Abstract
Objective
Homologous recombination (HR) proficient ovarian cancers, including CCNE1 (cyclin E)-amplified tumors, are resistant to poly (ADP-ribose) polymerase inhibitors (PARPi). Histone deacetylase inhibitors (HDACi) are effective in overcoming tumor resistance to DNA damaging drugs. Our goal was to determine whether panobinostat, a newly FDA-approved HDACi, can sensitize cyclin E, HR-proficient ovarian cancer cells to the PARPi olaparib.
Methods
Expression levels of CCNE1 (cyclin E), BRCA1, RAD51 and E2F1 in ovarian tumors and cell lines were extracted from The Cancer Genome Atlas (TCGA) and Broad-Novartis Cancer Cell Line Encyclopedia (CCLE). In HR-proficient ovarian cancer cell line models (OVCAR-3, OVCAR-4, SKOV-3, and UWB1.289 + BRCA1 wild-type), cell growth and viability were assessed by sulforhodamine B and xenograft assays. DNA damage and repair (pH2AX and RAD51 co-localization and DRGFP reporter activity) and apoptosis (cleaved PARP and cleaved caspase-3) were assessed by immunofluorescence and Western blot assays.
Results
TCGA and CCLE data revealed positive correlations (Spearman) between cyclin E E2F1, and E2F1 gene targets related to DNA repair (BRCA1 and RAD51). Panobinostat downregulated cyclin E and HR repair pathway genes, and reduced HR efficiency in cyclin E-amplified OVCAR-3 cells. Further, panobinostat synergized with olaparib in reducing cell growth and viability in HR-proficient cells. Similar co-operative effects were observed in xenografts, and on pharmacodynamic markers of HR repair, DNA damage and apoptosis.
Conclusions
These results provide preclinical rationale for using HDACi to reduce HR in cyclin E-overexpressing and other types of HR-proficient ovarian cancer as a means of enhancing PARPi activity.
Keywords: panobinostat, olaparib (Ola), homologous recombination, poly (ADP-ribose) polymerase inhibitors (PARPi), phosphorylated histone H2AX (pH2AX), high-grade serous ovarian cancer
INTRODUCTION
Ovarian cancer is the deadliest gynecologic malignancy and the fifth leading cause of cancer death among women in the US [1]. High-grade serous ovarian cancer is the most common and fatal subtype. Treatment options are limited for women with recurrent ovarian cancer, particularly those with chemoresistant disease. Poly ADP ribose polymerase inhibitors (PARPi) are promising new drugs that have shown clear advantage in BRCA-mutated ovarian cancer [2, 3] and in tumors with deficiencies in other homologous recombination (HR) DNA repair genes [4, 5]. By inhibiting single-strand break repair machinery, PARPi cause synthetic lethality in HR-deficient cells. Despite some activity, PARPi are far less effective in the 50% of high-grade serous tumors that retain HR proficiency [2, 6–8]. Developing strategies to expand the use of PARPi and other DNA damaging drugs to HR-proficient tumors is a critical clinical need.
Mutually exclusive to HR-deficient phenotypes are ~20% of high-grade serous HR-proficient ovarian tumors with CCNE1 (cyclin E) overexpression by amplification or upregulation. Ovarian tumors with cyclin E amplifications have high levels of HR proficiency, are relatively resistant to DNA damaging drugs, and have poor clinical outcomes in most studies [6, 9–14]. Amplified cyclin E is a known oncogenic driver of unchecked replication, which causes replicative stress and enormous genomic instability [6, 10–12, 15]. To escape sensors that detect and destroy cells with DNA damage, cyclin E amplified ovarian tumors depend on robust mechanisms to promote HR DNA repair. The major partner kinase of cyclin E, CDK2, phosphorylates Rb and displaces it from a complex with E2F1, which promotes E2F1-dependent transcription of BRCA1 and other DNA damage repair genes [6, 15]. To date, no drugs directly target cyclin E. Further, indirect targeting of cyclin E with currently available CDK2 inhibitors is limited by the development of chemoresistance that occurs in part through E2F1 upregulation [16, 17]. An alternate and emerging paradigm is to convert HR-proficient tumors to HR-deficient phenotypes by using epigenetic drugs [4, 9].
Our group has generated multiple lines of evidence demonstrating that histone deacetylase inhibitors (HDACi) improve responses to DNA damaging drugs in ovarian cancer cells [4, 18, 19]. We have shown that vorinostat downregulates HR gene expression in HR-proficient ovarian cancer cells and sensitizes chemoresistant cells to the PARPi olaparib both in vitro and in vivo [4]. The newest FDA-approved HDACi, panobinostat, is structurally similar to vorinostat but is more potent, with superior pharmacokinetics [20]. Here, we show that panobinostat treatment downregulated cyclin E, E2F1, and HR pathway genes. Consistent with this finding, established markers of HR repair efficiency were reduced in cyclin E amplified HR-proficient ovarian cancer cells with panobinostat treatment alone and in combination with olaparib. Panobinostat synergized with the cytotoxic effects of olaparib in HR-proficient ovarian cancer cells in vitro and in vivo. Further, panobinostat combined with olaparib induced robust and prolonged activation of pH2AX, indicative of deficient DNA damage repair and apoptosis. Our results indicate that targeting HR pathways with HDACi is a promising strategy for improving PARPi efficacy in cyclin E high and other types of HR-proficient ovarian cancer.
MATERIALS AND METHODS
Cell culture and compounds
The epithelial ovarian cancer cell lines SKOV-3, OVCAR-3, UWB1.289+BRCA1 wild-type (BRCA1 WT) and UWB1.289 BRCA1 null (BRCA1 Null) cell lines (American Type Culture Collection, Manassas, VA), and OVCAR-4 (National Cancer Institute, Bethesda, MD) were maintained in culture as previously described [4, 19, 21–23]. Cell lines were authenticated by the Vanderbilt VANTAGE Genomics Core using the GenePrint 10 kit (Promega, Madison, WI). All cell lines used tested negative for mycoplasma. Panobinostat was synthesized at the Broad Institute (Cambridge, MA) and AZD-2281 (olaparib) provided by Astra Zeneca Pharmaceuticals (Wilmington, DE). For in vitro experiments, combination panobinostat/olaparib treatment was as follows unless specifically noted: cells were pre-treated for 24h with vehicle (0.01% DMSO), followed by 24–72h treatment with vehicle (Con) or 10 µM olaparib (Ola); cells were also pre-treated for 24h with panobinostat (25nM), followed by 24–72h treatment with 25nM panobinostat (Pano) or 25nM panobinostat plus 10 µM olaparib (Pano+Ola). Clinically achievable doses of olaparib (10µM) were used in these experiments [24]. Separately, cells were treated with cisplatin (Sigma Chemical Co, St Louis, MO) and/or panobinostat.
Cell proliferation, cytotoxicity and clonogenic assays
Sulforhodamine B (SRB) assays were used to measure cell growth and viability as described [4]. The interaction between fixed ratios of panobinostat and olaparib was measured with the Combination Index (CI) method [25]. Clonogenic assays were performed and quantified as described [26].
