Abstract
Cognitive flexibility has traditionally been considered a frontal lobe function. However, converging evidence suggests involvement of a larger brain circuit which includes the cerebellum. Reciprocal pathways connecting the cerebellum to the prefrontal cortex provide a biological substrate through which the cerebellum may modulate higher cognitive functions, and it has been observed that cognitive inflexibility and cerebellar pathology co-occur in psychiatric disorders (e.g., autism, schizophrenia, addiction). However, the degree to which the cerebellum contributes to distinct forms of cognitive flexibility and rule learning is unknown. We tested lurcher↔wildtype aggregation chimeras which lose 0%–100% of cerebellar Purkinje cells during development on a touchscreen-mediated attentional set-shifting task to assess the contribution of the cerebellum to higher- and lower-order rule learning and cognitive flexibility. Purkinje cells, the sole output of the cerebellar cortex, ranged from 0 to 108, 390 in tested mice. Reversal learning and extradimensional set-shifting were impaired in mice with ≥ 95% Purkinje cell loss. Cognitive deficits were unrelated to motor deficits in ataxic mice. Acquisition of a simple visual discrimination and an attentional-set were unrelated to Purkinje cells. A positive relationship was observed between Purkinje cells and errors when exemplars from a novel, non-relevant dimension were introduced. Collectively, these data suggest that the cerebellum contributes to higher-order cognitive flexibility, lower-order cognitive flexibility, and attention to novel stimuli, but not the acquisition of higher- and lower-order rules. These data indicate that the cerebellar pathology observed in psychiatric disorders may underlie deficits involving cognitive flexibility and attention to novel stimuli.
Keywords: executive function, cognitive flexibility, touchscreen, set-shifting, reversal learning, attention, autism, novelty processing, chimera, lurcher, cerebellum
INTRODUCTION
Cognitive flexibility enables an organism to adapt learned behavior in the face of changing environmental demands. Deficits in this fundamental cognitive ability are proposed to underlie the maladaptive behaviors which characterize a wide range of neuropsychiatric disorders including autism (Hughes et al., 1994, Hill, 2004), schizophrenia (Pantelis et al., 1999, Floresco et al., 2009, Leeson et al., 2009), and drug addiction (Woicik et al., 2011, McCracken and Grace, 2013, Moreno-Lopez et al., 2015, Verdejo-Garcia et al., 2015, Miquel et al., 2016). Although cognitive flexibility is often discussed as a unitary construct, it can be subdivided into at least two dissociable cognitive processes. Lower-order cognitive flexibility is the ability to adapt behavior following changes in lower-order, stimulus specific rules (e.g., stimulus A is correct, stimulus B is not). Conversely, higher-order cognitive flexibility is the ability to adapt behavior following changes to higher-order rules (e.g., stimuli from category A provide task-relevant information, stimuli from category B do not). Deficits in higher- and lower-order cognitive flexibility co-occur (Sahakian et al., 1990), but may also occur independently (Downes et al., 1989, Lawrence et al., 1999, Ornstein et al., 2000, Ozonoff et al., 2004). The observation that these deficits may occur independently is consistent with findings that higher- and lower-order cognitive flexibility are subserved, at least in part, by distinct regions of the prefrontal cortex (PFC) (Dias et al., 1996, Birrell and Brown, 2000, McAlonan and Brown, 2003, Bissonette et al., 2008).
Cognitive flexibility has traditionally been considered a frontal lobe function, although more recent evidence suggests that this view is an oversimplification. Rather, converging evidence indicates that cognitive flexibility is dependent on a larger circuit encompassing multiple brain regions including the prefrontal cortex, striatum, nucleus accumbens, thalamus, and cerebellum (Ragozzino, 2007, De Bartolo et al., 2009, Floresco et al., 2009, Dickson et al., 2010, Klanker et al., 2013, Dalton et al., 2014). The Intra-Extra Dimensional Set-Shifting (IED) task, a computerized analog of the Wisconsin Card Sorting task, is commonly used to assess higher- and lower-order rule learning as well as the ability to adapt behavior following reversal of these rules (Sahakian and Owen, 1992). Using the IED task, the dissociable contributions of PFC subregions to higher- and lower-order cognitive flexibility have been deeply characterized in non-human primates (Dias et al., 1996, 1997, Crofts et al., 2001, Clarke et al., 2005, reviewed in Robbins and Roberts, 2007); similar findings have been reported in mice and rats using a maze-based version of the IED task (Birrell and Brown, 2000, McAlonan and Brown, 2003, Bissonette et al., 2008). However, the contribution of other brain regions to cognitive flexibility in general and cognitive flexibility subtypes specifically is only beginning to be explored. The cerebellum, in particular, has received little experimental attention in this regard, but may be a critical mediator of cognitive flexibility due to reciprocal connections with the prefrontal cortex and other regions which affect higher cognitive functions (Mittleman et al., 2008, Strick et al., 2009, Watson et al., 2009, Rogers et al., 2011, Rogers et al., 2013, Watson et al., 2014).
To assess the contribution of the cerebellum to higher- and lower-order rule learning and cognitive flexibility, we tested lurcher↔wildtype aggregation chimeras (Martin et al., 2003, Martin et al., 2004, Martin et al., 2006, Dickson et al., 2010, Martin et al., 2010) on a touchscreen version of the IED task that we (Dickson et al., 2014) and others (Brigman et al., 2005, Brigman et al., 2006) have adapted for mice. Purkinje cells, the sole output of the cerebellar cortex, die during the first month of development in lurcher mutants as a result of a gain-of-function mutation in Grid2 (Caddy and Biscoe, 1979, Zuo et al., 1997). Consequently, individual lurcher↔wildtype chimeras experience variable Purkinje cell loss ranging from 0% – 100% as a function of the incorporation of the wildtype lineage. A key advantage of this model is that the variable nature of Purkinje cell loss in individual chimeric mice enables correlational analysis of the relationship between cognitive function and cerebellar neuropathology. Moreover, precise Purkinje cell thresholds above which cognitive deficits do not occur can be identified. In the present study, lurcher↔wildtype chimeras were tested on a series of visual discriminations to assess acquisition of higher- and lower-order rules, as well as the ability to adapt responding following reversal of these rules. At the completion of cognitive testing, histological analysis of the cerebellum was performed and Purkinje cells were quantified. Subsequently, the relationship of Purkinje cell number and IED task performance was assessed.
