Abstract
Purpose
Although racial disparities in health-related quality of life (HRQOL) among women with breast cancer (BC) are well documented, less is known about HRQOL changes over time among women of different races. Our objective was to assess racial differences in HRQOL during active treatment and survivorship phases of BC care.
Methods
We used data from the third phase of the Carolina Breast Cancer Study (CBCS-III). CBCS-III enrolled 3,000 women in North Carolina aged 20-74 years diagnosed with BC between 2008 and 2013. HRQOL assessments occurred 5- and 25-months post-diagnosis, representing distinct phases of care. HRQOL measures included the Functional Assessment of Cancer Therapy for BC and Functional Assessment of Chronic Illness Therapy for Spiritual Well-Being. Analysis of covariance models were employed to assess racial differences in changes in HRQOL.
Results
The cohort included 2,142 Non-Hispanic White (n=1,105) and Black women (n=1,037) who completed both HRQOL assessments. During active treatment, Whites reported physical and functional scores 2-2.5 points higher than Blacks (p<0.0001). Spiritual HRQOL was 2.1 points higher for Blacks (p<0.0001). During survivorship, differences persisted. After adjusting for demographic, socioeconomic, tumor and treatment characteristics, physical and functional HRQOL gaps narrowed, but spiritual HRQOL gaps widened.
Conclusions
Racial differences in physical and functional HRQOL during active treatment and survivorship may be largely mediated by socioeconomic factors. However, our results suggest that among Black women, spiritual HRQOL is well supported throughout the BC care continuum. These results inform opportunities for improving the quality and equity of supportive services for women with BC.
Introduction
Over 3 million women in the United States (U.S) are currently living with breast cancer (BC) or have a history of BC [1]. Given that 5- and 10-year BC survival rates are 90% and 80%, respectively, most women diagnosed with BC become long-term survivors [2]. As the number of women surviving BC grows, monitoring and characterizing changes in health-related quality of life (HRQOL), as well as identifying women at high-risk for long-term HRQOL deterioration during survivorship, is critical to ensuring patient-centered care across the BC care continuum.
Thirty percent of women experience poor psychosocial and physical HRQOL before, during, and after treatments [3,4]. Women with BC report greater levels of treatment-related symptoms compared to similarly aged healthy women [5-7]. Studies have shown BC is a risk factor for poor mental health, and rates of depression are twice as high among adults with cancer than those without cancer [7,8].
BC risk and burden varies systemically between Black and White women [9]. Although BC incidence rates do not differ significantly between Black and Whites, Blacks are more likely to die from BC [2]. Black women are more likely to be diagnosed at younger ages with more aggressive tumors [10]. As Blacks are over-represented in lower socioeconomic groups, they may be more likely to experience diagnosis and treatment delays associated with worse survival [11] [12].
Racial gaps in HRQOL are well-documented in BC, but differences are not uniform across ages or HRQOL domains [3,13-17]. Specifically, compared with their White counterparts, Black women with BC report worse physical and functional HRQOL [17,18]. However, this may not be the case at different phases of BC care. A retrospective Medicare study found that while racial disparities in HRQOL were prevalent before cancer diagnosis, gaps in certain domains narrowed following exposure to the cancer care system [15]. Once Black women are diagnosed with BC and engage with the health care system, Black-White differences may narrow because previously unaddressed needs are met, which positively impacts HRQOL [15].
To our knowledge, no study has assessed racial disparities in changes in individual HRQOL domains over time in a large, multi-payer, population-based cohort. Our objective was to determine whether or not HRQOL varied between White and Black women during active treatment, survivorship, and in changes between these two time points. To do this, racial differences in six HRQOL domains (i.e., physical, social, emotional, functional, and spiritual well-being, and BC-specific concerns) were examined between 5- and 25-months after diagnosis among Black and White women with BC in North Carolina.
Methods
Data
Data for this study came from the third phase of the Carolina Breast Cancer Study (CBCS-III). CBCS-III enrolled 3,000 women diagnosed with incident, invasive, pathologically confirmed BC between 2008 and 2013. Participants spanned 44 counties in North Carolina and were identified using rapid case ascertainment [12]. To ensure representation of young and Black women, eligible participants were sampled from four strata (sampling fractions in parentheses): Blacks under 50 years (100%), Blacks 50 years and older (60%), Whites under 50 years (40%), and Whites 50 years and older (15%) [12]. As such, 50% of CBCS-III consists of Black women, of whom approximately half are under 50. CBCS-III includes women residing in rural and urban settings, with private and/or public insurance (and uninsured), and those of varying income levels [12]. In-person surveys were administered to women within 9-months of diagnosis, and at a median of 5.2 months post-diagnosis (referred to as the “5-month survey” and ranging from 1.8-8.9 months) [12]. Surveys collected data on demographics, socioeconomics and HRQOL [12,19]. Twenty-five months post-diagnosis women completed a follow-up mail-in survey, which included HRQOL questionnaires. Participants completed informed consent, including permission for researchers to abstract medical records [12,19]. This study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Participants
As the study was interested in changes in HRQOL between active treatment and survivorship (in terms of diagnosis and primary treatment), the cohort was restricted to women who completed both 5-and 25-month surveys. Furthermore, as the focus of this study was in Black-White differences in HRQOL, only Non-Hispanic Black and White women were included. Of the 2,998 women who completed a 5-month survey, 2,561 (85%) completed a 25-month survey. Additionally, 97 (4%) women were excluded because they identified as “other race” or Hispanic. Women with distant stage BC represented less than 3% of the cohort and were excluded to ensure tumor and treatment homogeneity. The final cohort included 2,142 women.