Immunofluorescence
Following drug treatment and/or transient transfection with the HR reporter plasmid pDRGFP and endonuclease encoding pCBASce1 (I-Sce1) (both gifts from Maria Jasin; Addgene plasmids #26475 and #26477, respectively) [27, 28] plasmids using Lipofectamine 2000 according to manufacturer’s instructions (Invitrogen Corp., Carlsbad, CA), ovarian cancer cells were fixed, permeabilized and visualized for GFP expression, or stained with mouse monoclonal anti-phospho(p)-H2AX (Ser139) (pH2AX) (Millipore, Billerica, MA), rabbit polyclonal anti-RAD51 (Millipore), and rabbit polyclonal anti-cleaved caspase-3 (Cell Signaling Technology, Beverly, MA) as described [19]. Secondary antibodies, and image acquisition and analysis was as described [19].
Western blotting
Whole cell protein isolation from cultured cells and harvested tumors, hydrochloric acid extraction of histones, western blotting and signal detection were as described [19]. Antibodies used were rabbit polyclonal anti-cyclin E (Abcam, Cambridge, MA), rabbit polyclonal anti-E2F1 (DBA Acris Antibodies, Inc, Rockville, MD), rabbit polyclonal anti-RAD51 (Millipore), mouse monoclonal anti-BRCA1 (Millipore), rabbit polyclonal anti-PARP (Cell Signaling Technology), mouse monoclonal anti-PCNA (Santa Cruz Biotechnology, Inc., Dallas, TX), rabbit polyclonal anti-cleaved caspase-3 (Cell Signaling Technology), and mouse monoclonal anti-pH2AX (Ser139) (Millipore). Loading controls were mouse monoclonal anti-histone H3 (Millipore) and mouse monoclonal β-actin (Sigma) for histones and total proteins, respectively.
Animals
Experiments performed were approved by the Vanderbilt University Institutional Animal Use and Care Committee, and female athymic Nude-Foxn1nu mice (Harlan Laboratories, Indianapolis, IN) maintained in accordance to guidelines of the American Association of Laboratory Animal Care. 5 × 106 SKOV-3 tumor cells in a 200 µL of mixture of PBS and Matrigel (1:1 v/v) (BD Biosciences, San Jose, CA) were injected subcutaneously into the right flank. After the tumors reached approximately 200mm3, mice were randomized into one of 4 treatment groups (n=10). Two treatment groups received panobinostat pre-treatment over one week (2.5mg/kg five times weekly IP) and two received vehicle only (0.01% DMSO in PBS five times weekly IP). Following pre-treatment, mice were treated for 3 weeks with: Vehicle (vehicle pre-treatment & 0.01% DMSO five times weekly IP and PO); Panobinostat (panobinostat pre-treatment & panobinostat 2.5mg/kg five times weekly IP, 0.01% DMSO five times weekly PO; Olaparib (vehicle pre-treatment & olaparib 100mg/kg five times weekly PO & 0.01% DMSO five times weekly IP); and the Panobinostat/Olaparib combination (panobinostat pre-treatment & panobinostat 2.5mg/kg five times weekly IP, olaparib 100mg/kg five times weekly PO). Animals were examined biweekly for the effects of tumor burden and tumor growth, and tumor measurements were performed weekly. Weekly tumor volume measurements were calculated from caliper measurements of the smallest (SD) and largest diameter (LD) volume = [LD × SD2] × π/6 [4]. 24h after the final dose of drug, mice were euthanized according to protocol and tumors excised and weighed.
Statistics
Unless otherwise specified, the Student’s t test was used for comparisons between groups for in vitro and in vivo experiments. Gene expression correlations for TCGA and CCLE data were performed using the Spearman test. In all cases, p < 0.05 was considered statistically significant.
RESULTS
Cyclin E overexpression in high-grade serous ovarian tumors is linked to increased E2F1 and key E2F1-associated HR pathway genes
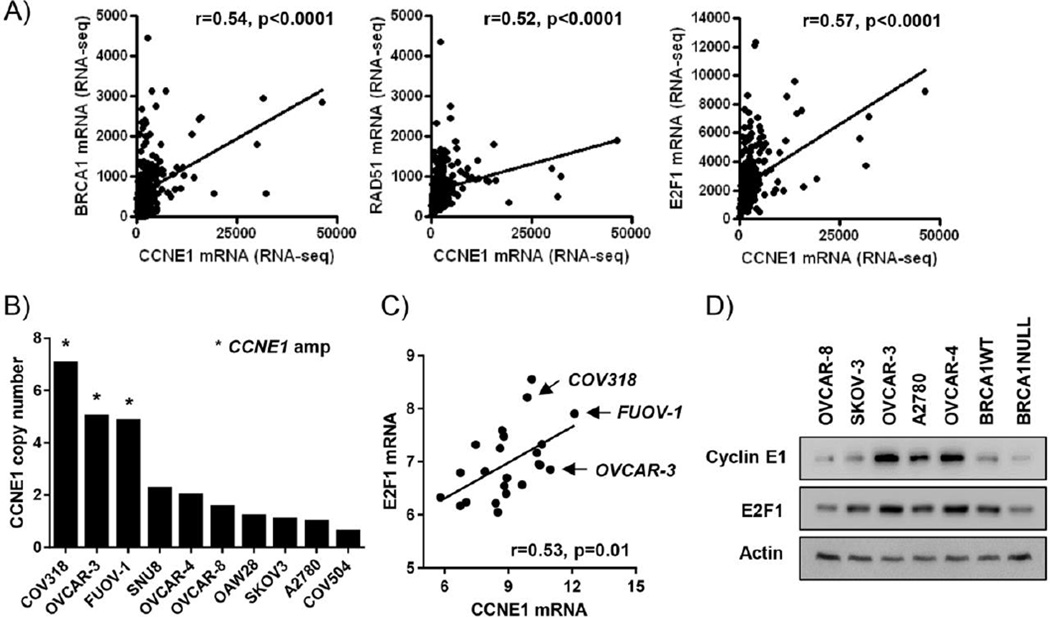
One major subtype of HR-proficient ovarian serous tumors are those harboring CCNE1 (cyclin E) amplifications [6, 9]. Upregulation of signaling through cyclin E and its partner kinase CDK2 has been implicated in increasing transcriptional activity of E2F1, leading to upregulation of DNA repair genes such as BRCA1 and RAD51 [6, 15]. In raw TCGA RNA-seq data (www.cbioportal.org), cyclin E expression levels positively correlated with E2F1, BRCA1 and RAD51 (Fig 1A). We also obtained data from the Cancer Cell Line Encyclopedia (CCLE) resource (www.broadinstitute.org/ccle) to validate these results in representative cell lines. The ovarian cancer cell lines COV318, OVCAR-3, and FUOV-1 have cyclin E amplifications and OVCAR-4 and SNU8 have cyclin E copy number gains (Fig 1B). When we filtered this dataset based on a serous ovarian cancer similarity score (score > 1) [29], we found positive correlations between cyclin E and E2F1 mRNA expression levels in 23/47 (49%) of high-grade serous ovarian cancer cell lines (Fig 1C). We confirmed that OVCAR-3 and OVCAR-4 have high cyclin E and E2F1 protein expression levels among a panel of cell lines (Fig 1D). As expected, BRCA1 WT (HR-proficient) ovarian cancer cells had higher levels of cyclin E and E2F1 protein expression than BRCA1 NULL (HR-deficient) counterparts.
