Abstract
Absence of stearoyl-CoA desaturase-1 (SCD1) in mice leads to chronic inflammation of the skin and increased susceptibility to atherosclerosis, while also increasing plasma inflammatory markers. A recent report suggested that SCD1 deficiency also increases disease severity in a mouse model of inflammatory bowel disease, induced by dextran sulfate sodium (DSS). However, SCD1-deficient mice are known to consume increased amounts of water, which would also be expected to increase the intake of DSS-treated water. The aim of this study was to determine the effect of SCD1 deficiency on DSS-induced acute colitis with DSS dosing adjusted to account for genotype differences in fluid consumption. Wild-type controls were treated with 3.5% DSS for 5 days to induce moderately severe colitis, while the concentration of DSS given to SCD1-deficient mice was lowered to 2.5% to control for increased fluid consumption. Colonic inflammation was assessed by clinical and histological scoring. Although SCD1-deficient mice consumed a total intake of DSS that was greater than that of wild-type controls, colonic inflammation, colon length and fecal blood were not altered by SCD1-deficiency in DSS-induced colitis, while diarrhea and total weight loss were modestly improved. Despite SCD1 deficiency leading to chronic inflammation of the skin and increased susceptibility to atherosclerosis, it does not accelerate inflammation in the DSS-induced model of acute colitis when DSS intake is controlled. These observations suggest that SCD1 deficiency does not play a significant role in colonic inflammation in this model.
Keywords: Inflammation, Dextran sulfate, Lipid, Dose–response relationship
In chronic inflammatory diseases, including atherosclerosis, inflammatory skin disorders (psoriasis and eczema) and inflammatory bowel disease (Crohn disease and ulcerative colitis), cytokines recruit leukocytes to the site of the lesions, thereby amplifying the inflammatory state and perpetuating the tissue damage. Genetic and environmental factors, specific cytokine involvement, and inflammatory cell infiltrate differ with the tissue involved, but the inflammatory processes are common to all chronic inflammatory diseases [1].
Stearoyl-CoA desaturase (SCD) is a lipogenic enzyme that has been recently implicated in inflammatory disease. SCD activity creates a cis-double bond in the Δ-9 position of palmitic (16:0) and stearic acid (18:0), converting them to palmitoleic (16:1n7) and oleic acid (18:1n9), respectively [2]. However, the mechanism whereby the specific lipids altered by SCD activity contribute to inflammatory processes in vivo remains unknown.
Similar to other lipogenic genes, the expression of mammalian SCD genes is highly regulated [3,4]. Human SCD is expressed most abundantly in adipose tissue [5], with lower expression in the liver and brain [6]. Of the SCD co-orthologs in mice, Scd1 is the most relevant murine ortholog for studying the metabolic and inflammatory functions of SCD activity in humans, due to its expression in adipose tissue and liver [7,8].
SCD1-deficient mice exhibit extreme sebaceous gland hypoplasia, which leads to hair fiber perforation of the follicle base and a foreign body response to fragments of hair fiber in the dermis [9]. In addition to this chronic inflammation of the skin [10,11], the absence of SCD1 increases markers of systemic inflammation and susceptibility to atherosclerosis [12]. The absence of SCD1 has also been reported to exacerbate acute colitis in a mouse model for human inflammatory bowel disease (IBD), i.e. dextran sulfate sodium (DSS)-induced colitis [13]. These findings may have implications for the ongoing development of SCD inhibitors for treatment of the metabolic syndrome in humans, and invite further exploration of pro-inflammatory mechanisms in SCD1-deficient mice.
DSS is a sulfated heparin-like polysaccharide; depending on the time course of oral administration in drinking water, it can induce both acute and chronic colitis, inhibiting epithelial cell proliferation and promoting apoptosis, which leads to epithelial injury, crypt loss and extensive ulceration and inflammation, predominantly localized to the distal colon [14–17]. The extent of colon damage increases with the amount of DSS administered [18,19].
SCD1-deficient mice with DBA/1LacJ and B6129S1F2 genetic backgrounds have been observed to consume increased amounts (~62% to 145%) of water, possibly due to increased trans-epidermal water loss [9,20]. This observation raised the question of whether the consequent increase in total DSS dose imbibed by the SCD1-deficient mice might be responsible for the accelerated colitis in these mice reported by Chen et al. [13]. One group has concluded that minor variations in fluid consumption do not affect the severity of DSS-induced colitis, based on Pearson correlation analyses between clinical or histological results and total DSS intake [19]. However, a more recent study reported significant correlations between histology scores or neutrophil recruitment and total DSS intake, supporting a conclusion that severity of DSS-induced colitis is dependent on total DSS intake in mice [18]. Furthermore, these authors also found that a minimum DSS intake of 30 mg/g body weight over seven days was required to reliably induce colitis in mice [18].
