Abstract
Generating the correct balance of inhibitory and excitatory neurons in a neural network is essential for normal functioning of a nervous system. The neural network in the dorsal spinal cord functions in somatosensation where it modulates and relays sensory information from the periphery. PTF1A is a key transcriptional regulator present in a specific subset of neural progenitor cells in the dorsal spinal cord, cerebellum and retina that functions to specify an inhibitory neuronal fate while suppressing excitatory neuronal fates. Thus, the regulation of Ptf1a expression is critical for determining mechanisms controlling neuronal diversity in these regions of the nervous system. Here we identify a sequence conserved, tissue-specific enhancer located 10.8 kb 3′ of the Ptf1a coding region that is sufficient to direct expression to dorsal neural tube progenitors that give rise to neurons in the dorsal spinal cord in chick and mouse. DNA binding motifs for Paired homeodomain (Pd-HD) and zinc finger (ZF) transcription factors are required for enhancer activity. Mutations in these sequences implicate the Pd-HD motif for activator function and the ZF motif for repressor function. Although no repressor transcription factor was identified, both PAX6 and SOX3 can increase enhancer activity in reporter assays. Thus, Ptf1a is regulated by active and repressive inputs integrated through multiple sequence elements within a highly conserved sequence downstream of the Ptf1a gene.
Keywords: dorsal spinal cord, PTF1A, neuronal specification, inhibitory neurons, transcriptional regulation, tissue-specific enhancer
Graphical Abstract
INTRODUCTION
The proper functioning of the central nervous system (CNS) is a result of the intricate balance between the activity of excitatory and inhibitory neurons. Improper specification of these neuronal subtypes during development can disrupt this balance and lead to neurological disorders or lethality (Fields et al., 1991; McCormick and Contreras, 2001). Previous studies have identified basic helix-loop-helix (bHLH) transcription factors as crucial regulators of neuronal subtype specification in the dorsal spinal cord where somatosensory information is processed (Glasgow et al., 2005; Gowan et al., 2001; Helms et al., 2005; Mizuguchi et al., 2006; Muller et al., 2005; Ross et al., 2010; Wildner et al., 2013). PTF1A is one such factor, initially identified as critical for pancreas development that is also required for specification of inhibitory neurons in various regions of the developing CNS including the spinal cord, brainstem, cerebellum, and retina (Dullin et al., 2007; Fujitani et al., 2006; Glasgow et al., 2005; Hoshino et al., 2005; Iskusnykh et al., 2016; Kawaguchi et al., 2002; Millen et al., 2014; Nakhai et al., 2007; Pascual et al., 2007; Yamada et al., 2007). In the dorsal spinal cord, PTF1A is induced in a population of progenitor cells that will give rise to inhibitory neurons when PTF1A is present, but in its absence the progenitors differentiate into excitatory neurons (Glasgow et al., 2005). Thus, identifying how Ptf1a expression is induced within these progenitors in the dorsal neural tube will reveal key mechanisms for generating the balance of inhibitory and excitatory neurons required for normal somatosensory function.
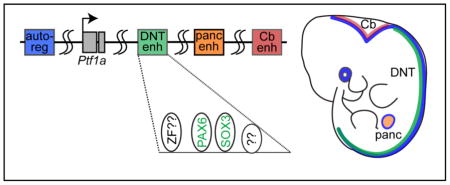
Available evidence from multiple species suggests that the complex regulation of Ptf1a expression is through multiple distal cell-type specific cis-elements spread across a large genomic region (see Graphical Abstract). The cerebelless mouse mutant (cbll) has a specific loss of Ptf1a expression in the cerebellum but not other Ptf1a domains. The cbll mutation is a 313 kb deletion 60 kb downstream of Ptf1a (Hoshino et al., 2005) suggesting the presence of a cerebellar-specific enhancer within this deletion, an enhancer that has not been further delineated. Whole-genome sequencing of humans with isolated pancreatic agenesis identified a 400 bp region 25 kb downstream of PTF1A that functions as an enhancer for PTF1A in the pancreas (Weedon et al., 2014). No function for this enhancer was reported in neural tissues. Zebrafish transgenic reporter studies revealed multiple large genomic regions in a 48 kb interval encompassing the Ptf1a locus had distinct activities directing expression of GFP to subsets of the Ptf1a expression domain including the spinal cord, cerebellum, retina, and pancreas (Pashos et al., 2013). In transgenic mice, a regulatory sequence comprising a 12.4 kb highly conserved genomic region downstream of the Ptf1a stop codon was shown to direct expression of a reporter gene to the dorsal neural tube encompassing the endogenous Ptf1a domain. This transgene did not efficiently function in pancreas or cerebellum (Masui et al., 2008; Meredith et al., 2009). And finally, a highly conserved 2.3 kb auto-regulatory enhancer was identified 13.4 kb upstream of the Ptf1a coding region that activates transcription of Ptf1a in all Ptf1a expressing domains including the pancreas and the nervous system (Masui et al., 2008; Meredith et al., 2009). Thus, multiple enhancers appear to work combinatorially to regulate the diverse expression pattern of Ptf1a during development.
Given the importance of Ptf1a expression in generating the correct composition of inhibitory and excitatory neurons in the dorsal spinal cord, we set out to delineate the dorsal neural tube (DNT) enhancer downstream of the Ptf1a coding region. The requirement for cis-elements within the enhancer identifies transcription factor binding motifs providing insight into the upstream regulatory factors controlling Ptf1a expression. In this study, we identify a 1.1 kb DNT enhancer that functions in chick and mouse neural tube and receives activating and repressive signals that generate the tissue-specific properties of this enhancer. Activity of the DNT enhancer depends on the integrity of a motif matching the consensus binding site for the Paired-homeodomain family of transcription factors, and PAX6 is shown to increase enhancer activity. SOX3 is also identified as a potential DNT enhancer activator. Thus, a DNT-specific enhancer that integrates activities from multiple transcription factor families is implicated in the control of Ptf1a expression.