Figure 1. Cyclin E overexpression is associated with high expression of E2F1 and E2F1 gene targets in high-grade serous ovarian cancer.
A) Raw RSEM RNA-Seq counts for 265 TCGA ovarian tumors. CCNE1 (cyclin E) expression was compared to E2F1, BRCA1 and RAD51 (Spearman correlation). B) CCNE1 copy number in CCLE ovarian cancer cell lines. C) Spearman correlation of cyclin E and E2F1 mRNA levels in CCLE cell lines most representative of serous ovarian cancer (similarity score >1) based on ref [29]. D) Western blot analysis of cyclin E and E2F1 expression in representative CCLE cell lines. Actin was the loading control.
The potent HDACi panobinostat downregulates cyclin E, E2F1, and HR gene expression in HR-proficient OVCAR-3 and SKOV-3 ovarian cancer cells
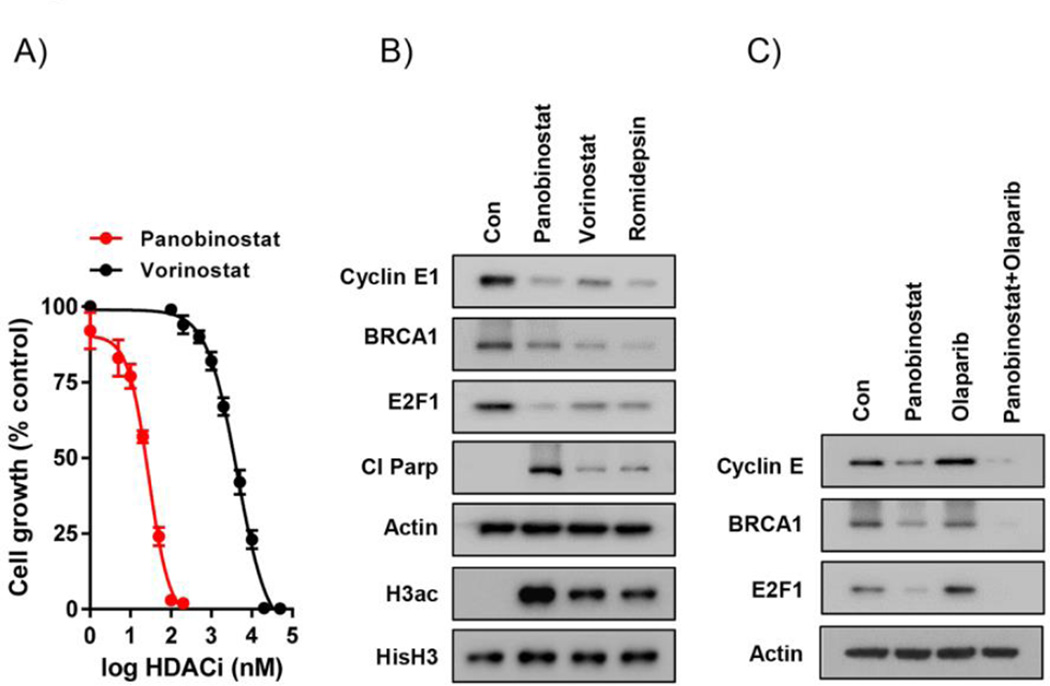
We have previously shown that treatment with the HDACi vorinostat downregulates HR pathway genes [4]. Because of its potency and superior pharmacokinetics (20), we tested the recently FDA-approved HDACi panobinostat in our expanded investigation of HDACi in HR-proficient ovarian cancer. Sulfurhodamine (SRB) cytotoxicity assays in cyclin E-amplified OVCAR-3 cells revealed that panobinostat induced potent cytotoxic effects at the low nM range (IC50: 27.1 ± 3.2 nM), which is several orders of magnitude lower than vorinostat (5.5 ± 0.5 µM) (Fig 2A). Panobinostat also reduced protein expression levels of cyclin E, E2F1 and BRCA1 in OVCAR-3 cells, and increased cleaved PARP, a marker of apoptosis, to a greater extent than vorinostat at their respective IC50 doses (Fig 2B). Romidepsin, another potent HDACi (19), showed similar downregulation of cyclin E, E2F1 and E2F1-related HR pathway genes. Similar potency of panobinostat (IC50: 20.1 ± 2.26 nM) relative to vorinostat (IC50: 2.7 ± 0.4 µM) was also seen in SRB assays (Fig 2A), and in western blot analyses of cyclin E, E2F1 and BRCA1 expression, in HR-proficient SKOV-3 cells (Supplementary Fig S1).
Figure 2. Panobinostat potently inhibits cell viability and reduces expression of cyclin E, E2F1 and BRCA1 in HR-proficient ovarian cancer cells.
A) Concentration-dependent effects of panobinostat and vorinostat in SRB viability assays in OVCAR-3 cells (72h). Values are mean+SE of 3 independent experiments. B) Western blot analysis of the effects of panobinostat (25nM), vorinostat (5µM) and romidepsin (10nM) on expression of cyclin E1, E2F1, BRCA1, cleaved PARP and acetylated histone H3 in OVCAR-3 cells. Actin and histone H3 were loading controls. C) Effects of the panobinostat pre-treatment (25nM; 24h)/panobinostat (25nM; 24h) and olaparib (10µM) co-treatment combination regimen (24h) on cyclin E, E2F1 and BRCA1 expression in OVCAR-3 cells (24h). Actin was the loading control.
