Abstract
N-methyl-D-aspartate receptors (NMDARs) are profound regulators of glutamate neurotransmission and behavior. To coordinate components of the limbic system, the dorsal and ventral striatum integrate cognitive and emotional information towards the execution of complex behaviors. Striatal outflow is conveyed by medium spiny neurons (MSNs), which can be dichotomized by expression of dopamine receptor subtype 1 (D1) or adenosine receptor subtype 2A (A2A). To examine how striatal NMDAR function modulates reward-related behaviors, we generated D1- and A2A-specific genetic deletions of the obligatory GluN1 subunit. Interestingly, we observed no differences in any GluN1−/− genotype in reward learning as assessed by acquisition or extinction of cocaine conditioned place preference (CPP). Control and A2A-GluN−/− mice exhibited robust cocaine-primed reinstatement, however this behavior was markedly absent in D1-GluN−/− mice. Interestingly, dual D1-/A2A-GluN−/− mice displayed an intermediate reinstatement phenotype. Next, we examined models of exploration, anxiety, and despair, states often associated with relapse to addiction-related behavior, to determine NMDAR contribution in D1 and A2A cell types to these behaviors. D1-GluN1−/− mice displayed aberrant exploratory locomotion in a novel environment, but the phenotype was absent in dual D1/A2A-GluN1−/− mice. In contrast A2A-GluN1−/− mice displayed a despair-resistant phenotype, and this phenotype persisted in dual D1/A2A-GluN−/− mice. These data support the hypothesis that cell type-specific NMDAR signaling regulates separable behavioral outcomes related to locomotion, despair, and relapse.
Keywords: striatum, nucleus accumbens, cocaine, NMDAR, reinstatement, despair
1. Introduction
Adaptations involved in the development of addiction-like behaviors share common mechanisms with learning and memory processes (Grueter et al., 2012; Joffe et al., 2014; Koob and Volkow, 2010; Lüscher and Malenka, 2011). In particular, N-methyl-D-aspartate receptors (NMDARs) have been extensively studied as mediators of drug experience-dependent memory formation (Brown et al., 2011; Kalivas and Alesdatter, 1993; Karler et al., 1989; Wolf and Khansa, 1991; Zweifel LS, Argilli E, Bonci A, 2008). NMDARs are glutamate-gated, calcium-permeable channels that provide major regulation of synaptic plasticity throughout much of the central nervous system (Traynelis et al., 2010), transforming transient patterns of neurotransmission into persistent changes in synaptic strength that underlie cognitive functions (Paoletti et al., 2013). Because constitutive genetic deletion of GluN1 is lethal (Tsien et al., 1996), probing NMDAR function in vivo necessitates cell type- and/or region-specific approaches.
One key brain complex that subserves reward-related behaviors is the striatum, which is conceptualized as a gatekeeper of descending neurotransmission relating to motor control, reward, and reinforcement (Grueter et al., 2012; Joffe et al., 2014; Kreitzer and Malenka, 2008). Throughout the dorsal and ventral striatum, GABAergic medium spiny neurons (MSNs) comprise 90–95% of neurons and provide the output from the structures. Striatal MSNs are often dichotomized into two groups by biochemistry, anatomy, and function (Lobo and Nestler, 2011), commonly depicted by expression of dopamine receptor subtype 1 (D1) or adenosine receptor subtype 2A (A2A), which overlaps with dopamine receptor subtype 2 expressing (D2) MSNs. When selectively activated in vivo, D1 and A2A/D2 MSNs in the dorsal striatum and NAc have been shown to exert opposing or divergent effects on locomotor (Kravitz et al., 2010), stress-induced (Francis et al., 2014), and reward-related (Kravitz et al., 2012; Lobo et al., 2010) behaviors. For example, driving NAc D1 MSNs conferred a conditioned place preference (CPP) to a subthreshold cocaine regimen, whereas activation of NAc A2A/D2 MSNs blocked the preference induced by a rewarding dose.
Although much remains to be learned, NMDAR signaling in NAc D1 MSNs has been demonstrated to be necessary for the development of psychostimulant-conditioned behaviors (Beutler et al., 2011; Cahill et al., 2014; Heusner and Palmiter, 2005). However, these experiments did not assess whether D1-NMDAR signaling underlies reinstatement, a model of relapse to drug seeking. Additionally, how A2A MSN NMDARs modulate psychostimulant-conditioned behaviors has not been addressed. In fact, without limit to drug-related behaviors, the behavioral relevance of NMDAR signaling in A2A-expressing neurons remains unknown. Given that NMDAR signaling is important for experience-driven changes in D1 MSN function, a hypothesis is that A2A MSN NMDAR function regulates stress-related behaviors. Moreover, with recent efforts towards developing NMDAR antagonists and related pharmacotherapies as treatments for Major Depressive Disorder (Krystal et al., 2013; Maeng et al., 2008), the literature requires a better understanding of how cell type-specific NMDAR function modulates despair- and anxiety-like behaviors.
The GluN1 subunit is essential to the formation of functioning NMDARs (Paoletti et al., 2013), Although splicing variants are common, all GluN1 protein is produced from a single gene, Grin1. Therefore we ablated functional NMDARs in D1- and/or A2A-expressing neurons by generating mice homozygous for floxed Grin1 (Grinlox/lox) that co-expressed Cre recombinase under control of the D1 and/or A2A promotors. MSN expression of D1 and A2A is by and large mutually exclusive (Gangarossa et al., 2013; Lu et al., 1998). Although D1 is expressed in other components of the limbic system, like the prefrontal cortex and amygdala (Boyson et al., 1986; Zhou et al., 1990), the neuronal expression of A2A is highly localized to the striatum (Fink et al., 1992; Schiffmann et al., 1991), where co-expression with D1 or interneuron markers is minimal (Schiffmann and Vanderhaeghen, 1993). Therefore, relative to the D1-specific deletion, the double knockout from D1- and A2A-expressing cell types induces similar impairments in NMDAR signaling across MSN types while exerting minimal additional extra-striatal off-target effects. After generating D1-, A2A-, and dual D1/A2A-GluN1−/− mice, we used targeted whole-cell electrophysiology to validate cell type specificity in the NAc core. We then assessed reward-related associative learning through cocaine CPP. All genotypes acquired, expressed, and extinguished cocaine CPP, however cocaine-primed reinstatement was markedly absent in D1-GluN1−/− mice. Dual D1-/A2A-GluN1−/− mice displayed a partial rescue, suggesting that both D1 MSNs and extra-striatal D1-expressing neurons may be involved in cocaine-primed reinstatement. We then examined models of exploration, anxiety, and behavioral despair. D1-GluN1−/− mice displayed aberrant exploratory locomotion in a novel environment, but the phenotype was absent in dual D1/A2A-GluN1−/− mice. In contrast, A2A-GluN1−/− mice displayed an antidepressant-like phenotype that persisted in dual D1/A2A-GluN−/− mice. These data suggest that balanced NMDAR signaling across striatal MSNs underlies aspects of locomotion, but that A2A MSN NMDAR function regulates despair in a more complex manner.
