Abstract
Background
We sought to determine the concordance between the accumulating evidence about the impact of tight vs. less tight glycemic control in patients with type 2 diabetes since the publication of UKPDS in 1998 until 2015 with the views about that evidence published in journal articles and practice guidelines.
Methods and Results
We searched in top general medicine and specialty journals for articles referring to glycemic control appearing between 2006 and 2015 and identified the latest practice guidelines. To summarize the evidence, we included all published systematic reviews and meta-analyses of contemporary randomized trials of glycemic control measuring patient-important microvascular and macrovascular outcomes, and completed a meta-analysis of their follow-up extensions. We identified 16 guidelines and 328 statements. The body of evidence produced estimates warranting moderate confidence. This evidence reported no significant impact of tight glycemic control on the risk of dialysis/transplantation/renal death, blindness, or neuropathy. In the last decade, however, most published statements (77–100%) and guidelines (95%) unequivocally endorsed benefit. There is also no significant effect on all-cause mortality, cardiovascular mortality, or stroke; however, there is a consistent 15% relative-risk reduction of nonfatal myocardial infarction. Between 2006–2008, most statements (47–83%) endorsed the benefit; after 2008 (ACCORD), only a minority (21–36%) did.
Conclusions
Discordance exists between the research evidence and academic and clinical policy statements regarding the value of tight glycemic control to reduce micro- and macrovascular complications. This discordance may distort priorities in the research and practice agendas designed to improve the lives of patients with type 2 diabetes.
Keywords: glycemic control, type 2 diabetes, evidence-based medicine, microvascular complications, macrovascular complications
Background
Type 2 diabetes mellitus is a growing pandemic and a leading cause of morbidity and mortality.1 After the Diabetes Control and Complications Trial (DCCT)2 found that tight glycemic control – a glycohemoglobin A1c (HbA1c) < 7% (53 mmol/mol) – could prevent or slow the progression of nephropathy, retinopathy, and neuropathy in patients with type 1 diabetes, a consensus, extended to patients with type 1 and type 2 diabetes, emerged: normalizing glycemia prevents diabetes complications. Guidelines, quality improvement interventions, quality-of-care measures and patient-directed marketing have since focused on achieving tight glycemic control.3–5 Experts labeled clinicians’ failure to intensify therapy to achieve this target as clinical inertia and a quality gap.6–8
As large randomized clinical trials (RCTs) and their follow-up extensions accrued, experts have interpreted their results as confirming that tight glycemic control prevented microvascular complications of type 2 diabetes, but may only prevent cardiovascular complications and mortality in some patients, perhaps those newly diagnosed with this condition.9–14 The body of evidence, previously summarized in meta-analyses of large RCTs, appears to confirm this impression (Table S1).15–25 This evidence has contributed to a consensus reflected in universal guideline recommendations, quality improvement efforts, and clinical decisions all promoting tight glycemic control (HbA1c <6.5 or 7.0%).26–28 The same evidence, however, has led some critics to question this consensus.22, 29, 30
Given the impact on patients, healthcare delivery, and policymaking, the extent to which the consensus about the value of tight glycemic control is consistent with the body of evidence merits clarification. Accordingly, we sought to systematically examine the relationship between the body of evidence about glycemic control in type 2 diabetes and contemporary statements on the value of tight glycemic control, when compared to less tight control (HbA1c 7.0–8.5%), with regard to microvascular and macrovascular outcomes, published in the last decade in top medical journals and clinical practice guidelines.
Methods
Identification and selection of published statements referring to glycemic control
Based on the 2014 Journal Citation Reports31, we identified the five general medical journals (New England Journal of Medicine, the Lancet, the Journal of the American Medical Association (JAMA), the BMJ, and Annals of Internal Medicine), and clinical diabetes (Diabetes Care) and cardiology (Journal of the American College of Cardiology) journals with the highest impact factor within these categories.
Using the search engine for each journal’s online site, we searched for “glycemic control” and alternative spellings in original articles, reviews, letters, commentaries, and editorials appearing between January 2006 and March 2015. Eligible articles offered any statement about the effect of glycemic control on microvascular or macrovascular complications in patients with type 2 diabetes. We included all eligible articles from general medicine journals, and, because of the large volume of pertinent articles, only those published in the first trimester (January to March) of each year for the specialty journals. Article selection was reproducible: chance-adjusted agreement between the two reviewers (RRG, VMM) tested in 20% of the sample was κ = 0.93, 95% CI 0.85 – 1.00.
With the help of an experienced librarian, we developed an environmental scan strategy using terms for concepts of “diabetes”, “guidelines”, and “standards of care”, to identify clinical practice guidelines about diabetes without language restriction. This was strengthened with a search in the National Guideline Clearinghouse. We also consulted Mayo Clinic experts in the field to identify guidelines missed by our search strategy. Eligible guidelines were the latest version published and included statements about the effect of glycemic control on microvascular and/or macrovascular complications in patients with type 2 diabetes. Because practice standards from the American Diabetes Association (ADA) are issued yearly and we believe have broad impact, we included all published in the decade of interest. Chance-adjusted agreement for guideline selection between reviewers was perfect (k=1.0).