In the only previous study of the effect of SCD1 deficiency on experimental colitis, Chen et al. treated both SCD1-deficient mice and wild-type controls with 2% DSS for seven days [13], a concentration that might be expected to deliver a total DSS intake of less than 30 mg/g to many of the mice in their study, particularly the wild-type controls that are expected to drink lower volumes of water [9,20]. Under these experimental conditions, Chen et al. observed increased weight loss, shorter colon length, and more severe diarrhea and rectal bleeding with SCD1 deficiency [13]. In contrast, our results reveal that SCD1 deficiency does not accelerate inflammation in the DSS-induced model of acute colitis when total DSS intake is increased and DSS dosing is adjusted to account for genotype differences in fluid consumption. Therefore, these findings suggest that clinical use of SCD inhibitors should not impact on susceptibility to bowel inflammation.
1. Materials and methods
1.1. Animals and diet
Mice carrying the Scd1ab-2J [9] null allele were back-crossed to C57BL/6 for 10 generations to produce congenic mice [21]. Animals were allowed free access to a standard laboratory rodent chow diet (Prolab Isopro RMH 3000, PMI Nutrition International, Richmond, IN) with a maximum of five mice per cage. Animals were maintained in a specific pathogen-free barrier facility that is free of common murine viruses and Mycoplasma pulmonis on serology testing, free of significant bacterial pathogens on culture of trachea or intestine, and free of ectoparasites and endoparasites. Sentinel mice were also confirmed by polymerase chain reaction amplification to be free of common Helicobacter species in fecal samples. All studies were approved by the University of British Columbia Animal Care Committee.
To determine the average rate of water consumption over 24h, a bottle was filled with water and the change in water weight was measured over several days. Mice were 20–22 weeks of age. Females were housed 1–3 to a cage (Scd1+/+, n=6; Scd1−/−, n=5), and males were housed individually because siblings of the same genotype were not available (Scd1+/+, n=5; Scd1−/−, n=3).
1.2. Induction of colitis
Clinically healthy age-matched female mice were used for induction of experimental colitis. Experimental colitis was induced by giving sterile filtered DSS (mol wt 36,000–50,000; MP Biomedicals, Solon, OH) in drinking water for five days ad libitum. Treatment with 3.5% (wt/vol) DSS for five days has been previously established to induce moderate to severe colitis while minimizing mortality [15], and can be expected to exceed the minimum DSS intake of 30 mg/g body weight required to reliably induce colitis in mice [18]. In the initial evaluation of colitis severity in C57Bl/6 mice, wild-type mice were treated with 3.5% DSS (n=4) or water (n=3) for five days. To control for genotype differences in fluid consumption [9,20], mice were grouped by genotype (Scd1+/+, n=10 Scd1−/−, n=7) and water consumption was monitored over three consecutive days immediately prior to the 5-day treatment period. The drug concentration was adjusted to obtain equivalent daily dosages in each genotype. We employed DSS from the same lot (9135J) throughout this study. None of the mice in this study died before termination of the experiment after five days of DSS treatment.
1.3. General assessment of colitis
Animals were assessed daily and mean DSS/water consumption and body weights were recorded. Stool consistency was assessed daily using a 0 to 3 scale: 0 = normal well-formed fecal pellets, 1 = loosely shaped moist pellets that do not adhere to the anus, 2 = amorphous, moist, sticky pellets, 3 = liquid stool. The presence of blood in the stools was assessed by a guaiac paper test (ColoScreen Occult Blood Test, Helena Laboratories, Beaumont, TX) using a 0 to 4 scale: 0 = negative, 1 = faintly blue, 2 = moderately blue, 3 = dark blue, 4 = fecal blood visible to the eye. After 5 days of DSS treatment, the mice were anesthetized by intra-peritoneal injection of 250 mg/kg 2,2,2-tribromoethanol (Sigma-Aldrich, Oakville, ON, Canada) and euthanized by exsanguination via cardiac puncture and cervical dislocation.
1.4. Histological assessment of colitis
The entire colon was excised, extending from the ileocecal junction to the anus, and the length was recorded. Colons were subsequently opened longitudinally along the mesenteric border and fecal matter was removed. The colons were divided into three sections: distal, mid-colon, and proximal colon. The mid-colon section was rolled, formalin-fixed, embedded in paraffin, sectioned at 5 μm thickness, and stained with hematoxylin and eosin (H&E) in a standard manner.
Semi-quantitative assessment of colon damage was performed in a randomized and blinded fashion using a scoring system modified from Dieleman et al. [22], by estimating: 1) the severity of inflammation; 2) the extent of inflammation; and 3) the amount of crypt damage. For each of these features, the percentage of area involved by the disease process was scored on a 1 to 4 scale as follows: 1=1–25%; 2=26–50%; 3=51–75%; 4=76–100%. The severity of inflammation was scored on a scale from 0 to 3 as follows: 0 = none; 1 = slight; 2 = moderate; 3 = severe. The depth of inflammation was scored on a scale from 0 to 3 as follows: 0 = rare inflammatory cells in the lamina propria; 1 = increased numbers of granulocytes in the lamina propria; 2 = inflammatory cells extending into the submucosa; 3 = transmural extension of the infiltrate. For crypt damage: 0 = intact crypts; 1 = loss of basal one third of crypts; 2 = loss of basal two thirds of crypts; 3 = entire crypt loss with the surface epithelium remaining intact; and 4 = entire crypt loss with change of epithelial surface (erosion). Sections from each animal were scored for each feature separately by establishing the product of the grade for that feature and the percentage involvement (in a range from 0 to 12 for each of inflammation severity and infiltrate extent, and in a range from 0 to 16 for crypt damage). Combining all scores on the individual parameters could result in a total score ranging from 0 to 40.