2. Methods and materials
2.1. Mice
Bacterial artificial chromosome (BAC) adult (6–12 week) mice were used in all experiments and were housed together in groups of two to five per cage on a 12/12-hr light/dark cycle (lights on at 06:00), with food and water available ad libitum. Transgenic floxed GluN1 (Grin1lox/lox) mice possess loxP sites flanking the transmembrane domain and C-terminal region (Tsien et al., 1996). To generate cell type-specific genetic deletions of GluN1, Grin1lox/lox mice were crossed with bacterial artificial chromosome (BAC) transgenic mice expressing Cre recombinase under the regulation of the Drd1a (FK150, GENSAT; Gong et al., 2007) and/or Adora2a promoter (KG139, GENSAT). We elected to use A2A as a marker for “indirect pathway” MSNs because A2A is more selective than dopamine receptor D2, which is expressed by some striatal interneurons and as an autoreceptor on midbrain dopaminergic neurons. Mice used for electrophysiology also expressed tdTomato under the control of the Drd1a promoter (Gong et al., 2003; Shuen et al., 2008). Mice have been backcrossed onto a C57BL/6J background for >10 generations. The open field test and cocaine CPP were performed in the Vanderbilt Mouse Neurobehavioral Core, where only male mice were utilized. For the EPM, NIH, TST, and FST, both male and female mice were used. Sexes were housed, tested, and analyzed separately. Results generated from males and females were not statistically different, therefore the data was pooled and subsequently analyzed together. All experiments were done in accordance with the policies set out by the Institutional Animal Care and Use Committees (IACUC) at Vanderbilt University and in accordance with the National Institutes of Health guide for the care and use of Laboratory animals.
2.2. Electrophysiology
Parasagittal slices (250 μm) containing the NAc core were prepared from D1-tdTomato heterozygotic BAC transgenic mice and excitatory postsynaptic currents (EPSCs) were recorded as described (Grueter et al., 2013, 2010).
2.3. Cocaine place conditioning
The place conditioning procedure was conducted in activity chambers identical to those used for the open field. (ENV-510, Med Associates) Prior to the place conditioning pretest, mice were habituated to behavioral testing with a one-hour exposure to similar activity chambers. For place conditioning studies, a two-chambered insert with contextually distinct metal floors (ENV-3013-2, Med Associates) was placed in the activity chambers. All place conditioning sessions were 20 min in duration, and the amount of time spent in each zone was recorded. Conditioning sessions were conducted twice daily, separated by 4 hours, and treatment order was counterbalanced across groups. All mice received saline prior to confinement in one compartment (mesh grid floor) and cocaine (20 mg/kg) immediately prior to confinement in the other compartment (parallel bar floor). Following three days of conditioning, the place preference expression test was conducted. Mice then underwent extinction training, and subjects that had not expressed a place preference (< 10%/120 s) were removed from the study. On extinction days 1 and 3, mice underwent active extinction (saline) conditioning sessions on both sides of the apparatus. On extinction days 2 and 4, standard post-test sessions occurred and time spent on the side previously conditioned with cocaine was recorded. Mice that did not adequately extinguish their place preference (> 10%/120 s) on the second extinction test were removed prior to reinstatement. No difference in the proportion of mice removed from the study was observed across genotypes. To reinstate the place preference, mice received a priming dose of cocaine (10 mg/kg) immediately prior to the test session. Locomotor activity (distance traveled, stereotypic counts, vertical counts, and time at rest) was measured during all sessions.
2.4. Open field
Mice were placed in an open field activity chamber (ENV-520, Med Associates) equipped with infrared beams and detectors for one hour. Each chamber was housed within a sound attenuating cabinet (ENV-022MD, Med Associates). A software interface (Activity Monitor, Med Associates) monitored the two-dimensional horizontal position of the mouse as well as beam breaks of a bar raised 4cm from the floor.
2.5. Elevated plus maze
The elevated plus maze (EPM) was based on the model described by Lister (Lister, 1987). The EPM was comprised of two open and two closed arms (5 cm wide × 30 cm long) that meet in the center to form a plus. The floors were opaque and the walls were made of tinted black acrylic plastic. The arms of the EPM were elevated 40 cm above a platform. Animals were placed on an open platform facing the center and remained in the maze for 5 min. Visual recordings of mouse movement were obtained with an overhead video camera. Mouse location and movement were assessed in real time with automated software (EthoVision XT, Noldus). Subsequent analyses determined the duration of time the center-point of each mouse was in the open arms, closed arms, or center.
2.6. Novelty-induced hypophagia
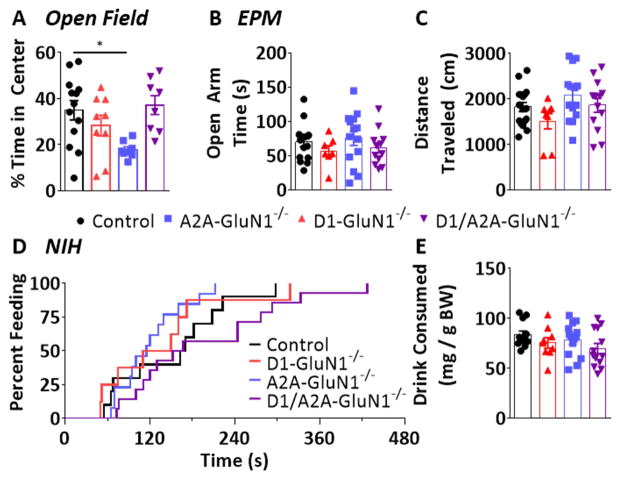
The novelty-induced hypophagia (NIH) assay was based on a previously published procedure (Louderback et al., 2013). Briefly, mice were conditioned for 4 days to have 30-min access to a highly palatable food (Ensure, home-made vanilla shake flavor) in their home cages while group housed. On the testing day, each mouse was transferred to an individual open field activity chamber (~150 lux) and given access to Ensure for up to 30 minutes. The latency to drink and amount consumed were measured.
