Abstract
Introduction
Bone marrow-derived mesenchymal stem cells (MSCs) can differentiate into multiple cell types, including osteoblasts, chondrocytes, and adipocytes. These pluripotent cells secrete hepatocyte growth factor (HGF), which regulates cell growth, survival, motility, migration, mitogenesis and is important for tissue development/regeneration. HGF has four splice variants, NK1, NK2, NK3, and NK4 which have varying functions and affinities for the HGF receptor, cMET. HGF promotes osteoblastic differentiation of MSCs into bone forming cells, playing a role in bone development, health and repair.
Areas Covered
This review will focus on the effects of HGF in osteogenesis, bone repair and bone health, including structural and functional insights into the role of HGF in the body.
Expert Opinion
Approximately 6.2 million Americans experience a fracture annually, with 5–10% being mal- or non-union fractures. HGF is important in priming MSCs for osteogenic differentiation in vitro and is currently being studied to assess its role during bone repair in vivo. Due to the high turnover rate of systemic HGF, non-classic modes of HGF-treatment, including naked-plasmid HGF delivery and the use of HGF splice variants (NK1 & NK2) are being studied to find safe and efficacious treatments for bone disorders, such as mal- or non-union fractures.
Keywords: Hepatocyte Growth Factor, HGF, Bone, Osteoblastic Differentiation, Osteoblasts, Osteoclasts, cMET
1. Introduction
Mesenchymal stem cells possess the potential to differentiate into multiple cell types, including osteoblasts, chondrocytes, and adipocytes. These pluripotent cells secrete hepatocyte growth factor (HGF), a growth factor reported to have regulatory effects on cell growth, survival, motility, migration, and mitogenesis 1–3. This cellular regulation by HGF generates an associated role in tissue regeneration and development, proliferation, and — in the case of tumor cells — invasion and metastasis4. Furthermore, the multi-domain protein, HGF, predominantly mediates cells of epithelial origin 5, 6. All the aforementioned activities are facilitated by HGF acting through its receptor, cMET 7. Moreover, HGF exists as four splice variants as a function of alternative splicing, identified as NK1, NK2, NK3, and NK4; all of which have varying functions on HGF/cMET signaling8, 9.
2.1 HGF
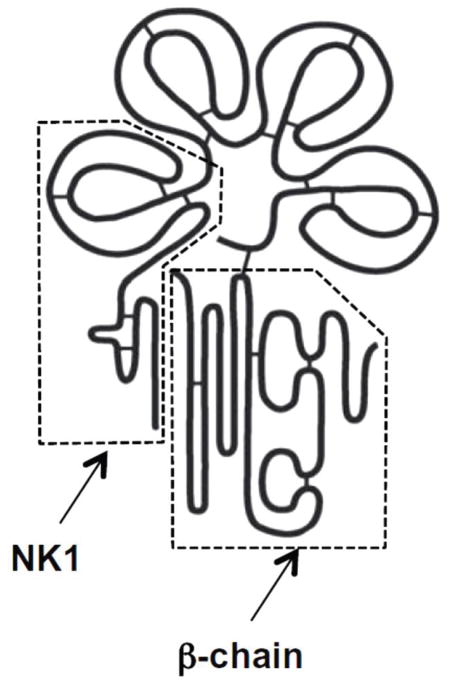
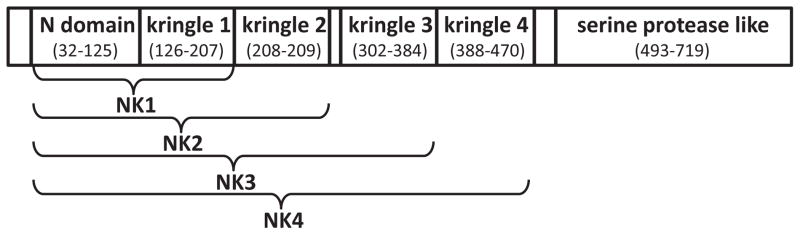
HGF is a potent growth factor that has been detected in a variety of tissues including the skin, lungs, liver, bone, muscle, pancreas, gastrointestinal tract, salivary glands, thyroid, brain, prostate, seminal vesicles, breast, uterus, placenta, and kidney 10. Within the brain HGF is expressed in the hippocampal, cortex, and cerebellum neurons, and acts to increase the growth of motor axons which function to transmit information through impulses 10. The 70 kb long human gene for HGF is positioned on chromosome 7q11.1-21, consisting of 18 exons and 17 introns 10, 11. The HGF precursor is produced as a biologically nonfunctional single chain consisting of 728 amino acids, referred to as pro-HGF 4. After cleavage, the full length functional HGF (~90kDa) consists of two polypeptide chains: the heavy chain (α: 69kDa) and the light chain (β: 34kDa) (figure 1). The heavy chain contains the N–terminal domain (n) and four kringle domains (k1, k2, k3, k4), which due to alternative splicing of the full-length transcript (HGF) can lead to four alternative splice variants (NK1-NK4) (figure 2) 10. Kringle domains are protein domains that form loops in a bridged structure and are stabilized by three disulfide bonds 10. Within the light chain is a serine protease homology domain 2.
Figure 1.
Structure of HGF. NK1: high-affinity Met binding and dimerization domain. β-chain: low-affinity Met binding and activation domain. Reused with permission from 7.
Figure 2.
Schematic diagram of HGF splice variants; NK1, NK2, NK3 and NK4.
Scatter factor (SF) was identified as a protein secreted by fibroblasts that promoted matrix invasion and mobility in epithelial cells6, 12, two functions similar to the effects of HGF5. When HGF and SF were purified individually they were found to be indistinguishable; equivalently binding to the MET receptor and stimulating the MET tyrosine phosphorylation, having nearly identical sequences, and equally functioning as a mitogen when measured using bioassays 5. Thus, full length HGF and SF are identical and are often referred to as HGF/SF.
The function of HGF as a critical developmental growth factor was determined through knock-out experiments in mice. HGF has a vital role in development of organs and disruption of HGF signaling can be embryonically lethal 7. For example, when the HGF signaling pathway is interrupted, the embryonic liver is smaller in size and presents widespread cell death7. Additionally, the essential mitogenic role of HGF was established through targeted disruption of HGF signaling in the context of muscle formation. An absence of HGF leads to reduced myogenic, or muscle cell migration during development, resulting in unformed skeletal muscles 7.
2.2 HGF Activation
The precursor of the functional HGF protein is a biologically inactive pre-pro-peptide, which is proteolytically cleaved near residue 31 before secretion as pro-HGF 10. Pro-HGF, the immediate precursor of HGF, is secreted by mesenchymal stem cells into the extracellular environment and remains inactive until activated through further proteolysis. Pro-HGF is a single chain 92 KDa precursor peptide that binds to the cellular surface or extracellular matrix following secretion 4. Upon binding, pro-HGF interacts with Urokinase-type plasminogen activator (uPA), a serine protease 7. The association between product yield of processed pro-HGF and total concentration of uPA has been demonstrated to be linear when the concentration of the uPA enzyme was greater than the concentration of the pro-HGF substrate. Consequently, it has been demonstrated that the concentration of the accumulated product equaled approximately one-half the molar amount of the uPA enzyme 13. Additionally, this reaction may be classified as a stoichiometric reaction due to the dependence on the level of enzyme reactant in determining the effective product yield. Under conditions of a greater amount of substrate compared to enzyme, as the concentration of uPA greatly increased in comparison to the concentration of the substrate, the curve plateaued, demonstrating that the concentration of the substrate, pro-HGF, is the limiting factor in this reaction. The high affinity between uPA and pro-HGF is demonstrated in the stable complex they form in vitro and upon the membrane of target receptor cells. Additionally, proteases such as plasma kallikrein, matriptase, hepsin, and HGF activator are a part of the system responsible for the activation of the precursor HGF 7. HGF-A is a kringle-containing serine protease that is predominantly synthesized by hepatocytes and exists as the non-functional single chain form proHGF-A 7. ProHGF-A is activated by a signaling cascade that is initiated in response to events that require the action of HGF, such as tissue damage.
2.3 Homology
HGF maintains strong homology with plasminogen and prothrombin, factors involved in blood coagulation and fibrinolysis 4. This homology is demonstrated by a 38% identity in amino acid sequence between plasminogen and HGF, and additional similar structural motifs 10. Similar to the synthesis pathway of HGF, plasminogen is secreted by the liver as an inactive single polypeptide enzyme that is activated by the actions of a serine-protease and is consequently converted into plasmin, a disulfide linked heterodimer 14. The physiologic function of plasmin is the degradation of fibrin proteins present in blood clots 15. Additionally, it is hypothesized that HGF, macrophage stimulating protein (MSP), plasminogen, and apolipoprotein retain a shared genetic precursor. This premise is based upon the common structural elements of these proteins: an N-terminal domain containing a “loop within a loop” made up of four cysteine residues, multiple kringle domain copies, and a serine protease domain 4. MSP is the most related plasminogen protein to HGF with an amino acid identity of 44% 10. While similarly structured to members of the plasminogen family, HGF deviates in that it does not possess proteolytic activity 10.