MATERIALS AND METHODS
Generation of reporter constructs
The UCSC genome browser was used to assess the mammalian conservation within the 12.4 kb regulatory region from 3′ of the Ptf1a gene identified in Meredith et al., 2009. The homology regions R1 (chr2: 19448483–19449519); R2 (chr2: 194503415–19452622); R3 (chr2: 19453522–19454928); R4/5 (chr2: 19454941–19456589); R6 (chr2: 19457563–19458031); R7 (chr2: 19458593–19459718); 132 bp ECR (chr2: 19458902–19459007) were cloned into BG-nEGFP or BG-nmCherry reporter (Meredith et al., 2009). These reporter cassettes contain the β-globin minimal promoter, a nuclear localized fluorescence reporter, and the 3′ cassette from the human growth hormone. Genomic coordinates for the homologies used are based on the mouse mm10 genome build. Each region was PCR amplified from the original 12.4 kb construct, SalI and BamHI restriction sites introduced on each end, and products were cloned adjacent to the β-globin minimal promoter in the reporter cassettes. A PCR mutagenesis strategy was used to generate the R7Δ132, R7mZF, R7mPd-HD, R7m1Sox, R7m2Sox, R7m3Sox, R7m12Sox, R7m13Sox, R7m23Sox and R7m123Sox mutations. The SOX1, SOX2, SOX3, PAX3, PAX6 and PAX7 expression constructs were generated by cloning the respective cDNA into a modified pMiWIII expression vector (Matsunaga et al., 2001; Suemori et al., 1990) such that each transcription factor is Myc tagged. All constructs were sequence verified.
Generation of transgenic mice
Each transgene was liberated from the recombinant plasmid described above using SalI, XhoI, and ScaI, and gel purified. Each transgene was injected at 1–3 ng/μl into the pronuclei of fertilized eggs from B6SJLF1 (C57BL/6JxSJL) by the Transgenic Core facility at UT Southwestern (Dallas, TX, USA). Transgenic embryos were identified using DNA isolated from embryonic yolk sacs and PCR analysis to detect GFP or mCherry. For transient transgenic analysis, embryos were harvested at E10.5 and E12.5, and subsequently cryosectioned and immunostained as described below. A single stable transgenic line for R7-GFP was generated. Embryos were staged based on the assumed copulation at E0, halfway through the dark cycle.
In-ovo chick electroporation assay
Fertilized White Leghorn eggs were obtained from the Texas A&M Poultry Department (College Station, TX, USA) and incubated for 48 hours at 37°C. The supercoiled reporter plasmids described above were diluted to 1.5 mg/ml in H20/1X loading dye and injected individually into the lumen of the closed neural tube of chick embryos at stages HH13-15. A pMiWIII-Myc epitope tagged vector was injected along with reporter plasmids as an electroporation control. The injected embryos were then electroporated with 5 pulses of 25 mV each for 50 msec with intervals of 100 msec. Embryos were harvested 48 hours later at stages HH22-23, fixed with 4% paraformaldehyde for 45 minutes, and processed for cryosectioning and immunofluorescence as described below.
Tissue processing and immunofluorescence
Mouse embryos at E10.5 and E12.5 were dissected in ice-cold PBS, fixed in 4% paraformaldehyde for 1 and 2 hours respectively at 4°C, and washed three times in PBS. Fixed mouse and chick embryos were sunk in 30% sucrose in PBS, embedded in OCT, and cryosectioned at 30μm. Immunofluorescence was performed on sections incubated using the antibodies described below at the indicated dilutions in 1% goat serum/0.2% NP-40/1xPBS followed by incubation with the appropriate secondary antibodies conjugated with either Alexa Fluor 488, 567, or 647 (Invitrogen). The primary antibodies used include guinea pig anti-ASCL1 (1:10,000 (Battiste et al. 2007)); guinea pig anti-PTF1A (1:10,000 (Hori et al. 2008)); mouse anti-MYC1 (1:1000; Santa Cruz Biotechnology, Cat. #sc-789, A-14); rabbit anti-PAX2 (1:500, Invitrogen, Cat. #36-9200), and guinea pig anti-TLX1/3 (1:20,000; Muller et al., 2005). Secondary antibodies used include goat anti-guinea pig (1:500, Invitrogen, Cat. #A-11073/A-11076/A-21450), goat anti-rabbit (1:500, Invitrogen, Cat. # A-11034/A-11037/A-21445) and goat anti-mouse (1:500, Invitrogen, Cat. #A-110041/A-11005/A-21235). Fluorescence imaging was carried out on a Zeiss LSM 510 confocal microscope. In situ hybridization for cPtf1a mRNA were performed as previously described (Chang et al., 2013). Multiple sections from at least five embryos were analysed at upper limb levels for each set of experiments.
Quantification of GFP intensity
Transverse sections of chick neural tube were analysed using ImageJ software. All sections within in a comparison group were mounted on one slide for immunostaining to reduce variability. A set gain setting was used for imaging in each channel using Zen Microscope and Imaging software for comparative analysis of each neural tube. The average GFP intensity relative to MYC levels (to control for electroporation efficiency) was calculated for the electroporated side of each neural tube (N>5). Student T test was used to calculate the p-value for significance.
