Abstract
Approximately one-third of advanced squamous cell carcinoma of the head and neck (HNSCC) recur within two years of treatment. Due to ease of collection, saliva is of interest to monitor changes that correlate with treatment. Previously this was a challenge due to xerostomia following conventional radiation. The emergence of gland-sparing radiation has made it possible to collect saliva post-treatment.
Objective
This study investigated changes in cytokines in saliva pre- to post-treatment to provide foundational knowledge for future studies exploring the use of saliva to monitor treatment response.
Study Design
Pre- and post-treatment saliva was evaluated for eight cytokines by multiplex assay and ELISA.
Results
In oropharyngeal HNSCC, secretion of EGF, GROα, IL-1α, IL-1β, IL-6, IL-8, TNFα, and VEGF increased significantly post-treatment. In additional patients, significant increases of GROα and IL-6 were validated but EGF showed no change.
Conclusions
The uniqueness of this study is the comparison of salivary cytokines from HNSCC patients pre- and post-treatment.
Keywords: squamous cell carcinoma, pre- and post-treatment, GROα, IL-6, EGF
Introduction
Saliva has been eagerly pursued as a diagnostic fluid since its composition reflects disease or physiologic conditions.1 Saliva has several advantages, including non-invasive collection that does not require specialized training. In addition to secretions from the parotid, submandibular, sublingual and minor salivary glands, and proteins detectable in serum, whole saliva also contains proteins derived from desquamated epithelial cells, leukocytes, gingival sulcular fluid, and bronchial and nasal secretions.2 Therefore, saliva may be more sensitive than serum for detection of disease.
Salivary biomarkers, including proteins, RNA, metabolites and DNA, are active areas of interest for detection of head and neck cancer (HNSCC).1 HNSCC includes cancers that originate in the oral cavity, OP, larynx and hypopharynx.3 Cytokines, particularly inflammatory and angiogenic cytokines, have been investigated in saliva as potential protein biomarkers of HNSCC.4, 5 Sources of cytokines in HNSCC may be tumor cells and the immune response to the tumor.4 Cytokines, such as IL-6 and VEGF, have a role in cell growth, angiogenesis, invasion, immunosuppression and survival.6–8 Elevated levels of NF-κB-related cytokines such as IL-6, IL-8, HGF, and VEGF, have been detected in saliva of patients with HNSCC; the NF-κB pathway plays an important role in HNSCC development and progression.6, 9–14
Saliva studies have focused on relative levels of cytokines in saliva between HNSCC patients and healthy individuals.1 However, changes in saliva from pre- to post-treatment have not been extensively explored due to destruction of salivary glands and subsequent xerostomia with conventional radiation. This stalemate changed with the emergence of salivary gland-sparing radiation that allows saliva recovery.15–18 In a recent study on pre- and post-radiation parotid saliva from HNSCC patients, we showed qualitative recovery in post-treatment salivary EGF.19 Recent investigations suggest that a multiplex approach (e.g. evaluating multiple cytokines concurrently), will be more informative than a single biomarker.20 Hence, in this study we investigated a panel of cytokines in whole saliva from HNSCC patients, pre- and post-treatment. As the use of gland-sparing, intensity modulated radiation therapy (IMRT) increases, the findings of the present study will provide foundational knowledge for subsequent studies with larger patient populations.
