Abstract
Nanos is a translational regulator required for the survival and maintenance of primordial germ cells. In the sea urchin, Strongylocentrotus purpuratus (Sp), Nanos 2 mRNA is broadly transcribed but accumulates specifically in the small micromere (sMic) lineage, in part because of the 3′UTR element GNARLE leads to turnover in somatic cells but retention in the sMics. Here we found that the Nanos 2 protein is also selectively stabilized; it is initially translated throughout the embryo but turned over in the future somatic cells and retained only in the sMics, the future germ line in this animal. This differential stability of Nanos protein is dependent on the open reading frame (ORF), and is independent of the sumoylation and ubiquitylation pathways. Manipulation of the ORF indicates that 68 amino acids in the N terminus of the Nanos protein are essential for its stability in the sMics whereas a 45 amino acid element adjacent to the zinc fingers targets its degradation. Further, this regulation of Nanos protein is cell autonomous, following formation of the germ line. These results are paradigmatic for the unique presence of Nanos in the germ line by a combination of selective RNA retention, distinctive translational control mechanisms (Oulhen et al., 2013), and now also by defined Nanos protein stability.
Keywords: Nanos, Pumilio, Cyclin B, Sumo, Ubiquitin, zinc finger, sea urchin, small micromeres, germ line, Strongylocentrotus purpuratus
Introduction
Nanos is a RNA binding protein which was first identified in Drosophila as a translational repressor required in anterior-posterior patterning (Cho et al., 2006; Irish et al., 1989). Subsequently it was found to be essential for germ line determination and Nanos orthologs have been found in the germ line of all animals tested (e.g. C. elegans (Kraemer et al., 1999), Xenopus (Lai et al., 2011) and planarians (Wang et al., 2007)). Nanos functions through its interaction with Pumilio, which binds RNAs containing a conserved motif in their 3′UTR, the Pumilio Response Element (PRE) (Sonoda and Wharton, 1999; Wharton and Struhl, 1991). Although only a limited number of mRNAs have been identified as Nanos/Pumilio targets, these mRNAs encode proteins with key cellular and developmental functions. These Nanos-targeted mRNAs include cyclin B (Asaoka-Taguchi et al., 1999; Dalby and Glover, 1993; Kadyrova et al., 2007; Lai et al., 2011), hid (Hayashi et al., 2004; Sato et al., 2007), hunchback (Murata and Wharton, 1995; Wreden et al., 1997), fem 3 (Ahringer and Kimble, 1991; Zhang et al., 1997), VegT (Lai et al., 2012), and CNOT6 (Swartz et al., 2014). This list may expand significantly; using a database from Xenopus laevis, a series of additional candidates of Nanos/Pumilio targeted mRNAs in the germline have been generated based on scanning for PRE consensus sequences in the 3′UTR sequences (Lai and King, 2013).
The Nanos protein has two conserved Cys-Cys-His-Cys zinc-finger motifs that are indispensable for its function and it was found that each amino acid of the CCHC motif is important for Nanos function in Drosophila (Arrizabalaga and Lehmann, 1999; Curtis et al., 1997). This zinc-finger domain was crystallized for the first time using the zebrafish Nanos (Hashimoto et al., 2010), which revealed that its basic surface is directly involved in RNA binding. In contrast to Nanos, its binding partner protein, Pumilio, is not limited to the germ line and instead is uniformly distributed e.g. in the Drosophila embryo (Macdonald, 1992). Pumilio is a member of the Puf family (Pum and FBF), in which members typically contain eight Puf repeats, each 36 amino acids in length (Goodwin, 2001). The eight repeats together with the C terminal conserved sequence form the Puf domain, which is critical for binding both the PRE in RNAs, and its cofactors, such as Nanos (Edwards et al., 2001; Wang et al., 2001).
The expression of germ-line factors is essential for the reproductive future of the organism, and as such, these factors are highly regulated. Indeed, ectopic expression of these genes often induces cell cycle and developmental defects (Luo et al., 2011; Wu and Ruvkun, 2010) and Nanos is thought to be “toxic” outside of its normal domain (Lai and King, 2013). In Drosophila, C.elegans, Danio, Xenopus, sea urchin, and mouse, appropriate expression of Nanos in the germ line requires its unique 3′ untranslated region (UTR) (D'Agostino et al., 2006; Gavis et al., 1996a; Gavis et al., 1996b; Kloc et al., 2000; Koprunner et al., 2001; Oulhen et al., 2013; Saito et al., 2006; Suzuki et al., 2010). Furthermore, in Xenopus oocytes, the nanos mRNA contains a secondary structural element - just downstream of the AUG start site which is both necessary and sufficient to prevent ribosome scanning of this mRNA until after fertilization (Luo et al., 2011). Thus, many different levels of regulation are imposed upon Nanos expression, yet no post-translational steps of regulation of the Nanos protein have yet to be identified.
Three Nanos homologs are present in the genome of the sea urchin, Strongylocentrotus purpuratus (Sp). Nanos 1 mRNA is only present in the ovaries (Swartz, unpublished data). Nanos 2 and Nanos 3 accumulate in the small micromeres (sMics) (Juliano et al., 2010), cells that contribute to the germ line (Yajima and Wessel, 2011). Nanos 2 is the first de novo mRNA to accumulate selectively in the sMics, is required for adult rudiment formation (Fujii et al., 2009; Juliano et al., 2010), but whose gene is transcriptionally active in many cells of the embryo. Nanos 2 is the only Nanos paralog that accumulates in the sMics until gastrulation when the Nanos 3 transcript then accumulates specifically also in the small micromere lineage. Previous data indicated that in sea urchins, the stability and the translation of Nanos2 RNA depended on its UTRs, both 5′ and 3′ sequences (Oulhen et al., 2013). In this study, we found that Nanos 2 protein accumulation is regulated by an additional mechanism that is post-translational; Nanos is translated in all cells of the embryo but the protein accumulates only in the germ line. This is the first documented case of such a germ cell specific, post-translational event for Nanos and may reflect the key and selective roles that Nanos plays in early development and in germ line determination.
Material and Methods
Animals
The sea urchin Strongylocentrotus purpuratus and the starfish Patiria miniata adults were housed in aquaria with artificial seawater (ASW) at 16°C (Coral Life Scientific Grade Marine Salt; Carson, CA). Sea urchin gametes were acquired by either 0.5M KCl injection or by shaking. Eggs were collected in ASW or filtered seawater and sperm was collected dry. Embryos were cultured in filtered seawater at 16°C. Sea star gametes were acquired by opening up the animals. Oocytes were collected in filtered seawater and sperm was collected dry. To obtain mature oocytes, the full-grown immature oocytes were incubated for an hour in filtered sea water containing 2 μM 1-methyladenine. After addition of sperm, fertilized eggs were cultured in filtered seawater at 16°C (Foltz et al., 2004; Wessel et al., 2010).
Immunofluorescence
Untreated embryos were cultured as described above and samples were collected at indicated stages of development for immunofluorescence. To test the ubiquitylation and sumoylation pathways, embryos were treated respectively with 25μM MG132 (C2211, Sigma Aldrich, St. Louis, MO) added 10 hours after fertilization or 10μM Gingkolic acid (345887, EMD Millipore, Billerica, MA) added 12 hours after fertilization. These treated embryos were fixed at mesenchyme blastula stage. Immunofluorescence was performed as described earlier; the Sp Nanos 2 affinity-purified antibody was diluted to 1:500 and used as described (Juliano et al., 2010). The Sp Vasa affinity-purified antibody was diluted to 1:500 (Voronina et al., 2008) . Sumo antibody was purchased from Cell Signaling Technology (4930), (Danvers, MA) and from Abcam (ab3742) (Cambridge, MA), and both were used at 1:100 dilution. Ubc9 antibody (AV43021) was purchased from Sigma Aldrich (St. Louis, MO) and used at dilution 1:100. For each immunofluorescence, the primary antibody was incubated at 4°C overnight, and an anti-rabbit Alexa Fluor 488 was used as the secondary antibody (Life Technologies, Carlsbad, CA), diluted by 1:500 in blocking buffer, for two hours at room temperature. Images were captured using a LSM 510 laser scanning confocal microscope (Carl Zeiss, Inc.; Thornwood, NY).
Plasmid constructions
The Renilla luciferase constructs, Xenopus β-globin 3′ UTR and Sp cyclin B 3′UTRs were amplified (Supplemental figure S1A) and cloned into a plasmid containing the Sp Nanos 5′UTR, Renilla luciferase open reading frame, and a SP6 promotor. The GFP constructs, Sp Nanos 2 5′ and ΔGNARLE 3′UTRs, were amplified and cloned next to the GFP open reading frame, and the T7 promotor as described previously (Wessel et al., 2013). Nanos 2 ORF was amplified and inserted in frame in C-terminal of the GFP ORF (Supplemental figure S1B). A mutant control was made by adding a T between the GFP ORF and the Nanos 2 ORF (Supplemental figure S1C), to create a stop codon after the GFP ORF and to change the open reading frame of Nanos 2. Mutations of the seven lysines and the zinc finger domains found in Nanos 2 ORF were accomplished using the QuickChange II Site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA), with the primers listed in Supplemental figure S2. The deletion constructs of Nanos ORF were made using the primers listed in Supplemental figure S3.
In vitro RNA synthesis
Capped sense RNA was synthesized using the mMessage mMachine® T7 or Sp6 Kit (Ambion, Austin, TX) yielding RNA concentrations between 0.5 and 2μg/μl. Each RNA was co-injected with mCherry flanked with β-globin UTRs. Injection solutions contained: 20% glycerol, 1×1012 copies of either a GFP RNA, or of the mCherry RNA. Approximately 2 pl of each RNA mixture was injected into each fertilized egg.
Microinjections
Microinjections of zygotes were performed as previously described (Cheers and Ettensohn, 2004). In brief, eggs were de-jellied with acidic sea water (pH 5.0) for 10 min, washed with filtered sea water three times, rowed with a mouth pipette onto protamine sulfate-coated 60 × 15 mm petri dishes, fertilized in the presence of 1 mM 3-AT, and injected using the Femto Jet ® injection system (Eppendorf; Hamburg, Germany). 1 × 90 mm glass capillaries with filaments (Narishige; Tokyo, Japan) were pulled on a vertical needle puller for injections (Narishige; Tokyo, Japan). Injected embryos were cultured in sea water at 16 °C.
