Abstract
Androgenesis is a form of quasi-sexual reproduction in which a male is the sole source of the nuclear genetic material in the embryo. Two types of androgenesis occur in nature. Under the first type, females produce eggs without a nucleus and the embryo develops from the male gamete following fertilization. Evolution of this type of androgenesis is poorly understood as the parent responsible for androgenesis (the mother) gains no benefit from it. Ultimate factors driving the evolution of the second type of androgenesis are better understood. In this case, a zygote is formed between a male and a female gamete, but the female genome is eliminated. When rare, androgenesis with genome elimination is favoured because an androgenesis-determining allele has twice the reproductive success of an allele that determines sexual reproduction. Paradoxically, except in hermaphrodites, a successful androgenetic strain can drive such a male-biased sex ratio that the population goes extinct. This likely explains why androgenesis with genome elimination appears to be rarer than androgenesis via non-nucleate eggs, although both forms are either very rare or remain largely undetected in nature. Nonetheless, some highly invasive species including ants and freshwater clams are androgenetic, for reasons that are largely unexplained.
This article is part of the themed issue ‘Weird sex: the underappreciated diversity of sexual reproduction’.
Keywords: Cupressus dupreziana, Corbicula spp., Wasmannia auropunctata, Vollenhovia emeryi, Paratrechina longicornis, Bacillus spp.
1. Introduction
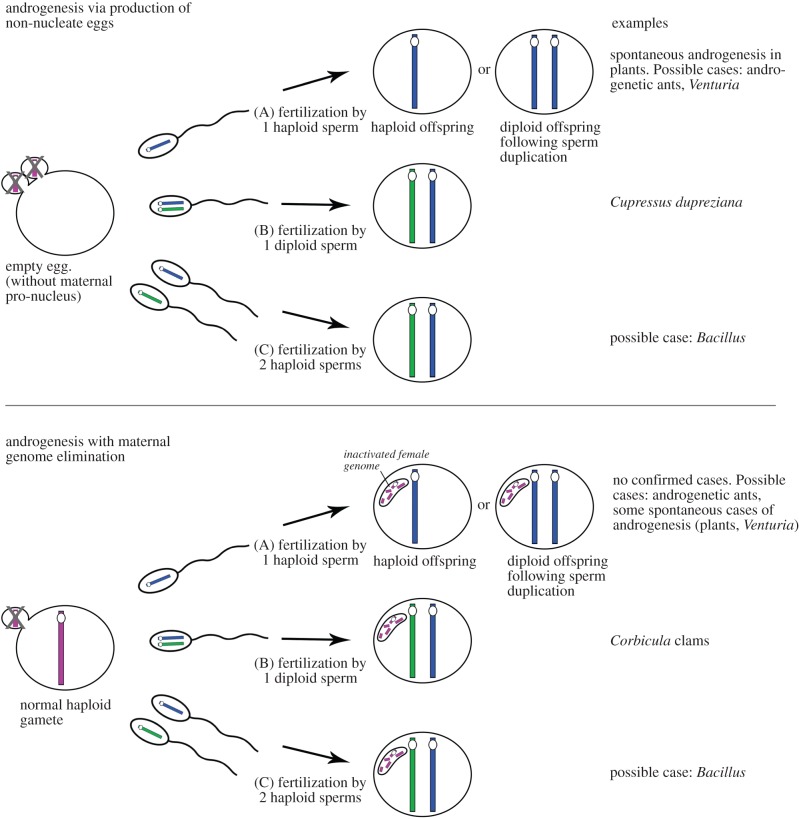
Androgenesis is a genuinely weird form of quasi-sexual reproduction of some eukaryote species in which a male's gamete develops to produce a new male, a clone or sub-clone of his father. In its original definition, androgenesis referred to when a sperm or pollen nucleus fuses with an egg nucleus, but the nuclear genome of the mother is eliminated by the paternal genome (figure 1) [1]. Alternative mechanisms of androgenesis are, however, recognized [2], particularly the development of an embryo from a male gamete in an egg that lacks a maternal nucleus (see §2; figure 1). When such a ‘non-nucleate’ egg is fertilized by sperm or by pollen it develops into an offspring whose entire nuclear genome is of paternal origin. Both types of androgenesis (with and without selfish elimination of the female genome driven by the male) require diploidization of the paternal genome, or the ability of the gamete to develop and survive as a haploid male. As we explain in the following sections, whether and how diploidization occurs, and under which form of sex determination, greatly affects the predicted fate of androgenetic lineages.
Figure 1.
Different forms of androgenesis.
While androgenesis is rare in nature, it is ‘normal’ in some natural populations, including clams of the genus Corbicula [3–5], a conifer [6–8], a few ants [9–12] and stick insects [13–16], and most likely an Australian carp gudgeon [17] (table 1). Androgenesis is also seen sporadically in some species, particularly hymenopterans (ants, bees and wasps), and in some monocots, dicots and gymnosperms (table 1). Interestingly, androgenesis appears to be overrepresented among taxa that feature other unusual forms of reproduction such as hybridogenesis (elimination of a parental genome, usually the paternal one, from the germline) and parthenogenesis (asexual reproduction by females). Natural androgenesis is also commonly associated with inter-species hybridization. Finally, androgenesis can be induced artificially under laboratory settings, and is used in plant, fish and silkworm breeding to propagate desirable genotypes and to develop mono-sexual populations.
Table 1.
Documented cases of asexual reproduction by males. ‘?’ stands for unknown or uncertain cases. Drawings courtesy of Loren Bes. (Online version in colour.)
| image | species | androgenesis mechanism | diploidization mechanism | characteristics of the sexual system | reproductive polymorphism |
|---|---|---|---|---|---|
 |
Cupressus dupreziana [6–8,18–20] | non-nucleate oocytes | diploid pollen | hermaphrodites | androgenesis fixed |
 |
Corbicula clams [3–5,21–25] | genome elimination | diploid sperm | hermaphrodites | androgenetic and sexual strains/populations |
 |
Wasmannia auropunctata [9] | ? | n.a. (male haploidy) | haplo-diploidy | androgenetic and sexual strains/populations |
| Vollenhovia emeryi [10,11] | ? | n.a. (male haploidy) | haplo-diploidy | ? | |
| Paratrechina longicornis [12] | ? | n.a. (male haploidy) | haplo-diploidy | ? | |
 |
Bacillus rossius-grandii hybrids [13–16,26] | non-nucleate oocytes? | fusion of two sperm cells and sperm duplication | separate male and female sexes, male heterogamety | spontaneous/facultative androgenesis |
 |
Hypseleotris carp gudgeons [17,27] | genome elimination (in germline only) | n.a. (maternal genome used in somatic cells) | separate male and female sexes | sexual lineages, female and male hybridogens |
 |
Apis mellifera [28] | ? | n.a. (male haploidy); fusion of two sperm cells for female tissues | haplo-diploidy | spontaneous androgenesis |
 |
Venturia canescens [29] | non-nucleate oocytes? | n.a. (male haploidy) | haplo-diploidy | spontaneous androgenesis |
 |
Hybrids between different Nicotiana species [30–35] | ? | absent (haploid androgens in a normally diploid species) | hermaphrodites | spontaneous androgenesis |
 |
Hybrids between Poa arachnifera and related species [30] | ? | absent (haploid androgens in a normally diploid species) | hermaphrodites | spontaneous androgenesis |
 |
Hybrids between Solanum verrucosum and related species [30,36] | ? | absent (haploid androgens in a normally diploid species) | hermaphrodites | spontaneous androgenesis |
 |
Pripsacum dactyloides × Zea mays hybrids & Zea mays [37–39] | ? | sperm duplication or absent (haploid androgens in a normally diploid species) | hermaphrodites | spontaneous androgenesis |
| Drosophila laboratory mutants [40] | ? | sperm duplication | separate male and female sexes, male heterogamety |
A form of incomplete androgenesis occurs rarely but spontaneously in some haplo-diploid insects. Some individuals, known as gynandromorphs, are mosaics of diploid female tissue (arising from the zygote) and haploid male tissue (arising from the division of a secondary sperm cell) (figure 1) [41,42]. As discussed in §3, androgenesis in mosaics may be mechanistically linked to androgenesis sensu stricto and could function as a first step towards androgenesis.
In this review, we examine the fascinatingly weird phenomenon of androgenesis. We survey the distribution of androgenesis in nature and explore the kinds of conditions that favour and constrain its emergence. We then speculate about why natural androgenesis is often associated with interspecific hybridization. We conclude with a discussion of the role of androgenesis in the generation of invasive species, and conversely, how we might use androgenesis to promote conservation efforts.
2. Androgenesis sensu stricto
(a). The conifer Cupressus dupreziana
Cupressus dupreziana is a very rare, hermaphroditic conifer that occurs only in the mountains of the Tassili N'Ajjer desert, in the Algerian Sahara [18]. Androgenesis is its sole form of reproduction, with diploid pollen providing all nuclear genes for the diploid offspring [6–8]. Because C. dupreziana also features paternal inheritance of mitochondria and chloroplasts (as do all species in the subfamily Cupressoideae), the reproductive system of C. dupreziana comes closest to the term ‘male asexuality’, which is sometimes used synonymously for androgenesis. The case of C. dupreziana is frequently cited as the prime example of selfish maternal genome elimination by males (e.g. [43,44]). However, androgenesis in C. dupreziana does not involve genome elimination. Instead, ovules are non-nucleate: they do not have a nucleus but only the tissues that eventually give rise to the endosperm (the nutritious tissue of the seed) and other components of the future seed [6,19]. The embryo itself, including mitochondria and chloroplasts, is entirely derived from diploid pollen. How such diploid pollen is produced (i.e. meiotically or ameiotically) remains unknown [7].