Panobinostat synergizes with olaparib to reduce cell growth and clonogenicity in HR-proficient ovarian cancer cells in vitro
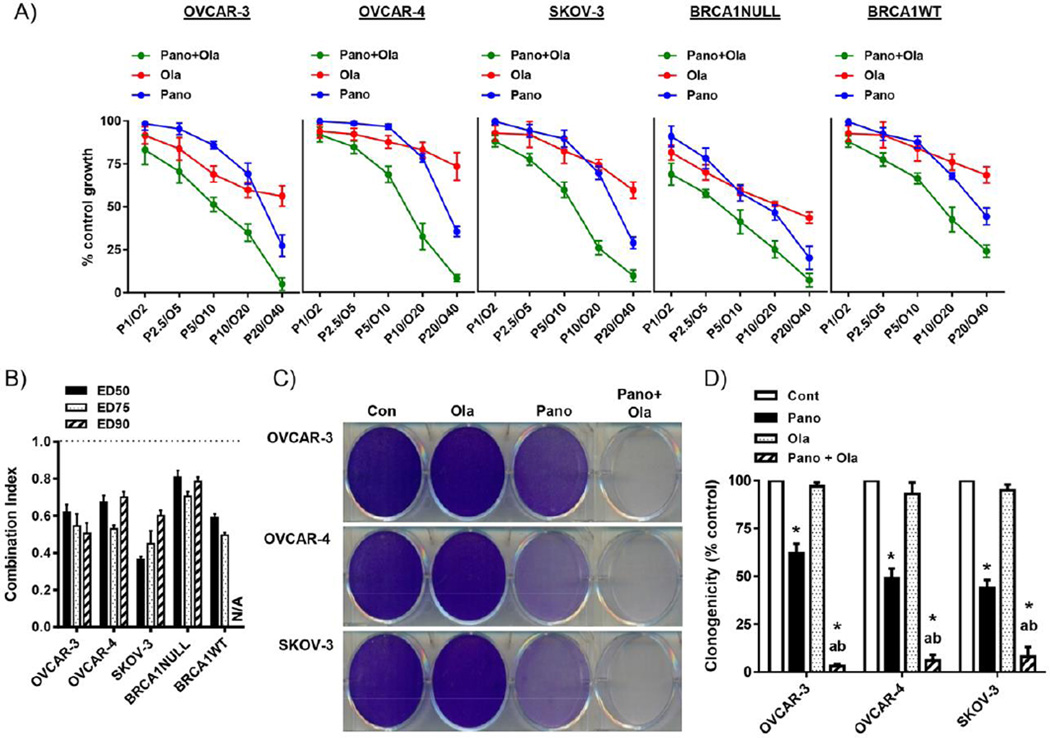
Having established the relative potency of panobinostat compared to vorinostat in models of HR-proficient ovarian cancer, we then studied the combinatorial effects of panobinostat with olaparib. First, timing of the panobinostat/olaparib combination was tested in SRB cell growth assays in OVCAR-3 cells. Cells pre-treated with panobinostat for 24 hours, followed by co-treatment with fixed molar ratios of panobinostat and olaparib was the most effective in reducing cell growth in a synergistic manner, as shown by Combination Index analysis (Supplementary Fig S2). This treatment regimen was chosen for subsequent combination drug experiments in additional models of HR-proficient ovarian cancer, cyclin E-overexpressing OVCAR-4 cells, SKOV-3 and BRCA1 WT and NULL cells. As shown in Fig 3A&B, panobinostat reduced cell viability at increasing concentrations and synergistically enhanced the effects of olaparib in our HR-proficient ovarian cancer cells. We observed a similar pattern of panobinostat-induced sensitization to olaparib in independent clonogenic assays (Fig 3C&D).
Figure 3. Panobinostat enhances the growth inhibitory effects of olaparib in HR-proficient ovarian cancer cells.
A) SRB assay showing combination effects the panobinostat pre-treatment (1–20nM; 24h)/panobinostat (1–20nM) and olaparib (2–40µM) co-treatment combination regimen (72h) in HR-proficient ovarian cancer cell lines, OVCAR-3, OVCAR-4, SKOV-3 and BRCA1 WT cells. B) Combination Index (CI) for ED(Effective Dose)50, ED75 and ED90 was calculated by isobologram analysis. CI < 1 is synergistic. All CI’s were p<0.05 compared to a CI of 1, Student’s t test. N/A not applicable. C) Effects of the panobinostat pre-treatment (25nM; 24h)/panobinostat (25nM; 24h) and olaparib (10µM) co-treatment combination regimen (24h) in clonogenic assays 7–10 days after drug withdrawal. D) Clonogenicity was measured by cumulative staining intensity in triplicate wells. Values in A), B) and D) are mean+SD of 3 independent experiments. *p<0.01 compared to vehicle; ap<0.01 relative to olaparib alone; bp<0.01 relative to panobinostat alone, all Student’s t test.
Combined panobinostat and olaparib treatment reduces HR efficiency in HR-proficient ovarian cancer cells
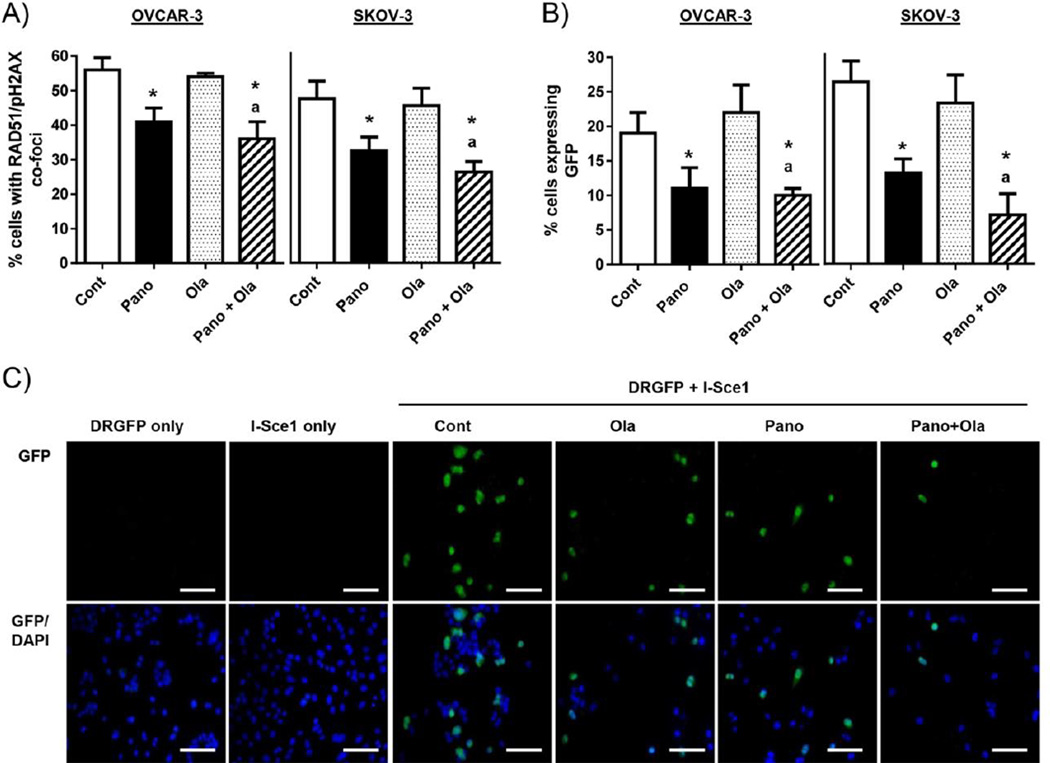
We have previously shown that vorinostat downregulates HR pathway genes and sensitizes ovarian cancer cells to the cytotoxic effects of the PARPi olaparib [4]. To determine if panobinostat had similar cooperative effects with olaparib, we confirmed reduction of cyclin E and BRCA1 expression in OVCAR-3 and SKOV-3 cells treated with the combination of panobinostat and olaparib (Fig 2C and Supplementary Fig S1). Second, we established a direct link between panobinostat inhibition of HR pathway genes and HR efficiency in response to DNA damage using an established IF assay measuring HR by the nuclear co-expression of RAD51 and pH2AX [4]. In cells pre-treated for 6h with the known inducer of double-strand DNA breaks, cisplatin (0.5 µM), there was an approximately 40% reduction in the frequency of cells co-expressing RAD51 and pH2AX with panobinostat as a single agent or in combination with olaparib in OVCAR-3 and SKOV-3 cells (Fig 4A and Supplementary Fig S3).