Morpholino approach
Morpholinos against Sp Sumo (5′-ACTTCCTTGTGTTCAGTGGTCATGA-3′) were purchased from Genetools (Philomath, OR). Morpholino injection solutions include 20% glycerol and 1 mM 10,000 MW Dextran conjugated to Texas Red ® (Life technologies, Carlsbad, CA). A morpholino against the sea star Patiria miniata dysferlin (5′-TCGACACAATCACCATCAGCGACAT-3′) was used as a non-relevant control (Oulhen et al., 2014). For injection in sea star oocytes, the sea urchin, Sp SCP2 morpholino was used as a control (5'-GGACATACTTGTCAGGTCGTGCCAA-3')
Dual luciferase assay
Strongylocentrotus purpuratus fertilized eggs were injected, as described above, with a solution containing 1×1012 copies of each RNA, 20% glycerol, and 1mM Alexafluor 488-dextran to allow visualization of injected eggs. For each measurement, 100 injected embryos were collected at the blastula stage. Renilla and Firefly luminescence were measured using the Dual luciferase assay kit (Promega) in a Lumat LB 9501 luminometer (Berthold Technologies, Germany).
GFP Reporter Fluorescence
Injected embryos were cultured as described above and samples were collected at indicated stages of development. Live embryos were imaged on an LSM 510 laser scanning confocal microscope (Carl Zeiss, Inc.; Thornwood, NY). Approximately one hundred blastulae were visualized for each construct and time point.
Whole mount RNA in situ hybridization (WMISH)
Sequences used to make antisense WMISH probes for Sp Sumo and Sp Ubc9 were amplified from a cDNA library and cloned into pGEMT-EZ. The Sp Sumo probe template (789 bases) includes the entire open reading frame plus 522 bases of the 3′UTR, the primer set is as follows: F (5′-ATGCGTTTAGAAGTGAAGCCAAGTAATG-3′) and R (5′-ACCTACATGTACAATACAT GTCAATGAGTTAG-3′). The Sp Ubc9 probe template (510 bases) includes the entire ORF and was amplified with the following primers: F (5′-ATGTCGGGTATCGCGATCACACGACTTTC-3′) and R (5′-CTACGCCTTGCTGTTTGACTGGTGGTG-3′). A probe against GFP (Oulhen and Wessel, 2013) was used to visualize GFP RNA and GFP Sp Nanos 2 RNA (Figure 1).
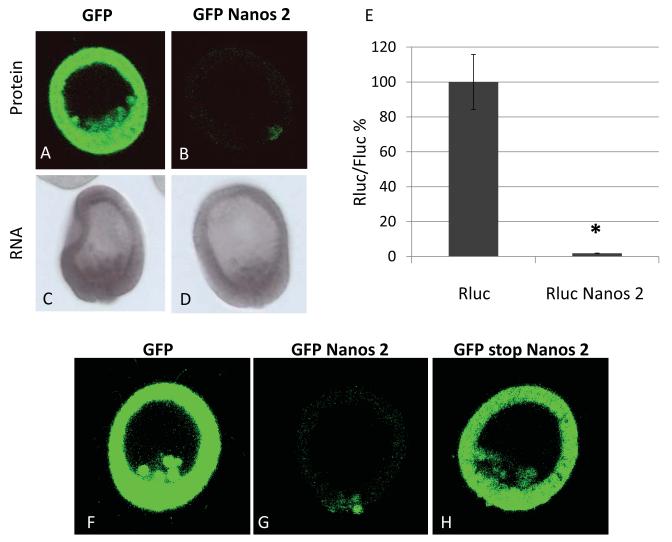
Figure 1.
Nanos 2 protein is enriched in the small micromeres. Synthetic mRNAs containing the GFP ORF alone (A) or the GFP ORF fused in frame with the Nanos 2 ORF (B) were injected in Sp fertilized eggs. In each case, the GFP alone, or the GFP Nanos 2, the ORF was surrounded by Nanos 2 5′ and ΔGNARLE 3′UTRs. GFP fluorescence was assayed 18 hours post-fertilization at mesenchyme blastula. A and B were obtained using the same settings (e.g. laser intensity, pin-hole opening) at 400x magnification. Approximately one hundred blastulae were visualized after injection of each construct. After injection of each construct, whole mount in situ hybridization was also performed using probes against GFP (C and D; not necessarily the same embryos for both tests) at the mesenchyme blastula stage. Synthetic mRNAs containing Renilla luciferase (Rluc) ORF alone or Rluc ORF fused in frame with the Nanos 2 ORF (E) were injected in Sp fertilized eggs. An mRNA containing the Firefly luciferase (Fluc) ORF flanked by Xenopus β-globin 5′ and 3′UTRs was co-injected. Luminescence was measured in mesenchyme blastulae. The ratio Rluc/Fluc was determined and the results are shown in percentages considering the ratio obtained for Rluc ORF as 100%. Error bars indicate the standard deviation from three technical replicates. Significance was assessed with the use of Student t test (P<0.05). Synthetic mRNAs containing the GFP ORF alone (F), the GFP ORF fused in frame with the Nanos 2 ORF (G) or the GFP ORF followed by a stop codon and an unframed Nanos 2 ORF (H) were injected in Sp fertilized eggs. In each case, the ORF was surrounded by Nanos 2 5′ and ΔGNARLE 3′UTRs. GFP fluorescence was assayed 20 hours post-fertilization at mesenchyme blastula. F, G and H were obtained using the same settings (laser intensity, pin-hole opening).
Antisense DIG-labeled RNA probes were constructed using a DIG RNA labeling kit (Roche; Indianapolis, IN). WMISH experiments were performed as previously described (Minokawa et al., 2004) and the alkaline phosphatase reaction was carried out for 5 hours. Samples were imaged on a Zeiss Axiovert 200M microscope equipped with a Zeiss color AxioCam MRc5 camera (Carl Zeiss, Inc.; Thornwood, NY).
Cell dissociation and measurement of autonomy
14 hours post fertilization embryos were dissociated as described in (Juliano et al., 2014), and cultured for 6 additional hours on a fluorodish. The single cells were then transferred to a protamine sulfate coated dish, and fixed with PFA 4% in filtered sea water. Immunofluorescence was used to co-label the cells using the affinity purified antibody against the Sp Vasa (as explained above), and an antibody against GFP (for Nanos-GFP) used at dilution 1:100 (A10262 from Invitrogen (Carlsbad, CA)).
Results
Nanos 2 protein is post-translationally enriched in the small micromeres
The sea urchin S. purpuratus Nanos 2 protein accumulates exclusively in the small micromeres as seen by a Sp Nanos-specific antibody (Juliano et al., 2010). This exclusivity may be explained by unique Nanos transcription in the sMics although preliminary results show that it is transcribed more broadly (Yajima, Swartz and Oulhen, personal communication). Alternatively, a broadly transcribed Nanos 2 mRNA may only be retained in the sMics since many mRNAs appear to be degraded selectively in the soma by a CNOT6-dependent mechanism, one that is lacking in the sMics (Gustafson and Wessel, 2010; Oulhen and Wessel, 2013; Swartz et al., 2014). Indeed, Nanos 2 contains an element in its 3′UTR, GNARLE (Global Nanos Associated RNA Lability Element), essential for the selective enrichment of its mRNA in the small micromeres (Oulhen and Wessel, 2014; Oulhen et al., 2013). Removal of GNARLE however, results in uniform RNA retention, and translation of the GFP reporter protein throughout the embryo. For example, injecting into fertilized eggs, the mRNA synthesized in vitro containing the GFP ORF fused to Nanos 2 5′UTR and ΔGNARLE 3′UTR, resulted in robust and uniform GFP accumulation throughout the embryo (Figure 1A). However, when the Nanos 2 ORF is also fused in frame to the C terminus of the GFP in this construct surrounded by Nanos 2 5′UTR and ΔGNARLE 3′UTR, low levels of protein were detected throughout the somatic cells during early development, followed by selective retention only within the small micromeres (Figure 1B). In situ hybridization indicates that both of these injected RNAs were present uniformly in embryos (Figure 1C and 1D), suggesting that an ectopic expression of a Nanos 2 reporter mRNA does not lead to an ectopic expression of its protein. These data indicate that even though the expression of the endogenous Nanos 2 is regulated by differential mRNA stability as previously shown, the embryos use an additional, post-translational mechanism that maintains a high protein retention in the PGCs and a low protein level in the somatic cells.
Nanos protein expression was then tested using a luciferase assay for quantitation independent of confocal imaging (Figure 1E). The Renilla luciferase ORF was used alone, or in frame with Nanos 2, and injected in Sp fertilized eggs. Both in vitro transcribed mRNAs were co-injected with a control RNA containing the Firefly luciferase ORF, flanked by the Xenopus β-globin 5′ and 3′UTRs. Both Rluc and Fluc activities were then measured at the mesenchyme blastula stage. Rluc was highly expressed and the corresponding ratio of Rluc/Fluc was set to 100%. In contrast, Rluc Nanos 2 gave a significantly lower luciferase activity, indicating that the Nanos 2 open reading frame caused decreased accumulation in the embryo. These results supported the conclusions obtained with the in situ GFP fusion protein dynamics (Figure 1A and 1B). Moreover, insertion of a nucleotide to create a stop codon after the GFP ORF, also changed the frame of the following Nanos 2 ORF. This led to GFP expression throughout the embryo (Figure 1H), indicating that the sequence of Nanos 2 open reading frame itself does not alter accumulation or translation dynamics of the selective expression in the small micromeres. The regulation for selective reporter accumulation instead functions once the Nanos protein is translated. In contrast to the GFP protein that continued to accumulate during development (Supplemental figure S4), the GFP Nanos 2 protein is maintained at overall low levels in the soma but is highly enriched in the small micromeres.
