Abstract
Fungi are a diverse group of organisms with a huge variation in reproductive strategy. While almost all species can reproduce sexually, many reproduce asexually most of the time. When sexual reproduction does occur, large variation exists in the amount of in- and out-breeding. While budding yeast is expected to outcross only once every 10 000 generations, other fungi are obligate outcrossers with well-mixed panmictic populations. In this review, we give an overview of the costs and benefits of sexual and asexual reproduction in fungi, and the mechanisms that evolved in fungi to reduce the costs of either mode. The proximate molecular mechanisms potentiating outcrossing and meiosis appear to be present in nearly all fungi, making them of little use for predicting outcrossing rates, but also suggesting the absence of true ancient asexual lineages. We review how population genetic methods can be used to estimate the frequency of sex in fungi and provide empirical data that support a mixed mode of reproduction in many species with rare to frequent sex in between rounds of mitotic reproduction. Finally, we highlight how these estimates might be affected by the fungus-specific mechanisms that evolved to reduce the costs of sexual and asexual reproduction.
This article is part of the themed issue ‘Weird sex: the underappreciated diversity of sexual reproduction’.
Keywords: mating systems, inbreeding, sex, asexual reproduction, linkage disequilibrium, Ascomycota
1. Introduction
Sexual reproduction is one of the most diverse characteristics in nature, both in the modes by which reproduction occurs [1] but also the frequency at which species reproduce sexually or asexually [2,3]. Only a small number of species are considered to be truly asexual (e.g. 1 in 100 for angiosperms [4] and 1 in 1000 for animals [5]). The other species reproduce either obligatorily sexually or go through a sexual cycle with some frequency. Moreover, sex is pervasive across the eukaryotic tree, evolving near the root and widespread throughout and common within all of the major groups [1].
The dominance of sexual reproduction in nature is an evolutionary problem that has fascinated evolutionary biologists for years [6,7]. Sexual reproduction has many advantages, such as removing deleterious mutations and generating genotypic diversity [8], but it also has many costs that when added together seem to outweigh the benefits [9]. Sex involves the obstacle of having to find a partner, preferably one of good quality, the dangers of sexually transmitted diseases, investment of time and energy in courtship, and the errors such as translocations that can occur during meiosis. And when males and females exist, there is the infamous twofold cost of sex, that is, the cost of producing males, which parthenogenetic females do not incur, combined with the cost of only half of a diploid parent's genes being transferred to each offspring [7]. Although no asexual mammals are known, many other animals, plants, and other taxa reproduce asexually, occasionally, regularly, or sometimes almost exclusively (for a recent review, see [2]). The freshwater crustaceans in the genus Daphnia, for example, alternate asexual reproduction during summer with a sexual cycle towards the end of the growth season [10]. Similar cycles can be seen in rotifers as well as the aquatic angiosperm duckweed [11]. To understand how the frequency of sex evolves, we need to understand the costs and benefits of sexual versus asexual reproduction.
Fungi are great models for addressing these questions, possessing a wide variety of reproductive strategies, ranging from fully asexual to almost exclusively sexual species and including those that perform both strategies to varying degrees [12]. Often different strategies can be observed within the same genus, which provide a comparative framework for testing hypotheses on the frequency, advantages and costs of sexual reproduction. Owing to their small genome sizes a lot of population-level genomic data are becoming available that can shed light on these questions. However, as we will show, some more or less fungal-specific mechanisms in reproductive strategies might muddle our interpretation of these data.
The fungal kingdom is an old, large and highly diverse clade [13]. In this review, we will limit our discussion primarily to the Ascomycota, the group of fungi that contains over half of the described fungal species (in the remainder of this manuscript when mentioning fungi we are referring to Ascomycota, except when explicitly indicated otherwise). It is also one of the best studied groups of organisms, which contains famous model species such as budding yeast (Saccharomyces cerevisiae) and fission yeast (Schizosaccharomyces pombe), but also important human pathogens (e.g. Candida albicans), plant pathogens (e.g. Fusarium oxysporum), and important industrial species that produce a wide variety of substances (e.g. Aspergillus niger). All of the aforementioned species have great capacity for asexual reproduction, through budding, fission or asexual sporulation. To biologists this might seem a given; they are microbes after all. Yet, each of these organisms also has a sexual or at least parasexual cycle (see §6 and §7c), and in the last two decades fungal biologists have come to appreciate that essentially all fungal species have some means by which they recombine. Nonetheless, it has been shown that there is large variation in the frequency of sex between ascomycete species, which can range from an estimated one for every thousand asexual generations in budding yeast to every generation in pathogenic Sclerotinia species. To understand why there are such large differences in the frequency of sex, we need to understand the consequences of either form of reproduction.
In this review, we will give an overview of the costs and benefits of sexual versus asexual reproduction in ascomycete fungi and ask how these costs and benefits are shaped by life history of different fungi. We will argue that it is the balance between these costs and benefits that determine the frequency of sexual reproduction. We will start by describing the general life cycle of fungi, beginning with the first cost of sex in the sexual part of the cycle, finding a mate. We will continue by discussing other aspects of sexual reproduction and show that there are many benefits, some that are specific for fungi, but also many costs. We then discuss the costs and benefits of asexual reproduction. As we will show, evolution has not only occurred by optimization of the frequency between sexual and asexual reproduction, but also by adaptations that reduce specific parts of the costs. Finally, we will go into the methods that can be used to measure the rate of sex in fungi, describe what has been observed in nature, and discuss how observations might be affected through the different aspects of fungal life cycles.
2. Life cycles in Ascomycota
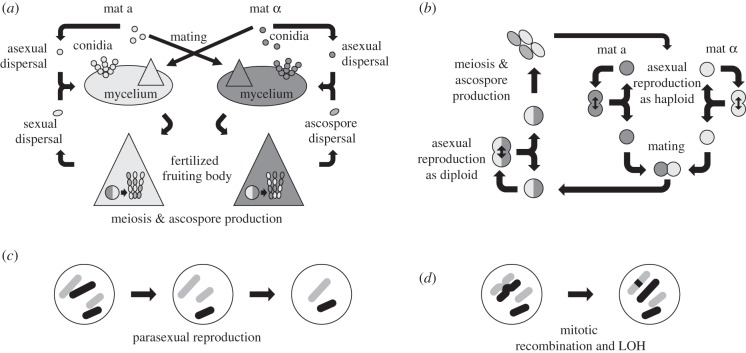
Even though there are many variations on the themes, ascomycete life cycles roughly come in two flavours: yeasts and filamentous fungi. Most filamentous fungi are predominantly haploid throughout their life (figure 1a). They start their life cycle by germination of a meiotic haploid spore to form a mycelium that grows vegetatively and can reproduce asexually by fragments or by specifically formed dispersal units, the conidia. Sexual reproduction is initiated by production of gametes that are capable of cross-fertilization between different mating types. Gametes are typically individual nuclei contained within hyphae or spores that function as gametangia. In Sordariomycetes, such as Neurospora, where sexual reproduction is best known, a female proto-fruiting body develops on a haploid mycelium that becomes fertilized by a male nucleus from a conidium produced by a compatible mate. After fertilization, the female mycelium produces a fruiting body within which grows very shortly the zygote as a heterokaryotic mycelium of male and female nuclei. After only a few mitotic divisions, meiotic spores are produced. These fungi generally produce both male and female structures, and can thus be considered hermaphrodites for which the aforementioned ‘twofold cost of sex’ is reduced to a 1.5-fold cost [14]. Haploid yeast (e.g. fission yeast and Pichia pastoris; figure 1b) have a similar life cycle, but perform asexual growth by mitotic cell divisions of the haploid cells and instead of producing fruiting bodies form diploid cells by fusion of two haploids that immediately initiate meiosis. Fusion occurs between cells of equal size and function, and these species are thus isogamous. Consequently, there is no ‘twofold cost of sex’; however, as we discuss below, other costs of sex do apply. Budding yeast and other predominantly diploid species have, after germination of the meiotic spore, no haploid phase of cell divisions, or only a very short one, and as soon as a partner is found will fuse to form a diploid cell. These diploid cells will continue asexual growth and will on induction finish the sexual cycle by producing meiotic offspring. The moment of mating and meiosis in these species can thus be separated from each other for significant amounts of time.
Figure 1.
Cartoon of the generalized life cycles of filamentous and single-celled Ascomycota and the parasexual cycle. (a) Filamentous fungi are generally hermaphroditic and reproduction occurs by fertilization of the female fruiting bodies (large triangles) in which a short-lived diploid phase yields haploid spores after meiosis. These spores can germinate to form a new haploid mycelium. When fertilization occurs by propagules (conidia) these often can also be used for asexual reproduction. (b) Haploid yeast cells can multiply by cell division. Mating occurs by fusion of two isogamous haploid cells forming a diploid cell that can multiply asexually or go into meiosis producing haploid offspring. Mating in both (a) and (b) is generally only possible between cells of opposite mating type (see the main text). (c) Schematic of parasexual reproduction and (d) mitotic recombination and loss of heterozygosity (LOH). (c) and (d) might occur whenever there is a diploid phase, e.g. after mating or genome duplication.