1.5. Statistical analysis
Data are presented as means plus or minus standard error. Initial analyses were performed by the unpaired two-tailed Student’s t test. Data that did not follow a normal distribution as judged by Kolmogorov–Smirnov tests were analyzed with the Mann–Whitney test for unpaired data. Body weight data were analyzed by two-way ANOVA (time, within subjects; genotype, between subjects) using repeated-measures, followed by Bonferroni post-tests. Statistical analysis was performed with GraphPad Prism software and with the open-source R-package (GraphPad, San Diego, CA; R Development Core Team, 2006 [23]). p<0.05 was considered significant.
2. Results
2.1. SCD1 deficiency increases water consumption in C57BL/6 mice
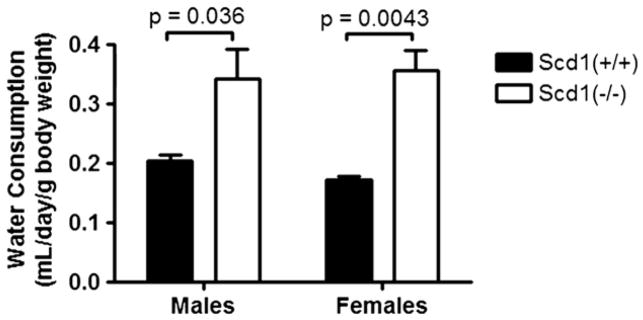
Water consumption was evaluated in male and female congenic C57BL/6 mice homozygous for a spontaneous deletion in Scd1 (Scd1−/−). Both male and female SCD1-deficient mice consumed significantly more water compared to controls (Fig. 1). Water consumption was increased by 67% in males (p=0.036; Scd1+/+, n=5; Scd1−/−, n=3) and by 100% in females (p=0.0043; Scd1+/+, n=6; Scd1−/−, n=5).
Fig. 1.
Water consumption in C57BL/6 mice lacking SCD1. SCD1-deficient (Scd1−/−) and wild-type control (Scd1+/+) mice were housed individually (males; Scd1+/+, n=5; Scd1−/−, n=3) or in cages of 1–3 mice (females; Scd1+/+, n=6; Scd1−/−, n=5) and given free access to water.
2.2. SCD1 deficiency does not accelerate DSS-induced acute colitis
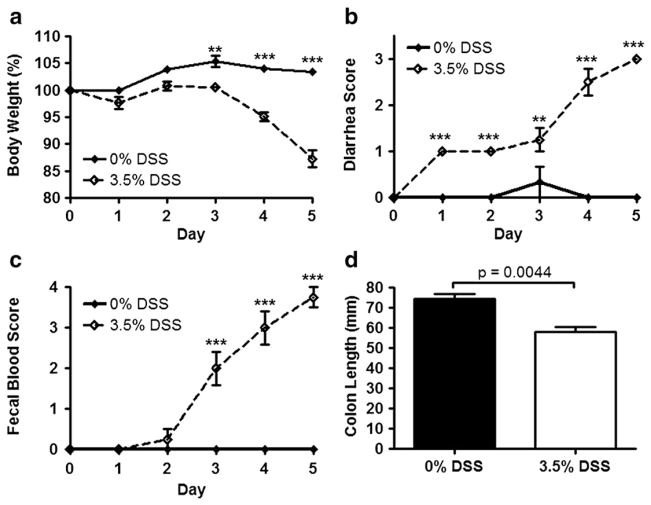
Wild-type mice (n=4) were treated with 3.5% DSS for five days to confirm that this dose can reliably induce acute colitis in the C57BL/6 strain, which has previously been demonstrated to be relatively resistant to DSS-induced colitis [15]. Control wild-type mice (n=3), receiving water only, did not exhibit any of the clinical signs associated with acute colitis (diarrhea, fecal occult blood, and weight loss). After a 5-day course of 3.5% DSS, wild-type mice lost 12.7% of their initial body weight, while wild-type mice receiving water only showed no significant change in body weight (Fig. 2a). DSS-treated mice all developed diarrhea (Fig. 2b) and hemoccult-positive stools (Fig. 2c), which often were overtly bloody. Colon length was decreased by 21% in DSS-treated mice compared to controls (p=0.0044; Fig. 2d). All animals survived until euthanized on day 5. Histological analysis revealed a patchy pattern of inflammatory cell inflltration in the lamina propria and submucosa with affected areas showing mucosal damage that ranged from erosion of surface epithelium to total loss of crypts (Fig. 3a vs. 3b).
Fig. 2.