Selective Nanos 2 protein stability is independent of the sumoylation and ubiquitylation pathways
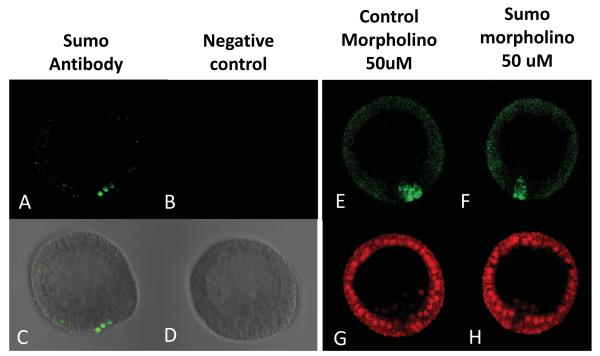
Protein modifications such as sumoylation (Geiss-Friedlander and Melchior, 2007; Johnson, 2004) or ubiquitylation (Komander and Rape, 2012) could be respectively stabilizing Nanos 2 in the small micromeres, or degrading it in the non-small micromere cells. One gene coding for Sumo was found in the sea urchin (SPU_018833). By immunofluorescence, we found that the Sumo protein is enriched in the small micromeres in blastulae (Figure 2A, 2C and Supplemental figure S5), coincident with the selective accumulation of Nanos 2 protein. The sequence of the sumoylation E2-conjugating enzyme Ubc9 (SPU_005107) was also found in the Sp genome. In contrast to Sumo, the Ubc9 protein was found uniformly throughout development (Supplemental figure S6). In situ hybridizations using probes against Sumo and Ubc9 indicate that both transcripts are expressed throughout the embryos (Supplemental figure S7). To test if the enrichment of Nanos 2 in the small micromeres depends on Sumo expression, embryos were co-injected with the transcript coding for the GFP Nanos 2 protein, and a morpholino against Sumo. This morpholino significantly decreased the level of Sumo protein in the small micromeres at blastula stage (Supplemental figure S8), although it had no effect on the enrichment of GFP Nanos 2 in the small micromeres (Figure 2F).
Figure 2.
Sumo protein accumulates in the small micromeres coincident with Nanos2 in blastula. Immunofluorescence using the antibody against Sumo (Abcam) was tested in blastula (A,C). Images using only the secondary antibody were used as a control (B,D), and were taken using the same microscope settings (laser intensity, pin-hole opening) at 400x magnification. Co-injection of Sp Sumo morpholino (50uM) with the GFP Nanos 2 transcript does not affect the enrichment of the GFP Nanos 2 protein in the small micromeres (F). A morpholino against Pm dysferlin was co-injected with the GFP Nanos 2 transcript as a non-relevant MO control (E). Images (E and F) were taken using the same microscope settings (laser intensity, pin-hole opening) at 400x magnification. In each case, a third transcript coding for mCherry and surrounded by Xenopus β-globin, was co-injected as a control reporter. Images (G and H) were taken using the same microscope settings (laser intensity, pin-hole opening) at 400x magnification.
To test a potential role of Sumo and Ubiquitin on Nanos 2 expression, embryos were incubated with a sumoylation inhibitor, Ginkgolic acid (Fukuda et al., 2009), and the level of the endogenous Nanos 2 protein was analyzed by immunofluorescence. Inhibition of the sumoylation pathway also did not affect the enrichment of the endogenous Nanos protein in the small micromeres (data not shown) nor did inhibition of the ubiquitylation pathway with MG132 (Supplemental figure S9).
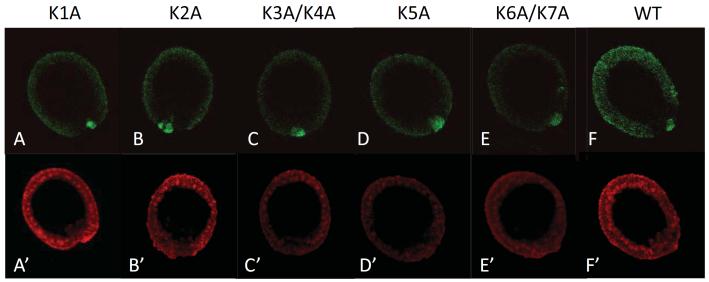
Both sumoylation and ubiquitylation occur on the lysines of the target proteins and seven lysines are present in the Nanos 2 protein. As another test for sumoylation or ubiquitylation on Nanos 2 enrichment in the small micromeres, five mutant constructs were crafted and injected (Figure 3). Mutations of the first (Fig 3A), the second (Fig 3B), the third and fourth (Fig 3C), the fifth (Fig 3D), or mutations of the sixth and seventh lysines (Fig 3E) did not affect the expression of GFP Nanos 2 in the small micromeres. Mutations of the seven lysines together led to an abnormally low expression of Nanos 2 protein throughout the embryo, perhaps as a result of misfolded protein structure that is quickly targeted for degradation in all cells (data not shown). Altogether, these results suggest that Nanos 2 enrichment in the small micromeres is not driven by protein modifications such as sumoylation or ubiquitylation.
Figure 3.
Mutations of the lysines in Nanos 2 ORF did not affect the relative enrichment of the protein in the small micromeres. Synthetic mRNAs containing the GFP ORF fused in frame with the Nanos 2 ORF were injected in Sp fertilized eggs. In each case, the ORF was surrounded by Nanos 2 5′ and ΔGNARLE 3′UTRs. The first lysine (A), the second lysine (B), the third and fourth lysines (C), the fifth lysine (D), the sixth and seventh lysine (E) present in Nanos 2 protein were mutated to alanine. The wild type Nanos 2 was used as a control (F). Each GFP Nanos 2 RNA was co-injected with a mCherry mRNA containing the Xenopus β-globin 5′ and 3′UTRs (A’ to F’). GFP and mCherry fluorescence were assayed 18 hours post-fertilization at mesenchyme blastula. A and A’ represent the same embryo, B and B’ represent another one, etc. For GFP images, A to F were obtained using the same settings (e.g. laser intensity, pin-hole opening). For mCherry images, A’ to F’ were also taken using the same settings.
Selective Nanos 2 accumulation is regulated by elements in its N-terminal region
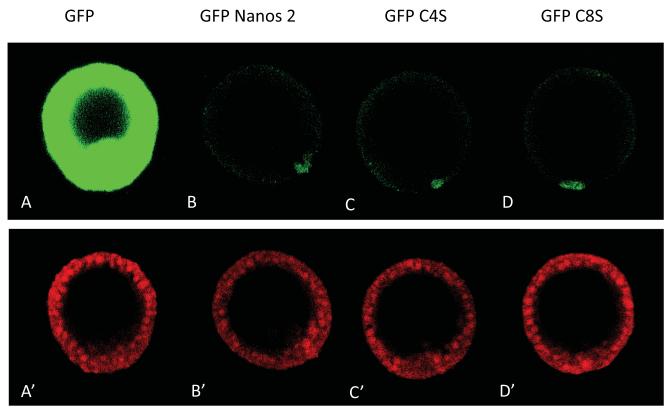
To test which domain of the Nanos 2 ORF may be involved in enrichment of the protein in the small micromeres, each of the two zinc fingers motifs were first mutated. It was previously shown that mutation of either the fourth cysteine of the first zinc-finger (C4), or the fourth cysteine of the second zinc-finger (C8) into a serine leads to a strong abdominal phenotype (Nanos-null) in Drosophila embryos (Arrizabalaga and Lehmann, 1999). Following a similar mutagenesis strategy for the GFP Nanos 2 protein, and then injecting the cognate synthetic transcripts of these sequences, we found that the Nanos 2 accumulation remained unchanged. The signal still remained enriched in the small micromeres (Figure 4) relative to the wild type Nanos 2 protein and appeared independent of Nanos function.
Figure 4.
Mutations of the zinc finger domains did not affect the expression of Nanos 2 in the small micromeres. Synthetic mRNAs containing the GFP ORF alone (A), or fused in frame with the Nanos 2 ORF (B,C,D) were injected in Sp fertilized eggs. In each case, the ORF was surrounded by Nanos 2 5′ and ΔGNARLE 3′UTRs. The wild type Nanos 2 was used as a control (B). The last cysteine of the first zinc finger domain (C), or the second zinc finger domain (D) present in Nanos 2 protein were mutated into a serine. Each GFP Nanos 2 RNA was co-injected with a mCherry mRNA containing the Xenopus β-globin 5′ and 3′UTRs (A’ to D’). GFP and mCherry fluorescence were assayed 18 hours post-fertilization at mesenchyme blastula. A and A’ represent the same embryo, B and B’ represent another one, etc. For GFP images, A to D were obtained using the same settings (laser intensity, pin-hole opening). For mCherry images, A’ to D’ were also taken using the same settings.
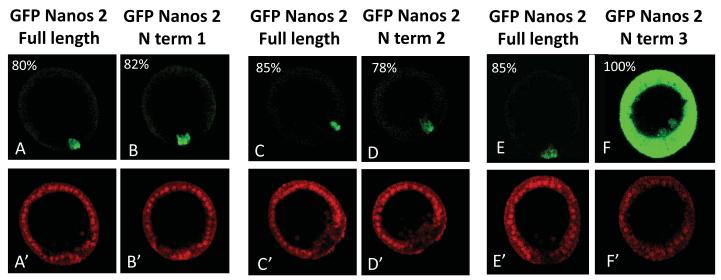
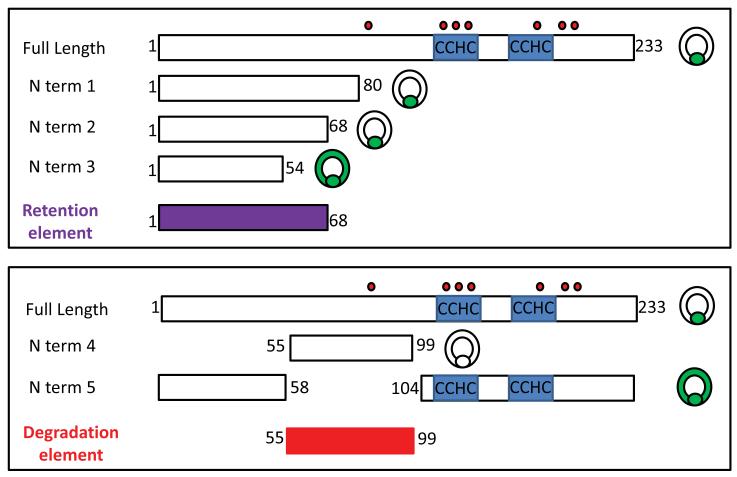
To determine what parts of the Nanos protein may be responsible for the selective accumulation in the germ line, we re-employed the GFP fusion protein strategy. We constructed deletion mutants whose cognate mRNAs were injected after fertilization, and the absence or presence of the green fluorescence in the somatic cells was analyzed in blastulae. Interestingly, GFP fused to the first 68 amino acids of the Sp Nanos 2 (N term 2) is specifically retained in the PGCs, similarly to Sp Nanos 2 full length (Figure 5 and Figure 7). However, the first 54 amino acids (N term 3) are not sufficient to degrade Sp Nanos 2 in the somatic cells. These results indicate that the first 68 amino acids of Sp Nanos 2 represent the minimal region required for the selective accumulation of the protein in the PGCs. This element will be then referred as NPRE (Nanos Protein Retention Element).
Figure 5.