RESULTS & DISCUSSION
A 1.1 kb 3′ distal enhancer is sufficient to drive transcription to a Ptf1a-specific domain in the neural tube
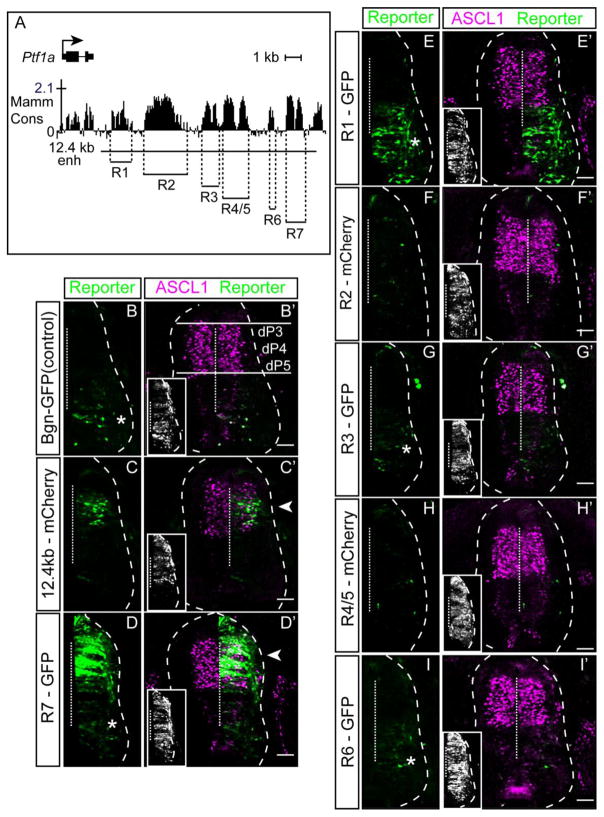
The spinal cord arises during embryogenesis from the caudal neural tube. The neural tube comprises progenitor cells residing in the ventricular zone and cells differentiating into post-mitotic neurons located laterally. Combinatorial expression of multiple transcription factors, largely homeodomain (HD) and bHLH factors, has led to the description of multiple distinct progenitor domains that give rise to defined neuronal populations. In mouse E10.5 or chick HH20-22, the dorsal neural tube has six of these (dorsal interneuron 1-6, dI1-6). The neural progenitors residing in dorsal domains 3-5 (dP3-5) express the bHLH factor ASCL1. During neurogenesis, PTF1A is induced in a subset of these cells and defines those cells as dorsal progenitor domain 4 (dP4). Importantly, these PTF1A+ cells give rise to inhibitory neurons (PAX2+, dI4), while the neighbouring ASCL1+/PTF1A− cells become excitatory neurons (TLX3+, dI3 and dI5) (Glasgow et al., 2005; Hori et al., 2008; Mizuguchi et al., 2006). In previous work from our lab, we demonstrated that a 12.4 kb region downstream of Ptf1a directs reporter gene expression to the dorsal neural tube encompassing the Ascl1 and Ptf1a expression domains (Meredith et al., 2009). The 12.4 kb sequence contains several distinct regions that are highly conserved between multiple mammalian species (Fig. 1A). Indeed, the genomic region downstream of the gene is more highly conserved than that found upstream. Based on this conservation, the 12.4 kb region can be divided into seven separate regions defined R1-R7 (Fig. 1A). To assess which of these conserved regions, if any, are sufficient to drive Ptf1a-specific expression in the dorsal neural tube, each region was tested in the chick neural tube using electroporation of heterologous reporter plasmids.
Figure 1. Identification of a neural specific-Ptf1a enhancer directing dorsal neural tube expression.
(A) Top: Graphical representation of sequence conservation across mammals including the Ptf1a coding and 3′ non-coding genomic regions (adapted from the UCSC Genome Browser). Bottom: Position of genomic regions within a previously reported 12.4 kb enhancer tested here for enhancer activity (R1-R7). The conserved region located beyond the 3′ extent of the 12.4 enhancer was not tested. (B–I′) Transverse sections from chick neural tubes electroporated with fluorescence reporter constructs as indicated at HH13-14 and harvested 24 hours later. Reporter is shown in green, and ASCL1 immunofluorescence (marking progenitors dP3-dP5) is magenta. (C–C′) 12.4 kb regulatory region drives expression to a Ptf1a-specific domain (dP4) (arrowhead). (D–D′) R7 is sufficient to drive reporter expression in the dP4 (arrowhead) although expression is more broad than the full 12.4 kb enhancer. (E–I′) R1, R2, R3/4, R5 and R6 do not direct expression of the reporter in the dorsal neural tube. (B, D, E, G, I) Asterisks indicate the GFP reporter cassette has intrinsic activity in the ventral neural tube. Insets show immunofluorescence for a MYC-epitope tag vector used to confirm electroporation efficiency. Dashed vertical line indicates the midline. Scale bar: 50 μM.
Enhancer-reporter plasmids were electroporated unilaterally into the chick neural tube at stage HH13-15 and the embryos harvested 48 hours later. To control for electroporation efficiency, an expression vector pMiWIII expressing a MYC-epitope tag was included in all experiments (Fig. 1, insets). To provide a reference marker for the dorsal neural tube progenitor domains, co-localization of the reporter genes with ASCL1 (defining dP3-5) was performed (see Fig. 1B′). Immunofluorescence for PTF1A that would mark dP4 was not done because the available antibodies for PTF1A do not recognize chick PTF1A. The candidate enhancer regions R1-R7 were tested for their ability to direct expression of a GFP or mCherry containing reporter to the dorsal neural tube in a similar manner to the 12.4 kb originally tested (Fig. 1C). Only the most distal of these regions, R7, was sufficient to direct expression of the reporter to the dorsal neural tube (Fig. 1D–I). R2-R6 did not have activity outside of the basal level intrinsic to the GFP reporter cassette (Fig. 1B; asterisk). R1 consistently exhibited ventral GFP expression above the basal cassette levels, an activity that must be repressed in the context of the full length 12.4 kb enhancer (Fig. 1E). The expression driven by R7 extends dorsally beyond that of the full length 12.4 kb regulatory region, also suggesting that one or more of R1-R6 regions is necessary to repress aberrant dorsal activation of Ptf1a. Nonetheless, these results demonstrate that the 1.1 kb R7 sequence located approximately 10.8 kb downstream of the Ptf1a coding sequence is sufficient to drive expression to the domain expressing endogenous PTF1A in the dorsal neural tube.
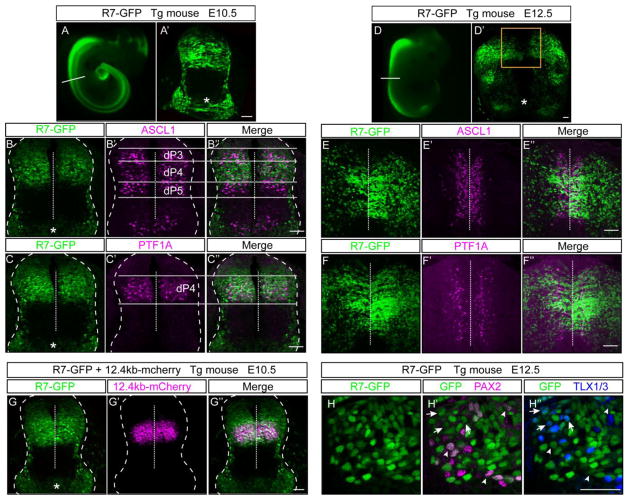
To support the activity of R7 as a neural specific enhancer that can direct reporter gene expression to the dorsal neural tube, we generated transgenic mice using the R7-GFP reporter construct. In transgenic embryos, the R7 enhancer directed robust neural specific GFP expression in the dorsal neural tube with no expression detected in forebrain regions as would be predicted from the endogenous Ptf1a pattern (Fig. 2A, D). Notably, as was reported for the 12.4 kb enhancer that includes R7 (Meredith et al., 2009), activity in the rostral regions that give rise to the cerebellum is much reduced relative to the enhancer activity in the more caudal regions. Also detected is the basal activity of the GFP cassette in the ventral neural tube consistent with that seen in the chick electroporation experiments (Fig. 2A′, D′, asterisks). To define the dorsal progenitor domains where R7 is active, transverse sections through the neural tube at E10.5 and E12.5 of R7-GFP transgenic embryos were co-localized with ASCL1 (Fig. 2B, E) and PTF1A (Fig. 2C, F). ASCL1 marks progenitor domains dP3-dP5 at E10.5 whereas PTF1A is restricted to dP4. At this stage, the R7 enhancer directs expression to the dorsal neural tube with a ventral boundary matching dP4 but a dorsal boundary extending into dP2, congruent with the expanded expression seen in chick (Fig, 1D). At E12.5 R7-GFP co-labels with both ASCL1 and PTF1A positive dorsal progenitor domains as well as extensively with PAX2 and TLX1/3 positive post-mitotic neurons derived from these progenitors (Fig. 2H). Direct comparison of R7 with the full length 12.4kb enhancer in transgenic mice carrying both the 12.4kb-mcherry and the R7-GFP transgenes further highlights their similar activity in dP4 and the expanded expression domain of R7 (Fig. 2G). Thus, as previously reported for the entire 12.4 kb regulatory region (Meredith et al., 2009), the 1.1 kb R7 directs expression to the dorsal neural tube caudal to the cerebellum, but its activity is broader than the Ptf1a-expressing progenitors.