DSS-induced acute colitis in C57BL/6 mice. a–c, Body weight (a), stool consistency (b), and fecal blood (c) were measured in wild-type control (Scd1+/+) C57BL/6 mice treated with 0% (n=3) or 3.5% DSS (n=4) in the drinking water over the course of 5 days (a, p=0.0008; b, c, p<0.0001; repeated-measures ANOVA). 0% DSS is indicated with a solid line, and 3.5% DSS is indicated with a broken line. **p<0.01. ***p<0.001. d, Colon lengths at the end of 5 days are shown.
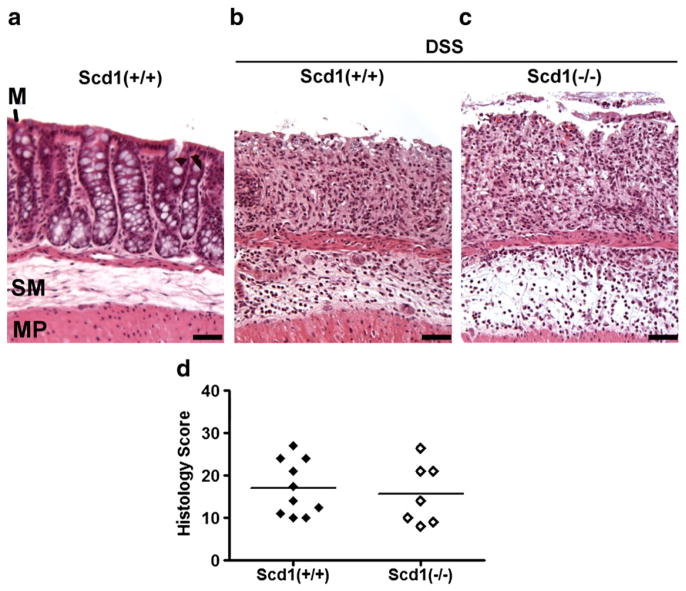
Fig. 3.
DSS-induced colon damage in SCD1-deficient mice. a–c, No colon damage was observed in control wild-type mice that received water only (a), but a patchy pattern of severe mucosal damage characterized by a loss of crypts and infiltration of inflammatory cells into both the mucosa and submucosa was observed in hematoxylin and eosin-stained colon tissues from wild-type control (b; Scd1+/+; DSS=3.5%; n=10) and SCD1-deficient (c; Scd1−/−; DSS=2.5%; n=7) mice after 5 day DSS treatment. Mucosa (M), submucosa (SM), and muscularis propria (MP). d, Semi-quantitative histological assessment of disease severity.
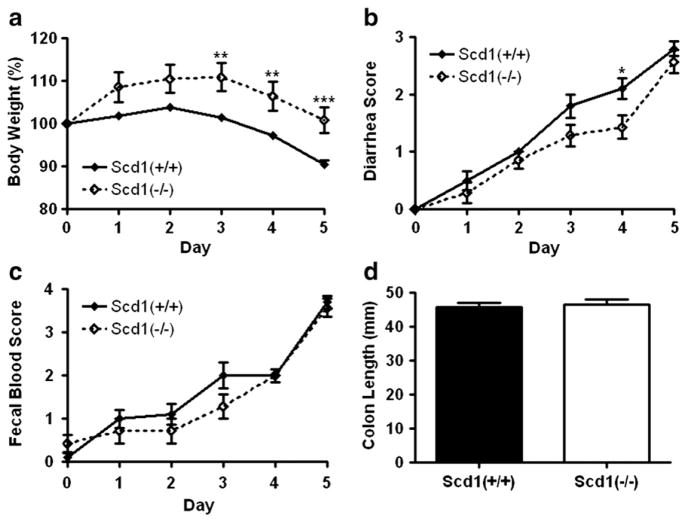
9-week-old female SCD1-deficient mice (n=7) and wild-type control mice (n=10) were treated with different DSS concentrations adjusted to control for total DSS intake. In the three days prior to treatment, SCD1-deficient mice consumed ~38% more water than control mice, so DSS concentration was accordingly decreased to 2.5% for SCD1-deficient mice. After 5 days of ad libitum exposure to DSS, all mice treated with DSS displayed clinical and macroscopic signs of acute colitis.
Prior to DSS consumption, SCD1-deficient mice tended to weigh more than wild-type controls (19.3 ± 0.5 g vs. 18.5 ± 0.2 g; p=0.075), but this difference was not significant. Over the course of DSS treatment, SCD1 deficient mice lost less weight than controls (p=0.0079; Fig. 4a). After reaching a peak body weight at day 3 of treatment, weights of SCD1-deficient mice decreased by 9.0% (19.4± 0.2 g, p<0.0001; Fig. 4a), a similar proportion of weight as that lost by wild-type mice over the 5-day course of DSS treatment (9.5% decrease; 16.7±0.3 g, p=0.0002). DSS treatment reduced stool consistency to a lesser extent in SCD1-deficient mice than wild-type mice (p=0.042; Fig. 4b), but fecal occult blood was similar between the two groups (p=0.16; Fig. 4c). Colon length was not significantly different between the two genotypes at the end of DSS treatment (46±1 mm vs. 47±2 mm; p=0.71; Fig. 4d).
Fig. 4.