Material and Methods
Patient Population
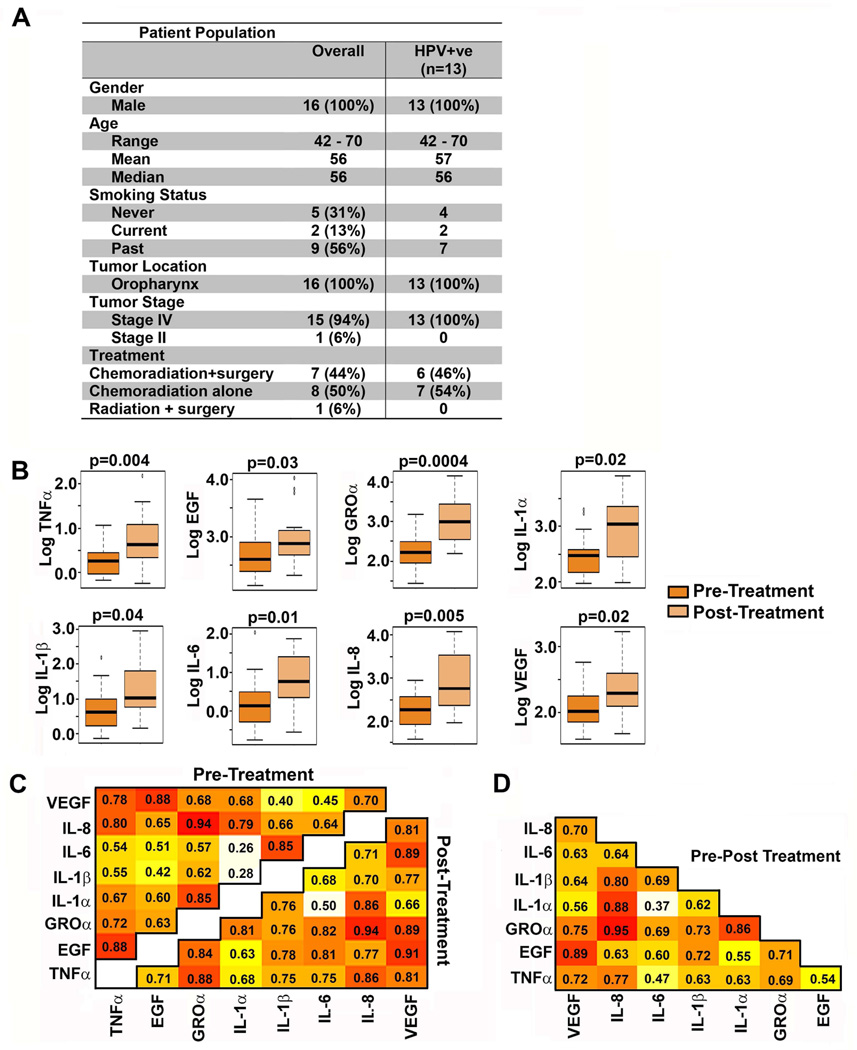
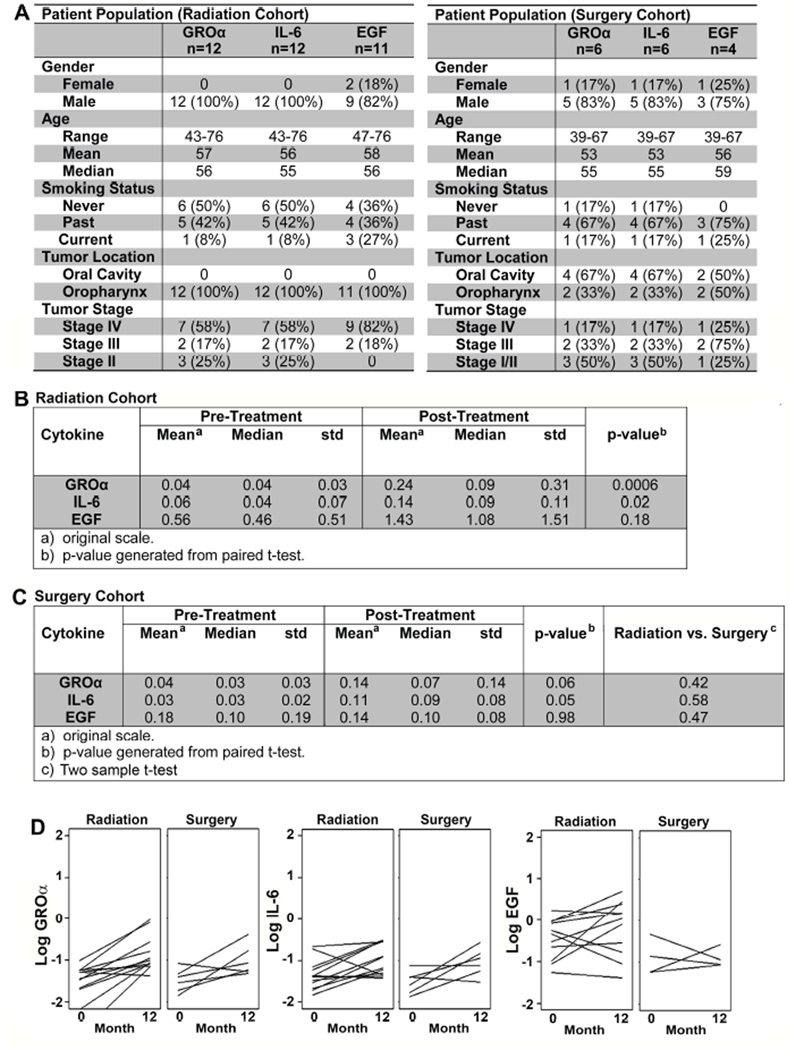
Approval was obtained from the Institutional Review Board (IRB) at the University of Michigan prior to the study. Saliva from patients from the University of Michigan SPORE prospective Epidemiology project who had signed an informed consent form, was evaluated (Fig. 1A). No patients with distant metastases at diagnosis were included. All patients received the treatment shown (Figs. 1A, 3A) and were alive and recurrence-free at 12 months. Saliva from sixteen patients was used for the initial cohort (Fig. 1A) and twelve and six treated with radiation and surgery, respectively, for the validation cohort (Fig. 3A).