Above, we quickly skimmed over the mating part in the descriptions of the life cycles, which, as in most organisms is not a trivial matter (see also Beekman et al. [15,16]). Two individuals must first find each other physically, either by growing at the same location, or by finding another individual through fertilizing propagules—often via conidia that are also the most common asexual propagules—comparable to pollen dispersal or sperm casting [17]. Sperm and pollen are costly to produce, and ‘lost’ when no female gamete is found, contributing to the twofold cost of sex [9]. In most fungi, however, conidia are capable of germination giving rise to a new clone, thus strongly reducing this cost. Other species produce smaller microconidia specialized for fertilization that do not readily germinate and thus do carry costs similar to sperm and pollen [18]. But mating requires more than merely finding a partner.
3. Mating types and finding a compatible mate
Mating in fungi is highly regulated and in general only possible between cells of opposite mating type. Mating types are genetically defined incompatibility groups that at the haploid level regulate if successful mating is possible between individuals. Mating types have evolved multiple times in eukaryotes (e.g. in fungi, green algae, diatoms [1,19]), and the forces explaining their origins remain debated [19,20]. Their function has been well studied especially in fungi [21]. The genetic basis of mating type is best understood in Ascomycota, where a single mating-type locus exists with two alleles. Interestingly, the mating-type alleles include genes encoding for completely different proteins, so are technically not alleles, and instead termed idiomorphs [22]; for simplicity, we refer to them as alleles nevertheless. A zygote is thus always heterozygous at the mating-type locus. The alleles at this locus are highly diverged and often one or both of the alleles incorporate one (e.g. in the Saccharomycotina) or a few (e.g. in Sordariomycetes) additional genes tightly linked to the mating-type locus [23]. The mating-type genes regulate downstream functions that, before mating, mainly regulate mate recognition and mating competence of the haploid cell [24]. Note that in contrast with self-incompatibility in, for instance, angiosperms, mating types do not prevent inbreeding by selfing of diploid genotypes, because after meiosis half of a tetrad is compatible with the other half [25] and instead will only be able to prevent selfing of haploid genotypes.
As a consequence of mating types, the chance that two individuals of a species with two mating types that meet randomly are of opposite mating type and thus compatible is 50%. Especially when densities are low, this will greatly decrease the chance for a successful mating between individuals. Similar to plants losing self-incompatibility under low densities (known as Baker's law [26]), fungi can evolve to become universally compatible, which is known as homothallism. Different forms of homothallism are known: same mating-type (or unisexual) mating (where mating occurs between cells of the same mating type, see also §7b), mating-type switching, and ‘true homothallism’ [27]. In the latter, fungi incorporate the alleles from both mating types into the same haploid genome, and as a consequence are compatible with all other individuals, including self and haploid clone-mates [28]. True homothallism is the most common form of homothallism and evolved repeatedly, sometimes multiple times independently within the same genus (e.g. [29]). Many yeasts and some filamentous Ascomycota are homothallic by switching between mating types during asexual growth (e.g. fission yeast, budding yeast or the plant pathogen Sclerotinia sclerotiorum; reviewed in [27]). These mating-type switchers have the genes for both mating types in their haploid genome, but only the genes at one specific physical location are expressed, while the genes of the other mating type at another locus are silenced [30,31]. During asexual growth, the genes from the silent locus are moved into the active locus, such that the daughter cell becomes the opposite mating type, and thus now is compatible with the mother cell [31]. A single individual can thus reproduce sexually after a single round of asexual reproduction without the need of another genotype.
Selfing in mating-type switchers and homothallic species will result in reproduction between individuals with identical haploid genomes, for which the assumed main driver of sex—recombination [32]—does not seem to yield any benefits [25]. We will first have a look at the importance of recombination and will then discuss other benefits of sexual reproduction.
4. Sexual reproduction and recombination
One of the main consequences of sexual reproduction is the mixing of genetic material between individuals, which by recombination and segregation during meiosis leads to new haplotypes. Alleles will thus become associated with different ones, potentially increasing variation in the population. Weismann [33] was the first to appreciate that the main function of sexual reproduction was the ‘mingling of the hereditary tendencies of father and mother’ and he suggested that it is this increased diversity on which selection can act. Recombination works at two levels: first, it can bring together beneficial alleles that are not present in the same genome, thereby increasing the speed of adaptation [34,35]; second, it can separate beneficial mutations from deleterious ones that are linked to it [36]. A variety of experimental studies have shown that adaptation in sexual populations occurs faster than in asexual populations (e.g. [8,37]). A recent study that tracked novel mutations in an evolution experiment using baker's yeast confirmed that sex improves the speed of adaptation to a novel environment both by combining beneficial mutations and removing deleterious mutations from genotypes with beneficial ones [8].
Even though environments in nature are never stable, they are generally predictable and thus species tend to undergo local adaptation. Under such semi-stable circumstances, there is little benefit of variation and selection will remove genotypes that are not locally adapted. In a population, most individuals will thus carry a combination of genes that together have a relatively high fitness in their local environment [6]. Recombination will break up these good combinations and will generate offspring with reduced fitness compared with the optimal genotype, which is known as recombination load [6,38]. A recent study in Aspergillus nidulans with artificially introduced, mainly deleterious, mutations found that overall outcrossing indeed confers a cost over selfing, but also rare high-fitness recombinants occurred [39].
To avoid destruction of good genotypes by sex, recombination can be blocked between beneficial gene combinations [40,41]. Many fungi are known to have evolved inversions of parts of their chromosomes, which can block recombination between chromosomes during meiosis [42,43]. Such recombination blocks are most easily observed when the effects are severe, which is the case in meiotic drive elements as seen in S. pombe [44] or the spore killers in Neurospora intermedia [45]. In both cases, rearrangements cause linkage between elements that together are beneficial. In some heterothallic fungi the chromosomes carrying the mating-type genes show suppressed recombination over a large region, sometimes almost the entire chromosome's length [43]; however, it is unclear if this evolved to maintain beneficial gene combinations. Alternatively, this could be driven by meiotic segregation mechanisms that maintain heterozygosity at the mating-type locus; because most of these species are diploid throughout their life, any heterozygous sites linked to the mating-type locus—which is per definition heterozygous—will be maintained [43]. Even though linkage will maintain beneficial gene combinations, at the regions with suppressed recombination Muller's ratchet can start turning and deleterious mutations will accumulate, leading to degeneration similar to that seen in sex chromosomes [46].
More extremely, some fungi have an alternative reproductive cycle that removes recombination at all chromosomes, but allows reassortment of the chromosomes, called the parasexual cycle [47]. Parasex occurs by fusion of haploid individuals to become diploid, or by fusion of diploid individuals to become tetraploid [48]. What is remarkable is what happens next. During vegetative growth, one by one the chromosomes, of which multiple copies are present, will be shed, going through stages of aneuploidy until after some time only one copy of each will remain (or two in diploid species; see figure 1c). Which chromosome will be retained appears to be random, and any combination of the different chromosomes is thus possible. C. albicans, a pathogenic yeast species that is not known to naturally mate but does show population genetic signs of recombination [49], can be induced to mate under certain circumstances using the parasexual cycle [48]. A recent study using mouse model experiments showed that novel parasexually produced genotypes can be obtained that are fitter than the parental strains [50]. Unfortunately it is not known if parasex occurs in nature, because in most Ascomycota it may be limited to species which are vegetatively compatible (usually genetically identical mycelia) and thus is difficult to detect. Whether it evolved to induce recombination suppression between beneficial gene combinations or is a by-product of heterokaryosis also is unknown.
5. Sexual reproduction with benefits
Even though recombination is assumed the most important function of sexual reproduction, there are other characteristics of sex that together can compensate for the costs. These might explain why many fungi reproduce sexually by haploid selfing with little or none of the benefits of recombination.
First, sexual reproduction is often associated with an alternative cell cycle that results in production of survival structures—generally, the haploid ascospores that are the result of meiosis themselves [13]. Association of survival and sex in facultative asexuals is common also outside of fungi (e.g. in Daphnia spp. [10]) and is often associated with low resource availability, crowding or some other form of stress, or seasonal changes. Sexual reproduction under unfavourable circumstances can be beneficial, because newly generated variation can yield genotypes that are locally fit, which is especially likely to evolve in haploid species [51]. The association of sexual reproduction and survival will also be beneficial in this circumstance, especially when germination is environment dependent: the locally good genotypes can germinate and grow, and the bad ones can be maintained as resting spores. It has been argued that the maintenance of sex in facultative asexuals can be explained by developmental constraints between sex and survival structures when selection for the latter occurs, even when sex is by selfing [52,53]. Especially, when sex is limited to unfavourable environments, selection to separate sex and survival structures from each other is reduced, because in these circumstances asexual reproduction itself is difficult and the relative cost of sex might be strongly reduced [54].