2. EXPERIMENTAL PROCEDURES
2.1. Subjects
Lurcher mutant (B6CBACa Aw-J/A-Grid2Lc/J) and wildtype mice were obtained from the Jackson Laboratory (Bar Harbor, Maine) and maintained at the Centre for Molecular Medicine and Therapeutics at the University of British Columbia (UBC). Aggregation chimeras were produced at UBC and shipped to the University of Memphis for behavioral testing. Mice were allowed to acclimate for at least 2 weeks prior to testing. Mice were housed in groups of 3 – 5 and provided free access to food until they entered the experiment at 12 weeks of age, at which point they were individually housed and food restricted to 90% of baseline weight. Mice were provided free access to water throughout the study.
2.2. Production of aggregation chimeras
Using previously described methods (Martin et al., 2003), aggregation chimeras were produced by fusing two 4–8 cell embryos derived from the mating of a lurcher mutant mouse and a wildtype mouse. All surgical procedures and animal care were performed in accordance with the National Institutes of Health guidelines for animal welfare.
2.3. IED task
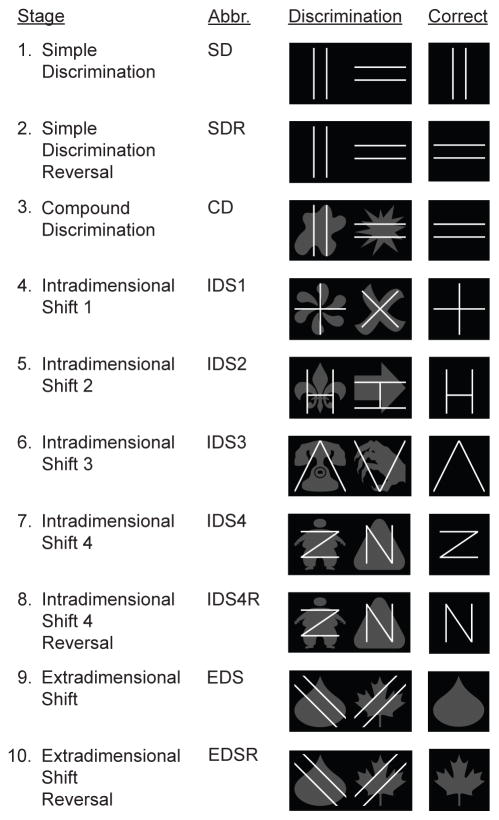
Behavioral training and testing was conducted in previously described operant conditioning chambers (Dickson et al., 2013). Mice were individually housed the day before training began and were trained for at least 7 days prior to the beginning of the simple discrimination phase. Mice were tested on ten IED stages as previously described (Dickson et al., 2014). Visual stimuli used at each stage of the IED task are provided in Figure 1. The lines dimension was relevant during the SD – IDS4R stages, and the shapes dimension was relevant during the EDS – EDSR stages. As we have done in previous studies, we prevented the development of side bias by using a correction procedure (Dickson et al., 2010, Dickson et al., 2013, Dickson et al., 2014). Sessions lasted for 60 minutes or until the mouse completed 64 trials.
Figure 1.
Visual stimuli used at each stage of the Intra-Extra Dimensional Set-Shifting task.
2.4. Dependent Variables
The following dependent variables were collected at each stage of the attentional set-shifting task: errors to criterion (non-correction trials only), perseverative errors, learning errors, latency to stimulus choice, latency to collect a reward, propensity to collect a reward. These variables have been described in detail previously (Dickson et al., 2014).
2.5. Purkinje cell quantification
Histology and Purkinje cell counting were performed as previously described (Cairns et al., 2016). Briefly, Purkinje cell nuclei were identified with the aid of a standard brightfield microscope equipped with 10x eyepieces and a 25x objective. Five brain sections spaced 20 sections apart were used for estimation of the total number of Purkinje cells in the left cerebellum of each mouse. The Abercrombie correction factor (Abercrombie, 1946) was used to account for split nuclei, section thickness, and the average diameter of each Purkinje cell. Total numbers of Purkinje cells for the entire cerebellum were then estimated from this sampling.
2.6. Statistical Methods
Analysis of Variance (ANOVA) and Covariance (ANCOVA) were used to assess performance on the attentional set-shifting task. Normality of all measures was assessed by inspecting normal probability plots. The assumption of homogeneity of variance across groups was assessed using Mauchly’s test of sphericity. The Huynh–Feldt correction was used when this assumption was violated. The criterion for statistical significance was p < .05. When performing multiple comparisons, Fisher’s Least Significant Difference procedure was used. Pearson product moment correlations were used to assess covariation of behavior and Purkinje cells.
3. RESULTS
3.1. Histological analysis
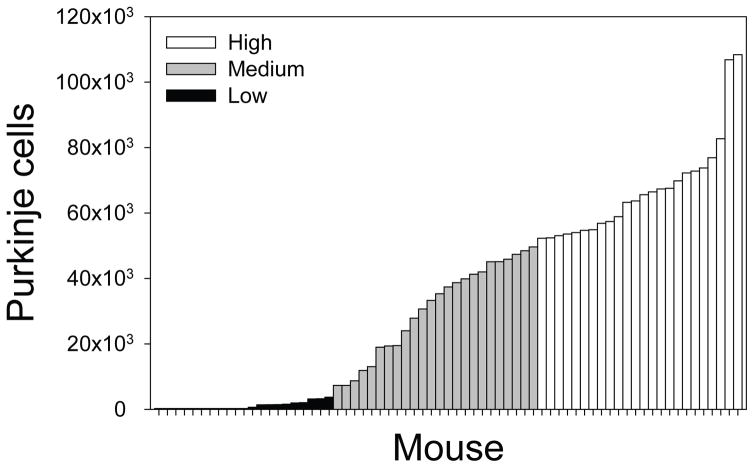
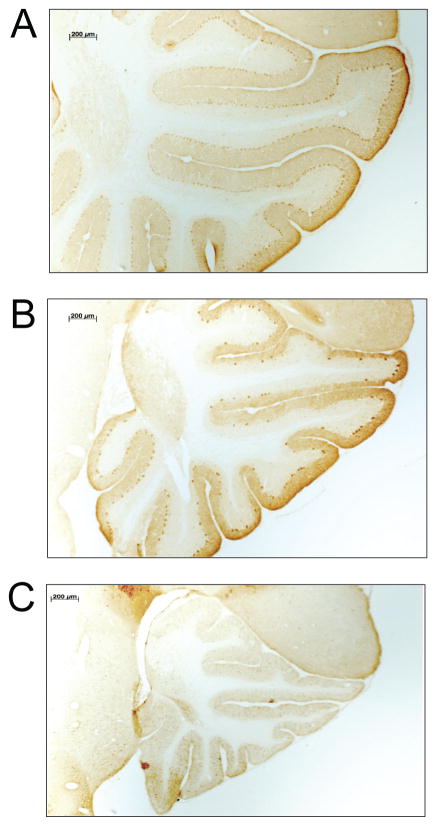
Following behavioral testing, histological analysis was performed and mice were assigned to three experimental groups (low, medium, high) reflecting cerebellar Purkinje cell numbers (Figure 2). Chimeric mice that were ataxic (n = 21) had very few Purkinje cells (0 – 3,711) and were assigned to the low group. Ataxic mice were easily identified by sight due to their lurching gait. The remaining chimeras exhibited a normal motor phenotype and were evenly divided into two groups: chimeras (n = 24) with 7,306 – 49,618 Purkinje cells were assigned to the medium group, and chimeras (n = 24) with 52,302 – 108,390 Purkinje cells were assigned to the high group. Photomicrographs depicting cerebellar sections from chimeras in the high, medium, and low groups are provided in Figures 3A, 3B, and 3C, respectively.