Figure 4. Panobinostat alone and combined with olaparib reduces HR repair in HR-proficient ovarian cancer cells.
A) The percentage of OVCAR-3 and SKOV-3 cells showing co-expression of RAD51 and pH2AX (positive was >5 foci). Cells were pre-treated with 0.5µM cisplatin (6h), then effects of the panobinostat pre-treatment (25nM; 24h)/panobinostat (25nM; 24h) and olaparib (10µM) co-treatment combination regimen (24h) determined by IF. B) IF analysis of GFP expression in cells co-transfected with pDRGFP and I-Sce1 plasmids (both 1µg), then treated with panobinostat and/or olaparib as above. At least 100 cells were counted in 3 independent fields. Values in A) and B) are mean+SD for 3 independent experiments. * p<0.01 compared to vehicle; ap<0.01 relative to olaparib alone, bp<0.01 relative to panobinostat alone, Student’s t test. C) Representative images of GFP-positive SKOV-3 cells (green) and DAPI-stained nuclei (blue). Scale bars are 20µm.
We then used the well-characterized DRGFP HR reporter plasmid as an independent assay to validate our results. HR events were measured by the production of GFP in cells transiently co-transfected with the DRGFP and I-Sce1 endonuclease plasmids [27, 28], which was assessed by immunofluorescence analysis. Consistent with the RAD51/pH2AX co-localization results, there was a reduction in GFP-positive cells in the panobinostat and panobinostat plus olaparib treatment groups (Fig 4B&C). The specificity of GFP production to cells undergoing HR was demonstrated by evidence that 100% of cells with single transfection of DRGFP or I-Sce1 were GFP-negative (Fig 4B&C).
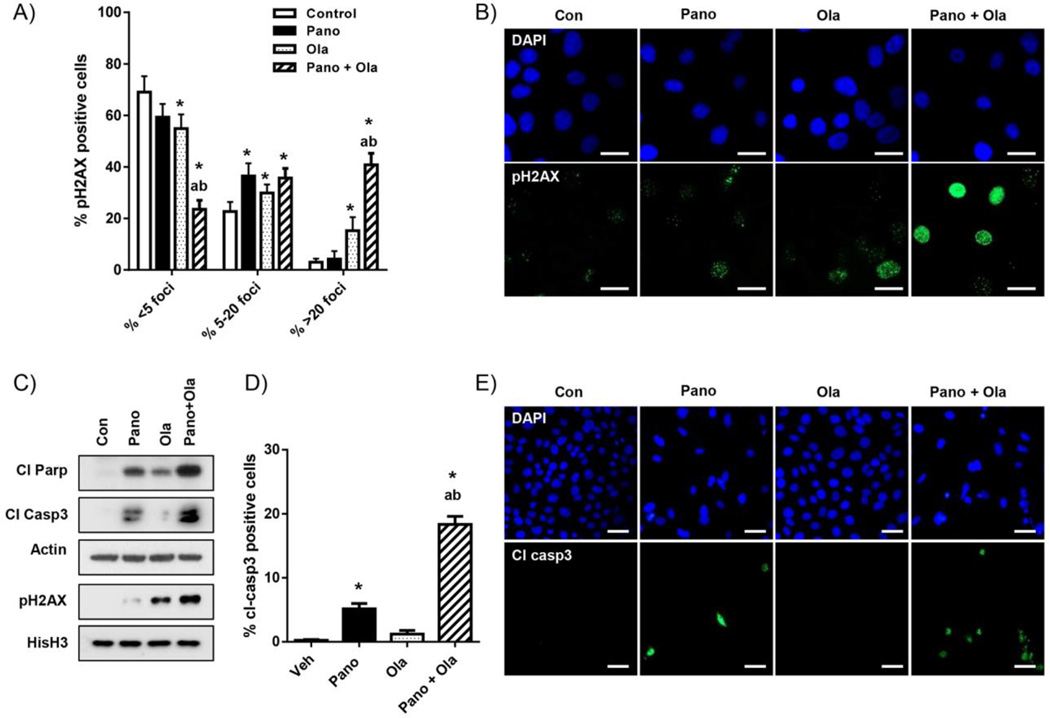
Panobinostat combined with olaparib induces DNA damage and apoptosis more than olaparib alone in HR-proficient ovarian cancer cells
Having demonstrated synergistic reduction in cell growth and viability accompanied by reduced HR efficiency in cells treated with the panobinostat/olaparib combination, we next determined whether these effects resulted in increased DNA damage and cell apoptosis. We have identified persistent pH2AX activation, which is associated with DNA damage-induced apoptosis (30), as a sensitive surrogate marker of cytotoxicity in ovarian cancer cells [18, 19]. First, we quantified drug effects on nuclear pH2AX expression by counting the number of pH2AX-negative cells (less than 6 foci), and with 6–20 and greater than 20 foci (including pan-nuclear staining where individual foci are not countable) in IF assays in OVCAR-3 cells [4] (Fig 5A). Compared to vehicle, olaparib and panobinostat alone, the combination treatment significantly reduced the number of pH2AX-negative cells, and robustly increased the proportion of cells displaying greater than 20 pH2AX foci. These results were confirmed by western blot analysis of purified histone extracts, which showed enhanced pH2AX expression in cells treated with panobinostat and olaparib combined and compared to each drug alone (Fig 5B). Similar results were seen in SKOV-3 cells (Supplementary Fig S4).
Figure 5. Panobinostat enhances the stimulatory effects of olaparib on DNA damage and apoptosis.
A) IF analysis of the effects of panobinostat pre-treatment (25nM; 24h)/panobinostat (25nM; 24h) and olaparib (10µM) co-treatment combination regimen (24h) panobinostat (25nM)/olaparib (10µM) combination (24h) on pH2AX expression in OVCAR-3 cells. B) Representative images showing pH2AX staining (green) and DAPI-stained nuclei (blue). C) Western blot analysis pH2AX, cleaved PARP and cleaved caspase-3 expression in OVCAR-3 cells treated as above. Actin and total histone H3 were loading controls. D) IF analysis of cleaved caspase-3 expression in cells treated as above. Values for A) and D) are mean+SD for 3 independent experiments. At least 100 cells were counted (×40) for each drug treatment per experiment. *p<0.01 compared to vehicle; ap<0.01 relative to olaparib alone; bp<0.01 relative to panobinostat alone, all Student’s t test. E) Representative images of cleaved caspase-3 staining (green) and DAPI-stained nuclei (blue). Scale bars in B) are 20µm and in E) are 10µm.
To assess apoptosis, we measured the expression of the established apoptotic markers, cleaved PARP and cleaved caspase-3 [4]. Consistent with the pH2AX data, there was increased expression of these markers with combined panobinostat and olaparib treatment compared to olaparib alone by western blot (Fig 5C) and IF analyses (Fig 5D&E) in OVCAR-3 cells. We obtained similar results using SKOV-3 cells (Supplementary Fig S4).