Figure 2. Activity of the R7 regulatory element in transgenic mice.
R7-GFP was used to generate mouse transgenic embryos assayed at embryonic day E10.5 (N=6) (A) or E12.5 (N=4) (D). A representative whole mount image shows R7 directs neural specific GFP expression. (A′, D′) Transverse sections showing activity of the transgene in the neural tube. ASCL1 (B–B″, E–E″) and PTF1A (C–C″, F–F″) immunostaining in magenta show co-localization of the GFP in the correct domain with a dP4 ventral boundary but GFP expression extends more dorsally and more laterally than PTF1A. (G–G″) R7-GFP and 12.4kb-mcherry transgenic mice were crossed to generate double heterozygous embryos. At E10.5, activity of these enhancers co-localize in the dP4 domain but R7 has a broader dorsal expression. (H–H″) Immunostaining for PAX2 (H′, arrowheads) in magenta and TLX1/3 (H″, arrows) in blue show co-localization with the GFP (H) at E12.5. The asterisk indicates the ventral neural tube expression intrinsic to the GFP reporter construct as seen in the chick electroporations. Dashed vertical line indicates the midline. Scale bar: 50 μM.
A 132 bp evolutionarily conserved region (ECR) is required for activity of the R7 enhancer
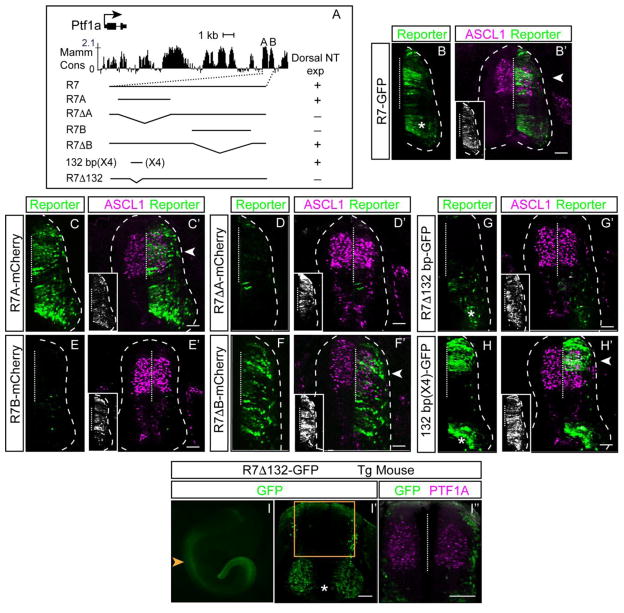
A closer look at the mammalian conservation in the R7 sequence revealed two sub-regions we termed R7A and R7B. To further define the minimal region required for activity of the dorsal neural tube enhancer, we sub-cloned R7A and R7B into the reporter cassette and tested them by chick electroporation (Figs. 3, 4). R7A has activity in the dorsal neural tube similar to but broader than the activity of the full length R7-GFP. Supporting the importance of the R7A region for enhancer activity, the R7 enhancer with R7A deleted lost activity (Fig. 4B–D). In contrast, the R7B homology region has no activity on its own in the neural tube, and deleting it from R7 resulted in unrestricted activity of the enhancer across the dorsal ventral extent in the neural tube (Fig. 4E, F). Taken together, these results demonstrate R7 contains 1) enhancer sequences (R7A) sufficient to drive expression throughout the neural tube, and 2) silencer sequences (R7B) that restrict R7A activity to the dorsal neural tube. The strategy to utilize repressor elements in controlling dorsal/ventral restricted expression in the neural tube is a common design principle. This principle for regulating spatially patterned expression in the neural tube was recently exemplified and extended in two studies on the function of the ventral specification factors NKX2.2, NKX6.1, and OLIG2 (Kutejova et al., 2016; Nishi et al., 2015). In these studies, the importance of the ventral neural tube specification transcription factors in repressing whole batteries of dorsal specific gene expression through combinatorial cis-regulatory elements was demonstrated. Even within the 1.1 kb R7 enhancer of Pt1a, we find evidence for multiple repressive elements that when lost alter the dorsal restricted expression of the enhancer.
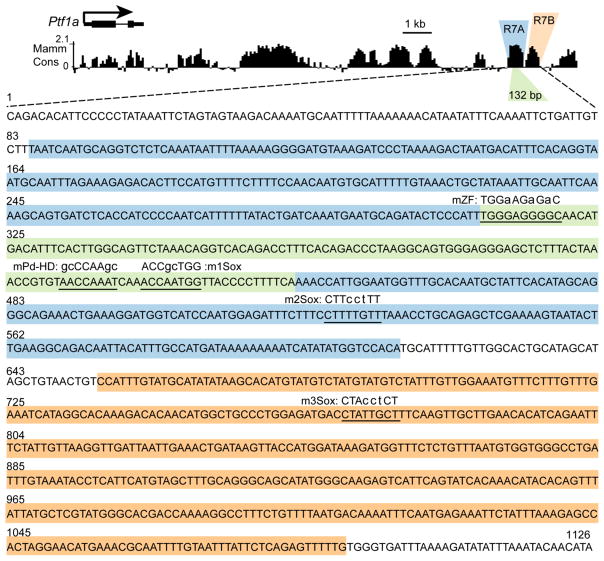
Figure 3. Sequence of the R7 Ptf1a DNT enhancer.