DSS-induced acute colitis in SCD1-deficient mice. a–c, Body weight (a), stool consistency (b), and fecal blood (c)were measured in SCD1-deficient (Scd1−/−; n=7) and wild-type control (Scd1+/+; n=10) mice treated with DSS in the drinking water over the course of 5 days (a, p=0.0079; b, p=0.042; c, p=0.16; repeated-measures ANOVA. *p<0.05. **p<0.01. ***p<0.001. d, Colon lengths at the end of the 5 days are shown.
Microscopic examination of colon tissues indicated that DSS-induced colon damage was similar in SCD1-deficient mice (Fig. 3c) and controls (Fig. 3b). A patchy pattern of severe mucosal damage characterized by a loss of crypts and surface epithelium and infiltration of inflammatory cells into both the mucosa and submucosa was observed in H&E-stained colon tissues from both SCD1-deficient and wild-type mice after 5 day DSS treatment. The inflammatory cell infiltrate within the mucosa consisted of a mix of mononuclear cells and granulocytes, while significant edema was observed in the submucosa.
Semi-quantitative histological assessment of sections stained with H&E was used to assign histological colon damage scores on a 0 to 14+ scale based on the following parameters: the percentage of involved area, the amount of inflammatory infiltrate, the depth of inflammation, and the degree of crypt damage. When examined in a blinded fashion, there was no significant difference between the histological colon damage scores of SCD1-deficient mice and wild-type control mice (Fig. 3d and Table 1).
Table 1.
Histological assessment of DSS-induced colon damage in SCD1-deficient mice.
| Scd1+/+ | Scd1−/− | p | |
|---|---|---|---|
| Inflammation severity | 4.3±0.4 (10) | 3.6±0.7 (7) | 0.36 |
| Inflammation extent | 5.7±0.6 (10) | 6.0±1.1 (7) | 0.89 |
| Crypt damage | 7.2±1.2 (10) | 6.0±1.1 (7) | 0.60 |
| Total histological score | 17.1±2.0 (10) | 15.6±2.7 (7) | 0.47 |
Data represent mean±SEM. The number of animals in each subgroup is indicated in parentheses.
Over the 5-day treatment period, the average volume of DSS-treated water consumed in two cages of SCD1-deficient mice was 32.3 mL/mouse, while the average volume consumed in the two cages of wild-type control mice was 17.7 mL/mouse. This resulted in a total intake of 808 mg DSS (41.9 mg/g initial body weight) by SCD1-deficient mice, and 621 mg DSS (33.7 mg/g initial body weight) by wild-type controls. These observations demonstrate that the beneficial effect of SCD1-deficiency on DSS-induced weight loss and diarrhea cannot be explained on the basis of decreased intestinal exposure to DSS. Indeed, SCD1-deficient mice may even be somewhat protected from DSS-induced colitis.
3. Discussion
Despite causing chronic inflammation of the skin and increased susceptibility to atherosclerosis, SCD1 deficiency does not accelerate inflammation in the DSS-induced model of acute colitis. The total amount of DSS-supplemented water per gram body weight is known to affect the induction and severity of experimental colitis [18]. Therefore we used a total DSS intake greater than 30 mg/g body weight and adjusted dosing to account for increased fluid consumption in SCD1-deficient mice. In contrast to a prior report [13], the absence of SCD1 does not worsen fecal occult blood, colon shortening, or colonic damage, and in fact may ameliorate other colitis-induced sequelae, including weight loss and diarrhea.
We have recently shown that SCD1-deficient mice have chronic inflammation of the skin, and when exposed to a pro-inflammatory dietary stimulus these mice also demonstrate increased susceptibility to atherosclerosis and increased plasma markers of systemic inflammation [12]. Several pro-inflammatory markers, including IL-6, IL-1β, IL-12p70, soluble intercellular adhesion molecule-1, and serum amyloid A are elevated in these mice [12], and intriguingly, most are also upregulated in both human IBD [24,25] and the DSS model of colitis in mice [26–29].
Our previous observations [12] suggested that the systemic pro-inflammatory profile in SCD1-deficient mice might contribute to inflammation at other sites, including the gastrointestinal tract, and one recent study has reported that the absence of SCD1 exacerbates DSS-induced colitis [13], an observation attributed to reduced SCD1-mediated hepatic oleic acid biogenesis and subsequent reduction in oleoyl-lysophosphatidylcholine (LPC) [13]. Chen et al. [13] used a low dose of DSS that may not reliably induce colitis [18] and did not control for the increased fluid consumption associated with SCD1-deficiency [9,20]. This study raised the question of whether colonic inflammation is also exacerbated in SCD1-deficient mice when total DSS intake is controlled.
Our results show that the absence of SCD1 does not exacerbate DSS-induced colitis when total DSS intake is increased and DSS dosing is adjusted to account for genotype differences in normalized to fluid consumption. Colonic inflammation, colon length and fecal blood are not altered by SCD1-deficiency in DSS-induced colitis, while diarrhea and total weight loss are ameliorated by SCD1 deficiency. Therefore, although SCD activity and oleoyl-LPC levels are decreased by DSS treatment [13], reduced SCD activity does not play a significant causal role in DSS-induced colonic inflammation.