In addition to the structural resemblance between multiple proteins, HGF shows strong homology between species. HGF is highly conserved: humans and chimpanzees uphold 99.9% homology between primary sequences, and humans and rodents maintain 91% homology 10. Moreover, it has been determined that the gene for HGF is conserved in the dog16, cow17, chicken18, 19, zebrafish20, and frog21. However, there is a divergence in the homolog of humans and the previously mentioned species with an amino acid identity of 75% between human and chicken, and 50% between human and zebra fish 10.
2.4 HGF Splice Variants
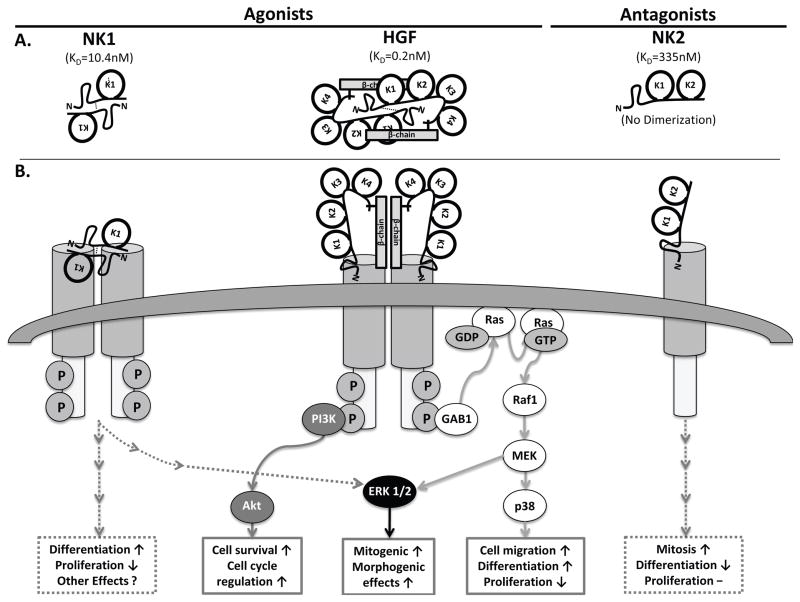
HGF is differentially spliced into two predominant naturally occurring splice variants, NK1 (25kDa) and NK2 (35kDa). In mice, serum HGF and NK2 levels are about 3.9ng/ml22 and 7.8ng/ml23 respectively. These natural isoforms consist of the N-terminal domain and the first kringle domain (NK1) or first 2 kringle domains (NK2) 24. NK1 acts as a cMET (the HGF receptor) agonist, promoting cMET activity through the formation of a head-to-tail dimer complex with the receptor. Conversely, NK2 demonstrates antagonistic behavior, inhibiting the activity of the cMET receptor.
The agonistic mechanism of NK1 is attributed to the structure of the isoform. Similar to full length HGF, the truncated NK1 isoform creates a dimer complex and the interface between the domains of the dimer is central to the functionality of NK1 as an activator of the cMET signaling 24. The essential nature of the dimer interface of NK1 is exemplified through the finding that when the interface is mutated, the activity of NK1 in relation to cMET is transformed from agonistic to antagonistic 24. NK1/cMET dimerization is facilitated by heparin 25, with an approximate 800 fold increase in NK1-cMET binding affinity in the presence of heparin (10.4±1.1nM) versus without heparin (8.5±4.6μM)24. NK1 has a lower binding affinity to cMET (10.4nM24) as compared to HGF (0.2nM26). NK1 is a known agonist of the HGF/cMET pathway and has been shown to induce a morphogenic response in both epithelial and endothelial cells27, and mitogenic response in hepatocytes in culture25.
NK2 differs from NK1 both structurally and operationally. The NK2 isoform exists in a closed triangle formation, consisting of the N-terminal at the top and the two kringle domains below 4. While the structure of the N-terminal domain and kringle domains are analogous between NK1 and NK2, the structures differ at the linkage between the domains, suggesting a functional importance of the links within the structure. The first kringle domain of NK2 is rotated approximately 180° compared to the corresponding position in NK1. The flexible link between the N-terminal domain and the first kringle domain enable this rotation, occurring at residues 122-127, the area of NK1, which is significant to formation of a dimer interface. NK2 is a known antagonist of the HGF/cMET pathway8, 9, 24. It is thought that, unlike NK1, NK2 cannot dimerize with cMET and in turn does not induce cMET activation. Additionally, NK2 does not require heparin to bind cMET and has a lower cMET binding affinity as compared to NK1 or HGF; NK2 - 335nM versus NK1 - 10.4±1.1nM versus HGF - 0.2nM, respectively24, 26. In review, unlike NK1, NK2 does not dimerize nor require heparin to bind cMET, acts as a competitive inhibitor for both NK1 and HGF-cMET binding, and has been shown to directly oppose the effects of HGF in kidney24, epithelial and endothelial cells27, 28.
NK3, an additional splice variant has been identified, but there is no significant data for it. This is a new area of research and nothing is yet definitively known about its function.
NK4, which consists of the N-terminal domain and four kringle domains, acts as an inhibitor of the HGF/cMET signaling pathway 7, 29. Due to the inhibitory activity of NK4, this splice variant suppresses tumor growth and diminishes tumor blood vessels in a variety of tumor types. In vivo studies found that NK4 treatment inhibited gallbladder cancer growth and invasion in mice 7. Thus NK4 acts as a bifunctional molecule: both an HGF/cMET antagonist and an angiogenesis inhibitor 29. The HGF splice variant NK4 is homologous to angiostatin, an isoform of plasminogen that also acts as an inhibitor of angiogenesis 29. The dual functions of NK4 lend it to being a potential treatment for various types of cancers. In the context of cell-cycle progression, the overexpression of NK4 resulted in an increase in the number of cells in the G1 phase, and decreased the number of cells in the S and G2/M phases 29. This demonstrates that NK4 inhibits the progression of cells from the G1 phase to the S phase, therefore causing arrest of cells at G1. Additionally, NK4 blocks the HGF/cMET pathway through inhibition of the downstream PI3K/Akt pathway. This was elucidated through the finding that treatment with NK4 prevented the tyrosine phosphorylation of Akt 29. The inhibition of the PI3K/Akt pathway results in a decrease in cell proliferation, migration, and invasion.
3.1 The cMET Receptor
HGF initiates cell signaling through binding to the MET tyrosine kinase receptor, also referred to as the cellular MET receptor (cMET). HGF is the only known ligand to activate cMET, and the cMET receptor is the only receptor of HGF 7. HGF can bind to cMET, a membrane receptor, using either of the two HGF polypeptide chains at different affinities; the α chain with high affinity and the β chain at low affinity 7. The binding of the α chain does not activate the cMET receptor, it acts as a binding domain for cMET, thus encouraging the low affinity binding of the β chain, which then activates cMET 7. The cMET receptor, a proto-oncogene, belongs to a family of heterodimeric tyrosine kinases including Ron, Ryk, and Sea 4. The Ron receptor binds Macrophage stimulatory protein (MSP), which maintains a sequence homology of 45% with HGF. Despite this similarity in sequences, MSP and HGF do not demonstrate functionality on the converse receptor 4. Cells of epithelial and endothelial origin — including those in the liver, kidney, prostate, pancreas, kidney, muscle, and bone marrow — express cMET 30. The cMET receptor is also expressed in the brain and the HGF/cMET pathway has been identified as a key neurotrophic factor for brain development by advancing the survival and maturation of neurons 10, 31. Additionally, both osteoclasts and osteoblasts produce a fully functional HGF receptor 32.
Following synthesis as an inactive polypeptide, the cMET receptor is cleaved producing two disulfide-bonded chains; an extracellular α chain and a transmembrane β chain 30. The 900 amino acid extracellular domain of the cMET receptor, comprised of residues 25-932, contains the Sema domain, a cysteine-rich PSI domain, and four immunoglobulin-like IPT domains 24, 33. The Sema domain consists of 519 amino acids and acts as a ligand/receptor recognition site. The 7-bladed β-propeller structure of the Sema domain is important for both the homodimerization of the MET receptor and the recognition and binding of the HGF ligand 33, 34. Researchers determined that MET receptors lacking the Sema domain are unable to crosslink, an action that is crucial to MET signaling33. The β chain encompasses domains in the extracellular, transmembrane, and cytoplasmic areas. Intracellularly, cMET contains the juxtamembrane and tyrosine kinase domains, which cause the cMET activity 7. The cMET receptor depends entirely upon the two-tyrosine docking sites. In conditions where the docking tyrosines were transformed into phenylalanine, the receptor no longer maintained its oncogenic potential 35.