2.7. Tail suspension and forced swim tests
The tail suspension test (TST) and forced swim test (FST) were based on a previously published procedure (Lim et al., 2012). For the TST each mouse was suspended 20 cm above a surface with tape placed 1–2 cm from the base of the tail. For the FST, mice were placed in 2-L beakers half-filled with room temperature water that was changed between sessions. All trials were videotaped and scored by one-of-two trained observers. The interrater correlation (Pearson Product-Moment Correlation Coefficient, r) was calculated to be 0.978 (TST) and 0.941 (FST) by linear regression. Approximately 75% of analyses were performed blinded to genotype. Mice were considered immobile when, for more than 0.5 s, they: (TST) exhibited no body movement and hung passively or (FST) engaged in minimal movements to stay afloat with no escape behavior. Total immobility was scored as the sum of all immobility over the 6 minute trial. Latency was scored as the time of completion of the first uninterrupted 10-s bout of immobility.
2.8. Data analysis
Data are expressed as mean ± SEM (n = number of animals for behavior and number of cells for electrophysiology), or as [minimum, lower quartile, median, upper quartile, maximum] for box and whiskers plots. One- or two-way ANOVA or two-tailed Student’s t-test were used when indicated. Repeated measures or paired tests were performed when appropriate. The Mantel-Cox log-rank test was used to assess differences between cumulative distributions. All post-tests employed Bonferroni corrections for multiple comparisons and p < 0.05 was considered statistically significant.
2.9. Drugs
Cocaine HCl and picrotoxin were purchased from Sigma-Aldrich.
3. Results
3.1. Functional verification of GluN1 genetic deletions
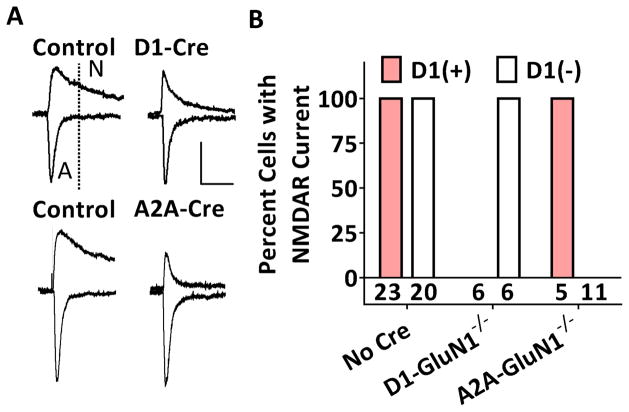
To confirm selectivity of the genetic deletions, we measured excitatory postsynaptic currents (EPSCs) in targeted NAc core MSNs from control animals which expressed either Cre or Grin1lox/lox, but not both and D1-GluN1−/− and A2A-GluN1−/− mice. MSNs were identified as either D1-expressing (+) or D1-non-expressing (−) by presence or absence of td-Tomato fluorescence. In control MSNs held at −70 mV, the EPSC exhibits fast decay kinetics and is mediated by AMPARs (Figure 1A: left). When held at +40 mV, the dual component EPSC is much slower and at 50 ms post EPSC onset, reflects current passing solely through NMDARs. In control slices, measurable NMDAR currents (>20% of peak) were obtained in all NAc core MSNs (D1(+): n = 23/23, D1(−): n = 20/20). In contrast, in slices prepared from D1-GluN1−/− mice, NMDAR currents were obtained from D1(−) MSNs (n = 6/6) but not from D1(+) MSNs (n = 0/6, 1A: top right). In A2A-GluN1−/− mice, the reverse was observed: NMDAR currents were not observed in D1(−) MSNs (n = 0/11, 1A: bottom right), but remained intact in D1(+) MSNs (n = 5/5). Fisher’s exact test revealed a significant deviation from observed outcome frequencies across groups (p < 0.001 Figure 1B).
Figure 1. Selectivity of cell type-specific GluN1 deletions.
(A) Representative traces of dual component EPSCs obtained from control (left) and GluN1−/− (right) NAc core MSNs. D1(+) MSNs are displayed on the top and D1(−) MSNs on the bottom. Scale bars denote 100 pA and 50 ms. The EPSC component at 50 ms or later is mediated by NMDARs. (B) Summary graph displaying percent D1(+) (red) and D1(−) (white) neurons with measurable NMDAR currents (p < 0.001, Fisher’s exact test).
3.2. Cocaine place conditioning in GluN1 deletion models
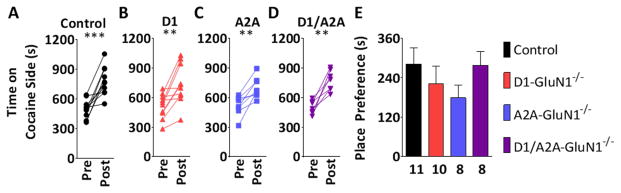
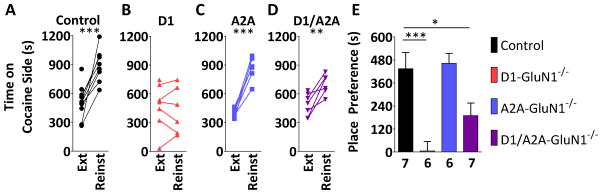
To determine contribution of NMDAR signaling at D1- and A2A-cell types in the rewarding properties of cocaine, we performed cocaine place conditioning. To test that genetic deletion of NMDARs might enhance cocaine CPP, we performed a sub-threshold conditioning paradigm. Mice were exposed to a single pairing of 5 mg/kg cocaine, which did not induce a place preference in control mice (76 ± 72 s, n.s. data not shown). Neither D1- nor A2A-GluN1−/− group expressed a place preference to that single pairing (D1: 29 ± 56 s, n.s., A2A: 3 ± 82 s, ns., data not shown), and no main effect of genotype was observed (F(2,23) = 0.2747, n.s., data not shown). We then proceeded to assess CPP to a maximal conditioning regimen, 3 pairings of 20 mg/kg cocaine. During the conditioning sessions, locomotor activity was also recorded. Consistent with Beutler et al., D1-GluN1−/− mice display diminished cocaine-induced hyperactivity (D1: 2837 ± 666 cm vs. control: 5170 ± 592 cm, p < 0.05, Figure S1), however the phenotype was absent in D1/A2A-GluN1−/− mice (5222 ± 704 cm, data not shown). The controls and each group of GluN1−/− mice exhibited robust place preferences on the expression test day (Figures 2A–D), and no difference across genotypes was observed (F(3,33) = 0.9733, n.s., Figure 2E). Mice then underwent extinction training, and no differences were observed ( F(3,26) = 1.161, n.s., Figure S2). Finally, to examine a facet of CPP related to relapse, mice were administered 10 mg/kg cocaine immediately prior to a place preference session. Control mice exhibited robust cocaine-primed reinstatement of CPP (432 ± 75 s, p < 0.001, Figure 3A). In contrast, D1-GluN1−/− mice exhibited a pronounced lack of reinstatement (−5 ± 136 s, n.s, Figure 3B.). Like the control controls, the A2A-GluN1−/− reinstated the place preference (463 ± 54 s, p < 0.001, Figure 3C), and the D1/A2A-GluN1−/− mice also displayed intact cocaine-induced reinstatement (274 ± 62 s, p < 0.01, Figure 3D), rescuing the phenotype displayed by the D1-GluN−/− animals. Although the D1/A2A-GluN−/− mice displayed cocaine-primed reinstatement, the magnitude of the preference was less relative to the control group so we performed a between-groups analysis (Figure 3E). A one-way ANOVA revealed a main effect of genotype on reinstatement (F(3,22) = 10.17, p < 0.001). Subsequent Bonferonni post-tests against controls confirmed the significant impairment of reinstatement in D1-GluN1−/− mice (t = 4.550, p < 0.001) and also revealed a blunted phenotype in the D1/A2A-GluN1−/− group (t = 2.698, p < 0.05). This suggests that drug-primed reinstatement of cocaine CPP requires NMDARs in D1-expressing cells, and that balanced striatal signaling is, at most, influential over the behavior.