Figure 2.
Cerebellar Purkinje cells in each of the 69 lurcher↔wildtype chimeras tested on the Intra-Extra Dimensional Set-Shifting task. Each bar represents Purkinje cells in a single mouse.
Figure 3.
Cerebellar morphology in representative mice from the (A) high, (B) medium, and (C) low Purkinje cells groups.
3.2 IED task
3.2.1. Sessions and errors to criterion
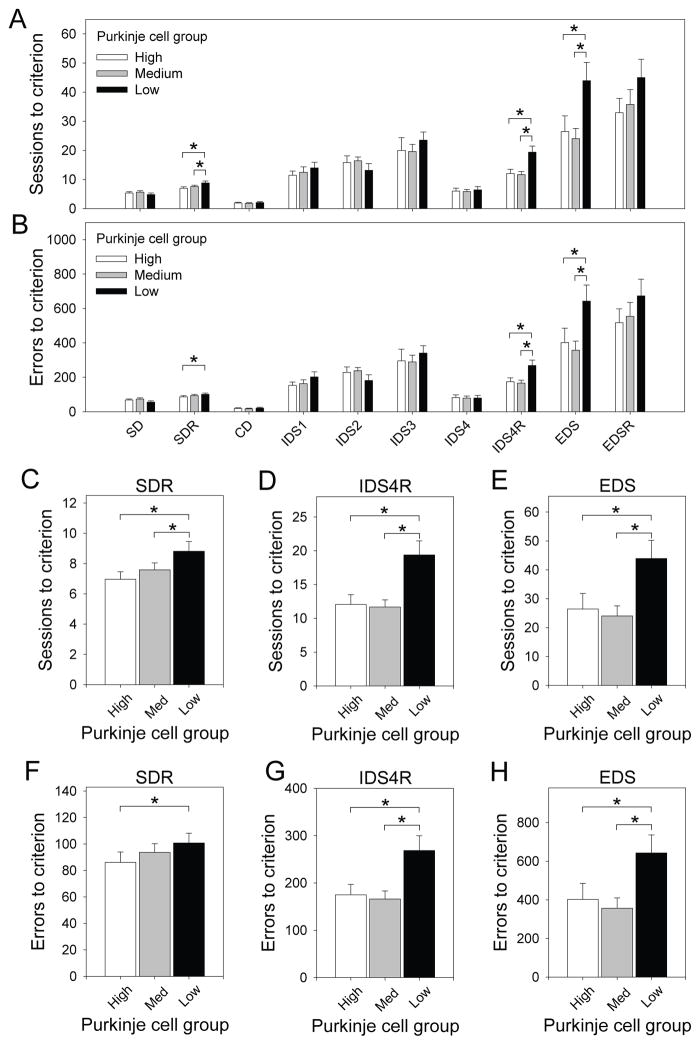
To determine if experimental groups differed at any of the IED stages, we performed ANCOVA for the three reversal stages and ANOVA for all other stages. For reversal stages (SDR, IDS4R, EDSR), performance on the preceding stage (SD, IDS4, EDS, respectively) was used as the covariate. We examined both the number of sessions to criterion and errors to criterion at each stage. For all tests, Purkinje cell group (low, medium, high) was the between subjects factor. Performance at all stages for the three Purkinje cells groups is depicted in Figure 4A (sessions to criterion) and Figure 4B (errors to criterion).
Figure 4.
(A) Sessions to criterion and (B) errors to criterion on all stages of the Intra-Extra Dimensional Set-Shifting task. (C–E) Mice from the low Purkinje cell group required significantly more sessions to reach criterion on the simple discrimination reversal, the compound discrimination reversal following the fourth intradimensional shift, and the extradimensional shift relative to mice from the other two Purkinje cell groups. (F–H) Similar results were observed when examining errors to criterion. Performance of Purkinje cell groups did not differ significantly on other stages. Data points and errors bars represent group means and standard error, respectively.
* p < .05.
3.2.1.1. SD and SDR stage
ANOVA revealed no main effect of Purkinje cell group on sessions or errors to criterion on the SD stage. ANCOVA revealed a significant main effect of Purkinje cell group on sessions to criterion [F (2, 65) = 5.24, p < .01] and a marginally significant main effect of Purkinje cell group on errors to criterion [F (2, 65) = 2.77, p < .07] on the SDR stage. Post hoc tests revealed that these effects were driven by impaired performance of mice from the low Purkinje cell group relative to mice from the other two Purkinje cell groups (Figure 4C, F).
3.2.1.2. CD, IDS1, IDS2, IDS3, and IDS4 stages
ANOVA revealed no main effect of Purkinje cell group on sessions or errors to criterion on the CD, IDS1, IDS2, IDS3, or IDS4 stages. To determine if performance improved across the four intradimensional shifts, we performed a repeated measures ANOVA using stage (IDS1– IDS4) and Purkinje cell group as the within and between subjects factors. We observed a main effect of stage [F (3, 198) = 38.51, p < .0001], but no main effect of Purkinje cell group. Post hoc tests revealed that mice as a group committed significantly fewer errors on IDS4 relative to IDS1, IDS2, and IDS3 (all comparisons p < .0001).