Unless C. dupreziana mostly self-fertilize, it is puzzling that these hermaphroditic trees continue to produce seeds, since no genes (nuclear or cytoplasmic) are contributed to future generations via seeds. Seeds are a resource shared at the population level that can be exploited by other individuals. Trees with reduced investment in ovules should be favoured, which should eventually lead to the extinction of the species (see §5). Consistent with this view, seed production in C. dupreziana is greatly reduced relative to other conifers. The majority of seeds produced by C. dupreziana are empty, only 20% contain an endosperm, and only half of the functional seeds contain an embryo [19]. Unfortunately, many aspects of the biology of C. dupreziana remain unknown, including, for example, why and how solely non-nucleate ovules are produced and whether the species is generally selfing or outcrossing.
One phenomenon that may contribute to the (at least transient) persistence of androgenesis in C. dupreziana is that C. dupreziana pollen can use other species as seed hosts [43]. For example, C. dupreziana can produce androgenetic offspring by pollinating ovules of the related species C. sempervirens, suggesting that C. sempervirens also produces a significant fraction of non-nucleate ovules, or that C. dupreziana is able to eliminate the C. sempervirens genome from the ovule [20,43]. Cupressus sempervirens can also generate haploid and diploid androgenetic offspring from C. dupreziana ovules [6], although C. sempervirens is only a spontaneous androgen and it remains unknown how offspring diploidy is restored in this case—C. sempervirens produces only haploid pollen [6].
(b). Clams of the genus Corbicula
Corbicula is a genus of freshwater clams and comprises a mixture of hermaphrodite species and species with separate male and female sexes [21,45]. Within the current (and largely unresolved) taxonomy, at least four morphologically distinct, hermaphroditic species reproduce via androgenesis. For two of the four species (C. leana and C. fluminea), androgenesis was identified through cytological examination of fertilization events [3–5]. For two additional species (C. australis and C. fluminalis), androgenesis is inferred from the observation of extremely low genetic diversity, polyploidy and biflagellate sperm (a presumed biomarker of androgenesis [46]).
The mechanisms behind androgenetic reproduction in C. leana and C. fluminea are well characterized and have been reviewed several times [2,47,48]. After fertilization by a diploid (or polyploid) sperm, the maternal nuclear genome is eliminated from the oocyte via two polar bodies [3–5] so that the only maternal genetic material retained resides in the mitochondria. The diploid or polyploid sperm have the same DNA content as somatic cells and presumably arise by an ameiotic process, the details of which are unknown [22].
Most androgenetically produced offspring are derived from self-fertilization, but outcrossing occurs at least occasionally [49]. Outcrossing occurs not only between individuals within a species. Eggs of other species can also be parasitized, a strategy that is especially efficient in androgens with widespread geographical distributions [23,50]. Androgenetic parasitism of other species does not always result in complete maternal genome elimination. Indeed, partial retention of different maternal genomes is likely to have contributed to the considerable genetic divergence between androgenetic strains and species [2,24,48]. In the most extreme case, maternal genome elimination fails completely and the haploid maternal nucleus fuses with the diploid sperm, resulting in polyploid strains [25].
The number of independent origins of androgenesis in Corbicula is unclear. Reliable phylogenetic inference is difficult given that multiple introgression events can generate polyphyletic gene trees for androgenetic strains that actually share a single origin [24,48].
(c). Androgenetic ants
Androgenesis has evolved independently in at least three ant species: Wasmannia auropunctata (the little fire ant) [9], Vollenhovia emeryi [10,11] and Paratrechina longicornis (the longhorn crazy ant) [12]. In all these species, sterile workers are produced via normal sexual reproduction involving queens and males. Daughter queens are produced via thelytokous parthenogenesis and are clones or sub-clones of their mothers, while males are produced androgenetically and are genetically identical to their fathers. The production of queens via parthenogenesis, workers via sex and males via androgenesis is obligate in at least some populations. This bizarre form of reproduction means that the queen and male lineages diverge genetically over time, as the only sexually produced individuals (workers) do not reproduce.
The mechanisms underlying androgenesis in these ant species are unknown. It is possible that sexual reproduction between queens and males (which usually generates workers) generates males if the queen's genome is successfully eliminated from the eggs [9]. Alternatively, it has been suggested that queens of at least one species (W. auropunctata) produce a small proportion of eggs that are non-nucleate (similar to the mechanism in Cupressus), and that these non-nucleate eggs give rise to androgenetic males if fertilized by sperm [51]. Given that queens of most ant species are highly fecund, the costs of producing non-nucleate eggs may be negligible if their number is low. It may even be possible that queens have control over the number of non-nucleate eggs that they lay, and adjust the number according to colony need, but there are currently no data available to test these hypotheses.
Ants, like all Hymenoptera, are characterized by haplo-diploid sex determination, where males develop from haploid (unfertilized) eggs and females from diploid (fertilized) ones. Therefore, androgenesis in ants does not require a mechanism to generate a diploid genome from a usually haploid sperm; an egg comprising a haploid genome will automatically develop into a male. Haplo-diploidy, therefore, provides an exaptation that increases the likelihood that androgenesis will emerge (see also §5).
(d). Androgenetic stick insects
A genus of Mediterranean stick insects (Bacillus spp.) is characterized by several unusual reproductive modes, one of which is androgenesis (reviewed in [52]). In Sicily, different hybridization events between females of the species B. rossius and males of the species B. grandii gave rise to several F1 hybrid lineages consisting almost exclusively of females (the few males that occur in natural populations are sterile). Genetic and cytological analyses have revealed that the typical reproductive mode of these females is hybridogenesis [13–16]. The paternal B. grandii genome is eliminated from the oocytes of F1 hybrid females, so that only the maternal B. rossius genome is transmitted to the next generation. The hybrid females then mate with sympatric B. grandii males, which restores the F1 hybrid ancestry in their offspring.
In addition to the elimination of the paternal genome from all oocytes, the maternal genome is eliminated in up to 20% of a hybrid female's eggs, resulting in non-nucleate eggs [14]. It is not known whether the elimination (or inactivation) of the maternal genome is controlled by the sperm, or whether it may happen accidentally as a consequence of an imperfect mechanism underlying the elimination of the paternal genome. If a non-nucleate egg is fertilized, it can develop into a diploid progeny comprising solely the genome of the B. grandii father. If a single sperm fertilizes a non-nucleate egg, the sperm can undergo duplication and generate a diploid but fully homozygous zygote. At least a subset of these homozygous individuals successfully reproduce under laboratory conditions, but their viability in natural populations is unknown. However, because stick insects are polyspermic (multiple spermatozoa enter every egg [53]), it is common for two sperm cells to fuse in a non-nucleate egg and give rise to a heterozygous diploid zygote [13,14].
Because stick insects are male heterogametic, the fusion of two sperm cells can generate either a female or male androgenetic offspring. The production of both sexes via androgenesis facilitates the persistence of androgenesis over evolutionary timescales; females do not disappear from androgenetic populations. Both males and females that are produced via androgenesis carry a diploid B. grandii genome and reproduce via normal sexual reproduction with other B. grandii individuals. The only difference between androgenetically produced B. grandii individuals and individuals from the normal B. grandii sexual population is that the former carry mitochondria from the species A. rossius instead of B. grandii. In natural populations, individuals with a complete B. grandii nuclear genome and a mitochondrial A. rossius genome are rare [26], suggesting that androgenetic reproduction is only sporadic in Bacillus.
Based on karyotype and genotype data from field-caught insects, similar forms of androgenesis may occur in the stick insect genera Leptynia and Clonopsis [54–56]. These suggestions remain speculative, as there are no data available (e.g. from crosses) that confirm androgenetic reproduction in these taxa.
(e). Carp gudgeons of the genus Hypseleotris
There are three distinct, co-occurring carp gudgeon lineages in the Murray River in South Australia [17,27]. One of the lineages consists almost exclusively of males, suggesting some form of asexual reproduction by males [17]. Detailed analyses of genotypes suggest that the male lineage most likely reproduces via hybridogenesis [17,27]. Males most likely eliminate their maternal genome during spermatogenesis and mate with females of a co-occurring sexual lineage. The paternal haploid male genome is thus clonally transmitted between generations.
There are two differences between male hybridogenesis and androgenesis. The first is that under hybridogenesis, males are not clones or sub-clones of their father because hybridogenetic males develop from fertilized eggs. These eggs comprise the clonally transmitted paternal genome and a sexual (i.e. variable) maternal genome. The second difference is the type of genome elimination. Under androgenesis there is either no genome elimination (in the case of non-nucleate eggs) or the maternal genome is eliminated upon fertilization. Under male hybridogenesis, the maternal genome is eliminated during spermatogenesis in the male gametes, but the maternal genome present in the egg is maintained. Despite these differences, the carp gudgeon example is included here as androgenesis and hybridogenesis are clearly related forms of reproduction given the asexual transmission of the paternal genome and the occurrence of genome elimination in some cases.