Classification of statements in articles and guidelines
We classified the statements in each article and guideline about the causal relationship between achieving tight glycemic control and the prevention of microvascular and macrovascular complications in patients with type 2 diabetes as either clearly favorable or uncertain/skeptical. For example, we classified as clearly favorable for microvascular complications and uncertain for macrovascular complications the following statement: “Data from randomized trials indicate early and aggressive antihyperglycemic therapy significantly reduces the risk of long-term microvascular outcomes. Although the effects of tight glucose control on macrovascular disease are less clear”.32 This classification was reproducible (κ=.87 for journal articles, κ=1.0 for guidelines). Within each year – 2006 to 2015 – we estimated the proportion of articles with statements clearly in favor of tight glycemic control to prevent micro- and macrovascular outcomes.
Body of evidence about glycemic control
The body of large randomized trial evidence about glycemic control has been previously summarized, except for the published follow-up extensions of these RCTs. Table S1 describes contemporary large RCTs, their corresponding follow-up extensions and the meta-analyses that include these RCTs. At the individual trial level, we excluded trials that did not test contemporary treatment approaches (e.g. Kumamoto)33, tested multifactorial risk factor reduction (e.g. Steno-2)34, or evaluated specific antihyperglycemic agents (e.g. PROactive)35. However, some reviews included some or all of these studies, and we retained their summaries in our analyses of the body of evidence, subject to sensitivity analyses. When necessary data were not discernible from published studies, as was the case with two extension studies, we attempted to contact authors without success. We did not impute any data.
Because the follow-up extension studies have not been summarized, we conducted a meta-analysis. To this end, we extracted the reported hazard or risk ratios and their 95% confidence intervals (CI) from each extension study and conducted a random-effects (DerSimonian and Laird) meta-analysis on each outcome of interest. As UKPDS 33 control patients also participated as controls in UKPDS 34, we constructed two pooled estimates including either one or the other study. Analyses were conducted using the OpenMeta Analyst Software.36
Examined outcomes were those patients experience and consider important.37–39 We selected the following microvascular outcomes as important to patients: end-stage renal disease (ESRD) or dialysis, renal death, blindness, and clinical neuropathy. We also included microalbuminuria and retinal photocoagulation as they are often cited as surrogate outcomes of patient-important microvascular complications and are consistently reported in RCTs. We selected the following macrovascular outcomes as important to patients: all-cause mortality, cardiovascular (CV) mortality, nonfatal myocardial infarction (MI), fatal and nonfatal stroke, and peripheral vascular events or amputations. To rate the confidence (high, moderate, low or very low) in the estimates about the impact of glycemic control on each micro- and macrovascular outcome from this body of evidence,38, 40 reviewers worked together using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, taking into account risk of bias (methodological quality), directness, consistency, precision of estimates, and risk of biased reporting.
Results
Study Identification
We identified 328 journal articles (Table S2), 16 guidelines (including 10 American Diabetes Association standards from 2006 to 2015, Table S3 and S4), 11 meta-analyses published between 2009 and 2014, and 5 RCTs10–14 and their extension studies41–44 (Figures S1a and S1b and Table S1, S2 and S3).
Reliability of the body of evidence about microvascular and macrovascular outcomes
Using GRADE, we rated the body of evidence as warranting moderate confidence in estimates; it rendered precise (with >400 events for most outcomes) and consistent estimates of direct applicability at moderate risk of bias (due to lack of blinding, loss to follow-up in long-term studies, Tables S9–S11).45 However, results were inconsistent for mortality outcomes; also the evidence was sparse for ESRD, renal death and amputations46 (Table S12).
Relationship between statements in favor of tight glycemic control and the body of evidence
Microvascular complications
Figure 1A (Table S5) shows the relation between estimates of treatment effect for each of the included studies and contemporaneous statements about the value of tight glycemic control on microvascular complications. Since 1998, evidence warranting moderate confidence reports no significant impact of tight glycemic control on the risk of ESRD, renal death, blindness, and clinical neuropathy. The exception was the ADVANCE trial that reported a reduction of 65% (95% CI 17 – 85) in the risk of ESRD or dialysis. These estimates are imprecise (very wide CI): important but small benefits, i.e., up to 5 fewer ESRD events per 1000 patients treated with tight glycemic control, are still consistent with the data. This imprecision may be due to lack of effect, the enrollment of low-risk patients or brief duration of follow-up.45 Figure 2, panel A also shows a very low (<6%) incidence of all microvascular outcomes and no apparent HbA1c threshold effect on microvascular complications. In contrast, practice guidelines and published statements offer a consistent and confident consensus, with 100% of the guidelines, and 77% to 100% of the statements in favor of tight glycemic control to prevent microvascular complications (Figure 1, panel A, tables S2 and S3).
Figure 1.