Nanos 2 N terminal domain contains an element that is essential for its selective retention in the PGCs at blastula stage. Synthetic mRNAs containing the GFP ORF fused with the Nanos 2 full length ORF (A,C, E) were injected in Sp fertilized eggs. Deletion mutants were also injected: GFP fused to the sequence coding for the first 80 (N term 1), 68 (N term 2), or 54 (N term 3) amino acids of Sp Nanos 2. In each case, the ORF was surrounded by Nanos 2 5′ and ΔGNARLE 3′UTRs. A control RNA containing mCherry flanked with β-globin UTRs was co injected with each deletion construct. Images were taken at mesenchyme blastula stage. The GFP and mCherry fluorescence were imaged using respectively the same settings: GFP (A to F), and mCherry (A’ to F’) at 400x magnification.
Figure 7.
Summary of the expression of GFP Nanos 2 fusion proteins imaged at mesenchyme blastula stage. The names of the injected constructs are indicated on the left, the corresponding amino acids fused in frame to the C terminal of the GFP are presented by rectangles on the right. The number surrounded the rectangles indicate the amino acid present at the beginning at the end of each construct. The location of the zinc finger motifs (CCHC) is shown in blue. Nanos 2 contains 7 lysines, their location is indicated by the small red circles, above the full length sequence. A schematic blastula at the end of the construct indicates where the corresponding GFP fluorescence was detected.
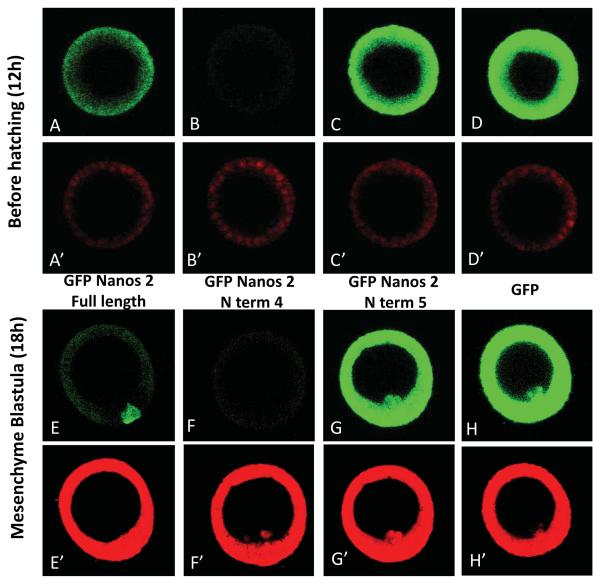
Importantly, GFP fused to as little as 45 amino acids (amino acid 55 to 99) of Sp Nanos 2 (N term 4) is degraded in every cell of the blastula (Figure 6, Figure 7, and Supplemental figure S10). The protein is translated, and accumulates early in development throughout the embryo but became undetectable before reaching the blastula stage (Supplemental figure S11). In contrast, deletion of this element (N term 5) led to Nanos 2 stabilization, with the protein accumulating abundantly throughout the entire blastula. These results indicate that the N terminal domain of Sp Nanos 2 (amino acid 55 to 99) is necessary and sufficient for the degradation of the protein. This 45 amino acid element (MNNNMVSGLPVSTSQSSTTASTGFMPLQLPLNIAEITELSKVMRG) will be then referred as NPDE (Nanos Protein Degradation Element). Interestingly, the NPDE leads to the degradation of Nanos 2 in both the somatic cells and the PGCs, showing the importance of the NPRE to prevent the degradation of the protein specifically in the PGCs. Several additional deletion mutants were tested; fusion proteins containing only the last 133 amino acids (C term 1), were highly expressed in the entire blastula, just as seen with GFP alone (Supplemental figure S12 and S13). However, the construct N term 9 (amino acid 81 to 140; Supplemental figure S12 and S14), like the N term 2 (amino acid 1 to 68; Figure 7) that do not contain the complete NPDE, were effectively degraded in the somatic cells, suggesting that the NPDE contains at least two independent functional motifs (amino acid 55 to 68, and 81 to 99). Altogether, these data suggest that multiple elements are present in the N terminal region of Nanos 2 to target its degradation in the soma, but to protect the protein from degradation in the PGCs.
Figure 6.
Nanos 2 N terminal domain contains an element that causes its degradation at the blastula stage. Synthetic mRNAs containing the GFP ORF alone (D,H) or fused with the Nanos 2 full length ORF (A,E) were injected in Sp fertilized eggs. Deletion mutants were also injected: GFP fused to the NPDE (N term 4: B,F) and GFP fused to Nanos 2 ORF deleted in the NPDE (N term 5:C,G). In each case, the ORF was surrounded by Nanos 2 5′ and ΔGNARLE 3′UTRs. A control RNA containing mCherry flanked with β-globin UTRs was co injected with each deletion construct. Images were taken at mesenchyme blastula stage. The GFP and mCherry fluorescence were imaged using respectively the same settings: GFP (A to F), and mCherry (A’ to F’) at 400x magnification.
Autonomous accumulation of Nanos 2 protein in the PGCs
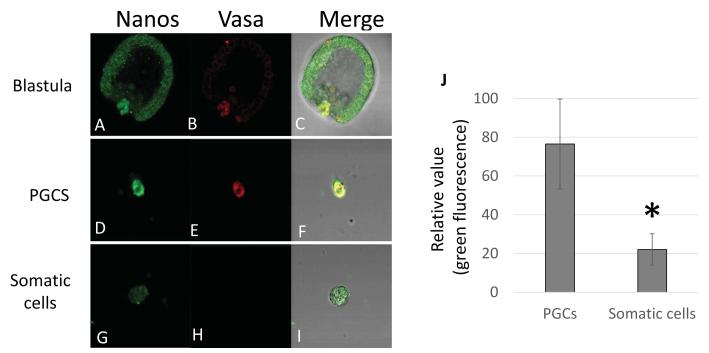
It was previously shown that Nanos mRNA autonomously accumulates in the small micromere lineage after dissociation of 16 cell stage embryos and in vitro culture. Nanos transcription was similarly regulated in whole embryos and in isolated cells (Yajima and Wessel, 2012). To test if Nanos 2 protein also accumulates autonomously in the PGCs, the GFP Nanos 2 full length mRNA surrounded by Sp Nanos 2 5′UTR and ΔGNARLE 3′UTR was injected in fertilized eggs. The embryos were dissociated into single cells at 14h, before Nanos 2 protein selectively accumulates in the small micromeres (Supplemental figure S4). At 20h, the Nanos 2 protein was identified by immunofluorescence and found selectively and highly expressed in the PGCs (Figure 8). Similar observations were obtained by directly observing the Nanos-GFP fluorescence in live dissociated cells (data not shown). Altogether these results indicate that Nanos 2 expression is autonomously regulated, not only at the transcription level as previously shown, but also at the protein stability level.
Figure 8.
The selective retention of Nanos 2 protein is autonomous. Synthetic mRNAs containing the GFP Nanos 2 full length ORF were injected in Sp fertilized eggs. The resulting early blastulae (14h post fertilization), were dissociated into single cells and cultured for an additional 6 hours. At 20h, cells were fixed to test the presence or absence of Nanos protein in the PGCs (D) and the somatic cells (G). Vasa was used as a control to identify the PGCs (E,H). Undissociated mesenchyme blastulae were used as a control (A,B). Images were taken using respectively the same microscope settings (laser intensity, pin-hole opening) for (A,D,G), and for (B,E,H) at 400x magnification. With Metamorph, the green fluorescence (Nanos expression) was quantified in 11 PGCs and 34 somatic cells (J). Significance was assessed with the use of Student t test (P<0.05).
Discussion
The specific expression and function of Nanos in germ line development is seen widespread: in the planarian flatworms (Wang et al., 2007), the nematode Caenorhabditis elegans (Kraemer et al., 1999; Subramaniam and Seydoux, 1999), Drosophila (Bhat, 1999; Forbes and Lehmann, 1998; Kobayashi et al., 1996; Sato et al., 2007), sea urchin (Juliano et al., 2010; Yajima and Wessel, 2011), zebrafish (Beer and Draper, 2013; Draper et al., 2007; Koprunner et al., 2001), Xenopus (Houston and King, 2000; Lai et al., 2012; Luo et al., 2011; Mosquera et al., 1993), mouse (Haraguchi et al., 2003; Saga, 2010; Tsuda et al., 2003), and human (Julaton and Reijo Pera, 2011; Wu et al., 2013). Loss of Nanos expression induces cell cycle and developmental defects resulting in the loss of PGCs through apoptosis (Forbes and Lehmann, 1998; Kobayashi et al., 1996; Koprunner et al., 2001; Sato et al., 2007; Tsuda et al., 2003). In contrast, ectopic expression of Nanos often leads to embryonic lethality (Luo et al., 2011). Furthermore, Nanos expression is also observed in cancer cells. A study in Drosophila showed that ectopic expression of germline genes such as the orthologs of Piwi, Vasa, Nanos and Aubergine contributed significantly to growth and survival of malignant brain tumors (Janic et al., 2010); inactivation of these germ cell genes even resulted in tumor regression. In humans, some genes that are predominantly expressed in germline cells become aberrantly activated in various malignancies, and these genes could be potential targets to treat tumors in humans (Wu and Ruvkun, 2010). Understanding the mechanisms regulating Nanos expression will address basic processes in germ cell development and likely contribute more broadly than originally anticipated.
In the sea urchin embryo, we find that Nanos 2 protein accumulation depends on the UTRs of the transcript for mRNA stability (Oulhen et al., 2013), on translational regulation (Oulhen et al., 2013), and now on the selective stability of the protein in the sMics, post translationally. Mutations of either the zinc finger motifs present in the C terminal region of Nanos 2, or the 7 lysines, that could be targeted by Sumo or Ubiquitin, do not affect the stability of the protein in the germ line. However, the Nanos Protein Degradation Element (NPDE) present in the N terminal region of Nanos 2 destabilizes the protein in both the somatic cells and the PGCs. This element is well conserved between urchin species, the amino acid sequence of the NPDE in Sp is 93% identical to the corresponding sequence found in the urchin Hemicentrotus pulcherimus that diverged from Sp approximately 20 million years ago (Lee, 2003). Thus we conclude that the post-translational mechanism of selective Nanos protein accumulation and degradation is a broad phenomenon. The NPDE in somatic cells is probably recognized by one or multiple proteases starting between 12 to 13h (blastula) until at least 18h (mesenchyme blastula) after fertilization. Potential proteases for this region were suggested using PROSPER (Supplemental figure S15) and predicted protease sites were predicted within the two motifs identified functionally in the NPDE (either between the amino acids 55 to 68, or 81 to 99). Identification of the protease(s) that regulates Nanos 2 stability in the somatic cells is currently under investigation. The Nanos Protein Retention Element present in the N terminal region also appears required to protect the protein from degradation in the PGCs; this element could interact with one or more proteins specifically localized in the small micromeres to sterically block the binding or the activity of the protease responsible for Nanos 2 degradation. The NPDE and NRPE may also be useful technically in that at least in this embryo and its close relatives, one could target rapid turnover of proteins by attaching the NPDE, or enhance selective protein accumulation into the PGCs by attaching the NPRE.