In addition to the differences in DSS dosing between this study and that reported by Chen et al. [13], it should be noted that there were also differences in age, sex, diet, and genetic background between mouse cohorts used in the two studies, which could have effects on colonic inflammation or the immune response. Both studies used inbred C57BL/6 mice carrying Scd1 null alleles, but the DSS-treated mice used in this study were 9-week-old females carrying the Scd1ab-2J allele [9], while Chen et al. [13] used 5- to 8-week-old males carrying the Scd1tm1Ntam allele [30]. No comparisons of these two alleles on the C57BL/6 genetic background have been reported, however, a male bias for severity of DSS-induced colitis has been previously described [15,31] and might have played a role in exacerbating the accelerated colitis in SCD1-deficient mice reported previously [13].
While SCD1-deficient mice are protected from weight gain in studies of high-fat feeding or genetic models of obesity [32,33], studies of chow-fed lean female but not male mice have demonstrated increased body weight with SCD1 deficiency [33,34]. In this study, female SCD1-deficient mice tended to weigh more than wild-type controls at the start of DSS treatment, suggesting that differences in body weight may be influenced by phenotypic effects of SCD1 deficiency other than susceptibility to DSS-induced colitis. The modest beneficial effect of SCD1-deficiency is not due to the lower percentage of DSS in their water, since SCD1-deficient mice consumed a total intake of DSS that was actually greater than that of wild-type controls. These observations suggest that chronic inflammation of the skin and a modest systemic pro-inflammatory profile do not play a significant role in colonic inflammation in this model.
The SCD1-deficient mouse continues to provide a useful model in which to explore inflammatory pathways as they relate to lipid synthesis and metabolic characteristics. However, the specific characteristics of each disease model must be taken into consideration when drawing extensions to human pathology. While this manuscript was in preparation, two different studies reported on the relationship between SCD1 deficiency and inflammatory liver disease [35,36]. SCD1 deficiency provided protection from concanavalin-A-induced nonalcoholic steatohepatitis [35], but SCD1 deficiency increased liver damage in a methionine-choline-deficient dietary model of nonalcoholic steatohepatitis [36]. In the former model, steatohepatitis is induced by a T lymphocyte mitogen, while phosphatidylcholine synthesis and VLDL secretion are blocked in the latter model [37]. Additional experiments are needed to test the effect of SCD1 overexpression and inhibition on inflammation in various cell types, but these conflicting results and the different mechanisms of liver damage in these two models suggest that SCD1 deficiency may protect against fatty liver and liver inflammation under normal or high-fat dietary conditions, and that SCD1 activity plays an important role in preventing lipotoxicity in the context of hepatic accumulation of free fatty acids.
Various mechanisms have been suggested to explain a pro-inflammatory response in cells and tissues exposed to increased saturated fatty acids and/or reduced unsaturated fatty acids, the conditions induced by endogenous SCD activity [38,39]. These proposed mechanisms include altered toll-like receptor signalling [40,41], activation of nuclear factor-κB [42–44], and altered production and fatty acid saturation of LPC [13,45,46]. SCD1-deficient mice have also been demonstrated to have increased tissue levels of arachidonic acid [38,43,47,48], a key intermediate in the production of pro-inflammatory eicosanoids [49]. Further studies are needed to fully explore the role of SCD activity in inflammation and the in vivo contribution of these mechanisms to the chronic inflammation observed in SCD1 deficient mice.
This study indicates that despite chronic inflammation of the skin [10–12], SCD1 deficiency does not accelerate inflammation in the DSS-induced model of acute colitis when total DSS intake is increased and DSS dosing is adjusted to account for genotype differences in fluid consumption. A previous report of accelerated DSS-induced colitis with SCD1-deficiency [13] may now be attributed to a genotype-related increase in the intake and delivery of DSS to the gut, rather than to a role for SCD1 in intestinal inflammation.
Acknowledgments
We thank Baoping Song for her expert technical assistance with colon histology. We also thank Michael D. Winther at Xenon Pharmaceuticals for the insightful comments and discussion. Special thanks to Mark Wang, Qingwen Xia and the staff of the Centre for Molecular Medicine and Therapeutics Transgenic Core Facility for their expert care of mice. M.L.E.M. and M.R.H. designed the research and wrote the paper; M.L.E.M. And B.A.V. analyzed the data. M.L.E.M. and N.B. performed the research.
Sources of funding: M.L.E.M. was a recipient of a Canadian Institutes for Health Research Canada Graduate Scholarship. M.R.H. holds a Canada Research Chair in Human Genetics and is a University Killam Professor.
Abbreviations
- SCD
stearoyl-CoA desaturase
- DSS
dextran sulfate sodium
- H&E
hematoxylin and eosin
- IBD
inflammatory bowel disease
- LPC
lysophosphatidylcholine
Footnotes
Disclosure: M.R.H is a founder and serves on the board of directors of Xenon Pharmaceuticals.