Figure 2. Expression of cocaine conditioned place preference.
(A–D) Absolute time spent by control, D1-GluN1−/−, A2A-GluN1−/−, or D1/A2A-GluN1−/− mice on cocaine-paired side before (Pre) and after (Post) cocaine conditioning (**: p < 0.01, ***: p < 0.001, paired t-test). (E) Place preference following conditioning with 20 mg/kg i.p. cocaine in control (black), D1-GluN1−/− (red), A2A-GluN1−/− (blue), or D1/A2A-GluN1−/− (purple) mice.
Figure 3. Reinstatement of cocaine conditioned place preference.
(A–D) Absolute time spent by WT, D1-GluN1−/−, A2A-GluN1−/−, or D1/A2A-GluN1−/− mice on cocaine-paired side after extinction (Ext) and priming with 10 mg/kg cocaine (Reinst) (**: p < 0.01, ***: p < 0.001, paired t-test). Data points without paired reinstatement values represent mice that did not meet extinction criterion and were subsequently excluded from the study. (E) Place preference in control (black), D1-GluN1−/− (red), A2A-GluN1−/− (blue), or D1/A2A-GluN1−/− (purple) mice following reinstatement primed by 10 mg/kg i.p. cocaine (different from control, *: p < 0.05, ***: p < 0.001, Bonferroni post-test).
3.3. Open field test in GluN1 deletion models
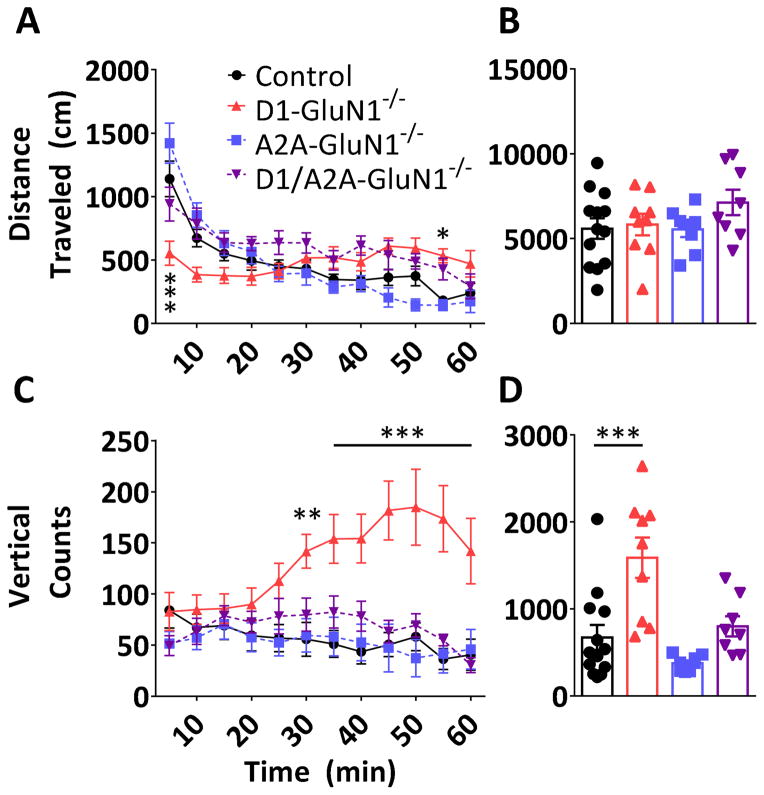
To examine a range of locomotor-based behaviors, we assessed spontaneous activity in an open field chamber. When placed in a novel environment, mice display a burst of horizontal locomotor activity during the first 5–10 minutes that declines substantially over time (F(11,374) = 34.06, p < 0.0001, Figure 4A). A two-way repeated measures ANOVA revealed a significant interaction between the locomotor time course and genotype (F(33,374) = 6.818, p < 0.0001). Post-tests determined that D1-GluN1−/− mice exhibited attenuated novelty-induced hyperlocomotion (0–5 min, 553 ± 95 cm vs. control: 1139 ± 140 cm). On the other hand, this behavior remained intact in A2A-GluN1−/− mice. D1-GluN1−/− mice also displayed atypical habituation to the novel environment, as evidenced by elevated locomotor activity towards the end of the hour session (50–55 min, 532 ± 55 cm vs. control: 238 ± 77 cm). Both of these phenotypes were rescued in the D1/A2A-GluN−/− mice, suggesting that imbalanced striatal signaling underlies the D1-GluN1−/− locomotor phenotypes. When summated over the entire hour-long test, no differences in total locomotion were observed (F(3,34) = 1.244, n.s., Figure 4B).
Figure 4. Open field assay.