Figure 1. Cytokine levels pre- and post-radiation.
A) Patient population demographics and treatment B) Boxplots of pre-treatment and post-treatment cytokines on a log-transformed scale. p-values from a paired t-test. C) Pearson’s correlation coefficients between cytokines pre-treatment (upper left corner) or post-treatment (lower right corner). D) Correlation coefficients from pre- to post-treatment. Darker shading signifies stronger positive correlation.
Figure 3. Validation of GROα, IL-6 and EGF in an independent radiation cohort.
A) Patient demographics within radiation (left panel) and surgery (right panel) cohorts. B and C) Mean, and standard deviation (std) of pre- and post-treatment GROα, IL-6 and EGF in: B) Radiation cohort. The original samples were analyzed using the Multiplex cytokine array and the validation cohort was analyzed via ELISA, hence the difference in scale. C) Surgery cohort. p-values from paired t-test. D) Pre-treatment and post-treatment cytokines in radiation and surgery cohorts. Each line represents a different patient.
Saliva Collection
Whole stimulated saliva was collected pre- and post-treatment (12 months post-diagnosis). Prior to collection, the patient rinsed with water (discarded). Salivary flow was stimulated by sugarless, flavorless gum by chewing for 2 minutes (no collection). For the next five minutes, under continued stimulation, saliva that accumulated in the mouth was expectorated into a tube on ice. The total volume was quantified and the amount secreted per minute (flow rate) was calculated. Saliva was centrifuged at 10,000xg for 20min. Protease and phosphatase inhibitors (1e−4U/ml aprotinin, 1.2mM Na3OV4, 0.1mg/ml PMSF) were added to the supernatant, which was aliquoted and frozen at −80°C.
Cytokine Multiplex Assay
Saliva was evaluated for EGF, GROα, IL-1α, IL-1β, IL-6, IL-8, TNFα, and VEGF using a MILLIPLEX MAP Cytokine/Chemokine Magnetic Bead Panel Immunology Multiplex Assay (Millipore, Billerica, MA) according to the manufacturer’s instructions. Briefly, standard controls or sample, and magnetic beads were added to each well. After incubation the wells were washed and detection antibodies were added. The plate was analyzed on a Luminex200 system (Millipore) by the Immunology Core (University of Michigan).
ELISA
GROα, IL-6 and EGF were analyzed by ELISA (Peprotech, Rocky Hill, NJ) per the manufacturer’s instructions. One sample was excluded from EGF analysis due to inadequate sample volume for the validation ELISA. Statistical analysis was performed as described below.