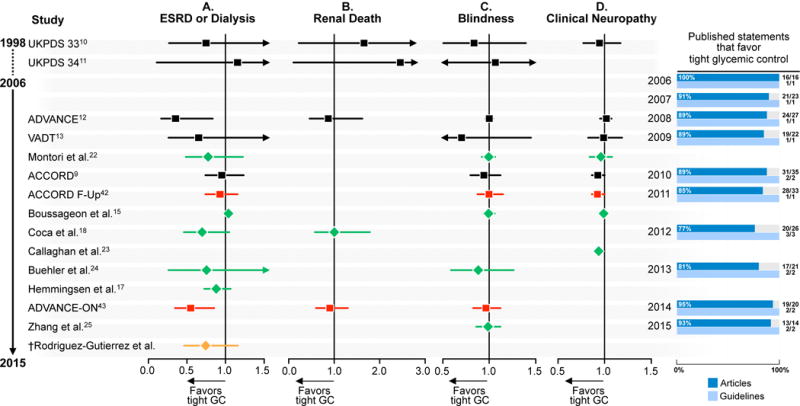
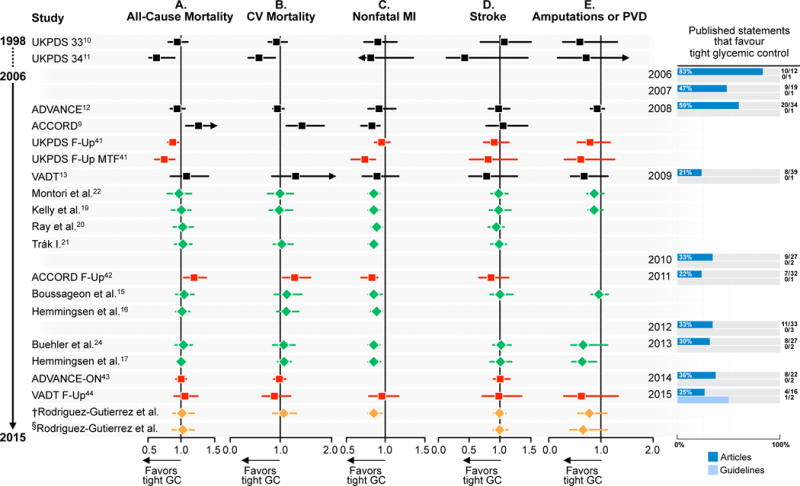
Body of evidence and statements published in journals and guidelines in favor of tight glycemic control in patients with type 2 diabetes mellitus. A. Microvascular complications. The number of guideline statements is presented in parenthesis. ESRD, end-stage renal disease; GC, glycemic control. Black, randomized clinical trials; red, follow-up studies of included randomized clinical trials; green, meta-analyses; orange; meta-analyses of follow-up studies. † Meta-analysis including follow-up UKPDS 3310 and excluding follow-up UKPDS 3411. B. Macrovascular complications. The number of guideline statements is presented in parenthesis. CV, cardiovascular; GC, glycemic control; MI, myocardial infraction; PVE, peripheral vascular events. Black, randomized clinical trials; red, follow-up studies of included randomized clinical trials; green, meta-analyses; orange; meta-analyses of follow-up studies. As UKPDS 3310 control patients also participated as controls in UKPDS 3411, two pooled estimates were constructed including either one or the other study. †Meta-analysis including follow-up UKPDS 3341 and excluding follow-up UKPDS 3441 §Meta-analysis including follow-up UKPDS 3441 and excluding follow-up UKPDS 3341. *UKPDS 3341 Follow-up (c) includes both fatal and nonfatal-MI. *UKPDS 3310, UKPDS 3411, Kelly et al.19, Trák I.21, Boussageon et al.15, Hemmingsen et al.16, Buehler et al.24 and VADT Follow-Up44 report only nonfatal strokes. *UKPDS 3411 was not included in the 2015 meta-analysis of follow-up studies for cardiovascular mortality and nonfatal MI outcomes, as these outcomes were not reported.
Figure 2.
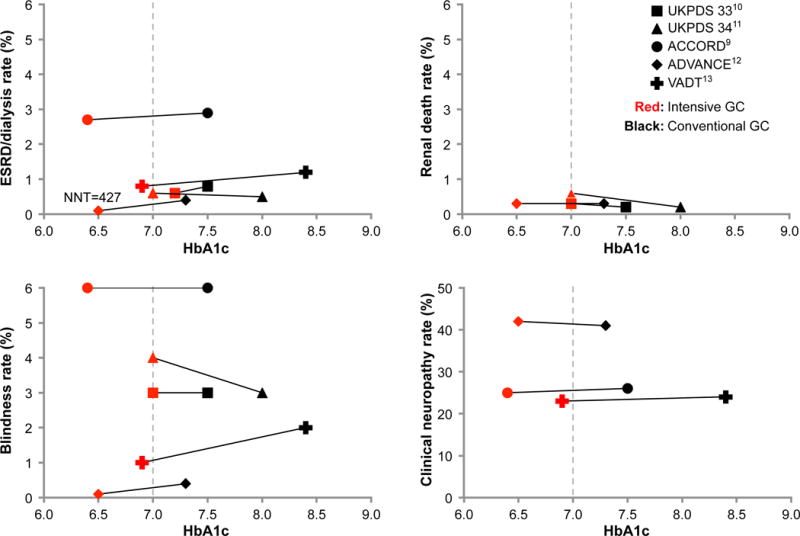
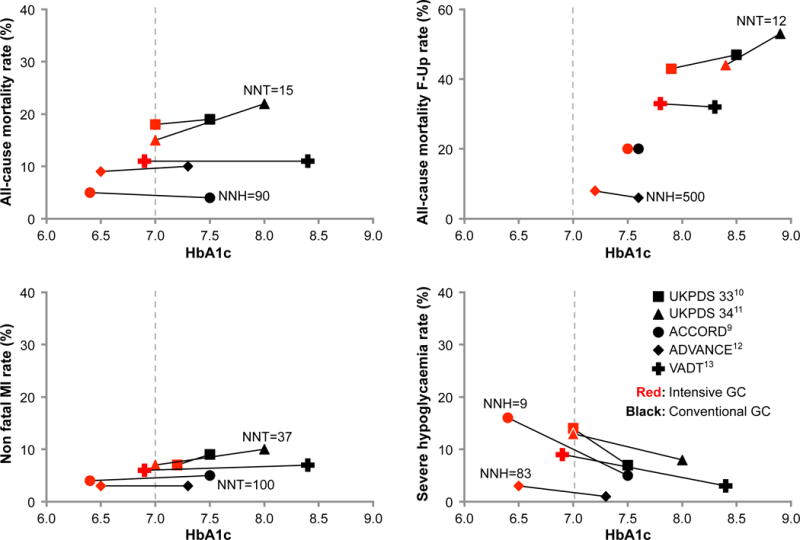
A. End-of-study mean HbA1c and rate of microvascular complications and macrovascular complications in the tight (red) and less tight (black) glycemic control groups (square: UKPDS 3310; triangle: UKPDS 3411; circle: ACCORD9; diamond: ADVANCE12; Cross: VADT13). GC, glycemic control; ESRD, end-stage renal disease; NNH, Number needed to harm; NNT, number needed to treat. B. End-of-study mean HbA1c and risk of macrovascular complications and severe hypoglycemia (Panel B) in the tight (red) and conventional (black) glycemic control groups of included studies (square: UKPDS 3310; triangle: UKPDS 3411; circle: ACCORD9; diamond: ADVANCE12; Cross: VADT13). GC, glycemic control; MI, myocardial infarction; NNH, number needed to harm; NNT, number needed to treat.
