Abstract
Centroacinar cells (CACs) are ductal Notch-responsive progenitors that in the larval zebrafish pancreas differentiate to form new islets and ultimately contribute to the majority of the adult endocrine mass. Uncovering the mechanisms regulating CAC differentiation will facilitate understanding how insulin-producing β cells are formed. Previously we reported retinoic acid (RA) signaling and Notch signaling both regulate larval CAC differentiation, suggesting a shared downstream intermediate. Sox9b is a transcription factor important for islet formation whose expression is upregulated by Notch signaling in larval CACs. Here we report that sox9b expression in larval CACs is also regulated by RA signaling. Therefore, we hypothesized that Sox9b is an intermediate between both RA- and Notch-signaling pathways. In order to study the role of Sox9b in larval CACs, we generated two cre/lox based transgenic tools, which allowed us to express full-length or truncated Sox9b in larval CACs. In this way we were able to perform spatiotemporal-controlled Sox9b gain- and loss-of-function studies and observe the subsequent effect on progenitor differentiation. Our results are consistent with Sox9b regulating CAC differentiation by being a downstream intermediate of both RA- and Notch-signaling pathways. We also demonstrate that adult zebrafish with only one functional allele of sox9b undergo accelerated β-cell regeneration, an observation consistent with sox9b regulating CAC differentiation in adults.
Keywords: Pancreas, Progenitors, differentiation, endocrine, Sox9
1. Introduction
A cure for diabetes needs to include a method to replace lost insulin-producing β cells of the pancreas. If a pharmacological method to induce β-cell neogenesis from human endogenous progenitors can be found, this therapeutic route has the potential to be more accessible to the global patient population than alternative approaches, such as transplantation of cells derived from donors or differentiated embryonic stem cells. The zebrafish has many attributes that lend itself to the challenge of finding factors that influence β-cell neogenesis (Kinkel and Prince, 2009; Tiso et al., 2009). The transparency and small size of zebrafish larvae facilitates carrying out chemical screens to find drugs that induce β-cell neogenesis (Rovira et al., 2011; Wang et al., 2015). For example, a hit from one such screen indicated that the morphogen retinoic acid (RA) is a molecule that is involved in regulating the differentiation of endocrine progenitors (Huang et al., 2014; Rovira et al., 2011). Other chemical screens in zebrafish larvae identified additional roles for RA in regulating β-cell proliferation (Tsuji et al., 2014; Wang et al., 2015).
The cellular mechanisms of β-cell neogenesis used during zebrafish development and adult regeneration are the same (Beer et al., 2016; Delaspre et al., 2015; Wang et al., 2011); specialized ductal cells called centroacinar cells (CACs) differentiate to form new insulin-producing cells. CACs differentiate throughout the pancreas but can be most easily distinguished in the tail of the pancreas where they give rise to distinct secondary (2°) islets (Parsons et al., 2009). In adult fish CACs are located in a terminal duct position within the lumen of the acinus – the functional unit of the exocrine pancreas. An enigmatic cell type with distinct ultrastructure (Ekholm et al., 1962; Parsons et al., 2009), CACs possess specialized morphology with long cytoplasmic extensions that line the pancreatic ducts and extend into the parenchyma (Beer et al., 2016; Leeson and Leeson, 1986; Pour, 1994). Following β-cell ablation, CACs leave their location in the acinus (Pour, 1994) before either forming new small islets or contributing to preexisting islets (Delaspre et al., 2015).
There are several known molecular attributes that distinguish CACs from other cells in the pancreatic epithelia, including their active responsive to RA signaling and persistent Notch signal transduction (Huang et al., 2014; Parsons et al., 2009). Notch signaling plays a crucial and well documented role in mammalian pancreas development (Apelqvist et al., 1999; Esni et al., 2004; Hald et al., 2003; Jensen et al., 2000a; Jensen et al., 2000b; Murtaugh et al., 2003). Notch is also important in the zebrafish pancreas. To date, the most potent method to induce precocious differentiation of CACs into 2° islets is to inhibit Notch signaling (Lorent et al., 2004; Ninov et al., 2012; Parsons et al., 2009; Zecchin et al., 2007). However, from a chemical screen and subsequent follow up work, endogenous RA signaling was also shown to play a role in maintaining CAC potency (Huang et al., 2014; Rovira et al., 2011). Furthermore, it was shown that inhibiting both RA and Notch lead to synergy in induction of endocrine differentiation (Huang et al., 2014). Accordingly, we hypothesized that both the Notch and RA signaling pathways act through a common downstream effector.
Other molecular characteristics of CACs include the expression of Sox9 transcription factors. Sox9 is expressed in multipotent pancreatic progenitors in the mouse (Seymour et al., 2007; Seymour and Sander, 2011) where it plays a vital function in pancreas development and homeostasis. There is considerable evidence that the level of Sox9 activity is critical for normal pancreas biology (Seymour et al., 2008): 1) Total loss of Sox9 activity in the pancreas anlagen of mouse models demonstrates it is required in endocrine and duct cell formation (Seymour et al., 2007). 2) Heterozygosity leading to a partial loss of SOX9 activity in humans or mice causes Campomelic dysplasia, a syndrome that includes malformed, smaller islets and glucose intolerance (Dubois et al., 2011; Piper et al., 2002; Seymour et al., 2008). 3) Over-expression of Sox9 in adult mice is sufficient to cause complete dedifferentiation of mature β cells (Puri et al., 2013). In the zebrafish there are two homologues of SOX9, sox9a and sox9b (Chiang et al., 2001); however, only sox9b is expressed in the pancreas (Manfroid et al., 2012). Larva homozygous for the null allele, sox9bfh313, have reduced CAC number, decreased ability to produce endocrine cells (Delous et al., 2012; Manfroid et al., 2012), and compromised β-cell regeneration following β-cell ablation (Manfroid et al., 2012). However, a haploinsufficient phenotype has yet to be described in zebrafish sox9bfh313 heterozygotes.
Consistent with CAC expression, Sox9 transcription factors are directly regulated by Notch signaling in the pancreatic progenitors of both mammals and zebrafish (Delous et al., 2012; Manfroid et al., 2012; Shih et al., 2012). Other reports have shown that the expression of Sox9 can be highly induced by the treatment of exogenous RA in human cell lines (Afonja et al., 2002; Laursen et al., 2013; Muller et al., 2010). This data led us to hypothesize that Sox9b is a common downstream target of both RA- and Notch-signaling pathways. In the study described in this paper we explored the relationship between Notch and RA signaling, and how these pathways converge on Sox9 to control CAC potency. We show that partial loss of Sox9b function facilitates differentiation in both larvae and adult zebrafish.
2. Material and Methods
2.1. Transgenic/mutant Lines
Tg(Tp1glob:creERT2)jh12 drives creERT2 in cells undergoing Notch signaling and is referred to as a ‘Notch-responsive creERT2 driver’. Tg(β-actin:GFP-F2A-creERT2)jh29 drives creERT2 ubiquitously and is referred to as a ‘ubiquitous creERT2 driver’ (Wang et al., 2011). Two new ubiquitous cre-responder lines utilizing the ubiquitous promoter/enhancer element from ubiquitin B (ubb) (Mosimann and Zon, 2011) were generated: 1) Tg(ubb:loxP-eCFP-loxP-sox9b-2A-mCherry)jh47, abbreviated to ‘sox9b cre responder’; 2) Tg(ubb:loxP-eCFP-loxP-trsox9b-2A-mCherry)jh48, shortened to ‘truncated sox9b cre responder’. Following cre recombination, these 2 transgenes express full-length Sox9b and mCherry or the first 289 residues of Sox9b and mCherry, respectively. The truncated version of Sox9b is equivalent to 304 residues of human SOX9 shown to lack an essential transactivation domain (Lefebvre et al., 1997). The Tg(neuroD:GFP)nl1 transgenic line (Obholzer et al., 2008) labels nascent pancreatic endocrine. Tg(Tp1glob:eGFP)um14 - shortened to Tp1:eGFP - labels Notch-responsive cells including the CACs with green fluorescence and Tg(Tp1glob:hmgb1-mCherry)jh32 - Tp1:nuc-mCherry - labels CAC nuclei with red fluorescence (Parsons et al., 2009). The TgBAC(cftr:cftr-gfp)pd1041 transgenic line expresses a Cftr-GFP fusion protein in the endogenous cftr expression domain (Navis et al., 2013). sox9bfh313 is a null allele of sox9b (Manfroid et al., 2012); hence, wildtype, heterozygotes and homozygotes are referred to as sox9b+/+, sox9b+/− and sox9b−/−. These fish were genotyped as described elsewhere (Delous et al., 2012). Tg(HS4-sst2:CFP;ins:PhiYFP-m-dest1-2TA-nfsB)lmc009 (abbreviated to ins:NTR) was used to perform β-cell ablation (Delaspre et al. 2015).
2.2. In situ Hybridization and Immunofluorescence
5 dpf larvae were fixed in 4% PFA at 4°C overnight (Parsons et al., 2009). In situ hybridization was performed on dissected larval pancreata. The primer sequences used to generate the ribo probes as follows: col2a1aF, GATTGCTGGATTCACGGACTCT; col2a1aR, TCACCGGGCAGACCACGAGGAC; neuroDF, TCACAGCAGCTCCCAAGACGAAC; neuroDR, AGACCCGCTGCCTGATAGTGC; sox9bF, TCGATGTTAATGAGTTCGA; sox9bR, CTATGGAAACCCTCAGGTTA. Images were recorded using a Nikon AZ100 microscope.
Immunofluorescent staining was performed on dissected larval pancreata and on adult paraffin sections (Parsons et al., 2009). The following antibodies were used: anti-Nkx6.1 (1:100, Developmental Studies Hybridoma Bank, F55A1), anti-insulin (1:500, Dako), anti-glucagon (1:500, Dako), anti-GFP (1:500, Life Technologies), anti-dsRed (1:500, Clontech), anti-Sox9b (1:100, this study), and fluorescently conjugated 2° antibodies (1:400, Jackson ImmunoResearch Labs Inc).