Several results converge to indicate that the selective accumulation of Nanos 2 in the germ cells is controlled by post translational mechanisms. First, the GFP is fused to the N terminal of Nanos 2 ORF, exempting a translational regulation. As shown in Xenopus, Nanos 1 mRNA contains a Translation Control Element (TCE) downstream of its ATG (Luo et al., 2011). This repression happens when the Nanos 1 ORF is on the N terminal of the Myc ORF reporter, and does not occur when the Nanos 1 ORF is on the C terminus of the Myc ORF; the ribosomes presumably are already recruited to the mRNA by the Myc ORF and the full Myc Nanos ORF was translated despite the TCE. Secondly, Nanos 2 ORF does not seem to contain a consensus sequence or a structural element that could inhibit its translation. As demonstrated in Figure 1H, the transcript containing the GFP ORF followed by a stop and an unframed Nanos 2 ORF can be translated in the whole blastula, suggesting that the sequence of the ORF does not inhibit translation, and that it’s the protein itself after being synthetized who is the target of this regulation. Thirdly, we see overall Nanos protein expression throughout the early embryo and then rapid degradation everywhere except in the germ line. Finally, we see the same results using luciferase as a reporter as well as multiple Nanos-deletion constructions to provide confidence in this conclusion of post-translational regulation.
These multiple control steps in Nanos accumulation emphasizes its functional importance at many different levels. Recently it was shown in this embryo that Nanos regulates selective mRNA turnover in the egg – to - embryo transition. Nanos 2 accomplishes this feat by interacting with its partner Pumilio to degrade transcripts in the PGCs such as CNOT6, encoding a deadenylase that is responsible for clearing of the maternally stored mRNAs in the future somatic cells, while retaining these transcripts in the germ line (Swartz et al., 2014). Mis-regulation of CNOT6 by interfering with Nanos function is lethal. In Drosophila, the Nanos/Pumilio complex is known to control the translation of specific mRNAs like cyclin B (Asaoka-Taguchi et al., 1999). A common sequence motif is found in the 3′UTR of Pumilio mRNA targets, the consensus includes a highly conserved 8 nucleotide core motif: UGUA(A/U/C)AUA (Gerber et al., 2006). We found that in at least two sea urchin species, Strongylocentrotus purpuratus, and Lytechinus variegatus, the cyclin B 3′UTR contains 7 out of these 8 highly conserved nucleotides (Supplemental figure S16A). Using a luciferase assay, we also found that Nanos 2 specifically inhibits the translation of the RNA containing the cyclin B 3′UTR (Supplemental figure S16B). Together, these results demonstrate that the restriction of Nanos 2 protein to the PGCs is indeed essential to prevent degradation of transcripts such as CNOT6 or cyclin B, in somatic cells.
Vasa protein is also enriched in the sMics post-translationally (Gustafson et al., 2011; Voronina et al., 2008). This protein appears to be rapidly degraded in the somatic cells, but is retained in the sMics by differential ubiquitylation mechanisms. Thus, we surmised that the Nanos 2 protein may be regulated by a similar strategy that might form a unifying concept of germ cell selection. However, having deleted each of the lysines in Nanos individually or in combination, and use of inhibitors of ubiquitylation suggests that ubiquitylation is unlikely a mechanism in the regulation of Nanos 2 selective accumulation. We did however discover that sumo is enriched in the germ cell lineage in sea urchin. It was previously reported in Drosophila that transcripts of five genes involved in the sumoylation pathway were found throughout the early embryo, and then predominantly expressed in the nuclei of pole cells in late stage embryos and then in gametogenic cells (Hashiyama et al., 2009). In the green mud crab, results suggest that Sumo-1 may have special import during gametogenesis. Sumo-1 was shown to be more abundantly expressed in the crab ovary than in other tissues, and it was also expressed in testis development (Dai et al., 2012). Although the role of Sumo is not well understood, studies with mutant animals have demonstrated that the sumoylation pathway is also critical for embryonic development (Lomeli and Vazquez, 2011). For example in C. elegans, Sumo-1 is required for germline development and the authors described the transcription factor Lin-11 as a substrate for sumoylation (Broday et al., 2004). Sumoylation of transcription factors has been correlated with inhibition of transcription (Gill, 2005) and evidence also suggests that sumo has multiples roles in chromatin regulation (Srikumar et al., 2013). Sumoylation of histones appears to mediate gene silencing through recruitment of histone deacetylase and heterochromatin protein 1 (Shiio and Eisenman, 2003). In most organisms, the specification of the germ line depends on mechanisms that also inhibit the expression of somatic genes (Seydoux and Braun, 2006). Thus, the enrichment of Sumo in the small micromere lineage could be crucial to inhibit transcription in the PGCs, especially when considering that these cells appear to be transcriptionally silent (Swartz et al., 2014).
Supplementary Material
Supplemental Figure S1: List of primers used to make (A) the cyclin B constructs and (B) the Nanos 2 constructs.
Supplemental Figure S2: List of primers used to make (A) the Nanos 2 lysine mutants constructs and (B) the Nanos 2 zinc finger mutants.
Supplemental Figure S3: List of primers used to make the Nanos 2 deletion mutants (A and B)
Supplemental Figure S4: GFP Nanos 2 protein expression remains low during the early development until its enrichment in the small micromeres. Synthetic mRNAs containing the GFP ORF alone (A to E), or the GFP ORF fused in frame with the Nanos 2 ORF (K to O) were injected in Sp fertilized eggs. In each case, the ORF was surrounded by Nanos 2 5′ and ΔGNARLE 3′UTRs. Injected embryos were imaged at the following times after fertilization: (A,K) 8H, (B,L) 9H, (C,M) 13H, (D,N) 16H, (E,O) 20H. Images were taken using respectively the same microscope settings (laser intensity, pin-hole opening) from A to E and K to O, at 400x magnification. The corresponding differential interference contrast images are shown from A’ to E’, and K’ to O’.
Supplemental Figure S5: Sumo protein accumulates in the small micromeres coincident with Nanos 2 in blastula. Immunofluorescence using the antibody against Sumo was tested in unfertilized eggs (A,A’), 2-cell stage (B,B’), and 4-cell stage embryos (C,C’), morula (D,D’), mesenchyme blastula (E,E’), gastrula (F,F’) and pluteus (G,G’). Two antibodies were tested, one from Abcam (A to G), and one from Cell Signaling Technology (A’,G’), Images (A to G), and (A’ to G’) were taken using the same microscope settings (laser intensity, pin-hole opening) at 400x magnification. Immunofluorescences using only the secondary antibody were used as controls and didn’t give any detectable signal (data not shown).
Supplemental Figure S6: The protein Ubc9 is ubiquitously expressed during the early development in sea urchin. Immunofluorescence using the antibody against Ubc9 was tested in unfertilized eggs (A), 2-cell stage (B), and 4-cell stage embryos (C), in blastula (D), gastrula (E) and pluteus (F). Images were taken using the same microscope settings (laser intensity, pin-hole opening) at 400x magnification. A control was performed using only the secondary antibody, on unfertilized eggs (G), 2-cell stage (H), 4-cell stage (I), blastula (J), gastrula (K), and pluteus (L).
Supplemental Figure S7: Sumo and Ubc9 mRNA are uniformly expressed during sea urchin early development. Whole mount in situ hybridization in the sea urchin using probes against Sumo (a to h), and Ubc9 (p to v). Embryos were fixed from fertilized eggs (a,i,p), to pluteus (h,o,v). A probe against the Neomycin resistance gene was used as a negative control (i to o).
Supplemental Figure S8: A morpholino against Sumo decreases the abundance of Sumo protein in the small micromeres at blastula stage. Dose response of Sumo morpholino (MO) during development. Fertilized eggs were injected with 25 to 500 uM of Sumo morpholino (C to G), and imaged 18 hours after fertilization. Uninjected embryos (A), and embryos injected with a control morpholino against Pm dysferlin (B) were used as controls. Concentrations higher than 50uM of Sumo morpholino led to embryonic lethality. The Sp Sumo morpholino was injected (200uM) in the germinal vesicle oocytes of the sea star (Pm), to test its potential toxicity during the embryonic development. After maturation and fertilization, healthy late gastrulae were obtained from these injected oocytes (I). An irrelevant morpholino (Sp SCP2) was used as a control (H). To test if the Sp Sumo morpholino decreases the abundance of the Sumo protein in the sea urchin embryo, immunofluorescence, using the antibody against Sumo (Abcam), was performed on blastulae injected by either the Sp Sumo morpholino (50uM), or the control morpholino (against Pm dysferlin, 50uM). With Metamorph, the fluorescence was quantified in the small micromeres, using 10 blastulae for each morpholino (J). Significance was assessed with the use of Student t test (P<0.05).
Supplemental Figure S9: Inhibition of the proteasome does not affect Nanos 2 enrichment in the small micromeres. Ten hours after fertilization, embryos were treated with DMSO only (A to D), or with 25μM MG132 (E to H) for ten hours, and were fixed at mesenchyme blastula stage. Immunofluorescence was performed using the purified antibodies against Vasa (A,E), or Nanos (C,G). Pictures were taken using respectively the same microscope settings (laser intensity, pin-hole opening) in A and E, and in C and G, at 400x magnification. The corresponding differential interference contrast images are shown in B and F, and from D and H. The expression of each protein was quantified (I), using Metamorph, in the somatic cells and in the small micromeres. For each type of cells, significance was assessed between 10 control and 11 treated embryos with the use of Student t test (P<0.05).
Supplemental Figure S10: Nanos 2 N terminal domain contains an element that is essential for its degradation at blastula stage. These are the same images as in figure 6, except that the mCherry fluorescence was taken with a lower intensity laser (E’,F’,G’,H’) to test if the expression of this control is the same in all the blastulae that were imaged.