Outcomes
HRQOL instruments included the Functional Assessment of Cancer Therapy for BC (FACT-B) and the Functional Assessment of Chronic Illness Therapy for Spiritual Well-Being (FACIT-SP). The FACT-B includes 5 domains: Physical Well-Being, Social Well-Being, Emotional Well-Being, Functional Well-Being, and BC-specific Concerns [20]. The FACT-B has been validated and shown to be responsive among women with BC [20]. Minimally important differences (MID) or smallest differences in HRQOL perceived as clinically meaningful are 2-points per domain [21].
The FACIT-SP is a validated chronic disease specific and includes the Spiritual Well-Being domain [20]. The FACIT-SP is the most commonly used instrument to measure spiritual well-being in cancer [22]. As with the FACT-B, higher scores indicate better HRQOL [20] [22].
Independent Variable
Self-reported race (Non-Hispanic Black or White).
Covariates
Demographic and lifestyle characteristics included age at diagnosis, smoking status, body mass index, and comorbid conditions (e.g., diabetes, chronic obstructive pulmonary disease, obesity, hypertension, and heart disease). Socioeconomics included marital status, education, family income, urban/rural residence, and insurance status at 5-months. Given a high degree of collinearity between socioeconomic factors (r>0.80), only education and insurance status were included in our models. Treatment covariates included surgery, radiation, chemotherapy, and Herceptin. Although we examined racial differences by tumor stage, size, grade, nodal status, and hormone receptor (HR) status, due to collinearity (r>0.80), we only controlled for tumor stage and HR status.
Statistical analysis
We compared characteristics between Black and White women using t-tests for continuous covariates and chi-square tests for categorical variables. We tested differences in unadjusted mean HRQOL scores for FACT-B and FACIT-SP domains between Black and White women. We also compared physical, functional, social and emotional well-being domains [23]. Changes in HRQOL domains between 5- and 25-months were calculated as 5-month scores subtracted from 25-month scores. Positive changes show improvements and negative changes denote decrements.
Analysis of covariance (ANCOVA) models were used to examine predictors of HRQOL at 25-months, adjusting for 5-month scores, demographics, comorbidities, socioeconomics, treatment, and tumor characteristics. Each model's outcome was the 25-month HRQOL score. We ran one model per domain to isolate domain-specific predictors that may be masked by overall HRQOL score. Given a sample size of 2,142 women and significance level of 5%, we were powered at nearly 100% to detect minimally important differences (MIDs) for each domain. Analyses were performed in SAS 9.3 with two-sided statistical tests and a significance level of 5% for all analyses.
Assessing Racial Disparities
We explored three approaches to assessing racial disparities to better understand if racial gaps in HRQOL existed and to identify potential mediators of HRQOL disparities. ANCOVA models controlling for race, treatment and tumor characteristics, but not socioeconomic factors were estimated. Differences in adjusted Least Square Means (LSMs) between Black and White women were tested for each domain. This was the study's primary approach and is consistent with the Institute of Medicine's (IOM) operationalization of racial disparities in health care, which conceptualizes race as a social construct linked to a range of mediating factors, including socioeconomic status [24]. The IOM considers a disparity as the difference in quality of care between two racial groups that cannot be explained by clinical differences, health status or patient preferences [25]. The motivation behind this approach is that minority groups are overrepresented in low socioeconomic groups; thus, including both race and socioeconomics in a model may underestimate racial differences [24]. The following two approaches were implemented as sensitivity analyses to explore the extent to which racial differences were mediated by measurable socioeconomic factors.
ANCOVA models controlling for race and socioeconomic factors (and tumor and treatment characteristics) were estimated. Differences in adjusted LSMs between Blacks and Whites were tested. This approach is the residual direct effect (RDE) of race on health care, because it controls for all measurable mediators of the relationship between race and the outcome, thereby resulting in a “race” parameter estimate reflecting the unmediated effect of race on HRQOL [25] [26].
Models testing interaction terms between race and socioeconomic factors were estimated. This final approach treated race and socioeconomic status as distinct constructs which have independent and interactive effects on HRQOL [24]. That is, socioeconomic status is on the causal pathway between race and HRQOL and may moderate associations [27].
As additional sensitivity analyses, we ran race-stratified models to determine if race modified relationships between individual characteristics and HRQOL. An age-stratified model was also run to evaluate if disparities existed among women below or above age 40, which is the age at which women begin mammography screening. Finally, models stratifying women by menopausal status were estimated to determine if relationships between race and HRQOL varied.
Results
Participant characteristics
Table 1 presents race-stratified characteristics of the cohort. Age at diagnosis, surgery type, receipt of Herceptin, and prevalence of COPD and heart disease did not differ significantly between groups. Compared to Whites, Blacks were less likely to be married, have public insurance, and less education. Diabetes, obesity and hypertension were twice as common among Blacks. Blacks were more likely than Whites to be diagnosed with later stage BC and receive aggressive treatments such as chemotherapy and radiation.