Other non-recombination benefits of sex occur at the genetic level, just before and during meiosis. Fungi have two mechanisms that act at these moments in which (i) genomic regions that are present only in one of the parental chromosome copies are targeted (meiotic silencing by unpaired DNA or MSUD [55]) or (ii) regions that are present in multiple copies in the same genome are targeted (repeat-induced point mutations or RIP [56]). Both are thought to have evolved to suppress transposons, retroviruses and similar genomic parasites. During meiosis, when transposons are most active, MSUD silences through an unknown mechanism all homologous sequences that are not present in one copy per chromosome, using one of the RNA interference pathways [57]. RIP acts at the DNA level, and introduces point mutations in regions that show high (approx. 85% or more) sequence similarity [56]. Where MSUD prevents spread, RIP breaks down active transposons, but additionally increases the background mutation rate. The signature of RIP, an over-accumulation of G : C to A : T mutations, is found in many different fungal species [58]. Because RIP occurs during meiosis, the loss of sexual reproduction will make it harder to fight off transposable elements from spreading through the genome.
6. Reducing the costs of asexual reproduction
So far, we have discussed the benefits and the costs of sexual reproduction and some adaptations that evolved to reduce costs associated with sex (e.g. reduced frequency of sex, homothallism and suppression of recombination). Implicitly, we discussed the benefits (e.g. increased reproductive rate and maintenance of beneficial haplotypes) and costs (e.g. Muller's ratchet and Hill–Robertson interference) of asexual reproduction too, which appear to be the inverse of those during sexual reproduction. However, in Ascomycota costs of asexual reproduction might be reduced because of fungal life histories and specific evolved mechanisms that work in concordance.
First, it needs to be noted that because most Ascomycota are haploid, deleterious mutations are purged more easily than in diploid organisms, as these mutations are not sheltered [59]. The mutation load in haploids is thus expected to be reduced relative to diploids and mutations that do arise can be selected against efficiently [60,61].
Second, selection can already act between genomes within the individual. Most fungi have a multinucleate state in which one cell or hyphal compartment contains a number of mitotically derived cells [62]. Owing to their mitotic nature, these cells are generally identical, but mutations that occur during genome duplication can lead to nuclear differences known as heterokaryosis [63]. Experiments with artificially created heterokaryons have shown that during growth a less adapted nucleus can be lost due to competition between the nuclei [64]. These factors will not stop Muller's ratchet, but they will slow down the rate of decay, further reducing the need for frequent sexual reproduction to purge deleterious mutations.
Even stronger, owing to mitotic recombination between chromosomes from different nuclei, it is possible, without sex, to remove deleterious mutations from a population of nuclei within the individual, as well as to bring together beneficial mutations that arose independently within the individual. Recombination within heterokaryons has been shown many times in laboratory experiments for different species using selectable markers (e.g. A. nidulans, N. crassa [65]), where recombination presumably occurs through a parasexual cycle. However, it is unknown how important this function is in nature, where naturally occurring heterokaryons might be less common due to the vegetative incompatibility system.
7. How to measure the frequency of sexual versus asexual reproduction
Given the clear costs and advantages of sexual reproduction and the means by which costs of asexuality can be avoided, we now turn to the question of how often fungi reproduce sexually or asexually and the frequency of asexual species. It may be surprising that most fungi harvest the benefits of both, while examples of pure asexuality are extremely rare.
(a). Experimental evidence
Until very recently, fungi were considered to contain a large percentage of species that lacked sexual reproduction altogether. This inference was based on classical mycological studies where certain asexual growth forms (so-called anamorphs) were not known to be associated with sexual growth forms (so-called teleomorphs). Some species, however, were known to be pleomorphic, i.e. produced both sexual and asexual stages, and these presumably had facultative sex. Making the linkage between an anamorph and teleomorph typically relied on culturing studies where one form could be derived from spores of the other form, for example, which suffered greatly from biases in experimental conditions or self-sterility in heterothallic species. Species for which no sexual stage was known and which could not be allied with taxa based on asexual sporulation were placed in phylum Deuteromycota (Fungi imperfecti).
The pre-molecular era of mycology nonetheless had established that only half of Ascomycota are meiosporic, and of these most are obligately sexual and do not produce asexual spores [66]. The rest are presumably mitosporic and, in theory, obligately asexual, but may actually be facultatively sexual with heretofore undetected teleomorphs. Only about 5% of Ascomycota were known to be pleomorphic and thus facultatively sexual, making mixed-mating systems seem rare [66]. Despite the considerable variation in life cycles among the major groups of Ascomycota, mating systems appear to be similar across the phylum. A survey of the lichens of Britain and Ireland found that species were mostly sexually competent (90%), while fewer (29%) had lichen-specific vegetative reproductive strategies, such as production of small fragments containing both fungal and algal partners such as soredia [67]. These morphological data would seem to suggest that obligate sex is more normal than mixed- (meiotic and mitotic) mating systems.
Molecular data have led to massive revisions to fungal systematics, and one major change was the unification of anamorphs and teleomorphs, such that the group Deuteromycetes was abandoned altogether and the majority of the 15 000 or so asexual fungi embedded within the Ascomycota classification [68,69]. This revision provides a greater appreciation of the commonness of facultative sex in Ascomycota. On the other hand, some presumably anamorphic fungi, such as the ectomycorrhizal fungus Cenococcum, the anamorphic yeast Candida, the mould Aspergillus fumigatus, the vegetatively cloned cultivar of leaf cutter ants Leucoagaricus gongylophorus and the ubiquitous arbuscular mycorrhizal fungi Glomeromycota had been studied extensively for decades with no known morphological evidence of sex or meiosis, and some of these were labelled ancient asexual scandals [70]. However, each of these scandals, which presumably had been asexual for a very long time, would ultimately fail the tests of asexuality through molecular evidence.
(b). Mating-type genes
When the mating-type loci of the model species Saccharomyces and Neurospora were cloned, it opened up the possibility that the secret sex lives of fungi could be investigated for a number of species that either had no obvious teleomorph or were non-amenable to laboratory manipulation [71]. Fortunately for fungal geneticists, the mating-type gene families throughout the Dikarya appear to be homologous, with homeodomain or high mobility group transcription factors universally used as master regulatory switches [23,72]. The assumption for asexual species or anamorphs, based on the prevailing notions at the time, was that they would either not contain any mating-type genes or that they would be fixed for a single mating-type allele. Contrary to these assumptions, the supposed asexuals in Dikarya actually all have genes homologous to mating-type loci in their genome. With the exception of Lodderomyces elongisporus, a homothallic yeast in the Candida clade, all genome sequences of Dikarya have identifiable homologues of the mating-type genes [73] including anamorphic taxa formerly placed in Deuteromycota [74]. These mating-type data provided the clue that either these so-called asexuals might be having a form of ‘cryptic sex’ or that they have recently arisen from sexual species, and therefore are not long-term asexual lineages. Many of the classic anamorphic genera such as Aspergillus and Candida were shown to have mating-type genes in their genome [73,75], and the genes were polymorphic and not pseudo-genes, implying that multiple mating types existed in the population [76,77]. Such was the case with A. fumigatus, which was considered a prime example of a purely asexually reproducing species because it was so well studied and had no evidence of a sexual stage. Yet, population genetics suggested recombination and determined that populations were polymorphic for the two mating types in roughly equal proportions [78]. Assuming that these hallmarks of sexual reproduction were not red herrings, the researchers persisted, and after 100+ years of study and no teleomorphic links, the authors were able to produce a teleomorph by pairing appropriate isolates of A. fumigatus on oatmeal agar [79]. Other examples of presumed asexuals with recently discovered sexual cycles are C. albicans and Septoria passerinii (reviewed in [80]).
Outside of the Ascomycota, molecular data have had considerably less of an impact on the understanding of sex in the early diverging fungal lineages, which lack conspicuous fruiting bodies. Many of these groups, such as Glomeromycota, Cryptomycetes, Spizellomycetales and Neocallimastigomycota have no known sexual cycle. Heterokaryosis versus clonality in arbuscular mycorrhizal fungi (Glomeromycota) has been particularly controversial and this group was considered the best example of an ancient asexual fungal ‘scandal’ [81]. This debate seems to finally have been resolved by genome sequencing, where the best-known evidence for sex was obtained after sequencing the genomes of multiple strains of the best-known species Rhizophagus irregularis. It, like essentially all fungi, has a genome with a standard meiotic toolkit and mating-type gene homologues whose allelism is correlated with heterokaryosis [82,83]. Thus, the myth of the most scandalous ancient fungal asexual needs a retraction.