To produce the Sox9b antibody, cDNA from 27 hour post fertilization (hpf) embryos was used to amplify a 356 bp fragment of sox9b cDNA using the following primers: 5’-gtacggatccatggggccggccggtgcag-3’, and 5’-gtacgaatcctcagggtctggacagctgtgtg-3’. The amplified sox9b cDNA was cloned into the pGEX4T1 vector, followed by the purification of recombinant Sox9b protein (amino acids 290–407) from E. coli. The purified recombinant Sox9b protein was used as an antigen to generate anti-Sox9b antibody in rabbit (Pacific Immunology, CA), followed by affinity purification. For anti-Sox9b immunofluorescence, 5 dpf larvae were fixed in fresh 4% PFA overnight at 4°C. Following PBST washes, pancreata were dissected and permeabilized in acetone for 7 minutes at −20°C. Pancreata were then washed in PBST and staining proceeded as above.
2.3. Confocal Imaging
Dissected larval pancreata were mounted in fluorescent mounting medium (Dako) and imaged with a Nikon A1-si Laser Scanning Confocal Head mounted on a TiE inverted microscope through a Plan Apo 20× VC (N.A. 0.75) (Nikon). Acquisition and analysis was performed using NIS-Elements software (Ver 3.22, build 710). All the fluorescent images are merged to DIC view. Cell number was quantified by counterstaining nuclei with DAPI (1:2000) and counted using NIS-Elements software (Nikon) or ImageJ.
2.4. Drug Treatments
Unless otherwise stated in the text, drug treatments were performed as follows. Compounds were made into 10 mM stock (in DMSO) and diluted in E3 media (Westerfield, 2000) to 50 µM All-trans retinoic acid (RA; Sigma, R2625), 50 µM N-[N-(3, 5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT; Sigma, D5942), and 10 µM Diethylaminobenzaldehyde (DEAB; D86256, Sigma). Larvae were incubated in drug until 5 dpf in the dark at 28 °C. 4-Hydroxytamoxifen (4OHT; Sigma, T176) was dissolved in 100% ethanol to make a 10 mM stock solution. Embryos were incubated in E3 medium with 5 µM 4OHT and kept in the dark at 28°C for 24 hours, then washed in fresh E3. To ablate β cells, 30 mM metronidazole (MTZ, M3761, Sigma) in PBS was intra-coelomically (ic) injected into adult ins:NTR fish at a dose of 0.25 g/kg of body weight. Control fish were injected with PBS.
2.5. Quantitative RT-PCR
Zebrafish larvae were incubated in the dark with 50 µM RA from 4–5 dpf, and DMSO treated larvae were used as control. pPCR was performed on pools of 10 pancreata in 3 independent experiments. Total RNA was extracted from dissected larval pancreata and purified using RNeasy Mini Kit (Qiagen). For qRT-PCR from whole embryos, ~15–20 embryos were pooled and RNA was extracted using TRIzol (Thermo Fisher Scientific). In both experiments, cDNA was generated using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific) with random hexamer primers. Quantitative PCR was run using iQ SYBR Green Supermix (Bio-Rad) on a CFX96 Real-Time System (Bio-Rad) and fold change in expression was calculated using the ΔΔCt method. rpl13a, tbp, and rnap were used as reference genes for dissected pancreata (McCurley and Callard, 2008; Tang et al., 2007), and elfa was used as a reference gene for whole embryos (McCurley and Callard, 2008).
2.6 Western Blotting
For western blotting, genotyped 9 dpf wildtype or sox9bfh313 heterozygous larvae were pooled and lysed in Laemmli loading buffer. The equivalent of 1.7 larvae were loaded into each lane. Following blotting, anti-Sox9b (1°, 1:1000) and anti-rabbit-IgG-HRP (2°, 1:100,000) were detected using Supersignal Femto substrate (Thermo).
2.7 Glucose assays
Adult fish were fasted for 24 hours, sacrificed, and blood glucose measured using a OneTouch Ultra (LifeScan) glucose meter (Eames et al., 2010).
2.8 Statistical analysis
Significance was assessed using Student's t test and probability (p) is provided.
3. Results
3.1. RA and Notch signaling regulate sox9b expression in larval CACs
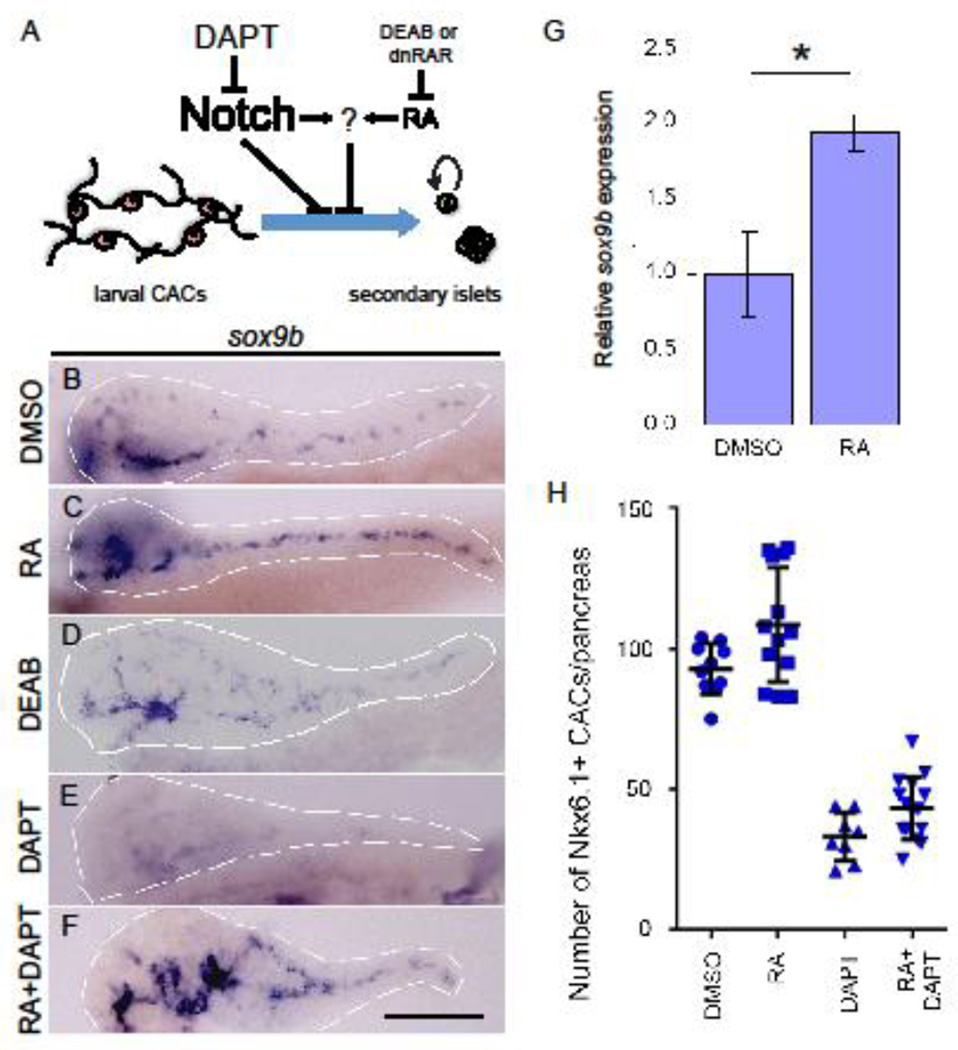
To better understand the mechanism mediating the actions of RA on CAC differentiation (Figure 1A), we tested the effects of RA treatment on the expression of a number of transcription factors known to be important in pancreas development. Using whole mount in situ hybridization we found that expression of sox9b, but not pdx1, hb9 or neuroD (data not shown) was significantly increased in larval pancreata treated with RA from 3–5 days post fertilization (dpf) compared to control (Figure 1B–C). This effect on sox9b expression was verified by quantitative RT-PCR (Figure 1G). Conversely, expression of sox9b was unchanged in larvae treated with the RA-signaling inhibitor DEAB from 3–5 dpf (Figure 1D). As previously reported, treating larvae with the Notch inhibitor DAPT reduced sox9b mRNA levels (Delous et al., 2012) (Figure 1E) but co-treatment with RA and DAPT restored sox9b expression in the larval CACs (Figure 1F) (Huang et al., 2014). Using detection of Nkx6.1, a marker of larval CACs (Huang et al., 2014), we quantified CAC numbers (Figure 1H) and found that loss of Notch-signaling by DAPT treatment leads to loss of this cell type. CAC proliferation was unaffected by DAPT and/or RA treatment (Supplemental Figure 1). Although addition of RA to the DAPT treatment rescues sox9b expression, RA does not prevent the differentiation of Nkx6.1-positive CACs. This observation is consistent with CACs differentiating to become cells of a non-endocrine fate.
Figure 1. RA and Notch signaling both influence larval CAC expression of sox9b.
(A) Model of the regulation controlling larval CAC differentiation towards an endocrine fate. Notch signaling blocks differentiation that can be overcome by the Notch-inhibitor, DAPT. RA also impedes differentiation, an action that can be inhibited either by blocking RA synthesis with diethylaminobenzaldehyde (DEAB) or signal transduction with a dominant negative RA receptor (dnRAR). DAPT and DEAB work synergistically to inhibit CAC differentiation, suggesting a shared mechanism downstream of Notch and RA signaling (Huang et al., 2014). (B–F) Images of dissected larval foregut following whole mount in situ hybridization to detect sox9b expression. Larvae were treated from 3–5 dpf with DMSO (B), RA (C), DEAB (D), DAPT (E) or RA+DAPT (F). White dashed lines outline pancreata. Scale bar = 100 µm (F). (G) Quantitative RT-PCR results indicate that sox9b expression is significantly increased in dissected larval pancreata following RA treatment from 4–5 dpf; t-test *p<0.01. (H) Quantification of the number of Nkx6.1+ CACs in larval pancreata at 5 dpf after drug treatment from 3–5 dpf. Error bars indicate SD.