Table 1.
Cohort Characteristics by Race
| Whites N=1,105 |
Blacks N=1,037 |
P-value | |||
|---|---|---|---|---|---|
| Demographics | |||||
| Age at diagnosis | 0.1327 | ||||
| <35 | 39 | 4% | 40 | 4% | |
| 35-50 | 482 | 44% | 440 | 42% | |
| 50-64 | 363 | 33% | 382 | 37% | |
| 65+ | 221 | 20% | 175 | 17% | |
| Smoking status | <0.0001*** | ||||
| Never | 593 | 54% | 607 | 59% | |
| Former | 346 | 31% | 231 | 22% | |
| Current | 166 | 15% | 199 | 19% | |
| Socioeconomics | |||||
| Married | 802 | 73% | 441 | 43% | <0.0001*** |
| Education | <0.0001*** | ||||
| <High School | 47 | 4% | 119 | 11% | |
| Completed High School | 514 | 47% | 594 | 57% | |
| College+ | 544 | 49% | 324 | 31% | |
| Insurance status | <0.0001*** | ||||
| None | 30 | 3% | 78 | 8% | |
| Private | 917 | 83% | 618 | 60% | |
| Public | 158 | 14% | 341 | 33% | |
| Comorbidities | |||||
| Diabetes | 91 | 8% | 231 | 22% | <0.0001*** |
| COPD | 29 | 3% | 24 | 2% | 0.6443 |
| Heart Disease | 51 | 5% | 55 | 5% | 0.4629 |
| Obesity | 100 | 9% | 190 | 18% | <0.0001*** |
| Hypertension | 350 | 32% | 619 | 60% | <0.0001*** |
| Treatments | |||||
| Surgery | 0.2337 | ||||
| Not specified | 11 | 1% | 6 | <1% | |
| Lumpectomy | 709 | 63% | 696 | 66% | |
| Mastectomy | 385 | 34% | 335 | 32% | |
| Chemotherapy | 627 | 57% | 709 | 68% | <0.0001*** |
| Radiation | 785 | 71% | 785 | 76% | 0.0149* |
| Herceptin | 143 | 13% | 165 | 16% | 0.0502 |
| Tumor characteristics | |||||
| Stage | <0.0001*** | ||||
| I | 570 | 52% | 416 | 40% | |
| II | 416 | 38% | 466 | 45% | |
| III | 119 | 11% | 155 | 15% | |
| Hormone Receptor Status | <0.0001*** | ||||
| Positive/Borderline | 909 | 81% | 690 | 65% | |
| Negative | 196 | 18% | 347 | 33% | |
p<0.05 *, p<0.01 **, p<0.0001*
Unadjusted HRQOL
During active treatment (5-month survey), mean physical well-being scores were 1.2-3.7 points below U.S norm scores (Table 2) [23]. White women reported physical and functional well-being scores 2.5 and 1.9 points higher, respectively, than Blacks (p<0.0001). Blacks reported spiritual well-being scores 2.1 points higher than Whites at 5-months (p<0.0001).
Table 2.
Unadjusted HRQOL Scores by Race and U.S Norms [23]
| 5-Month Scores | 25-Month Scores | 5 to 25-month Changes | |||||
|---|---|---|---|---|---|---|---|
| U.S Norms | White | Black | White | Black | White | Black | |
| Physical | 22.1 (5.4) | 20.9 (6.0) | 18.4 (6.8) | 23.2 (5.3) | 20.9 (6.3) | 2.3 | 2.5 |
| Social | 19.8 (6.8) | 23.7 (4.5) | 22.6 (5.1) | 22.8 (5.3) | 21.5 (6.2) | −0.9 | −1.1 |
| Emotional | 19.4 (5.1) | 19.4 (3.7) | 19.3 (4.5) | 19.5 (3.9) | 19.5 (4.6) | 0.1 | 0.2 |
| Functional | 18.3(6.9) | 20.1 (5.8) | 18.2 (6.7) | 21.8 (5.6) | 19.4 (7.0) | 1.7 | 1.2 |
| BC-Specific | N/A | 23.9 (6.1) | 23.6 (6.9) | 25.0 (6.3) | 23.9 (7.5) | 1.1 | 0.3 |
| Spiritual | N/A | 39.3 (7.9) | 41.4 (7.1) | 38.5 (8.7) | 40.5 (8.1) | −0.8 | −0.9 |
Means (standard deviations). Change was calculated as 5-month scores subtracted from 25-month scores.