Interestingly, there is also a female-only carp gudgeon lineage that most likely also reproduces via hybridogenesis [17]. Females of this lineage eliminate the paternal genome during oogenesis and clonally transmit the haploid maternal genome, similar to female hybridogenesis in Bacillus stick insects (see §2d). Eggs are then fertilized via matings with the same sexual lineage whose eggs are parasitized by the hybridogenetic males.
Hybridogenesis by males and females in gudgeons remains to be confirmed via controlled crosses combined with genetic analyses of offspring. It will be interesting to see whether genome elimination of the male and female genomes occurs via the same or similar mechanisms. A common mechanism seems likely as hybridogenesis by both males and females suggests that the genetic factors controlling hybridogenesis introgressed from one genome into the other. Such introgression may be ongoing, as F1 hybrids between the male and female hybridogens are observed at low frequency [17]. These ‘hybrids of hybridogens’ are mostly, though not exclusively, female, but their mode of reproduction remains unknown [17].
(f). Spontaneous androgenesis in Apis mellifera and Venturia canescens
Koeniger et al. [28] identified three honeybee (Apis mellifera) queens from a population of 60 that produced gynandromorph (see Glossary and §3) offspring at high frequency (13–67%). In addition to their tendency to produce gynandromorphs, these queens were homozygous for a mutation, cordovan, which causes a reddish-brown body colour. A small number of males produced by these queens showed no evidence of female tissue (i.e. they were not cordovan in whole or part) and were uniformly black. Koeniger et al. concluded that these black males had developed from sperm nuclei via androgenesis. The possibility that the males had been laid by workers was excluded because after the queens were fostered into colonies of yellow workers they again produced some black males.
Spontaneous androgenesis also occurs in another haplo-diploid insect, the parasitoid wasp Venturia canescens. Scheider et al. [29] mated thelytokous females to males of a sexual strain to determine whether thelytokously produced eggs retain the ability to incorporate a paternal genome. In 70% of crosses, all offspring were females that did not carry any paternal alleles, as expected given thelytoky in their mothers [29]. In the remaining crosses, 16% of offspring carried paternal alleles indicating that some ‘thelytokous’ females were capable of fertilizing their eggs. The same pattern is seen in Cape honeybee queens [57], although in the vast majority of thelytokous species females are unable to fertilize their eggs [58]. Interestingly, two offspring produced by mated thelytokous female wasps were male, carrying solely paternal alleles. Although Schneider et al. had no explanation for this finding, we speculate that these androgenetic males developed because the thelytokous females lay a small proportion of non-nucleate eggs that can develop into males carrying the paternal genome if successfully fertilized. Alternatively, Venturia may represent a case of spontaneous, male-mediated maternal genome elimination.
These remarkable findings serve to show that androgenesis can occur spontaneously, at least in haplo-diploid species. Such spontaneous cases could be co-opted into androgenesis sensu stricto, as described above, provided that there is genetic variation for the rate of spontaneous androgenesis among individuals. This would require the production of non-nucleate eggs at high frequency or maternal genome elimination by males.
(g). Spontaneous androgenesis in flowering plants
Several cases of spontaneous androgenesis are known from plants. In the majority of botanical cases, the androgenetically produced offspring are haploid, even though species that feature spontaneous androgenesis are typically diploid (reviewed in [30]). Thus, there is generally no male genome duplication associated with spontaneous androgenesis in plants, although exceptions exist as, for example, in C. sempervirens as described above (§2a). Furthermore, few cases are known where haploid pollen cells duplicate and give rise to diploid offspring that are completely homozygous (e.g. in maize, Zea mays [37]). The frequency of spontaneous production of haploid (or rarely diploid), androgenetic offspring is typically low, ranging from 0.04% in Nicotiana tabacum (tobacco) to 0.0005–0.001% in maize [31,38,39].
In plants, spontaneous haploid androgenesis is often associated with inter-population or interspecific hybridization. For example, androgenetic offspring are produced by several hybrids of Capsicum frutescens (pepper) [59], crosses between widely divergent strains of Cicer arietinum (chick pea) [60] and Brassica napus (oilseed rape) [61] and by interspecific Nicotiniana hybrids: N. digluta × N. tabacum [32], N. tabacum × N. langsdorfii [33], N. glutinosa × N. repanda [34] and N. sylvestris × N. tabacum [35]. We speculate that hybrid individuals produce androgenetic offspring because genetic divergence between genomic regions greatly reduces the local rate of recombination [62]. As a consequence, genome divergence between parental genomes in F1 hybrid females may impair meiosis and lead to a higher proportion of non-nucleate eggs than in non-hybrid females—a situation ripe for the androgenetic development of a pollen cell.
3. Androgenesis in gynandromorphs and other mosaics
Gynandromorphy (mosaics of male and female tissue in one organism) can arise as an incomplete form of androgenesis. In Hymenoptera, gynandromorphy sometimes arises as a consequence of polyspermy [42]. That is, an egg is fertilized by a sperm, giving rise to female tissue. The male tissue arises from division of a second (or perhaps more) sperm cell(s) so that part of the embryo derives from the zygote, and is therefore diploid and female, whereas other parts derive from a second sperm and are haploid and male [63,64]. Clearly, the male tissue is androgenetic [63], though this is not usually of evolutionary consequence as such individuals are unlikely to reproduce. Nevertheless, if gynandromorphy goes ‘all the way’, as it were, then we would have a case of androgenesis. It is, therefore, appropriate to consider species where gynandromorphy occurs spontaneously and at high frequency as a possible evolutionary route to androgenesis, and to explore the factors that increase the frequency of gynandromorphy [65].
Although factors increasing gynandromorphy are poorly understood, new insights could be developed from comparative studies as gynandromorphs are regularly reported across a broad range of Hymenoptera, including bees (e.g. [66–72]), wasps (e.g. [73–78]) and more than 40 ant species (e.g. [65,79–81]). These cases do not include a further variant of mosaic androgenesis that arises in honeybees when two sperm pronuclei fuse within an egg [82]. In these individuals, some female tissue is derived from the zygote (the union of an egg and sperm pronucleus), whereas other female tissue is derived from the union of two accessory sperm cells.
Gynandromorphs are also reported in fish (e.g. [83]), lepidopterans (e.g. [84]), birds (e.g. [85]) and flies [86], but the male tissue in these creatures does not arise androgenetically, but from the zygote, with errors in the expression of sex determining genes. Gynandromorphy of this kind does not provide an easy evolutionary route to androgenesis and is not further discussed here.
4. Artificial induction of androgenesis
(a). Induction of androgenesis in fishes
Androgenesis (and gynogenesis) can be artificially induced in some fishes that have external fertilization [87]. Such artificial androgenesis is used in aquaculture to produce clonal lineages featuring commercially interesting traits. Briefly, eggs or sperm are irradiated to fragment DNA and render the gamete non-nucleate. Next, the treated gamete is fused with an egg or sperm to produce a haploid embryo. Diploidy is then restored by heat or pressure shock during the first mitotic division, which induces the mitotic products to fuse and generate a diploid and fully homozygous individual [87]. In salmonids, approximately 30% of eggs so treated result in viable diploid offspring [88].
(b). Induction of androgenesis in plants
In plant breeding it is often desirable to obtain inbred lines that are then outcrossed to produce hybrids for farmers' fields. Creation of inbred lines by repeated backcrossing or selfing is tedious, so induction of androgenesis and the production of doubled haploids can provide a welcomed shortcut [36].
Androgenesis can sometimes be induced in vivo via irradiation or temperature-shock treatments in plants (e.g. in Crepis tectorum [89] and Antirrhinum majus [90], cited in [30]). More often, androgenesis is induced in vitro via a process known as anther culture (reviewed in [91]). The process begins by pre-treating anthers with cold or heat shock to help initiate mitotic cell division, followed by removal of the microspores (immature pollen cells) so treated, and culture of the microspores on synthetic media. Diploidization often occurs spontaneously due to abnormal mitosis in the callus that forms in the media from each microspore [91]. Artificial androgenesis requires induction of the developmental switch from the gametophytic to the saprophytic developmental pathway.
5. The rise and fall of new androgenetic lineages
(a). Androgenesis is transient for most systems with genome elimination
Theory predicts that androgenesis with genome elimination is likely to be transient over evolutionary time. McKone & Halpern [47] modelled the invasion of an allele causing androgenesis via maternal genome elimination under various scenarios. When rare, an androgenesis-causing allele is expected to spread in a population of gonochorists (separate male and female sexes) because a male carrying such an allele produces offspring that carry twice the number of paternal alleles relative to the offspring of a male that does not carry the androgenesis-causing allele. Under most circumstances, androgenesis-causing alleles can spread in a population even if they are detrimental to female function. When androgenesis-causing alleles reach high frequencies, egg hosts become a limiting resource because most individuals in the androgenetic population are male. If androgenesis drives extinction of females in a population, then the population itself will go extinct.
In contrast with gonochorists, androgenesis in hermaphrodites may persist at least over ecological time frames [47]. Indeed, the spread of androgenesis-causing alleles will not necessarily lead to extinction in hermaphrodites because all individuals carry a female function. There may be selection for reallocation of resources from female to male functions but the female function is less likely to be completely lost in hermaphrodites than in species with separate sexes. The hermaphrodite clam Corbicula (§2b), for example, is invasive worldwide, suggesting that the cost to female function imposed by androgenesis is low.
