Abstract
Background
Under-recognition of angina by physicians may result in under-treatment with revascularization or medications that could improve patients’ quality of life. We sought to describe characteristics associated with under-recognition of patients’ angina.
Methods and Results
Patients with coronary disease from 25 US cardiology outpatient practices completed the Seattle Angina Questionnaire (SAQ) prior to their clinic visit, quantifying their frequency of angina over the prior month. Immediately following the clinic visit, physicians independently quantified their patients’ angina. Angina frequency was categorized as none, monthly, and daily/weekly. Among 1257 patients, 411 reported angina in the prior month, of whom 173 (42%) were under-recognized by their physician, defined as the physician reporting a lower frequency category of angina than the patient. In a hierarchical logistic model, heart failure (OR 3.06, 95% CI 1.89-4.95) and less frequent angina (OR for monthly angina [vs. daily/weekly] 1.69, 95% CI 1.12-2.56) were associated with greater odds of under-recognition. No other patient or physician factors were associated with under-recognition. Significant variability across physicians (MOR 2.06) was observed.
Conclusions
Under-recognition of angina is common in routine clinical practice. While patients with less frequent angina and those with heart failure more often had their angina under-recognized, most variation was unrelated to patient and physician characteristics. The large variation across physicians suggests that some physicians are more accurate in assessing angina frequency than others. Standardized prospective use of a validated clinical tool, such as the SAQ, should be tested as a means to improve recognition of angina and, potentially, improve appropriate treatment of angina.
Keywords: angina, coronary artery disease, quality of care
Chronic angina is exceedingly common1 and substantially worsens patients’ quality of life 2, 3. Furthermore, while physicians often focus on managing ischemia, it is the patient-reported symptoms of angina that drives healthcare utilization 4. A unique feature of angina is that laboratory and imaging tests cannot measure it. Instead, effective history taking by the physician is required to quantify the patient's burden of angina and guide diagnostic and management decisions. As such, the evaluation of angina is subject to limitations inherent in history taking, including pre-existing biases and time constraints on the part of physicians and patients 5.
We quantified this difficulty by asking patients with documented coronary artery disease to complete the Seattle Angina Questionnaire—a patient-reported standardized assessment of angina burden over the prior 4 weeks—and then compared responses with clinical impressions of their cardiologist after the visit. While physicians effectively recognized the absence of angina among patients who reported being asymptomatic, we found that 25% patients who reported daily or weekly angina were thought to be chest pain free by their physician 6.
Under-recognition of angina by physicians could affect patients’ quality of life (by failing to intensify antianginal treatment) as well as increase costs to the healthcare system (due to increased hospital admissions) 4. Variability in physician assessment of angina has been described 7, 8; however, the patient and physician factors that contribute to under-recognition are unknown. Understanding those factors that drive under-recognition could support efforts to improve physicians’ recognition and therefore effective treatment of angina.
Methods
Study Protocol and Population
We enrolled consecutive patients with a diagnosis of ischemic heart disease (defined as stable angina, prior myocardial infarction, prior percutaneous coronary intervention, or prior coronary artery bypass graft surgery) seen in cardiology practices in the Angina Prevalence and Provider Evaluation of Angina Relief (APPEAR) study. APPEAR was a cross-sectional observational study designed to assess the frequency of angina and its impact on quality of life among outpatients with coronary artery disease. US outpatient cardiology practices that are currently participating in the PINNACLE registry (a national practice-based cardiovascular quality improvement registry sponsored by the American College of Cardiology National Cardiovascular Data Registry) were invited to participate in APPEAR. We selected cardiology practices for analysis as we believed that this would represent the best-case scenario when physicians are assessing chest pain in routine practice. Patients were then recruited from the 25 participating practices between April 2013 and July 2015 (Supplemental Figure 1, Supplemental Table 1). For 1-2 weeks of active enrolment per site, local study coordinators recruited 25-50 consecutive adults with a history coronary artery disease, irrespective of the reason for their appointment.