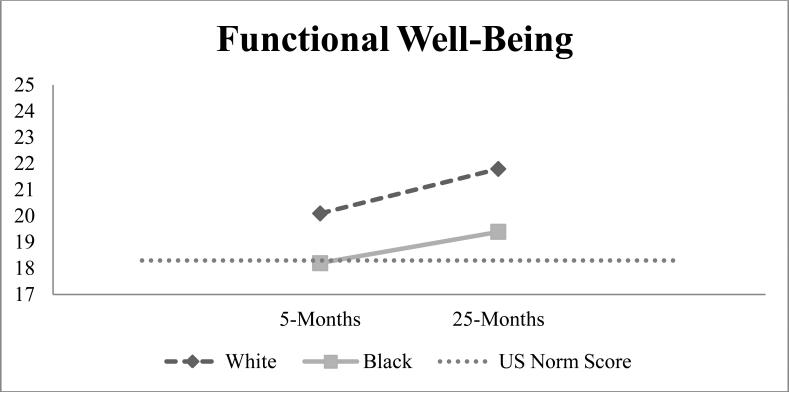
During survivorship (25-month survey), Whites reported HRQOL scores above U.S norms across physical, social, emotional and functional well-being domains (Table 2, Figures 1-4) [23]. Blacks, however, reported physical well-being scores 1.2 points below U.S norms and 2.3 points below their White BC counterparts (p<0.0001). Whites reported functional well-being scores 2.4 points higher on average than Blacks (p<0.0001), exceeding MID thresholds. Black women reported spiritual well-being scores 2-points higher than Whites (p<0.0001).
Figure 1.
HRQOL at 5- and 25-months by race with dotted line representing U.S norms
Figure 4.
HRQOL at 5- and 25-months by race with dotted line representing U.S norms
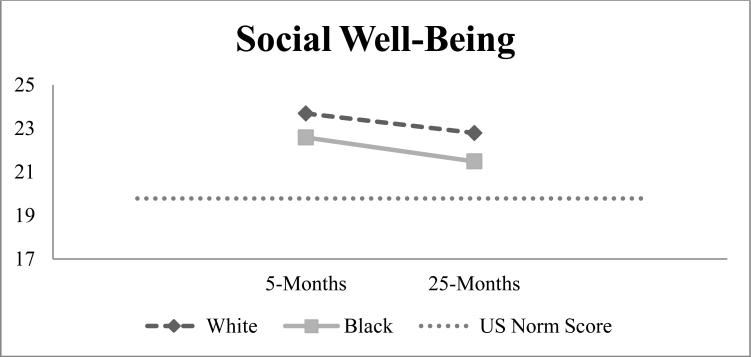
As women entered survivorship, MIDs of 2.3-2.5 points (improvements over time) were seen in physical well-being for Whites and Blacks. We did not observe meaningful changes for other domains (Table 2). On average, social well-being declined 1-point from 5- to 25-months for both groups, but was 1.7-3.0 points above U.S norms (Figure 2). From active treatment to survivorship, spiritual well-being declined 1-point for both groups while emotional well-being remained unchanged. On average, Black-White differences in HRQOL changes from 5- to 25-months ranged from 0.1-0.8 points. Differences between Blacks and White in functional well-being and BC-specific concerns changes from 5- to 25- months were statistically significant, but did not exceed MID thresholds.
Figure 2.
HRQOL at 5- and 25-months by race with dotted line representing U.S norms
Adjusted HRQOL
In models adjusting for characteristics in Table 1, older age was significantly associated with better HRQOL at 25-months across all domains (Table 3). Being a smoker was associated with 1.2-1.9-point decreases in HRQOL across domains except for social well-being. Higher education was associated with the largest increase in physical and functional HRQOL. Obesity was significantly associated with decreases in BC-specific concerns.
Table 3.
Analysis of Covariance Models By HRQOL Domain
| 25-Month HRQOL | ||||||
|---|---|---|---|---|---|---|
| Physical | Social | Emotional | Functional | BC-Specific | Spiritual | |
| 5-Month HRQOL | 0.46*** | 0.68*** | 0.54**** | 0.52*** | 0.58*** | 0.68*** |
| Demographics | ||||||
| Black race (ref=white) | −0.17 | −0.18 | 0.54** | −0.52* | 0.04 | 1.12** |
| Age at diagnosis | 0.04** | 0.03* | 0.04*** | 0.03* | 0.06*** | 0.04** |
| Smoking status (ref=never) | ||||||
| Former | 0.08 | −0.27 | −0.02 | −0.21 | −0.31 | 0.31 |
| Current | −1.34** | −0.77* | −1.32*** | −1.84*** | −2.03*** | −2.25*** |
| Socioeconomics | ||||||
| Marital status (ref=not married) | −0.08 | −0.31 | 0.12 | 0.24 | 0.02 | 0.51 |
| Education level (ref=<HS) | ||||||
| HS & Post HS | 0.76 | 0.85* | 0.56 | 1.75*** | 0.39 | 0.36 |
| College+ | 1.53** | 0.77 | 0.63 | 1.95*** | 0.57 | 0.16 |
| Insurance status (ref=private) | ||||||
| Public | −1.55*** | 0.01 | −0.43* | −1.65*** | −1.35*** | −0.69 |
| Uninsured | −1.72** | −0.25 | −0.01 | −2.02*** | −2.14*** | −0.25 |
| Comorbid Conditions | ||||||
| Diabetes (ref=no) | −0.34 | −0.48 | −0.23 | −0.33 | 0.52 | 0.34 |
| COPD (ref=no) | −0.93 | −0.59 | −0.34 | −1.45* | −0.91 | −0.01 |
| Heart Disease (ref=no) | −1.04* | −0.29 | −0.88* | −1.22* | −0.51 | −1.44* |
| Obesity (ref=no) | −0.90*** | −0.05 | −0.33* | −0.66** | −1.03*** | −0.33 |
| Hypertension (ref=no) | −0.11 | −0.24 | −0.25 | −0.21 | −0.25 | −0.34 |
| Treatments | ||||||
| Surgery (ref=lumpectomy) | ||||||
| Mastectomy | −0.29 | −0.11 | −0.07 | −0.01 | −0.56 | −0.15 |
| Not specified | 0.36 | 0.15 | 1.41 | 0.65 | −0.57 | 1.89 |
| Chemotherapy (ref=none) | 0.24 | −0.13 | 0.03 | 0.65* | 0.52 | −0.08 |
| Radiation (ref=none) | 0.03 | −0.03 | −0.02 | 0.18 | 0.04 | −0.12 |
| Herceptin (ref=none) | −0.43 | −0.11 | −0.35 | −0.51 | −0.51 | −0.35 |
| Tumor characteristics | ||||||
| Stage (ref=Stage I) | ||||||
| II | −0.36 | −0.23 | −0.28 | −0.31 | −0.94** | −0.13 |
| III | −0.15 | 0.04 | −0.18 | −0.15 | −1.09* | −0.11 |
| HR negative (ref=positive) | 0.29 | −0.31 | 0.21 | 0.27 | −0.27 | 0.18 |
p<0.05 *, p<0.01 **, p<0.0001* 25-month HRQOL scores are adjusted for HRQOL at 5-months.