Rapid extinction of new androgenetic lines in gonochorists is most likely part of the reason why androgenesis is rare in nature. It may arise from time to time, but when it does, it goes to fixation and drives extinction. Unfortunately, the frequency of androgenesis cannot be estimated from the few known cases described here. Detection of rare or spontaneous androgenesis requires laboratory crosses and detailed genetic analyses that have simply not been done in the vast majority of species. Furthermore, genotype patterns indicative of androgenesis may often be misinterpreted or discarded as ‘technical errors’.
There is currently no theoretical framework for population dynamics of androgenesis from non-nucleate oocytes (i.e. without male-controlled genome elimination). Although male alleles also benefit from increased transmission rates under androgenesis with non-nucleate oocytes, this form of androgenesis cannot occur at high frequencies because it is not caused by alleles expressed in males. Females that produce non-nucleate eggs may spread in a population via drift or if there is a (currently unknown) benefit associated with the production of such eggs. However, this will only happen while the proportion of non-nucleate eggs remains low (or the costs for females will be too high). As a consequence, systems relying on non-nucleate oocyte production are less likely to go extinct than androgenesis systems with genome elimination.
Indeed, the production of non-nucleate oocytes has to be regulated by alleles expressed in females. Because such expression reduces female fitness, androgenesis via non-nucleate eggs should usually be maladaptive. Cases of spontaneous androgenesis would thus represent accidental meiotic errors during oocyte production. This idea is supported by the observation that androgenesis (and especially spontaneous cases) are more common in hybrids (table 1) where meiotic errors are expected to be more frequent because genetic divergence causes problems during homologous recombination [62]. Even if maladaptive, there may be segregating genetic variation for the proportion of non-nucleate eggs females produce. If this is the case, then we predict that spontaneous androgenesis should be more frequent in highly fecund species, as the costs associated with the production of a small number of non-nucleate eggs would be low.
One could also imagine a scenario where the production of non-nucleate ovules is a trait that evolved after androgenesis with genome elimination has become fixed. For example, in the hermaphrodite conifer C. dupreziana, where androgenesis arises from the production of non-nucleate ovules, it is difficult to see how such a system can reach fixation and persist. No alleles are transmitted via non-nucleate ovules; hence investment in such ovules should be reduced and a reallocation of resources to the male function favoured, leading to loss of female function. A possible scenario of androgenesis in C. dupreziana is that androgenesis at its origin would have involved genome elimination. If individuals are largely selfing, the production of non-nucleate ovules may endure fewer costs than genome elimination in virtually all ovules. As such, non-nucleate ovule production would have followed the evolution and fixation of androgenesis via genome elimination.
(b). Interaction between androgenesis and sex determination systems in species with separate sexes
For androgenesis from non-nucleate oocytes/ovules, we do not expect differences in the types of sex determination to strongly affect the spread and persistence of androgenesis. By contrast, the sex determination system has a strong effect on the spread and fate of androgenesis with genome elimination. As we saw in §5a, in diploid species with separate sexes, alleles that cause androgenesis with genome elimination can spread even if there is a cost to females [47]. The fate of such species depends on how diploidy is restored in offspring without the contribution from females. If androgenetic offspring are diploid because males produce diploid gametes without meiosis, all offspring of males are male. This will rapidly drive extinction of the population due to a lack of females to produce eggs [47]. As expected, androgenesis of this form is unknown from natural systems.
If androgenetic offspring are diploid because two sperm cells fuse or because a single sperm cell duplicates, androgenesis is more likely to be maintained, at least in species with male heterogamety (XX females and XY or XO males). When diploidy arises via doubling of meiotic products, males will only have female (XX) offspring because YY or OO offspring are inviable. This greatly reduces or eliminates the likelihood that androgenesis-causing alleles can invade, and reduces the likelihood of extinction [47]. When diploidy arises via fusion of two meiotic products, androgenetic males produce one-third (XX) female offspring. The expected proportion of female offspring is one-third because there are three possible random fusions between meiotic products of heterogametic males: one-fourth XX, half XY and one-fourth non-viable YY (or OO). Nonetheless, when androgenesis is fixed, females become very rare and the overall reproductive output of the population is decreased to 5% of that of its ancestral sexual population [47]. Under most situations such a population would also go extinct, but more slowly than under the mitotic production of diploid sperm. This type of scenario (fusion or duplication of sperm cells and androgenetic production of sons and daughters) is currently only known in Bacillus stick insects where it is unknown whether androgenesis stems from genome elimination or from the production of non-nucleate oocytes. If Bacillus androgenesis proves to be a case of genome elimination, it is possible that it has been maintained thus far because it occurs in hybridogenetic females that feature selfish elimination of the male genome. Male genome elimination will reduce or impede the spread of genome elimination alleles expressed in males.
McKone & Halpern [47] did not develop their fitness functions for haplo-diploid species. It also remains undetermined whether the known androgenic ant species (see §2c) would be relevant to the spread of selfish male genes considered by McKone and Halpern, as the ant examples may involve production of non-nucleate oocytes rather than genome elimination. Independently of the androgenesis mechanism in the ants, androgenesis is more widespread among haplo-diploids relative to species with other sex determination mechanisms (five out of eight gonochorist species in table 1 are haplo-diploid), suggesting that the costs of androgenesis may be less in haplo-diploids than in diploid species, and/or that there may be fewer developmental/genetic constraints hampering the emergence of new androgenetic lines in haplo-diploids. Haplo-diploidy acts as an exaptation for the evolution of androgenesis because males develop from a haploid, unfertilized egg. The implications are that no novel mechanisms for generating a diploid offspring from haploid sperm are needed for androgenesis to evolve in haplo-diploids. Normally the egg nucleus is maternally derived, but if there is no intrinsic reason why a sperm pronucleus should not divide and produce a male, then this explains why androgenesis (and gynadromorphy) is more common in haplo-diploids than it is in diploid species.
(c). Other constraints on androgenesis
As we have seen, under most scenarios androgenesis will spread to fixation in species with separate sexes, but this will then drive extinction. This most likely explains the rarity of androgenesis in nature. However, there are other important reasons why androgenesis is rare among multicellular species. These reasons share some of the genetic and developmental constraints that hamper the evolution of thelytoky from sexual ancestors in species with separate sexes (reviewed in [92]).
The ability to survive as a haploid, or the ability to generate diploid genomes from normally haploid sperm may be an important constraint that prevents the emergence of new androgenetic lineages. Even if a diploid offspring can be generated from haploid sperm, the offspring will suffer from inbreeding depression in many cases. Notably, if diploidy is restored by sperm duplication, offspring will be fully homozygous. Restoration of diploidy via fusion of different sperm cells of the same father also results in a loss of heterozygosity and expression of recessive deleterious alleles.
Another constraint may stem from genomic imprinting. Genomic imprinting is a well-established phenomenon in which genes are modified according to the sex of the individual producing the gamete [93,94]. That is, a gene inherited from a female will differ epigenetically from the same gene inherited from a male. Although the DNA sequence itself is the same, heritable epigenetic modifications to DNA result in changes in gene expression in offspring, depending on whether the allele was inherited from the mother or the father (e.g. [93,94]). In species where sex-specific epigenetic modifications are standard (e.g. mammals [95]), androgenesis could be lethal because a zygote formed from the union of two male gametes or two female gametes is non-viable [92,96]. The same argument has been proposed to constrain the evolution of parthenogenesis; two copies of the maternal genome may result in non-balanced gene expression in zygotes [97].
How the various genetic and developmental constraints to androgenesis are overcome are poorly understood because so little is known about the genetic changes required for the initial emergence of new androgenetic lines. In laboratory strains of Drosophila, mutations in oocyte–early-embryo-specific α-tubulin can result in haploid embryos because the maternal pronucleus fails to fuse with the paternal pronucleus. The paternal pronucleus then divides to produce a haploid androgenetic embryo [40]. Diploidy can be restored by fusion of two haploid nuclei or by non-disjunction [40]. Diploidization is enhanced in Drosophila by a second mutation Nonclaret disjuctional [40]. In combination, these two mutations induce classic androgenesis: loss of the maternal genome, and diploidization of the paternal genome. Whether these two mutations are present in natural populations remains unknown.
6. Androgenesis and conservation
Success with androgenetic cloning of fishes has led to calls for the use of these technologies in fish conservation [98,99]. In particular, in salmonids it is often possible to fertilize eggs with heterospecific sperm. Incubation of the milt with CaCl2 or ethylene glycol increases the frequency of polyspermy. The F1 hybrid embryo fails to develop, but secondary sperm may fuse and reconstitute the father(s), especially after cold shock [100]. There has been some success with this technique with sperm harvested from cadavers (e.g. [101,102]), and one could imagine that in an emergency situation one might clone an endangered species in the eggs of a common farmed one [103].
Another potential application is in biological control. A proposal to introduce an exotic fish species, for example, for control of an aquatic weed, needs careful evaluation of the likely ecological consequences. The trouble is, after the fish is introduced, it may be impossible to eradicate it should its introduction have unforeseen adverse consequences. Stanley et al. [104] proposed that an androgenetic or gynogenetic single-sex population might be used to test the waters, so to speak, with little chance of a feral population becoming established. We are unaware of any application of this intriguing idea.