In order to be included in the study, patients had to be ≥18 years of age, have coronary artery disease, and have had at least 1 prior office visit to the practice. Patients who declined participation, had dementia, were unable to speak or read English, and were prisoners were excluded. For this specific analysis, as we were interested in under-recognition of angina by physicians, we included only patients who reported some angina in the prior 4 weeks. Clinical data were collected from chart abstraction, while patients’ assessments of their angina was captured immediately prior to the visit, and physicians’ reports of angina frequency were collected immediately after the clinic visit. Each participating site obtained Institutional Research Board approval, and all patients provided written informed consent.
Patient-Reported and Physician-Assessed Angina
Prior to the visit with the cardiologist, patients were asked to complete a questionnaire that included socio-demographic characteristics and patient-reported health status measures. Patients completed the Seattle Angina Questionnaire (SAQ), a 19-item self-administered reliable and valid questionnaire that measures 5 dimensions of health in patients with coronary artery disease 9, 10. The SAQ has a 4-week recall period, and domain scores range from 0 to 100, with higher scores indicating less disease burden. The primary domain of interest for our study was the SAQ angina frequency domain, which has been shown to correlate well with patient-reported daily diaries of angina 11. To facilitate clinical interpretability, scores were categorized, congruent with prior work, as none (SAQ score=100), monthly (SAQ score=61-99), weekly (SAQ score=31-60), and daily (SAQ score=0-30) 12.
Immediately after the visit, physicians were asked: “In the past 4 weeks, has the patient had chest pain, angina or angina-equivalent symptoms?” If yes, the physician was then asked to describe the character of the patient's chest pain (typical angina, atypical angina, non-cardiac chest pain), frequency (daily, weekly, monthly, less than monthly), location (chest, arm, back, neck, stomach, shoulder, leg, jaw, other), associated symptoms (dyspnea, nausea, confusion, light-headedness, sweating, other), and whether the symptoms were provoked by exertion or by emotional stress and if they were relieved by rest or by short-acting nitrates.
Definition of Physician Under-recognition
The SAQ-generated categories of none, monthly, or daily/weekly angina were used to represent the patients’ reports of their angina frequency and the physicians’ documentation of angina frequency was used to quantify the physicians’ perceptions of angina frequency. Using these categories, under-recognition was defined as the physician rating the patient's angina at a lower frequency category than what the patient reported.
Statistical Analysis
Demographic and clinical characteristics were compared between patients who were and were not under-recognized using t-tests for continuous variables and chi-square tests or Fisher's exact test for categorical variables. We then constructed a hierarchical logistic model to examine patient and physician factors associated with under-recognition by the physician. Variables for the model were selected a priori based on clinical judgement. Patient factors included age, sex, race, self-reported avoidance of care due to costs, chronic lung disease, chronic heart failure, diabetes mellitus, history of coronary artery bypass graft surgery, and category of angina frequency. Physician-level variables included sex and years since finishing cardiology training. Both physician and site were included as random effects, to account for patient clustering within physician and within site. Non-linear spline terms were tested for all continuous variables, but none were significant and were therefore not retained in the model. Physician-level variability was explored with a median odds ratio (MOR), which estimates the average relative difference in 2 hypothetical patients being under-recognized if seen by 2 different physicians. Also, we calculated the intraclass correlation coefficient, which estimates the proportion of the total variance in under-recognition that is accounted for by physician variation (as compared with patient factors).13 The intraclass correlation coefficient was calculated from the variance components of the hierarchical logistic regression model; this quantifies variation in an underlying continuous latent variable representing the propensity for recognition of patients’ angina.
Goodness of fit of the model was examined with the c-index (to test discrimination) and by plotting predicted versus observed values and comparing these against the line y=x (to test calibration). Collinearity was determined not significant as the variance inflation factor was ≤1.20 for all variables and the condition index was <30. Baseline data had a high rate of completion, with only 2 patients missing 1 data element, which were estimated using a single imputation dataset (IVEware; Institute for Social Research, University of Michigan, Ann Arbor, MI). All statistical analyses were performed with SAS, version 9.4 (SAS Institute, Inc., Cary, NC) and R version 3.2.0 14.