Equal frequencies of mating types is the norm across the majority of studies, and where unequal ratios are observed, this is often a phenomenon of small populations [84]. Nonetheless, some sexual species have a strong mating-type skew, such as the basidiomycete pathogen of humans Cryptococcus neoformans where the alpha mating type is found in 95% of clinical and environmental isolates [85]. Such a skew is consistent with rare sexual reproduction and/or strong selection on pleiotropic effects of a mating-type allele. Interestingly, the C. neoformans mating-type locus includes many more genes than the average fungal mating-type locus and is known to have effects on virulence and hyphal growth. Cryptococcus is also special in that under certain circumstances mating occurs between cells of the same mating type [86], which counteracts Fisher's principle of equal sex and mating-type ratios. In summary, although the presence of multiple mating types is strong evidence of sex, this should be coupled with population level tests that demonstrate recombination is actually occurring.
(c). Population-level variation
The most robust evidence for identifying a sexual cycle in a species without obvious morphological sex is to identify population-level signatures of meiotic recombination. In haploid fungi, these signatures are relatively straight forward as they involve looking for recombination between alleles at linked or unlinked loci. Soon after the advent of PCR markers, it was observed that the many presumably asexual species and species with no morphological evidence of sex were recombining. Examples of well-known species with no known meiotic stage but population structures indicating some level of recombination included: Coccidioides immitis [87], Aspergillus flavus [88], F. oxysporum [89] and Cenococcum geophilum [90]. Indeed finding and demonstrating a truly asexual species began to be considered nearly impossible [91].
What have the population genetic studies really been demonstrating though? The aforementioned studies all showed evidence of both clonality and recombination, and therefore the take-home-messages on population structure has varied from one publication to the next. Tests for recombination, such as the four-gamete test, which tests for the existence of all allele combinations at multiple loci within populations, can detect rare recombination [92], while tests such as the Index of Association address genomic levels of linkage disequilibrium (LD) and might be expected to be less sensitive [93]. However, both the frequency and nature of recombination cannot be distinguished by these methods. For example, parasexual recombination (see above) cannot be distinguished from meiotic recombination. Importantly, parasexuality does not involve teleomorph production and the production of survival structures, and therefore distinguishing parasexuality from meiosis would be very relevant information when characterizing fungal life cycles as might be desired by plant pathologists. Diploid fungi present additional problems. Detecting a largely clonal population is straightforward by demonstrating heterozygosity excess, which is expected to increase over time due to mutations, known as the Meselson effect [94]. When rates of mitotic recombination are high, this can influence the interpretation because it creates homozygous genotypes from heterozygous ones, so-called loss of heterozygosity (LOH; figure 1d), and could be confounded with rare sex [95]. Additionally, gene conversion will leave a similar signature [96]. In L. gongylophorus—the basidiomycete fungal symbiont of the leaf cutter ant—recombination has been clearly demonstrated among cultivars on the islands of Guadeloupe [97], but the population genetic patterns are consistent with either selfing of a single introduced diploid genotype or LOH during asexual growth by mitotic recombination or related mechanisms. For Batrachochytrium dendrobatidis and C. albicans, tests of recombination have shown heterozygosity excess at some loci and not others [98–101], leading to ongoing debate in both species regarding the role of mitotic versus meiotic recombination. One take home is that neither testing against a null model of random segregation nor linkage equilibrium are satisfactory for addressing just how often sex occurs in these systems, which undoubtedly have very rare sex.
Perhaps counterintuitively, high genome-wide levels of heterozygosity should be taken as the signature of sex, rather than evidence of ancient asexuality [102]. This follows because experimental studies show that the genome-wide rate of mutation is much lower than the genome-wide rate of LOH [103–105]. Therefore, anciently asexual species are expected to be largely homozygous with the caveat that there are mechanisms that buffer from LOH across genomic regions, such as chromosomal rearrangements. Given that it is now hypothesized that the greatest asexual scandals, the bdelloid rotifers, are sexual [106], though this interpretation may also be confounded with mitotic gene conversion [11], then the Meselson effect may not be possible, given the high rate LOH observed in most organisms. On the other hand, the footprint of LOH can be used to understand the frequency of sex. Magwene et al. [107] used regions of LOH to estimate the time between sexual outcrossing events for 11 S. cerevisiae genotypes. Their logic was that heterozygosity was generated by an initial outcross and LOH then proceeded to remove heterozygosity at a steady rate: one calculated in the laboratory. That these values (one sexual generation for every 30 000 mitotic) agreed well with another measure of the rate of sexual reproduction in yeast using a coalescence approach (1 in 50 000) [108] confirms the clock-like decay of heterozygosity during mitotic divisions.
In Ascomycota, facultative sexuality should now be seen as a dominant mode of reproduction, particularly in the myriad microscopic fungi. Yet, other than the aforementioned studies with S. cerevisiae, little is known about the actual rates of sexual versus mitotic reproduction in facultative species, though understanding the ratio of the two would add a great deal to the ongoing debate about the role of sexual reproduction in evolution. Perhaps, the most comprehensive study of alternative reproductive strategies comes again from Saccharomyces, where Tsai et al. [109] developed a model to calculate the ratio of sexual generations to mitotic generations and distinguished various types of sexual processes in S. paradoxus. Their strategy leveraged the differential effects of inbreeding on standing genetic diversity at the nucleotide level, often denoted θ, and on the correlation in allele frequencies among loci, or the linkage disequilibrium (LD) parameter ρ. The logic used was that the mode of reproduction has only a modest effect on θ, but will have a strong effect on ρ because mitotic division or severe inbreeding generates LD in contrast with outcrossing which reduces it. Tsai et al. then used effective population sizes with respect to θ and ρ and showed that the ρ effective population size was severely reduced, with only one meiotic generation per 1000 mitotic generations. Furthermore, selfing was distinguished from outcrossing, the latter of which had a rate of only 1 in 105 generations or one half that estimated for S. cerevisiae. This approach could readily be extended to other fungi, and has been recently done so for fission yeast, where the outcrossing rate was a mere 1 in 600 000 mitotic generations [110], but does require having independent estimates of recombination and mutation rates per basepair. With the onset of next generation sequencing to measure these rates, such approaches are likely to be readily adopted.
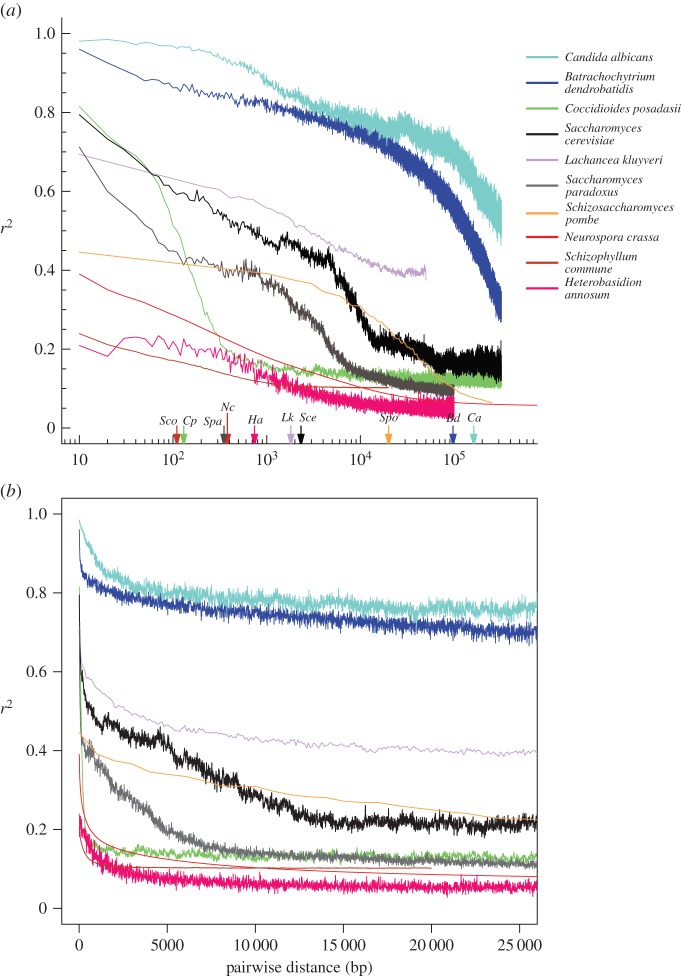
Taylor et al. [111] proposed that LD decay, the decrease in LD between pairs of sites increasing distances apart in the genome, could be a useful means of addressing the relative importance of recombination in a fungal life cycle across the diversity of species, and this seems like a useful approximation. Although LD can be influenced by a number of factors, such as intrinsic differences in recombination rates, background selection and demographic history, focusing on genome-wide estimates and categories of nearly neutral mutations could avoid region-specific problems with selection and recombination rate variation. We attempted to test this by comparison of LD decay in some of the better-known fungal species (figure 2). The distances at which LD is half decayed generally tracks the standard interpretation of sexual frequency across these species (table 1). The most sexual species of the group is the obligately outcrossing mushroom Schizophyllum commune with an abrupt LD half-decay distance at 110 bp. Yeast species show intermediate decay values, revealing a mixed reproductive mode with clonality and occasional sexual reproduction. Finally, those species that are widely accepted as being highly clonal, B. dendrobatidis and C. albicans, show very slow rates of LD decay, with half-decay points at greater than 100 kb. Both of these are emerging pathogens, which may reflect the brevity of strict clonality due to the costs mentioned above.