In plants, it is possible to produce sterile haploids via pollen culture [91]. One might imagine situations where it is desirable to keep a plant lineage permanently sterile because it has been genetically engineered to produce a toxin, drug or other bioactive compound. If so, haploid lines produced by microspore embryogenesis could prove useful.
Plant conservation increasingly relies on long-term storage of germplasm, with consequent need for large, temperature-controlled facilities, with regular turnover of seeds. Such seed banks are expensive to maintain. Storage of pollen, as an alternative to seeds, may offer a cost-saving solution [105,106]. However, to be successful it will be necessary to develop utterly reliable tissue culture techniques so that entire plants can be regenerated from pollen—via androgenesis.
7. Androgenesis and invasiveness
At first sight androgenesis seems like an inefficient mode of reproduction that should be outcompeted by normal thelytokous parthenogenesis or sexual reproduction. From a genetic point of view androgenesis is form of asexual reproduction. Yet its ecological costs are similar to normal sexual reproduction. Like a sexual species, an androgenetic species must pay the costs of finding appropriate partners and bringing fertilization about. Mating is often associated with increased risk of predation and exposure to pathogens, including sexually transmitted diseases [107]. Further, it is impossible for gonochorist androgenetic species to colonize empty or new niches since they need to co-occur with the sexual species that is exploited as an egg source. Despite these ecological costs, several androgenetic species are highly successful and have become invasive across large parts of the globe.
Corbicula clams originate from Asia, Australia, Africa and the Middle East where both sexual and androgenetic strains occur. By contrast, in areas where they are invasive in North America and Europe, the populations are exclusively androgenetic [2,108,109]. Invasive strains of the ant W. auropunctata are also androgenetic, while some native populations are sexual [110,111]. Androgenetic strains of the ants P. longicornis and V. emeryi are spreading to new areas [112,113], but it is not known whether sexual reproduction occurs in native populations.
These examples suggest that androgenesis can be beneficial to invasive populations but be disfavoured in native populations. A possible benefit of androgenesis in invasive populations is that some forms of androgenesis reduce or eliminate inbreeding [12]. Invasive populations typically derive from very few individuals. Inbreeding among these individuals can lead to inbreeding depression and may constrain the establishment of the invasive population.
In invasive populations of Corbicula, offspring are derived from diploid sperm that are produced ameiotically (table 1). This means that there is no loss of heterozygosity between generations, and therefore, no inbreeding depression. Heterozygosity is also maintained in the three androgenetic ants. Because there is no gene flow between queens and males, the sexually produced workers are always highly heterozygous [9,10,12]. Heterozygosity is maintained in queens via a type of parthenogenesis that is functionally ameiotic [114], and males are haploid in both sexual and androgenetic populations.
8. Does androgenesis arise as a consequence of inter-sexual competition?
In the little fire ant, W. auropunctata, workers arise sexually, queens arise by thelytoky, and males arise androgenetically [9,11,51]. It is tempting to regard this situation as a classic evolutionary arms race in which males retaliate against the absence of their genomes in queen-destined embryos by androgenesis. However, a more nuanced consideration of this system suggests that androgenesis is controlled by females, which most likely lay a small number of non-nucleate eggs [111]. In a population where all queens are produced clonally, and no males are produced, daughter queens have no mating partners, so queens would be obliged to produce worker offspring asexually. Social insect colonies greatly benefit from genetic heterogeneity among workers, which can increases disease resistance [115,116] and enhance the task allocation system [117,118]. These benefits may be sufficient to ensure that males are not lost altogether.
It is interesting to note that two examples of clonal or hemi-clonal reproduction by males come from species with female hybridogenesis (Bacillus stick insects and Hypseleotris carp gudgeons). Males that mate with female hybridogens have zero fitness as their genome is excluded from the germ line. Clonal reproduction by males in such a system is strongly favoured—even more strongly than in a normal sexual population. Thus, the increased intergenomic conflict generated by unusual sexual systems such as hybridogenesis may favour the emergence of additional unusual strategies in the ‘losing’ genome.
9. Conclusion
Androgenesis encompasses several forms of male asexuality that can only persist under rare circumstances. Androgenesis via non-nucleate oocytes is probably maladaptive in the vast majority of cases and is only evolutionarily stable under special conditions as, for example, those hypothesized for the androgenetic ants. Androgenetic systems with male-driven genome elimination are generally unstable because they drive distortion in the sex ratio towards males, and males cannot reproduce without females (or without the female function being retained in hermaphrodites). Thus, for an androgenetic population to persist there must be an alternative source of females. Often the source of females is a related species, but even here, a successful androgenetic strain runs the danger of driving its host species extinct. More stable systems can arise when there are sublineages that specialize in either gynogenesis or androgenesis, with each lineage exploiting the other as a source of sperm or eggs, respectively. Although such systems mirror sexual systems in terms of ecology, they differ from sexual species in that there is no mixing of male and female genomes. Androgenetic systems may thus serve to understand genetic consequences of asexuality without confounding ecological differences between sexual and asexual systems.
Glossary
- androgenesis
asexual reproduction by males
- gonochorist
having separate male and female sexes
- gynandromorphy
mosaics of male and female tissue in one organism
- gynogenesis
sperm-dependent parthenogenesis; a form of parthenogenesis where sperm are required to trigger embryo development but where the sperm does not contribute nuclear genetic material to offspring
- haplo-diploidy
a form of sex determination where males develop from haploid, unfertilized eggs and females from diploid, fertilized eggs
- hybridogenesis
a form of asexual reproduction by hybrid individuals involving the systematic elimination of one parental genome from the germline
- parthenogenesis
reproduction by females in the absence of males
- ‘sub-clone’
an asexually produced offspring that has lost some of the heterozygosities present in its parent
- thelytoky (thelytokous parthenogenesis)
female-producing parthenogenesis
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Australian Research Council project DP150101985 to B.P.O. and by grant PP00P3_139013 from the Swiss National Science Foundation to T.S.
References
- 1.Rieger R, Michaelis A, Green MM. 1968. A glossary of genetics and cytogenetics: classical and molecular. Berlin, Germany: Springer. [Google Scholar]
- 2.Pigneur LM, Hedtke SM, Etoundi E, Van Doninck K. 2012. Androgenesis: a review through the study of the selfish shellfish Corbicula spp. Heredity 108, 581–591. ( 10.1038/hdy.2012.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komaru A, Kawagishi T, Konishi K. 1998. Cytological evidence of spontaneous androgenesis in the freshwater clam Corbicula leana Prime. Dev. Genes Evol. 208, 46–50. ( 10.1007/s004270050152) [DOI] [PubMed] [Google Scholar]