Supplemental Figure S11: The fusion protein GFP N term 4 is expressed in early development before its degradation. Synthetic mRNA GFP Nanos 2 N term 4 ORF was injected in Sp fertilized eggs. The ORF was surrounded by Nanos 2 5′ and ΔGNARLE 3′UTRs. This RNA was co-injected with a control RNA containing mCherry flanked with β-globin UTRs. Injected embryos were imaged at the following times after fertilization: 8 hours, 10 hours and 12 hours after fertilization. Images were taken using respectively the same microscope settings (laser intensity, pin-hole opening) from A to C and A’ to C’ at 400x magnification. The corresponding differential interference contrast images are shown from A” to C”.
Supplemental Figure S12: Summary of the additional GFP Nanos 2 fusion constructs. The names of the injected constructs are indicated on the left, the corresponding amino acids fused in frame to the C terminal of the GFP are presented by rectangles on the right. The number surrounded the rectangles indicate the amino acid present at the beginning at the end of each construct. The location of the zinc finger motifs (CCHC) is shown in blue. Nanos 2 contains 7 lysines, their location is indicated by the small red circles, above the full length sequence.
Supplemental Figure S13: The expression of Nanos 2 protein is regulated by its N terminal domain. Synthetic mRNAs containing the GFP ORF fused in frame with the full length Nanos 2 ORF (A) were injected in Sp fertilized eggs. Deletion mutants were also injected and compared to the full length protein: C term 1 (B, C), C term 2 (D,E). In each case, the ORF was surrounded by Nanos 2 5′ and ΔGNARLE 3′UTRs. A control RNA containing mCherry flanked with β-globin UTRs was co injected with each deletion construct (A’, B’, D’). The GFP fluorescence was assayed 18 hours post-fertilization at mesenchyme blastula. (A, B, D). When the GFP fluorescence was overexposed, a second image of the same blastula was taken using a lower laser intensity (C, E). For each group, the respective mCherry fluorescence was also imaged with the same settings: (A’, B’,D’).
Supplemental Figure S14: The expression of Nanos 2 protein is regulated by its N terminal domain. Synthetic mRNAs containing the GFP ORF fused in frame with the full length Nanos 2 ORF (A,E,H) were injected in Sp fertilized eggs. Deletion mutants were also injected and compared to the full length protein: N term 6 (B), N term 7 (C,D), N term 8 (F,G), and N term 9 (I,J). In each case, the ORF was surrounded by Nanos2 5′ and ΔGNARLE 3′UTRs. A control RNA containing mCherry flanked with β-globin UTRs was co injected with each deletion construct (A’ to J’). The GFP fluorescence was assayed 18 hours post-fertilization at mesenchyme blastula. A, B, C, D were obtained using the same settings (laser intensity, pinhole opening) at 400x magnification, E, F, G were taken with the same settings, H, I, J were taken with the same settings. For each group, the respective mCherry fluorescence was also imaged with the same settings: (A’, B’,C’, D’), (E’,F’,G’), (H’,I’,J’).
Supplemental Figure S15: Analysis of the potential proteases that could target Nanos 2 NPDE, using PROSPER (PROtease Specificity Prediction servER).
Supplemental Figure S16: Nanos 2 regulates cyclin B translation. (A) The Pumilio Response Element is present in the 3′UTR of the cyclin B in at least two sea urchin species: Sp (Strongylocentrotus purpuratus), and Lv (Lytechnius variegatus). (B) Synthetic mRNAs, containing Renilla luciferase (Rluc) ORF preceded by Xenopus β-globin 5′UTR (a,b,c,d), and followed by either Xenopus β-globin 3′UTR (a,b) or cyclin B 3′UTR were each co-injected with an RNA containing the Firefly luciferase (Fluc) ORF surrounded by Xenopus β-globin 5′ and 3′UTR. A third RNA was coinjected: coding for either mCherry or Nanos 2 protein. Both ORF were surrounded by Xenopus β-globin 5′ and 3′UTR. Luminescence was measured 6 hours after fertilization. The ratio of Rluc/Fluc was determined, and the results are shown in percentages considering the ratio obtained with mCherry protein as 100% (a and c). Error bars indicate the standard deviation from three technical replicates. Significance was assessed with the use of Student t test (P<0.005).
Highlights.
The regulation of Nanos accumulation is largely post-translational in this embryo
Over – expression of Nanos mRNA throughout the embryo results in protein accumulation only in the germ line
Nanos has seven lysines (important for ubiquitylation or sumoylation), but none are relevant to the selective protein accumulation.
However, SUMO accumulates selectively in the germ line
This is the first documented case of such a germ cell specific, post-translational event
Acknowledgments
The authors are grateful to the NIH (2R01HD028152,GMW) for supporting this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahringer J, Kimble J. Control of the sperm-oocyte switch in Caenorhabditis elegans hermaphrodites by the fem-3 3' untranslated region. Nature. 1991;349:346–348. doi: 10.1038/349346a0. [DOI] [PubMed] [Google Scholar]
- Arrizabalaga G, Lehmann R. A selective screen reveals discrete functional domains in Drosophila Nanos. Genetics. 1999;153:1825–1838. doi: 10.1093/genetics/153.4.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka-Taguchi M, Yamada M, Nakamura A, Hanyu K, Kobayashi S. Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nature cell biology. 1999;1:431–437. doi: 10.1038/15666. [DOI] [PubMed] [Google Scholar]
- Beer RL, Draper BW. nanos3 maintains germline stem cells and expression of the conserved germline stem cell gene nanos2 in the zebrafish ovary. Developmental biology. 2013;374:308–318. doi: 10.1016/j.ydbio.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Bhat KM. The posterior determinant gene nanos is required for the maintenance of the adult germline stem cells during Drosophila oogenesis. Genetics. 1999;151:1479–1492. doi: 10.1093/genetics/151.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broday L, Kolotuev I, Didier C, Bhoumik A, Gupta BP, Sternberg PW, Podbilewicz B, Ronai Z. The small ubiquitin-like modifier (SUMO) is required for gonadal and uterine-vulval morphogenesis in Caenorhabditis elegans. Genes & development. 2004;18:2380–2391. doi: 10.1101/gad.1227104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers MS, Ettensohn CA. Rapid microinjection of fertilized eggs. Methods Cell Biol. 2004;74:287–310. doi: 10.1016/s0091-679x(04)74013-3. [DOI] [PubMed] [Google Scholar]
- Cho PF, Gamberi C, Cho-Park YA, Cho-Park IB, Lasko P, Sonenberg N. Cap-dependent translational inhibition establishes two opposing morphogen gradients in Drosophila embryos. Current biology : CB. 2006;16:2035–2041. doi: 10.1016/j.cub.2006.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D, Treiber DK, Tao F, Zamore PD, Williamson JR, Lehmann R. A CCHC metal-binding domain in Nanos is essential for translational regulation. The EMBO journal. 1997;16:834–843. doi: 10.1093/emboj/16.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino I, Merritt C, Chen PL, Seydoux G, Subramaniam K. Translational repression restricts expression of the C. elegans Nanos homolog NOS-2 to the embryonic germline. Developmental biology. 2006;292:244–252. doi: 10.1016/j.ydbio.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Dai Y, Han K, Zou Z, Yan S, Wang Y, Zhang Z. SUMO-1 of mud crab (Scylla paramamosain) in gametogenesis. Gene. 2012;503:260–268. doi: 10.1016/j.gene.2012.04.056. [DOI] [PubMed] [Google Scholar]
- Dalby B, Glover DM. Discrete sequence elements control posterior pole accumulation and translational repression of maternal cyclin B RNA in Drosophila. The EMBO journal. 1993;12:1219–1227. doi: 10.1002/j.1460-2075.1993.tb05763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper BW, McCallum CM, Moens CB. nanos1 is required to maintain oocyte production in adult zebrafish. Developmental biology. 2007;305:589–598. doi: 10.1016/j.ydbio.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TA, Pyle SE, Wharton RP, Aggarwal AK. Structure of Pumilio reveals similarity between RNA and peptide binding motifs. Cell. 2001;105:281–289. doi: 10.1016/s0092-8674(01)00318-x. [DOI] [PubMed] [Google Scholar]
- Foltz KR, Adams NL, Runft LL. Echinoderm eggs and embryos: procurement and culture. Methods in cell biology. 2004;74:39–74. doi: 10.1016/s0091-679x(04)74003-0. [DOI] [PubMed] [Google Scholar]
- Forbes A, Lehmann R. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development. 1998;125:679–690. doi: 10.1242/dev.125.4.679. [DOI] [PubMed] [Google Scholar]
- Fujii T, Sakamoto N, Ochiai H, Fujita K, Okamitsu Y, Sumiyoshi N, Minokawa T, Yamamoto T. Role of the nanos homolog during sea urchin development. Dev Dyn. 2009;238:2511–2521. doi: 10.1002/dvdy.22074. [DOI] [PubMed] [Google Scholar]
- Fukuda I, Ito A, Hirai G, Nishimura S, Kawasaki H, Saitoh H, Kimura K, Sodeoka M, Yoshida M. Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem Biol. 2009;16:133–140. doi: 10.1016/j.chembiol.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Gavis ER, Curtis D, Lehmann R. Identification of cis-acting sequences that control nanos RNA localization. Developmental biology. 1996a;176:36–50. doi: 10.1006/dbio.1996.9996. [DOI] [PubMed] [Google Scholar]
- Gavis ER, Lunsford L, Bergsten SE, Lehmann R. A conserved 90 nucleotide element mediates translational repression of nanos RNA. Development. 1996b;122:2791–2800. doi: 10.1242/dev.122.9.2791. [DOI] [PubMed] [Google Scholar]
- Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nature reviews. Molecular cell biology. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Gerber AP, Luschnig S, Krasnow MA, Brown PO, Herschlag D. Genome-wide identification of mRNAs associated with the translational regulator PUMILIO in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4487–4492. doi: 10.1073/pnas.0509260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. Something about SUMO inhibits transcription. Current opinion in genetics & development. 2005;15:536–541. doi: 10.1016/j.gde.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Goodwin EB. Translational repression: not just a Puf of smoke. Current biology : CB. 2001;11:R607–609. doi: 10.1016/s0960-9822(01)00364-5. [DOI] [PubMed] [Google Scholar]
- Gustafson EA, Wessel GM. Exogenous RNA is selectively retained in the small micromeres during sea urchin embryogenesis. Molecular reproduction and development. 2010;77:836. doi: 10.1002/mrd.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson EA, Yajima M, Juliano CE, Wessel GM. Post-translational regulation by gustavus contributes to selective Vasa protein accumulation in multipotent cells during embryogenesis. Developmental biology. 2011;349:440–450. doi: 10.1016/j.ydbio.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi S, Tsuda M, Kitajima S, Sasaoka Y, Nomura-Kitabayashid A, Kurokawa K, Saga Y. nanos1: a mouse nanos gene expressed in the central nervous system is dispensable for normal development. Mechanisms of development. 2003;120:721–731. doi: 10.1016/s0925-4773(03)00043-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Hara K, Hishiki A, Kawaguchi S, Shichijo N, Nakamura K, Unzai S, Tamaru Y, Shimizu T, Sato M. Crystal structure of zinc-finger domain of Nanos and its functional implications. EMBO reports. 2010;11:848–853. doi: 10.1038/embor.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiyama K, Shigenobu S, Kobayashi S. Expression of genes involved in sumoylation in the Drosophila germline. Gene expression patterns : GEP. 2009;9:50–53. doi: 10.1016/j.gep.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Hayashi M, Kobayashi S. Nanos suppresses somatic cell fate in Drosophila germ line. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10338–10342. doi: 10.1073/pnas.0401647101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DW, King ML. Germ plasm and molecular determinants of germ cell fate. Current topics in developmental biology. 2000;50:155–181. doi: 10.1016/s0070-2153(00)50008-8. [DOI] [PubMed] [Google Scholar]