Macrovascular complications
The picture with regard to macrovascular complications is more complex. Tight glycemic control reduces the risk of nonfatal MI by 15%, a consistent finding across the included studies (Figure 1, panel B and Table S6), although there is no significant effect of tight glycemic control on all-cause and CV mortality. That a reduction in the risk of nonfatal MI is not associated with a concomitant reduction in the risk of CV death complicates its interpretation. In fact, the ACCORD study reported significant increases in the risks of all cause-mortality (by 26%, 95% CI 6–51) and of CV mortality (by 43%, 95% CI 11–86) while reporting a significant reduction in the risk of non-fatal MI (by 21%, 95% CI 5–34). These is evidence of no significant effect of tight glycemic control on the risk of strokes. The effect on amputations is imprecise in part due to very few events. Recent RCTs have enrolled lower risk participants, reducing the chance of detecting differences if they exist (Figure 2). Long-term follow-up studies that accrued more events, however, could not maintain HbA1c < 7% in the intervention arm limiting their relevance to current guideline targets (Figure 2, panel B, Figure S4).
Before the ACCORD trial, a majority of statements declared valuable to achieve tight glycemic control to prevent macrovascular complications (47% to 59%). Uncertainty clearly emerged after the publication of the results of ACCORD in 2008:14 only 21% of statements favored tight glycemic control in 2009. While biological reasons, including hypoglycemia (Figure S2 and Table S7) have been proposed and rejected,14, 47, 48 chance remains an explanation, the estimate likely an exaggeration produced by the trialists’ decision to truncate the trial.49, 50 After ACCORD, the consensus about the value of tight glycemic control to prevent macrovascular complications withered, with most statements (64% to 79%) expressing uncertainty and skepticism. Only two of the guidelines examined, the ADA standards published in 2003 and in 2004, declared valuable to achieve tight glycemic control to reduce macrovascular complications.
Discussion
Our findings
While no significant impact of tight glycemic control on the risks of patient-important nephropathy, retinopathy, or neuropathy is evident, most published statements and practice guidelines endorse its value to prevent microvascular complications. It is possible that these statements rely on indirect evidence (i.e., on surrogates of these patient-important outcomes such as microalbuminuria), but such reliance should reduce their confidence in the value of glycemic control. While the evidence supports similar cautious skepticism about the impact of glycemic control on mortality and cardiovascular endpoints, a similarly favorable consensus existed prior to the ACCORD trial (2008). Since then, the prevailing skepticism, while appropriate, may have failed to account for the consistent apparent benefits of tight glycemic control on the risk of nonfatal MI.
The use of composite endpoints that include both patient-important and surrogate outcomes may have contributed to this consensus. The UKPDS was a landmark study that reported a significant decrease in the risk of the composite “any diabetes-related endpoint” with tight glycemic control,10 although 85% of the effect was limited to one component: retinal photocoagulation. Similarly, ADVANCE investigators reported a 14% relative reduction in the risk of a composite microvascular outcome, with almost all of the effect limited to reductions in the risk of new micro- and macroalbuminuria.9, 12 ADVANCE researchers also reported a 65% reduction in the risk of ESRD, but this was based on very few endpoints (20 vs. 7 events) which renders statistical inference fragile.45, 51 ACCORD reported benefits such as delayed onset of macroalbuminuria, of 3-line worsened visual acuity, of loss of ankle jerk, and of loss of sensation to light touch, but no significant reduction in the risk of patient-important microvascular complications. The VADT also reported reduction in the risk of progression to albuminuria, but no significant impact on important microvascular outcomes.13 This pattern persists in meta-analyses of these RCTs and of their extensions (Figure 1A). Thus, the consensus favoring glycemic control may narrowly reflect evidence of benefit of tight glycemic control on surrogate markers of microalbuminuria and retinal photocoagulation.