(A) Novelty-induced hyperactivity and habituation in control (black circles), D1-GluN1−/− (red triangles), A2A-GluN1−/− (blue squares), or D1/A2A-GluN1−/− (purple triangles) mice (different from control, *: p<0.05, ***: p<0.001, Bonferroni post-test). (B) Total one-hour locomotor activity in control (black), D1-GluN1−/− (light grey), A2A-GluN1−/− (dark grey), or D1/A2A-GluN1−/− (white) mice. (C) Vertical beam breaks binned over 5 minute periods (different from control, **: p<0.01, ***: p<0.001, Bonferroni post-test). (D) Total vertical beam breaks obtained during the open field assay (different from control, ***: p<0.001, Bonferroni post-test).
During the same open field session, vertical beam breaks were also measured, having been described previously in relation to exploratory behavior (Fonio et al., 2009). We found that vertical counts varied significantly by time (F(11,374) = 2.455, p < 0.01, genotype (F(3,34) = 6.681, p < 0.01), and an interaction (F(33,374) = 5.657, p < 0.0001). Subsequent analyses revealed that D1-GluN1−/− mice displayed increased vertical counts during the second half of the hour session (Figure 4C). The increase in vertical counts is also evident when summated across the entire session (D1: 1588 ± 698 vs control: 673 ± 143, p < 0.001, Figure 4D). Similar to the aberrations in horizontal locomotion, the vertical count phenotype was rescued by the D1/A2A-GluN1−/− manipulation, without significant contribution of the A2A-specific knockout alone. Together these data suggest that D1-NMDAR function can modulate locomotor activity by disrupting the balance of signaling across MSN cell types.
We assessed the time spent in the center of the chamber during the open field test and found significant differences across genotypes (F(3,34) = 4.050, p < 0.05, Figure 5A). Control and D1-GluN1−/− mice both spent approximately 35% and 29% of the session in the center zone, respectively (control: 1260 ± 151 s, D1: 1034 ± 184 s). In contrast, A2A-GluN1−/− mice displayed increased thigmotactic behavior and spent only 18% of the session in the center zone (643 ± 46s, p < 0.05). Like the altered locomotor behaviors in the D1-GluN1−/− mice, the center time phenotype of the A2A-GluN1−/− was absent in the D1/A2A-GluN1−/− mice (37%, 1340 ± 149 s). Because decreases in center time often reflect increased anxiety, we hypothesized that A2A-GluN1−/− mice display a high-anxiety-like phenotype and proceeded to assess anxiety- and depressive-like behaviors in more targeted assays.
Figure 5. Anxiety-like behavior assays.
(A) Time spent in center of arena during first open field assay in control (black circles), D1-GluN1−/− (red triangles), A2A-GluN1−/− (blue squares), or D1/A2A-GluN1−/− (purple triangles) mice (different from control, *: p<0.05, Bonferroni post-test). (B) Time spent in open arms during elevated plus maze. (C) Total distance traveled during elevated maze session. (D) Cumulative distribution of latency to consume palatable drink during novelty-induced hypophagia test session. (E) Total amount of drink consumed (normalized to bodyweight) during test session.
3.4. Elevated plus maze and novelty-induced hypophagia in GluN1 deletion models
To examine anxiety-like behavior, we first utilized the elevated plus maze (EPM). The EPM takes advantage of the innate tendency of rodents to avoid open spaces. No significant differences in open arm time during the EPM session were observed across genotypes (F(3,45) = 0.8393, n.s.) (Figure 5B): control and all GluN1−/− mice also spent similar amounts of time in the closed arms (58%, control: 176 ± 7 s, D1: 192 ± 11 s, A2A: 164 ± 15 s, D1/A2A: 188 ± 6 s; F(3,45) = 1.386, n.s.; data not shown). No significant differences in locomotor activity during the EPM session were observed across genotypes (F(3,45) = 2.163, n.s., Figure 5C). As a complimentary assessment of anxiety-like behavior, we examined novelty-induced hypophagia (NIH). We employed a version of NIH in which non-food restricted mice must approach a highly palatable drink in a novel environment, and the latency to consume the liquid is taken as a measure of anxiety-like behavior. A Mantel-Cox log-rank test determined that control and all GluN1−/− mice exhibited similar latency distributions (χ2 = 5.281, df = 3, n.s., Figure 5D). Furthermore no difference in mean latency (control: 164 ± 22 s, D1: 136 ± 31s, A2A: 120 ± 13s, D1/A2A: 196 ± 29 s; F(3,43) = 2.119, n.s.) or overall drink consumption was observed (F(3,43) = 1.617, n.s., Figure 5E). Neither specific assessment of anxiety-like behavior revealed any genotypic differences, therefore we conclude that the center time phenotype is not related to an anxiogenic state, and instead reflects aberrant locomotor behavior.
3.5. Tail suspension and forced swim tests in GluN1 deletion models
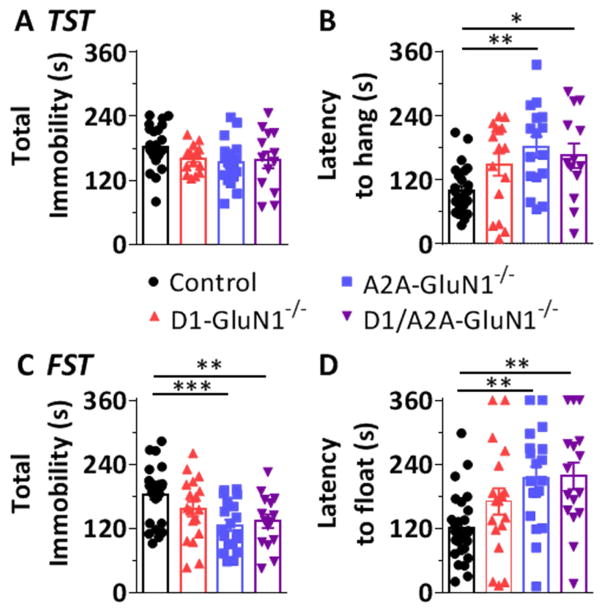
We performed the tail suspension and forced swim tests, models of behavioral despair. In each of these tests, mice attempt to deliver themselves from compromising, yet inescapable positions. The animals eventually assume an immobile posture, and the latency to hang/float is taken as a behavioral correlate of despair. No differences in immobility between controls and any GluN1−/− mice were observed in the TST when measured in total (F(3,69) = 2.129, n.s., Figure 6A). However a significant effect of genotype on the latency to immobility was observed (F(3,67) = 5.415, p < 0.05, Figure 6B), and post-tests revealed an increased mean latency in the A2A-GluN1−/− mice (181 ± 20 s vs control: 100 ± 9 s, p < 0.01). Under conditions of duress in the FST (Solich et al., 2008), significant genotypic differences in total immobility were observed (F(3,75) = 5.722, p < 0.01, Figure 6C). Specifically, A2A-GluN1−/− mice exhibited a decrease in total immobility (125 ± 10 s vs control: 183 ± 10 s, p < 0.001) consistent with an increased mean latency to float (214 ± 21 s vs control: 121 ± 12 s, p < 0.01, Figure 6D). In each of these tests and measures, D1-GluN1−/− mice did not differ from controls (TST total: 161 ± 6 s, latency: 149 ± 21 s; FST total: 156 ± 14 s, latency: 170 ± 25 s). Interestingly, D1/A2A-GluN1−/− mice behaved similarly to A2A-GluN1−/− mice and different from controls on all measures. That is, restoring striatal NMDAR balance did not rescue the A2A-GluN1−/− phenotype: D1/A2A-GluN1−/− mice exhibited an increased latency to hang during the TST (165 ± 23 s), p < 0.05 and to float during the FST (218 ± 25 s, P < 0.01), as well as decreased total immobility during the FST (133 ± 13 s, p < 0.01).