IOM
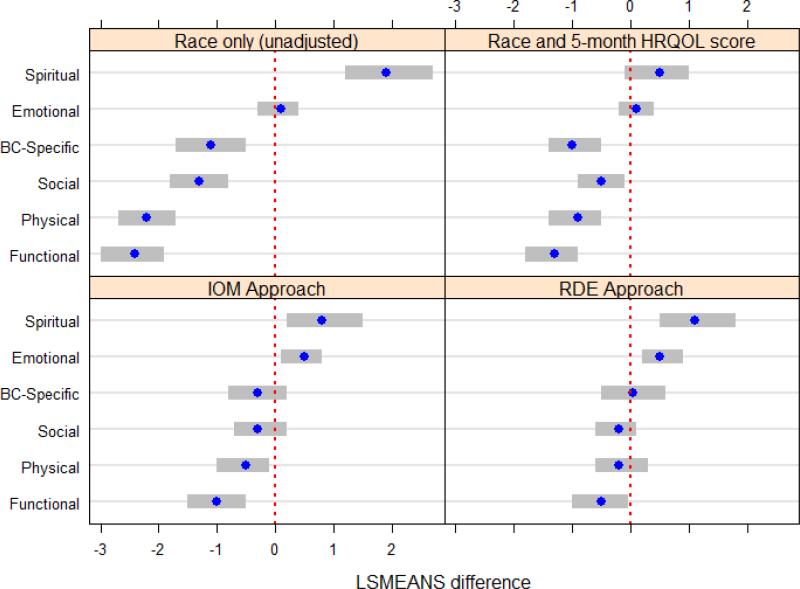
We observed statistically significant racial differences in physical, emotional, functional and spiritual well-being (Figure 5) during survivorship. Specifically, Whites had physical and functional well-being scores 0.5 (p<0.05) and 1.0 (p<0.0001) points higher, respectively, than Blacks. However, Blacks had emotional and spiritual well-being scores 0.5 (p<0.05) and 0.8 (p<0.01) points, respectively, higher than Whites. Differences were statistically significant, but none exceeded 2-point MID thresholds.
Figure 5.
Adjusted Least Square Mean Differences in 25-Month Scores by HRQOL Domain
Note: Blue dots represent adjusted LSM differences. Grey bars represent 95% confidence intervals. Differences calculated as White scores subtracted from Black scores. Negative differences indicate White women have higher HRQOL than Black women.
RDE
Once socioeconomics were added to adjusted models, emotional, functional, and spiritual well-being domains remained significantly different between Black and White women (Figure 5) during survivorship. Black race was significantly associated with a 0.5-point (p<0.01) increase in emotional well-being and a 1.2-point (p<0.01) increase in spiritual well-being, while White race was associated with a 0.5-point (p<0.05) increase in functional HRQOL. Although differences were statistically significant, none exceeded 2-point MID thresholds.
Race and Socioeconomic Interactions
Interaction terms between race and socioeconomic factors were not statistically significant at the 0.05 level.
Sensitivity Analyses
In race-stratified models, patient and treatment characteristics had similar associations with HRQOL in terms of estimates’ direction, magnitude and significance for Blacks and Whites (results not shown). One notable difference was that among Black women, receipt of Herceptin was significantly associated with 0.8-1.2 point lower physical, emotional, functional and BC-specific concerns scores (p<0.01), but this association was not seen among Whites. White-Black Differences in HRQOL by age or menopausal status were not observed.