Figure 2.
Plot of linkage disequilibrium (r2) as a function of distance between nucleotides across 10 fungal species. Arrows indicate half-decay values of LD (table 1; LD502). (a) Log-linear plot, (b) zoomed in linear–linear plot showing steep decay. Note that the asymptotic value of LD at increasing distances varies across species because of differing underlying population structure.
Table 1.
Linkage disequilibrium statistics for 10 diverse fungi. Max LD and min LD are maximum and minimum LD values observed. LD501 is the physical distance in basepairs of the LD value halfway between the maximum and zero. LD502 is the physical distance in basepairs of the LD value halfway between max LD and min LD. All LD values are calculated as r2 [112]. Values for S. commune, N. crassa, L. kluyveri and S. pombe were obtained from original publications, and the remaining values were calculated using the software plink [113].
| species | max LD | min LD | LD501 | LD502 | reference |
|---|---|---|---|---|---|
| Schizophyllum commune (USA) | 0.2392 | 0.1011 | 730 | 110 | [114] |
| Coccidioides posadasii | 0.8151 | 0.0937 | 150 | 130 | [115] |
| Saccharomyces paradoxus (European) | 0.7123 | 0.0776 | 910 | 350 | [116] |
| Neurospora crassa (LA) | 0.3904 | 0.0573 | 700 | 380 | [117] |
| Heterobasidion annosum | 0.2341 | 0.0208 | 750 | 740 | [118] |
| Lachancea kluyveri | 0.6935 | 0.3749 | n.a. | 1810 | [119] |
| Saccharomyces cerevisiae (Wine/European) | 0.7940 | 0.0736 | 5180 | 2320 | [116] |
| Schizosaccharomyces pombe | 0.4457 | 0.0634 | 27 010 | 20 010 | [120] |
| Batrachochytrium dendrobatidis (GPL) | 0.9601 | 0.0967 | 126 420 | 97 400 | [121] |
| Candida albicans MLST 1 | 0.9849 | 0.1823 | 286 740 | 162 100 | [122] |
Finally, the numerous population genetic studies of fungi reveal a great deal about genotypic frequencies, such as the recovery of clonal genotypes [123,124]. These data could be useful for estimating the frequency of sex using a coalescence-based framework for a single temporal sample or using a probabilistic model based on resampling of identical genotypes across generations [125]. Sexual rate in mixed mating species can also be explored in experimental field studies such as the inoculate-reisolate-genotype procedure where 10 isolates of Zymoseptoria triciti were inoculated into a field of winter wheat over one growing season. After reisolation, 30% of non-immigrant isolates were sexual recombinants of the original 10 genotypes, and the remaining were clonal derivatives [126].
8. Concluding remarks
Molecular tools have given much insight into the reproductive strategies of fungi and have shifted the paradigm from one where most fungi reproduce sexually to one in which there are very few truly asexual species, let alone ancient asexual lineages. Rather, reproduction in Ascomycota generally includes a combination of asexual reproduction interspersed with sex. Signatures of sex, such as balanced mating-type ratios and signs of recombination between loci, show that sex in most species occurs regularly, but not always frequently (e.g. once per 1000 asexual cell divisions in S. paradoxus [109]). However, our estimates might be muddled by a variety of phenomena, of which mitotic recombination might lead to overestimation, and haploid selfing lead to a strong underestimation of sex.
Some open questions need answering to make better estimates on the frequency of sex in fungi. What is the frequency of mitotic recombination and parasexual processes in nature? The occurrence of heterokaryons in nature—a requirement for mitotic recombination to generate new associations between alleles—for basidiomycetes is well known [127], but how common is it in Ascomycota, where heterokaryons are rarely formed due to vegetative incompatibility systems [128]. Could it be that ‘asexual’ species generally retain all the genes from the so-called meiosis toolkit primarily to function during parasexual reproduction? Understanding if these recombination-associated genes have multiple functions and, if so, when these novel functions evolved within the fungi might give insight into the importance of recombination without sex.
We speculate that the frequency of sex in self-compatible haploids might be strongly underestimated when only LD is analysed. In budding yeast, where this has been specifically analysed, there is an estimated 100-fold difference in the number of sexual events and the number of outcrossing events [109] and this might be even greater in fission yeast [110]. LD around the mating-type genes in heterothallic species is informative to estimate the frequency of sex because it is expected to be under balancing selection; unfortunately, this is not possible for homothallics.
Nonetheless, the analysis of genome-wide LD has much to offer for providing a comparable measure of frequency of sex across the fungi. Population genomics of many species promise to provide more and more information on the hidden sex life of fungi, but we must take into account the many tricks that fungi evolved to enjoy all the benefits of sex, without giving in to its many costs.
Acknowledgements
The authors are very grateful to Maria Andrianova, Christina Cuomo, Chris Ellison, Anne Friedrich, Kajsa Himmelstrand, Daniel Jeffares, Gianni Liti and Åke Olson for providing datasets used to produce figure 2. Two anonymous reviewers provided helpful comments on an earlier version of the manuscript.
Data accessibility
Data used to generate figure 2 is available in the online repository figshare under doi:10.6084/m9.figshare.3472754 [129].
Authors' contributions
Both authors contributed equally to the manuscript and approved of the final version for publication.
Competing interests
The authors declare no competing interests.
Funding
T.Y.J. is supported by the US National Science Foundation (DEB 1441677) and the National Institute of Health (AI105167-02) and B.P.S.N. by a grant from the Carl Tryggers Foundation.
References
- 1.Beukeboom L, Perrin N. 2014. The evolution of sex determination. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Hartfield M. 2016. Evolutionary genetic consequences of facultative sex and outcrossing. J. Evol. Biol. 29, 5–22. ( 10.1111/jeb.12770) [DOI] [PubMed] [Google Scholar]
- 3.Roze D. 2012. Disentangling the benefits of sex. PLoS Biol. 10, e1001321 ( 10.1371/journal.pbio.1001321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitton J, Sears CJ, Baack EJ, Otto SP. 2008. The dynamic nature of apomixis in the angiosperms. Int. J. Plant Sci. 169, 169–182. ( 10.1086/523369) [DOI] [Google Scholar]
- 5.Vrijenhoek RC. 1998. Animal clones and diversity. BioScience 48, 617–628. ( 10.2307/1313421) [DOI] [Google Scholar]
- 6.Otto SP. 2009. The evolutionary enigma of sex. Am. Nat. 174, S1–S14. ( 10.1086/599084) [DOI] [PubMed] [Google Scholar]
- 7.Maynard Smith J. 1978. The evolution of sex. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.McDonald MJ, Rice DP, Desai MM. 2016. Sex speeds adaptation by altering the dynamics of molecular evolution. Nature 531, 233–236. ( 10.1038/nature17143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehtonen J, Jennions MD, Kokko H. 2012. The many costs of sex. Trends Ecol. Evol. 27, 172–178. ( 10.1016/j.tree.2011.09.016) [DOI] [PubMed] [Google Scholar]
- 10.Hebert PDN, Ward RD, Weider LJ. 1988. Clonal-diversity patterns and breeding-system variation in Daphnia pulex, asexual-sexual complex. Evolution 42, 147–159. ( 10.2307/2409123) [DOI] [PubMed] [Google Scholar]
- 11.Debortoli N, Li X, Eyres I, Fontaneto D, Hespeels B, Tang CQ, Flot J-F, Van Doninck K. 2016. Genetic exchange among bdelloid rotifers is more likely due to horizontal gene transfer than to meiotic sex. Curr. Biol. 26, 723–732. ( 10.1016/j.cub.2016.01.031) [DOI] [PubMed] [Google Scholar]
- 12.Billiard S, López-Villavicencio M, Hood ME, Giraud T. 2012. Sex, outcrossing and mating types: unsolved questions in fungi and beyond. J. Evol. Biol. 25, 1020–1038. ( 10.1111/j.1420-9101.2012.02495.x) [DOI] [PubMed] [Google Scholar]
- 13.Stajich JE, Berbee ML, Blackwell M, Hibbett DS, James TY, Spatafora JW, Taylor JW. 2009. Primer–the fungi. Curr. Biol. CB 19, R840 ( 10.1016/j.cub.2009.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlesworth B. 1980. The cost of sex in relation to mating system. J. Theor. Biol. 84, 655–671. ( 10.1016/S0022-5193(80)80026-9) [DOI] [PubMed] [Google Scholar]