In addition, we tested the ability of panobinostat to sensitize models of HR-proficiency to a second clinically relevant DNA damaging drug, cisplatin. As shown in Supplementary Fig S5A&B, panobinostat synergized with cisplatin in SRB assays, and the combination of panobinostat and cisplatin induced higher levels of pH2AX and apoptosis than either drug alone by western blot and IF assays (Supplementary Fig S5C–E).
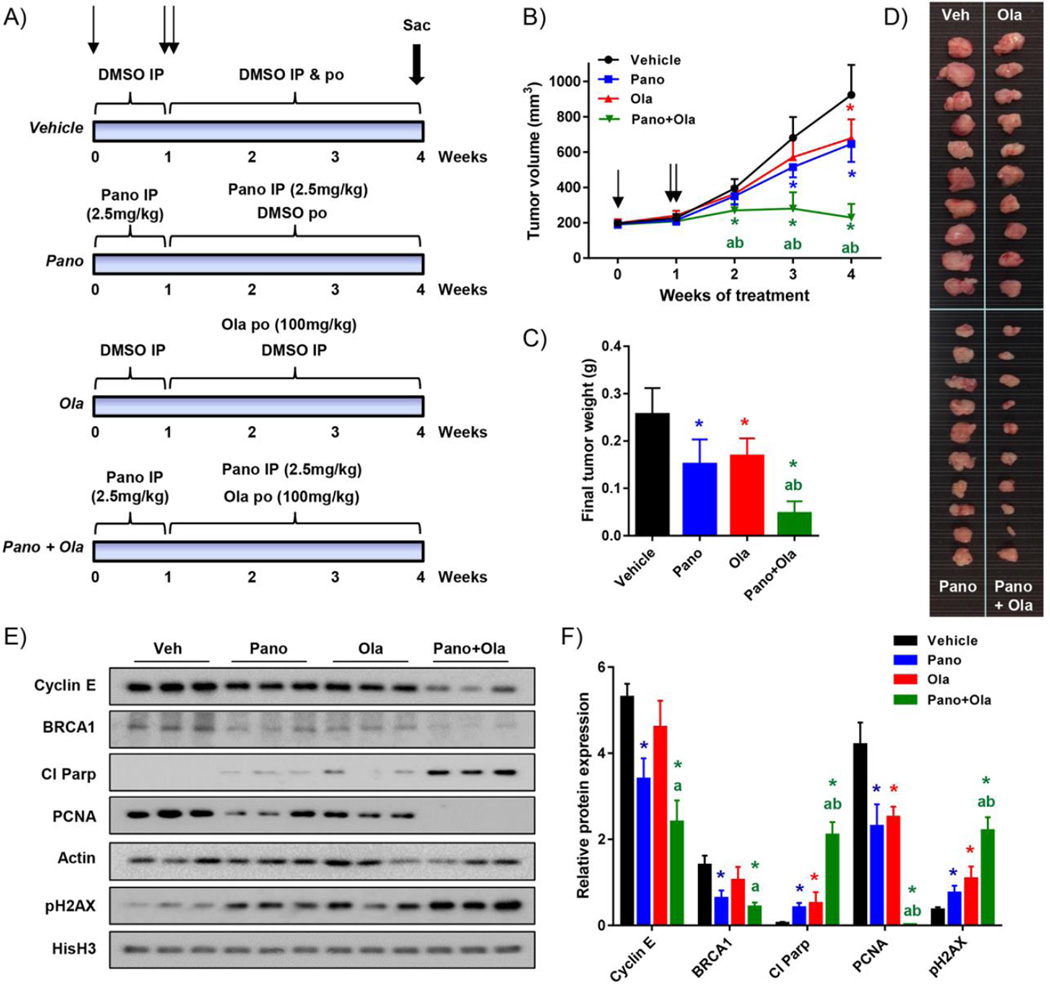
Combination treatment with panobinostat and olaparib shows enhanced robust inhibitory effects in HR-proficient ovarian cancer cells grown as xenograft tumors
Nude mice injected subcutaneously with SKOV-3 cells were treated with olaparib alone and in combination with panobinostat for 3 weeks following an initial 7 day panobinostat pre-treatment (Fig 6A). There was similar tumor growth in panobinostat or vehicle pre-treatment groups over the 7-day pre-treatment phase (Fig 6B). Both panobinostat and olaparib led to significant tumor growth inhibition as single agents compared to vehicle controls (Fig 6B–D), and tumors in the panobinostat/olaparib combination group were significantly smaller compared to vehicle and each drug alone. Further, the combination led to a greater than 80% reduction in tumor volume and weight at sacrifice, compared to these indices in control mice (Fig 6B–D). These differences were unlikely to be a result of generalized toxicity of drug treatment, since mouse weights were similar over the treatment period for all treatment groups (Supplementary Fig S6). We confirmed down-regulation of cyclin E and BRCA1 protein expression in tumors treated with panobinostat alone or combined with olaparib (Fig 6E&F). Finally, consistent with the relative effects on tumor size, the combination treatment reduced expression of the pharmacodynamic marker of proliferation (PCNA), and increased expression of apoptosis (cleaved PARP), and DNA damage (pH2AX) markers to a significantly greater extent than either panobinostat or olaparib alone (Fig 6E&F).
Figure 6. Combination panobinostat/olaparib treatment markedly reduces growth of HR-proficient ovarian tumor xenografts.
A) Schematic of drug treatment experiment in nude mice injected subcutaneously with SKOV-3 cells. Mice (10 per group) were pre-treated for one week with vehicle or panobinostat, and then subsequently with vehicle, panobinostat and/or olaparib for three weeks as shown (IP: intraperitoneal; PO: per os, oral gavage). B) Time course measurements of tumor volume at weekly intervals (single arrow: start of panobinostat pre-treatment; double arrow: start of full drug regimen). C) Tumor weights at sacrifice. Tumors are shown in D). E) Western blot analysis of cyclin E, BRCA1, cleaved PARP, PCNA and pH2AX protein expression in harvested tumors. Actin and histone H3 were loading controls. F) Densitometry analysis of expression relative to corresponding actin or histone H3 levels. Values are mean+ SD; *p<0.01 single drug effect relative to vehicle; ap<0.01 combination drug effect relative to olaparib; bp<0.01 combination drug effect relative to panobinostat, Student’s t test.
DISCUSSION
CCNE1 (cyclin E) amplification/gain HR-proficient ovarian cancer is associated with poor clinical outcomes and chemoresistance [6, 9–14]. Extending the efficacy of PARPi to these HR-proficient tumors is an unmet clinical need. An emerging paradigm in multiple tumor types is to convert HR-proficient tumors to HR-deficient phenotypes by using epigenetic drugs [4, 9]. Our group has previously shown that the HDACi vorinostat sensitizes HR proficient SKOV-3 cells to the cytotoxic effects of the PARPi, olaparib [4]. In this study, we focused on the HDACi panobinostat because of its potency and superior pharmacokinetics to vorinostat [20] as a means of sensitizing cyclin E-overexpressing, HR-proficient ovarian cancer cells to olaparib. We confirmed that panobinostat induced potent growth inhibitory and pro-apoptotic effects at nanomolar concentrations in ovarian cancer cell lines in vitro. In contrast, similar effects required vorinostat concentrations that were several orders of magnitude higher, likely due to reduced cellular bioavailability and potency of inhibition of HDAC activity compared to panobinostat.