Limitations and strengths of our analysis
Several concerns may reduce confidence in our analyses. We limited our review of journals to those with highest impact factor, which may capture a consensus that exists only among an elite of researchers and clinicians. That these statements agree with contemporary guidelines strengthen our sense that they represent dominant and influential, rather than fringe, views. Our focus on the last decade (2006–2015), while arbitrary, offers complete coverage: after the UKPDS, before the ACCORD, ADVANCE and VADT, before and after the publication of the respective extension reports, and present time. As others, we have excluded the Kumamoto (which tested the DCCT intervention in patients with type 2 diabetes) and UGDP trials (which was stopped early due to harm marred in controversy over its data handling) as individual trials, although their data were represented in some of the included meta-analyses. In addition, the timeframe of our systematic search for meta-analyses of RCTs (2009 to present time) offers complete coverage of all major trials: UKPDS, ACCORD, ADVANCE, and VADT, the latter published last in January 2009.
There is some concern about placing the UKPDS trial alongside the ACCORD, VADT or ADVANCE trials because the populations are different in terms of duration of diabetes, glycemic targets, antihyperglycemic agents used, comorbidities, and use of statins. Described as supportive of early aggressive intervention to reduce macrovascular complications, statements often expressed uncertainty about the applicability of the UKPDS results to older patients with comorbidities and more advanced disease.48, 52 Inspection of Figure 2, however, reveals how most results of UKPDS, particularly of UKPDS 33, are similar to those of more recent RCTs for both micro- and macrovascular outcomes. Furthermore, a consistent beneficial effect on nonfatal MI is seen across all of these trials, even ACCORD. These findings should reduce our confidence that the results of the UKPDS trial are different to those of latter trials and enable us to consider these trials together as forming a body of evidence.
Our focus on outcomes important to patients may have artificially introduced uncertainty that perhaps is not as evident when one focuses on the effect of glycemic control on surrogate markers or composite endpoints (e.g., any diabetes-related complications) (Figure S3, Table S8). Surrogate markers, to be valid, need to capture all of the effect of treatment on the outcomes of interest.53, 54 In the ON-TARGET trial, for example, dual renin-angiotensin-aldosterone blockade prevented albuminuria, but worsened renal outcomes and mortality.55 The inconsistencies observed in diabetes trials between the effect of glycemic control on surrogates and on outcomes important to patients should lower our confidence in relying on these surrogates for decision-making and support the case for larger and longer-term investigations.
Composite endpoints have been used in all diabetes trials and may have contributed to obfuscate their interpretation. The UKPDS used the endpoint “any diabetes-related endpoint” which included 14 components and was reduced significantly with glycemic control by 3.2%. Almost all of this reduction, 2.7%, was on the retinal photocoagulation endpoint, with almost no effect on the other components of greater importance to patients such as mortality, stroke, amputation, blindness, or need for dialysis.10 Composite endpoints that exhibit large gradients of treatment effects and of importance to patients (death and cataract extraction while very different in their importance to patients were both included in the same UKPDS endpoint) cannot be interpreted, i.e., the statement that glycemic control significantly reduced all-diabetes related complications is potentially misleading.56, 57 It is plausible that reliance on surrogate and composite endpoints has contributed to the observed consensus.
Implications for policy and practice
We find the overwhelming consensus in favor of tight glycemic control to prevent microvascular complications to be stronger than warranted by the evidence. This consensus likely drives guidelines and quality-of-care interventions focused on glycemic control. It also supports the US Food and Drug Administration policy to approve diabetes drugs only on the basis of their antihyperglycemic effect without requiring evidence of reduction in the risk of complications. This consensus is also driving studies like the NIH-funded GRADE trial comparing antihyperglycemic drugs on their ability to reduce HbA1c, rather than to reduce the risk of diabetes complications.58 Given the uncertain relationship between tight glycemic control and outcomes that matter to patients, this consensus and its downstream consequences to practice, policy and research deserve review.
As of 2015, the evidence suggests that a skeptical view may be necessary to move diabetes care forward. The notion that tight glycemic control is clearly beneficial does not hold in the face of the evidence accrued over the last decade, evidence that has fallen short of confirming this notion. The contributions of more than 27,000 patients participating in RCTs of glycemic control, their clinicians, and the investigators that designed and conducted these trials, question our confident reliance on tight glycemic control as the main or, in some cases only, strategy to prevent complications in patients with type 2 diabetes. Perhaps as a result, guideline developers are now advocating for selecting less stringent HbA1c targets in patients with recurrent severe hypoglycemia, high-comorbidity burden, or limited-life expectancy.52, 59
Embracing this skeptical view may spur research to discover new therapeutic approaches to prevent diabetes complications. Consider the list of evidence-based therapies recommended in guidelines, subject of quality metrics, or routinely prescribed to patients with type 2 diabetes to prevent retinopathy or neuropathy beyond glycemic control: none. Beyond interventions to improve vascular health which may be helpful,60 our narrow focus on hyperglycemia has kept this list empty. In this sense, we could not find in clinicaltrials.gov any ongoing trials exploring interventions to prevent microvascular complications. In contrast, where we are skeptical, there is at least one NIH program announcement calling for RCTs to reduce cardiovascular risk in older adults with diabetes.61
Moderation in expectations about the value of tight glycemic control may help advance the individualization of diabetes care protocols, whispered in recent guidelines,52 using shared decision making to select glycemic targets and treatments.62, 63 This, however, will require further research to clarify the tradeoffs involved when selecting different targets (below the point of symptomatic hyperglycemia), and when selecting glycemic control to reduce residual risk for complications (e.g., nonfatal MI) after implementing other evidence-based interventions, such as statins.64 Recognizing the nature of the evidence also requires the revision of model-based estimates of the economic impact of tight glycemic control65, 66 and moderation of the exuberant support for policies of tight glycemic control with consequent overtesting and overtreatment.67, 68 Any moderation will have to be delicately balanced against the risk of therapeutic nihilism. A careful and thoughtful recalibration is likely to promote patient trust in our efforts to advance their best interest. Today, patients with type 2 diabetes, at least in certain parts of the world, seem to live longer lives with fewer complications.69–72 The evidence summarized here requires us to explore factors other than tight glycemic control to explain this improvement and better address the diabetes epidemic. Exciting new questions and new answers may surface as we look beyond glycemic control.