- 15.Aanen D, Beekman M, Kokko H. 2016. Weird sex: the underappreciated diversity of sexual reproduction. Phil. Trans. R. Soc. B 371, 20160262 ( 10.1098/rstb.2016.0262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beekman M, Nieuwenhuis B, Ortiz-Barrientos D, Evans JP. 2016. Sexual selection in hermaphrodites, sperm and broadcast spawners, plants and fungi. Phil. Trans. R. Soc. B 371, 20150541 ( 10.1098/rstb.2015.0541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson JB, Kohn LM. 2007. Dikaryons, diploids, and evolution. In Sex in fungi: molecular determination and evolutionary implications (eds Heitman J, Kronstad JW, Taylor JW, Casselton LA), pp. 333–348. Washington, DC: ASM Press. [Google Scholar]
- 18.Fukumori Y, Nakajima M, Akutsu K. 2004. Microconidia act the role as spermatia in the sexual reproduction of Botrytis cinerea. J. Gen. Plant Pathol. 70, 256–260. ( 10.1007/s10327-004-0124-9) [DOI] [Google Scholar]
- 19.Billiard S, López-Villavicencio M, Devier B, Hood ME, Fairhead C, Giraud T. 2011. Having sex, yes, but with whom? Inferences from fungi on the evolution of anisogamy and mating types. Biol. Rev. 86, 421–442. ( 10.1111/j.1469-185X.2010.00153.x) [DOI] [PubMed] [Google Scholar]
- 20.Perrin N. 2012. What uses are mating types? The ‘developmental switch’ model. Evolution 66, 947–956. ( 10.1111/j.1558-5646.2011.01562.x) [DOI] [PubMed] [Google Scholar]
- 21.Heitman J, Kronstad JW, Taylor JW, Casselton LA (eds). 2007. Sex in fungi: molecular determination and evolutionary implications. Washington, DC: ASM Press. [Google Scholar]
- 22.Glass NL, Grotelueschen J, Metzenberg RL. 1990. Neurospora crassa A mating-type region. Proc. Natl Acad. Sci. USA 87, 4912–4916. ( 10.1073/pnas.87.13.4912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler G. 2007. The evolution of MAT: the Ascomycetes. In Sex in fungi: molecular determination and evolutionary implications (eds Heitman J, Kronstad JW, Taylor JW, Casselton LA), pp. 3–18. Washington, DC: ASM Press. [Google Scholar]
- 24.Rogers DW, Denton JA, McConnell E, Greig D. 2015. Experimental evolution of species recognition. Curr. Biol. 25, 1753–1758. ( 10.1016/j.cub.2015.05.023) [DOI] [PubMed] [Google Scholar]
- 25.Giraud T, Yockteng R, Lopez-Villavicencio M, Refregier G, Hood ME. 2008. Mating system of the anther smut fungus Microbotryum violaceum: selfing under heterothallism. Eukaryot. Cell 7, 765–775. ( 10.1128/EC.00440-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pannell J, Barrett S. 1998. Baker's law revisited: reproductive assurance in a metapopulation. Evolution 52, 657–668. ( 10.2307/2411261) [DOI] [PubMed] [Google Scholar]
- 27.Wilson AM, Wilken PM, van der Nest MA, Steenkamp ET, Wingfield MJ, Wingfield BD. 2015. Homothallism: an umbrella term for describing diverse sexual behaviours. IMA Fungus 6, 207–214. ( 10.5598/imafungus.2015.06.01.13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paoletti M, Seymour FA, Alcocer MJC, Kaur N, Calvo AM, Archer DB, Dyer PS. 2007. Mating type and the genetic basis of self-fertility in the model fungus Aspergillus nidulans. Curr. Biol. 17, 1384–1389. ( 10.1016/j.cub.2007.07.012) [DOI] [PubMed] [Google Scholar]
- 29.Nygren K, Strandberg R, Gioti A, Karlsson M, Johannesson H. 2012. Deciphering the relationship between mating system and the molecular evolution of the pheromone and receptor genes in Neurospora. Mol. Biol. Evol. 29, 3827–3842. ( 10.1093/molbev/mss193) [DOI] [PubMed] [Google Scholar]
- 30.Klar AJS. 2007. Lessons learned from studies of fission yeast mating-type switching and silencing. Annu. Rev. Genet. 41, 213–236. ( 10.1146/annurev.genet.39.073103.094316) [DOI] [PubMed] [Google Scholar]
- 31.Hanson SJ, Byrne KP, Wolfe KH. 2014. Mating-type switching by chromosomal inversion in methylotrophic yeasts suggests an origin for the three-locus Saccharomyces cerevisiae system. Proc. Natl Acad. Sci. USA 111, E4851–E4858. ( 10.1073/pnas.1416014111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartfield M, Keightley PD. 2012. Current hypotheses for the evolution of sex and recombination. Integr. Zool. 7, 192–209. ( 10.1111/j.1749-4877.2012.00284.x) [DOI] [PubMed] [Google Scholar]
- 33.Weismann A. 1892. Amphimixis or the essential meaning of conjugation and sexual reproduction, 1891. In Essays upon heredity and kindred biological problems (trans EB Poulton, S Schonland, AE Shipley), pp. 99–222. Oxford, UK: Clarendon Press. [Google Scholar]
- 34.Fisher RA. 1930. Sexual reproduction and sexual selection. In The genetical theory of natural selection, pp. 121–145. Oxford, UK: Oxford University Press. [Google Scholar]
- 35.Hill WG, Robertson A. 1966. The effect of linkage on limits to artificial selection. Genet. Res. 8, 269–294. ( 10.1017/S001667230800949X) [DOI] [PubMed] [Google Scholar]
- 36.Peck JR. 1994. A ruby in the rubbish: beneficial mutations, deleterious mutations and the evolution of sex. Genetics 137, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becks L, Agrawal AF. 2012. The evolution of sex is favoured during adaptation to new environments. PLoS Biol. 10, e1001317 ( 10.1371/journal.pbio.1001317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charlesworth B, Barton NH. 1996. Recombination load associated with selection for increased recombination. Genet. Res. 67, 27–41. ( 10.1017/S0016672300033450) [DOI] [PubMed] [Google Scholar]
- 39.López-Villavicencio M, Debets AJM, Slakhorst M, Giraud T, Schoustra SE. 2013. Deleterious effects of recombination and possible nonrecombinatorial advantages of sex in a fungal model. J. Evol. Biol. 26, 1968–1978. ( 10.1111/jeb.12196) [DOI] [PubMed] [Google Scholar]
- 40.Charlesworth D. 2016. The status of supergenes in the 21st century: recombination suppression in Batesian mimicry and sex chromosomes and other complex adaptations. Evol. Appl. 9, 74–90. ( 10.1111/eva.12291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirkpatrick M. 2010. How and why chromosome inversions evolve. PLoS Biol. 8, e1000501 ( 10.1371/journal.pbio.1000501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown WRA, et al. 2011. A geographically diverse collection of Schizosaccharomyces pombe isolates shows limited phenotypic variation but extensive karyotypic diversity. G3 Genes Genomes Genet. 1, 615–626. ( 10.1534/g3.111.001123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Idnurm A, Hood ME, Johannesson H, Giraud T. 2015. Contrasted patterns in mating-type chromosomes in fungi: hotspots versus coldspots of recombination. Fungal Biol. Rev. 29, 220–229. ( 10.1016/j.fbr.2015.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanders SE, Eickbush MT, Yu JS, Kang J-W, Fowler KR, Smith GR, Malik HS. 2014. Genome rearrangements and pervasive meiotic drive cause hybrid infertility in fission yeast. eLife 3, e02630 ( 10.7554/eLife.02630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammond TM, Rehard DG, Xiao H, Shiu PKT. 2012. Molecular dissection of Neurospora spore killer meiotic drive elements. Proc. Natl Acad. Sci. USA 109, 12 093–12 098. ( 10.1073/pnas.1203267109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fraser JA, Heitman J. 2005. Chromosomal sex-determining regions in animals, plants and fungi. Curr. Opin. Genet. Dev. 15, 645–651. ( 10.1016/j.gde.2005.09.002) [DOI] [PubMed] [Google Scholar]
- 47.Pontecorvo G, Sermonti G. 1954. Parasexual recombination in Penicillium chrysogenum. Microbiology 11, 94–104. ( 10.1099/00221287-11-1-94) [DOI] [PubMed] [Google Scholar]
- 48.Forche A, Alby K, Schaefer D, Johnson AD, Berman J, Bennett RJ. 2008. The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol. 6, e110 ( 10.1371/journal.pbio.0060110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McManus BA, Coleman DC. 2014. Molecular epidemiology, phylogeny and evolution of Candida albicans. Infect. Genet. Evol. 21, 166–178. ( 10.1016/j.meegid.2013.11.008) [DOI] [PubMed] [Google Scholar]
- 50.Zhang N, Magee BB, Magee PT, Holland BR, Rodrigues E, Holmes AR, Cannon RD, Schmid J. 2015. Selective advantages of a parasexual cycle for the yeast Candida albicans. Genetics 200, 1117–1132. ( 10.1534/genetics.115.177170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hadany L, Otto SP. 2007. The evolution of condition-dependent sex in the face of high costs. Genetics 176, 1713–1727. ( 10.1534/genetics.107.074203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nunney L. 1989. The maintenance of sex by group selection. Evolution 43, 245–257. ( 10.2307/2409205) [DOI] [PubMed] [Google Scholar]