Results
Study Population
APPEAR enrolled 1257 patients, of whom 411 patients (32.6%) reported angina in the prior month and formed the analytic cohort. The mean age of the analytic cohort was 69.0 years, 60.3% were male, 91.4% were white, and 14.1% were current smokers at the time of their clinic visit. A history of prior myocardial infarction was noted in 38.2%, prior coronary stenting in 57.2%, and prior bypass graft surgery in 31.6%. Among the patients who reported having some angina in the prior month, daily angina was reported in 4.6% of patients, weekly angina in 18.7%, and monthly angina in 76.6%. Most patients were on 1 medication that reduces angina, which was most often a beta-blocker.
There were 155 cardiologists from 25 sites located in 19 US states who participated in APPEAR (Supplemental Figure 1, Supplemental Table 1). After excluding patients who reported no angina in the month prior to their clinic visit, the physician pool was reduced to 121 cardiologists. Each physician saw a median of 5 patients who reported angina, with a range of 1-21, and 26 physicians contributed 5 or more patients to the analytic cohort. Physicians were mostly male (83.5%) and had practiced for a median of 18 years (IQR 10-25).
Rate and Predictors of Under-recognition
Among the 411 patients who reported angina in the prior month, 173 (42.1%) were under-recognized by their physician. Demographic and clinical characteristics of the patients who were and were not under-recognized are shown in Table 1. Patients whose angina was under-recognized (vs. not) were more likely to have a diagnosis of chronic heart failure (26.0% vs. 9.2%, p<0.001), have lower burdens of angina (monthly angina: 82.7% vs. 72.3%, p=0.01), and were on fewer antianginal medications (31.2% vs. 45.8% were taking ≥2 antianginal medications, p=0.002). In a multivariable model, chronic heart failure (OR 3.06, 95% CI 1.89-4.95) and monthly angina (vs. weekly/daily: OR 1.69, 95% CI 1.12-2.56) were independently associated with a greater odds of under-recognition (Table 2). Age, sex, race, socioeconomic status, and other comorbidities, importantly, were not associated with under-recognition of the patient's angina by the physician.
Table 1.
Baseline characteristics according to recognition of angina during clinic visit
| Under-recognized n=173 | Recognized n=238 | p-value | |
|---|---|---|---|
| Patient factors | |||
| Age (y) | 68.9±12.3 | 69.0±11.2 | 0.916 |
| Male sex | 61.8% | 59.2% | 0.593 |
| White race | 89.2% | 92.9% | 0.198 |
| Insurance for medications | 95.3% | 97.0% | 0.360 |
| Avoidance of care due to cost | 1.7% | 1.2% | 0.374 |
| High school education | 83.9% | 87.4% | 0.326 |
| Hypertension | 83.2% | 77.7% | 0.167 |
| Chronic heart failure | 26.0% | 9.2% | <0.001 |
| Prior myocardial infarction | 43.9% | 34.0% | 0.041 |
| Prior coronary stenting | 56.6% | 57.6% | 0.853 |
| Prior bypass graft surgery | 31.2% | 31.9% | 0.877 |
| Diabetes mellitus | 39.9% | 37.0% | 0.548 |
| Chronic lung disease | 9.2% | 13.9% | 0.153 |
| Chronic kidney disease | 15.6% | 9.7% | 0.068 |
| Smoking status | 0.484 | ||
| Current | 13.5% | 14.6% | |
| Former | 56.7% | 60.9% | |
| Never | 29.8% | 24.5% | |
| Number of antianginal medications | 0.002 | ||
| 0 | 8.1% | 10.9% | |
| 1 | 60.7% | 43.3% | |
| ≥2 | 31.2% | 45.8% | |
| Class of antianginal medications | |||
| Beta-blocker | 82.7% | 78.2% | 0.258 |
| Calcium channel blocker | 22.7% | 28.2% | 0.211 |
| Long-acting nitrate | 20.8% | 31.1% | 0.020 |
| Ranolazine | 6.4% | 17.6% | <0.001 |
| Patient-reported angina | 0.015 | ||
| Daily | 1.7% | 6.7% | |
| Weekly | 15.6% | 21.0% | |
| Monthly | 82.7% | 72.3% |
Table 2.