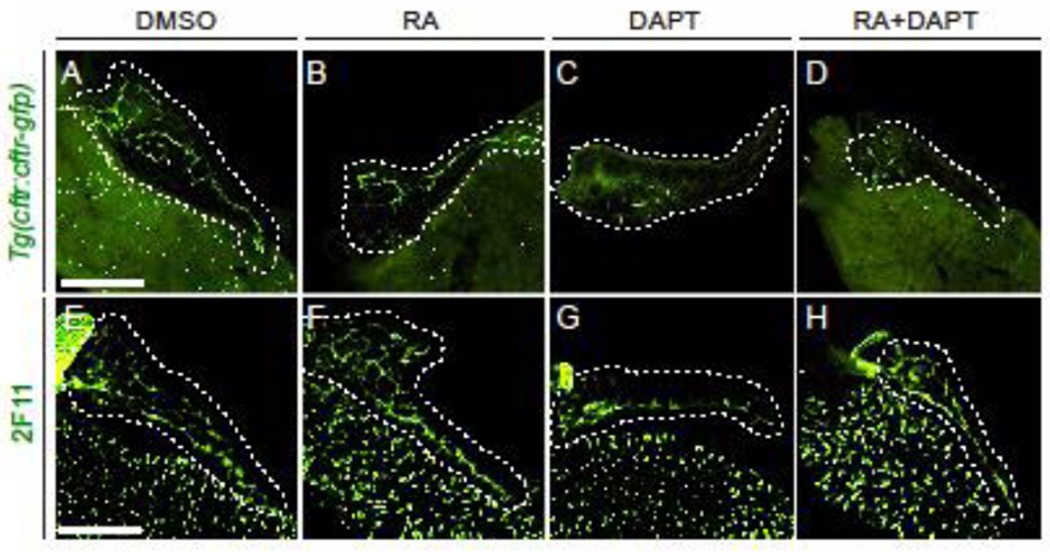
To further explore the fate of CAC progenitors differentiating to a non-endocrine fate, we examined expression of cftr:cftr-gfp, another CAC marker (Delaspre et al., 2015; Navis and Bagnat, 2015), and 2F11, a marker of ductal epithelium (Zhang et al., 2014), following treatment with RA and/or DAPT from 3–5 dpf. Compared to DMSO control, expression of cftr:cftr-gfp in the intrapancreatic duct was unaffected by treatment with RA alone (Figure 2A–B), but was abolished in DAPT treated larva (Figure 2C). cftr:cftr-gfp expression could not be rescued by co-treatment with DAPT and RA (Figure 2D). These observations parallel the loss of Nkx6.1 expression in CACs under the same conditions. Conversely, expression of the ductal marker 2F11 was unaffected by treatment with DAPT and/or RA (Figure 2E–H). We conclude that, following inhibition of Notch signaling, CACs that do not differentiate toward an endocrine fate lose their endocrine progenitor status and retain ductal epithelium identity. RA signaling may thus act downstream of Notch signaling during endocrine differentiation of CACs.
Figure 2. RA and Notch signaling have separate effects on CAC differentiation.
(A–D) Images of 5 dpf cftr:cftr-gfp dissected foregut following drug treatment. GFP signal is abolished in larvae treated with DAPT (C) and RA+DAPT (D). (E–H) Images of anti-2F11 staining in 5 dpf dissected foregut following drug treatment. Expression of the ductal epithelial marker 2F11 is maintained under all treatment conditions. Larvae were incubated from 3–5 dpf with DMSO (A, E), RA (B, F), DAPT (C, G), or RA+DAPT (D, H). White dashed lines outline pancreata. Scale bar = 100 µm.
3.2. Generation of new cre-responder lines to study gain and loss of Sox9b function
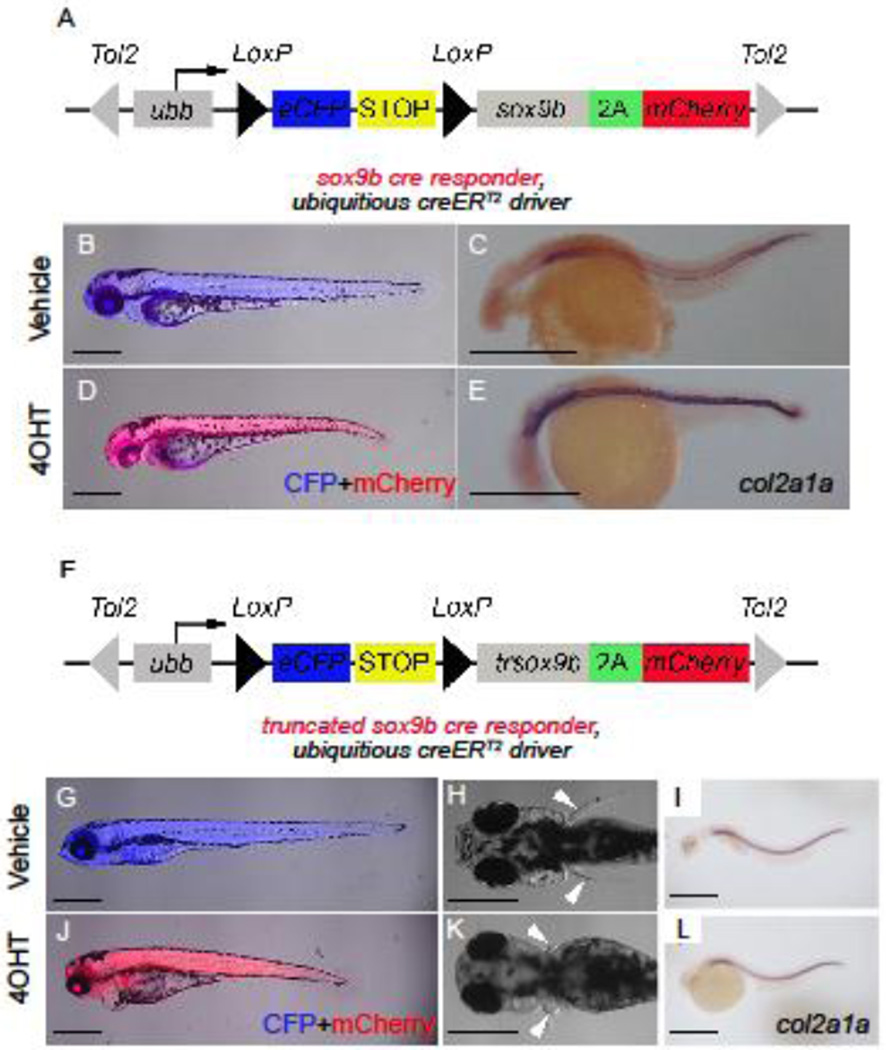
Our initial results showed that both RA levels and Notch activity influence sox9b expression. Sox9 is a known downstream target of Notch signaling in the pancreas (Delous et al., 2012; Shih et al., 2012) and from our new observations, we hypothesized that RA signaling in CACs influences Sox9b activity. To test this hypothesis, we set out to study gain and loss of Sox9b function in larval CACs using an inducible cre/lox system. We first created sox9b cre responder and truncated-sox9b cre responder transgenic lines. Following cre-directed recombination, the sox9b cre responder line is designed to constitutively expresses Sox9b and mCherry (Figure 3A and Supplemental Figure 2A); whereas, the truncated-sox9b cre responder expresses a truncated form of Sox9b (trSox9b) designed to act in a dominant-negative fashion (Lefebvre et al., 1997) and mCherry (Figure 3F and Supplemental Figure 2A).
Figure 3. Inducible systems to overexpress Sox9b or dominant-negative Sox9b.
(A) sox9b and (F) trsox9b cre responder constructs used to make new lines. Tol2 arms (grey triangles) facilitate transgenesis. Following cre-recombination between LoxP sites (black triangles), both the eCFP gene (blue) and stop sequences (yellow) are removed allowing ubiquitous expression of either sox9b-2A-mCherry (A) or trsox9b-2A-mCherry (F) to be driven off the ubb promoter/enhancer. Due to 2A sequence (green), either Sox9b (A) or trSox9b (F) will be expressed as separate proteins along with mCherry. Double transgenic larvae for the ubiquitous creERT2 driver and either sox9b cre-responder (B–E) or trsox9b cre-responder (G–J). (B, D, G, J) Merged fluorescence images of the larvae at 72 hpf to detect both eCFP in blue and mCherry in red. (C, E, I, L) Results of whole mount in situ hybridization on 24 hpf larvae to detect the known Sox9 target gene col2a1a. (H, K) Bright field dorsalimages at 72 hpf. From 4 hpf, fish in (B, C, G–I) were treated with vehicle and fish in (D, E, J–L) were treated with 5 µM 4OHT. 4OHT treatment leads to induction of mCherry expression (D and J) concomitant with abnormal gross development due to Sox9b overexpression (D) and trSox9b expression (J–K). Overexpression of Sox9b also caused increased expression of the Sox9 target gene col2a1a (E). Scale bars = 500 µm.
Before testing CAC-autonomous phenotypes, we first validated both the new cre-responder lines by observing the effects of widespread cre-activation by generating fish transgenic for our cre-responder lines and our ubiquitous creERT2 driver [Tg(β-actin:GFP-F2A-creERT2)jh29] (Wang et al., 2011). These fish were treated continuously with 5 µM 4-hydroxytamoxifen (4OHT) starting in the first hour of development to induce constant and widespread cre activity. Observations from both cre-responder lines demonstrated widespread mCherry expression by 24 hpf, indicating successful excision of the loxP flanked sequences and the co-expression of a fluorescent reporter and either full length or truncated Sox9b (Figure 3D, I). Continuous expression of ectopic Sox9b was shown to be deleterious to development. Ubiquitous Sox9b expression during the first 72 hours of development led to an aberrant phenotype including pericardial edema, heart malfunction, and underdevelopment of the jaw (Figure 3B, D). This aberrant morphology is concomitant with increased expression of the well-known Sox9b-target gene, col2a1a (Yan et al., 2002; Yan et al., 2005) (Figure 3C, E). As predicted, ectopic expression of truncated Sox9b also leads to abnormal development. By 5 dpf, fish expressing widespread trSox9b displayed pericardia edema and underdeveloped jaws and pectoral fins (Figure 3G, H, J, K). This phenotype is more severe than is seen in null mutations of sox9b but is reminiscent of double null sox9a/sox9b phenotype (Yan et al., 2005). This result suggests that truncated Sox9b does act as a dominant negative form but most likely also inhibits the actions of its paralog, Sox9a, and potentially other closely related Sox transcription factors. However, over expression of neither full length nor truncated Sox9b affected sox9a expression levels (Supplemental Figure 2B). Additionally, we were unable to observe that expression of col2a1a was affected by expression of the truncated Sox9b (Figure 3I, L), indicating that other Sox9b-independent factors may maintain its expression.