Statistical Analysis
The analysis comparing pre- to post-treatment groups was pre-specified by a statistician prior to analysis. The experiment was designed to have at least 80% power to detect change in cytokine levels of at least 1 standard deviation in magnitude allowing for a wide range of intra-subject correlation scenarios. Cytokine measures were log-transformed for statistical testing after normalization by time point specific volume (log(crude level/volume)). Grubbs test was applied to each measure to test for extreme outliers. One subject was identified as an outlier (extremely high) pretreatment in multiple cytokines (EGF, TNFa), however removal of this subject from the analysis did not affect any significance of results reported among all 16 subjects. The final analysis was performed on n=16 subjects (32 specimens). Pearson correlation coefficients were used to describe correlations among cytokines. Cytokine change from pre-treatment to post-treatment (12 months post-diagnosis) was first tested using unadjusted paired t-tests of log-transformed measures. Next, multivariable analyses controlling for stage, age and smoking were performed using linear mixed models with unstructured covariance matrices to account for within subject correlation of repeated measurements. Since each cytokine was analyzed individually, but was likely correlated with other cytokines, formal multiple testing corrections of the p-values may be overly conservative and were not performed. Instead, crude two-sided p-values are reported for interpretation by the reader. A two-sided p-value of 0.05, corresponding to a Type I error rate of 0.05, was considered significant for the purposes of discussion in the manuscript. All analyses were performed in SAS v.9.3 (SAS Institute Inc., Cary, NC).
Validation
An independent cohort of 12 patients (Fig. 3A, left panel) was chosen to validate the original cytokine array for GROα, IL-6 and EGF. One sample was excluded from EGF analysis due to inadequate sample volume for the validation ELISA. All patients with lesions in the OP location had radiation. Statistical analysis was performed as described above in the original cohort. To explore whether cytokine changes were dependent on treatment modality, an additional validation cohort (Fig. 3A, right panel) was selected independent of all previous patients. This cohort was composed of 6 patients who received only surgery. These saliva samples were also analyzed by ELISA for GROα, IL-6 and EGF. Similar to the previous two analyses, cytokine change from pre-treatment to post-treatment were tested in this cohort using unadjusted paired t-tests of log-transformed measures. Next, the two validation cohorts were combined and a t-test performed on the pre-post differences by treatment cohort.
Results
To determine treatment-related changes in cytokines, pre- and post-treatment saliva was collected (Fig. 1A). In initial studies, eight cytokines, TNFα, EGF, GROα, IL-1α, IL-1β, IL-6, IL-8, and VEGF were analyzed concurrently by multiplex analysis. Analyses were on 16 patients (pre- and post-treatment, i.e., 32 specimens). Mean cytokine levels, standard deviations, ranges and distributions are shown (Supplementary Fig 1). For all eight cytokines, log-transformed values were significantly higher post-treatment than pre-treatment (Fig. 1B).
To determine whether changes in salivary cytokines are a result of reduced fluid in post-treatment saliva, differential changes and correlations between cytokines were investigated pre- and post-treatment and normalized to volume. Pearson’s Correlation Coefficients (rho) were calculated to summarize the degree of linear dependence between two variables, if any, between two cytokines at pre- and post-treatment (Fig. 1C) and for the difference from pre- to post-treatment of the log-transformed measures (Fig. 1D). Strong pre-treatment correlations (rho≥0.8) were evident between TNFα and GROα with IL-8 (Fig. 1C). The pre-treatment correlations between TNFα and GROα with IL-8 were also strong post-treatment (Fig. 1C). In contrast, positive correlations such as those between TNFα and GROα with VEGF were evident post-treatment but were not strong pre-treatment. VEGF and EGF had strong positive correlations pre- and post-treatment (Fig. 1C). There were strong positive correlations between changes in VEGF with EGF as well as changes in IL-8 with IL-1β, IL-1α, and GROα (Fig. 1D). For example, patients with increasing IL-8 also tended to have increasing IL-1β, IL-1α, and GROα. Together these data showing differential changes between cytokines pre- and post-treatment.