Discussion
Domain-specific HRQOL patterns were examined to assess if Black and White women with BC are equally susceptible to poor HRQOL during active treatment and survivorship. Using the RDE approach, we found statistically significant, but not clinically meaningful Black-White differences in emotional and spiritual well-being at 25-months. Implementing the IOM approach, we again found small, but significant differences in physical, functional, emotional and spiritual well-being domains during survivorship. These findings are similar to other BC survivor studies that found racial differences in HRQOL following treatment were small and not clinically meaningful [28]. Previous work attributed racial differences in HRQOL to BC stage, treatments, comorbidities, and socioeconomics related to health care access [29].
Racial differences in physical and functional HRQOL appear to be mediated by socioeconomics. Race coefficients for physical and functional well-being domains attenuated by 50% once socioeconomics were controlled for, whereas emotional and social well-being coefficients remained unchanged. Gaps in spiritual well-being actually widened once socioeconomics were added to the model. Worse physical and functional HRQOL may be related to racial differences in access (e.g., financial barriers) to cancer care, which often emphasize management of physical and functional effects of cancer and its treatment [30]. Thus, racial differences in access to care are more likely to impact physical and functional well-being. Furthermore, physical and functional HRQOL may be impacted by comorbid conditions such as obesity, diabetes and hypertension, all of which were more prevalent in Black women in our study. A systematic review of BC survivors concluded that, in general, Black women report worse physical and functional well-being than their White counterparts, however, once demographic, socioeconomic and medical characteristics were adjusted for, physical and functional well-being differences between Black and White women attenuated, suggesting differences may be largely mediated by demographic and socioeconomic factors [31]. Socioeconomic-related barriers to physical and functional well-being must be addressed to help narrow gaps in these domains. Potential ways to improve physical and functional HRQOL could be to help manage other comorbidities and promote greater physical activity. Black women with BC tend to report lower levels of physical activity, which has been associated with poorer physical well-being and overall HRQOL [3,32]. Studies have also confirmed that although BC survivors report less physical activity compared to the general population, Whites with BC have higher levels of physical activity compared to Blacks, which may facilitated by physical well-being and vitality [17,18,32].
Our findings are consistent with previous work concluding that women with BC report greater levels of social support compared to healthy women their age [33]. Increased social support among women with BC has been associated with better adjustment following primary BC treatments [30,34]. As such, it is not surprising that women in our study reported social well-being scores well above U.S norms during active treatment. Black women were less likely to be married, suggesting that their social support may have come from sources other than partners (i.e., community, religious organizations) [30]. Previous studies also suggest Blacks experience positive growth following BC diagnosis and treatment, which may support social and emotional well-being [33,35].
Black women consistently report the importance of spirituality in coping with BC [18,36,37]. Spirituality has been described as a source of hope and comfort for Black women struggling with the psychosocial burden of BC diagnosis and treatment [35]. In fact, clinicians have been recommended to promote spirituality as a coping mechanism among Black women with BC, as it may mitigate effects of BC on psychosocial health [31] [35]. This is consistent with our findings that Blacks reported significantly higher levels of emotional and spiritual well-being than Whites, even after adjusting for patient characteristics.
Emotional and spiritual well-being were the only HRQOL domains not attenuated by socioeconomic factors. Racial differences in spiritual well-being actually widened after adjusting for socioeconomics. At first, it seems this suggests emotional and spiritual well-being domains may not be impacted by socioecological burden Black women more often endure [18]. Financial stressors, urban residence, job insecurity, and balancing multiple responsibilities (e.g., house, parental, job) make dealing with BC more difficult for Blacks [17,18,31]. Following diagnosis, Black women struggle with these challenges, exacerbating physical and functional well-being, which often decline following BC treatment [17,18]. This is in line with our observations that physical and functional HRQOL were lower among Blacks during active treatment. Emotional and spiritual well-being domains, however, appear more resistant to life stressors affecting other domains due to increased spirituality and community support. Another possibility is that BC and socioeconomic factors actually impact emotional and spiritual well-being, but in a way that reinforces these domains. The Superwoman role, a theory stating Black women feel they must exude strength, suppress emotions, and demonstrate perseverance when faced with adversity, may partially explain this [38]. If Black women do not allow themselves to acknowledge weakness or stress related to BC, self-reported emotional and spiritual well-being may not appear to suffer [38].
Limitations
First, we only included Non-Hispanic Black and White women in North Carolina, limiting generalizability to women outside of the state and of other racial groups. As most women have initiated primary BC treatments by 5-months, there is no pre-treatment HRQOL measure. However, BC treatments (from medical records) were adjusted for in analyses. We were unable to control for unmeasured confounders such as patient preferences, culture, economic, political, and legal factors that may impact HRQOL.[27] As such, even in conservative models, race coefficients encompassed unmeasurable factors such as institutional racism, different care seeking behavior and access to health care networks that are not determined by insurance status, education and income, introducing potential bias.
Conclusion
The richness of CBCS-III made it an ideal platform to understand racial disparities in HRQOL among women with BC in North Carolina. Statistically significant racial differences in HRQOL changes (from active treatment to survivorship) were identified, but differences were not considered clinically meaningful. Results suggest socioeconomic factors may considerably mediate racial gaps in physical and functional HRQOL between White and Black women with BC. Supportive services should consider domain-specific findings to provide targeted HRQOL management across socio-demographic groups of women with BC.
Figure 3.