3.2 HGF/cMET Signaling Pathways
The interaction between HGF and the cMET receptor causes a downstream signaling cascade that works through multiple pathways to produce a multiplicity of biological effects. The binding of HGF to cMET generates two initial responses: receptor phosphorylation and initiation of downstream signaling pathways. This is enacted through the autophosphorylation of tyrosine residues Y1234 and Y1235 by the tyrosine kinase domain of the cMET receptor 36. Subsequently, the phosphorylation of tyrosine residues Y1349 and Y1356 causes the induction of downstream signals. The activation of the cMET receptor and consequent pathways is cell-type specific and can result in such actions as: cell migration, decreased proliferation, and loss of stem cell markers 1, 37, 38. After activation, cMET is degraded by MET-specific proteases. Furthermore, the cMET signal is negatively regulated by the intracellular juxtamembrane domain of the cMET receptor. This domain, consisting of 47 highly conserved amino acids, terminates the cMET signal when bound by the E3 ubiquitin ligase Cbl at the phosphorylation tyrosine in position Y1003 on the cMET receptor 7. Following the binding of Cbl to cMET, cMET is ubiquinated and degraded, preventing a constant signal by HGF/cMET. The constitutive activation of cMET due to degradation pathway deregulation or cMET overexpression results in increased cell motility and the invasive characteristics of cancer 36. The HGF/cMET system primarily functions through three prominent signaling pathways: ERK1/2, PI3K/Akt and p38-MAPK (figure 3).
Figure 3.
Schematic diagram of HGF signaling pathways; A) Representative structures, cMET binding affinities (KD) and dimerization of HGF, NK1 (cMET agonists) and NK2 (cMET antagonists. B) HGF signaling pathways known to be involved in bone maturation; Ras-Raf1-MEK-ERK1/2 pathway, Ras-Raf1-MEK-p38 MAPK pathway, and PI3K/Akt pathway.
3.2.1 The Ras-ERK1/2 Pathway
The Ras-dependent extracellular signal-regulated kinase (Ras-ERK1/2) pathway has been shown to be influential in cell proliferation, and specifically in the case of HGF/cMET this pathway predominantly promotes mitogenic and morphogenic effects 1. The binding of HGF to the cMET receptor causes the response of the signal transducers Src homology 2 domain containing protein (SHC) and Growth factor receptor-bound protein 2 (GRB2) 1. Signaling by HGF/cMET leads to propagation of a signal via the ERK1/2 pathway, signified by rapid phosphorylation of ERK1/2. In a study of HGF-treated murine mesenchymal stem cells (MSC), HGF treatment led to a peak in ERK1/2 phosphorylation within ten minutes 1. Furthermore, when HGF/cMET signaling was blocked using an siRNA specific to cMET, the phosphorylation of ERK1/2 was attenuated, indicating that HGF maintains a positive regulatory effect on the phosphorylation and subsequent signal transduction of ERK1/2 1.
3.2.2 The p38 MAPK Pathway
The mitogen-activated protein kinase (MAPK) is known to direct a variety of cellular responses, including osteogenesis. The binding of HGF to the cMET receptor initiates the activation of the small membrane-attached GTPase, Ras. Ras activation leads to the activation of Raf, which induces activity of the MAP kinase/Extracellular signal regulated kinase (MEK). The signaling cascade continues with the phosphorylation of the p38 MAPK by MEK3/63 4. p38, a class of mitogen-activating protein kinases most commonly thought of as being involved in stress-response consists of four isoforms p38α, p38β, p38δ, and p38γ. Depending on cell type and the nature of stimuli, different isoforms may have redundant, specific, or even opposing functions 39, 40. Knockout studies in mice have shown that p38α and p38β are required for skeletogenesis and bone homeostasis 41. p38 has also been found to regulate the interaction of RUNX2 (early activator of osteogenesis) and Osterix (a late stage marker of osteogenic differentiation) 42, 43. However, the specific p38 isoform(s) mediating the interaction between RUNX2 and Osterix was not studied. Although the α and β isoforms of p38 are believed to have different effects on osteogenesis, both p38α and p38β knock-out mice were shown to have reduced bone mass41, suggesting both isoforms maintain key roles in bone growth.
HGF treatment leads to the rapid activation of the p38 pathway in mouse MSCs 1, 44 and rat hepatocytes 45. Our group was the first to show that HGF treatment leads to the rapid activation of total p38 in hMSC 37. Moreover, using phospho-p38α specific antibodies we found that HGF treatment rapidly activated and phosphorylated p38α in hMSC in a dose- and time-dependent manner 37. Interestingly, HGF treatment of hMSC also induced an increase in protein levels of p38α, p38β, p38δ, and p38γ, with the peak at 24hrs post-HGF treatment 37. An increase in p38α, p38β and p38γ RNA was also seen within 48hrs after HGF treatment, in contrast to a decrease in p38δ 37. The data suggest that the HGF-induced rapid phosphorylation of p38 leads to the downstream maintenance of p38 protein expression. At the same time, HGF is activating a yet to be determined signal transduction cascade leading to an increase or inhibition (p38δ) of the transcription of p38 isoforms. Further follow-up siRNA knock-down of p38α and p38β demonstrated a reduced HGF induction of key osteogenic markers 37. The combined effect of p38 on bone development and maintenance, together with HGF activation of p38 in hMSC provides potential for therapeutic treatment for osteoporosis and bone fractures.
3.2.3 The PI3K/AKT Pathway
Phosphatidylinositol-3 kinase (PI3K) in conjunction with protein kinase B (AKT), together with additional signaling molecules, create a pathway known to affect cellular survival, growth, and cycle regulation 46. This is consistent in the context of HGF/cMET, to which PI3K/AKT demonstrates motogenic and anti-apoptotic regulation 1. Following the binding of HGF to the cMET receptor and subsequent receptor dimerization and auto-phosphorylation, the activated receptor recruits a signaling molecule, Gab-1, which associates with PI3K. Gab-1 is critical to the HGF signaling pathway by virtue of the strong interaction between the Gab-1 cMET binding site and the active phosphorylated cMET receptor. In mouse models with knock-out Gab-1, the phenotype was comparable to that of HGF/cMET knock-out mice 7. This suggests that Gab-1 is essential to the function and signal produced by HGF, and a deficiency of Gab-1 diminishes the signal of the HGF/cMET pathway. The association with PI3K initiates the production of phosphatidylinositol 3,4-biphosphate (PIP2) and phosphatidylinositol 3,4,5-triphosphate (PIP3) and further generates the second messenger inositol-triphosphate (IP3) 4. Downstream signaling from PI3K activates AKT, also known as Protein Kinase B, which acts to prevent apoptosis through increased cell survival factors 47.
3.3 HGF cell cycle effects
It has been established that HGF promotes the differentiation of stem cells, thus reducing stem cell like characteristics. In order to understand the potential of HGF as a pleiotrophic growth factor, it is important to identify the effects of HGF on the cell’s progression through the phases of the cell cycle. Proteins such as p21, p27, and p53 regulate cell cycle progression. When treated with HGF, human mesenchymal stem cells (hMSCs) have higher levels of these cell cycle regulators in both mRNA and protein than control cells 48. Furthermore, when the HGF/cMET pathway was disrupted through the use of a specific siRNA, the levels of p21, p27, and p53 decreased and the anti-proliferative effects were reduced 48. This suggests HGF increases cell cycle regulatory proteins, resulting in a decrease in cell proliferation. Despite an effect on p21, p27, p53, HGF treatment has not been shown to have a significant effect on the levels of additional cell-cycle regulators such as RB, cyclin D1, CDK2, CDK4, or CDK6 48. Using an apoptosis assay, it was determined that HGF does not promote apoptosis in hMSCs 48. Accordingly, it appears HGF does not regulate the cell cycle through induced senescence, but rather it supports the inhibitory regulation by p21/p53.
4.1 Osteogenesis
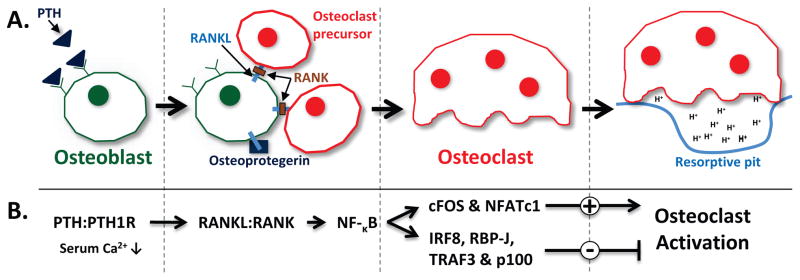
The skeletal system maintains a dynamic homeostasis by virtue of a coupled process of bone formation and bone resorption. Two types of cells balance this dynamic process, osteoblasts (formation) and osteoclasts (resorption). Osteoblasts, which are found at the surface of bone, primarily function to promote the synthesis of bone 49. After their formation from the maturation of proliferating MSCs, osteoblasts can be stimulated to further differentiate as osteocytes. Furthermore, osteoblasts indirectly alter and regulate the differentiation of osteoclasts through paracrine signaling via the expression and release of receptor activator of nuclear factor-κB ligand (RANKL) 49. Osteoclasts are multinucleated cells derived from the maturation of hematopoietic stem cells, mononuclear cells, which express the RANKL receptor RANK. The signaling between RANKL and cell surface associated RANK, leads to NF-κB signaling pathway activation which is involved in both the downstream positive (c-Fos, NFAT) and negative (IRF8, RBP-J & TRAF3, p100) regulation of cytokine-mediated development and activation of osteoclasts (figure 4) 50–52. In order to actively break down bone, osteoclasts physically attach to the surface of bone. The membrane of the osteoclast that is in contact with the bone surface becomes a ruffled membrane, producing an adhesive seal between the osteoclast and the bone surface 53. The ruffled membrane of the osteoclast produces H+-adenosine triphosphatase which acts like a proton pump to acidify the resorptive pit, a chamber created between bone surface and the membrane of the osteoclast 53. The acidic environment in the resorptive pit dissolves bone minerals. Further enzymatic digestion of type I collagen and associated proteins completes this process known as bone resorption, which concludes with the apoptosis of osteoclasts.