- 4.Komaru A, Ookubo K, Kiyomoto M. 2000. All meiotic chromosomes and both centrosomes at spindle pole in the zygotes discarded as two polar bodies in clam Corbicula leana: unusual polar body formation observed by antitubulin immunofluorescence. Dev. Genes Evol. 210, 263–269. ( 10.1007/s004270050313) [DOI] [PubMed] [Google Scholar]
- 5.Ishibashi R, Ookubo K, Aoki M, Utaki M, Komaru A, Kawamura K. 2003. Androgenetic reproduction in a freshwater diploid clam Corbicula fluminea (Bivalvia: Corbiculidae). Zool. Sci. 20, 727–732. ( 10.2108/zsj.20.727) [DOI] [PubMed] [Google Scholar]
- 6.Pichot C, Liens B, Nava JLR, Bachelier JB, El Maataoui M. 2008. Cypress surrogate mother produces haploid progeny from alien pollen. Genetics 178, 379–383. ( 10.1534/genetics.107.080572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pichot C, El Maâtaoui M. 2000. Unreduced diploid nuclei in Cupressus dupreziana A. Camus pollen. Theor. Appl. Genet. 101, 574–579. ( 10.1007/s001220051518) [DOI] [Google Scholar]
- 8.Pichot C, Fady B, Hochu I. 2000. Lack of mother tree alleles in zymograms of Cupressus dupreziana A. Camus embryos. Ann. For. Sci. 57, 17–22. ( 10.1051/forest:2000108) [DOI] [Google Scholar]
- 9.Fournier D, Estoup A, Orivel RM, Foucaud J, Jourdan H, Le Breton J, Keller L. 2005. Clonal reproduction by males and females in the little fire ant. Nature 435, 1230–1234. ( 10.1038/nature03705) [DOI] [PubMed] [Google Scholar]
- 10.Ohkawara K, Makayama M, Satoh A, Trindl A, Heinze J. 2006. Clonal reproduction and genetic caste differences in a queen-polymorphic ant, Vollenhovia emeryi. Biol. Lett. 2, 359–363. ( 10.1098/rsbl.2006.0491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi K, Hasegawa E, Ohkawara K. 2008. Clonal reproduction by males of the ant Vollenhovia emeryi (Wheeler). Entomol. Sci. 11, 167–172. ( 10.1111/j.1479-8298.2008.00272.x) [DOI] [Google Scholar]
- 12.Pearcy M, Goodisman MAD, Keller L. 2011. Sib mating without inbreeding in the longhorn crazy ant. Proc. R. Soc. B 278, 2677–2681. ( 10.1098/rspb.2010.2562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantovani B, Scali V. 1992. Hybridogenesis and androgenesis in the stick-insect Bacillus rossius-grandi benazzi (Insecta, Phasmatodea). Evolution 46, 783–796. ( 10.2307/2409646) [DOI] [PubMed] [Google Scholar]
- 14.Tinti F, Scali V. 1995. Allozymic and cytological evidence for hemiclonal, all-paternal, and mosaic offspring of the hybridogenetic stick insect Bacillus rossius-grandii grandii. J. Exp. Biol. 273, 149–159. ( 10.1002/jez.1402730208) [DOI] [Google Scholar]
- 15.Tinti F, Scali V. 1992. Genome exclusion and gametic DAPI-DNA content in the hybridogenetic Bacillus rossius-grandii benazzii complex (Insecta Phasmatodea). Mol. Reprod. Dev. 33, 235–242. ( 10.1002/mrd.1080330302) [DOI] [PubMed] [Google Scholar]
- 16.Tinti F, Scali V. 1993. Chromosomal evidence of hemiclonal and all-paternal offspring production in Bacillus rossius-grandii benazzii (Insecta Phasmatodea). Chromosoma 102, 403–414. ( 10.1007/BF00360405) [DOI] [Google Scholar]
- 17.Schmidt DJ, Bond NR, Adams M, Hughes JM. 2011. Cytonuclear evidence of hybridogenetic reproduction in natural populations of the Australian carp gudgeon (Hypseleotris: Eleotidae). Mol. Ecol. 20, 3367–3380. ( 10.1111/j.1365-294X2011.05206.x) [DOI] [PubMed] [Google Scholar]
- 18.Abdoun F, Beddiaf M. 2002. Cupressus dupreziana A. Camus: distribution, decline and regeneration on the Tassili n'Aijer, Central Sahara. C. R. Biol. 325, 617–627. [DOI] [PubMed] [Google Scholar]
- 19.Pichot C, Borrut A, El Maataoui M. 1998. Unexpected DNA content in the endosperm of Cupressus dupreziana A. Camus seeds and its implications in the reproductive process. Sex Plant Reprod. 11, 148–152. ( 10.1007/s004970050132) [DOI] [Google Scholar]
- 20.Pichot C, El Maâtauoui M, Raddi S, Raddi P. 2001. Surrogate mother for endangered Cupressus. Nature 412, 39 ( 10.1038/35083687) [DOI] [PubMed] [Google Scholar]
- 21.Houki S, Yamada M, Honda T, Komaru A. 2011. Origin and possible role of males in hermaphroditic androgenetic Corbicula clams. Zool. Sci. 28, 526–531. ( 10.2108/zsj.28.526) [DOI] [PubMed] [Google Scholar]
- 22.Komaru A, Konishi K. 1999. Non-reductional spermatozoa in three shell color types of the freshwater clam Corbicula fluminea in Taiwan. Zool. Sci. 16, 105–108. ( 10.2108/zsj.16.105) [DOI] [Google Scholar]
- 23.Hedtke SM, Stanger-Hall K, Baker RJ, Hillis DM. 2008. All-male asexuality: origin and maintenance of androgenesis in the Asian clam Corbicula. Evolution 62, 1119–1136. ( 10.1111/j.1558-5646.2008.00344.x) [DOI] [PubMed] [Google Scholar]
- 24.Hedtke SM, Glaubrecht M, Hillis DM. 2011. Rare gene capture in predominantly androgenetic species. Proc. Natl Acad. Sci. USA 108, 9520–9524. ( 10.1073/pnas.1106742108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komaru A, Kumamoto A, Kato T, Ishibashi R, Obata M, Nemoto T. 2006. A hypothesis of ploidy elevation by formation of a female pronucleus in the androgenetic clam Corbicula fluminea in the Tone River Estuary, Japan. Zool. Sci. 23, 529–532. ( 10.2108/zsj.23.529) [DOI] [PubMed] [Google Scholar]
- 26.Mantovani B, Passamonti M, Scali V. 2001. The mitochondrial cytochrome oxidase II gene in Bacillus stick insects: ancestry of hybrids, androgenesis, and phylogenetic relationships. Mol. Phylogenet. Evol. 19, 157–163. ( 10.1006/mpev.2000.0850) [DOI] [PubMed] [Google Scholar]
- 27.Bertozzi T, Adams M, Walker KF. 2000. Species boundaries in carp gudgeons (Eleotrididae: Hypseleotris) from the River Murray, South Australia: evidence for multiple species and extensive hybridization. Mar. Freshw. Res. 51, 805–815. ( 10.1071/MF00039) [DOI] [Google Scholar]
- 28.Koeniger N, Hemmling C, Yoshida T. 1989. Drones as sons of drones in Apis mellifera. Apidologie 20, 391–394. ( 10.1051/apido:19890503) [DOI] [Google Scholar]
- 29.Schneider MV, Driessen G, Beukeboom LW, Boll R, van Eunen K, Selzner A, Talsma J, Lapchin L. 2003. Gene flow between arrhenotokous and thelytokous populations of Venturia canescens (Hymenoptera). Heredity 90, 260–267. ( 10.1038/sj.hdy.6800245) [DOI] [PubMed] [Google Scholar]
- 30.Seguí-Simarro JM. 2010. Androgenesis revisited. Bot. Rev. 76, 377–404. ( 10.1007/s12229-010-9056-6) [DOI] [Google Scholar]
- 31.Burk LG. 1962. Haploids in genetically marked progenies of tobacco. J. Hered. 53, 222–225. [Google Scholar]
- 32.Clausen RE, Lammerts WE. 1929. Interspecific hybridisation in Nicotiana. X. Haploid and diploid merogony. Am. Nat. 63, 279–282. ( 10.1086/280261) [DOI] [Google Scholar]
- 33.Kostoff D. 1929. An androgenic Nicotiniana haploid. Z. Zellforschung Mikrosk. Anat. 9, 640–642. ( 10.1007/BF02450775) [DOI] [Google Scholar]
- 34.Kehr AE. 1951. Monoploidy in Nicotiana. J. Hered. 42, 107–112. [DOI] [PubMed] [Google Scholar]
- 35.Kostoff D. 1942. The problem of haploidy (Cytogenetic studies in Nicotiana haploids and their bearing on some other cytogenetic problems). Bibliogr. Genet. 13, 1–148. [Google Scholar]
- 36.Segui-Simarro JM, Corral-Martinez P, Parra-Vega V, Gonzalez-Garcia B. 2011. Androgenesis in recalcitrant solanaceous crops. Plant Cell Rep. 30, 765–778. ( 10.1007/s00299-010-0984-8) [DOI] [PubMed] [Google Scholar]
- 37.Kermicle JL. 1974. Origin of androgenetic haploids and diploids induced by the indeterminate gametophyte (ig) mutation in maize. In Haploids in higher plants: advances and potential (ed. Kasha KJ.). Guelph, Canada: University of Guelph. [Google Scholar]
- 38.Chase SS. 1969. Monoploids and monoploid derivatives of maize (Zea mays L.). Bot. Rev. 35, 117–168. ( 10.1007/BF02858912) [DOI] [Google Scholar]
- 39.Goodsell SF. 1961. Male sterility in corn by androgenesis. Crop Sci. 1, 227–228. ( 10.2135/cropsci1961.0011183X000100030022x) [DOI] [Google Scholar]
- 40.Komma DJ, Endow SA. 1995. Haploidy and androgenesis in Drosophila. Proc. Natl Acad. Sci. USA 92, 11 884–11 888. ( 10.1073/pnas.92.25.11884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milne CPJ, Rothenbuhler WC. 1983. Polarization of the honey bee gynandromprphic blastoderm. Can. J. Genet. Cytol. 25, 561–566. ( 10.1139/g83-085) [DOI] [Google Scholar]