Here, we found that panobinostat led to downregulation of cyclin E, E2F1 and HR pathway genes, such as BRCA1, in HR proficient, cyclin E-amplified OVCAR-3 ovarian cancer cells, and in SKOV-3 cells. Moreover, there was synergistic enhancement of cytotoxicity in these cell lines and in other models of HR-proficiency (BRCA1 WT and cyclin E-overexpressing OVCAR-4 cells) when panobinostat was combined with the PARPi olaparib. Optimal effects of the combination occurred when cells were pre-treated with panobinostat prior to treatment with both drugs. This combination regimen also reduced tumor growth to a greater extent than olaparib alone in HR-proficient SKOV-3 xenografts in vivo. Our experimental design for the in vivo experiments were changed in two important ways from our previous study combining vorinostat and olaparib [4]: olaparib was delivered orally and mice were “primed” by panobinostat pre-treatment prior to exposure to olaparib. Along with the superior pharmacokinetics of panobinostat compared to vorinostat [20], these factors likely contributed to the superior performance of the panobinostat/olaparib combination in the present study. The overall anti-tumor effects of the panobinostat/olaparib were strongly replicated in vivo. Enhanced cytotoxicity of the combination was associated with pharmacodynamic marks of proliferation, apoptosis, prolonged activation of pH2AX, and reduced HR efficiency in response to DNA damage. However, some differences were observed. For example, downregulation of cyclin E differed in SKOV-3 cells grown in vitro and in vivo. The most likely factors include the longer treatment time in vivo (3 weeks compared to 24 hours) and inherent differences in the growth of cells as a monolayer in vitro versus as tumors, which rely on systemic circulation for drug delivery.
We acknowledge several limitations of our study. The SKOV-3 cell line is HR-proficient but does not harbor gain or amplification in cyclin E. However, it was chosen for the xenograft experiments because SKOV-3 cells readily form reproducible subcutaneous tumors in nude mice [4, 18]. In contrast, the cyclin E-overexpressing OVCAR-3 cell line has an unpredictable growth rate in vivo. We acknowledge that intra-peritoneal (IP) xenograft models of ovarian cancer mimic the peritoneal disease spread in humans better than subcutaneous tumors. IP models require in vivo imaging modalities to monitor treatment effects longitudinally, which will be pursued in future studies now that dose and schedule have been optimized. Another potential limitation of combining panobinostat and olaparib treatment is toxicity, as toxic side-effects of both panobinostat and olaparib in the clinic are well-recognized [2, 7, 8, 24, 31–33]. Our in vivo studies showed that there was minimal overall effect of drug treatment on mouse weight. Further, panobinostat is approved in a triple-drug regimen for multiple myeloma, suggesting that the therapeutic benefit may outweigh risks in selected patients [33].
The main strength of this study is that relatively low doses of panobinostat HDACi enhanced responses to the DNA damaging drugs olaparib and cisplatin. We speculate that sub-lethal doses of HDACi induce transcriptional reprogramming that is permissive for synthetic lethality with the PARPi olaparib, specifically through downregulation of HR pathway genes. This transcriptional effect likely contributes to the requirement for pre-treatment with panobinostat, for optimal combinatorial effects with olaparib, and thus, has important implications for clinical development of this combination. The potential to harness transcriptional reprogramming as a therapeutic anti-cancer strategy is emphasized by studies showing enhanced anti-tumor effects of HDACi combined with various chromatin-modulating drugs, including DNA methyltransferase and bromodomain inhibitors [34, 35]. Finally, it is possible that lower doses of panobinostat administered with therapeutic doses of olaparib will have significant benefit with minimal added toxicity.
An additional mechanism by which HDACi lead to synthetic lethality in transformed cells is through induction of replicative stress [36]. The contribution of replicative stress to the combinatory effects of panobinostat and olaparib will be investigated in future studies. Future studies will also determine the contribution of inhibition of specific isoforms, particularly HDAC3, which is known to promote DNA repair and protect cells from replicative stress [36–39]. Identification and targeting of specific HDAC isoforms involved in DNA damage and repair processes may improve both treatment efficacy and reduce toxicity associated with broad inhibition of homeostatic HDAC functions.
In conclusion, the response of cyclin E-overexpressing, HR-proficient ovarian cancer cells to the PARPi olaparib is enhanced by pre-treatment, followed by co-treatment with the HDACi, panobinostat. Our preclinical studies indicate that the efficacy of PARPi in cyclin E high, HR-proficient ovarian cancer is improved by downregulating HR gene expression and efficiency with HDACi drugs. Therefore, these preclinical studies provide strong rationale for extending the use of PARPi, in combination with HDACi, to a significant proportion of women diagnosed with cyclin E-overexpressing and other types of HR-proficient ovarian cancer.
Supplementary Material
Research Highlights.
Panobinostat downregulates DNA damage repair genes and homologous recombination (HR) efficiency in cyclin E-overexpressing, HR-proficient ovarian cancer cells.
Panobinostat synergizes with the poly (ADP-ribose) polymerase inhibitor olaparib to inhibit growth and viability in HR-proficient ovarian cancer cells.
Panobinostat combined with olaparib promotes DNA damage and apoptosis in HR-proficient ovarian cancer cells in vitro and in vivo.
Acknowledgments
We acknowledge the Vanderbilt University High-Throughput Screening Core Facility, the Vanderbilt Translational Pathology Shared Resource, and the members of and donors to the Vanderbilt Ovarian Cancer Alliance (VOCAL). Olaparib was provided by Astra Zeneca Pharmaceuticals.