Figure 4.
Schematic diagram of osteoclastogenesis. A) Osteoblasts, bone forming cells, also regulate the differentiation of osteoclasts through the RANKL/RANK signaling system. Osteoclasts cause bone resorption through the creation of a resorptive pit and production of an acidic environment (Adapted from 52). B) RANKL/RANK signaling pathway involved in osteoclastogenesis and osteoclast activation.
Under hypocalcemia — low serum calcium conditions — parathyroid hormone (PTH) indirectly induces bone resorption through the development of new osteoclasts. PTH binds to a receptor on osteoblasts and signals for the expression of RANKL, which promotes the degradation of bone by activating osteoclastic differentiation 38. Conversely, osteoblasts produce a protein to protect against rampant bone resorption, appropriately named Osteoprotegrin (OPG), which acts as a “decoy” receptor to the RANKL, thus preventing it from binding to its receptor RANK 52. It is believed that the RANKL/OPG ratio — measured in the serum — is demonstrative of bone mass/resorption in individuals 54. In the context of the skeletal system, HGF is recognized to stimulate proliferation of osteoblasts and migration of osteoclasts 38, modulate the proliferation, differentiation and/or apoptosis of growth plate chondrocytes3, as well as promote skeletal muscle formation 55.
4.2 Effects of HGF in the context of osteogenesis
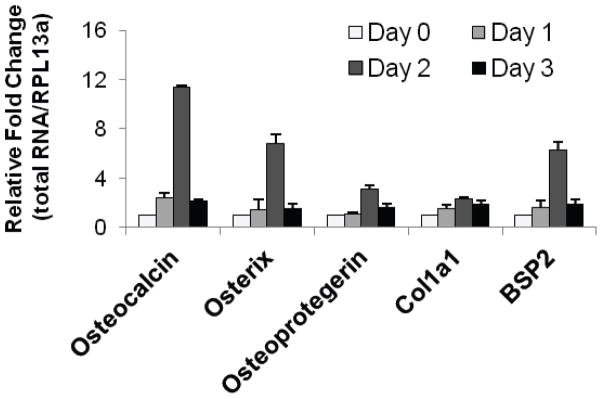
There is growing evidence both in vivo and in vitro establishing that HGF promotes osteogenesis56, and has been demonstrated in vitro by the increase in expression of primary osteogenic markers such as osteocalcin (OCN), osterix (OSX), osteoprotegerin (OPG), bone sialoprotein 2 (BSP2), and collagen 1a1 (COL1a1) in hMSC 37 (figure 5). After treatment with HGF (40 ng/mL), these five markers all experienced a significant increase in expression by day 2, supporting the action of HGF to promote osteogenesis. While HGF is known to have effects on osteogenesis as a whole, it functions differently in the context of osteoclasts versus osteoblasts. In human osteoclastic cells, the binding of HGF to its receptor promotes an increase in the intracellular concentration of calcium, due to the associated induced signaling of phospholipase C-γ 4, 32. The relationship between HGF and the intracellular calcium level is dose-dependent, and has been found to hold true in osteoclasts and osteoclast-like cells of several species32. The increase in calcium levels functions both to induce further signaling events and to act as a form of negative regulation for the HGF receptor. An increase in the calcium level due to treatment with HGF was not found in osteoblasts. Additionally, HGF elicits a change in the morphology of osteoclasts, while having no effect on the shape of osteoblasts. Under HGF treatment, osteoclasts undergo modifications, being converted from flat to spindle shaped, thought to encourage functional motility and effective migration to the bone matrix to perform bone resorption 32. Furthermore, HGF causes an increase in DNA synthesis in osteoclasts and osteoblasts 32, 57. Using an HGF-neutralizing antibody to block HGF signaling, a recent study found a significant dose-dependent decrease in mineralization with increasing concentrations of the HGF-neutralizing antibody 37. The reduction in mineralization due to the disruption of HGF signal transduction suggests that HGF plays a crucial role in the formation of bone. Additionally, treatment by HGF stimulates the production of osteopontin (OPN), a phosphoglycoprotein secreted by osteoblasts 38. OPN is a factor shown to be involved in proliferation and apoptosis, and an increase in OPN expression due to HGF treatment promotes osteogenesis. In regards to inhibition of skeletal HGF production, one study found that NF-κB activation (required for osteoclast formation) suppressed HGF expression and activity, thus decreasing skeletal muscle repair and levels of circulating HGF 55. This NF-κB – HGF inhibitory mechanism is further supported by a promoter analysis study in adipose tissue demonstrating that NF-κB inhibited the HGF gene promoter through suppression of PPAR γ 58, suggesting that NF-κB (RANKL/RANK) signaling may inhibit tissue specific levels of HGF.
Figure 5.
HGF treatment increases the expression of key osteogenic markers. Expression of mRNA was assessed using RT-qPCR using primer pairs for osteocalcin, osterix, osteoprotegerin, collagen-1a1 (Col1a1) and bone sialoprotein (BSP2), and normalized against RPL13a. Reproduced with permission from 37.
In vivo studies have shown that HGF treatment promotes osteogenic processes. The local expression of HGF in the microenvironment of a bone fracture showed enhanced healing of the bone fracture in rabbits 59. Furthermore, a rabbit model of a bone defect showed that HGF in combination with β-tricalcium phosphate (β-TCP) and collagen was more effective than the β-TCP and collagen treatments alone or the untreated control 60. β-TCP is used as a bone substitute when repairing bone defects due to its resorbability and biocompatibility, allowing it to enable surface bone growth. Increased β-TCP resorption indicates greater repair of the bone defect. After eight weeks a slight amount of bone formation was visible in the untreated controls, while both treatment groups showed a substantial increase in bone density and volume 60. Additionally, the group treated with the combination of β-TCP, collagen, and HGF had a larger area of new bone formation at weeks 2, 4, and 6 and smaller area of β-TCP, suggesting more β-TCP was resorbed and replaced by newly formed bone 60. HGF also significantly improved tissue self-repair in an animal model of traumatic osteonecrosis of the femora 61; however these studies did not explore the mechanism mediating HGF’s effects.
In contrast, several papers have reported that HGF inhibits osteogenic differentiation. For example, a supra physiological dose of HGF (100ng/ml) inhibited BMP induced ALP and mineralization62. Moreover, a recent article reported that high levels of HGF found in osteoarthritis patients decreased BMP2 activity and osteogenic capacity of osteoblasts63. While interesting these studies fail to identify the effect of supraphysiological levels of HGF on cMet levels and downstream signaling pathways. Additionally, these studies fail to discuss the role of chronic high levels of HGF. For example, a recent study reported a dose-dependent effect of HGF treatment, with higher doses of HGF decreasing cMet levels and MAPK and PI3K signaling pathways 64. Moreover, the timing of HGF treatment can affect its role on BMP activity. HGF treatment before BMP2 administration does not affect BMP-induced ALP65. Additionally, the role of HGF on hMSC osteogenic differentiation may be dependent on the stage of hMSC, the less mature the hMSC the more likely that HGF will promote osteogenic differentiation66. Therefore, endogenous and physiological levels of HGF, when given appropriately appear to promote osteogenic differentiation.
5.1 The Role of HGF in Cancer
HGF/cMET signaling has been linked to many types of cancer in humans. While we appreciate the vast amount of research being done on the role of HGF/cMET in cancer progression and as a therapeutic target for cancer treatment, the main focus of this review is to bring to light the research findings and recent endeavors’ concerning HGF in bone development, repair and its potential therapeutic applications. However, in brief review, cancers such as hepatocellular carcinoma are associated with an overexpression of HGF10. Despite this established link between HGF/cMET and hepatocellular carcinoma, there are inconsistent results as to whether HGF serum level is correlated to survival time or pathological factors 10. Conversely, the HGF levels within the tumors of breast cancer patients are associated with survival time; high HGF levels are associated with significantly shorter survival times than low levels of HGF 10. Additionally, one-third of breast cancer patients, in comparison to cancer-free individuals, had higher levels of serum HGF 10. Upon surgical removal of the tumor, serum HGF levels decreased, evidence suggesting that the rise in HGF levels is directly associated with the tumor.