Supplementary Material
What Is Known.
Tight glycemic control is considered an essential strategy to prevent chronic complications in patients with type 2 diabetes.
Practice guideline recommendations, quality improvement programs, and clinical care all promote tight glycemic control.
What the Study Adds.
The evidence accrued in the last two decades consistently demonstrates no significant benefit of tight glycemic control on patient-important micro- and macrovascular outcomes, with the exception of a 15% relative risk reduction in nonfatal myocardial infarction.
Despite this, most published statements and all guidelines unequivocally endorse tight glycemic control to prevent microvascular complications, although the benefits for macrovascular outcomes have been tempered after one trial was stopped early because of increased cardiovascular mortality.
The widespread consensus about the value of tight glycemic control to prevent complications in patients with type 2 diabetes needs to be recalibrated.
Acknowledgments
We would like to thank our colleagues at the Knowledge and Evaluation Research Unit at Mayo Clinic for their encouragement and generous review of this work.
Sources of Funding: This publication was made possible by Center for Clinical and Translational Science Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Journal Subject Terms: Complications; Meta Analysis; Statements and Guidelines; Quality and Outcomes
Conflict of Interest Disclosures: None.
References
- 1.International Diabetes Federation. International Diabetes Federation diabetes atlas. 2014;2015 [Google Scholar]
- 2.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The diabetes control and complications trial research group. New Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Pogach L, Aron DC. Sudden acceleration of diabetes quality measures. JAMA. 2011;305:709–710. doi: 10.1001/jama.2011.153. [DOI] [PubMed] [Google Scholar]
- 4.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez G, Davidson MH. Aace comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19:327–336. doi: 10.4158/endp.19.2.a38267720403k242. [DOI] [PubMed] [Google Scholar]
- 5.Standards of medical care in diabetes–2007. Diabetes care. 2007;30(Suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 6.Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, Miller CD, Ziemer DC, Barnes CS. Clinical inertia. Ann Intern Med. 2001;135:825–834. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 7.Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: A retrospective cohort study of more than 80,000 people. Diabetes care. 2013;36:3411–3417. doi: 10.2337/dc13-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: Do specialists differ from primary care physicians? Diabetes care. 2005;28:600–606. doi: 10.2337/diacare.28.3.600. [DOI] [PubMed] [Google Scholar]
- 9.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH, Jr, Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A, O’Connor P, Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the accord randomised trial. Lancet. 2010;376:419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (ukpds 33). Uk prospective diabetes study (ukpds) group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 11.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (ukpds 34). Uk prospective diabetes study (ukpds) group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 12.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 13.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. New Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 14.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. New Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: Meta-analysis of randomised controlled trials. Bmj. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, Wetterslev J. Intensive glycaemic control for patients with type 2 diabetes: Systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. Bmj. 2011;343:d6898. doi: 10.1136/bmj.d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal TP, Hemmingsen C, Wetterslev J. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2013;11:CD008143. doi: 10.1002/14651858.CD008143.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: Systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012;172:761–769. doi: 10.1001/archinternmed.2011.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: Glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151:394–403. doi: 10.7326/0003-4819-151-6-200909150-00137. [DOI] [PubMed] [Google Scholar]
- 20.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: A meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 21.Tkac I. Effect of intensive glycemic control on cardiovascular outcomes and all-cause mortality in type 2 diabetes: Overview and metaanalysis of five trials. Diabetes Res Clin Pract. 2009;86(Suppl 1):S57–62. doi: 10.1016/S0168-8227(09)70011-7. [DOI] [PubMed] [Google Scholar]
- 22.Montori VM, Fernandez-Balsells M. Glycemic control in type 2 diabetes: Time for an evidence-based about-face? Ann Intern Med. 2009;150:803–808. doi: 10.7326/0003-4819-150-11-200906020-00008. [DOI] [PubMed] [Google Scholar]
- 23.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543. doi: 10.1002/14651858.CD007543.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buehler AM, Cavalcanti AB, Berwanger O, Figueiro M, Laranjeira LN, Zazula AD, Kioshi B, Bugano DG, Santucci E, Sbruzzi G, Guimaraes HP, Carvalho VO, Bordin SA. Effect of tight blood glucose control versus conventional control in patients with type 2 diabetes mellitus: A systematic review with meta-analysis of randomized controlled trials. Cardiovasc Ther. 2013;31:147–160. doi: 10.1111/j.1755-5922.2011.00308.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Zhao J, Zhao T, Liu H. Effects of intensive glycemic control in ocular complications in patients with type 2 diabetes: A meta-analysis of randomized clinical trials. Endocrine. 2015;49:78–89. doi: 10.1007/s12020-014-0459-8. [DOI] [PubMed] [Google Scholar]