HRQOL at 5- and 25-months by race with dotted line representing U.S norms
Acknowledgments
Melissa Troester, Jessica Tse, and Mary Beth Bell
Funding: This study was supported by the University Cancer Research Fund of North Carolina, the National Cancer Institute Specialized Program of Research Excellence in Breast Cancer at UNC (NIH/NCI P50-CA58223), and the Susan G. Komen for the Cure Foundation.
Footnotes
Compliance with Ethical Standards:
Conflict of Interest: Authors Dr. Samuel and Dr. Wheeler have received a research grant from Pfizer for another unrelated study. All other authors (Ms. Pinheiro, Dr. Reeder-Hayes, Dr. Olshan and Dr. Reeve) declare that they have no conflicts of interest to disclose.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Informed consent was obtained from all individual participants included in the study.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians. 2014;64(4):252–271. doi: 10.3322/caac.21235. doi:10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N NA, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2012. National Cancer Institute; Bethesda, MD: 2015. SEER Cancer Statistics, Review -hscgc, based on, November 2014 SEER data submission pttSws, April 2015. Bethesda MNCI, 2015. [Google Scholar]
- 3.Smith AW, Alfano CM, Reeve BB, Irwin ML, Bernstein L, Baumgartner K, Bowen D, McTiernan A, Ballard-Barbash R. Race/ethnicity, physical activity, and quality of life in breast cancer survivors. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(2):656–663. doi: 10.1158/1055-9965.EPI-08-0352. doi:10.1158/1055-9965.epi-08-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegel MT, Moore CP, Collins ED, Kearing S, Gillock KL, Riggs RL, Clay KF, Ahles TA. Distress, psychiatric syndromes, and impairment of function in women with newly diagnosed breast cancer. Cancer. 2006;107(12):2924–2931. doi: 10.1002/cncr.22335. doi:10.1002/cncr.22335. [DOI] [PubMed] [Google Scholar]
- 5.Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, Bower JE, Belin TR. Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. Journal of the National Cancer Institute. 2004;96(5):376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA, Rowland JH, Meyerowitz BE, Desmond KA. Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 1998;152:396–411. doi: 10.1007/978-3-642-45769-2_38. [DOI] [PubMed] [Google Scholar]
- 7.Tomich PL, Helgeson VS. Five years later: a cross-sectional comparison of breast cancer survivors with healthy women. Psycho-oncology. 2002;11(2):154–169. doi: 10.1002/pon.570. [DOI] [PubMed] [Google Scholar]
- 8.Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psycho-oncology. 2007;16(8):691–706. doi: 10.1002/pon.1208. doi:10.1002/pon.1208. [DOI] [PubMed] [Google Scholar]
- 9.Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(1):121–131. doi: 10.1158/1055-9965.EPI-08-0679. doi:10.1158/1055-9965.epi-08-0679. [DOI] [PubMed] [Google Scholar]
- 10.Reeder-Hayes KE, Wheeler SB, Mayer DK. Health disparities across the breast cancer continuum. Semin Oncol Nurs. 2015;31(2):170–177. doi: 10.1016/j.soncn.2015.02.005. doi:10.1016/j.soncn.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gwyn K, Bondy ML, Cohen DS, Lund MJ, Liff JM, Flagg EW, Brinton LA, Eley JW, Coates RJ. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100(8):1595–1604. doi: 10.1002/cncr.20169. doi:10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 12.McGee SA, Durham DD, Tse CK, Millikan RC. Determinants of breast cancer treatment delay differ for African American and White women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(7):1227–1238. doi: 10.1158/1055-9965.EPI-12-1432. doi:10.1158/1055-9965.epi-12-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maly RC, Stein JA, Umezawa Y, Leake B, Anglin MD. Racial/ethnic differences in breast cancer outcomes among older patients: effects of physician communication and patient empowerment. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2008;27(6):728–736. doi: 10.1037/0278-6133.27.6.728. doi:10.1037/0278-6133.27.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews AK, Tejeda S, Johnson TP, Berbaum ML, Manfredi C. Correlates of quality of life among African American and white cancer survivors. Cancer nursing. 2012;35(5):355–364. doi: 10.1097/NCC.0b013e31824131d9. doi:10.1097/NCC.0b013e31824131d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinheiro LC, Wheeler SB, Chen RC, Mayer DK, Lyons JC, Reeve BB. The effects of cancer and racial disparities in health-related quality of life among older Americans: a case-control, population-based study. Cancer. 2015;121(8):1312–1320. doi: 10.1002/cncr.29205. doi:10.1002/cncr.29205. [DOI] [PubMed] [Google Scholar]
- 16.Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Alderman A, Hamilton AS, Graff J, Katz SJ. Racial/ethnic differences in quality of life after diagnosis of breast cancer. Journal of cancer survivorship : research and practice. 2009;3(4):212–222. doi: 10.1007/s11764-009-0097-y. doi:10.1007/s11764-009-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashing-Giwa K, Ganz PA, Petersen L. Quality of life of African-American and white long term breast carcinoma survivors. Cancer. 1999;85(2):418–426. doi: 10.1002/(sici)1097-0142(19990115)85:2<418::aid-cncr20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Ashing-Giwa K. Quality of Life and Psychosocial Outcomes in Long-Term Survivors of Breast Cancer. Journal of psychosocial oncology. 2000;17(3-4):47–62. doi:10.1300/J077v17n03_03. [Google Scholar]