Cancers are not only linked to the levels of HGF, but also associated with the cMET receptor. One mechanism in which cMET is involved in human cancers is through a mutation in the cMET gene67,68. In a recent clinical retrospective review 68, five unique cMET point mutations were identified from 134 tumors (6–7% of total tumors studied). Of those with cMET point mutations, 44% had co-mutations in 1–2 other known proto-oncogenes: p53, KRAS, NRAS, BRAF, PIK3CA, IDH1, KIT, AKT or EGFR. When comparing the survival outcomes of patients with surgically resected metastatic colorectal cancers containing (1) exclusively a cMET mutation versus (2) no mutation in cMET or other proto-oncogene detected, survival was slightly better in the patients with the cMET mutation present. This study suggested that the HGF/cMET pathway plays a role in cancer progression, possibly through the regulation of cancer stem cells.
As researchers gain a greater understanding of the HGF/cMET pathway, there is an opportunity for the use of this signaling pathway as a targeted cancer treatment 36, 69. In contrast to the above results suggesting that cMET mutations alone do not negatively impact survival, there are numerous HGF/cMET inhibitors currently in ongoing clinical trials to potentially be developed as cancer therapies 7 (http://clinicaltrials.gov/ct2/results?term=met).
5.2 Therapeutic implications
Due to the role of HGF in prevention of apoptotic cell death, HGF has been explored as a possible strategy for treatment of a variety of diseases. In animal models HGF has been demonstrated as an effective treatment for liver cirrhosis, chronic kidney disease, dilated cardiomyopathy, and lung fibrosis 7. HGF enables the reorganization of tissues, promoting the recovery of chronic fibrotic diseases. Despite these findings in animals, HGF has not yet been found effective in the therapeutic treatment of chronic fibrotic diseases in humans 7. However, HGF is being investigated as a possible treatment for renal failure due to its role as a mitogenic and motogenic factor, which may help stimulate kidney regeneration 70. Studies in a mouse model of Duchenne muscular dystrophy demonstrated that HGF is being suppressed by NF-κB signaling, and that knock-out of the NF-κB gene, or use of NF-κB chemical inhibitors leads to an increase in HGF transcription and skeletal HGF levels stimulating HGF-induced muscle repair 55. Additionally, a recent human phase I clinical trial used intramuscular injections of plasmid encoded HGF (pUDK-HGF) to treat and ameliorate the symptoms of critical limb ischemia. While the study was small (21-patients) and shows varied degrees of symptom reduction between patients, it does set the stage for further phase II trials using plasmid-based HGF71.
There are potential limits to the applicability of HGF as a therapeutic agent due to the possibility of exceeding appropriate doses of HGF, which may have negative effects. In order to use HGF as a treatment it is important to understand the regulatory clearance of HGF from the body. HGF has a relatively short half-life in plasma of 3–4 minutes, and full degradation is shown to occur within about 15 minutes 70, 72, 73, thus rendering IV HGF administration problematic. The chief organ for the clearance of HGF is the liver 70. There are two identified elimination mechanisms for HGF: receptor-mediated endocytosis through HGF/cMET, and endocytosis through heparin on the cell surface 70. However, the use of plasmid DNA delivery of HGF has been and is being successfully used in clinical models71, 74, 75. There are also reports of HGF being administered via hydrogels60, 76, 77 and using small molecule mimetics of HGF78 to produce positive effects in vitro and in vivo. One such small HGF-like molecule called BB3 (Refanalin) is currently in phase 3 clinical trials to treat delayed graft function in renal transplant patients (clinicaltrials.gov; NCT01561599), is under development for a phase 2 clinical trial to prevent acute kidney injury following cardiac surgery, and has recently been shown to improve neurological function in a preclinical model of cerebral ischemia in rats and mice78. Non-traditional modes of drug delivery have been found to be clinical beneficially for HGF treatment.
6.0 Conclusions
The HGF/cMET signaling system is vital to many cellular processes such as chondrogenesis, osteogenesis, tissue regeneration, and cell proliferation. The high molecular weight, multi-domain protein HGF mediates these processes through its interactions with the membrane bound cMET receptor. However, overexpression of HGF or cMET, mutation of cMET, and increased signaling has the potential to create cancer(s). After secretion by mesenchymal cells, HGF requires activating cleavage in order to function. Additionally, HGF has been identified to exist as four splice variants — NK1, NK2, NK3, and NK4 — as a function of alternative splicing. The splice variants are not well understood, but increasing research has demonstrated there is a difference in the function of each splice variant due to the structural distinctions between them.
HGF is a growth factor that has potential as a targeted therapeutic treatment for a variety of diseases and conditions due to the extensive effects of the HGF/cMET pathway. As the base of knowledge on HGF grows in different contexts — including the skeletal system, tumor formation, and kidney disease — HGF could become a strong player in the development of pharmaceuticals. Additionally, future research targeted towards the structure, functions and effects of the various HGF splice variants could allow drugs to target the intrinsic agonistic or antagonistic roles of NK1 and NK2, respectively, and potentially promote our understanding of the activities of NK3 and NK4. Further research into the mechanisms of HGF will contribute greatly to various fields of biomedical research and the use of the HGF/cMET pathway will prove valuable to benefiting human health.
7. Expert Opinion
Therapeutic targeting of HGF signaling pathway in bone repair and health
It is estimated that 6.2 million Americans experience a fracture annually and of those fractures 5–10% are mal- or non-union fractures 79. Bone regeneration and repair necessitates the production/activation of proteins or cells specific to the promotion of osteogenesis. Therefore, using growth factors or osteogenic cells to induce bone regeneration is a possible therapeutic technique for use on bone defects due to trauma or the removal of a tumor. A novel approach to treat fractures, particularly mal- or non-union bone fractures, includes the use of the growth factor HGF. HGF has robust effects on hMSC; blocking HGF bioactivity reduces hMSC migration 80 and blocking cMET diminishes both proliferation and differentiation56. While previous studies have found an important role for HGF in priming hMSCs for osteogenic differentiation in an in vitro model of bone repair, recent research has established a definitive role of HGF during osteogenic differentiation and bone repair 37, 59, 61.
Naked-HGF-Plasmid
Recent work supports the role of HGF in the bone repair using a rabbit model 59,61; however these studies did not explore the mechanism mediating HGF’s effects. In a human in vitro model of hMSC osteogenic differentiation and mineralization, HGF promoted the transcription of key osteogenic markers. Moreover, blocking HGF signaling through chemical inhibition of the cMET receptor or using antibodies to neutralized HGF reduced hMSC mineralization and alkaline phosphatase activity (ALP) in differentiated cells 37. These results along with in vivo studies of bone repair solidify the potential therapeutic role of HGF in osteogenic differentiation and promoting bone repair.
The use of HGF for treatment in animal models and in the clinical setting has been limited by its rapid metabolism and degradation in the liver. Therefore, to maintain a therapeutic level of hHGF a new approach has been developed using a naked-plasmid-based delivery of hHGF, systemically increasing HGF levels, which appears to be a safe, effective in vivo method 81–83. Plasmid-based delivery of hHGF should promote (through the p38 pathway) hMSC osteogenic differentiation and offers a safe and effective therapeutic treatment for bone repair. This new technology is currently being used in phase II clinical trials and is shown to be safe and therapeutically beneficial 71, 81, 83–85. Animal models using naked-plasmid hHGF delivery show safe and efficient transient delivery of exogenous HGF, leading to activation of growth-related signal transduction events and promotion of cell proliferation 84 in a mouse model of liver regeneration. Naked-plasmid HGF delivery in combination with hMSC has yet to be examined in bone repair models, and offers a unique method for a safe and efficacious treatment modality for bone disorders. Moreover, understanding and utilizing the role of the HGF/p38 pathway during hMSC differentiation and bone repair will have important translational implications for the treatment of bone diseases and promotion of bone homeostasis.
HGF Variants: NK1 & NK2
Increasing the stability and bioavailability of HGF would allow for implementation of localized or systemic recombinant HGF-mediated therapies. The naturally occurring splice variants NK1 and NK2 are both known to bind cMET, yet have opposing agonistic (NK1) and antagonistic (NK2) 8, 28 effects, respectively 27. Though lower then HGF (0.2nM26), NK1 has a higher affinity (10.4nM24) then NK2 (335nM24) for the cMET receptor. Suggestively, NK1 or an NK1-derived peptide could be administered IP/IV, or by local injection, and due to its greater stability, provide increased systemic or local levels of NK186. Thus an NK1-based treatment would still target cMET-expressing cells activating downstream pro-osteogenic HGF/cMET signaling pathways, such as p38 MAPK, and overcome the stability hurdle and high turn-over rate of endogenous and recombinant HGF.