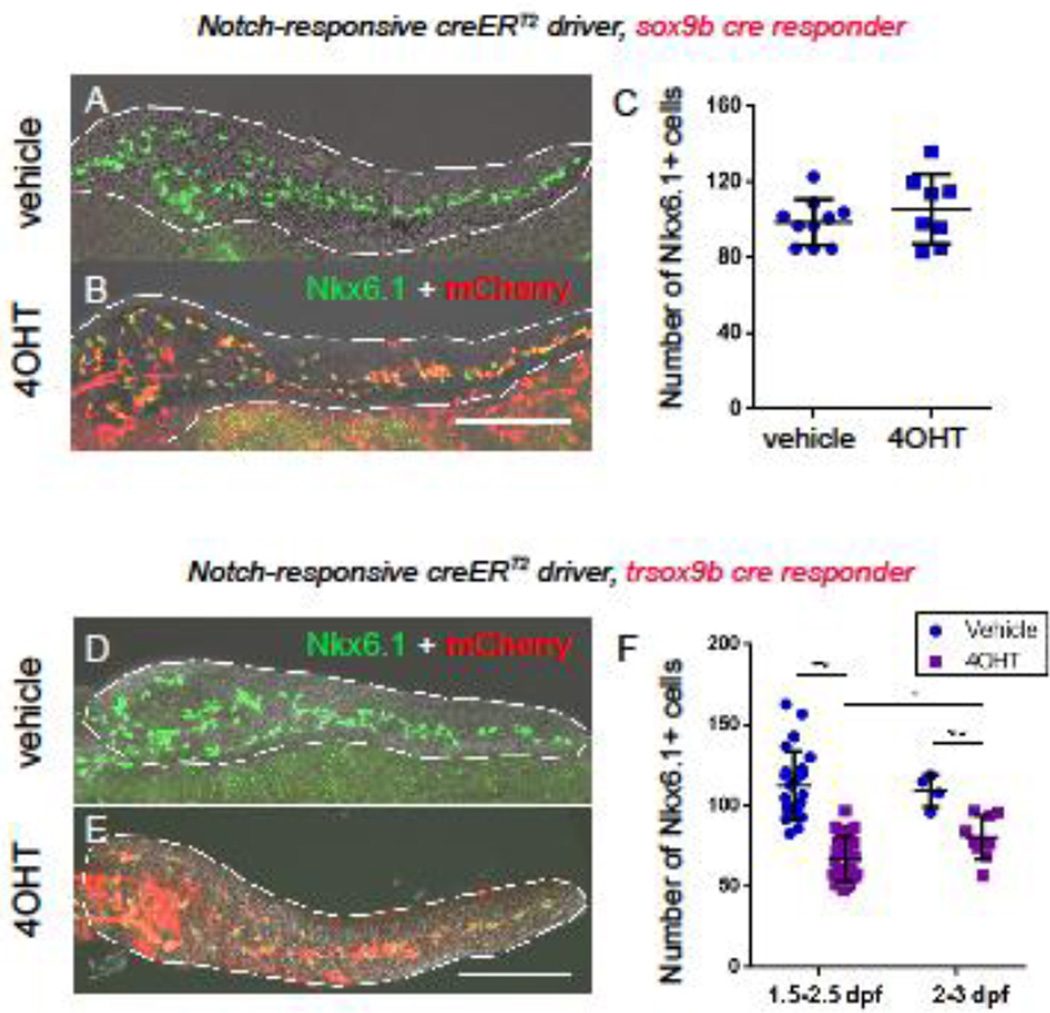
Having validated our ability to cause cre-dependent expression of both full length and truncated Sox9b in a temporally controlled manner, we next tested limiting expression to just the pancreatic progenitors. To overexpress either Sox9b or trSox9b in CACs, we crossed our new cre-responder lines with our Notch-responsive creERT2 driver fish (Delaspre et al., 2015; He et al., 2014; Wang et al., 2011). The resulting double transgenic larvae were incubated with 4OHT from 2–3 dpf to induce overexpression of either Sox9b or trSox9b in larval CACs. At 5 dpf in both sets of fish the mCherry label was co-expressed with the CAC marker Nkx6.1 (Figure 4A–B, D–E). By quantifying the percentage of Nkx6.1 positive CACs that were labeled with mCherry, we showed that both our new cre-responder lines were efficient in directing CAC-specific 4OHT-dependent cre-recombination (sox9b cre-responder - 96.5%±3% SD, 8 pancreata imaged and trsox9b cre-responder 93.1%±3.2% SD, 9 pancreata imaged). By counting numbers of Nkx6.1 positive CACs in double transgenic larvae in 4OHT treated and untreated controls, we concluded that overexpression of Sox9b in larval CACs from 2 – 3 dpf does not significantly affect CAC number compared to vehicle controls (105.9±18.4 SD vs 98.8±12.1 SD, p>0.05, Figure 4C). Ectopic expression of trSox9b lead to a significant reduction in CAC number (80.7±13.5 SD vs 109.5±10.1 SD in vehicle controls, p<0.001, Figure 4F). CAC numbers could be even further reduced by inducing expression of truncated Sox9b earlier in development between 1.5 – 2.5 dpf (67.1±13.5 SD vs 112.5±21.3 SD in controls, p<0.001, Figure 4F). A reduction in CAC numbers following induction of trSox9b expression is reminiscent of the pancreatic phenotype in sox9b null larvae where CAC numbers are reduced (Delous et al., 2012; Manfroid et al., 2012). Our observations are consistent with trSox9b expression inhibiting Sox9b function, which is required for pancreatic progenitor specification and maintenance
Figure 4. Inducible expression of Sox9b and truncated Sox9b in CAC pancreatic progenitors.
(A, B and D, E) Confocal images of 5 dpf pancreata dissected from double transgenic larvae for the Notch-responsive creERT2 driver and either sox9b cre-responder (A, B) or trsox9b cre-responder (D, E) showing localization of Nkx6.1 (green) and mCherry (red). Transgenic larvae were treated with vehicle (A, D) or 4OHT (B, E) from 2–3 dpf. The numbers of double positive (green/red) cells were quantified (C, F). Overexpression of Sox9b did not significantly affect numbers of Nkx6.1 positive CACs (C); however, 4OHT induction of trSox9b significantly reduced CAC numbers, an effect that was heightened if 4OHT treatment was performed even earlier in development (1.5–2.5 dpf, F). Scale Bar = 100 µm, *p<0.05, **p<0.001.
3.3 The effect of exogenous RA treatment on larval CACs is dependent on Sox9b activity
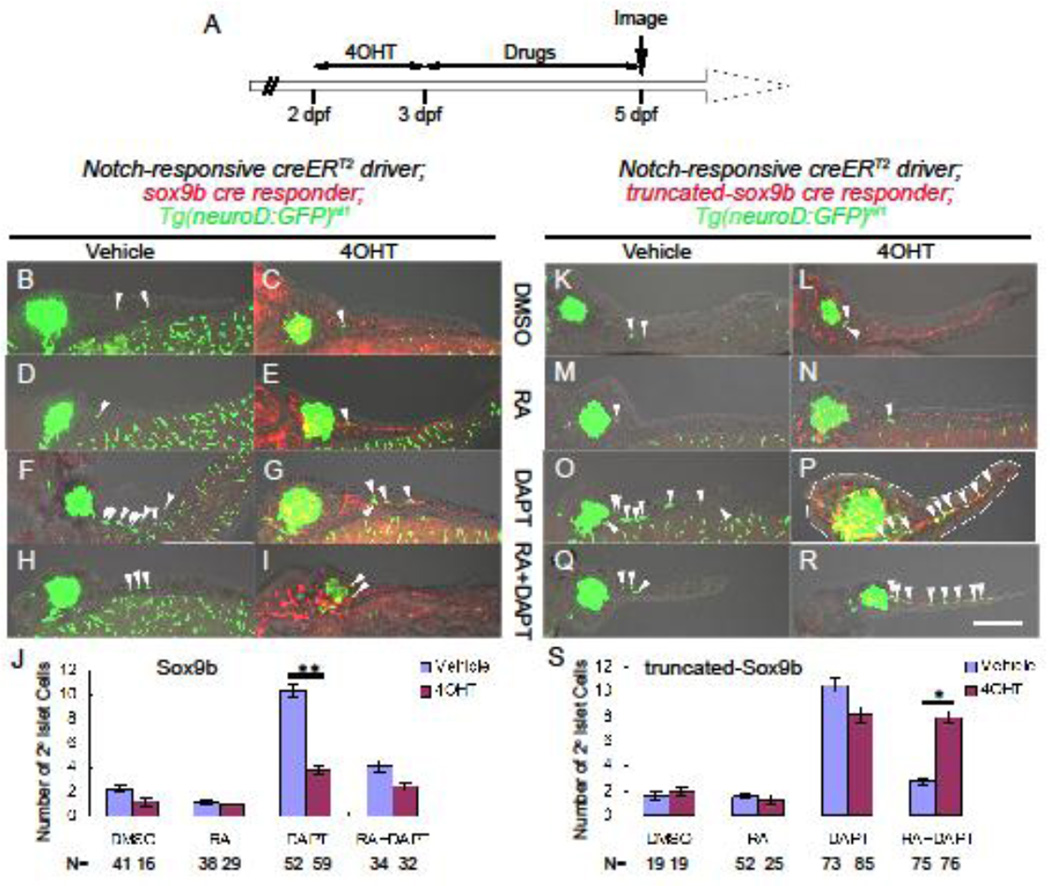
2° islet production can be induced by DAPT treatment and blocked by exogenous RA treatment (Huang et al., 2014; Rovira et al., 2011). We set out to test if changing Sox9b activity in CACs would alter the actions of DAPT and RA. To facilitate studying the effects of either Sox9b or trSox9b overexpression on 2° islet formation, we crossed our double transgenic fish conditionally expressing either Sox9b or trSox9b with the neuroD:GFP transgenic line, which carries a pan-endocrine GFP reporter allowing visualization of 2° islet cells in the tail of the pancreas (Huang et al., 2014). As outlined in Figure 5A, triple transgenic larvae were first treated with 4OHT from 2–3 dpf to induce expression of mCherry and either Sox9b or trSox9b. Larvae displaying red fluorescence were collected and further incubated from 3–5 dpf with one of the following treatments: DMSO (negative control), DAPT, RA or DAPT+RA. Vehicle treated larvae, in which no cre-recombination was induced were treated with the same drug regime from 3–5 dpf for comparison.