- 42.Drescher W, Rothenbuhler WC. 1963. Gynandromorph production by egg chilling: cytological mechanisms in honey bees. J. Hered. 54, 195–201. [Google Scholar]
- 43.Burt A, Trivers R. 2006. Genes in conflict: the biology of selfish genetic elements. Cambridge, MA: Harvard University Press. [Google Scholar]
- 44.Lehtonen J, Schmidt DJ, Heubel K, Kokko H. 2013. Evolutionary and ecological implications of sexual parasitism. Trends Ecol. Evol. 28, 297–306. ( 10.1016/j.tree.2012.12.006) [DOI] [PubMed] [Google Scholar]
- 45.Glaubrecht M, von Rintelen T, Korniushin AV. 2003. Toward a systematic revision of brooding freshwater Corbiculidae in Southeast Asia (Bivalvia, Veneroida): on shell morphology, anatomy and molecular phylogenetics of endemic taxa from islands in Indonesia. Malacologia 45, 1–40. [Google Scholar]
- 46.Korniushin AV. 2004. A revision of some Asian and African freshwater clams assigned to Corbicula fluminalis (Muller, 1774) (Mollusca: Bivalvia: Corbiculidae), with a review of anatomical characters and reproductive features based on museum collections. Hydrobiologia 529, 251–270. ( 10.1007/s10750-004-9322-x) [DOI] [Google Scholar]
- 47.McKone MJ, Halpern SL. 2003. The evolution of androgenesis. Am. Nat. 161, 641–656. ( 10.1086/368291) [DOI] [PubMed] [Google Scholar]
- 48.Hedtke SM, Hillis DM. 2011. The potential role of androgenesis in cytoplasmic-nuclear phylogenetic discordance. Syst. Biol. 60, 87–96. ( 10.1093/sysbio/syq070) [DOI] [PubMed] [Google Scholar]
- 49.Kraemer LR, Swanson C, Galloway M, Kraemer R. 1986. Biological basis of behavior in Corbicula fluminea, 2. Functional morphology of reproduction and development and review of evidence for self-fertilization. Am. Malacolog. Bull. 2, 193–201. [Google Scholar]
- 50.Lee T, Siripattrawan S, Ituarte CF, Foighil DO. 2005. Invasion of the clonal clams: Corbicula lineages in the New World. Am. Malacolog. Bull. 20, 113–122. [Google Scholar]
- 51.Foucaud J, Fournier D, Orivel J, Delabie JHC, Loiseau A, Le Breton J, Kergoat GJ, Estoup A. 2007. Sex and clonality in the little fire ant. Mol. Biol. Evol. 24, 2465–2473. ( 10.1093/molbev/msm180) [DOI] [PubMed] [Google Scholar]
- 52.Scali V. 2009. Metasexual stick insects: model pathways to losing sex and bringing it back. In Lost sex: the evolutionary biology of parthenogenesis (eds Schon I, Martens K, VanDijk P), pp. 317–345. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 53.Snook RR, Hosken DJ, Karr TL. 2011. The biology and evolution of polyspermy: insights from cellular and functional studies of sperm centrosomal behaviour in the fertilized egg. Reproduction 142, 779–792. ( 10.1530/REP-11-0255) [DOI] [PubMed] [Google Scholar]
- 54.Ghiselli F, Milani L, Scali V, Passamonti M. 2007. The Leptynia hispanica species complex (Insecta Phasmida): polyploidy, parthenogenesis, hybridization and more. Mol. Ecol. 16, 4256–4268. ( 10.1111/j.1365-294X.2007.03471.x) [DOI] [PubMed] [Google Scholar]
- 55.Milani L, Scali V, Passamonti M. 2009. The Clonopsis gallica puzzle: Mendelian species, polyploid parthenogens with karyotype re-diploidization and clonal androgens in Moroccan stick insects (Phasmida). J. Zool. Syst. Evol. 47, 132–140. ( 10.1111/j.1439-0469.2008.00489.x) [DOI] [Google Scholar]
- 56.Scali V, Milani L. 2009. New clonopsis stick insects from Morocco: the amphigonic C. felicitatis sp.n., the parthenogenetic C. soumiae sp.n., and two androgenetic taxa. Ital. J. Zool. 76, 291–305. ( 10.1080/11250000802649750) [in Italian] [DOI] [Google Scholar]
- 57.Beekman M, Allsopp MH, Lim J, Goudie F, Oldroyd BP. 2011. Asexually produced Cape honeybee queens (Apis mellifera capensis) reproduce sexually. J. Hered. 102, 562–566. ( 10.1093/jhered/esr075) [DOI] [PubMed] [Google Scholar]
- 58.van der Kooi CJ, Schwander T. 2014. On the fate of sexual traits under asexual reproduction. Biol. Rev. 89, 805–819. ( 10.1111/brv.12078) [DOI] [PubMed] [Google Scholar]
- 59.Campos FF, Morgan DTJ. 1958. Haploid pepper from a sperm. J. Hered. 49, 135–137. [Google Scholar]
- 60.Nalini M, Deepak J, Clarke H, Coyne C, Muehlbauer F. 2005. Induction of androgenesis as a consequence of wide crossing in chickpea. J. SAT Agric. Res. 1, 1–3. [Google Scholar]
- 61.Chen BY, Heneen WK. 1989. Evidence for spontaneous diploid androgenesis in Brassica napus L. Sex Plant Reprod. 2, 15–17. ( 10.1007/BF00190114) [DOI] [Google Scholar]
- 62.Waldman AS. 2008. Ensuring the fidelity of recombination in mammalian chromosomes. Bioessays 30, 1163–1171. ( 10.1002/bies.20845) [DOI] [PubMed] [Google Scholar]
- 63.Rothenbuhler WC, Gowen JW, Park OW. 1952. Androgenesis with zoogenesis in gynandromorphic honey bees (Apis mellifera L.). Science 115, 637–638. ( 10.1126/science.115.2998.637) [DOI] [PubMed] [Google Scholar]
- 64.Morgan TH. 1916. The Euguster gynandromorph bees. Am. Nat. 50, 39–45. ( 10.1086/279521) [DOI] [Google Scholar]
- 65.Dobata S, Shimoji H, Ohnishi H, Hasegawa E, Tsuji K. 2012. Paternally inherited alleles in male body parts of an ant (Diacamma sp.) sex mozaic: implication for androgenetic male production in the Hymenoptera. Insect. Soc. 59, 55–59. ( 10.1007/s00040-011-0187-5) [DOI] [Google Scholar]
- 66.Wcislo WT, Gonzalez VH, Arneson L. 2004. A review of deviant phenotypes in bees in relation to brood parasitism, and a gynandromorph of Megalopta genalis (Hymenoptera: Halictidae). J. Nat. Hist. 38, 1443–1457. ( 10.1080/0022293031000155322) [DOI] [Google Scholar]
- 67.Engel MS. 2007. A lateral gynandromorph in the bee genus Thyreus and the sting mechanism in the melectini (Hymenoptera: Apidae). Am. Mus. Nov. 3553, 1–11. ( 10.1206/0003-0082(2007)530%5B1:algitb%5D2.0.co;2) [DOI] [Google Scholar]
- 68.Hopwood JL. 2007. A ‘cyclops’ of the bee Lasioglossum (Dialictus) bruneri (Hymenoptera: Halictidae). J. Kans. Entomol. Soc. 80, 259–261. ( 10.2317/0022-8567(2007)80%5B259:acotbl%5B2.0.co;2) [DOI] [Google Scholar]
- 69.Lucia M, Abrahamovich AH, Alvarez LJ. 2009. A gynandromorph of Xylocopa nigrocincta Smith (Hymenoptera: Apidae). Neotrop. Entomol. 38, 155–157. ( 10.1590/S1519-566X2009000100020) [DOI] [PubMed] [Google Scholar]
- 70.Hinojosa-Diaz IA, Gonzalez VH, Ayala R, Merida J, Sagot P, Engel MS. 2012. New orchid and leaf-cutter bee gynandromorphs, with an updated review (Hymenoptera, Apoidea). Zoosyst. Evol. 88, 205–214. ( 10.1002/zoos.201200017) [DOI] [Google Scholar]
- 71.Lucia M, Gonzalez VH. 2013. A new gynandromorph of Xylocopa frontalis with a review of gynandromorphism in Xylocopa (Hymenoptera: Apidae: Xylocopini). Ann. Entomol. Soc. Am. 106, 853–856. ( 10.1603/an13085) [DOI] [Google Scholar]
- 72.Suzuki KM, Giangarelli DC, Ferreira DG, Frantine-Silva W, Augusto SC, Sofia SH. 2015. A scientific note on an anomalous diploid individual of Euglossa melanotricha (Apidae, Euglossini) with both female and male phenotypes. Apidologie 46, 495–498. ( 10.1007/s13592-014-0339-5) [DOI] [Google Scholar]
- 73.Kinomura K, Yamauchi K. 1994. Frequent occurrence of gynandromorphs in the natural population of the ant Vollenhovia emeryi (Hymenoptera, Formicidae). Insect. Soc. 41, 273–278. ( 10.1007/bf01242298) [DOI] [Google Scholar]
- 74.Pereira RAS, Prado AP, Kjellberg F. 2003. Gynandromorphism in pollinating fig wasps (Hymenoptera: Agaonidae). Entomol. News 114, 152–155. [Google Scholar]
- 75.Beukeboom LW, Kamping A, Louter M, Pijnacker LP, Katju V, Ferree PM, Werren JH. 2007. Haploid females in the parasitic wasp Nasonia vitripennis. Science 315, 206 ( 10.1126/science.1133388) [DOI] [PubMed] [Google Scholar]
- 76.Kamping A, Katju V, Beukeboom LW, Werren JH. 2006. Inheritance of gynandromorphism in the parasitic wasp Nasonia vitripennis. Genetics 175, 1321–1333. ( 10.1534/genetics.106.067082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tulgetske GM, Stouthamer R. 2012. Characterization of intersex production in Trichogramma kaykai infected with parthenogenesis-inducing Wolbachia. Naturwissenschaften 99, 143–152. ( 10.1007/s00114-011-0880-2) [DOI] [PubMed] [Google Scholar]