Even though the utilization of the more stable HGF variant NK1 circumvents the stability and high turn-over rate issues of HGF/SF, NK1 would still affect other cMET-expressing somatic tissues (such as liver, kidney) as well as any malignant tissues which also express cMET. For this reason, prior to utilizing these therapeutic modalities to increase systemic or local HGF levels, clinicians would need to take into account a patient’s predisposition to cancer. In these cases, injection of HGF, NK1 or naked-HGF-plasmid directly into the site of damage, such as into the muscle around or bone-callus within a mal- or non-union fracture, might mitigate the risk of off-target HGF effects while still promoting bone repair. Further research needs to be done to determine 1) the effects of the HGF splice variants, NK1, NK2 and NK4 on hMSC osteogenic differentiation, 2) serum half-life of HGF as compared to its variants NK1 and NK2, as well as 3) the efficacy of using a naked-plasmid-based system for HGF overexpression in vivo.
Article Highlights.
Accumulating evidence supports the concept that HGF promotes bone maturation and is involved in maintenance and osteoblastic differentiation of bone derived mesenchymal stem cells.
Hepatocyte Growth Factor (HGF) consists of full-length HGF (also known as scatter factor), along with alternative splice variants; endogenously expressed NK1 and NK2, together with NK3 and NK4.
The reported increased stability and low turn-over rate of NK1 suggest that NK1 could provide novel therapeutic modalities in combination with stem cells for treatment of bone diseases.
Local expression of HGF in the microenvironment of a bone fracture showed enhanced healing of the bone fracture in animal models.
To maintain a therapeutic level of HGF a new approach has been developed using a naked-plasmid-based delivery of HGF, systemically increasing HGF levels, which appears to be a safe, effective in vivo method – currently being used in phase II clinical trials for critical limb ischemia.
Footnotes
Declaration of Interest
The authors were supported by National Institute of Health/National Institute of Arthritis and Musculoskeletal Diseases (GRANT # 5F32AR062990-04), Miami VA Medical Center, University of Miami School of Medicine. KM Curtis is a postdoctoral fellow funded in part by NIH/NIAMS F32 Postdoctoral Grant #5F32AR062990-04. GA Howard is funded in part through veterans affairs career scientist award and veterans affairs merit review grant. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1*.Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, et al. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006 Jan;24(1):23–33. doi: 10.1634/stemcells.2004-0176. Experiments show that HGF acts through different pathways to determine its complex effects on MSCs. [DOI] [PubMed] [Google Scholar]
- 2.Ross J, Gherardi E, Mallorqui-Fernandez N, Bocci M, Sobkowicz A, Rees M, et al. Protein engineered variants of hepatocyte growth factor/scatter factor promote proliferation of primary human hepatocytes and in rodent liver. Gastroenterology. 2012 Apr;142(4):897–906. doi: 10.1053/j.gastro.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Grumbles RM, Howell DS, Wenger L, Altman RD, Howard GA, Roos BA. Hepatocyte growth factor and its actions in growth plate chondrocytes. Bone. 1996 Sep;19(3):255–61. doi: 10.1016/8756-3282(96)00180-9. [DOI] [PubMed] [Google Scholar]
- 4.Stuart KA, Riordan SM, Lidder S, Crostella L, Williams R, Skouteris GG. Hepatocyte growth factor/scatter factor-induced intracellular signalling. Int J Exp Pathol. 2000 Feb;81(1):17–30. doi: 10.1046/j.1365-2613.2000.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991 Oct;10(10):2867–78. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Gherardi E, Gray J, Stoker M, Perryman M, Furlong R. Purification of scatter factor, a fibroblast-derived basic protein that modulates epithelial interactions and movement. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5844–8. doi: 10.1073/pnas.86.15.5844. Historic manuscript describing the initial purification and characterization of scatter factor, which later came to be recognized as hepatocyte growth factor (HGF) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura T, Sakai K, Matsumoto K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J Gastroenterol Hepatol. 2011 Jan;26( Suppl 1):188–202. doi: 10.1111/j.1440-1746.2010.06549.x. [DOI] [PubMed] [Google Scholar]
- 8.Chan AM, Rubin JS, Bottaro DP, Hirschfield DW, Chedid M, Aaronson SA. Identification of a competitive HGF antagonist encoded by an alternative transcript. Science. 1991 Nov 29;254(5036):1382–5. doi: 10.1126/science.1720571. [DOI] [PubMed] [Google Scholar]
- 9.Miyazawa K, Kitamura A, Naka D, Kitamura N. An alternatively processed mRNA generated from human hepatocyte growth factor gene. Eur J Biochem. 1991 Apr 10;197(1):15–22. doi: 10.1111/j.1432-1033.1991.tb15876.x. [DOI] [PubMed] [Google Scholar]
- 10.Athauda GCF, Ito T, Giubellino A, Rabe D, Raffensperger K, Lee Y, Bottaro DP. HGF (hepatocyte growth factor (hepapoietin A; scatter factor)) Atlas of Genetics and Cytogenetics in Oncology and Haematology. 2010;14(10):1008–25. [Google Scholar]
- 11.Seki T, Hagiya M, Shimonishi M, Nakamura T, Shimizu S. Organization of the human hepatocyte growth factor-encoding gene. Gene. 1991 Jun 30;102(2):213–9. doi: 10.1016/0378-1119(91)90080-u. [DOI] [PubMed] [Google Scholar]
- 12.Stoker M, Gherardi E. Scatter factor and other regulators of cell mobility. Br Med Bull. 1989 Apr;45(2):481–91. doi: 10.1093/oxfordjournals.bmb.a072336. [DOI] [PubMed] [Google Scholar]
- 13.Naldini L, Vigna E, Bardelli A, Follenzi A, Galimi F, Comoglio PM. Biological activation of pro-HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction. J Biol Chem. 1995 Jan 13;270(2):603–11. doi: 10.1074/jbc.270.2.603. [DOI] [PubMed] [Google Scholar]
- 14.Cioce V, Csaky KG, Chan AM, Bottaro DP, Taylor WG, Jensen R, et al. Hepatocyte growth factor (HGF)/NK1 is a naturally occurring HGF/scatter factor variant with partial agonist/antagonist activity. J Biol Chem. 1996 May 31;271(22):13110–5. doi: 10.1074/jbc.271.22.13110. [DOI] [PubMed] [Google Scholar]
- 15.Kuiper J, Van’t Hof A, Otter M, Biessen EA, Rijken DC, van Berkel TJ. Interaction of mutants of tissue-type plasminogen activator with liver cells: effect of domain deletions. Biochem J. 1996 Feb 1;313( Pt 3):775–80. doi: 10.1042/bj3130775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyake M, Saze K, Yaguchi T, Wang J, Suzuta Y, Haga Y, et al. Canine hepatocyte growth factor: molecular cloning and characterization of the recombinant protein. Vet Immunol Immunopathol. 2003 Oct 15;95(3–4):135–43. doi: 10.1016/s0165-2427(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 17.Cai H, Lan X, Li A, Zhou Y, Sun J, Lei C, et al. SNPs of bovine HGF gene and their association with growth traits in Nanyang cattle. Res Vet Sci. 2013 Oct;95(2):483–8. doi: 10.1016/j.rvsc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Thery C, Sharpe MJ, Batley SJ, Stern CD, Gherardi E. Expression of HGF/SF, HGF1/MSP, and c-met suggests new functions during early chick development. Dev Genet. 1995;17(1):90–101. doi: 10.1002/dvg.1020170110. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Bell AW, Michalopoulos GK, Zarnegar R. The mouse hepatocyte growth factor-encoding gene: structural organization and evolutionary conservation. Gene. 1994 Jul 8;144(2):179–87. doi: 10.1016/0378-1119(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 20.Elsen GE, Choi LY, Prince VE, Ho RK. The autism susceptibility gene met regulates zebrafish cerebellar development and facial motor neuron migration. Dev Biol. 2009 Nov 1;335(1):78–92. doi: 10.1016/j.ydbio.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koibuchi N, Kaneda Y, Taniyama Y, Matsumoto K, Nakamura T, Ogihara T, et al. Essential role of HGF (hepatocyte growth factor) in blood formation in Xenopus. Blood. 2004 May 1;103(9):3320–5. doi: 10.1182/blood-2003-02-0352. [DOI] [PubMed] [Google Scholar]