The 1.1 kb sequence of R7 is shown. The blue/green and orange highlighted regions represent R7A and R7B, respectively, tested for enhancer activity in chick electroporation assay (see Fig. 4). The evolutionarily conserved 132 bp region within R7A is highlighted in green. The transcription factor binding motifs that were identified and tested in this study are underlined and the mutated sequence is given. The smaller letter indicates the specific mutations introduced.
Figure 4. Delineation of sequence within the R7 enhancer required for its activity.
(A) Top: Graphical representation of sequence conservation across mammals (adapted from the UCSC Genome Browser). Bottom (left): Position of genomic region within R7 tested for enhancer activity and (right) summary of the reporter expression directed by the enhancer in the chick dorsal neural tube. (B–H′) Transverse sections from chick neural tubes electroporated with fluorescence reporter constructs as indicated at HH13-14 and harvested 24 hours later. Reporter is in green, and ASCL1 immunofluorescence (marking progenitors dP3-dP5) is magenta. A 132 bp within R7A is sufficient to direct dorsal expression and is necessary for R7 activity (G, H). (B, G, H) Asterisks indicate the GFP reporter cassette has intrinsic activity in the ventral neural tube. Insets show immunofluorescence for a MYC-epitope tag vector used to confirm electroporation efficiency. (I–I″) 132 bp within R7 is required for its activity in the Ptf1a expression domain in E10.5 transgenic embryos (N=3). Compare to R7-GFP transgenic embryos in Fig. 2A. Orange arrowhead and box notes the dorsal but not ventral expression is absent in these transgenic mouse embryos. PTF1A immunofluorescence (marking progenitors dP4) is magenta. Dashed vertical line indicates the midline. Scale bar: 50 μM.
Extending analysis of the R7A sequence to conservation between zebrafish and mammals, identified a 132 bp highly conserved sequence (Figs. 3, 4). Deletion of this 132 bp from R7 completely disrupted dorsal neural tube enhancer activity of R7 when tested both in chick neural tube and in transgenic mouse embryos (Fig. 4G, I). Strikingly, in mouse, the dorsal neural tube expression encompassing the PTF1A domain was completely absent in R7Δ132-GFP transgenic embryos (Fig. 4I), the non-specific activity attributed to the reporter cassette was retained. Furthermore, to test the sufficiency of the 132 bp for directing dorsal neural tube specific expression, we cloned four copies of 132 bp in tandem in the reporter vector and performed the chick electroporation assay. The 132 bp (X4) reporter construct drove GFP expression in the dorsal neural tube, although expression was shifted dorsally relative to the full-length R7 (Fig. 4H). Together these data support the importance of the regulatory motifs within the highly conserved 132 bp sequence for activity of the R7 enhancer.
Zinc Finger and Paired homeodomain transcription factor binding motifs are essential for activity of the 1.1 kb Ptf1a enhancer R7
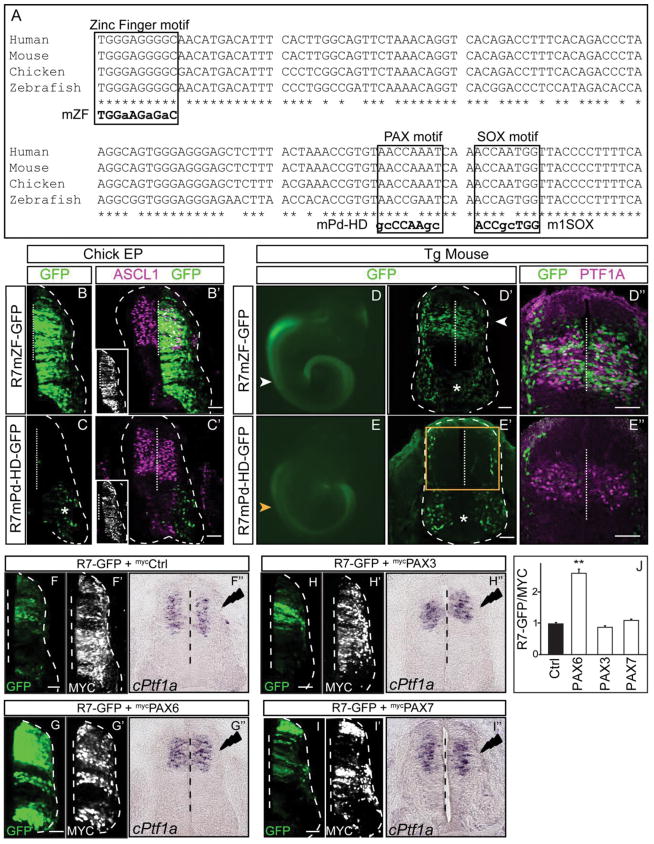
To resolve the transcriptional machinery involved in the regulation of Ptf1a through the 1.1 kb enhancer, we used bioinformatic analysis to predict transcription factor binding motifs. Multiple evolutionarily conserved binding motifs were predicted within the essential 132 bp region including motifs predicted to be binding sites for zinc finger (ZF) transcription factors, Paired homeodomain (Pd-HD) factors, and SOX factors (Fig. 3, 5A). To determine the requirement of these sequences within R7 for regulatory function we generated reporter constructs with mutated motifs utilizing PCR site-directed mutagenesis. These mutant reporter constructs were assessed for enhancer activity in comparison to R7-GFP in chick neural tube by electroporation. Mutation of a putative zinc finger binding motif, R7mZF, resulted in broad expression across the dorsal/ventral axis of the neural tube (Fig. 5B). This suggests the sequence mutated is a binding site for a repressor that serves to restrict activity of the R7 enhancer to the dorsal neural tube. Because the motif matches sequence found in zinc finger transcription factor binding sites, a zinc finger family factor, or multiple factors, may serve to restrict expression dorsally. In contrast, mutation of a putative Paired homeodomain binding motif, R7mPd-HD dramatically attenuated enhancer activity (Fig. 5C). Thus, a Paired homeodomain family transcription factor is predicted to be an activator of Ptf1a expression through this Paired homeodomain motif in R7.
Figure 5. Identification of transcription factor binding motifs within R7 required for its activity.