- 26.(6) Glycemic targets. Diabetes care. 2015;38(Suppl):S33–40. doi: 10.2337/dc15-S009. [DOI] [PubMed] [Google Scholar]
- 27.Diabetes TRACoGPa. General practice management of type 2 diabetes 2014–15. 2014;2015 [Google Scholar]
- 28.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez G, Davidson MH. Aace/ace comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21:438–447. doi: 10.4158/EP15693.CS. [DOI] [PubMed] [Google Scholar]
- 29.Yudkin JS, Richter B, Gale EA. Intensified glucose lowering in type 2 diabetes: Time for a reappraisal. Diabetologia. 2010;53:2079–2085. doi: 10.1007/s00125-010-1864-z. [DOI] [PubMed] [Google Scholar]
- 30.McCormack J, Greenhalgh T. Seeing what you want to see in randomised controlled trials: Versions and perversions of ukpds data. United kingdom prospective diabetes study. Bmj. 2000;320:1720–1723. doi: 10.1136/bmj.320.7251.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomson Reuters. Journal citation reports. 2014;2015 http://thomsonreuters.com/en/products-services/scholarly-scientific-research/research-management-and-evaluation/journal-citation-reports.html. [Google Scholar]
- 32.Inzucchi SE. Clinical practice. Diagnosis of diabetes. New Engl J Med. 2012;367:542–550. doi: 10.1056/NEJMcp1103643. [DOI] [PubMed] [Google Scholar]
- 33.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in japanese patients with non-insulin-dependent diabetes mellitus: A randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 34.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. New Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 35.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the proactive study (prospective pioglitazone clinical trial in macrovascular events): A randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 36.University B. Openmeta analyst software, brown university. 2015:2015. [Google Scholar]
- 37.Montori VM, Gandhi GY, Guyatt GH. Patient-important outcomes in diabetes–time for consensus. Lancet. 2007;370:1104–1106. doi: 10.1016/S0140-6736(07)61489-5. [DOI] [PubMed] [Google Scholar]
- 38.Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. Grade guidelines: A new series of articles in the journal of clinical epidemiology. J Clin Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Gandhi GY, Murad MH, Fujiyoshi A, Mullan RJ, Flynn DN, Elamin MB, Swiglo BA, Isley WL, Guyatt GH, Montori VM. Patient-important outcomes in registered diabetes trials. JAMA. 2008;299:2543–2549. doi: 10.1001/jama.299.21.2543. [DOI] [PubMed] [Google Scholar]
- 40.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. Grade guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. New Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 42.Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH, Jr, Byington RP, Rosenberg YD, Friedewald WT. Long-term effects of intensive glucose lowering on cardiovascular outcomes. New Engl J Med. 2011;364:818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, Arima H, Monaghan H, Joshi R, Colagiuri S, Cooper ME, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Lisheng L, Mancia G, Marre M, Matthews DR, Mogensen CE, Perkovic V, Poulter N, Rodgers A, Williams B, MacMahon S, Patel A, Woodward M. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. New Engl J Med. 2014;371:1392–1406. doi: 10.1056/NEJMoa1407963. [DOI] [PubMed] [Google Scholar]
- 44.Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, McCarren M, Duckworth WC, Emanuele NV. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. New Engl J Med. 2015;372:2197–2206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 45.Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW, Jr, Murad MH, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schunemann HJ. Grade guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol. 2011;64:1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA, Norris S, Vist G, Dahm P, Shukla VK, Higgins J, Falck-Ytter Y, Schunemann HJ. Grade guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS. Intensive glycemic control and the prevention of cardiovascular events: Implications of the accord, advance, and va diabetes trials: A position statement of the american diabetes association and a scientific statement of the american college of cardiology foundation and the american heart association. Diabetes care. 2009;32:187–192. doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bloomgarden ZT. Glycemic control in diabetes: A tale of three studies. Diabetes care. 2008;31:1913–1919. doi: 10.2337/dc08-zb09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montori VM, Devereaux PJ, Adhikari NK, Burns KE, Eggert CH, Briel M, Lacchetti C, Leung TW, Darling E, Bryant DM, Bucher HC, Schunemann HJ, Meade MO, Cook DJ, Erwin PJ, Sood A, Sood R, Lo B, Thompson CA, Zhou Q, Mills E, Guyatt GH. Randomized trials stopped early for benefit: A systematic review. JAMA. 2005;294:2203–2209. doi: 10.1001/jama.294.17.2203. [DOI] [PubMed] [Google Scholar]