Figure 6. Behavioral despair assays.
(A) Total time spent immobile during tail suspension in control (black circles), D1-GluN1−/− (red triangles), A2A-GluN1−/− (blue squares), or D1/A2A-GluN1−/− (purple triangles) mice. (B) Latency to hang immobile for 10 s (different from control, *: p<0.05, **: p<0.01, Bonferroni post-test). (C) Total time spent immobile during forced swim test (different from control, **: p<0.01, ***: p<0.001, Bonferroni post-test). (D) Latency to float immobile for 10 s (different from control, **: p<0.01 Bonferroni post-test).
4. Discussion
Striatal NMDAR signaling has been implicated in the pathophysiology of drug addiction and other psychiatric diseases with motivational or affective components (Beutler et al., 2011; Cahill et al., 2014; Lim et al., 2012; Pascoli et al., 2014; Schwartz et al., 2014). In this report, we demonstrate separable roles for cell type-specific NMDAR function in the regulation of complex behaviors. These data support the hypothesis that balanced signaling across MSN subtypes regulates some locomotor-dependent behaviors, while providing further evidence that a more complex model is required to describe reward-related and depressive-like outcomes.
We observed no effect of any genotype on the acquisition of cocaine CPP. These findings may appear to disagree with published literature, where similar impairments of NMDAR function resulted in blunted psychostimulant CPP (Beutler et al., 2011; Heusner and Palmiter, 2005), however specific methodological differences (i.e. background strain, apparatus, and drug) can readily explain minor discrepancies. To summarize, the published accounts and present data collectively demonstrate that a moderate dose of cocaine does not require D1-NMDAR function to generate reward learning. We did observe that D1-GluN1−/− mice exhibited a stark impairment of drug-primed reinstatement of cocaine CPP. These data are in accordance with recent findings, where putative NMDAR-dependent modifications on NAc D1 MSNs were demonstrated to be essential for cue-induced reinstatement of cocaine seeking (Pascoli et al., 2014). Our data corroborate the mechanistic underpinnings of those findings and support the importance of D1 MSN NMDARs in drug-primed reinstatement. Moreover we observed that dual D1/A2A-GluN1−/− mice retained cocaine-primed reinstatement, suggesting that balanced NMDAR signaling across striatal projection neurons may underlie this phenomenon. On the other hand, the dual knockout mice did exhibit blunted reinstatement relative to control mice, suggesting that NMDARs in extra-striatal D1-expressing neurons may contribute to drug-primed reinstatement. Consistently, Sanchez and Sorg (Sanchez et al., 2003) demonstrated that reinstatement of cocaine CPP was blocked by infusion of a D1 antagonist into the PFC. D1-expressing neurons in the orbitofrontal cortex (Capriles et al., 2003; Lasseter et al., 2013) and amygdala (Alleweireldt et al., 2006; Erb et al., 2001; Mashhoon et al., 2009) represent other intriguing candidate substrates.
To assess movement-based adaptive learning, we examined locomotor activity in a novel environment. D1-GluN1−/− mice displayed a substantial attenuation of hyperactivity during the first five minutes, and did not habituate normally as evidenced by increased activity after 50 minutes. During the same task, D1-GluN1−/− mice displayed a robust increase in vertical counts, which have been described as a form of exploratory behavior (Fonio et al., 2009). Neither of these locomotor phenotypes were present in D1/A2A-GluN1−/− mice, supporting the conclusion that balanced MSN NMDAR signaling regulates exploration and habituation. We also observed that A2A-GluN1−/− mice spent less time in the center of the arena than controls. Since thigmotactic behavior can be indicative of an anxiety-like phenotype, we followed up with more targeted assays. Contrary to our working hypothesis, A2A-GluN1−/− mice performed similarly to controls on both the EPM and NIH. These two independent assays suggest that genetic deletion of NMDARs does not generate an anxiety-like state, We conclude that A2A-GluN1−/− mice display atypical patterns of horizontal locomotion (i.e. thigmotaxis) that are not related to anxiety. For example, certain drugs including MDMA, are known to induce perseverative thigmotaxis (Gold et al., 1988; Powell et al., 2004; Risbrough et al., 2006) without having any demonstrated inherent anxiogenic effects. We hypothesize that more rigorous assessments of movement patterns (for example, see Paulus and Geyer, 1991) would reveal subtle differences in unconditioned locomotion in A2A-GluN1−/− mice.