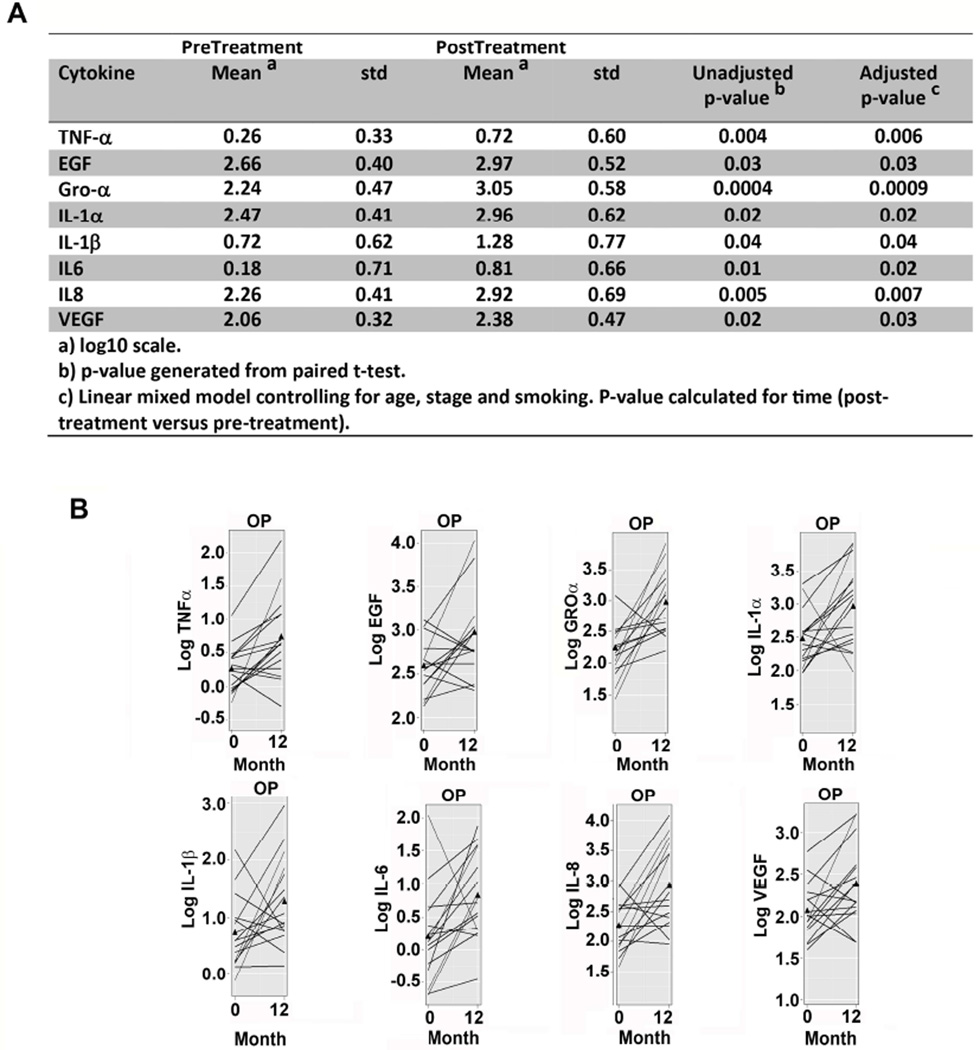
Cytokine changes observed earlier (Fig. 1B) were still significant after controlling for age, stage and smoking (Fig. 2A, linear mixed model). Individual patients were plotted to monitor for outliers (Fig. 2B).
Figure 2. Change in cytokines pre- to post-treatment.
A) Univariate and multivariate analyses for pre- to post-treatment change from linear mixed model. Information about the model is provided in the footnote. B) Pre-treatment to post-treatment volume normalized cytokine levels. Each line represents a different patient and values are log-transformed.
To validate the original dataset, an independent cohort of samples from patients with OP-HNSCC was used to investigate changes in GROα, EGF and IL-6. Age and gender distribution (Fig. 3A, left panel) were similar to the original group (Fig. 1A). Both GROα and IL-6 significantly increased in saliva post-treatment compared to pre-treatment (Fig. 3B, 3D) whereas there was no significant change in EGF (Fig. 3B).
The original patient cohort received radiation. To determine if radiation induces changes in cytokines, a third cohort of samples (Fig. 3A, right panel) from patients receiving surgery only was tested for GROα, IL-6 and EGF. Due to surgery alone being an unusual treatment option in OP-HNSCC, oral cavity cancers were included in this group. Results in the surgery cohort were similar to those in the original and validation cohorts. There was no significant change in EGF between pre- and post-treatment (Fig. 3C, 3D). GROα (p=0.06) and IL-6 (p=0.05) trended towards an increase but did not attain significance. Importantly, there were no significant differences between radiation and surgery cohorts (Fig. 3C). Together the data support that increases in cytokines in post-treatment saliva are independent of treatment modality.
Discussion
This study reports a post-treatment increase in multiple cytokines in stimulated whole saliva from patients with HNSCC. GROα, IL-1α, IL-1β, Il-6, IL-8, TNFα, and VEGF increased pre- to post-treatment. These findings may serve as the foundation of studies exploring the use of saliva as a biofluid to monitor response to treatment.