Financial support: DK was supported by NIH grants 5P30 CA068485, CA091408 5 U54, 1UL1 RR024975 and K08CA148887. The Vanderbilt Translational Pathology Shared Resource is supported by NCI/NIH Cancer Center Support Grant 2P30 CA068485.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: DK received olaparib from AstraZeneca and is a co-investigator for a clinical trial of olaparib. MAC is the local principal investigator for a clinical trial of olaparib.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott CL, Swisher EM, Kaufmann SH. Poly (adp-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33(12):1397–1406. doi: 10.1200/JCO.2014.58.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konstantinopoulos PA, Wilson AJ, Saskowski J, Wass E, Khabele D. Suberoylanilide hydroxamic acid (SAHA) enhances olaparib activity by targeting homologous recombination DNA repair in ovarian cancer. Gynecol. Oncol. 2014;133(3):599–606. doi: 10.1016/j.ygyno.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner N, Tutt A, Ashworth A. Hallmarks of 'BRCAness' in sporadic cancers. Nature reviews Cancer. 2004;4(10):814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 8.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 9.Wiedemeyer WR, Beach JA, Karlan BY. Reversing Platinum Resistance in High-Grade Serous Ovarian Carcinoma: Targeting BRCA and the Homologous Recombination System. Front Oncol. 2014;4:34. doi: 10.3389/fonc.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akli S, Keyomarsi K. Cyclin E and its low molecular weight forms in human cancer and as targets for cancer therapy. Cancer biology & therapy. 2003;2(4 Suppl 1):S38–S47. [PubMed] [Google Scholar]
- 11.Topp MD, Hartley L, Cook M, Heong V, Boehm E, McShane L, et al. Molecular correlates of platinum response in human high-grade serous ovarian cancer patient-derived xenografts. Mol. Oncol. 2014;8(3):656–668. doi: 10.1016/j.molonc.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karst AM, Jones PM, Vena N, Ligon AH, Liu JF, Hirsch MS, et al. Cyclin E1 deregulation occurs early in secretory cell transformation to promote formation of fallopian tube-derived high-grade serous ovarian cancers. Cancer Res. 2014;74(4):1141–1152. doi: 10.1158/0008-5472.CAN-13-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etemadmoghadam D, George J, Cowin PA, Cullinane C, Kansara M, Gorringe KL, et al. Australian Ovarian Cancer Study Group. Amplicon-dependent CCNE1 expression is critical for clonogenic survival after cisplatin treatment and is correlated with 20q11 gain in ovarian cancer. PloS One. 2010;5(11):e15498. doi: 10.1371/journal.pone.0015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 15.Etemadmoghadam D, Weir BA, Au-Yeung G, Alsop K, Mitchell G, George J, et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. PNAS. 2013;110(48):19489–19494. doi: 10.1073/pnas.1314302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etemadmoghadam D, Au-Yeung G, Wall M, Mitchell C, Kansara M, Loehrer E, et al. Resistance to CDK2 inhibitors is associated with selection of polyploid cells in CCNE1-amplified ovarian cancer. Clin Cancer Res. 2013;19(21):5960–5971. doi: 10.1158/1078-0432.CCR-13-1337. [DOI] [PubMed] [Google Scholar]
- 17.Taylor-Harding B, Aspuria PJ, Agadjanian H, Cheon DJ, Mizuno T, Greenberg D, et al. Cyclin E1 and RTK/RAS signaling drive CDK inhibitor resistance via activation of E2F and ETS. Oncotarget. 2015;6(2):696–714. doi: 10.18632/oncotarget.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson AJ, Lalani AS, Wass E, Saskowski J, Khabele D. Romidepsin (FK228) combined with cisplatin stimulates DNA damage-induced cell death in ovarian cancer. Gynecol. Oncol. 2012;127(3):579–586. doi: 10.1016/j.ygyno.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson AJ, Holson E, Wagner F, Zhang YL, Fass DM, Haggarty SJ, et al. The DNA damage mark pH2AX differentiates the cytotoxic effects of small molecule HDAC inhibitors in ovarian cancer cells. Cancer Biol. Ther. 2011;12(6):484–493. doi: 10.4161/cbt.12.6.15956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anne M, Sammartino D, Barginear MF, Budman D. Profile of panobinostat and its potential for treatment in solid tumors: an update. OncoTargets and Therapy. 2013;6:1613–1624. doi: 10.2147/OTT.S30773. Epub 2013/11/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenzi PL, Reinhold WC, Varma S, Hutchinson AA, Pommier Y, Chanock SJ, et al. DNA fingerprinting of the NCI-60 cell line panel. Mol. Cancer Ther. 2009;8(4):713–724. doi: 10.1158/1535-7163.MCT-08-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scudiero DA, Monks A, Sausville EA. Cell line designation change: multidrug-resistant cell line in the NCI anticancer screen. J Nat. Cancer Inst. 1998;90(11):862. doi: 10.1093/jnci/90.11.862. [DOI] [PubMed] [Google Scholar]
- 23.DelloRusso C, Welcsh PL, Wang W, Garcia RL, King MC, Swisher EM. Functional characterization of a novel BRCA1-null ovarian cancer cell line in response to ionizing radiation. Mol Cancer Res. 2007;5(1):35–45. doi: 10.1158/1541-7786.MCR-06-0234. [DOI] [PubMed] [Google Scholar]
- 24.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 25.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 26.Wilson AJ, Fadare O, Beeghly-Fadiel A, Son DS, Liu Q, Zhao S, et al. Aberrant overexpression of COX-1 intersects multiple pro-tumorigenic pathways in high-grade serous ovarian cancer. Oncotarget. 2015:21353–21368. doi: 10.18632/oncotarget.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierce AJ, Johnson RD, Thompson LH, Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13(20):2633–2638. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson C, Moynahan ME, Jasin M. Double-strand break repair by interchromosomal recombination: suppression of chromosomal translocations. Genes Dev. 1998;12(24):3831–3842. doi: 10.1101/gad.12.24.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banath JP, Klokov D, MacPhail SH, Banuelos CA, Olive PL. Residual gammaH2AX foci as an indication of lethal DNA lesions. BMC Cancer. 2010;10:4. doi: 10.1186/1471-2407-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30(21):2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 33.Richardson PG, Harvey RD, Laubach JP, Moreau P, Lonial S, San-Miguel JF. Panobinostat for the treatment of relapsed or relapsed/refractory multiple myeloma: pharmacology and clinical outcomes. Exp. Review Clin. Pharmacol. 2016;9(1):35–48. doi: 10.1586/17512433.2016.1096773. [DOI] [PubMed] [Google Scholar]
- 34.Shahbazi J, Liu PY, Atmadibrata B, Bradner JE, Marshall GM, Lock RB, et al. The Bromodomain Inhibitor JQ1 and the Histone Deacetylase Inhibitor Panobinostat Synergistically Reduce N-Myc Expression and Induce Anticancer Effects. Clin. Cancer Res. 2016;22(10):2534–2544. doi: 10.1158/1078-0432.CCR-15-1666. [DOI] [PubMed] [Google Scholar]
- 35.Fiskus W, Buckley K, Rao R, Mandawat A, Yang Y, Joshi R, et al. Panobinostat treatment depletes EZH2 and DNMT1 levels and enhances decitabine mediated de-repression of JunB and loss of survival of human acute leukemia cells. Cancer Biol. Ther. 2009;8(10):939–950. doi: 10.4161/cbt.8.10.8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wells CE, Bhaskara S, Stengel KR, Zhao Y, Sirbu B, Chagot B, et al. Inhibition of histone deacetylase 3 causes replication stress in cutaneous T cell lymphoma. PloS one. 2013;8(7):e68915. doi: 10.1371/journal.pone.0068915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhaskara S, Chyla BJ, Amann JM, Knutson SK, Cortez D, Sun ZW, et al. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol. Cell. 2008;30(1):61–72. doi: 10.1016/j.molcel.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhaskara S, Knutson SK, Jiang G, Chandrasekharan MB, Wilson AJ, Zheng S, et al. Hdac3 is essential for the maintenance of chromatin structure and genome stability. Cancer Cell. 2010;18(5):436–447. doi: 10.1016/j.ccr.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khabele D. The therapeutic potential of class I selective histone deacetylase inhibitors in ovarian cancer. Front Oncol. 2014;4:111. doi: 10.3389/fonc.2014.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.