(A) Sequence conservation of the 132 bp compared across 4 species from human to zebrafish. Zinc finger (ZF), Pd-HD, and SOX family consensus binding motifs are indicated. The smaller letter indicates the mutation introduced in the binding motifs and tested in reporter assays. (B–B′) Mutation in the ZF binding motif broadens the R7 activity dorso-ventrally across the chick neural tube. (C–C′) Pd-HD binding motif mutation disrupts R7 activity in the dorsal neural tube. ASCL1 immunofluorescence (labelling progenitors dP3-dP5) is magenta. Insets show immunofluorescence for a MYC-epitope tag vector used to confirm electroporation efficiency. (D–D″) The ZF motif mutation in R7 does not alter activity of the reporter in transgenic mouse embryos (N=3). (E–E″) the Pd-HD motif mutation in R7 dramatically attenuates enhancer activity in transgenic mouse embryos (N=3). Compare to R7-GFP transgenic embryos in Fig. 2A. Orange arrowhead and box notes the dorsal but not ventral expression is absent in these transgenic embryos. (D″–E″) PTF1A immunofluorescence (magenta) highlights the dP4. (C, D, E) Asterisk indicates the GFP reporter cassette has intrinsic activity in the ventral neural tube. (F–I) Over expression of PAX6 (G) but not PAX3 (H) or PAX7 (I) is sufficient to increase the activity of the R7 enhancer. (G′–I′) MYC-epitope tag is used to confirm the overexpression of the respective transcription factor and confirm electroporation efficiency. (G″–I″) In situ hybridization for mRNA shows no change in endogenous Ptf1a mRNA levels upon PAX6 (G″), PAX3 (H″) or PAX7 (I″) over expression on the electroporated side (right) compared to the control side (left). (J) Quantification of the GFP intensity compared when the PAX factors are overexpressed with R7-GFP. MYC intensity is used as an internal control. Dashed vertical line indicates the midline. **p <0.01. Scale bar: 50 μM.
To gain additional support for the role of these two motifs for R7 activity, we generated transgenic mouse embryos with R7mZF and R7mPd-HD reporter constructs. The R7mPd-HD-GFP transgenic embryos analysed at E10.5 showed no GFP expression in the dorsal neural tube similar to deletion of 132 bp region (Fig. 5E compare to Fig. 4I) confirming the importance of the Pd-HD motif for R7 enhancer activity. In contrast, transgenic embryos containing R7mZF-GFP were not distinguishable from those with R7-GFP (Fig. 5D compare to Fig. 2A). This difference in the requirement for the ZF motif could reflect a species difference, but more likely reflects a difference in enhancer activity when tested as an episomal transgene in the chick assay versus integration of the transgene in the mouse genome. Nevertheless, the Pd-HD motif is critical for activity of the R7 enhancer in both mice and chick, but the requirement for the ZF motif for repressing activity outside the dorsal neural tube is revealed only in the chick electroporation assay.
To identify the transcription factors that bind the Pd-HD or ZF motifs, we tested candidate PAX family and ZF transcription factors known to be expressed in the ventricular zone of the dorsal neural tube. Co-electroporation of a PAX6 expression vector, but not PAX3 or PAX7, along with the R7 reporter plasmid increased reporter expression significantly, suggesting a role for PAX6 in activating Ptf1a expression in the neural tube (Fig. 5F–J). However, over expression of PAX6 was not sufficient to induce ectopic expression of endogenous cPtf1a mRNA (Fig. 5F″–I″). The latter result highlights a complexity in the endogenous locus that is not evident in the reporter assay, and suggests PAX6 likely functions in combination with other transcription factors to activate transcription of Ptf1a.
Although ZF containing DNA binding factors represent a hugely diverse group, we tested the effect of overexpressing a known ZF transcription factor expressed in the dorsal neural tube, ZIC1 (McMahon and Merzdorf, 2010; Nagai et al., 1997). ZIC1 was able to repress endogenous chick Ptf1a mRNA and R7-GFP activity in the chick neural tube, however, the repression did not require the ZF motif identified in the R7 enhancer (data not shown). The latter result suggests this repression by ZIC1 is not through the identified ZF motif, but through other unidentified sequences that will require further analysis to address the role of ZIC1 in repressing Ptf1a. Another candidate ZF containing repressor in the neural tube is PRDM13 (Chang et al., 2013). Prdm13 electroporation in chick did not alter R7-GFP activity, and PRDM13 is not localized to the R7 region in the mouse neural tube based on ChIP-Seq data (data not shown). Other factors may work through this site such as a broadly expressed factor like CNBP that binds G-rich motifs similar to the ZF motif in R7 (Armas et al., 2013), however, these were not tested here. Taken together, although we identify multiple sequences with distinct requirements for DNT enhancer activity, only PAX6 was found to modulate enhancer activity while other transcription factors that function through these cis-elements remain elusive.