Association of patient and physician characteristics with under-recognition of the patient's angina by the physician
| Odds Ratio (95% CI) | p-value | |
|---|---|---|
| Patient factors | ||
| Age (per 5 years) | 1.00 (0.93-1.08) | 0.973 |
| Male sex | 1.13 (0.80-1.59) | 0.499 |
| White race | 0.63 (0.39-1.04) | 0.070 |
| Self-reported avoidance of care due to cost | 1.48 (0.51-4.28) | 0.464 |
| Chronic lung disease | 0.61 (0.34-1.09) | 0.093 |
| Chronic heart failure | 3.06 (1.89-4.95) | <0.001 |
| Diabetes mellitus | 1.02 (0.73-1.44) | 0.891 |
| Prior CABG | 0.98 (0.68-1.42) | 0.922 |
| Monthly angina (vs. daily/weekly angina) | 1.69 (1.12-2.56) | 0.012 |
| Physician factors | ||
| Male sex | 0.66 (0.33-1.35) | 0.258 |
| Years of practice | 0.99 (0.97-1.02) | 0.678 |
| Median odds ratio* | 2.06 | 0.001 |
Assessment of variability across physicians. Confidence intervals are not appropriate, due to the nature of the model.
c-index=0.67. Calibration plot R2=0.91
Physician Factors and Variability
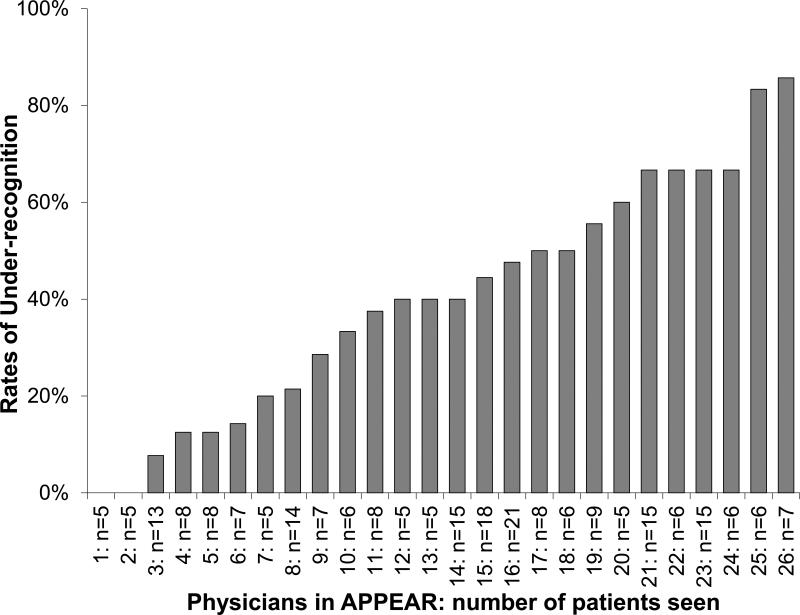
Neither the sex of the physician nor the number of years of experience of the physician were associated with better recognition of angina, either in univariable or multivariable analyses (Table 2). Typical symptom presentations were not the only symptoms that were recognized. Among patients whose angina was appropriately recognized, physicians reported that these patients had a wide range of presenting symptoms, with fewer than half presenting with typical angina (Supplemental Table 2). There was substantial variability in under-recognition across physicians, indicating that while patient demographics, comorbidities, and presenting symptoms largely were not associated with better recognition, some physicians were much better at recognizing the frequency of patients’ angina than others. In the model that accounted for patient and physician factors, the MOR was 2.06, indicating that, on average, 2 statistically identical patients would have a 2-fold greater odds of having their angina under-recognized if seen by 1 random physician as compared with another. Furthermore, the amount of variability in under-recognition that could be attributed to physician-level variation (as opposed to patient-level differences), as measured with the intraclass correlation coefficient, was 15%. Among the 26 physicians who saw 5 or more patients who reported angina in the month prior to their clinic visit, the rate of under-recognition ranged broadly from 0-86% (Figure 1).
Figure 1.