- 19.Newman B, Moorman PG, Millikan R, Qaqish BF, Geradts J, Aldrich TE, Liu ET. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast cancer research and treatment. 1995;35(1):51–60. doi: 10.1007/BF00694745. [DOI] [PubMed] [Google Scholar]
- 20.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 21.Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2002;11(3):207–221. doi: 10.1023/a:1015276414526. [DOI] [PubMed] [Google Scholar]
- 22.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy--Spiritual Well-being Scale (FACIT-Sp). Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2002;24(1):49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 23.Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G). Eval Health Prof. 2005;28(2):192–211. doi: 10.1177/0163278705275341. doi:10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 24.Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health affairs (Project Hope) 2005;24(2):343–352. doi: 10.1377/hlthaff.24.2.343. doi:10.1377/hlthaff.24.2.343. [DOI] [PubMed] [Google Scholar]
- 25.McGuire TG, Alegria M, Cook BL, Wells KB, Zaslavsky AM. Implementing the Institute of Medicine definition of disparities: an application to mental health care. Health Serv Res. 2006;41(5):1979–2005. doi: 10.1111/j.1475-6773.2006.00583.x. doi:10.1111/j.1475-6773.2006.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Cook B, McGuire TG, Zuvekas SH. Measuring trends in racial/ ethnic health care disparities. Med Care Res Rev. 2009;66(1):23–48. doi: 10.1177/1077558708323607. doi:10.1177/1077558708323607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams DR. Race and health: basic questions, emerging directions. Ann Epidemiol. 1997;7(5):322–333. doi: 10.1016/s1047-2797(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 28.Bradley CJ, Wilk A. Racial differences in quality of life and employment outcomes in insured women with breast cancer. Journal of cancer survivorship : research and practice. 2014;8(1):49–59. doi: 10.1007/s11764-013-0316-4. doi:10.1007/s11764-013-0316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. The Prostate. 2011;71(9):985–997. doi: 10.1002/pros.21314. doi:10.1002/pros.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashing-Giwa KT, Padilla G, Tejero J, Kraemer J, Wright K, Coscarelli A, Clayton S, Williams I, Hills D. Understanding the breast cancer experience of women: a qualitative study of African American, Asian American, Latina and Caucasian cancer survivors. Psycho-oncology. 2004;13(6):408–428. doi: 10.1002/pon.750. doi:10.1002/pon.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell KM, Von Ah DM, Giesler RB, Storniolo AM, Haase JE. Quality of life of African American breast cancer survivors: how much do we know? Cancer nursing. 2008;31(6):E36–45. doi: 10.1097/01.NCC.0000339254.68324.d7. doi:10.1097/01.NCC.0000339254.68324.d7. [DOI] [PubMed] [Google Scholar]
- 32.Mandelblatt JS, Luta G, Kwan ML, Makgoeng SB, Ergas IJ, Roh JM, Sternfeld B, Adams-Campbell LL, Kushi LH. Associations of physical activity with quality of life and functional ability in breast cancer patients during active adjuvant treatment: the Pathways Study. Breast cancer research and treatment. 2011;129(2):521–529. doi: 10.1007/s10549-011-1483-5. doi:10.1007/s10549-011-1483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Von Ah DM, Russell KM, Carpenter J, Monahan PO, Qianqian Z, Tallman E, Ziner KW, Storniolo AM, Miller KD, Giesler RB, Haase J, Otte J, Champion VL. Health-related quality of life of african american breast cancer survivors compared with healthy African American women. Cancer nursing. 2012;35(5):337–346. doi: 10.1097/NCC.0b013e3182393de3. doi:10.1097/NCC.0b013e3182393de3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Northouse LL. Social support in patients' and husbands' adjustment to breast cancer. Nurs Res. 1988;37(2):91–95. [PubMed] [Google Scholar]
- 35.Hamilton JB, Powe BD, Pollard AB, 3rd, Lee KJ, Felton AM. Spirituality among African American cancer survivors: having a personal relationship with God. Cancer nursing. 2007;30(4):309–316. doi: 10.1097/01.NCC.0000281730.17985.f5. doi:10.1097/01.NCC.0000281730.17985.f5. [DOI] [PubMed] [Google Scholar]
- 36.Gibson LM, Parker V. Inner resources as predictors of psychological well-being in middle-income african american breast cancer survivors. Cancer control : journal of the Moffitt Cancer Center. 2003;10(5 Suppl):52–59. doi: 10.1177/107327480301005s08. [DOI] [PubMed] [Google Scholar]
- 37.Holt CL, Schulz E, Caplan L, Blake V, Southward VL, Buckner AV. Assessing the role of spirituality in coping among African Americans diagnosed with cancer. J Relig Health. 2012;51(2):507–521. doi: 10.1007/s10943-011-9453-0. doi:10.1007/s10943-011-9453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woods-Giscombe CL. Superwoman schema: African American women's views on stress, strength, and health. Qualitative health research. 2010;20(5):668–683. doi: 10.1177/1049732310361892. doi:10.1177/1049732310361892. [DOI] [PMC free article] [PubMed] [Google Scholar]