-wide Functional Analysis of CREB/Long-Term Memory-Dependent Transcription Reveals Distinct Basal and Memory Gene Expression Programs. Neuron. 2015;85:330–345. doi: 10.1016/j.neuron.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langheinrich U. Zebrafish: A new model on the pharmaceutical catwalk. Bioessays. 2003;25:904–912. doi: 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- Lau HL, Timbers TA, Mahmoud R, Rankin CH. Genetic dissection of memory for associative and non-associative learning in Caenorhabditis elegans. Genes Brain Behav. 2013;12:210–223. doi: 10.1111/j.1601-183X.2012.00863.x. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- McNamara AM, Magidson PD, Linster C, Wilson DA, Cleland TA. Distinct neural mechanisms mediate olfactory memory formation at different timescales. Learn Mem. 2008;15:117–125. doi: 10.1101/lm.785608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medan V, Preuss T. The Mauthner-cell circuit of fish as a model system for startle plasticity. J Physiol Paris. 2014;108:129–140. doi: 10.1016/j.jphysparis.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Kandel ER, Schacher S. Long-term heterosynaptic inhibition in Aplysia. Nature. 1988;333:171–174. doi: 10.1038/333171a0. [DOI] [PubMed] [Google Scholar]
- Moore FE, Reyon D, Sander JD, Martinez SA, Blackburn JS, Khayter C, Ramirez CL, Joung JK, Langenau DM. Improved somatic mutagenesis in zebrafish using transcription activator-like effector nucleases (TALENs) PLoS ONE. 2012;7:e37877. doi: 10.1371/journal.pone.0037877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Smith SJ. Functional imaging reveals rapid development of visual response properties in the zebrafish tectum. Neuron. 2005;45:941–951. doi: 10.1016/j.neuron.2005.01.047. [DOI] [PubMed] [Google Scholar]
- O’Malley DM, Kao YH, Fetcho JR. Imaging the functional organization of zebrafish hindbrain segments during escape behaviors. Neuron. 1996;17:1145–1155. doi: 10.1016/s0896-6273(00)80246-9. [DOI] [PubMed] [Google Scholar]
- Pellicano MP, Siciliano F, Sadile AG. NMDA receptors modulate long-term habituation to spatial novelty: Dose- and genotype-dependent differential effects of posttrial MK-801 and CPP in rats. Physiology & Behavior. 1993;54:563–568. doi: 10.1016/0031-9384(93)90250-j. [DOI] [PubMed] [Google Scholar]
- Pinsker HM, Hening WA, Carew TJ, Kandel ER. Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science. 1973;182:1039–1042. doi: 10.1126/science.182.4116.1039. [DOI] [PubMed] [Google Scholar]
- Portugues R, Severi KE, Wyart C, Ahrens MB. Optogenetics in a transparent animal: circuit function in the larval zebrafish. Curr Opin Neurobiol. 2013;23:119–126. doi: 10.1016/j.conb.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Bill BR, Glanzman DL. Learning and memory in zebrafish larvae. Front Neural Circuits. 2013;7:126. doi: 10.3389/fncir.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Reichl J, Song MY, Dearinger AD, Moridzadeh N, Lu ED, Pearce K, Esdin J, Glanzman DL. Habituation of the C-start response in larval zebrafish exhibits several distinct phases and sensitivity to NMDA receptor blockade. PLoS One. 2011;6:e29132. doi: 10.1371/journal.pone.0029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Kaun KR, Rankin CH. A new group-training procedure for habituation demonstrates that presynaptic glutamate release contributes to long-term memory in Caenorhabditis elegans. Learn Mem. 2002;9:130–137. doi: 10.1101/lm.46802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor TC. PKMzeta, LTP maintenance, and the dynamic molecular biology of memory storage. Prog Brain Res. 2008;169:27–40. doi: 10.1016/S0079-6123(07)00002-7. [DOI] [PubMed] [Google Scholar]
- Schacher S, Kandel ER, Montarolo P. cAMP and arachidonic acid simulate long-term structural and functional changes produced by neurotransmitters in Aplysia sensory neurons. Neuron. 1993;10:1079–1088. doi: 10.1016/0896-6273(93)90056-w. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Sison M, Gerlai R. Associative learning in zebrafish (Danio rerio) in the plus maze. Behav Brain Res. 2010;207:99–104. doi: 10.1016/j.bbr.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AM, Kalueff AV. The developing utility of zebrafish models for cognitive enhancers research. Current neuropharmacology. 2012;10:263–271. doi: 10.2174/157015912803217323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Masters SE, Bagnall MW, Carew TJ. Molecular mechanisms underlying a unique intermediate phase of memory in Aplysia. Neuron. 2001;31:143–154. doi: 10.1016/s0896-6273(01)00342-7. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Timbers TA, Rankin CH. Tap withdrawal circuit interneurons require CREB for long-term habituation in Caenorhabditis elegans. Behav Neurosci. 2011;125:560–566. doi: 10.1037/a0024370. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Warp E, Agarwal G, Wyart C, Friedmann D, Oldfield Claire S, Conner A, Del Bene F, Arrenberg Aristides B, Baier H, Isacoff Ehud Y. Emergence of Patterned Activity in the Developing Zebrafish Spinal Cord. Curr Biol. 2012;22:93–102. doi: 10.1016/j.cub.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams FE, White D, Messer WS. A simple spatial alternation task for assessing memory function in zebrafish. Behav Processes. 2002;58:125–132. doi: 10.1016/s0376-6357(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Wolman M, Granato M. Behavioral genetics in larval zebrafish-learning from the young. Dev Neurobiol. 2011 doi: 10.1002/dneu.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman MA, Jain RA, Liss L, Granato M. Chemical modulation of memory formation in larval zebrafish. Proc Natl Acad Sci USA. 2011;108:15468–15473. doi: 10.1073/pnas.1107156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, Goodspeed J, Suciu C, Tan J, Grimes C, Chung A, Rosenberg M, Gaikwad S, Denmark A, Jackson A, Kadri F, Chung KM, Stewart A, Gilder T, Beeson E, Zapolsky I, Wu N, Cachat J, Kalueff AV. Analyzing habituation responses to novelty in zebrafish (Danio rerio) Behav Brain Res. 2010;208:450–457. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, Baier H, Isacoff EY. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature. 2009;461:407–410. doi: 10.1038/nature08323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Sekiguchi T, Suzuki H, Mizukami A. Behavioral analysis of internal memory states using cooling-induced retrograde amnesia in Limax flavus. J Neurosci. 1992;12:729–735. doi: 10.1523/JNEUROSCI.12-03-00729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XD, Faber DS. Initial synaptic efficacy influences induction and expression of long-term changes in transmission. Proc Natl Acad Sci U S A. 1991;88:4299–4303. doi: 10.1073/pnas.88.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JC, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Zhu P, Narita Y, Bundschuh ST, Fajardo O, Scharer YP, Chattopadhyaya B, Bouldoires EA, Stepien AE, Deisseroth K, Arber S, Sprengel R, Rijli FM, Friedrich RW. Optogenetic dissection of neuronal circuits in zebrafish using viral gene transfer and the Tet System. Front Neural Circuits. 2009;3:21. doi: 10.3389/neuro.04.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]