Rates of under-recognition by physician. Includes only physicians who contributed 5 or more patients to the analytic cohort
Discussion
In a multicenter, cross-sectional sample of patients with coronary artery disease, we found that under-recognition of angina was common in routine clinical practice. Furthermore, there was large variation in the rates of under-recognition across physicians but few patient factors associated with under-recognition. These data underscore that a more systematic approach is needed for eliciting a history and assessing angina in patients with coronary artery disease. As the physician's assessment of angina is key in guiding further testing and treatment, under-recognition of the patient's burden of angina could result in under-treatment. The use of a validated, patient-centered tool for eliciting patients’ angina, such as the SAQ, should be tested in routine clinical care to see if it improves angina recognition, treatment, and outcomes.
Prior Studies
Prior studies that have compared physician-reported and patient-reported angina also found discrepancies. Among 2031 stable angina patients treated at 207 primary care practices, physicians rated 61% of patients as having minimal angina, of whom 20% self-reported weekly and 12% reported daily angina 7. This discrepancy has also been observed in clinical trials. At 1-year follow-up in the Stent or Surgery trial, investigators systematically underestimated their subjects’ symptom burden, often rating a patient as angina-free when the patient was not 8. Interestingly, prior to coronary revascularization, physicians were more likely to over-estimate a patients’ burden of angina and misclassified angina in 63% (over-coding ~37%, under-coding ~26%). Even in this carefully conducted clinical trial, physicians were unable to accurately estimate the patients’ burden of angina. Our study supports these findings by demonstrating variation in the recognition of angina by cardiologists among patients with coronary artery disease and extends these studies by examining predictors of under-recognition and variability.
Implications and Future Directions
While an accurate estimation of angina is the goal, we focused on under-recognition, as we believe the implications are missed opportunities to intensify medical treatment. The burden of patient-reported chest pain (regardless of whether or not it is related to myocardial ischemia) is strongly and independently associated with quality of life, rehospitalization, and use of healthcare resources 4. It is certainly notable that not all chest pain reported by the patient is appropriately treated with antianginal medications or coronary revascularization, as the chest pain may be non-cardiac or the patient may be maximally treated (i.e., on maximally tolerated antianginal medications and no revascularization options). However, regardless of the decision of treatment escalation, we believe that it is important for the physician to understand the burden of chest pain experienced by the patient.
The routine assessment and documentation of patients’ symptoms was highlighted in 2011 by the American College of Cardiology/American Heart Association/Physician Consortium for Performance Improvement, which advocated for the routine use of patient-reported outcomes, such as the SAQ 15. In addition, the appropriateness of coronary revascularization for stable angina relies heavily on the assessment of the severity of patients’ angina 16. Moreover, the Center for Medicare and Medicaid Services has highlighted the importance of patients’ perceptions of their disease in quantifying quality and has begun developing performance measures based upon patient-reported outcomes 17. In fact, they are in the midst of developing a patient-reported outcome-based performance measure for percutaneous coronary intervention, and these data highlight the potential for this measure to more accurately assess patients’ angina than physicians’ reports 18.
Despite the importance of assessing angina burden in patients with coronary disease, we still routinely depend solely on an unstructured interview, instead of directly asking patients using standardized assessments. The primary reason for this likely lies in the barriers of implementing a patient-reported outcome into regular clinical care 19. However, our data highlight the limitations of relying on traditional physician/patient interactions to accurately assess angina burden. Moving patient-reported outcomes into routine clinical care requires creative implementation strategies to successfully integrate such measures into routine practice, including novel mechanisms to collect, score, and interpret patient-reported outcomes data. Towards that end, a shorter, 7-item version of the SAQ has been introduced that retained the entire angina frequency domain of the original instrument 20. Future studies should examine the feasibility of using such instruments as a means to improve recognition of angina in the outpatient setting and the impact that this may have on the treatment of angina and subsequent outcomes.