Figure 5. RA regulates CAC differentiation through action of Sox9b.
(A) Experimental timeline of 4OHT-dependent induction of either Sox9b or truncated-Sox9b expression. 4OHT was applied from 2–3 dpf, and drugs (either DMSO (vehicle), RA, DAPT or RA+DAPT) from 3–5 dpf. (B–I, and K–R) Confocal images of 5 dpf dissected pancreata from larvae transgenic for the Notch-responsive creERT2 driver, a pan-endocrine marker neuroD:GFP and either the sox9b cre responder (B–I) or the truncated sox9b cre responder (K–R). All larvae were incubated from 2–3 dpf with either vehicle or 4OHT (as indicated). Next, vehicle and 4OHT treated larvae were incubated from 3–5 dpf with either: DMSO (B, C, K, L), RA (D, E, M, N), DAPT (F, G, O, P), or RA+DAPT (H, I, Q, R). 2° islet cells are marked by GFP (white arrow heads) and seen in the tail of the pancreas. White dashes outline the pancreata. Scale bar = 100 µm. Average number of 2° islet cells/pancreas in larvae transgenic for the Notch-responsive creERT2 driver and Tg(neuroD:GFP)nl1 and either the sox9b cre responder (J) or the truncated sox9b cre responder (S). N = number of larval pancreata quantified. Error bar represents SE; *p<0.01, **p<0.001.
As expected, larvae treated with just DMSO (Figure 5B, 5K) or exogenous RA (Figure 5D, 5M) had very few 2° islet cells. Additionally, 4OHT-dependent expression of neither Sox9b (Figure 5C, 5E) nor trSox9b (Figure 5L, 5N) had any influence on 2° islet number. Conversely, Notch-signaling via DAPT treatment dramatically increased the number of 2° islet cells, as expected (Figure 5F and 5O).
Two different experimental paradigms attenuated the DAPT induction of 2° islet formation. First, 4OHT-directed over-expression of Sox9b expression prior to Notch signaling inhibition (4OHT+DAPT) significantly reduced the average number of 2° islet cells/pancreas (3.9±0.3 SE 2° islet cells vs 10.4±0.5 SE in DAPT treated controls, p<0.001, Figure 5F–G). Second, as previously shown (Huang et al., 2014; Rovira et al., 2011), adding RA to the DAPT treatment blocked CAC differentiation towards an endocrine fate (vehicle+RA+DAPT, Figure 5H and 5Q). These two observations demonstrated that Sox9b overexpression in larval CACs has the same outcome as exogenous RA treatment.
This capacity of RA to mitigate the effects of DAPT treatment was unaffected by Sox9b over-expression (Figure 5I) but was abolished in the presence of the dominant negative trSox9b form. In larvae induced to express trSox9b and treated with both RA and DAPT (Figure 5R), the average number of 2° islet cells per pancreas was similar to larvae treated with DAPT alone and significantly higher than detected in vehicle+RA+DAPT treated controls (8.0 ±0.5 SE vs 2.9 ±0.3 SE, p<0.001 Figure 5Q). Overall, these results are summarized in Figure 5J and Figure 5S.
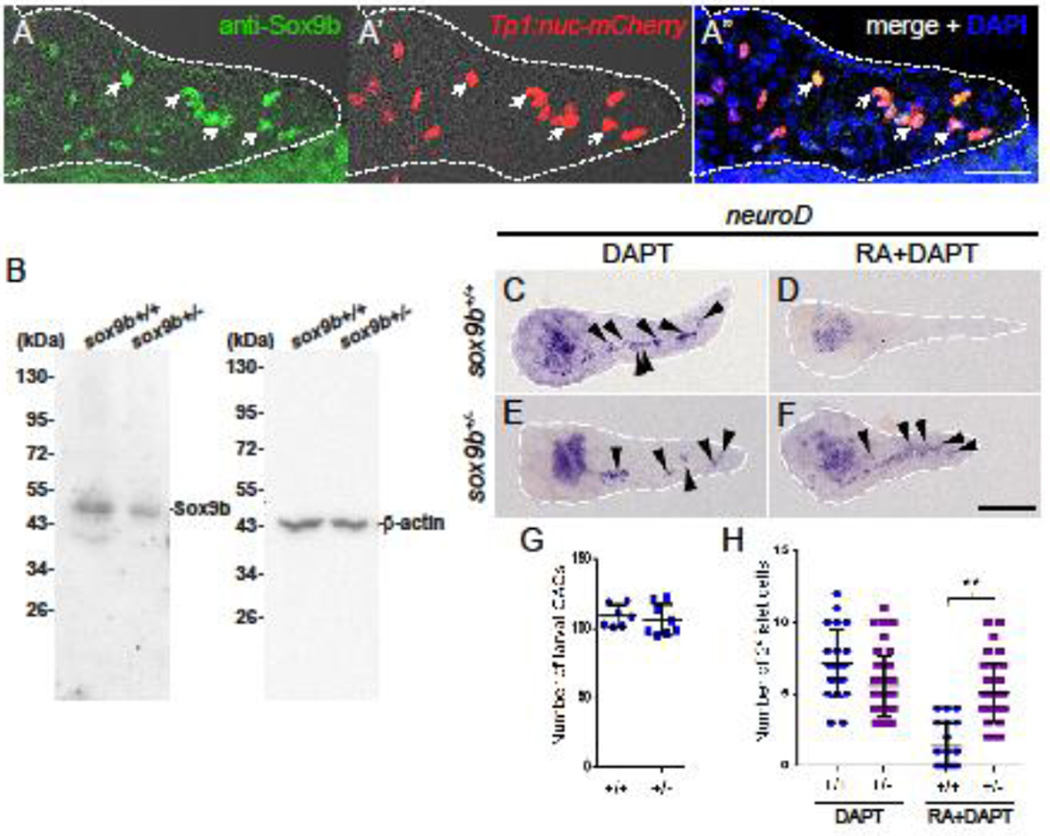
From our work with the transgenic lines, we conclude that normal levels of Sox9b activity are required in CACs to mediate the effects of exogenous RA on 2° islet formation. To verify these results using an independent method, we took advantage of fish carrying a nonsense allele of sox9b, soxbfh313 (Delous et al., 2012; Manfroid et al., 2012). Blocking Sox9b activity either in sox9bfh313 homozygous mutant fish or by over expression of truncated sox9b results in fewer CAC progenitors available to produce new endocrine cells (Delous et al., 2012; Manfroid et al., 2012). Therefore, we focused on examining sox9b heterozygous larvae. To examine whether sox9bfh313 heterozygous larvae have a reduced dosage of Sox9b protein we first generated an antibody specific to Sox9b (Supplemental Figure 3). To demonstrate the specificity of this antibody, we performed immunofluorescence staining on 5 dpf pancreata in which the nuclei of Notch-responsive cells (including CACs) are labelled with mCherry (Tp1:nuc-mCherry). As can be seen in Figure 6A, Sox9b co-localized to nuclear mCherry within CACs. Using this new antibody on Western blots of whole larval lysate showed that sox9bfh313 heterozygous larvae express significantly less Sox9b when compared to wildtype (Figure 6B, 9 dpf, pool of 10 larvae each).
Figure 6. sox9b haploinsufficiency impedes the effects of RA on CAC differentiation.
(A) Immunofluorescence staining using a Sox9b antibody in 5 dpf pancreata expressing Tp1:nuc-mCherry. Sox9b expression co-localizes with nuclear mCherry in CACs (white arrows). Pancreas is outlined in dashed line. Scale bar = 20 µm. (B) Western blot demonstrating that Sox9b expression is reduced in sox9bfh313 heterozygous larvae. (C–F) Images of dissected pancreata from 5 dpf larvae with the following genotype: (C–D) wildtype (sox9b+/+) (E–F) sox9bfh313 heterozygous (sox9b+/−). in situ hybridization for neuroD was used to detect 2° islets. Larvae of differing genotypes (as indicated) were treated from 3–5 dpf with either DAPT (C, E) alone or RA+DAPT (D, F). 2° islets are indicated by black arrow heads. White dashes outline the pancreata. Scale bar = 100 µm. (G) Numbers of CACs quantified using expression of Tp1:eGFP in pancreata from 5 dpf larvae, genotyped as indicated. Heterozygotes do not have significantly different numbers of CACs compared to wildtype. (H) Numbers of 2° islets/pancreas from genotyped larvae after being treated with DAPT alone or RA+DAPT. Error bar represents SD; **p<0.001.
Next, we incubated wild-type and sox9bfh313 heterozygous larvae with DAPT from 3–5 dpf and used whole mount in situ hybridization to detect neuroD expression. Since this method is not suitable for counting numbers of individual 2° islet cells, we instead counted numbers of 2° islets to gauge levels of endocrine differentiation. As previously observed, DAPT treatment induced precocious 2° islet formation in the wild-type controls. Additionally, as expected, sox9bfh313 heterozygotes developed with wild-type numbers of CACs (Figure 6C, E, G).
To further test the hypothesis that Sox9b function is required for RA to block CAC differentiation, we incubated wild-type and sox9bfh313 heterozygous larvae with RA+DAPT from 3–5 dpf. As we have previously shown, wild-type larvae treated with RA+DAPT had fewer 2° islets per pancreas than fish treated with DAPT alone (1.4±1.60 SD vs 7.13±2.32 SD, p<0.001 Figure 6C–D, H) (Huang et al., 2014). On the other hand, the ability of RA to mitigate DAPT-induced differentiation was significantly diminished in sox9b+/− heterozygotes compared to wildtype (5.1±2.0 vs 1.4±1.6 SD, p<0.001, Figure 6E–F, H). This finding is consistent with our previous result using our truncated-sox9b cre responder fish to express a dominant negative form of Sox9b in larval CACs (Figure 5Q, S). Taken together, our results show that impeding Sox9b function prevents exogenous RA from blocking CAC differentiation into endocrine cells. Along with our observations of RA induced expression of sox9b, we conclude that Sox9b mediates the CAC response to RA stimulation. As such, Sox9b function is pivotal in transducing both Notch and RA signaling in pancreatic endocrine progenitors.