- 53.Gouyon. 1999. Sex: a pluralist approach includes species selection. (One step beyond and it's good.) J. Evol. Biol. 12, 1029–1030. ( 10.1046/j.1420-9101.1999.00130.x) [DOI] [Google Scholar]
- 54.Aanen DK, Hoekstra RF. 2007. Why sex is good: on fungi and beyond. In Sex in fungi: molecular determination and evolutionary implications (eds Heitman J, Kronstad JW, Taylor JW, Casselton LA), pp. 527–534. Washington, DC: ASM Press. [Google Scholar]
- 55.Shiu PKT, Raju NB, Zickler D, Metzenberg RL. 2001. Meiotic silencing by unpaired DNA. Cell 107, 905–916. ( 10.1016/S0092-8674(01)00609-2) [DOI] [PubMed] [Google Scholar]
- 56.Irelan JT, Hagemann AT, Selker EU. 1994. High frequency repeat-induced point mutation (RIP) is not associated with efficient recombination in Neurospora. Genetics 138, 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quoc NB, Nakayashiki H. 2015. RNA silencing in filamentous fungi: from basics to applications. In Genetic transformation systems in fungi, vol. 2 (eds van den Berg MA, Maruthachalam K), pp. 107–124. Berlin, Germany: Springer International. [Google Scholar]
- 58.Selker EU. 2002. Repeat-induced gene silencing in fungi. In Advances in genetics, vol. 46 (eds C. -ting Wu, JC Dunlap), pp. 439–450. New York, NY: Academic Press. [DOI] [PubMed] [Google Scholar]
- 59.Orr HA, Otto SP. 1994. Does diploidy increase the rate of adaptation? Genetics 136, 1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otto SP, Marks JC. 1996. Mating systems and the evolutionary transition between haploidy and diploidy. Biol. J. Linn. Soc. 57, 197–218. ( 10.1111/j.1095-8312.1996.tb00309.x) [DOI] [Google Scholar]
- 61.Kondrashov AS, Crow JF. 1991. Haploidy or diploidy: which is better? Nature 351, 314–315. ( 10.1038/351314a0) [DOI] [PubMed] [Google Scholar]
- 62.Gladfelter A, Berman J. 2009. Dancing genomes: fungal nuclear positioning. Nat. Rev. Microbiol. 7, 875–886. ( 10.1038/nrmicro2249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caten CE, Jinks JL. 1966. Heterokaryosis: its significance in wild homothallic ascomycetes and fungi imperfecti. Trans. Br. Mycol. Soc. 49, 81–93. ( 10.1016/S0007-1536(66)80038-4) [DOI] [Google Scholar]
- 64.Pittenger TH, Atwood KC. 1956. Stability of nuclear proportions during growth of Neurospora heterokaryons. Genetics 41, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orr-Weaver TL, Szostak JW. 1985. Fungal recombination. Microbiol. Rev. 49, 33–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reynolds DR. 1993. The fungal holomorph: an overview. In The Fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics (eds Reynolds DR, Taylor JW), pp. 15–25. Wallingford, UK: CAB International. [Google Scholar]
- 67.Seymour FA, Crittenden PD, Dyer PS. 2005. Sex in the extremes: lichen-forming fungi. Mycologist 19, 51–58. ( 10.1017/s0269915%D705002016) [DOI] [Google Scholar]
- 68.Taylor JW. 1995. Making the Deuteromycota redundant—a practical integration of mitosporic and meiosporic fungi. Can. J. Bot. 73, S754–S759. ( 10.1139/b95-319) [DOI] [Google Scholar]
- 69.Taylor JW. 2011. One fungus=one name: DNA and fungal nomenclature twenty years after PCR. IMA Fungus 2, 113–120. ( 10.5598/imafungus.2011.02.02.01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Judson OP, Normark BB. 1996. Ancient asexual scandals. Trends Ecol. Evol. 11, 41–46. ( 10.1016/0169-5347(96)81040-8) [DOI] [PubMed] [Google Scholar]
- 71.Turgeon BG, Christiansen SK, Yoder OC. 1993. Mating type genes in Ascomycetes and their imperfect relatives. In The Fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics (eds Reynolds DR, Taylor JW), pp. 199–215. Wallingford, UK: CAB International. [Google Scholar]
- 72.Martin T, Lu S-W, van Tilbeurgh H, Ripoll DR, Dixelius C, Turgeon BG, Debuchy R. 2010. Tracing the origin of the fungal α1 domain places its ancestor in the HMG-box superfamily: implication for fungal mating-type evolution. PLoS ONE 5, e15199 ( 10.1371/journal.pone.0015199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butler G, et al. 2009. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459, 657–662. ( 10.1038/nature08064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turgeon BG. 1998. Application of mating type gene technology to problems in fungal biology. Annu. Rev. Phytopathol. 36, 115–137. ( 10.1146/annurev.phyto.36.1.115) [DOI] [PubMed] [Google Scholar]
- 75.Galagan JE, et al. 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438, 1105–1115. ( 10.1038/nature04341) [DOI] [PubMed] [Google Scholar]
- 76.Lockhart SR, Pujol C, Daniels KJ, Miller MG, Johnson AD, Pfaller MA, Soll DR. 2002. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 162, 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramirez-Prado JH, Moore GG, Horn BW, Carbone I. 2008. Characterization and population analysis of the mating-type genes in Aspergillus flavus and Aspergillus parasiticus. Fungal Genet. Biol. 45, 1292–1299. ( 10.1016/j.fgb.2008.06.007) [DOI] [PubMed] [Google Scholar]
- 78.Paoletti M, et al. 2005. Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Curr. Biol. 15, 1242–1248. ( 10.1016/j.cub.2005.05.045) [DOI] [PubMed] [Google Scholar]
- 79.O'Gorman CM, Fuller HT, Dyer PS. 2009. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457, 471–474. ( 10.1038/nature07528) [DOI] [PubMed] [Google Scholar]
- 80.Kück U, Pöggeler S. 2009. Cryptic sex in fungi. Fungal Biol. Rev. 23, 86–90. ( 10.1016/j.fbr.2009.10.004) [DOI] [Google Scholar]
- 81.Sanders IR, Croll D. 2010. Arbuscular mycorrhiza: the challenge to understand the genetics of the fungal partner. Annu. Rev. Genet. 44, 271–292. ( 10.1146/annurev-genet-102108-134239) [DOI] [PubMed] [Google Scholar]
- 82.Ropars J, et al. 2016. Evidence for the sexual origin of heterokaryosis in arbuscular mycorrhizal fungi. Nat. Microbiol. 1, 16033 ( 10.1038/nmicrobiol.2016.33) [DOI] [PubMed] [Google Scholar]
- 83.Halary S, Malik S-B, Lildhar L, Slamovits CH, Hijri M, Corradi N. 2011. Conserved meiotic machinery in Glomus spp., a putatively ancient asexual fungal lineage. Genome Biol. Evol. 3, 950–958. ( 10.1093/gbe/evr089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh G, Dal Grande F, Cornejo C, Schmitt I, Scheidegger C. 2012. Genetic basis of self-incompatibility in the lichen-forming fungus Lobaria pulmonaria and skewed frequency distribution of mating-type idiomorphs: implications for conservation. PLoS ONE 7, e51402 ( 10.1371/journal.pone.0051402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan Z, Li X, Xu J. 2002. Geographic distribution of mating type alleles of Cryptococcus neoformans in four areas of the United States. J. Clin. Microbiol. 40, 965–972. ( 10.1128/JCM.40.3.965-972.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin X, Hull CM, Heitman J. 2005. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434, 1017–1021. ( 10.1038/nature03448) [DOI] [PubMed] [Google Scholar]
- 87.Burt A, Carter DA, Koenig GL, White TJ, Taylor JW. 1996. Molecular markers reveal cryptic sex in the human pathogen Coccidioides immitis. Proc. Natl Acad. Sci. USA 93, 770–773. ( 10.1073/pnas.93.2.770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geiser DM, Pitt JI, Taylor JW. 1998. Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc. Natl Acad. Sci. USA 95, 388–393. ( 10.1073/pnas.95.1.388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koenig RL, Ploetz RC, Kistler HC. 1997. Fusarium oxysporum f. sp. cubense consists of a small number of divergent and globally distributed clonal lineages. Phytopathology 87, 915–923. ( 10.1094/phyto.1997.87.9.915) [DOI] [PubMed] [Google Scholar]
- 90.LoBuglio KF, Taylor JW. 2002. Recombination and genetic differentiation in the mycorrhizal fungus Cenococcum geophilum Fr. Mycologia 94, 772–780. ( 10.2307/3761692) [DOI] [PubMed] [Google Scholar]
- 91.Taylor D, Trimble S, McCauley D. 1999. Ecological genetics of gynodioecy in Silene vulgaris: relative fitness of females and hermaphrodites during the colonization process. Evolution 53, 745–751. ( 10.2307/2640714) [DOI] [PubMed] [Google Scholar]
- 92.Stumpf MPH, McVean GAT. 2003. Estimating recombination rates from population-genetic data. Nat. Rev. Genet. 4, 959–968. ( 10.1038/nrg1227) [DOI] [PubMed] [Google Scholar]
- 93.Maynard Smith J, Smith NH, O'Rourke M, Spratt BG. 1993. How clonal are bacteria? Proc. Natl Acad. Sci. USA 90, 4384–4388. ( 10.1073/pnas.90.10.4384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Meeus T, Lehmann L, Balloux F. 2006. Molecular epidemiology of clonal diploids: a quick overview and a short DIY (do it yourself) notice. Infect. Genet. Evol. 6, 163–170. ( 10.1016/j.meegid.2005.02.004) [DOI] [PubMed] [Google Scholar]