Limitations
Our data should be viewed in light of the following potential limitations. First, although we were able to examine a large number of patients and physicians across geographically diverse US practices, it is unclear if our results are generalizable to the entirety of US cardiologists’ clinics or to non-cardiologists. Furthermore, the number of patients with angina per physician was small, making examination of unadjusted variability in under-recognition rates limited. Second, the number of predictor variables were limited by the sample size in order to avoid over-fitting and finding spurious associations. We selected covariates a priori based on clinical judgement, but we may have omitted important predictors of under-recognition. In addition, the distribution of particular covariates in our analytic cohort (e.g., race) may have limited the identification of important patient predictors. Third, physicians were aware of the study and that they would be asked to estimate the patient's chest pain after the clinic visit. As such, we expect that our findings represent the best-case scenario, and the rates of under-recognition likely would be higher outside of the confines of a structured study. Fourth, some may argue that assessment of angina with the patient-reported SAQ may not be accurate. However, the SAQ has been extensively validated against daily angina diaries11 and shown to be reliable, valid, and predictive of future cardiac events 21, 22. It is likely to be the best method for identifying the frequency and implications of angina from patients’ perspectives. Finally, while we were able to identify the prevalence and predictors (or lack thereof) of under-recognition of angina in this cross-sectional study, we were unable to determine whether this under-recognition resulted in under-testing or under-treatment. Further research investigating the implications of under-recognition on treatment and outcomes is needed.
Conclusions
Angina is frequently under-recognized in routine clinical practice and rates of under-recognition varied widely across physicians. Given the importance of assessing patient's angina frequency in order to properly apply testing and treatment, these data support an assessment of angina directly from the patient. By incorporating standard tools, such as the SAQ-7, as routine clinical assessments during office visits, a more consistent recognition of angina may occur. Further work is needed to understand the implications of under-recognition on outcomes and how standardized assessments of angina impact both recognition and outcomes.
Supplementary Material
What Is Known
Angina is common among patients with stable coronary artery disease and requires an effective patient/physician interview to quantify this and guide diagnostic and management decisions.
What This Study Adds
Among patients with CAD in cardiology outpatient practices who reported angina in the prior month, 42% were believed to have less (or no) angina by their physician
Most of the variation in under-recognition was unrelated to patient and physician characteristics, but there was large variation across physicians, suggesting some physicians are more accurate in assessing angina than others.
These data underscore that a more systematic approach is needed for eliciting a history and assessing angina in patients with coronary artery disease in order to appropriately guide further testing and treatment.
The use of a validated, patient-centered tool for eliciting patients’ angina should be tested in routine clinical care to see if it improves angina recognition, treatment, and outcomes.
Acknowledgments
Funding Sources: The Angina Prevalence and Provider Evaluation of Angina Relief (APPEAR) study was supported by an investigator-initiated grant from Gilead Sciences. Dr. Grodzinsky was supported by a T32 training grant from the National Heart Lung and Blood Institute (T32HL110837).
Footnotes
Conflict of Interest Disclosures: MK reports speaker fees from the American Association of Clinical Endocrinology, American Diabetes Association, HealthSciences Media, Inc., Heartland Mid America Chapter of AACE, and R & R Healthcare Communications, Inc.; consultant honoraria from Abbvie, AstraZeneca , Edwards Life Sciences, Gilead Sciences, Roche, St. Jude Medical, Genentech, Regeneron, Lilly, and ZS Pharma; and research grants the American Heart Association, Genentech, Gilead Sciences, Glumetrics, Maquet, and Sanofi Aventis. JB reports consultant honoraria from Servier Laboratories. JAS reports research grants from theNHLBI, AHA, ACCF, Gilead, Lilly, EvaHeart, and Amorcyte; consultant honoraria from United Healthcare, Genentech, and Amgen; and copyright of the Seattle Angina Questionnaire.