3.2.1.3. IDS4R stage
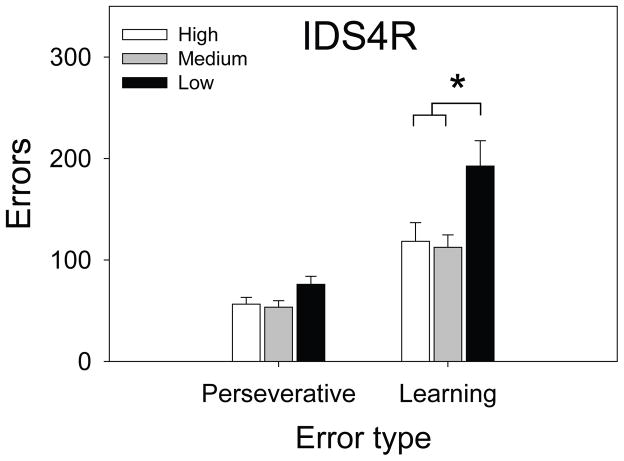
ANCOVA revealed a significant main effect of Purkinje cell group on sessions to criterion [F (2, 65) = 10.63, p < .001] and errors to criterion [F (2, 65) = 9.54, p < .001] on the IDS4R stage. Post hoc tests revealed that IDS4R performance of chimeric mice with the fewest Purkinje cells was significantly impaired relative to mice from the other two Purkinje cell groups (Figure 4D, G). To determine the nature of this impairment, we divided IDS4R errors into those committed on sessions during which performance was ≤ 40% correct (perseverative errors) and those committed on sessions during which performance was ≥ 41% correct (learning errors). Impairments at these early and late phases of a reversal learning stage reflect perseverative responding to the stimulus that was correct on the previous stage and attenuated learning of the new response rule, respectively (Bussey et al., 1997, Chudasama and Robbins, 2003, Dickson et al., 2010, Dickson et al., 2013). ANCOVA was conducted using perseverative errors or learning errors on the IDS4R stage as the dependent measure, Purkinje cell group as the between-subjects factor, and perseverative or learning errors on the IDS4 stage as the covariate. We observed a significant main effect of Purkinje cell group on learning errors, F (2, 65) = 7.46, p < .01. Post hoc tests revealed that chimeras in the low Purkinje cell group exhibited significantly impaired performance on the learning phase relative to mice from the other two groups (p < .01 for both comparisons, Figure 5). The main effect of Purkinje cell group approached significance on the perseverative phase, F (2, 65) = 2.99, p < .06. Post hoc tests indicated that this was driven by a trend towards elevated perseverative errors in mice from the low Purkinje cell group (Figure 5).
Figure 5.
Learning-phase but not perseverative-phase errors on the IDS4R stage were significantly greater in the low Purkinje cell group relative to the other Purkinje cell groups. Data points and errors bars represent group means and standard error, respectively.
* p < .05.
3.2.1.4. EDS and EDSR stages
ANOVA revealed a significant main effect of Purkinje cell group on sessions to criterion [F (2, 66) = 4.33, p < .05] and errors to criterion [F (2, 66) = 3.76, p < .05] on the EDS stage. Post hoc tests revealed that EDS performance of chimeric mice with the fewest Purkinje cells was significantly impaired relative to mice from the other two Purkinje cell groups (Figure 4E, H). ANCOVA revealed no main effect of Purkinje cell group on sessions or errors to criterion on the EDSR stage.
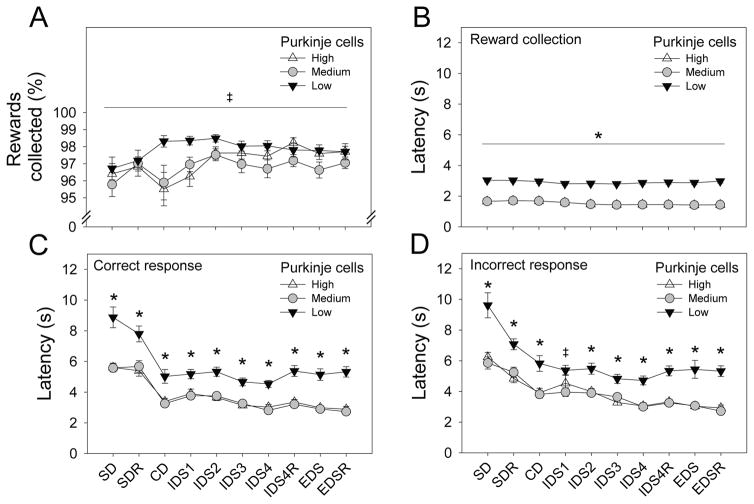
3.2.2. Reward collection propensity and latency
To determine the effect of Purkinje cells on reward collection propensity, we conducted a repeated measures ANOVA using the percentage of rewards that were collected as the dependent variable (Figure 6A). Stage (SD - EDSR) was the within subjects factors. Purkinje cell group was the between subjects factor. ANOVA revealed a significant main effect of stage [F (9, 594) = 3.87, p < .01] and Purkinje cell group [F (2, 66) = 3.38, p < .05]. Post hoc tests indicated that the percentage of rewards collected by ataxic mice in the low Purkinje cell group (M = 97.84, SD = 1.05) was modestly though significantly greater than the percentage collected by mice in the medium Purkinje cell group (M = 96.77, SD = 1.66). The percentage of rewards collected by mice in the high Purkinje cell group (M = 97.12, SD = 1.37) did not differ from that of the other two groups. The main effect of stage was driven by a small but significant increase in percentage of rewards collected following the first several stages.
Figure 6.
Percentage of rewards collected, reward collection latency, and response latency. (A) Mice from all groups collected > 95% of delivered rewards at all stages. (B) Latency to collect a reward was significantly greater in the low Purkinje cell group relative to other groups, but this did not vary across stages. (C and D) Latency to make a correct and incorrect response was consistently greater in the low Purkinje cell group relative to the other two groups. Effects of Purkinje cell group on reward collection and response latency were likely due to ataxia in these mice. Reward collection and response latency did not vary with cognitive deficits observed on the SDR, IDS4R, and EDS stages. Data points and errors bars represent group means and standard error, respectively.
* p < .05 (low Purkinje cell group vs medium and high Purkinje cell groups)
‡ p < .05 (low Purkinje cell group vs medium Purkinje cell group)
To determine the effect of Purkinje cells on reward collection latency, we conducted a repeated measures ANOVA using latency to collect a reward as the dependent variable (Figure 6B). Stage and Purkinje cell group were the within and between subjects factors, respectively. ANOVA revealed significant main effects of Purkinje cell group [F (2, 66) = 203.29, p < .001] and stage [F (9, 594) = 15.56, p < .001] on reward collection latency. Post hoc tests indicated that reward collection latency was significantly longer at all 10 stages of the IED task in the ataxic mice (low Purkinje cell group) relative to the non-ataxic mice comprising the other two groups. Observation of mice performing the task suggests that this effect was due specifically to the motor deficit in ataxic mice. The main effect of stage was driven by a small but significant reduction in food collection latency following the first several stages.