3.4 Sox9b haploinsufficiency affects β-cell regeneration
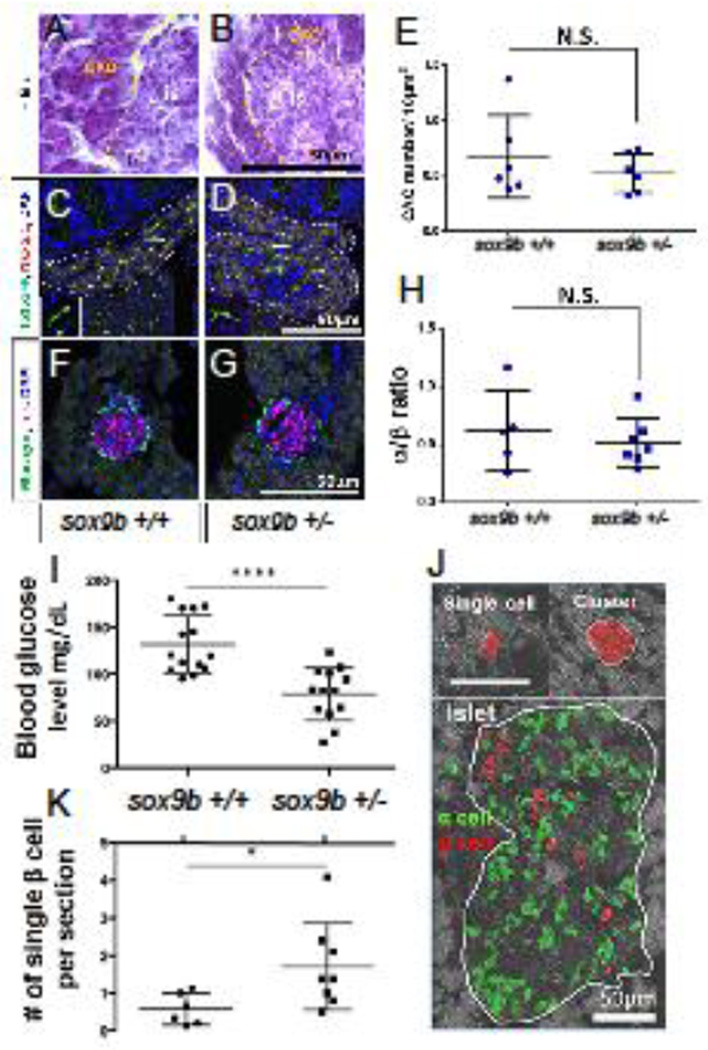
From our studies above, it was clear that sox9bfh313 heterozygous larvae possess a haploinsufficient phenotype affecting the regulation of CAC endocrine differentiation during development. Accordingly, we wondered whether Sox9b function might also be important during CAC differentiation in an adult setting. As sox9bfh313 homozygous adults display severe cholestasis (Delous et al., 2012), we focused on examining adult heterozygotes. We first examined whether there was a gross morphological phenotype in the pancreata of adult sox9bfh313 heterozygotes. According to gross histology, (Figure 7A–B), numbers of CACs (Figure 7C–D, E), or α to β cell ratio (Figure 7F–G) we detected no significant difference between wild-type and sox9bfh313 heterozygous pancreas (Figure 7E, H).
Figure 7. β-cell regeneration is accelerated in adult sox9b heterozygous fish.
Hematoxylin and eosin stained sections of adult pancreata from: A) wildtype (here on sox9b+/+); and, B sox9bfh313 heterozygotes (here on sox9b+/−). Exocrine (exo) and islet (is). (C, D) Detection of CACs using immunofluorescent detection of Nkx6.1 and GFP on pancreas sections from fish transgenic for the Notch-responsive reporter and either sox9b+/+ (C) or sox9b+/− (D). Double positive (Nkx6.1+/GFP+) cells were quantified and show no significant difference between sox9b+/+ and sox9b+/− (E). (F, G) Immunofluorescent detection of glucagon (green) and insulin (magenta) to label α and β cells in sox9b+/+ (F) and sox9b+/−(G) adult pancreata. (H) α to β ratio showed no significance difference between islet composition from sox9b+/+ and sox9b+/− fish. (I–K) After β-cell ablation, sox9b+/− adult fish showed better capability of β-cell regeneration compared to sox9b+/+. (I) Blood glucose at 7 days post β-cell ablation shows significantly lower blood glucose levels in sox9b+/− (N = 13) compared to sox9b+/+ (N = 14). (J) Representative immunofluorescent images of single β cells, small β-cell clusters and pre-existing islets. (K) Number of single β cells 7 days post β-cell ablation was significantly higher in sox9b+/− (n = 8) than that in sox9b+/+ (n = 6). Each dot represents the average number of single β cells observed per section from one fish. 18–20 transverse paraffin sections for each fish were examined. In this manner we analyzed the majority of pancreatic tissue for all 4 lobes. *p < 0.05, ****p < 0.0001.
To test if there is a sox9b haploinsufficient phenotype affecting CAC differentiation, we took advantage of our ins:NTR fish where β-cell-specific ablation can be induced by metronidazole (MZT) treatment (Delaspre et al., 2015; Moss et al., 2009; Pisharath et al., 2007). Following ablation, we tested if CAC contribution to β-cell regeneration was affected in sox9bfh313 heterozygous adult fish. The level of blood glucose in wild-type fish is an average of 63 mg/dL (±17.6 SD), rising to an average of 491 mg/dL (± 65 SD) three days after β-cell ablation (Delaspre et al., 2015; Eames et al., 2010). We performed β-cell ablation in ins:NTR; wild-type (control) and ins:NTR; sox9bfh313 heterozygous siblings and measured blood glucose after 7 days, a time point at the halfway mark for normal recovery (Delaspre et al., 2015). While the control fish still had elevated average blood glucose levels of 131.6 mg/dL (± 30 SD), sox9bfh313 heterozygous fish had a significantly lower average blood glucose concentration of 78.6 mg/dL (± 28 SD), consistent with a return to normal blood glucose levels (p<0.0001, Figure 7I).
We then sectioned recovering pancreata 7 days after β-cell ablation, and quantified numbers of regenerated β cells. Regenerated β cells can appear in three different structures; single cells, small clusters and within pre-existing islets (Figure 7J). We saw no difference in numbers of β cells reappearing in pre-existing islets from sox9bfh313 heterozygous and wild-type samples (data not shown); however, we did see significantly more single β cells and β-cell clusters in sox9bfh313 heterozygotes when compared to controls. Heterozygous fish showed 1.71 (± 1.15 SD) single β cells per analyzed section while controls showed 0.57 (± 0.42 SD) (p<0.05, Figure 7K). β cells presenting as single cells are consistent with β-cell neogenesis from progenitors and transiently appear during regeneration but are harder to find in uninjured pancreata (Delaspre et al., 2015). We conclude, therefore, that these physiological and morphological differences observed between wildtype and sox9bfh313 heterozygotes suggest sox9b haploinsufficiency is associated with accelerated regeneration and is consistent with enhanced differentiation of CACs.
4. Discussion
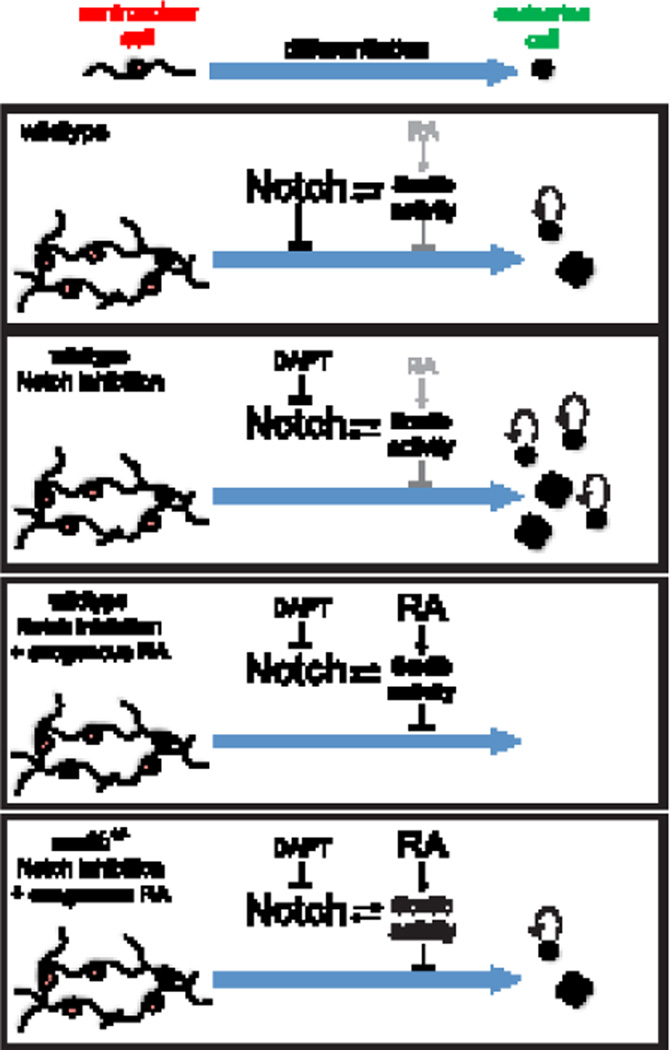
We have previously demonstrated that RA and Notch signaling pathways act in concert to regulate CAC differentiation into endocrine cells (Huang et al., 2014). We showed that RA could block DAPT-induced CAC differentiation into endocrine cells (Rovira et al., 2011). Together these observations suggested that there are common downstream targets shared by both RA and Notch signaling pathways. In this study, we concluded that Sox9b is a likely intermediate in the signal transduction from both the Notch and RA pathways in CAC pancreatic progenitors. Thus, we have developed a model for the control of endocrine differentiation of CAC progenitors in the larval zebrafish pancreas (Figure 8). In this model, Notch signaling is the dominant mechanism restricting differentiation. Notch signaling restricts differentiation in part by promoting a Sox9b positive feedback loop within progenitors, and DAPT inhibition of Notch signaling leads to precocious endocrine differentiation. In an independent secondary mechanism, RA signaling also promotes Sox9b activity to inhibit differentiation. Exogenous RA is enough to completely block precocious endocrine differentiation induced by DAPT Notch inhibition. Importantly, Sox9b activity is necessary and sufficient for RA signaling to impart this effect.
Figure 8. Model of Sox9b regulation of CAC differentiation.