References
- 1.Barnes PJ, Karin M. Nuclear factor-kappab: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki M, Ntambi JM. Role of stearoyl-coenzyme a desaturase in lipid metabolism. Prostaglandins Leukot Essent Fat Acids. 2003;68:113–121. doi: 10.1016/s0952-3278(02)00261-2. [DOI] [PubMed] [Google Scholar]
- 3.Bené H, Lasky D, Ntambi JM. Cloning and characterization of the human stearoyl-CoA desaturase gene promoter: transcriptional activation by sterol regulatory element binding protein and repression by polyunsaturated fatty acids and cholesterol. Biochem Biophys Res Commun. 2001;284:1194–1198. doi: 10.1006/bbrc.2001.5102. [DOI] [PubMed] [Google Scholar]
- 4.Chu K, Miyazaki M, Man WC, Ntambi JM. Stearoyl-coenzyme a desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol. 2006;26:6786–6798. doi: 10.1128/MCB.00077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Yu L, Schmidt RE, Su C, Huang X, Gould K, Cao G. Characterization of hscd5, a novel human stearoyl-CoA desaturase unique to primates. Biochem Biophys Res Commun. 2005;332:735–742. doi: 10.1016/j.bbrc.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Ge L, Parimoo S, Stenn K, Prouty SM. Human stearoyl-CoA desaturase: alternative transcripts generated from a single gene by usage of tandem polyadenylation sites. Biochem J. 1999;340(Pt 1):255–264. [PMC free article] [PubMed] [Google Scholar]
- 7.Ntambi JM, Buhrow SA, Kaestner KH, Christy RJ, Sibley E, Kelly TJJ, Lane MD. Differentiation-induced gene expression in 3t3-l1 preadipocytes. Characterization of a differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem. 1988;263:17291–17300. [PubMed] [Google Scholar]
- 8.Zheng Y, Prouty SM, Harmon A, Sundberg JP, Stenn KS, Parimoo S. Scd3—a novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics. 2001;71:182–191. doi: 10.1006/geno.2000.6429. [DOI] [PubMed] [Google Scholar]
- 9.Sundberg JP, Boggess D, Sundberg BA, Eilertsen K, Parimoo S, Filippi M, Stenn K. Asebia-2j (scd1(ab2j)): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067–2075. doi: 10.1016/S0002-9440(10)65078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown WR, Hardy MH. A hypothesis on the cause of chronic epidermal hyper-proliferation in asebia mice. Clin Exp Dermatol. 1988;13:74–77. doi: 10.1111/j.1365-2230.1988.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 11.Oran A, Marshall JS, Kondo S, Paglia D, McKenzie RC. Cyclosporin inhibits intercellular adhesion molecule-1 expression and reduces mast cell numbers in the asebia mouse model of chronic skin inflammation. Br J Dermatol. 1997;136:519–526. [PubMed] [Google Scholar]
- 12.MacDonald MLE, van Eck M, Hildebrand RB, Wong BWC, Bissada N, Ruddle P, Kontush A, Hussein H, Pouladi MA, Chapman MJ, Fievet C, van Berkel TJC, Staels B, McManus BM, Hayden MR. Despite antiatherogenic metabolic characteristics, scd1-deficient mice have increased inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:341–347. doi: 10.1161/ATVBAHA.108.181099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Shah YM, Morimura K, Krausz KW, Miyazaki M, Richardson TA, Morgan ET, Ntambi JM, Idle JR, Gonzalez FJ. Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab. 2008;7:135–147. doi: 10.1016/j.cmet.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 15.Mähler M, Bristol IJ, Leiter EH, Workman AE, Birkenmeier EH, Elson CO, Sundberg JP. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am J Physiol. 1998;274:G544–G551. doi: 10.1152/ajpgi.1998.274.3.G544. [DOI] [PubMed] [Google Scholar]
- 16.Vetuschi A, Latella G, Sferra R, Caprilli R, Gaudio E. Increased proliferation and apoptosis of colonic epithelial cells in dextran sulfate sodium-induced colitis in rats. Dig Dis Sci. 2002;47:1447–1457. doi: 10.1023/a:1015931128583. [DOI] [PubMed] [Google Scholar]
- 17.Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597–608. doi: 10.1016/s0002-9440(10)63853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vowinkel T, Kalogeris TJ, Mori M, Krieglstein CF, Granger DN. Impact of dextran sulfate sodium load on the severity of inflammation in experimental colitis. Dig Dis Sci. 2004;49:556–564. doi: 10.1023/b:ddas.0000026298.72088.f7. [DOI] [PubMed] [Google Scholar]
- 19.Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Büchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240–248. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 20.Binczek E, Jenke B, Holz B, Günter RH, Thevis M, Stoffel W. Obesity resistance of the stearoyl-CoA desaturase-deficient (scd1(−/−)) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biol Chem. 2007;388:405–418. doi: 10.1515/BC.2007.046. [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hyper-cholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by th1 and th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R: a language and environment for statistical computing. 2008. [Google Scholar]