The patient group was consistent with the demographics for OP-HNSCC, which has a male predilection.6, 21 Eight cytokines, TNFα, EGF, GROα, IL-1α, IL-1β, IL-6, IL-8, and VEGF were analyzed because these cytokine are secreted in saliva from HNSCC patients9, 11–13, 22 and were quantifiable concurrently in a multiplex assay. The concurrent increase in IL-6 and IL-8 in post-treatment saliva suggests a common regulatory mechanism, such as NF-κB, which plays an important role in development and progression of HNSCC. NF-κB-regulated cytokines are upregulated in saliva in HNSCC.10
The radiation validation cohort was used to test conclusions about three cytokines from the initial test group in an independent sample of OP-HNSCC. The findings for GROα and IL-6 were validated but EGF showed no change. Cytokines in the initial test group were quantified using a multiplex cytokine array and in the validation group by ELISA for individual cytokines. Therefore, data from the initial and validation groups was not combined.
A surprising finding in the present study was the post-treatment increase in cytokines. Several studies have reported increases in salivary IL-8, TNFa, IL-1, IL-6, basic fibroblast growth factor and IL-1β.1, 9, 10, 13, 22 in HNSCC relative to normal patients but did not quantify changes post-treatment. The present study quantified cytokines pre- and post-treatment. IL-6 and GROα showed an increase post-treatment. This may be related to response to treatment. Other factors to consider are post-radiation mucositis which correlates with increases in cytokines.23 However, IL-6 was increased in patients who received surgery alone suggesting that this increase is related to response to treatment not radiation-induced inflammation. Of note, we previously reported that EGF in stimulated parotid saliva from HNSCC patients returned to baseline levels at 12 months post-treatment after an initial decrease at 6 months.19 These findings are similar to the present study in whole stimulated saliva, supporting our findings in two independent patient cohorts.
The differential changes observed between different cytokines pre- and post-treatment support that the changes are not entirely a function of reduced fluid content of post-treatment saliva, which would increase all cytokines. Furthermore, changes are not due to variations in salivary flow rate alone since all samples were normalized to flow rate. Moreover, since ionizing radiation increases expression of pro-inflammatory and pro-angiogenic cytokines,5 differences in cytokines between patients treated with radiation or surgery were investigated. In the surgical cohort of this study, combination of samples from different disease sites was necessitated by limitations of sample availability for the surgical cohort. Changes in cytokines did not vary as a function of treatment modality.
HNSCC patients may develop recurrent or second primary tumors3 highlighting the importance of permanently monitoring patients after treatment. However, repeated clinical visits maybe inconvenient, expensive and time-consuming, emphasizing the importance of developing alternative monitoring approaches. Ease of collection and changes in salivary composition in health versus disease have escalated the importance of saliva as a diagnostic fluid. When applied to a high-risk population such as HNSCC survivors, a saliva-based test utilizing a panel of biomarkers for HNSCC could provide an accurate, non-invasive and relatively inexpensive monitoring method. The initial step in developing such a test is characterizing changes in saliva post-treatment. Unfortunately, this has been a challenge likely due to unavailability of saliva. This xerostomia (perception of dry mouth) or salivary hypofunction (decreased salivary flow) due to destruction of salivary glands is the most common long-term complication of conventional radiation. The impact is usually permanent. Consequently, information about post-treatment salivary changes is sparse. IMRT, an emerging standard-of-care in HNSCC,24 spares salivary glands15–18 and is associated with preferential recovery of stimulated saliva whereas unstimulated volumes remain depressed.17 To maximize the likelihood of detecting changes pre- to post-treatment, we took advantage of post-IMRT recovery of stimulated saliva.