Since depressive symptoms are often comorbid with drug abuse and addiction (Joffe et al., 2014; Volkow, 2010), we assessed behavioral despair in two parallel assays, the TST and the FST. Due to previously observed genotype-sex interactions in these behaviors (Kokras and Dalla, 2014) we ran separate cohorts of male and female mice. We observed similar results in both sexes: A2A-GluN1−/− mice displayed a prolonged latency to immobility in both assays and increased total immobility in the FST. These findings suggest that A2A-GluN1−/− mice exhibit a despair-resistant or “resilient” phenotype. Ionotropic glutamate receptor signaling in the NAc has been heavily implicated in depressive-like behaviors (Bagot et al., 2015; Francis et al., 2014; Lim et al., 2012; Robison et al., 2014; Schwartz et al., 2014; Vialou et al., 2010). Our data suggest that these characterized physiological processes may proceed largely through A2A MSNs, consistent with recent findings that the development of amotivation induced by chronic pain proceeds through NMDAR signaling on NAc A2A MSNs (Schwartz et al., 2014). Together these data support the hypothesis that A2A MSN NMDARs signaling promotes depressive-like behavior. Conversely, the antidepressant efficacy of fluoxetine requires downregulation of the NMDAR signaling partner, CaMKII (Robison et al., 2005; Shonesy et al., 2014). Whether changes to A2A MSN NMDAR signaling are required for the behavioral efficacy of SSRIs and other antidepressants remains untested and merits further study. Interestingly D1/A2A-GluN1−/− mice also displayed the anti-despair phenotype exhibited by A2A-GluN1−/− mice. Unlike the locomotor-based assays, these results suggest that A2A MSNs contribute to despair-like behavior in a manner that cannot be distilled to balanced MSN NMDAR signaling. One intriguing possibility is that A2A MSNs, or their targets, project to a region outside of the canonical basal ganglia that modulates negative affective behaviors.
If indeed striatal balance is required for the expression of some psychostimulant and novelty-related behaviors, it begs the question: why did A2A-GluN1−/− mice not display unusual drug-related behaviors? We propose two, non-exclusive explanations. For one, from a practical perspective, we may have encountered ceiling effects in the selected classical conditioning paradigms. Operant behavioral paradigms would be expected to provide a greater signal window for detecting increases in drug reinforcement and susceptibility to relapse. Accordingly, following chemogenetic inhibition of NAc A2A MSNs, Bock et al. observed enhanced motivation to obtain cocaine as assessed by lever-pressing behavior. Alternatively, following a sub-threshold priming dose or stressor, A2A-GluN1−/− mice might reinstate CPP under conditions where control mice would not.
The second explanation is that, for drug-related behaviors, the striatal balancing act may not be bidirectional. That is, changes in the A2A pathway may have little effect on drug-conditioned behaviors due to absence of subsequent circuit-level changes. For one, NMDARs may not generate the most prominent form of synaptic plasticity on A2A MSNs. In the NAc core and dorsal striatum, endocannabinoid (eCB)-mediated plasticity has been documented on A2A MSNs, whereas it is absent on D1 MSNs (Grueter et al., 2010; Kreitzer and Malenka, 2007). Cocaine-induced synaptic modifications may therefore proceed preferentially through NMDARs on D1 MSNs but through eCB signaling on A2A MSNs. Additionally, A2A MSNs may not play a major role in psychostimulant-related behaviors due to anatomy. A2A NAc MSNs send inhibitory projections primarily to the ventral pallidum (VP), but the VP also receives substantial innervation from D1 NAc MSNs (Kupchik et al., 2015). These projections may lie such that following psychostimulant administration, D1 MSN activity dominates the circuit-level logic and precludes small changes in A2A MSN function from being observed at the behavioral level. An exciting hypothesis is that VP neurons receiving projections from both MSN classes are involved in stimulant/locomotor-based behaviors, while those innervated strictly by A2A MSNs are involved in affective behaviors. Future experiments will need to be directed towards the targets of these A2A MSNs to examine the mechanistic basis of responses to stressful and reward-related stimuli. A combination of biochemistry and slice and in vivo electrophysiology, would be ideal to uncover how NMDAR signaling in each MSN population guides behavioral outcomes at the circuit level.
Supplementary Material
Figure S1. Locomotor response to cocaine. Distance traveled in the last cocaine conditioning session by control (black circles), D1-GluN1−/− (red triangles), A2A-GluN1−/− (blue squares), or D1/A2A-GluN1−/− (purple triangles) mice. D1-GluN1−/− exhibited attenuated locomotor response to cocaine relative to controls (different from control, *: p<0.05, Bonferroni post-test).
Figure S2. Extinction of cocaine conditioned place preference. The change in time spent on the cocaine-paired side was measured during a 4-day extinction protocol in control (black), D1-GluN1−/− (red), A2A-GluN1−/− (blue), or D1/A2A-GluN1−/− (purple) mice. No differences in extinction learning were observed.
Highlights.
Acquisition of cocaine CPP remains intact in D1-GluN1−/− and A2A-GluN1−/− mice
D1-GluN1−/− mice do not reinstate CPP following cocaine challenge
D1-GluN1−/− mice display abnormal exploration; rescued by A2A-GluN1−/− co-deletion
A2A-GluN1−/− mice and D1/A2A-GluN1−/− mice resist behavioral despair
Acknowledgments
We thank Christopher Olsen, Ph.D. and Patrick Rothwell Ph.D. for comments and Robert Malenka M.D., Ph.D. for providing the mouse lines. CPP, EPM, and the open field test were performed in part through the use of the Murine Neurobehavior Core lab at the Vanderbilt University Medical Center. We thank Katherine Holleran of Vanderbilt University for assistance with the NIH assay. This study was supported by National Institute on Drug Abuse R00DA031699 (B.A.G.).