- 22.Takayama H, La Rochelle WJ, Anver M, Bockman DE, Merlino G. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):5866–71. doi: 10.1073/pnas.93.12.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otsuka T, Jakubczak J, Vieira W, Bottaro DP, Breckenridge D, Larochelle WJ, et al. Disassociation of met-mediated biological responses in vivo: the natural hepatocyte growth factor/scatter factor splice variant NK2 antagonizes growth but facilitates metastasis. Mol Cell Biol. 2000 Mar;20(6):2055–65. doi: 10.1128/mcb.20.6.2055-2065.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Tolbert WD, Daugherty-Holtrop J, Gherardi E, Vande Woude G, Xu HE. Structural basis for agonism and antagonism of hepatocyte growth factor. Proc Natl Acad Sci U S A. 2010 Jul 27;107(30):13264–9. doi: 10.1073/pnas.1005183107. Using crystal structures of the HGF splice variants NK1 and NK2 the authors were able to determine for the first time the cMET-agonistic and antagonistic effects of NK1 and NK2, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pediaditakis P, Monga SP, Mars WM, Michalopoulos GK. Differential mitogenic effects of single chain hepatocyte growth factor (HGF)/scatter factor and HGF/NK1 following cleavage by factor Xa. J Biol Chem. 2002 Apr 19;277(16):14109–15. doi: 10.1074/jbc.M112196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basilico C, Arnesano A, Galluzzo M, Comoglio PM, Michieli P. A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met. J Biol Chem. 2008 Jul 25;283(30):21267–77. doi: 10.1074/jbc.M800727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montesano R, Soriano JV, Malinda KM, Ponce ML, Bafico A, Kleinman HK, et al. Differential effects of hepatocyte growth factor isoforms on epithelial and endothelial tubulogenesis. Cell Growth Differ. 1998 May;9(5):355–65. [PubMed] [Google Scholar]
- 28.Hartmann HA, Sun DY. Regional mRNA changes in brain stem motor neurons from patients with amyotrophic lateral sclerosis. Mol Chem Neuropathol. 1992 Dec;17(3):249–57. doi: 10.1007/BF03160014. [DOI] [PubMed] [Google Scholar]
- 29.Ge X, Wang Y, Li Q, Yu H, Miao L. NK4 gene therapy inhibits HGF/Met-induced growth of human cholangiocarcinoma cells. Dig Dis Sci. 2013 Jun;58(6):1636–43. doi: 10.1007/s10620-012-2523-7. [DOI] [PubMed] [Google Scholar]
- 30.Venepalli NK, Goff L. Targeting the HGF-cMET Axis in Hepatocellular Carcinoma. Int J Hepatol. 2013;2013:341636. doi: 10.1155/2013/341636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achim CL, Katyal S, Wiley CA, Shiratori M, Wang G, Oshika E, et al. Expression of HGF and cMet in the developing and adult brain. Brain Res Dev Brain Res. 1997 Sep 20;102(2):299–303. doi: 10.1016/s0165-3806(97)00108-9. [DOI] [PubMed] [Google Scholar]
- 32**.Grano M, Galimi F, Zambonin G, Colucci S, Cottone E, Zallone AZ, et al. Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):7644–8. doi: 10.1073/pnas.93.15.7644. The HGF receptor is expressed by human primary osteoclasts, by osteoclast-like cell lines, and by osteoblasts, strongly suggesting the possibility of an autocrine regulation of the osteoclast by HGF and a paracrine regulation of the osteoblast by the HGF produced by the osteoclast. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong-Beltran M, Stamos J, Wickramasinghe D. The Sema domain of Met is necessary for receptor dimerization and activation. Cancer Cell. 2004 Jul;6(1):75–84. doi: 10.1016/j.ccr.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Gherardi E, Youles ME, Miguel RN, Blundell TL, Iamele L, Gough J, et al. Functional map and domain structure of MET, the product of the c-met protooncogene and receptor for hepatocyte growth factor/scatter factor. Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12039–44. doi: 10.1073/pnas.2034936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comoglio PM. Pathway specificity for Met signalling. Nat Cell Biol. 2001 Jul;3(7):E161–2. doi: 10.1038/35083116. [DOI] [PubMed] [Google Scholar]
- 36.Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signalling pathway in cancer. Eur J Cancer. 2010 May;46(7):1260–70. doi: 10.1016/j.ejca.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Aenlle KK, Curtis KM, Roos BA, Howard GA. Hepatocyte growth factor and p38 promote osteogenic differentiation of human mesenchymal stem cells. Mol Endocrinol. 2014 May;28(5):722–30. doi: 10.1210/me.2013-1286. The data reported demonstrate the importance of p38 signaling in HGF regulation of osteogenic differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Chen HT, Tsou HK, Chang CH, Tang CH. Hepatocyte growth factor increases osteopontin expression in human osteoblasts through PI3K, Akt, c-Src, and AP-1 signaling pathway. PLoS One. 2012;7(6):e38378. doi: 10.1371/journal.pone.0038378. The findings provide the first evidence that HGF increased OPN expression, providing a link and molecular mechanism between the HGF family and OPN in the physiology of bone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nebreda AR, Porras A. p38 MAP kinases: beyond the stress response. Trends Biochem Sci. 2000 Jun;25(6):257–60. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- 40.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000 Jan;12(1):1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 41.Greenblatt MB, Shim JH, Zou W, Sitara D, Schweitzer M, Hu D, et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Invest. 2010 Jul;120(7):2457–73. doi: 10.1172/JCI42285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ortuno MJ, Ruiz-Gaspa S, Rodriguez-Carballo E, Susperregui AR, Bartrons R, Rosa JL, et al. p38 regulates expression of osteoblast-specific genes by phosphorylation of osterix. J Biol Chem. 2010 Oct 15;285(42):31985–94. doi: 10.1074/jbc.M110.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ortuno MJ, Susperregui AR, Artigas N, Rosa JL, Ventura F. Osterix induces Col1a1 gene expression through binding to Sp1 sites in the bone enhancer and proximal promoter regions. Bone. 2013 Feb;52(2):548–56. doi: 10.1016/j.bone.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Lee KH, Kim JR. Kiss-1 suppresses MMP-9 expression by activating p38 MAP kinase in human stomach cancer. Oncol Res. 2009;18(2–3):107–16. doi: 10.3727/096504009789954591. [DOI] [PubMed] [Google Scholar]
- 45.Limaye PB, Bowen WC, Orr AV, Luo J, Tseng GC, Michalopoulos GK. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology. 2008 May;47(5):1702–13. doi: 10.1002/hep.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004 Apr;30(2):193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A. 2001 Jan 2;98(1):247–52. doi: 10.1073/pnas.011532898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen K, Perez-Stable C, D’Ippolito G, Schiller PC, Roos BA, Howard GA. Human bone marrow-derived stem cell proliferation is inhibited by hepatocyte growth factor via increasing the cell cycle inhibitors p53, p21 and p27. Bone. 2011 Dec;49(6):1194–204. doi: 10.1016/j.bone.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 49.Nardone V, D’Asta F, Brandi ML. Pharmacological management of osteogenesis. Clinics (Sao Paulo) 2014 Jun;69(6):438–46. doi: 10.6061/clinics/2014(06)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyce BF, Xiu Y, Li J, Xing L, Yao Z. NF-kappaB-Mediated Regulation of Osteoclastogenesis. Endocrinol Metab (Seoul) 2015 Mar 27;30(1):35–44. doi: 10.3803/EnM.2015.30.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3540–5. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9( Suppl 1):S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000 Sep 1;289(5484):1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 54.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004 Jul 28;292(4):490–5. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 55.Proto JD, Tang Y, Lu A, Chen WC, Stahl E, Poddar M, et al. NF-kappaB inhibition reveals a novel role for HGF during skeletal muscle repair. Cell Death Dis. 2015;6:e1730. doi: 10.1038/cddis.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Ippolito G, Schiller PC, Perez-stable C, Balkan W, Roos BA, Howard GA. Cooperative actions of hepatocyte growth factor and 1,25-dihydroxyvitamin D3 in osteoblastic differentiation of human vertebral bone marrow stromal cells. Bone. 2002 Aug;31(2):269–75. doi: 10.1016/s8756-3282(02)00820-7. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto K, Tajima H, Nakamura T. Hepatocyte growth factor is a potent stimulator of human melanocyte DNA synthesis and growth. Biochem Biophys Res Commun. 1991 Apr 15;176(1):45–51. doi: 10.1016/0006-291x(91)90887-d. [DOI] [PubMed] [Google Scholar]