3.2.3. Response latencies
To determine the effect of Purkinje cells on latency to make correct and incorrect responses, we conducted a repeated measures ANOVA. Latency to make a response to the visual stimuli presented on the touchscreen was the dependent measure. Stage (SD - EDSR) and response type (correct, incorrect) were within subjects factors. Purkinje cell group was the between subjects factor. ANOVA revealed significant interactions of Purkinje cell group and stage [F (18, 594) = 2.59, p < .01] and response type and stage [F (9, 594) = 13.32, p < .001]. Significant main effects of response type [F (1, 66) = 29.08, p < .001], stage [F (9, 594) = 70.19, p < .001], and Purkinje cell group were observed [F (2, 66) = 34.90, p < .001]. Post hoc tests indicated that latencies to make a correct response were significantly longer at all 10 stages of the IED task in the ataxic mice (low Purkinje cell group) relative to the non-ataxic mice comprising the other two groups (Fig 6C). This was also true for latencies to make an incorrect response with the exception of the IDS1 stage on which the low and high Purkinje cell groups did not differ (Fig 6D). Importantly, the significantly longer response and reward collection latencies observed in ataxic mice at all stages of the IED task (Figure 6B, C, D) were most likely due to motor deficits in these mice as opposed to cognitive impairments which were restricted to reversal learning stages and the extradimensional set-shifting stage (Figure 4).
3.2.4. Associations of Purkinje cells and behavior in non-ataxic chimeric mice
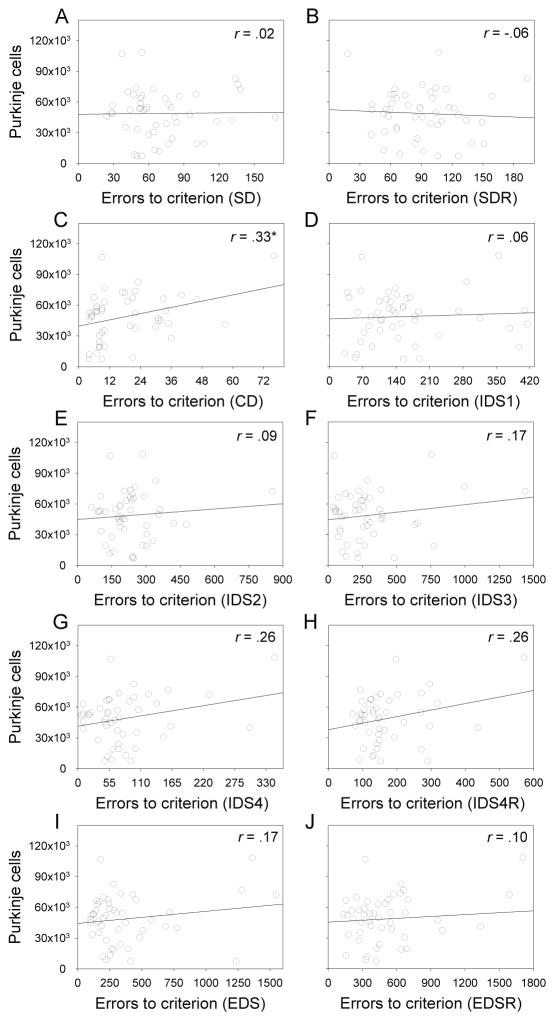
Means of the two non-ataxic Purkinje cell groups (medium, high) did not differ significantly on measures of learning, cognitive flexibility, response latency, or reward collection latency. To determine if more subtle relationships between Purkinje cells and behavior could be detected, we performed Pearson product moment correlations to examine the relationship of Purkinje cell number and performance measures at all stages of the IED task. On the CD stage, we observed significant positive correlations between Purkinje cells and errors to criterion (r = .33, p < .05), sessions to criterion (r = .32, p < .05), and response latency (r = .34, p < .05). These relationships indicate that on the CD stage non-ataxic chimeras with more Purkinje cells took longer to respond and committed more errors relative to chimeras with fewer Purkinje cells. Scatterplots of the errors to criterion measure at all IED stages are depicted in Figure 7.
Figure 7.
In non-ataxic mice, errors to criterion and sessions to criterion (data not shown) were significantly positively correlated with Purkinje cells on the CD stage but not on other stages.
4. DISCUSSION
We assessed the effect of developmental cerebellar Purkinje cell loss on visual discrimination, reversal learning, attentional set-acquisition, and attentional set-shifting in mice. Aggregation chimeras produced from lurcher mutant and wildtype embryos were tested on a touchscreen mediated attentional set-shifting task adapted from those used with humans and non-human primates (Figure 1). Following behavioral testing, Purkinje cells were quantified (Figures 2, 3). Mice with ≥ 95% Purkinje cell loss exhibited impaired reversal learning and extra dimensional set-shifting performance on the IED task (Figures 4, 5). Visual discrimination and intradimensional set-shifting performance of these mice was unimpaired. Propensity to collect a reward following a correct response was > 95% at all stages in all Purkinje cell groups (Figure 6A). In addition to exhibiting cognitive deficits, mice with ≥ 95% Purkinje cell loss were ataxic and exhibited significantly longer response and food collection latencies relative to non-ataxic chimeras (Figure 6B, C, D). Importantly, significantly-elevated food collection and response latencies in ataxic mice were observed at all stages and did not vary significantly across stages, whereas cognitive deficits were observed only on reversal learning and extradimensional set-shifting stages. In non-ataxic mice, positive associations between Purkinje cells and IED performance were observed on the CD stage.
4.1 Cerebellar contribution to higher- and lower-order rule learning and cognitive flexibility
In the present study, the low, medium, and high Purkinje cell groups exhibited equivalent performance on the SD stage (Figure 4). These data indicate that the ability of mice to acquire a food-motivated learning task and to discriminate between visual stimuli was not affected by Purkinje cell number. Moreover, equivalent performance of mice in all three groups indicates that the ataxic gait exhibited by all chimeras in the low Purkinje cell group did not affect their ability to perform the visual discrimination task. These conclusions are consistent with those of a previous study in which we found equivalent performance across Purkinje cell groups on the acquisition of a conditional visual discrimination (Dickson et al., 2010). In reversal learning and IED behavioral assays, performance on the SD stage is a measure of the ability to learn a lower-order rule (Wise et al., 1996). Equivalent performance of all Purkinje cell groups on the SD stage in the present study and in our previous study (Dickson et al., 2010) suggests that the cerebellum is not involved in lower-order rule learning.