- Irish V, Lehmann R, Akam M. The Drosophila posterior-group gene nanos functions by repressing hunchback activity. Nature. 1989;338:646–648. doi: 10.1038/338646a0. [DOI] [PubMed] [Google Scholar]
- Janic A, Mendizabal L, Llamazares S, Rossell D, Gonzalez C. Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science. 2010;330:1824–1827. doi: 10.1126/science.1195481. [DOI] [PubMed] [Google Scholar]
- Johnson ES. Protein modification by SUMO. Annual review of biochemistry. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- Julaton VT, Reijo Pera RA. NANOS3 function in human germ cell development. Human molecular genetics. 2011;20:2238–2250. doi: 10.1093/hmg/ddr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano C, Swartz SZ, Wessel G. Isolating specific embryonic cells of the sea urchin by FACS. Methods Mol Biol. 2014;1128:187–196. doi: 10.1007/978-1-62703-974-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano CE, Yajima M, Wessel GM. Nanos functions to maintain the fate of the small micromere lineage in the sea urchin embryo. Developmental biology. 2010;337:220–232. doi: 10.1016/j.ydbio.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyrova LY, Habara Y, Lee TH, Wharton RP. Translational control of maternal Cyclin B mRNA by Nanos in the Drosophila germline. Development. 2007;134:1519–1527. doi: 10.1242/dev.002212. [DOI] [PubMed] [Google Scholar]
- Kloc M, Bilinski S, Pui-Yee Chan A, Etkin LD. The targeting of Xcat2 mRNA to the germinal granules depends on a cis-acting germinal granule localization element within the 3'UTR. Developmental biology. 2000;217:221–229. doi: 10.1006/dbio.1999.9554. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Yamada M, Asaoka M, Kitamura T. Essential role of the posterior morphogen nanos for germline development in Drosophila. Nature. 1996;380:708–711. doi: 10.1038/380708a0. [DOI] [PubMed] [Google Scholar]
- Komander D, Rape M. The ubiquitin code. Annual review of biochemistry. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- Koprunner M, Thisse C, Thisse B, Raz E. A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes & development. 2001;15:2877–2885. doi: 10.1101/gad.212401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer B, Crittenden S, Gallegos M, Moulder G, Barstead R, Kimble J, Wickens M. NANOS-3 and FBF proteins physically interact to control the sperm-oocyte switch in Caenorhabditis elegans. Curr Biol. 1999;9:1009–1018. doi: 10.1016/s0960-9822(99)80449-7. [DOI] [PubMed] [Google Scholar]
- Lai F, King ML. Repressive translational control in germ cells. Molecular reproduction and development. 2013 doi: 10.1002/mrd.22161. [DOI] [PubMed] [Google Scholar]
- Lai F, Singh A, King ML. Xenopus Nanos1 is required to prevent endoderm gene expression and apoptosis in primordial germ cells. Development. 2012;139:1476–1486. doi: 10.1242/dev.079608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Zhou Y, Luo X, Fox J, King ML. Nanos1 functions as a translational repressor in the Xenopus germline. Mechanisms of development. 2011;128:153–163. doi: 10.1016/j.mod.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH. Molecular phylogenies and divergence times of sea urchin species of Strongylocentrotidae, Echinoida. Mol Biol Evol. 2003;20:1211–1221. doi: 10.1093/molbev/msg125. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Vazquez M. Emerging roles of the SUMO pathway in development. Cellular and molecular life sciences : CMLS. 2011;68:4045–4064. doi: 10.1007/s00018-011-0792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Nerlick S, An W, King ML. Xenopus germline nanos1 is translationally repressed by a novel structure-based mechanism. Development. 2011;138:589–598. doi: 10.1242/dev.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald PM. The Drosophila pumilio gene: an unusually long transcription unit and an unusual protein. Development. 1992;114:221–232. doi: 10.1242/dev.114.1.221. [DOI] [PubMed] [Google Scholar]
- Minokawa T, Rast JP, Arenas-Mena C, Franco CB, Davidson EH. Expression patterns of four different regulatory genes that function during sea urchin development. Gene Expr Patterns. 2004;4:449–456. doi: 10.1016/j.modgep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Mosquera L, Forristall C, Zhou Y, King ML. A mRNA localized to the vegetal cortex of Xenopus oocytes encodes a protein with a nanos-like zinc finger domain. Development. 1993;117:377–386. doi: 10.1242/dev.117.1.377. [DOI] [PubMed] [Google Scholar]
- Murata Y, Wharton RP. Binding of pumilio to maternal hunchback mRNA is required for posterior patterning in Drosophila embryos. Cell. 1995;80:747–756. doi: 10.1016/0092-8674(95)90353-4. [DOI] [PubMed] [Google Scholar]
- Oulhen N, Onorato TM, Ramos I, Wessel GM. Dysferlin is essential for endocytosis in the sea star oocyte. Developmental biology. 2014;388:94–102. doi: 10.1016/j.ydbio.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhen N, Wessel GM. Retention of exogenous mRNAs selectively in the germ cells of the sea urchin requires only a 5'-cap and a 3'-UTR. Molecular reproduction and development. 2013;80:561–569. doi: 10.1002/mrd.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhen N, Wessel GM. Every which way--nanos gene regulation in echinoderms. Genesis. 2014;52:279–286. doi: 10.1002/dvg.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhen N, Yoshida T, Yajima M, Song J, Sakuma T, Sakamoto N, Yamamoto T, Wessel GM. The 3'UTR of nanos2 directs enrichment in the germ cell lineage of the sea urchin. Developmental biology. 2013 doi: 10.1016/j.ydbio.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y. Function of Nanos2 in the male germ cell lineage in mice. Cellular and molecular life sciences : CMLS. 2010;67:3815–3822. doi: 10.1007/s00018-010-0456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Fujimoto T, Maegawa S, Inoue K, Tanaka M, Arai K, Yamaha E. Visualization of primordial germ cells in vivo using GFP-nos1 3'UTR mRNA. The International journal of developmental biology. 2006;50:691–699. doi: 10.1387/ijdb.062143ts. [DOI] [PubMed] [Google Scholar]
- Sato K, Hayashi Y, Ninomiya Y, Shigenobu S, Arita K, Mukai M, Kobayashi S. Maternal Nanos represses hid/skl-dependent apoptosis to maintain the germ line in Drosophila embryos. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7455–7460. doi: 10.1073/pnas.0610052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux G, Braun RE. Pathway to totipotency: lessons from germ cells. Cell. 2006;127:891–904. doi: 10.1016/j.cell.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Shiio Y, Eisenman RN. Histone sumoylation is associated with transcriptional repression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13225–13230. doi: 10.1073/pnas.1735528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Wharton RP. Recruitment of Nanos to hunchback mRNA by Pumilio. Genes & development. 1999;13:2704–2712. doi: 10.1101/gad.13.20.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikumar T, Lewicki MC, Costanzo M, Tkach JM, van Bakel H, Tsui K, Johnson ES, Brown GW, Andrews BJ, Boone C, Giaever G, Nislow C, Raught B. Global analysis of SUMO chain function reveals multiple roles in chromatin regulation. The Journal of cell biology. 2013;201:145–163. doi: 10.1083/jcb.201210019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Seydoux G. nos-1 and nos-2, two genes related to Drosophila nanos, regulate primordial germ cell development and survival in Caenorhabditis elegans. Development. 1999;126:4861–4871. doi: 10.1242/dev.126.21.4861. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Saba R, Sada A, Saga Y. The Nanos3-3'UTR is required for germ cell specific NANOS3 expression in mouse embryos. PloS one. 2010;5:e9300. doi: 10.1371/journal.pone.0009300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz SZ, Reich AM, Oulhen N, Raz T, Milos PM, Campanale JP, Hamdoun A, Wessel GM. Deadenylase depletion protects inherited mRNAs in primordial germ cells. Development. 2014;141:3134–3142. doi: 10.1242/dev.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, Saga Y. Conserved role of nanos proteins in germ cell development. Science. 2003;301:1239–1241. doi: 10.1126/science.1085222. [DOI] [PubMed] [Google Scholar]
- Voronina E, Lopez M, Juliano CE, Gustafson E, Song JL, Extavour C, George S, Oliveri P, McClay D, Wessel G. Vasa protein expression is restricted to the small micromeres of the sea urchin, but is inducible in other lineages early in development. Developmental biology. 2008;314:276–286. doi: 10.1016/j.ydbio.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zamore PD, Hall TM. Crystal structure of a Pumilio homology domain. Molecular cell. 2001;7:855–865. doi: 10.1016/s1097-2765(01)00229-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zayas RM, Guo T, Newmark PA. nanos function is essential for development and regeneration of planarian germ cells. Proc Natl Acad Sci U S A. 2007;104:5901–5906. doi: 10.1073/pnas.0609708104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel GM, Brayboy L, Fresques T, Gustafson EA, Oulhen N, Ramos I, Reich A, Swartz SZ, Yajima M, Zazueta V. The biology of the germ line in echinoderms. Mol Reprod Dev. 2013 doi: 10.1002/mrd.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel GM, Reich AM, Klatsky PC. Use of sea stars to study basic reproductive processes. Syst Biol Reprod Med. 2010;56:236–245. doi: 10.3109/19396361003674879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton RP, Struhl G. RNA regulatory elements mediate control of Drosophila body pattern by the posterior morphogen nanos. Cell. 1991;67:955–967. doi: 10.1016/0092-8674(91)90368-9. [DOI] [PubMed] [Google Scholar]
- Wreden C, Verrotti AC, Schisa JA, Lieberfarb ME, Strickland S. Nanos and pumilio establish embryonic polarity in Drosophila by promoting posterior deadenylation of hunchback mRNA. Development. 1997;124:3015–3023. doi: 10.1242/dev.124.15.3015. [DOI] [PubMed] [Google Scholar]
- Wu X, Ruvkun G. Cancer. Germ cell genes and cancer. Science. 2010;330:1761–1762. doi: 10.1126/science.1200772. [DOI] [PubMed] [Google Scholar]
- Wu X, Wang B, Dong Z, Zhou S, Liu Z, Shi G, Cao Y, Xu Y. A NANOS3 mutation linked to protein degradation causes premature ovarian insufficiency. Cell death & disease. 2013;4:e825. doi: 10.1038/cddis.2013.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Wessel GM. Small micromeres contribute to the germline in the sea urchin. Development. 2011;138:237–243. doi: 10.1242/dev.054940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Wessel GM. Autonomy in specification of primordial germ cells and their passive translocation in the sea urchin. Development. 2012;139:3786–3794. doi: 10.1242/dev.082230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Gallegos M, Puoti A, Durkin E, Fields S, Kimble J, Wickens MP. A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature. 1997;390:477–484. doi: 10.1038/37297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1: List of primers used to make (A) the cyclin B constructs and (B) the Nanos 2 constructs.