SOX3 but not SOX1 or SOX2 activates the 1.1 kb Ptf1a enhancer R7
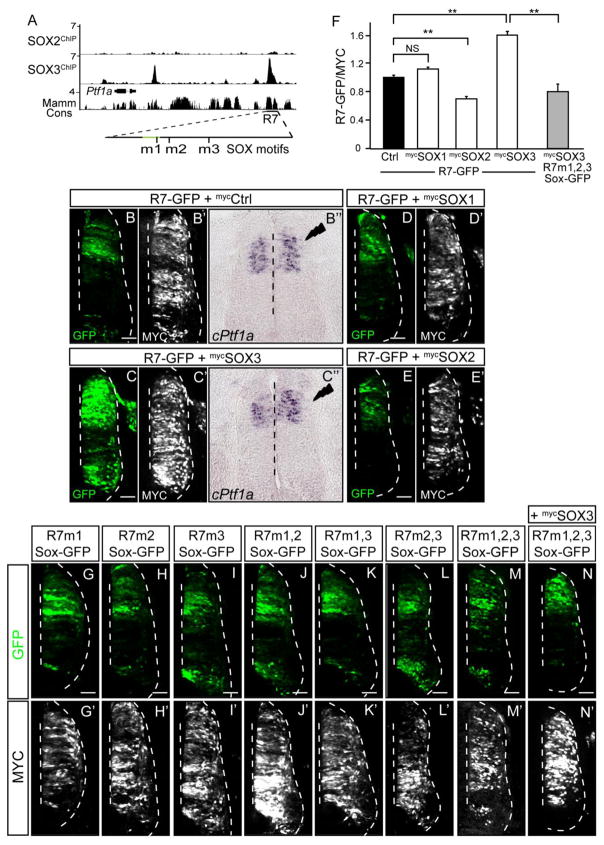
SoxB1 family members have been extensively studied in neurogenesis and are recognized as key factors in maintenance of neural progenitor populations. Analysis of ChIP-Seq for multiple SOX factors in neural progenitor cells in culture (Bergsland et al., 2011), available through the Cistrome database, reveal SOX3 but not SOX2 can bind to the 1.1 kb R7 locus at least in this cell population (Fig. 6A). This finding suggests that SOX3 might bind the R7 enhancer and regulate Ptf1a expression in vivo. SOX1-3 belong to the SOXB1 subclass, are co-expressed in neural progenitors in the developing neural tube, and can have redundant function (Bylund et al., 2003). We tested whether SOX1, SOX2 and/or SOX3 could alter the activity of the R7 enhancer in the chick neural tube. Co-electroporation of a SOX3 expression vector along with the R7-GFP reporter induced reporter expression ectopically in the cells expressing SOX3, suggesting SOX3 is sufficient to induce R7 activity (compare Fig. 6B with C; quantification in Fig. 6F). To isolate the binding motifs for SOX3 on the R7 enhancer we performed a bioinformatic analysis and identified three potential SOX binding motifs (Figs. 3, 6). When these motifs were mutated individually or in combination and tested in the chick reporter assay the activity was indistinguishable from that of the wildtype R7 enhancer (Fig. 6G–M). However, SOX3 regulated induction of R7 was attenuated when all three SOX motifs were mutated (compare Fig. 6N with Fig. 6C; quantification in Fig. 6F). The activation of the R7 enhancer by SOX3 was specific since the related SOX1 and SOX2 did not activate enhancer activity (Fig. 6D, E, F). Furthermore, over expression of SOX3 but not SOX1 or SOX2 resulted in a subtle increase in the endogenous cPtf1a mRNA levels within its normal domain (Fig. 6B″–C″, compare the electroporated side relative to the control side).
Figure 6. SOX3 activates the Ptf1a-R7 enhancer.
(A) Genome browser image of the ChIP-Seq data in cultured neural progenitors for SOX2 and SOX3 near the Ptf1a locus (Bergsland et al., 2011). The location of the 132 bp enhancer (green line) and the putative SOX binding motifs identified within R7 are indicated (also see Fig. 3). (B–N) Transverse sections from chick neural tubes electroporated with fluorescence reporter constructs and expression vectors as indicated at HH13-14 and harvested 24 hours later. (B′–N′) Immunofluorescence for a MYC-epitope tag vector used to confirm electroporation efficiency. (B–E) Over expression of SOX3 but not SOX1 or SOX2 is sufficient to drive R7 activity. (B″–C″) In situ hybridization for mRNA shows a subtle increase in endogenous cPtf1a mRNA levels upon SOX3 (C″) over expression on the electroporated side (right) compared to the control side (left). (F) Quantification of the GFP intensity compared when the SOX factors are over expressed with R7-GFP. MYC intensity is used as an internal control. (G–M) Mutations in putative SOX binding motifs individually or in combination do not change the activity of the R7 enhancer. (N) Over expression of SOX3 does not affect R7 activity when all three putative SOX binding motifs are mutated (quantified in F). (C′–E′, N′) MYC-epitope tag is used to confirm the overexpression of the respective transcription factor. Dashed vertical line indicates the midline. **p <0.01. Scale bar: 50 μM.
CONCLUSION
PTF1A is a key transcriptional regulator required for the specification of inhibitory neurons in various regions of the central nervous system. Teasing out the complex transcriptional machinery for the control of Ptf1a expression is essential knowledge for understanding mechanisms controlling the balance of inhibitory and excitatory neurons generated in multiple regional networks. Here, we identify a tissue-specific enhancer of Ptf1a that adds to the complexity of its regulation during development. This enhancer comprises multiple sequence elements required for activating the enhancer in the neural tube, combined with repressor elements required for silencing enhancer activity specifically in the ventral neural tube. Although the full cohort of transcriptional activators and repressors that function through the DNT enhancer remain elusive, PAX6 and SOX3 represent two factors that contribute to Ptf1a expression through this enhancer.
HIGHLIGHTS.
Identification of a dorsal neural tube specific enhancer for Ptf1a
An evolutionarily conserved region is required for activity of the Ptf1a enhancer.
The dorsal neural tube enhancer integrates activator and repressor activities.
SOX3 and PAX6 increase activity of the Ptf1a enhancer.
Acknowledgments
We acknowledge the many hours of helpful discussions with members of the Johnson laboratory and early contributions to the project by Ms. Maria Islam (Green Fellowship Program) and Katie Fife (STARS Program). We are grateful for the excellent transgenic mouse services provided by the UT Southwestern Transgenic Core. This work was supported by the National Institutes of Health R01 HD037932 to JEJ, T32 GM083831 to JMA, and F31 NS061440 to DMM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armas P, Margarit E, Mouguelar VS, Allende ML, Calcaterra NB. Beyond the binding site: in vivo identification of tbx2, smarca5 and wnt5b as molecular targets of CNBP during embryonic development. PloS one. 2013;8:e63234. doi: 10.1371/journal.pone.0063234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsland M, Ramskold D, Zaouter C, Klum S, Sandberg R, Muhr J. Sequentially acting Sox transcription factors in neural lineage development. Genes & development. 2011;25:2453–2464. doi: 10.1101/gad.176008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nature neuroscience. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Chang JC, Meredith DM, Mayer PR, Borromeo MD, Lai HC, Ou YH, Johnson JE. Prdm13 mediates the balance of inhibitory and excitatory neurons in somatosensory circuits. Developmental cell. 2013;25:182–195. doi: 10.1016/j.devcel.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dullin JP, Locker M, Robach M, Henningfeld KA, Parain K, Afelik S, Pieler T, Perron M. Ptf1a triggers GABAergic neuronal cell fates in the retina. BMC developmental biology. 2007;7:110. doi: 10.1186/1471-213X-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annual review of neuroscience. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Fujitani S, Luo H, Qiu F, Burlison J, Long Q, Kawaguchi Y, Edlund H, MacDonald RJ, Furukawa T, Fujikado T, Magnuson MA, Xiang M, Wright CV. Ptf1a determines horizontal and amacrine cell fates during mouse retinal development. Development. 2006;133:4439–4450. doi: 10.1242/dev.02598. [DOI] [PubMed] [Google Scholar]