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics--2013 Update: A Report From the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown N, Melville M, Gray D, Young T, Munro J, Skene AM, Hampton JR. Quality of life four years after acute myocardial infarction: short form 36 scores compared with a normal population. Heart. 1999;81:352–358. doi: 10.1136/hrt.81.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brorsson B, Bernstein SJ, Brook RH, Werko L. Quality of life of patients with chronic stable angina before and four years after coronary revascularisation compared with a normal population. Heart. 2002;87:140–145. doi: 10.1136/heart.87.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold SV, Morrow DA, Lei Y, Cohen DJ, Mahoney EM, Braunwald E, Chan PS. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circ Cardiovasc Qual Outcomes. 2009;2:344–353. doi: 10.1161/CIRCOUTCOMES.108.829523. [DOI] [PubMed] [Google Scholar]
- 5.Goldman L, Hashimoto B, Cook EF, Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation. 1981;64:1227–1234. doi: 10.1161/01.cir.64.6.1227. [DOI] [PubMed] [Google Scholar]
- 6.Shafiq A, Arnold SV, Gosch K, Kureshi F, Breeding T, Jones PG, Beltrame J, Spertus JA. Patient and physician discordance in reporting symptoms of angina among stable coronary artery disease patients: Insights from the Angina Prevalence and Provider Evaluation of Angina Relief (APPEAR) study. Am Heart J. 2016;175:94–100. doi: 10.1016/j.ahj.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltrame JF, Weekes AJ, Morgan C, Tavella R, Spertus JA. The prevalence of weekly angina among patients with chronic stable angina in primary care practices: The Coronary Artery Disease in General Practice (CADENCE) Study. Arch Intern Med. 2009;169:1491–1499. doi: 10.1001/archinternmed.2009.295. [DOI] [PubMed] [Google Scholar]
- 8.Appleby C, Kemp I, Stables RH. Patient vs physician reported angina before and after revascularisation of coronary artery disease: evidence from a large randomised controlled trial (the SOS trial). Heart. 2011;97:A27–A28. [Google Scholar]
- 9.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–1244. doi: 10.1016/0002-9149(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 10.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 11.Arnold SV, Kosiborod M, Li Y, Jones PG, Yue P, Belardinelli L, Spertus JA. Comparison of the Seattle Angina Questionnaire With Daily Angina Diary in the TERISA Clinical Trial. Circ Cardiovasc Qual Outcomes. 2014;7:844–850. doi: 10.1161/CIRCOUTCOMES.113.000752. [DOI] [PubMed] [Google Scholar]
- 12.Spertus JA, Salisbury AC, Jones PG, Conaway DG, Thompson RC. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110:3789–3794. doi: 10.1161/01.CIR.0000150392.70749.C7. [DOI] [PubMed] [Google Scholar]
- 13.Snjiders T, Bosker R. Multilevel Analysis; an Introduction to Basic and Advanced Multilevel Modelling. Sage; London: 1999. [Google Scholar]
- 14.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. URL http://www.R-project.org/ [Google Scholar]
- 15.Drozda J, Jr., Messer JV, Spertus J, Abramowitz B, Alexander K, Beam CT, Bonow RO, Burkiewicz JS, Crouch M, Goff DC, Jr., Hellman R, James T, 3rd, King ML, Machado EA, Jr., Ortiz E, O'Toole M, Persell SD, Pines JM, Rybicki FJ, Sadwin LB, Sikkema JD, Smith PK, Torcson PJ, Wong JB. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with coronary artery disease and hypertension: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. Circulation. 2011;124:248–270. doi: 10.1161/CIR.0b013e31821d9ef2. [DOI] [PubMed] [Google Scholar]
- 16.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 Appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2012;59:857–881. doi: 10.1016/j.jacc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Department of Health and Human Services Federal Register. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Proposed Rule. 2015;80:41198–41316. [PubMed] [Google Scholar]
- 18.Centers for Medicare & Medicaid Services Technical Expert Panel Summary Web Page Posting. [September 10, 2015];Hospital-Level Patient-Reported Outcome Performance Measure for Patients Undergoing Non-Emergent Percutaneous Coronary Intervention. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/TechnicalExpertPanels.html. ARCHIVE TEP - March 2014-August 2015. 2015.
- 19.Spertus J. Barriers to the use of patient-reported outcomes in clinical care. Circ Cardiovasc Qual Outcomes. 2014;7:2–4. doi: 10.1161/CIRCOUTCOMES.113.000829. [DOI] [PubMed] [Google Scholar]
- 20.Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes. 2014;7:640–647. doi: 10.1161/CIRCOUTCOMES.114.000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–49. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Bryson CL, Spertus JA, McDonell MB, Fihn SD. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015–1022. doi: 10.1016/S0002-8703(03)00436-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.