Supplemental Figure S2: List of primers used to make (A) the Nanos 2 lysine mutants constructs and (B) the Nanos 2 zinc finger mutants.
Supplemental Figure S3: List of primers used to make the Nanos 2 deletion mutants (A and B)
Supplemental Figure S4: GFP Nanos 2 protein expression remains low during the early development until its enrichment in the small micromeres. Synthetic mRNAs containing the GFP ORF alone (A to E), or the GFP ORF fused in frame with the Nanos 2 ORF (K to O) were injected in Sp fertilized eggs. In each case, the ORF was surrounded by Nanos 2 5′ and ΔGNARLE 3′UTRs. Injected embryos were imaged at the following times after fertilization: (A,K) 8H, (B,L) 9H, (C,M) 13H, (D,N) 16H, (E,O) 20H. Images were taken using respectively the same microscope settings (laser intensity, pin-hole opening) from A to E and K to O, at 400x magnification. The corresponding differential interference contrast images are shown from A’ to E’, and K’ to O’.
Supplemental Figure S5: Sumo protein accumulates in the small micromeres coincident with Nanos 2 in blastula. Immunofluorescence using the antibody against Sumo was tested in unfertilized eggs (A,A’), 2-cell stage (B,B’), and 4-cell stage embryos (C,C’), morula (D,D’), mesenchyme blastula (E,E’), gastrula (F,F’) and pluteus (G,G’). Two antibodies were tested, one from Abcam (A to G), and one from Cell Signaling Technology (A’,G’), Images (A to G), and (A’ to G’) were taken using the same microscope settings (laser intensity, pin-hole opening) at 400x magnification. Immunofluorescences using only the secondary antibody were used as controls and didn’t give any detectable signal (data not shown).
Supplemental Figure S6: The protein Ubc9 is ubiquitously expressed during the early development in sea urchin. Immunofluorescence using the antibody against Ubc9 was tested in unfertilized eggs (A), 2-cell stage (B), and 4-cell stage embryos (C), in blastula (D), gastrula (E) and pluteus (F). Images were taken using the same microscope settings (laser intensity, pin-hole opening) at 400x magnification. A control was performed using only the secondary antibody, on unfertilized eggs (G), 2-cell stage (H), 4-cell stage (I), blastula (J), gastrula (K), and pluteus (L).
Supplemental Figure S7: Sumo and Ubc9 mRNA are uniformly expressed during sea urchin early development. Whole mount in situ hybridization in the sea urchin using probes against Sumo (a to h), and Ubc9 (p to v). Embryos were fixed from fertilized eggs (a,i,p), to pluteus (h,o,v). A probe against the Neomycin resistance gene was used as a negative control (i to o).
Supplemental Figure S8: A morpholino against Sumo decreases the abundance of Sumo protein in the small micromeres at blastula stage. Dose response of Sumo morpholino (MO) during development. Fertilized eggs were injected with 25 to 500 uM of Sumo morpholino (C to G), and imaged 18 hours after fertilization. Uninjected embryos (A), and embryos injected with a control morpholino against Pm dysferlin (B) were used as controls. Concentrations higher than 50uM of Sumo morpholino led to embryonic lethality. The Sp Sumo morpholino was injected (200uM) in the germinal vesicle oocytes of the sea star (Pm), to test its potential toxicity during the embryonic development. After maturation and fertilization, healthy late gastrulae were obtained from these injected oocytes (I). An irrelevant morpholino (Sp SCP2) was used as a control (H). To test if the Sp Sumo morpholino decreases the abundance of the Sumo protein in the sea urchin embryo, immunofluorescence, using the antibody against Sumo (Abcam), was performed on blastulae injected by either the Sp Sumo morpholino (50uM), or the control morpholino (against Pm dysferlin, 50uM). With Metamorph, the fluorescence was quantified in the small micromeres, using 10 blastulae for each morpholino (J). Significance was assessed with the use of Student t test (P<0.05).
Supplemental Figure S9: Inhibition of the proteasome does not affect Nanos 2 enrichment in the small micromeres. Ten hours after fertilization, embryos were treated with DMSO only (A to D), or with 25μM MG132 (E to H) for ten hours, and were fixed at mesenchyme blastula stage. Immunofluorescence was performed using the purified antibodies against Vasa (A,E), or Nanos (C,G). Pictures were taken using respectively the same microscope settings (laser intensity, pin-hole opening) in A and E, and in C and G, at 400x magnification. The corresponding differential interference contrast images are shown in B and F, and from D and H. The expression of each protein was quantified (I), using Metamorph, in the somatic cells and in the small micromeres. For each type of cells, significance was assessed between 10 control and 11 treated embryos with the use of Student t test (P<0.05).
Supplemental Figure S10: Nanos 2 N terminal domain contains an element that is essential for its degradation at blastula stage. These are the same images as in figure 6, except that the mCherry fluorescence was taken with a lower intensity laser (E’,F’,G’,H’) to test if the expression of this control is the same in all the blastulae that were imaged.
Supplemental Figure S11: The fusion protein GFP N term 4 is expressed in early development before its degradation. Synthetic mRNA GFP Nanos 2 N term 4 ORF was injected in Sp fertilized eggs. The ORF was surrounded by Nanos 2 5′ and ΔGNARLE 3′UTRs. This RNA was co-injected with a control RNA containing mCherry flanked with β-globin UTRs. Injected embryos were imaged at the following times after fertilization: 8 hours, 10 hours and 12 hours after fertilization. Images were taken using respectively the same microscope settings (laser intensity, pin-hole opening) from A to C and A’ to C’ at 400x magnification. The corresponding differential interference contrast images are shown from A” to C”.
Supplemental Figure S12: Summary of the additional GFP Nanos 2 fusion constructs. The names of the injected constructs are indicated on the left, the corresponding amino acids fused in frame to the C terminal of the GFP are presented by rectangles on the right. The number surrounded the rectangles indicate the amino acid present at the beginning at the end of each construct. The location of the zinc finger motifs (CCHC) is shown in blue. Nanos 2 contains 7 lysines, their location is indicated by the small red circles, above the full length sequence.
Supplemental Figure S13: The expression of Nanos 2 protein is regulated by its N terminal domain. Synthetic mRNAs containing the GFP ORF fused in frame with the full length Nanos 2 ORF (A) were injected in Sp fertilized eggs. Deletion mutants were also injected and compared to the full length protein: C term 1 (B, C), C term 2 (D,E). In each case, the ORF was surrounded by Nanos 2 5′ and ΔGNARLE 3′UTRs. A control RNA containing mCherry flanked with β-globin UTRs was co injected with each deletion construct (A’, B’, D’). The GFP fluorescence was assayed 18 hours post-fertilization at mesenchyme blastula. (A, B, D). When the GFP fluorescence was overexposed, a second image of the same blastula was taken using a lower laser intensity (C, E). For each group, the respective mCherry fluorescence was also imaged with the same settings: (A’, B’,D’).
Supplemental Figure S14: The expression of Nanos 2 protein is regulated by its N terminal domain. Synthetic mRNAs containing the GFP ORF fused in frame with the full length Nanos 2 ORF (A,E,H) were injected in Sp fertilized eggs. Deletion mutants were also injected and compared to the full length protein: N term 6 (B), N term 7 (C,D), N term 8 (F,G), and N term 9 (I,J). In each case, the ORF was surrounded by Nanos2 5′ and ΔGNARLE 3′UTRs. A control RNA containing mCherry flanked with β-globin UTRs was co injected with each deletion construct (A’ to J’). The GFP fluorescence was assayed 18 hours post-fertilization at mesenchyme blastula. A, B, C, D were obtained using the same settings (laser intensity, pinhole opening) at 400x magnification, E, F, G were taken with the same settings, H, I, J were taken with the same settings. For each group, the respective mCherry fluorescence was also imaged with the same settings: (A’, B’,C’, D’), (E’,F’,G’), (H’,I’,J’).
Supplemental Figure S15: Analysis of the potential proteases that could target Nanos 2 NPDE, using PROSPER (PROtease Specificity Prediction servER).
Supplemental Figure S16: Nanos 2 regulates cyclin B translation. (A) The Pumilio Response Element is present in the 3′UTR of the cyclin B in at least two sea urchin species: Sp (Strongylocentrotus purpuratus), and Lv (Lytechnius variegatus). (B) Synthetic mRNAs, containing Renilla luciferase (Rluc) ORF preceded by Xenopus β-globin 5′UTR (a,b,c,d), and followed by either Xenopus β-globin 3′UTR (a,b) or cyclin B 3′UTR were each co-injected with an RNA containing the Firefly luciferase (Fluc) ORF surrounded by Xenopus β-globin 5′ and 3′UTR. A third RNA was coinjected: coding for either mCherry or Nanos 2 protein. Both ORF were surrounded by Xenopus β-globin 5′ and 3′UTR. Luminescence was measured 6 hours after fertilization. The ratio of Rluc/Fluc was determined, and the results are shown in percentages considering the ratio obtained with mCherry protein as 100% (a and c). Error bars indicate the standard deviation from three technical replicates. Significance was assessed with the use of Student t test (P<0.005).