- 58.Yin J, Lee JH, Zhang J, Gao Z, Polotsky VY, Ye J. Regulation of hepatocyte growth factor expression by NF-kappaB and PPARgamma in adipose tissue. Am J Physiol Endocrinol Metab. 2014 Apr 15;306(8):E929–36. doi: 10.1152/ajpendo.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59**.Matsubara H, Tsuchiya H, Watanabe K, Takeuchi A, Tomita K. Percutaneous nonviral delivery of hepatocyte growth factor in an osteotomy gap promotes bone repair in rabbits: a preliminary study. Clin Orthop Relat Res. 2008 Dec;466(12):2962–72. doi: 10.1007/s11999-008-0493-z. The data suggest delivery of hHGF plasmid into the osteotomy gap promotes fracture repair, and HGF could become a novel agent for fracture treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Goshima K, Nakase J, Xu Q, Matsumoto K, Tsuchiya H. Repair of segmental bone defects in rabbit tibia promoted by a complex of beta-tricalcium phosphate and hepatocyte growth factor. J Orthop Sci. 2012 Sep;17(5):639–48. doi: 10.1007/s00776-012-0262-4. The combined application of HGF in a β-TCP and collagen matrix promoted histological bone healing and augmented mechanical strength of the healing bone, particularly in the early stages. [DOI] [PubMed] [Google Scholar]
- 61**.Wen Q, Jin D, Zhou CY, Zhou MQ, Luo W, Ma L. HGF-transgenic MSCs can improve the effects of tissue self-repair in a rabbit model of traumatic osteonecrosis of the femoral head. PLoS One. 2012;7(5):e37503. doi: 10.1371/journal.pone.0037503. Transplantation of HGF-transgenic MSC one week after an experimentally induced osteonecrosis of the femoral head promoted recovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Standal T, Abildgaard N, Fagerli UM, Stordal B, Hjertner O, Borset M, et al. HGF inhibits BMP-induced osteoblastogenesis: possible implications for the bone disease of multiple myeloma. Blood. 2007 Apr 1;109(7):3024–30. doi: 10.1182/blood-2006-07-034884. [DOI] [PubMed] [Google Scholar]
- 63.Abed E, Bouvard B, Martineau X, Jouzeau JY, Reboul P, Lajeunesse D. Elevated hepatocyte growth factor levels in osteoarthritis osteoblasts contribute to their altered response to bone morphogenetic protein-2 and reduced mineralization capacity. Bone. 2015 Jun;75:111–9. doi: 10.1016/j.bone.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Walker N, Kahamba T, Woudberg N, Goetsch K, Niesler C. Dose-dependent modulation of myogenesis by HGF: implications for c-Met expression and downstream signalling pathways. Growth factors. 2015;33(3):229–41. doi: 10.3109/08977194.2015.1058260. [DOI] [PubMed] [Google Scholar]
- 65.Shibasaki S, Kitano S, Karasaki M, Tsunemi S, Sano H, Iwasaki T. Blocking c-Met signaling enhances bone morphogenetic protein-2-induced osteoblast differentiation. FEBS open bio. 2015;5:341–7. doi: 10.1016/j.fob.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawasaki T, Niki Y, Miyamoto T, Horiuchi K, Matsumoto M, Aizawa M, et al. The effect of timing in the administration of hepatocyte growth factor to modulate BMP-2-induced osteoblast differentiation. Biomaterials. 2010 Feb;31(6):1191–8. doi: 10.1016/j.biomaterials.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 67.Peruzzi B, Bottaro DP. Targeting the c-Met signaling pathway in cancer. Clin Cancer Res. 2006 Jun 15;12(12):3657–60. doi: 10.1158/1078-0432.CCR-06-0818. [DOI] [PubMed] [Google Scholar]
- 68.Zenali M, deKay J, Liu Z, Hamilton S, Zuo Z, Lu X, et al. Retrospective Review of MET Gene Mutations. Oncoscience. 2015;2(5):533–41. doi: 10.18632/oncoscience.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012 Jun;16(6):553–72. doi: 10.1517/14728222.2012.680957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sugiura T, Takahashi S, Sano K, Abe T, Fukuta K, Adachi K, et al. Pharmacokinetic modeling of hepatocyte growth factor in experimental animals and humans. J Pharm Sci. 2013 Jan;102(1):237–49. doi: 10.1002/jps.23337. [DOI] [PubMed] [Google Scholar]
- 71**.Cui S, Guo L, Li X, Gu Y, Fu J, Dong L, et al. Clinical Safety and Preliminary Efficacy of Plasmid pUDK-HGF Expressing Human Hepatocyte Growth Factor (HGF) in Patients with Critical Limb Ischemia. Eur J Vasc Endovasc Surg. 2015 Oct;50(4):494–501. doi: 10.1016/j.ejvs.2015.05.007. This manuscript shows the safety and efficacy of plasmid based delivery of HGF in the clincal setting, and may provide symptomatic relief for CLI patients, although a larger, randomized, double blinded phase II trial is most likely needed to provide more information on safety and efficacy. [DOI] [PubMed] [Google Scholar]
- 72.Appasamy R, Tanabe M, Murase N, Zarnegar R, Venkataramanan R, Van Thiel DH, et al. Hepatocyte growth factor, blood clearance, organ uptake, and biliary excretion in normal and partially hepatectomized rats. Lab Invest. 1993 Mar;68(3):270–6. [PubMed] [Google Scholar]
- 73.Zioncheck TF, Richardson L, DeGuzman GG, Modi NB, Hansen SE, Godowski PJ. The pharmacokinetics, tissue localization, and metabolic processing of recombinant human hepatocyte growth factor after intravenous administration in rats. Endocrinology. 1994 Apr;134(4):1879–87. doi: 10.1210/endo.134.4.8137756. [DOI] [PubMed] [Google Scholar]
- 74.Kaga T, Kawano H, Sakaguchi M, Nakazawa T, Taniyama Y, Morishita R. Hepatocyte growth factor stimulated angiogenesis without inflammation: differential actions between hepatocyte growth factor, vascular endothelial growth factor and basic fibroblast growth factor. Vascul Pharmacol. 2012 Aug 19;57(1):3–9. doi: 10.1016/j.vph.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Kitamura K, Fujiyoshi K, Yamane J, Toyota F, Hikishima K, Nomura T, et al. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS One. 2011;6(11):e27706. doi: 10.1371/journal.pone.0027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu P, Guo L, Huang L, Zhao D, Zhen R, Hu X, et al. Effect of semisynthetic extracellular matrix-like hydrogel containing hepatocyte growth factor on repair of femoral neck defect in rabbits. Int J Clin Exp Med. 2015;8(5):7374–80. [PMC free article] [PubMed] [Google Scholar]
- 77.Chiang CH, Wu WW, Li HY, Chien Y, Sun CC, Peng CH, et al. Enhanced antioxidant capacity of dental pulp-derived iPSC-differentiated hepatocytes and liver regeneration by injectable HGF-releasing hydrogel in fulminant hepatic failure. Cell Transplant. 2015;24(3):541–59. doi: 10.3727/096368915X686986. [DOI] [PubMed] [Google Scholar]
- 78.Chaparro RE, Izutsu M, Sasaki T, Sheng H, Zheng Y, Sadeghian H, et al. Sustained functional improvement by hepatocyte growth factor-like small molecule BB3 after focal cerebral ischemia in rats and mice. J Cereb Blood Flow Metab. 2015 Jun;35(6):1044–53. doi: 10.1038/jcbfm.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun D, Yuan D, Zhang X. A new hypothesis on the mechanism of atrophic non-union. Med Hypotheses. 2011 Jul;77(1):69–70. doi: 10.1016/j.mehy.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 80.Vogel S, Trapp T, Borger V, Peters C, Lakbir D, Dilloo D, et al. Hepatocyte growth factor-mediated attraction of mesenchymal stem cells for apoptotic neuronal and cardiomyocytic cells. Cell Mol Life Sci. 2010 Jan;67(2):295–303. doi: 10.1007/s00018-009-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81*.Shigematsu H, Yasuda K, Sasajima T, Takano T, Miyata T, Ohta T, et al. Transfection of human HGF plasmid DNA improves limb salvage in Buerger’s disease patients with critical limb ischemia. Int Angiol. 2011 Apr;30(2):140–9. This study showed the efficacy and safety of intramuscular injection of naked plasmid DNA encoding the human hepatocyte growth factor gene in Japanese patients with Buerger’s disease and critical limb ischemia. [PubMed] [Google Scholar]
- 82.Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, et al. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008 Jul 1;118(1):58–65. doi: 10.1161/CIRCULATIONAHA.107.727347. [DOI] [PubMed] [Google Scholar]
- 83.Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y, et al. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010 Sep;17(9):1152–61. doi: 10.1038/gt.2010.51. [DOI] [PubMed] [Google Scholar]
- 84.Yang J, Chen S, Huang L, Michalopoulos GK, Liu Y. Sustained expression of naked plasmid DNA encoding hepatocyte growth factor in mice promotes liver and overall body growth. Hepatology. 2001 Apr;33(4):848–59. doi: 10.1053/jhep.2001.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85*.Morishita R, Makino H, Aoki M, Hashiya N, Yamasaki K, Azuma J, et al. Phase I/IIa clinical trial of therapeutic angiogenesis using hepatocyte growth factor gene transfer to treat critical limb ischemia. Arterioscler Thromb Vasc Biol. 2011 Mar;31(3):713–20. doi: 10.1161/ATVBAHA.110.219550. Intramuscular injection of naked HGF plasmid is safe and feasible and can achieve successful improvement of ischemic limbs as sole therapy. [DOI] [PubMed] [Google Scholar]
- 86.Jones DS, 2nd, Tsai PC, Cochran JR. Engineering hepatocyte growth factor fragments with high stability and activity as Met receptor agonists and antagonists. Proc Natl Acad Sci U S A. 2011 Aug 9;108(32):13035–40. doi: 10.1073/pnas.1102561108. [DOI] [PMC free article] [PubMed] [Google Scholar]