- 78.Whiting PW. 1943. Androgenesis in the parasitic wasp Habrobracon. J. Hered. 34, 355–366. [Google Scholar]
- 79.Yoshizawa J, Mimori K, Yamauchi K, Tsuchida K. 2009. Sex mosaics in a male dimorphic ant Cardiocondyla kagutsuchi. Naturwissenschaften 96, 49–55. ( 10.1007/s00114-008-0447-z) [DOI] [PubMed] [Google Scholar]
- 80.Yang AS, Abouheif E. 2011. Gynandromorphs as indicators of modularity and evolvability in ants. J. Exp. Zool. B 316B, 313–318. ( 10.1002/jez.b.21407) [DOI] [PubMed] [Google Scholar]
- 81.Jones SR, Phillips SA. 1985. Gynandromorphism in the ant Pheidole dentata Mayr (Hymenoptera, Formicidae). Proc. Entomol. Soc. Wash. 87, 583–586. [Google Scholar]
- 82.Laidlaw HH, Tucker KW. 1964. Diploid tissue derived from accessory sperm in the honey bee. Genetics 50, 1439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O'Farrell MM, Peirce RE. 1989. The occurrence of a gynandromorphic migratory trout, Salmo trutta L. J. Fish Biol. 34, 327–327. ( 10.1111/j.1095-8649.1989.tb03313.x) [DOI] [Google Scholar]
- 84.Nielsen JE. 2010. A review of gynandromorphism in the genus Ornithoptera boisduval, (Lepidoptera: Papilionidae). Aust. Entomol. 37, 105–112. [Google Scholar]
- 85.Halverson JL, Dvorak J. 1993. Genetic control of sex determination in birds and the potential for its manipulation. Poult. Sci. 72, 890–896. ( 10.3382/ps.0720890) [DOI] [PubMed] [Google Scholar]
- 86.Janning W. 1978. Gynandromorph fate maps in Drosophila. In Genetic mosaics and cell differentiation (ed. Gehring WJ.), pp. 1–28. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 87.Komen H, Thorgaard GH. 2007. Androgenesis, gynogenesis and the production of clones in fishes: a review. Aquaculture 269, 150–173. ( 10.1016/j.aquaculture.2007.05.009) [DOI] [Google Scholar]
- 88.Parsons JE, Thorgaard GH. 1985. Production of androgenetic diploid rainbow trout. J. Hered. 76, 177–181. [DOI] [PubMed] [Google Scholar]
- 89.Gerassimova H. 1936. Experimentally produced haploid plant in Crepis tectorum. Biol. J. 5, 895–900. [Google Scholar]
- 90.Ehrensberger R. 1948. Versuche zur auslösung von haploidie bei Blütenpflanzen. Biol. Zentralbl. 67, 537–546. [Google Scholar]
- 91.Wang M, van Bergen S, van Duijn B. 2000. Insights into a key developmental switch and its importance for efficient plant breeding. Plant Physiol. 124, 523–530. ( 10.1104/pp.124.2.523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Engelstädter J. 2008. Constraints on the evolution of asexual reproduction. BioEssays 30, 1138–1150. ( 10.1002/bies.20833) [DOI] [PubMed] [Google Scholar]
- 93.Reik W, Walter J. 2001. Genomic imprinting: Parental influence on the genome. Nat. Rev. Genet. 2, 21–32. ( 10.1038/35047554) [DOI] [PubMed] [Google Scholar]
- 94.Wilkins JF, Haig D. 2003. What good is genomic imprinting: the function of parent-specific gene expression. Nat. Rev. Genet. 4, 359–368. ( 10.1038/nrg1062) [DOI] [PubMed] [Google Scholar]
- 95.Wilkins JF. 2005. Genomic imprinting and methylation: epigenetic canalization and conflict. Trends Genet. 21, 356–365. ( 10.1016/j.tig.2005.04.005) [DOI] [PubMed] [Google Scholar]
- 96.Rougier N, Werb Z. 2001. Minireview: parthenogenesis in mammals. Mol. Reprod. Dev. 59, 468–474. ( 10.1002/mrd.1054) [DOI] [PubMed] [Google Scholar]
- 97.Kono T. 2006. Genomic imprinting is a barrier to parthenogenesis in mammals. Cytok. Genome Res. 113, 31–35. ( 10.1159/000090812) [DOI] [PubMed] [Google Scholar]
- 98.Nagoya H, Sato S, Ohta H. 2010. Preservation of endangered salmonids using androgenesis. J. Nat. Taiwan Mus. 11, 71–78. [Google Scholar]
- 99.Pandian TJ, Kirankumar S. 2003. Androgenesis and conservation of fishes. Curr. Sci. 85, 917–931. [Google Scholar]
- 100.David CJ, Pandian TJ. 2006. Cadaveric sperm induces intergeneric androgenesis in the fish, Hemigrammus caudovittatus. Theriogenology 65, 1048–1070. ( 10.1016/j.theriogenology.2005.07.014) [DOI] [PubMed] [Google Scholar]
- 101.Clifton JD, Pandian TJ. 2008. Dispermic induction of interspecific androgenesis in the fish, Buenos Aires tetra using surrogate eggs of widow tetra. Curr. Sci. 95, 64–74. [Google Scholar]
- 102.Yasui GS, Fujimoto T, Arai K. 2010. Restoration of the loach, Misgurnus anguillicaudatus, from cryopreserved diploid sperm and induced androgenesis. Aquaculture 308, S140–S144. ( 10.1016/j.aquaculture.2010.05.041) [DOI] [Google Scholar]
- 103.Corley-Smith GE, Brandhorst BP. 1999. Preservation of endangered species and populations: a role for genome banking, somatic cell cloning, and androgenesis? Mol. Reprod. Dev. 53, 363–367. ( 10.1002/(sici)1098-2795(199907)53:3%3C363::aid-mrd12%3E3.0.co;2-0) [DOI] [PubMed] [Google Scholar]
- 104.Stanley JG, Biggers CJ, Schultz DE. 1976. Isozymes in androgenetic and gyandrogenic white amur, gynogenetic carp and carp-amur hybrids. J. Hered. 67, 129–134. [DOI] [PubMed] [Google Scholar]
- 105.Bajaj YPS. 1979. Technology and prospects of cryopreservation of germplasm. Euphytica 28, 267–285. ( 10.1007/BF00056584) [DOI] [Google Scholar]
- 106.Reed BM, Denoma J, Luo J, Chang J, Towill L. 1998. Cryopreservation and long-term storage of pear germplasm. In Vitro Cell Dev. Biol. Plant 34, 256–260. ( 10.1007/BF02822718) [DOI] [Google Scholar]
- 107.Lehtonen J, Jennions MD, Kokko H. 2012. The many costs of sex. Trends Ecol. Evol. 27, 172–178. ( 10.1016/j.tree.2011.09.016) [DOI] [PubMed] [Google Scholar]
- 108.Pigneur LM, Risterucci AM, Dauchot N, Li X, Van Doninck K. 2011. Development of novel microsatellite markers to identify the different invasive lineages in the Corbicula complex and to assess androgenesis. Mol. Ecol. Resour. 11, 573–577. ( 10.1111/j.1755-0998.2010.02963.x) [DOI] [PubMed] [Google Scholar]
- 109.Okawa T, Kurita Y, Kanno K, Koyama A, Onikura N. 2016. Molecular analysis of the distributions of the invasive Asian clam, Corbicula fluminea (O.F. Müller, 1774), and threatened native clam, C. leana Prime, 1867, on Kyushu Island, Japan. BioInvasions Rec. 5, 25–29. ( 10.3391/bir.2016.5.1.05) [DOI] [Google Scholar]
- 110.Foucaud J, Orivel J, Fournier D, Delabie JHC, Loiseau A, Le Breton J, Cerdan P, Estoup A. 2009. Reproductive system, social organization, human disturbence and ecological dominance in native populations of the little fire ant, Wasmannia auropunctata. Mol. Ecol. 18, 5059–5073. ( 10.1111/j.1365-294X.2009.04440.x) [DOI] [PubMed] [Google Scholar]
- 111.Rey O, Facon B, Foucaud J, Loiseau A, Estoup A. 2013. Androgenesis is a maternal trait in the invasive ant Wasmannia auropunctata. Proc. R. Soc. B 280, 20131181 ( 10.1098/rspb.2013.1181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kjar DS, Sunian TW. 2007. First records of invasion by the myrmicine Japanese ant Vollenhovia emeryi W. M. Wheeler (Hymenoptera: Formicidae) in the United States. Proc. Entomol. Soc. Wash. 109, 596–604. [Google Scholar]
- 113.Wetterer JK, Guenard B, Booher DB. 2015. Geographic spread of Vollenhovia emeryi (Hymenoptera: Formicidae). Asian Myrmecol. 7, 107–114. [Google Scholar]
- 114.Rey O, et al. 2011. Meiotic recombination dramatically decreased in thelytokous queens of the little fire ant and their sexually produced workers. Mol. Biol. Evol. 28, 2591–2601. ( 10.1093/molbev/msr082) [DOI] [PubMed] [Google Scholar]
- 115.Tarpy DR, Seeley TD. 2006. Lower disease infections in honeybee (Apis mellifera) colonies headed by polyandrous vs monandrous queens. Naturwissenschaften 93, 195–199. ( 10.1007/s00114-006-0091-4) [DOI] [PubMed] [Google Scholar]
- 116.Seeley TD, Tarpy DR. 2007. Queen promiscuity lowers disease within honeybee colonies. Proc. R. Soc. B 274, 67–72. ( 10.1098/rspb.2006.3702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oldroyd BP, Fewell JH. 2007. Genetic diversity promotes homeostasis in insect colonies. Trends Ecol. Evol. 22, 408–413. ( 10.1016/j.tree.2007.06.001) [DOI] [PubMed] [Google Scholar]
- 118.Jones J, Myerscough M, Graham S, Oldroyd BP. 2004. Honey bee nest thermoregulation: diversity promotes stability. Science 305, 402–404. ( 10.1126/science.1096340) [DOI] [PubMed] [Google Scholar]