In contrast to their unimpaired visual discrimination performance during the SD stage, chimeras in the low Purkinje cell group exhibited impaired performance at the SDR and IDS4R stages (Figure 4). Notably, the reversal learning impairment exhibited by low Purkinje cell chimeric mice was most robust on the late-phase of the IDS4R stage. We have observed profoundly impaired performance of low Purkinje cell chimeras on serial reversals of a conditional visual discrimination in a previous study (Dickson et al., 2010). Performance on reversal learning stages is a measure of lower-order cognitive flexibility (Wise et al., 1996, Clarke et al., 2005). Thus, the observation of impaired reversal learning performance in the present study and in our previous study (Dickson et al., 2010) suggests that the cerebellum contributes to lower-order cognitive flexibility. It is also possible that altered perception of stimulus salience in the low Purkinje cell group contributed to the performance impairments on reversal learning stages (Bussey et al., 1997, Brigman and Rothblat, 2008, Dickson et al., 2013).
In addition to impaired performance on reversal learning stages, mice from the low Purkinje cell group exhibited a profound impairment on the EDS stage (Figure 4). In contrast to reversal learning stages, performance on the extradimensional set-shifting stage is a measure of higher-order cognitive flexibility (Wise et al., 1996, Clarke et al., 2005). On this stage, novel stimuli are used, but the higher-order response requirement is reversed. These data suggest that the cerebellum contributes to higher-order cognitive flexibility. It is also possible that altered perception of stimulus salience in the low Purkinje cell group contributed to the EDS performance deficit (Dickson et al., 2014). The use of a counterbalanced design in which some mice learn to shift attention from shapes to lines and other mice learn to shift attention from lines to shapes would enable fully dissociating the effect of the cerebellum on stimulus salience attribution and attentional set-shifting.
In contrast to lower-order rule learning, the ability to learn a higher-order rule is measured by examining performance improvement across intradimensional shifts (Dias et al., 1997). As a group, mice committed significantly fewer errors on the IDS4 stage relative to the IDS1, IDS2, and IDS3 stages. With regard to cerebellar contribution to this effect, the low, medium, and high Purkinje cell groups all exhibited significantly improved performance on the IDS4 stage relative to the IDS1 stage, and this performance improvement was equivalent for all groups. Collectively, these data suggest that the cerebellum does not contribute to the acquisition of a higher-order rule. It should be noted, however, that we cannot exclude the possibility that the stimuli used on the IDS4 stage were intrinsically easier to discriminate than stimuli used on the other IDS stages. Thus, it remains possible that the use of different stimuli could reveal an effect of the cerebellum on the acquisition of a higher-order rule. It should be noted that differences between the U-maze and touchscreen versions of the IED task, such as differential salience of dimensions, could result in differential effects on attentional set development and shifting (Dickson et al., 2014).
Collectively, these data indicate that mice from the low Purkinje cell group exhibited a deficit on the extradimensional set-shifting stage and a late-phase reversal learning deficit. Extradimensional set-shifting deficits are observed on the maze-based version of the IED task following medial PFC lesion in mice (Bissonette et al., 2008) and rats (Birrell and Brown, 2000). Late-phase reversal learning deficits have been observed in operant reversal learning paradigms following medial PFC (mPFC) lesion in mice (Brigman and Rothblat, 2008) and rats (Bussey et al., 1997). The cerebellum sends projections to the mPFC (Watson et al., 2009, Watson et al., 2014), and mPFC neurotransmitter dynamics are partially mediated through cerebellar-mPFC circuitry. In lurcher mice, reduced Purkinje cell numbers result in multiple changes in mPFC neurotransmitter dynamics (Mittleman et al., 2008, Rogers et al., 2011, Rogers et al., 2013, McKimm et al., 2014). These data suggest a mechanism through which cerebellar Purkinje cell loss may affect cognitive flexibility.
4.2 Effects of motor ability and reward valence on task performance
When assessing cognitive function, effects of potentially confounding variables such as motor ability and reward valence should be considered. With regard to motor ability, ataxic mice in the low Purkinje cell group exhibited motor deficits in addition to cognitive deficits. Specifically, latency to respond following stimulus presentation and latency to collect a reward were modestly though significantly longer in ataxic mice. These effects were likely directly caused by the ataxia in these mice. Moreover, the pattern of these deficits suggests that they were unrelated to the observed reversal learning and attentional set-shifting deficits. Specifically, motor deficits in ataxic mice were observed on all IED stages, and this effect did not vary across stages (Figure 6B, C, D). In contrast, cognitive deficits were observed only on reversal learning stages and the extradimensional set-shifting stage (Figure 4). With regard to reward valence, mice from all groups collected > 95% of delivered rewards (Figure 6A). This suggests that both ataxic and non-ataxic mice were strongly motivated to perform the IED task. Collectively, these data suggest that neither motor deficits nor differential reward valence can explain the cognitive flexibility deficits observed in chimeric mice from the low Purkinje cell group.
4.3 Association of Purkinje cell number and IED performance in non-ataxic chimeric mice
Positive relationships between Purkinje cells and IED performance were observed in non-ataxic mice with variable Purkinje cell loss. Specifically, relative to mice with fewer Purkinje cells, mice with more Purkinje cells exhibited longer response latencies, committed more errors, and required more sessions to reach criterion on the CD stage. One explanation for this seemingly counterintuitive relationship is that the cerebellum influences selective attention to novel stimuli. Specifically, because exemplars from the shapes dimension were introduced for the first time on the CD stage and were not relevant, they served as novel distractor stimuli. Under these conditions, ignoring these novel and non-relevant stimuli would facilitate learning. Thus, it is possible that mice with greater numbers of Purkinje cells committed more errors on the CD stage because they were attending more strongly to newly introduced stimuli, not because they were exhibiting a learning impairment.