In the current study, cytokines were not investigated in healthy controls due to the focus on changes in cytokines in response to treatment, not as a diagnostic test for cancer. Indeed, a significant advantage of the current study was the longitudinal design, i.e. pre- and post-treatment comparison of the same patient. Most investigations of cancer biomarkers in saliva used a cross-sectional design making pre-treatment comparisons to healthy controls. Then patients were followed clinically to determine the prognostic value of pre-treatment biomarkers, but the biomarkers were not serially measured.6, 9–13, 25, 26 Pre-treatment levels of cancer biomarkers can vary widely in patients with the same type of cancer.27 Moreover, variations in cytokine secretion occur in individuals without cancer.1 For example, average IL-6 in whole unstimulated saliva in healthy individuals may vary from 1.4±0.9 to 47.46±18.74 pg/ml.1, 11 Therefore, we investigated differences in cytokines in pre- and post-treatment saliva in the same patient. IL-6 and IL-8 are elevated in common oral diseases such as periodontal disease and lichen planus.1 Although no dental findings were recorded as part of this study, an advantage was the comparison between saliva samples from the same patient.
Another limitation of this study was that cytokines in post-treatment saliva were measured at 12 months post-diagnosis. This may have been at different time points post-treatment if treatment was variably paced in different patients. We expect that most patients were treated at similar pace because of the single institution. However, if the cytokines varied with time post-treatment, this would not be taken into account.
Given the small sample size, the multivariable analyses (controlling for age, stage and smoking) of the original cohort were not intended to provide definitive conclusions, only evidence about whether the significant change reported is worthy of further exploration.
Although saliva exhibits protein changes in response to HNSCC,28 the mechanism is unclear. This is of particular interest when the biomarker decreases in cancer i.e. is there a negative feedback loop from HNSCC to the adjacent normal epithelium? Alternatively, is the protein secreted by normal salivary gland and does HNSCC provide feedback to salivary gland to decrease protein secretion? These issues remain to be answered.
Conclusions
Previously post-treatment saliva was not extensively explored because salivary glands are destroyed with conventional radiation. This will change as salivary gland-sparing radiation (IMRT) that allows saliva recovery, becomes more widely used. The uniqueness of the present study is the serial nature of the saliva samples from patients who have undergone salivary gland-sparing radiation. Results from a small group of patients (n=28) suggest that there are post-treatment increases in salivary cytokines. This study provides foundational knowledge for future large studies exploring the use of saliva as a diagnostic fluid to monitor response to treatment.
Supplementary Material
Statement of Clinical Relevance.
This study provides foundational knowledge for translational studies exploring the use of saliva to monitor response to treatment for patients with head and neck cancer.
Acknowledgments
Funding: This work was supported by NIDCR DE017977, DE019513 (NJD), Delta Dental Foundation, (NJD, CAM-K), NCI SPORE P50CA97248 (GTW) and NIH P30CA046592 (University of Michigan Cancer Center Immunologic Monitoring Core).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have no financial disclosures or conflicts of interest.
Presentation: This work was presented as a poster at the American Association of Dental Research meeting in March 2015.
References
- 1.Cheng YS, Rees T, Wright J. A review of research on salivary biomarkers for oral cancer detection. Clin Transl Med. 2014;3(1):3. doi: 10.1186/2001-1326-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castagnola M, et al. Potential applications of human saliva as diagnostic fluid. Acta Otorhinolaryngol Ital. 2011;31(6):347–357. [PMC free article] [PubMed] [Google Scholar]
- 3.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 4.Osman TA, Costea DE, Johannessen AC. The use of salivary cytokines as a screening tool for oral squamous cell carcinoma : A review of the literature. J Oral Maxillofac Pathol. 2012;16(2):256–261. doi: 10.4103/0973-029X.99083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Citrin DE, et al. Determination of cytokine protein levels in oral secretions in patients undergoing radiotherapy for head and neck malignancies. Radiat Oncol. 2012;7:64. doi: 10.1186/1748-717X-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy SA, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113(4):750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 7.Dendorfer U, Oettgen P, Libermann TA. Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol Cell Biol. 1994;14(7):4443–4454. doi: 10.1128/mcb.14.7.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee R, et al. The G protein-coupled receptor GALR2 promotes angiogenesis in head and neck cancer. Mol Cancer Ther. 2014;13(5):1323–1333. doi: 10.1158/1535-7163.MCT-13-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St John MA, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130(8):929–935. doi: 10.1001/archotol.130.8.929. [DOI] [PubMed] [Google Scholar]