- 95.Balloux F, Lehmann L, de Meeus T. 2003. The population genetics of clonal and partially clonal diploids. Genetics 164, 1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hartfield M, Wright SI, Agrawal AF. 2016. Coalescent times and patterns of genetic diversity in species with facultative sex: effects of gene conversion, population structure, and heterogeneity. Genetics 202, 297–312. ( 10.1534/genetics.115.178004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mikheyev AS, Mueller UG, Abbot P. 2006. Cryptic sex and many-to-one colevolution in the fungus-growing ant symbiosis. Proc. Natl Acad. Sci. USA 103, 10 702–10 706. ( 10.1073/pnas.0601441103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pujol C, Reynes J, Renaud F, Raymond M, Tibayrenc M, Ayala FJ, Janbon F, Mallie M, Bastide JM. 1993. The yeast Candida albicans has a clonal mode of reproduction in a population of infected human immunodeficiency virus-positive patients. Proc. Natl Acad. Sci. USA 90, 9456–9459. ( 10.1073/pnas.90.20.9456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gräser Y, Volovsek M, Arrington J, Schonian G, Presber W, Mitchell TG, Vilgalys R. 1996. Molecular markers reveal that population structure of the human pathogen Candida albicans exhibits both clonality and recombination. Proc. Natl Acad. Sci. USA 93, 12 473–12 477. ( 10.1073/pnas.93.22.12473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.James TY, et al. 2009. Rapid expansion of an emerging fungal disease into declining and healthy amphibian populations. PLoS Pathog. 5, e1000458 ( 10.1371/journal.ppat.1000458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Farrer RA, et al. 2011. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc. Natl Acad. Sci. USA 108, 18 732–18 736. ( 10.1073/pnas.1111915108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Welch DBM, Meselson M. 2000. Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science 288, 1211–1215. ( 10.1126/science.288.5469.1211) [DOI] [PubMed] [Google Scholar]
- 103.Mandegar MA, Otto SP. 2007. Mitotic recombination counteracts the benefits of genetic segregation. Proc. R. Soc. B 274, 1301–1307. ( 10.1098/rspb.2007.0056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Forche A, Magee PT, Selmecki A, Berman J, May G. 2009. Evolution in Candida albicans populations during a single passage through a mouse host. Genetics 182, 799–811. ( 10.1534/genetics.109.103325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tucker AE, Ackerman MS, Eads BD, Xu S, Lynch M. 2013. Population-genomic insights into the evolutionary origin and fate of obligately asexual Daphnia pulex. Proc. Natl Acad. Sci. USA 110, 15 740–15 745. ( 10.1073/pnas.1313388110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Signorovitch A, Hur J, Gladyshev E, Meselson M. 2015. Allele sharing and evidence for sexuality in a mitochondrial clade of bdelloid rotifers. Genetics 200, 581–590. ( 10.1534/genetics.115.176719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Magwene PM, Kayikci O, Granek JA, Reininga JM, Scholl Z, Murray D. 2011. Outcrossing, mitotic recombination, and life-history trade-offs shape genome evolution in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 108, 1987–1992. ( 10.1073/pnas.1012544108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruderfer DM, Pratt SC, Seidel HS, Kruglyak L. 2006. Population genomic analysis of outcrossing and recombination in yeast. Nat. Genet. 38, 1077–1081. ( 10.1038/ng1859) [DOI] [PubMed] [Google Scholar]
- 109.Tsai IJ, Bensasson D, Burt A, Koufopanou V. 2008. Population genomics of the wild yeast Saccharomyces paradoxus: quantifying the life cycle. Proc. Natl Acad. Sci. USA 105, 4957–4962. ( 10.1073/pnas.0707314105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Farlow A, Long H, Arnoux S, Sung W, Doak TG, Nordborg M, Lynch M. 2015. The spontaneous mutation rate in the fission yeast Schizosaccharomyces pombe. Genetics 201, 737–744. ( 10.1534/genetics.115.177329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taylor JW, Hann-Soden C, Branco S, Sylvain I, Ellison CE. 2015. Clonal reproduction in fungi. Proc. Natl Acad. Sci. USA 112, 8901–8908. ( 10.1073/pnas.1503159112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Slatkin M. 2008. Linkage disequilibrium—understanding the evolutionary past and mapping the medical future. Nat. Rev. Genet. 9, 477–485. ( 10.1038/nrg2361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. 2015. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4, 7 ( 10.1186/s13742-015-0047-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baranova MA, et al. 2015. Extraordinary genetic diversity in a wood decay mushroom. Mol. Biol. Evol. 32, 2775–2783. msv153 ( 10.1093/molbev/msv153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Neafsey DE, et al. 2010. Population genomic sequencing of Coccidioides fungi reveals recent hybridization and transposon control. Genome Res. 20, 938–946. ( 10.1101/gr.103911.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bergström A, et al. 2014. A high-definition view of functional genetic variation from natural yeast genomes. Mol. Biol. Evol. 31, 872–888. ( 10.1093/molbev/msu037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ellison CE, Hall C, Kowbel D, Welch J, Brem RB, Glass NL, Taylor JW. 2011. Population genomics and local adaptation in wild isolates of a model microbial eukaryote. Proc. Natl Acad. Sci. USA 108, 2831–2836. ( 10.1073/pnas.1014971108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dalman K, Himmelstrand K, Olson A, Lind M, Brandstrom-Durling M, Stenlid J. 2013. A genome-wide association study identifies genomic regions for virulence in the non-model organism Heterobasidion annosum s.s. PLoS ONE 8, 10 ( 10.1371/journal.pone.0053525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Friedrich A, Jung P, Reisser C, Fischer G, Schacherer J. 2015. Population genomics reveals chromosome-scale heterogeneous evolution in a protoploid yeast. Mol. Biol. Evol. 32, 184–192. ( 10.1093/molbev/msu295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jeffares DC, et al. 2015. The genomic and phenotypic diversity of Schizosaccharomyces pombe. Nat. Genet. 47, 235–241. ( 10.1038/ng.3215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rosenblum EB, et al. 2013. Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. Proc. Natl Acad. Sci. USA 110, 9385–9390. ( 10.1073/pnas.1300130110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hirakawa MP, et al. 2015. Genetic and phenotypic intra-species variation in Candida albicans. Genome Res. 25, 413–425. ( 10.1101/gr.174623.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anderson JB, Kohn LM. 1998. Genotyping gene genealogies and genomics bring fungal population genetics above ground. Trends Ecol. Evol. 13, 444–449. ( 10.1016/S0169-5347(98)01462-1) [DOI] [PubMed] [Google Scholar]
- 124.McDonald BA, Linde C. 2002. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 40, 349–379. ( 10.1146/annurev.phyto.40.120501.101443) [DOI] [PubMed] [Google Scholar]
- 125.Ali S, et al. 2016. CLONCASE: estimation of sex frequency and effective population size by clonemate resampling in partially clonal organisms. Mol. Ecol. Resour. 16, 845–861. ( 10.1111/1755-0998.12511) [DOI] [PubMed] [Google Scholar]
- 126.Zhan J, Mundt CC, McDonald BA. 1998. Measuring immigration and sexual reproduction in field populations of Mycosphaerella graminicola. Phytopathology 88, 1330–1337. ( 10.1094/phyto.1998.88.12.1330) [DOI] [PubMed] [Google Scholar]
- 127.Vreeburg S, Nygren K, Aanen DK. 2016. Unholy marriages and eternal triangles: how competition in the mushroom life cycle can lead to genomic conflict. Phil. Trans. R. Soc. B 371, 20150533 ( 10.1098/rstb.2015.0533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Glass NL, Kaneko I. 2003. Fatal attraction: nonself recognition and heterokaryon incompatibility in filamentous fungi. Eukaryot. Cell 2, 1–8. ( 10.1128/EC.2.1.1-8.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Timothy J, Nieuwenhuis B. 2016. Decay of linkage disequilibrium by physical distance between sites in basepairs for 10 fungal species. figshare. ( 10.6084/m9.figshare.3472754.v2) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used to generate figure 2 is available in the online repository figshare under doi:10.6084/m9.figshare.3472754 [129].