- 50.Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, Heels-Ansdell D, Walter SD, Guyatt GH, Flynn DN, Elamin MB, Murad MH, Abu Elnour NO, Lampropulos JF, Sood A, Mullan RJ, Erwin PJ, Bankhead CR, Perera R, Ruiz Culebro C, You JJ, Mulla SM, Kaur J, Nerenberg KA, Schunemann H, Cook DJ, Lutz K, Ribic CM, Vale N, Malaga G, Akl EA, Ferreira-Gonzalez I, Alonso-Coello P, Urrutia G, Kunz R, Bucher HC, Nordmann AJ, Raatz H, da Silva SA, Tuche F, Strahm B, Djulbegovic B, Adhikari NK, Mills EJ, Gwadry-Sridhar F, Kirpalani H, Soares HP, Karanicolas PJ, Burns KE, Vandvik PO, Coto-Yglesias F, Chrispim PP, Ramsay T. Stopping randomized trials early for benefit and estimation of treatment effects: Systematic review and meta-regression analysis. JAMA. 2010;303:1180–1187. doi: 10.1001/jama.2010.310. [DOI] [PubMed] [Google Scholar]
- 51.Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, Li Q, MacMahon S, Cooper ME, Hamet P, Marre M, Mogensen CE, Poulter N, Mancia G, Cass A, Patel A, Zoungas S. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83:517–523. doi: 10.1038/ki.2012.401. [DOI] [PubMed] [Google Scholar]
- 52.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the american diabetes association and the european association for the study of diabetes. Diabetes care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 53.Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. Bmj. 2011;343:d7995. doi: 10.1136/bmj.d7995. [DOI] [PubMed] [Google Scholar]
- 54.Yudkin JS, Eggleston EM. ‘Hard, ’ ‘soft’ and ‘surrogate’ endpoints in diabetes. J Epidemiol Commun Health. 2013;67:295–297. doi: 10.1136/jech-2012-201361. [DOI] [PubMed] [Google Scholar]
- 55.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsarinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ontarget study): A multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 56.Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: Greater precision but with greater uncertainty? JAMA. 2003;289:2554–2559. doi: 10.1001/jama.289.19.2554. [DOI] [PubMed] [Google Scholar]
- 57.Montori VM, Permanyer-Miralda G, Ferreira-Gonzalez I, Busse JW, Pacheco-Huergo V, Bryant D, Alonso J, Akl EA, Domingo-Salvany A, Mills E, Wu P, Schunemann HJ, Jaeschke R, Guyatt GH. Validity of composite end points in clinical trials. Bmj. 2005;330:594–596. doi: 10.1136/bmj.330.7491.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nathan DM, Buse JB, Kahn SE, Krause-Steinrauf H, Larkin ME, Staten M, Wexler D, Lachin JM. Rationale and design of the glycemia reduction approaches in diabetes: A comparative effectiveness study (grade) Diabetes care. 2013;36:2254–2261. doi: 10.2337/dc13-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez-Gutierrez R, Lipska KJ, McCoy RG, Ospina NS, Ting HH, Montori VM, Hypoglycemia as a Quality Measure in Diabetes Study G Hypoglycemia as an indicator of good diabetes care. Bmj. 2016;352:i1084. doi: 10.1136/bmj.i1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Do DV, Wang X, Vedula SS, Marrone M, Sleilati G, Hawkins BS, Frank RN. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;1:CD006127. doi: 10.1002/14651858.CD006127.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diabetes and cardiovascular disease in older adults. 2015;2015 Grants.gov. http://www.grants.gov/web/grants/search-grants.html?keywords=Diabetes%20and%20Cardiovascular%20Disease%20in%20Older%20Adults. Accessed July 2016. [Google Scholar]
- 62.Rodriguez-Gutierrez R, Gionfriddo MR, Ospina NS, Maraka S, Tamhane S, Montori VM, Brito JP. Shared decision making in endocrinology: Present and future directions. Lancet Diabetes Endocrinol. 4:706–716. doi: 10.1016/S2213-8587(15)00468-4. 201. [DOI] [PubMed] [Google Scholar]
- 63.Mullan RJ, Montori VM, Shah ND, Christianson TJ, Bryant SC, Guyatt GH, Perestelo-Perez LI, Stroebel RJ, Yawn BP, Yapuncich V, Breslin MA, Pencille L, Smith SA. The diabetes mellitus medication choice decision aid: A randomized trial. Arch Intern Med. 2009;169:1560–1568. doi: 10.1001/archinternmed.2009.293. [DOI] [PubMed] [Google Scholar]
- 64.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW. 2013 acc/aha guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Gray A, Raikou M, McGuire A, Fenn P, Stevens R, Cull C, Stratton I, Adler A, Holman R, Turner R. Cost effectiveness of an intensive blood glucose control policy in patients with type 2 diabetes: Economic analysis alongside randomised controlled trial (ukpds 41). United kingdom prospective diabetes study group. Bmj. 2000;320:1373–1378. doi: 10.1136/bmj.320.7246.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clarke PM, Gray AM, Briggs A, Stevens RJ, Matthews DR, Holman RR. Cost-utility analyses of intensive blood glucose and tight blood pressure control in type 2 diabetes (ukpds 72) Diabetologia. 2005;48:868–877. doi: 10.1007/s00125-005-1717-3. [DOI] [PubMed] [Google Scholar]
- 67.McCoy RG, Zhang Y, Herrin J, Denton BT, Mason JE, Montori VM, Smith SA, Shah ND. Changing trends in type 2 diabetes mellitus treatment intensification, 2002–2010. Am J Manag Care. 2015;21:e288–296. [PubMed] [Google Scholar]
- 68.Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362. doi: 10.1001/jamainternmed.2014.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention. Diabetes report card. 2012;2015 http://www.cdc.gov/diabetes/pubs/pdf/diabetesreportcard.pdf. Accessed July 2016. [Google Scholar]
- 70.Jansson SP, Andersson DK, Svardsudd K. Mortality trends in subjects with and without diabetes during 33 years of follow-up. Diabetes care. 2010;33:551–556. doi: 10.2337/dc09-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in ontario, canada 1995–2005: A population-based study. Lancet. 2007;369:750–756. doi: 10.1016/S0140-6736(07)60361-4. [DOI] [PubMed] [Google Scholar]
- 72.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147:149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