- Glasgow SM, Henke RM, Macdonald RJ, Wright CV, Johnson JE. Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development. 2005;132:5461–5469. doi: 10.1242/dev.02167. [DOI] [PubMed] [Google Scholar]
- Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- Helms AW, Battiste J, Henke RM, Nakada Y, Simplicio N, Guillemot F, Johnson JE. Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons. Development. 2005;132:2709–2719. doi: 10.1242/dev.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K, Cholewa-Waclaw J, Nakada Y, Glasgow SM, Masui T, Henke RM, Wildner H, Martarelli B, Beres TM, Epstein JA, Magnuson MA, Macdonald RJ, Birchmeier C, Johnson JE. A nonclassical bHLH Rbpj transcription factor complex is required for specification of GABAergic neurons independent of Notch signaling. Genes & development. 2008;22:166–178. doi: 10.1101/gad.1628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino M, Nakamura S, Mori K, Kawauchi T, Terao M, Nishimura YV, Fukuda A, Fuse T, Matsuo N, Sone M, Watanabe M, Bito H, Terashima T, Wright CV, Kawaguchi Y, Nakao K, Nabeshima Y. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47:201–213. doi: 10.1016/j.neuron.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Iskusnykh IY, Steshina EY, Chizhikov VV. Loss of Ptf1a Leads to a Widespread Cell-Fate Misspecification in the Brainstem, Affecting the Development of Somatosensory and Viscerosensory Nuclei. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36:2691–2710. doi: 10.1523/JNEUROSCI.2526-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nature genetics. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kutejova E, Sasai N, Shah A, Gouti M, Briscoe J. Neural Progenitors Adopt Specific Identities by Directly Repressing All Alternative Progenitor Transcriptional Programs. Developmental cell. 2016;36:639–653. doi: 10.1016/j.devcel.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui T, Swift GH, Hale MA, Meredith DM, Johnson JE, Macdonald RJ. Transcriptional autoregulation controls pancreatic Ptf1a expression during development and adulthood. Molecular and cellular biology. 2008;28:5458–5468. doi: 10.1128/MCB.00549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga E, Araki I, Nakamura H. Role of Pax3/7 in the tectum regionalization. Development. 2001;128:4069–4077. doi: 10.1242/dev.128.20.4069. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annual review of physiology. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- McMahon AR, Merzdorf CS. Expression of the zic1, zic2, zic3, and zic4 genes in early chick embryos. BMC Res Notes. 2010;3:167. doi: 10.1186/1756-0500-3-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith DM, Masui T, Swift GH, MacDonald RJ, Johnson JE. Multiple transcriptional mechanisms control Ptf1a levels during neural development including autoregulation by the PTF1-J complex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:11139–11148. doi: 10.1523/JNEUROSCI.2303-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen KJ, Steshina EY, Iskusnykh IY, Chizhikov VV. Transformation of the cerebellum into more ventral brainstem fates causes cerebellar agenesis in the absence of Ptf1a function. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1777–1786. doi: 10.1073/pnas.1315024111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi R, Kriks S, Cordes R, Gossler A, Ma Q, Goulding M. Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat Neurosci. 2006;9:770–778. doi: 10.1038/nn1706. [DOI] [PubMed] [Google Scholar]
- Muller T, Anlag K, Wildner H, Britsch S, Treier M, Birchmeier C. The bHLH factor Olig3 coordinates the specification of dorsal neurons in the spinal cord. Genes & development. 2005;19:733–743. doi: 10.1101/gad.326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Aruga J, Takada S, Gunther T, Sporle R, Schughart K, Mikoshiba K. The expression of the mouse Zic1, Zic2, and Zic3 gene suggests an essential role for Zic genes in body pattern formation. Developmental biology. 1997;182:299–313. doi: 10.1006/dbio.1996.8449. [DOI] [PubMed] [Google Scholar]
- Nakhai H, Sel S, Favor J, Mendoza-Torres L, Paulsen F, Duncker GI, Schmid RM. Ptf1a is essential for the differentiation of GABAergic and glycinergic amacrine cells and horizontal cells in the mouse retina. Development. 2007;134:1151–1160. doi: 10.1242/dev.02781. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Zhang X, Jeong J, Peterson KA, Vedenko A, Bulyk ML, Hide WA, McMahon AP. A direct fate exclusion mechanism by Sonic hedgehog-regulated transcriptional repressors. Development. 2015;142:3286–3293. doi: 10.1242/dev.124636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Abasolo I, Mingorance-Le Meur A, Martinez A, Del Rio JA, Wright CV, Real FX, Soriano E. Cerebellar GABAergic progenitors adopt an external granule cell-like phenotype in the absence of Ptf1a transcription factor expression. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5193–5198. doi: 10.1073/pnas.0605699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashos E, Park JT, Leach S, Fisher S. Distinct enhancers of ptf1a mediate specification and expansion of ventral pancreas in zebrafish. Developmental biology. 2013;381:471–481. doi: 10.1016/j.ydbio.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suemori H, Kadodawa Y, Goto K, Araki I, Kondoh H, Nakatsuji N. A mouse embryonic stem cell line showing pluripotency of differentiation in early embryos and ubiquitous beta-galactosidase expression. Cell Differ Dev. 1990;29:181–186. doi: 10.1016/0922-3371(90)90120-l. [DOI] [PubMed] [Google Scholar]
- Weedon MN, Cebola I, Patch AM, Flanagan SE, De Franco E, Caswell R, Rodriguez-Segui SA, Shaw-Smith C, Cho CH, Lango Allen H, Houghton JA, Roth CL, Chen R, Hussain K, Marsh P, Vallier L, Murray A, Ellard S, Ferrer J, Hattersley AT International Pancreatic Agenesis C. Recessive mutations in a distal PTF1A enhancer cause isolated pancreatic agenesis. Nature genetics. 2014;46:61–64. doi: 10.1038/ng.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildner H, Das Gupta R, Brohl D, Heppenstall PA, Zeilhofer HU, Birchmeier C. Genome-wide expression analysis of Ptf1a- and Ascl1-deficient mice reveals new markers for distinct dorsal horn interneuron populations contributing to nociceptive reflex plasticity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:7299–7307. doi: 10.1523/JNEUROSCI.0491-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Terao M, Terashima T, Fujiyama T, Kawaguchi Y, Nabeshima Y, Hoshino M. Origin of climbing fiber neurons and their developmental dependence on Ptf1a. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:10924–10934. doi: 10.1523/JNEUROSCI.1423-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]