Our hypothesis is that Sox9b mediates both Notch and RA signaling in a cell autonomous fashion to restrict CAC differentiation. In this model, Notch is the dominant mechanism restricting endocrine differentiation, and promotes a positive feedback loop of Sox9b activity. In a secondary mechanism, RA induces Sox9b activity to inhibit endocrine differentiation of CACs. Inhibition of Notch signaling using DAPT leads to the precocious differentiation of CACs toward an endocrine fate. However, addition of exogenous RA can completely abolish DAPT induced differentiation. Sox9b activity is necessary and sufficient for the ability of exogenous RA to block CAC differentiation.
It seems likely that Sox9 also plays a central role in integrating differentiation signals in pancreatic progenitors in other organisms as well. Classically, Notch signaling regulates progenitor differentiation through the action of members of the hairy and enhancer of split class of bHLH transcription factors (Bray, 2006). For instance, Notch signaling regulates pancreatic progenitor differentiation in the mouse, at least in some part through the actions of Hes1 (Apelqvist et al., 1999; Kopinke et al., 2011). We have identified several hairy related genes that are expressed in larval and adult zebrafish CACs, including: her6 and her15 (Delaspre et al., 2015). Ongoing work will attempt to establish if these genes are important in zebrafish CAC biology. Aside from Hes homologues, an ever-increasing body of work suggests that Sox9 plays a role in transducing Notch signaling in endocrine bipotent progenitors in the mouse (Apelqvist et al., 1999; Delous et al., 2012; Jensen et al., 2000b; Lynn et al., 2007; Seymour et al., 2007; Shih et al., 2012).
On the other hand, although RA is a well-known regulator of pancreas development, little is known about its downstream transcriptional effectors in pancreatic progenitors. Several studies in mouse and Xenopus suggest that RA signaling influences the expression of another important master regulator of pancreas development, Pdx1 (Chen et al., 2004; Kumar et al., 2003; Micallef et al., 2005). Interestingly, recent work in mouse has demonstrated that Pdx1 and Sox9 regulate the specification of pancreatic progenitors in concert and that a positive feedback loop between Pdx1 and Sox9 maintains the pancreas fate (Shih et al., 2015). Thus, RA signaling may influence Sox9b activity indirectly by regulating its cofactors. Alternatively, RA signaling is well known to broadly affect chromatin state, transcription factor activity, and ultimately cellular differentiation by directly regulating the expression of nuclear lamins (Swift and Discher, 2014). Consequently, during CAC differentiation RA may work to direct a large network of transcriptional changes, in which Sox9b plays a central role.
In previous work, we showed evidence that the acinar cells of the developing 5 dpf larval pancreas are enzymatically capable of generating a local source of RA and that larval CACs are actively responding to RA signaling (Huang et al., 2014; (Rovira et al., 2011). Later in larval development (15 dpf), specific epithelial cells express the enzyme, Aldehyde dehydrogenase1 (Aldh1). Aldh1 activity is integral in RA synthesis, hence, these Aldh1 positive cells are another potential source for local RA (Matsuda et al., 2013). We showed that loss of RA signaling leads to heightened CAC differentiation and endocrine production (Huang et al., 2014; Rovira et al., 2011). Alongside the work presented here, these results are consistent with local sources of RA acting upon larval CACs to regulate Sox9b activity that in turn impedes CAC differentiation to endocrine cells. However, other downstream effectors of RA signaling in CACs are likely also important during endocrine differentiation.
There are significant differences between the individual effects of Notch and RA on CAC differentiation. Using the CAC markers Nkx6.1, sox9b, and cftr:cftr-gfp, it is clear that Notch inhibition by DAPT treatment causes differentiation and loss of CAC identity. Yet, a minor portion of CACs differentiates to become endocrine cells. Co-treatment with RA and DAPT, however, blocks the production of any endocrine cells while still extinguishing Nkx6.1 and cftr-gfp expression. In contrast to DAPT alone, RA plus DAPT leads to retention in expression of both sox9b and the duct marker 2F11. These observations are consistent with Notch inhibition in combination with exogenous RA causing a loss of CAC endocrine progenitor capacity but retention of ductal epithelial identity. One interpretation is that Notch and RA influence different parts in a CAC differentiation pathway with RA downstream of Notch activity. An alternative hypothesis is that RA signaling only blocks CAC differentiation to endocrine fate whereas Notch signaling blocks all CAC differentiation.
In this report we used two different methods and showed that Sox9b function is required for RA-dependent restriction of CAC differentiation. Furthermore we diminished Sox9b activity in adult zebrafish leads to accelerated recovery of blood-glucose levels following β-cell ablation concomitant with enhanced progenitor differentiation. Discovering that diminished Sox9b activity leads to expedited differentiation is consistent with the findings of others that established Sox9 homologs as being essential for maintenance of progenitor populations (Belo et al., 2013; Seymour et al., 2007). We are now exploring a model where Sox9b, via its downstream targets, maintains CACs by promoting self-renewal and restricting differentiation. It is now important to identify these downstream targets and ascertain if they can be exploited to facilitate progenitor differentiation as part of a diabetes treatment.
Supplementary Material
Wild-type larvae were treated with RA and/or DAPT from 3–5 dpf, and incubated with EdU from 4–5 dpf. Following fixation and dissection, pancreata were stained with anti-Nkx6.1 to detect CACs. As expected, CAC number was significantly reduced in larvae treated with DAPT. No significant difference in the ratio of EdU+ CACs to total CACs was detected in any of the treatments. *p<0.05, ****p<0.0001.
Embryos carrying the sox9b responder and trsox9b responder transgenes were injected with 125 ng cre mRNA at the one-cell stage to induce expression of the sox9-2A-mCherry or sox9b-2A-mCherry cassette, respectively. At 48 hpf, following visual confirmation of mCherry expression, embryos were sacrificed for cDNA synthesis. (A) RT-qPCR confirms that sox9b is expressed at higher levels compared to uninjected controls. (B) RT-qPCR for sox9a shows that its expression is not significantly effected by sox9b or trsox9b expression compared to uninjected controls. *p<0.05.
Total lysate was prepared from HeLa cells transfected with vectors to express either zebrafish FLAG-Sox9a or zebrafish FLAG-Sox9b proteins, or from 6 dpf larvae. Western blot demonstrates that the anti-Sox9b antibody does not recognize the zebrafish FLAG-Sox9a fusion, but does bind the zebrafish FLAG-Sox9b protein (~60 kDa). Additionally, the anti-Sox9b antibody binds to a single band of ~50 kDa in 6 dpf larval lysate, corresponding to endogenous Sox9b. Note that the FLAG-Sox9b protein migrated slower compared to endogenous Sox9b, possibly due to the tag.
Highlights.
sox9b is downstream of Notch and RA signaling pathways in pancreas progenitors
Transgenic tools to express full length and dominant negative sox9b are created
sox9b is necessary and sufficient for RA to block progenitor differentiation
sox9b halploinsufficiency leads to accelerated endocrine regeneration
Acknowledgments
This work was supported by the Juvenile Diabetes Research Foundation, the NIH (R01DK080730, R01HD058530 and RC4DK090816) and a Maryland Stem Cell Research Fund 2013 Postdoctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonja O, Raaka BM, Huang A, Das S, Zhao X, Helmer E, Juste D, Samuels HH. RAR agonists stimulate SOX9 gene expression in breast cancer cell lines: evidence for a role in retinoid-mediated growth inhibition. Oncogene. 2002;21:7850–7860. doi: 10.1038/sj.onc.1205985. [DOI] [PubMed] [Google Scholar]
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Beer RL, Parsons MJ, Rovira M. Centroacinar cells: At the center of pancreas regeneration. Dev Biol. 2016;413:8–15. doi: 10.1016/j.ydbio.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belo J, Krishnamurthy M, Oakie A, Wang R. The role of SOX9 transcription factor in pancreatic and duodenal development. Stem Cells Dev. 2013;22:2935–2943. doi: 10.1089/scd.2013.0106. [DOI] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nature reviews. Molecular cell biology. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pan FC, Brandes N, Afelik S, Solter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. 2004;271:144–160. doi: 10.1016/j.ydbio.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Chiang EF, Pai CI, Wyatt M, Yan YL, Postlethwait J, Chung B. Two sox9 genes on duplicated zebrafish chromosomes: expression of similar transcription activators in distinct sites. Dev Biol. 2001;231:149–163. doi: 10.1006/dbio.2000.0129. [DOI] [PubMed] [Google Scholar]
- Delaspre F, Beer RL, Rovira M, Huang W, Wang G, Gee S, Vitery Mdel C, Wheelan SJ, Parsons MJ. Centroacinar Cells Are Progenitors That Contribute to Endocrine Pancreas Regeneration. Diabetes. 2015;64:3499–3509. doi: 10.2337/db15-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, Yin C, Shin D, Ninov N, Debrito Carten J, Pan L, Ma TP, Farber SA, Moens CB, Stainier DY. Sox9b is a key regulator of pancreaticobiliary ductal system development. PLoS genetics. 2012;8:e1002754. doi: 10.1371/journal.pgen.1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois CL, Shih HP, Seymour PA, Patel NA, Behrmann JM, Ngo V, Sander M. Sox9-haploinsufficiency causes glucose intolerance in mice. PloS one. 2011;6:e23131. doi: 10.1371/journal.pone.0023131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames SC, Philipson LH, Prince VE, Kinkel MD. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish. 2010;7:205–213. doi: 10.1089/zeb.2009.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm R, Zelander T, Edlund Y. The ultrastructural organization of the rat exocrine pancreas: II. Centroacinar cells, intercalary and intralobular ducts. Journal of Ultrastructure Research. 1962;7:73–83. doi: 10.1016/s0022-5320(62)80029-x. [DOI] [PubMed] [Google Scholar]
- Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, Pack MA, Ball DW, Leach SD. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol. 2003;260:426–437. doi: 10.1016/s0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- >He J, Lu H, Zou Q, Luo L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology. 2014;146:789–800. e788. doi: 10.1053/j.gastro.2013.11.045. [DOI] [PubMed] [Google Scholar]