- 10.Rhodus NL, et al. The feasibility of monitoring NF-kappaB associated cytokines: TNF-alpha, IL-1alpha, IL-6, and IL-8 in whole saliva for the malignant transformation of oral lichen planus. Mol Carcinog. 2005;44(2):77–82. doi: 10.1002/mc.20113. [DOI] [PubMed] [Google Scholar]
- 11.Rhodus NL, et al. NF-kappaB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect Prev. 2005;29(1):42–45. doi: 10.1016/j.cdp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Arellano-Garcia ME, et al. Multiplexed immunobead-based assay for detection of oral cancer protein biomarkers in saliva. Oral Dis. 2008;14(8):705–712. doi: 10.1111/j.1601-0825.2008.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katakura A, et al. Comparison of salivary cytokine levels in oral cancer patients and healthy subjects. Bull Tokyo Dent Coll. 2007;48(4):199–203. doi: 10.2209/tdcpublication.48.199. [DOI] [PubMed] [Google Scholar]
- 14.Allen C, et al. Nuclear factor-kappaB-related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clin Cancer Res. 2007;13(11):3182–3190. doi: 10.1158/1078-0432.CCR-06-3047. [DOI] [PubMed] [Google Scholar]
- 15.Eisbruch A, et al. Comprehensive irradiation of head and neck cancer using conformal multisegmental fields: assessment of target coverage and noninvolved tissue sparing. Int J Radiat Oncol Biol Phys. 1998;41(3):559–568. doi: 10.1016/s0360-3016(98)00082-0. [DOI] [PubMed] [Google Scholar]
- 16.Eisbruch A, et al. Parotid gland sparing in patients undergoing bilateral head and neck irradiation: techniques and early results. Int J Radiat Oncol Biol Phys. 1996;36(2):469–480. doi: 10.1016/s0360-3016(96)00264-7. [DOI] [PubMed] [Google Scholar]
- 17.Henson BS, et al. Two-year longitudinal study of parotid salivary flow rates in head and neck cancer patients receiving unilateral neck parotid-sparing radiotherapy treatment. Oral Oncol. 1999;35(3):234–241. doi: 10.1016/s1368-8375(98)00104-3. [DOI] [PubMed] [Google Scholar]
- 18.Ship JA, et al. Parotid sparing study in head and neck cancer patients receiving bilateral radiation therapy: one-year results. J Dent Res. 1997;76(3):807–813. doi: 10.1177/00220345970760031401. [DOI] [PubMed] [Google Scholar]
- 19.Murdoch-Kinch CA, et al. Recovery of salivary epidermal growth factor in parotid saliva following parotid sparing radiation therapy: a proof-of-principle study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(1):64–70. doi: 10.1016/j.tripleo.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Linkov F, et al. Early detection of head and neck cancer: development of a novel screening tool using multiplexed immunobead-based biomarker profiling. Cancer Epidemiology Biomarkers & Prevention. 2007;16(1):102–107. doi: 10.1158/1055-9965.EPI-06-0602. [DOI] [PubMed] [Google Scholar]
- 21.Carvalho AL, et al. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. International journal of cancer. 2005;114(5):806–816. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 22.Brinkmann O, et al. Oral squamous cell carcinoma detection by salivary biomarkers in a Serbian population. Oral oncology. 2011;47(1):51–55. doi: 10.1016/j.oraloncology.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein JB, et al. The correlation between epidermal growth factor levels in saliva and the severity of oral mucositis during oropharyngeal radiation therapy. Cancer. 2000;89(11):2258–2265. doi: 10.1002/1097-0142(20001201)89:11<2258::aid-cncr14>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 24.Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the U.S., 2004. Cancer. 2005;104(6):1296–1303. doi: 10.1002/cncr.21284. [DOI] [PubMed] [Google Scholar]
- 25.Kim CH, et al. Serum hepatocyte growth factor as a marker of tumor activity in head and neck squamous cell carcinoma. Oral Oncol. 2007;43(10):1021–1025. doi: 10.1016/j.oraloncology.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Franzmann EJ, et al. Soluble CD44 is a potential marker for the early detection of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1348–1355. doi: 10.1158/1055-9965.EPI-06-0011. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen ME, Partin AW. The impact of definitions of failure on the interpretation of biochemical recurrence following treatment of clinically localized prostate cancer. Rev Urol. 2007;9(2):57–62. [PMC free article] [PubMed] [Google Scholar]
- 28.Hu S, Wong DT. Oral cancer proteomics. Curr Opin Mol Ther. 2007;9(5):467–476. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.