4.4. Translational relevance
Cerebellar pathology and cognitive inflexibility co-occur in neuropsychiatric disorders including autism (Hughes et al., 1994, Hill, 2004, Fatemi et al., 2012), schizophrenia (Pantelis et al., 1999, Andreasen and Pierson, 2008, Floresco et al., 2009, Leeson et al., 2009), and drug addiction (Woicik et al., 2011, Fatemi et al., 2012, McCracken and Grace, 2013, Moreno-Lopez et al., 2015, Verdejo-Garcia et al., 2015, Miquel et al., 2016). Findings from the present study suggest that the nature of these relationships may be causal. In addition to effects on cognitive flexibility, the cerebellum may be involved in a host of other non-motor functions including behavioral and cognitive processes (Fatemi et al., 2012, Koziol et al., 2014, Baumann et al., 2015). Many of these effects have been examined using cerebellar mutant mice (Lalonde and Strazielle, 2007).
Due to consistent findings of reduced cerebellar Purkinje cells in autism (Palmen et al., 2004, Whitney et al., 2008), the lurcher↔wildtype chimeras used in the present study are a particularly appropriate model for studying the relationship between cognitive flexibility and neuropathology in autism. Moreover, deficits in extradimensional set-shifting may be the most precise index of cognitive inflexibility in autism (Geurts et al., 2009). Using the CANTAB IED subtest on which the mouse touchscreen version of the IED task is based, Ozonoff et al. (2004) conducted the largest study to date on the phenomenon of cognitive inflexibility in autism. Similar to mice from the low Purkinje cell group in the present study, participants with autism were impaired relative to controls on the EDS stage but not on the SD stage. The correlation of Purkinje cells and errors on the CD stage in the present study suggests that Purkinje cells are involved in attention to novel stimuli, and individuals with autism exhibit impairments in attending to novel visual stimuli in a touchscreen attentional set-shifting task (Maes et al., 2011). Collectively, these data suggest that cerebellar Purkinje cell loss is, at least in part, a causal factor driving cognitive inflexibility and inattention to novel stimuli in autism and other disorders in which cerebellar pathology is present.
5. Conclusion
Using a touchscreen attentional set-shifting task modeled on those used with humans and non-human primates, we assessed higher and lower-order rule learning and cognitive flexibility in chimeric mice with varying degrees of Purkinje cell loss. Higher- and lower-order rule learning were unaffected by Purkinje cell loss, whereas mice with low cerebellar Purkinje cell numbers exhibited profound cognitive flexibility deficits at reversal learning stages and the attentional set-shifting stage. Cognitive deficits in ataxic mice were not related to motor deficits. The positive relationship of Purkinje cells and errors on the CD stage in non-ataxic mice suggests that the cerebellum may facilitate attention to novel stimuli. Collectively, these data suggest that the cerebellum contributes to reversal learning, attentional set-shifting, and attention to novel stimuli, but not the acquisition of lower- or higher-order rules. These findings suggest that cerebellar pathology observed in psychiatric disorders including autism, schizophrenia, and drug addiction may cause the cognitive impairments which have been proposed to underlie the core symptoms of these disorders.
Highlights.
Reversal learning and set-shifting were impaired in mice with ≥ 95% Purkinje cell loss.
Cognitive deficits were unrelated to motor deficits in ataxic mice.
The cerebellum contributes to cognitive flexibility and attention to novel stimuli.
Acknowledgments
This project was made possible by NINDS grant 1R01NS063009. The authors gratefully acknowledge Erin Clardy for assistance with data collection.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. The Anatomical Record. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, Leggio M, Mattingley JB, Molinari M, Moulton EA, Paulin MG, Pavlova MA, Schmahmann JD, Sokolov AA. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum. 2015;14:197–220. doi: 10.1007/s12311-014-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Bussey TJ, Saksida LM, Rothblat LA. Discrimination of multidimensional visual stimuli by mice: intra- and extradimensional shifts. Behav Neurosci. 2005;119:839–842. doi: 10.1037/0735-7044.119.3.839. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Padukiewicz KE, Sutherland ML, Rothblat LA. Executive functions in the heterozygous reeler mouse model of schizophrenia. Behav Neurosci. 2006;120:984–988. doi: 10.1037/0735-7044.120.4.984. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Rothblat LA. Stimulus specific deficit on visual reversal learning after lesions of medial prefrontal cortex in the mouse. Behav Brain Res. 2008;187:405–410. doi: 10.1016/j.bbr.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Caddy KW, Biscoe TJ. Structural and quantitative studies on the normal C3H and Lurcher mutant mouse. Philos Trans R Soc Lond B Biol Sci. 1979;287:167–201. doi: 10.1098/rstb.1979.0055. [DOI] [PubMed] [Google Scholar]
- Cairns J, Swanson D, Yeung J, Sinova A, Chan R, Potluri P, Dickson P, Mittleman G, Goldowitz D. Abnormalities in the Structure and Function of Cerebellar Neurons and Neuroglia in the Lc/+ Chimeric Mouse Model of Variable Developmental Purkinje Cell Loss. Cerebellum. 2016 doi: 10.1007/s12311-015-0756-7. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Phillips AG, Floresco SB. Preferential involvement by nucleus accumbens shell in mediating probabilistic learning and reversal shifts. J Neurosci. 2014;34:4618–4626. doi: 10.1523/JNEUROSCI.5058-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bartolo P, Mandolesi L, Federico F, Foti F, Cutuli D, Gelfo F, Petrosini L. Cerebellar involvement in cognitive flexibility. Neurobiol Learn Mem. 2009;92:310–317. doi: 10.1016/j.nlm.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PE, Calton MA, Mittleman G. Performance of C57BL/6J and DBA/2J mice on a touchscreen-based attentional set-shifting task. Behav Brain Res. 2014;261:158–170. doi: 10.1016/j.bbr.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PE, Corkill B, McKimm E, Miller MM, Calton MA, Goldowitz D, Blaha CD, Mittleman G. Effects of stimulus salience on touchscreen serial reversal learning in a mouse model of fragile X syndrome. Behav Brain Res. 2013;252:126–135. doi: 10.1016/j.bbr.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PE, Rogers TD, Del Mar N, Martin LA, Heck D, Blaha CD, Goldowitz D, Mittleman G. Behavioral flexibility in a mouse model of developmental cerebellar Purkinje cell loss. Neurobiology of Learning and Memory. 2010;94:220–228. doi: 10.1016/j.nlm.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes JJ, Roberts AC, Sahakian BJ, Evenden JL, Morris RG, Robbins TW. Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson’s disease: evidence for a specific attentional dysfunction. Neuropsychologia. 