- 24.Niederau C, Backmerhoff F, Schumacher B, Niederau C. Inflammatory mediators and acute phase proteins in patients with Crohn’s disease and ulcerative colitis. Hepatogastroenterology. 1997;44:90–107. [PubMed] [Google Scholar]
- 25.Chambers RE, Stross P, Barry RE, Whicher JT. Serum amyloid a protein compared with c-reactive protein, alpha 1-antichymotrypsin and alpha 1-acid glycoprotein as a monitor of inflammatory bowel disease. Eur J Clin Invest. 1987;17:460–467. doi: 10.1111/j.1365-2362.1987.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 26.Hyams JS, Fitzgerald JE, Treem WR, Wyzga N, Kreutzer DL. Relationship of functional and antigenic interleukin 6 to disease activity in inflammatory bowel disease. Gastroenterology. 1993;104:1285–1292. doi: 10.1016/0016-5085(93)90336-b. [DOI] [PubMed] [Google Scholar]
- 27.Melgar S, Karlsson A, Michaëlsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in c57bl/6 but not in balb/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1328–G1338. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- 28.Oz HS, Zhong J, de Villiers WJS. Pattern recognition scavenger receptors, SR-A and CD36, have an additive role in the development of colitis in mice. Dig Dis Sci. 2009 doi: 10.1007/s10620-008-0673-4. Available from ([cited 2009 Jul 19]) p. URL: http://view.ncbi.nlm.nih.gov/pubmed/19117124. [DOI] [PMC free article] [PubMed]
- 29.Zhong J, Eckhardt ERM, Oz HS, Bruemmer D, de Villiers WJS. Osteopontin deficiency protects mice from dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2006;12:790–796. doi: 10.1097/00054725-200608000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 31.Kruidenier L, van Meeteren ME, Kuiper I, Jaarsma D, Lamers CBHW, Zijlstra FJ, Verspaget HW. Attenuated mild colonic inflammation and improved survival from severe DSS-colitis of transgenic cu/zn-sod mice. Free Radic Biol Med. 2003;34:753–765. doi: 10.1016/s0891-5849(02)01426-0. [DOI] [PubMed] [Google Scholar]
- 32.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald MLE, Singaraja RR, Bissada N, Ruddle P, Watts R, Karasinska JM, Gibson WT, Fievet C, Vance JE, Staels B, Hayden MR. Absence of stearoyl-CoA desaturase-1 ameliorates features of the metabolic syndrome in LDLR-deficient mice. J Lipid Res. 2008;49:217–229. doi: 10.1194/jlr.M700478-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng D, Wang Y, Mei Y, Xu Y, Xu H, Lu Y, Luo Q, Zhou S, Kong X, Xu L. Stearoyl-CoA desaturase 1 deficiency protects mice from immune-mediated liver injury. Lab Invest. 2009;89:222–230. doi: 10.1038/labinvest.2008.105. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiegs G, Hentschel J, Wendel A. A t cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132–30138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 39.Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR, Ntambi JM. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res. 2002;43:1899–1907. doi: 10.1194/jlr.m200189-jlr200. [DOI] [PubMed] [Google Scholar]
- 40.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 42.Weigert C, Brodbeck K, Staiger H, Kausch C, Machicao F, Haring HU, Schleicher ED. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappab. J Biol Chem. 2004;279:23942–23952. doi: 10.1074/jbc.M312692200. [DOI] [PubMed] [Google Scholar]
- 43.Bradley RL, Fisher FFM, Maratos-Flier E. Dietary fatty acids differentially regulate production of TNF-alpha and IL-10 by murine 3t3-l1 adipocytes. Obesity (Silver Spring) 2008;16:938–944. doi: 10.1038/oby.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coll T, Eyre E, Rodriguez-Calvo R, Palomer X, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem. 2008;283:11107–11116. doi: 10.1074/jbc.M708700200. [DOI] [PubMed] [Google Scholar]
- 45.Han MS, Park SY, Shinzawa K, Kim S, Chung KW, Lee J, Kwon CH, Lee K, Lee J, Park CK, Chung WJ, Hwang JS, Yan J, Song D, Tsujimoto Y, Lee M. Lysopho-sphatidylcholine as a death effector in lipoapoptosis of hepatocytes. J Lipid Res. 2008;49:84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Graham A, Zammit VA, Brindley DN. Fatty acid specificity for the synthesis of triacylglycerol and phosphatidylcholine and for the secretion of very-low-density lipoproteins and lysophosphatidylcholine by cultures of rat hepatocytes. Biochem J. 1988;249:727–733. doi: 10.1042/bj2490727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, Dobrzyn A, Dobrzyn P, Rahman SM, Miyazaki M, Ntambi JM. Lack of stearoyl-CoA desaturase 1 upregulates basal thermogenesis but causes hypothermia in a cold environment. J Lipid Res. 2004;45:1674–1682. doi: 10.1194/jlr.M400039-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Dobrzyn A, Dobrzyn P, Miyazaki M, Sampath H, Chu K, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases ctp:choline cytidylyltransferase translocation into the membrane and enhances phosphatidylcholine synthesis in liver. J Biol Chem. 2005;280:23356–23362. doi: 10.1074/jbc.M502436200. [DOI] [PubMed] [Google Scholar]
- 49.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]