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31:363–374. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Bagot RC, Parise EM, Peña CJ, Zhang HX, Maze I, Chaudhury D, Persaud B, Cachope R, Bolaños-Guzmán Ca, Cheer J, Deisseroth K, Han MH, Nestler EJ. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler LR, Wanat MJ, Quintana A, Sanz E, Bamford NS, Zweifel LS, Palmiter RD. Balanced NMDA receptor activity in dopamine D1 receptor (D1R)- and D2R-expressing medium spiny neurons is required for amphetamine sensitization. PNAS. 2011;108:4206–11. doi: 10.1073/pnas.1101424108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, Shen H, Kalivas PW, Sorg BA, Zukin RS, Nestler EJ, Dong Y, Schlüter OM. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31:8163–74. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill E, Pascoli V, Trifilieff P, Savoldi D, Kappès V, Lüscher C, Caboche J, Vanhoutte P. D1R/GluN1 complexes in the striatum integrate dopamine and glutamate signalling to control synaptic plasticity and cocaine-induced responses. Mol Psychiatry. 2014:1–10. doi: 10.1038/mp.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328X(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Fonio E, Benjamini Y, Golani I. Freedom of movement and the stability of its unfolding in free exploration of mice. PNAS. 2009;106:21335–21340. doi: 10.1073/pnas.0812513106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis T, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, Brooks JM, Iñiguez SD, O’Donnell P, Kravitz A, Kay Lobo M, Chase Francis T, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, Brooks JM, Iñiguez SD, O’Donnell P, Kravitz A, Kay Lobo M. Nucleus Accumbens Medium Spiny Neuron Subtypes Mediate Depression-Related Outcomes to Social Defeat Stress. Biol Psychiatry. 2014;77:1–10. doi: 10.1016/j.biopsych.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa G, Espallergues J, de Kerchove d’Exaerde A, El Mestikawy S, Gerfen CR, Hervé D, Girault JA, Valjent E. Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front Neural Circuits. 2013;7:22. doi: 10.3389/fncir.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxyamphetamine in rats. J Pharmacol Exp Ther. 1988;247:547–55. [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–25. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. ΔFosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci U S A. 2013;110:1923–8. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol. 2012;22:1–7. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusner CL, Palmiter RD. Expression of mutant NMDA receptors in dopamine D1 receptor-containing cells prevents cocaine sensitization and decreases cocaine preference. J Neurosci. 2005;25:6651–6657. doi: 10.1523/JNEUROSCI.1474-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Grueter CA, Grueter BA. Biological substrates of addiction. Wiley Interdiscip Rev Cogn Sci. 2014;5:151–171. doi: 10.1002/wcs.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- Karler R, Calder LD, Chaudhry IA, Turkanis SA. Blockade of “reverse tolerance” to cocaine and amphetamine by MK-801. Life Sci. 1989;45:599–606. doi: 10.1016/0024-3205(89)90045-3. [DOI] [PubMed] [Google Scholar]
- Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. Br J Pharmacol. 2014:1–58. doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–6. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–8. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–54. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–7. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–1233. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Arguello AA, Wells AM, Hodges MA, Fuchs Ra. Contribution of a Mesocorticolimbic Subcircuit to Drug Context-Induced Reinstatement of Cocaine-Seeking Behavior in Rats. Neuropsychopharmacology. 2013;39:660–669. doi: 10.1038/npp.2013.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–9. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science (80- ) 2010;330:385–90. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louderback KM, Wills TS, Muglia LJ, Winder DG. Knockdown of BNST GluN2B-containing NMDA receptors mimics the actions of ketamine on novelty-induced hypophagia. Transl Psychiatry. 2013;3:e331. doi: 10.1038/tp.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–80. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate Ca, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular Mechanisms Underlying the Antidepressant Effects of Ketamine: Role of α-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Mashhoon Y, Tsikitas LA, Kantak KM. Dissociable effects of cocaine-seeking behavior following D1 receptor activation and blockade within the caudal and rostral basolateral amygdala in rats. Eur J Neurosci. 2009;29:1641–1653. doi: 10.1111/j.1460-9568.2009.06705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Lüscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–64. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology (Berl) 1991;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- Powell SB, Lehmann-Masten VD, Paulus MP, Gainetdinov RR, Caron MG, Geyer MA. MDMA “ecstacy” alters hyperactive and perseverative behaviors in dopamine transporter knockout mice. Psychopharmacology (Berl) 2004;173:310–317. doi: 10.1007/s00213-003-1765-7. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer Ma. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Bartlett RK, Bass MA, Colbran RJ. Differential modulation of Ca2+/calmodulin-dependent protein kinase II activity by regulated interactions with N-methyl-D-aspartate receptor NR2B subunits and ? ?-actinin J Biol Chem. 2005;280:39316–39323. doi: 10.1074/jbc.M508189200. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Vialou V, Sun HS, Labonte B, Golden SA, Dias C, Turecki G, Tamminga C, Russo S, Mazei-Robison M, Nestler EJ. Fluoxetine Epigenetically Alters the CaMKIIα Promoter in Nucleus Accumbens to Regulate ΔFosB Binding and Antidepressant Effects. Neuropsychopharmacology. 2014;39:1178–86. doi: 10.1038/npp.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA. Manipulation of dopamine D1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience. 2003;119:497–505. doi: 10.1016/S0306-4522(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Vanderhaeghen JJ. Adenosine A2 receptors regulate the gene expression of striatopallidal and striatonigral neurons. J Neurosci. 1993;13:1080–1087. doi: 10.1523/JNEUROSCI.13-03-01080.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC. Chronic pain. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science (80- ) 2014;345:535–542. doi: 10.1126/science.1253994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonesy BC, Jalan-Sakrikar N, Cavener VS, Colbran RJ. CaMKII: A molecular substrate for synaptic plasticity and memory. Prog Mol Biol Transl Sci. 2014;122:61–87. doi: 10.1016/B978-0-12-420170-5.00003-9. [DOI] [PubMed] [Google Scholar]
- Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci. 2008;28:2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solich J, Pałach P, Budziszewska B, Dziedzicka-Wasylewska M. Effect of two behavioral tests on corticosterone level in plasma of mice lacking the noradrenaline transporter. Pharmacol Reports. 2008;60:1008–1013. doi: 10.1016/S0924-977X(08)70045-2. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/S0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, Watts EL, Wallace DL, Iñiguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolaños CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND. Comorbidity: Addiction and Other Mental Illnesses 2010 [Google Scholar]
- Wolf ME, Khansa MR. Repeated administration of MK-801 produces sensitization to its own locomoter stimulant effects but blocks sensitization to amphetamine. Brain Res. 1991;562:164–168. doi: 10.1016/0006-8993(91)91202-C. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Grandy DK, Thambi L, Kushner JA, Van Tol HH, Cone R, Pribnow D, Salon J, Bunzow JR, Civelli O. Cloning and expression of human and rat D1 dopamine receptors. Nature. 1990;347:76–80. doi: 10.1038/347076a0. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci APR. Role of NMDA Receptors in Dopamine Neurons for Plasticity and Addictive Behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Locomotor response to cocaine. Distance traveled in the last cocaine conditioning session by control (black circles), D1-GluN1−/− (red triangles), A2A-GluN1−/− (blue squares), or D1/A2A-GluN1−/− (purple triangles) mice. D1-GluN1−/− exhibited attenuated locomotor response to cocaine relative to controls (different from control, *: p<0.05, Bonferroni post-test).
Figure S2. Extinction of cocaine conditioned place preference. The change in time spent on the cocaine-paired side was measured during a 4-day extinction protocol in control (black), D1-GluN1−/− (red), A2A-GluN1−/− (blue), or D1/A2A-GluN1−/− (purple) mice. No differences in extinction learning were observed.