- Huang W, Wang G, Delaspre F, Vitery MD, Beer RL, Parsons MJ. Retinoic acid plays an evolutionarily conserved and biphasic role in pancreas development. Dev Biol. 2014 doi: 10.1016/j.ydbio.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 2000a;49:163–176. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000b;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Kinkel MD, Prince VE. On the diabetic menu: zebrafish as a model for pancreas development and function. BioEssays : news and reviews in molecular, cellular and developmental biology. 2009;31:139–152. doi: 10.1002/bies.200800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopinke D, Brailsford M, Shea JE, Leavitt R, Scaife CL, Murtaugh LC. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development. 2011;138:431–441. doi: 10.1242/dev.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol. 2003;259:109–122. doi: 10.1016/s0012-1606(03)00183-0. [DOI] [PubMed] [Google Scholar]
- Laursen KB, Mongan NP, Zhuang Y, Ng MM, Benoit YD, Gudas LJ. Polycomb recruitment attenuates retinoic acid-induced transcription of the bivalent NR2F1 gene. Nucleic acids research. 2013;41:6430–6443. doi: 10.1093/nar/gkt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson TS, Leeson R. Close association of centroacinar/ductular and insular cells in the rat pancreas. Histol Histopathol. 1986;1:33–42. [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorent K, Yeo SY, Oda T, Chandrasekharappa S, Chitnis A, Matthews RP, Pack M. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development. 2004;131:5753–5766. doi: 10.1242/dev.01411. [DOI] [PubMed] [Google Scholar]
- Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N, German MS. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc Natl Acad Sci U S A. 2007;104:10500–10505. doi: 10.1073/pnas.0704054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfroid I, Ghaye A, Naye F, Detry N, Palm S, Pan L, Ma TP, Huang W, Rovira M, Martial JA, Parsons MJ, Moens CB, Voz ML, Peers B. Zebrafish sox9b is crucial for hepatopancreatic duct development and pancreatic endocrine cell regeneration. Dev Biol. 2012;366:268–278. doi: 10.1016/j.ydbio.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Parsons MJ, Leach SD. Aldh1-expressing endocrine progenitor cells regulate secondary islet formation in larval zebrafish pancreas. PloS one. 2013;8:e74350. doi: 10.1371/journal.pone.0074350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurley AT, Callard GV. Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol Biol. 2008;9:102. doi: 10.1186/1471-2199-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef SJ, Janes ME, Knezevic K, Davis RP, Elefanty AG, Stanley EG. Retinoic acid induces Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes. 2005;54:301–305. doi: 10.2337/diabetes.54.2.301. [DOI] [PubMed] [Google Scholar]
- Mosimann C, Zon LI. Advanced zebrafish transgenesis with Tol2 and application for Cre/lox recombination experiments. Methods Cell Biol. 2011;104:173–194. doi: 10.1016/B978-0-12-374814-0.00010-0. [DOI] [PubMed] [Google Scholar]
- Moss JB, Koustubhan P, Greenman M, Parsons MJ, Walter I, Moss LG. Regeneration of the pancreas in adult zebrafish. Diabetes. 2009;58:1844–1851. doi: 10.2337/db08-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Crofts JD, Newman BS, Bridgewater LC, Lin CY, Gustafsson JA, Strom A. SOX9 mediates the retinoic acid-induced HES-1 gene expression in human breast cancer cells. Breast Cancer Res Treat. 2010;120:317–326. doi: 10.1007/s10549-009-0381-6. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navis A, Bagnat M. Loss of cftr function leads to pancreatic destruction in larval zebrafish. Dev Biol. 2015 doi: 10.1016/j.ydbio.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navis A, Marjoram L, Bagnat M. Cftr controls lumen expansion and function of Kupffer's vesicle in zebrafish. Development. 2013;140:1703–1712. doi: 10.1242/dev.091819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninov N, Borius M, Stainier DY. Different levels of Notch signaling regulate quiescence, renewal and differentiation in pancreatic endocrine progenitors. Development. 2012;139:1557–1567. doi: 10.1242/dev.076000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obholzer N, Wolfson S, Trapani JG, Mo W, Nechiporuk A, Busch-Nentwich E, Seiler C, Sidi S, Sollner C, Duncan RN, Boehland A, Nicolson T. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J Neurosci. 2008;28:2110–2118. doi: 10.1523/JNEUROSCI.5230-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MJ, Pisharath H, Yusuff S, Moore JC, Siekmann AF, Lawson N, Leach SD. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev. 2009;126:898–912. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper K, Ball SG, Keeling JW, Mansoor S, Wilson DI, Hanley NA. Novel SOX9 expression during human pancreas development correlates to abnormalities in Campomelic dysplasia. Mech Dev. 2002;116:223–226. doi: 10.1016/s0925-4773(02)00145-4. [DOI] [PubMed] [Google Scholar]
- Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pour PM. Pancreatic centroacinar cells. The regulator of both exocrine and endocrine function. Int J Pancreatol. 1994;15:51–64. [PubMed] [Google Scholar]
- Puri S, Garcia-Nunez A, Hebrok M, Cano DA. Elimination of von Hippel-Lindau function perturbs pancreas endocrine homeostasis in mice. PloS one. 2013;8:e72213. doi: 10.1371/journal.pone.0072213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira M, Huang W, Yusuff S, Shim JS, Ferrante AA, Liu JO, Parsons MJ. Chemical screen identifies FDA-approved drugs and target pathways that induce precocious pancreatic endocrine differentiation. Proc Natl Acad Sci U S A. 2011;108:19264–19269. doi: 10.1073/pnas.1113081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Dubois CL, Shih HP, Patel NA, Sander M. A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Dev Biol. 2008;323:19–30. doi: 10.1016/j.ydbio.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Sander M. Historical perspective: beginnings of the beta-cell: current perspectives in beta-cell development. Diabetes. 2011;60:364–376. doi: 10.2337/db10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HP, Kopp JL, Sandhu M, Dubois CL, Seymour PA, Grapin-Botton A, Sander M. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development. 2012;139:2488–2499. doi: 10.1242/dev.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HP, Seymour PA, Patel NA, Xie R, Wang A, Liu PP, Yeo GW, Magnuson MA, Sander M. A Gene Regulatory Network Cooperatively Controlled by Pdx1 and Sox9 Governs Lineage Allocation of Foregut Progenitor Cells. Cell Rep. 2015;13:326–336. doi: 10.1016/j.celrep.2015.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift J, Discher DE. The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J Cell Sci. 2014;127:3005–3015. doi: 10.1242/jcs.149203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang R, Dodd A, Lai D, McNabb WC, Love DR. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin (Shanghai) 2007;39:384–390. doi: 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiso N, Moro E, Argenton F. Zebrafish pancreas development. Molecular and cellular endocrinology. 2009;312:24–30. doi: 10.1016/j.mce.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Tsuji N, Ninov N, Delawary M, Osman S, Roh AS, Gut P, Stainier DY. Whole organism high content screening identifies stimulators of pancreatic Beta-cell proliferation. PloS one. 2014;9:e104112. doi: 10.1371/journal.pone.0104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Rajpurohit SK, Delaspre F, Walker SL, White DT, Ceasrine A, Kuruvilla R, Li RJ, Shim JS, Liu JO, Parsons MJ, Mumm JS. First quantitative high-throughput screen in zebrafish identifies novel pathways for increasing pancreatic beta-cell mass. Elife. 2015;4 doi: 10.7554/eLife.08261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rovira M, Yusuff S, Parsons MJ. Genetic inducible fate mapping in larval zebrafish reveals origins of adult insulin-producing beta-cells. Development. 2011;138:609–617. doi: 10.1242/dev.059097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. A guide for the laboratory use of zebrafish (Danio rerio. 4. Eugene: University of Oregon Press; 2000. The zebrafish book. [Google Scholar]
- Yan YL, Miller CT, Nissen RM, Singer A, Liu D, Kirn A, Draper B, Willoughby J, Morcos PA, Amsterdam A, Chung BC, Westerfield M, Haffter P, Hopkins N, Kimmel C, Postlethwait JH. A zebrafish sox9 gene required for cartilage morphogenesis. Development. 2002;129:5065–5079. doi: 10.1242/dev.129.21.5065. [DOI] [PubMed] [Google Scholar]
- Yan YL, Willoughby J, Liu D, Crump JG, Wilson C, Miller CT, Singer A, Kimmel C, Westerfield M, Postlethwait JH. A pair of Sox: distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development. 2005;132:1069–1083. doi: 10.1242/dev.01674. [DOI] [PubMed] [Google Scholar]
- Zecchin E, Filippi A, Biemar F, Tiso N, Pauls S, Ellertsdottir E, Gnugge L, Bortolussi M, Driever W, Argenton F. Distinct delta and jagged genes control sequential segregation of pancreatic cell types from precursor pools in zebrafish. Dev Biol. 2007;301:192–204. doi: 10.1016/j.ydbio.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Zhang D, Golubkov VS, Han W, Correa RG, Zhou Y, Lee S, Strongin AY, Dong PD. Identification of Annexin A4 as a hepatopancreas factor involved in liver cell survival. Dev Biol. 2014;395:96–110. doi: 10.1016/j.ydbio.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wild-type larvae were treated with RA and/or DAPT from 3–5 dpf, and incubated with EdU from 4–5 dpf. Following fixation and dissection, pancreata were stained with anti-Nkx6.1 to detect CACs. As expected, CAC number was significantly reduced in larvae treated with DAPT. No significant difference in the ratio of EdU+ CACs to total CACs was detected in any of the treatments. *p<0.05, ****p<0.0001.
Embryos carrying the sox9b responder and trsox9b responder transgenes were injected with 125 ng cre mRNA at the one-cell stage to induce expression of the sox9-2A-mCherry or sox9b-2A-mCherry cassette, respectively. At 48 hpf, following visual confirmation of mCherry expression, embryos were sacrificed for cDNA synthesis. (A) RT-qPCR confirms that sox9b is expressed at higher levels compared to uninjected controls. (B) RT-qPCR for sox9a shows that its expression is not significantly effected by sox9b or trsox9b expression compared to uninjected controls. *p<0.05.
Total lysate was prepared from HeLa cells transfected with vectors to express either zebrafish FLAG-Sox9a or zebrafish FLAG-Sox9b proteins, or from 6 dpf larvae. Western blot demonstrates that the anti-Sox9b antibody does not recognize the zebrafish FLAG-Sox9a fusion, but does bind the zebrafish FLAG-Sox9b protein (~60 kDa). Additionally, the anti-Sox9b antibody binds to a single band of ~50 kDa in 6 dpf larval lysate, corresponding to endogenous Sox9b. Note that the FLAG-Sox9b protein migrated slower compared to endogenous Sox9b, possibly due to the tag.