1989;27:1329–1343. doi: 10.1016/0028-3932(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, Chauhan V, Dager SR, Dickson PE, Estes AM, Goldowitz D, Heck DH, Kemper TL, King BH, Martin LA, Millen KJ, Mittleman G, Mosconi MW, Persico AM, Sweeney JA, Webb SJ, Welsh JP. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11:777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends Cogn Sci. 2009;13:74–82. doi: 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32:477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Front Neurosci. 2013;7:201. doi: 10.3389/fnins.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C, Parker K, Pezzulo G, Ramnani N, Riva D, Schmahmann J, Vandervert L, Yamazaki T. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014;13:151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. Spontaneous and induced mouse mutations with cerebellar dysfunctions: behavior and neurochemistry. Brain Res. 2007;1140:51–74. doi: 10.1016/j.brainres.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Sahakian BJ, Rogers RD, Hodge JR, Robbins TW. Discrimination, reversal, and shift learning in Huntington’s disease: mechanisms of impaired response selection. Neuropsychologia. 1999;37:1359–1374. doi: 10.1016/s0028-3932(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, Joyce EM. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes JH, Eling PA, Wezenberg E, Vissers CT, Kan CC. Attentional set shifting in autism spectrum disorder: differentiating between the role of perseveration, learned irrelevance, and novelty processing. J Clin Exp Neuropsychol. 2011;33:210–217. doi: 10.1080/13803395.2010.501327. [DOI] [PubMed] [Google Scholar]
- Martin LA, Escher T, Goldowitz D, Mittleman G. A relationship between cerebellar Purkinje cells and spatial working memory demonstrated in a lurcher/chimera mouse model system. Genes Brain Behav. 2004;3:158–166. doi: 10.1111/j.1601-183x.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G. The cerebellum and spatial ability: dissection of motor and cognitive components with a mouse model system. The European journal of neuroscience. 2003;18:2002–2010. doi: 10.1046/j.1460-9568.2003.02921.x. [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G. Sustained attention in the mouse: a study of the relationship with the cerebellum. Behav Neurosci. 2006;120:477–481. doi: 10.1037/0735-7044.120.2.477. [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G. Repetitive behavior and increased activity in mice with Purkinje cell loss: a model for understanding the role of cerebellar pathology in autism. Eur J Neurosci. 2010;31:544–555. doi: 10.1111/j.1460-9568.2009.07073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. Persistent cocaine-induced reversal learning deficits are associated with altered limbic cortico-striatal local field potential synchronization. J Neurosci. 2013;33:17469–17482. doi: 10.1523/JNEUROSCI.1440-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimm E, Corkill B, Goldowitz D, Albritton LM, Homayouni R, Blaha CD, Mittleman G. Glutamate dysfunction associated with developmental cerebellar damage: relevance to autism spectrum disorders. Cerebellum. 2014;13:346–353. doi: 10.1007/s12311-013-0541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel M, Vazquez-Sanroman D, Carbo-Gas M, Gil-Miravet I, Sanchis-Segura C, Carulli D, Manzo J, Coria-Avila GA. Have we been ignoring the elephant in the room? Seven arguments for considering the cerebellum as part of addiction circuitry. Neurosci Biobehav Rev. 2016;60:1–11. doi: 10.1016/j.neubiorev.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Goldowitz D, Heck DH, Blaha CD. Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse. 2008;62:544–550. doi: 10.1002/syn.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Perales JC, van Son D, Albein-Urios N, Soriano-Mas C, Martinez-Gonzalez JM, Wiers RW, Verdejo-Garcia A. Cocaine use severity and cerebellar gray matter are associated with reversal learning deficits in cocaine-dependent individuals. Addict Biol. 2015;20:546–556. doi: 10.1111/adb.12143. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, McMahon WM, Minshew N, Munson JA, Pennington BF, Rogers SJ, Spence MA, Tager-Flusberg H, Volkmar FR, Wrathall D. Performance on Cambridge Neuropsychological Test Automated Battery subtests sensitive to frontal lobe function in people with autistic disorder: evidence from the Collaborative Programs of Excellence in Autism network. J Autism Dev Disord. 2004;34:139–150. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res. 1999;37:251–270. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex. 2007;17(Suppl 1):i151–160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- Rogers TD, Dickson PE, Heck DH, Goldowitz D, Mittleman G, Blaha CD. Connecting the dots of the cerebro-cerebellar role in cognitive function: neuronal pathways for cerebellar modulation of dopamine release in the prefrontal cortex. Synapse. 2011;65:1204–1212. doi: 10.1002/syn.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TD, Dickson PE, McKimm E, Heck DH, Goldowitz D, Blaha CD, Mittleman G. Reorganization of circuits underlying cerebellar modulation of prefrontal cortical dopamine in mouse models of autism spectrum disorder. Cerebellum. 2013;12:547–556. doi: 10.1007/s12311-013-0462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakian BJ, Downes JJ, Eagger S, Evenden JL, Levy R, Philpot MP, Roberts AC, Robbins TW. Sparing of attentional relative to mnemonic function in a subgroup of patients with dementia of the Alzheimer type. Neuropsychologia. 1990;28:1197–1213. doi: 10.1016/0028-3932(90)90055-s. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Verdejo-Roman J, Albein-Urios N, Martinez-Gonzalez JM, Gutierrez B, Soriano-Mas C. Neural substrates of cognitive flexibility in cocaine and gambling addictions. Br J Psychiatry. 2015 doi: 10.1192/bjp.bp.114.152223. [DOI] [PubMed] [Google Scholar]
- Watson TC, Becker N, Apps R, Jones MW. Back to front: cerebellar connections and interactions with the prefrontal cortex. Front Syst Neurosci. 2014;8:4. doi: 10.3389/fnsys.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TC, Jones MW, Apps R. Electrophysiological mapping of novel prefrontal - cerebellar pathways. Front Integr Neurosci. 2009;3:18. doi: 10.3389/neuro.07.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum. 2008;7:406–416. doi: 10.1007/s12311-008-0043-y. [DOI] [PubMed] [Google Scholar]
- Wise SP, Murray EA, Gerfen CR. The frontal cortex-basal ganglia system in primates. Crit Rev Neurobiol. 1996;10:317–356. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]
- Woicik PA, Urban C, Alia-Klein N, Henry A, Maloney T, Telang F, Wang GJ, Volkow ND, Goldstein RZ. A pattern of perseveration in cocaine addiction may reveal neurocognitive processes implicit in the Wisconsin Card Sorting Test. Neuropsychologia. 2011;49:1660–1669. doi: 10.1016/j.neuropsychologia.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in delta2 glutamate receptor gene. Nature. 1997;388:769–773. doi: 10.1038/42009. [DOI] [PubMed] [Google Scholar]